Abstract
Semiaphis heraclei Takahashi (Hemiptera: Aphididae) serves as a vital resource for natural enemies from functional plant Cnidium monnieri (L.) Cusson (Apiaceae), playing a crucial role in ecological dynamics. Endosymbionts influence the performance of their hosts. Here, we determined the communities of facultative endosymbionts in aphids from Lonicera japonica Thunb. (Caprifoliaceae), Apium graveolens L. (Apiaceae), and C. monnieri and assessed the performance of four aphid clones. The infection rates of Serratia symbiotica Moran (Gammaproteobacteria: Enterobacteriaceae) and Regiella insecticola Moran (Enterobacteriales: Enterobacteriaceae) reached 100%. Notably, the infection rates of Spiroplasma and Rickettsia varied across host plants. Fitness assessment revealed that aphids performed better on their natal hosts, exhibiting shorter nymphal development times and higher fecundity. S. symbiotica had contrasting effects on aphids based on their origin. It prolonged the development duration and decreased the intrinsic rate of increase (rm), net reproductive rate (R0), and finite rate of increase (λ) in aphids collected from plant A. graveolens. However, for aphids collected from plant C. monnieri, it shortened the doubling time (DT) and improved rm, R0, and λ, while prolonging the mean generation time. Our studies are the first to investigate the infection status and role of facultative endosymbionts in aphid S. heraclei, extending the documented effects of plant diversity to fluctuations in the infection rate, with potentially far-reaching consequences for related endosymbionts’ ecosystem processes.
1. Introduction
Semiaphis heraclei (Takahashi) (Hemiptera: Aphididae), a holocyclic aphid, utilizes Lonicera spp. as its primary host and plants from the Apiaceae family (e.g., Apium graveolens (L.) (Apiaceae), Cnidium monnieri L. Cusson (Apiaceae), Foeniculum Vulgare Mill (Apiaceae), etc.) as secondary hosts. It has been recorded in several provinces in China (e.g., Liaoning, Shaanxi, Hebei, Shandong, Henan, Sichuan, Hubei, Guangxi, etc.) [1,2]. The infestation of S. heraclei inflicts serious harm on its host plants (i.e., it can be harmful to the growth of C. monnieri by depleting plant phloem sap through its piercing–sucking mouthparts), causing leaf shriveling, yellowing, and withering in plants [3]. Additionally, the honeydew excreted by S. heraclei promotes the growth of sooty mold, seriously hindering the photosynthetic ability of the plants [4]; the transmission of plant viruses recruits more herbivores and poses a significant threat to plant health [5,6,7,8]. Aphids prefer virus- or fungal-infected plants, thereby increasing plant damage [6]. The host range of S. heraclei is restricted to the Caprifoliaceae and Apiaceae plants. Consequently, it does not pose a threat to grain crops (e.g., wheat, maize), economic crops (e.g., cotton, pepper), and fruit crops (e.g., apples and pear) [9,10]. Beyond its impacts as a dominant pest for C. monnieri, S. heraclei also plays a vital role in various ecological processes. It serves as a vital food source for numerous predators attracted to C. monnieri, such as ladybugs (e.g., Propylaea japonica Thunberg (Coleoptera: Coccinellidae), Harmonia axyridis Pallas (Coloptera: Coccinellidae), and Hippodamia variegata Goeze (Coloptera: Coccinellidae)), hoverflies (e.g., Episyrphus balteatus De Geer (Diptera: Syrphidae)), and lacewings (e.g., Chrysoperla sinica Tjeder (Neuroptera: Chrysopidae)) [1,11], contributing to the overall diversity and stability of natural ecosystems.
As a potential functional plant, C. monnieri not only attracts predatory natural enemies of insect pests [9] but also serves as a food source and shelter for natural enemies and pollinators, which can migrate into crops and orchards to facilitate plant pollination and enhance biological pest control [12,13,14]. The colonization of natural enemies (e.g., ladybugs, hoverflies, lacewings) of pests on C. monnieri by S. heraclei might influence their biocontrol ability. The population dynamics and corresponding control strategies for aphid S. heraclei on honeysuckle have received increased attention due to their medical value [15,16,17,18]. Currently, studies on the adaptability of aphid S. heraclei to functional plant C. monnieri are still limited.
The host plant quality, including its defensive compounds and physical properties, significantly influences the performance of herbivorous insects. Long-term interaction between aphid feeding habits and host plants involves a range of induced, reciprocal, and responsive mechanisms. Some species of aphids feed on a variety of host plants, but most species of aphids are specialized or even highly specialized to one specific host plant or different individuals of the same host plant species, exhibiting variable fitness levels depending on the host plant species [19]. Aphid fitness, including the developmental time, fecundity, and average lifespan, have been shown to vary among genetically distinct clonal lines in response to plant stress [20], different host plants [21], and feeding experience [22]. Additionally, leaf surface characteristics, nutrient content, and plant age often influence aphid feeding behavior on host plants [23]. Some Serratia genera not only positively influence plant quality but also suppress the plant defense via aphid saliva transmission [24,25]. The interactions of endosymbionts and host plants influence the feeding behavior of aphids [26].
Symbionts have evolved symbiotic relationships with aphids, profoundly influencing their host’s biology, notably impacting their dietary breadth and fitness [27]. Almost all aphids harbor an obligate endosymbiont, Buchnera aphidicola Buchnera (Gammaproteobacteria: Enterobacteriaceae), which provides essential amino acids and enhances the resistance to heat stress [28]. Apart from the obligate endosymbiont, some aphids harbor one or more facultative symbionts. These endosymbionts are not essential for host survival and reproduction and can be inherited both vertically and horizontally [29]. Facultative endosymbionts enhance aphid host performance and fitness under external conditions by altering amino acid requirements, shortening development times, and improving fecundity [30,31,32]. Facultative endosymbiont Serratia symbiotica Moran (Gammaproteobacteria: Enterobacteriaceae) improves the performance of pea aphid Acyrthosiphon pisum Harris (Hemiptera: Aphididae) to withstand unfavorable conditions (e.g., improving heat tolerance, protecting against parasitoids, and mitigating plant defense mechanisms) [33]. However, S. symbiotica does not affect adult body mass or fecundity but increases nymph weights and temporarily boosts B. aphidicola titers; it also inhibits winged-to-wingless morph regression to enhance its spread [34]. When introduced to plants, cultured S. symbiotica colonizes the tissue, mediates horizontal transfer among aphids, and enhances plant growth, most notably in the root [24]. Currently, little research has been conducted on endosymbionts of the aphid S. heraclei.
The aphid S. heraclei, primarily feeding on Apiaceae and Caprifoliaceae plants, adapts with significant performance variation across different host plants and various aphid clones. S. symbiotica also plays an important role in aphid fitness. However, the performance of aphid S. heraclei on C. monnieri and the role of S. symbiotica remain largely unexplored. This study investigated the prevalence of facultative endosymbionts in aphid populations collected from L. japonica, C. monnieri, and A. graveolens through multiplex diagnostic PCR. Four aphid clones were obtained for fitness experiments on original and novel hosts. This study pursued two primary objectives: (1) to systematically characterize the prevalent patterns of facultative endosymbionts in aphid populations of S. heraclei, and (2) to evaluate host plant-dependent fitness trade-offs associated with S. symbiotica. Our findings advance the mechanistic understanding of tripartite symbiosis dynamics in herbivore–microbe–plant systems.
2. Results
2.1. Presence of Facultative Endosymbionts of Semiaphis heraclei
A total of 87 aphid samples were collected from three host plants, namely L. japonica (52), A. graveolens (20), and C. monnieri (15). Four species of facultative endosymbionts, S. symbiotica, Regiella insecticola Moran (Gammaproteobacteria: Enterobacteriaceae), Spiroplasma spp., and Rickettsia spp., were detected in aphid S. heraclei at varying infection rates. Among these, R. insecticola and S. symbiotica were ubiquitous in all aphid populations, irrespective of the host plants on which they were reared. Rickettsia was detected in all aphids, except those on A. graveolens, which showed only a 5% infection rate. Among aphids reared on C. monnieri, the infection rate of Spiroplasma was 100%, whereas those on L. japonica and A. graveolens had much lower rates of 55.77% and 5%, respectively. A chi-squared test revealed significantly higher infection rates of Spiroplasma in aphids feeding on C. monnieri compared to those on L. japonica (χ2 = 67.00, p < 0.001) or A. graveolens (χ2 = 48.26, p < 0.001). Similarly, the infection rate of Rickettsia was also higher on C. monnieri than on A. graveolens (χ2 = 48.26, p < 0.001) (Figure 1).
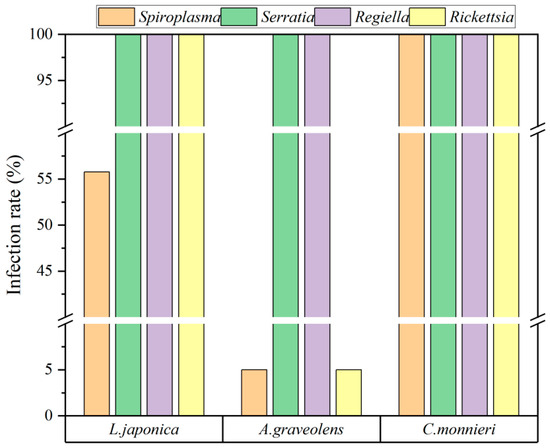
Figure 1.
The presence and infection rates of facultative endosymbionts in aphid S. heraclei on three host plants.
2.2. Developmental Duration of Nymph Stage of S. heraclei on Two Host Plants
We predetermined four aphid clones to confirm the absence or presence of S. symbiotica and R. insecticola by diagnostic PCR before conducting fitness experiments. As shown in Table 1 and Table 2, aphid clones and host plants significantly affected the nymphal development time and three other development parameters (last molt, last molt to reproduction, and start of reproduction), except for the development time of the second and fourth instars, not being related to the host plant. Their interaction significantly influenced the development time of the first to third instars and the time of last molt and start of reproduction.

Table 1.
Effects of aphid clones, host plants, and their interactions on developmental time of S. heraclei.

Table 2.
Effects of aphid clones, host plants, and their interactions on the developmental parameters of S. heraclei.
The developmental durations of the nymph stage in the four aphid clones on C. monnieri and A. graveolens are presented in Figure 2. The nymphal developmental duration varied significantly among aphid clones and nymph instars. Compared to feeding on A. graveolens, Q1 clones on C. monnieri exhibited longer first-instar (t = 8.68, p < 0.001) and second-instar durations (t = 3.83, p < 0.001) but shorter third-instar development (t = 3.98, p < 0.001), while Q2 clones showed accelerated development for the second (t = 2.05, p = 0.043) and third instars (t = 2.25, p = 0.025). Aphid clone S1 developed more slowly on C. monnieri in the first instar (t = 2.78, p = 0.006) and fourth instar (t = 2.20, p = 0.03), but faster in the second instar (t = 4.16, p < 0.001) and third instar (t = 7.67, p < 0.001), compared to that on A. graveolens. Aphid clone S2 took less time only in the second instar (t = 2.57, p = 0.011) on C. monnieri.
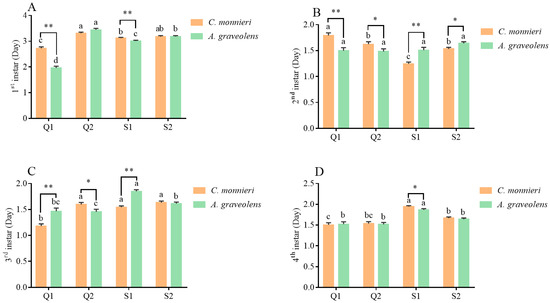
Figure 2.
The development durations of aphid S. heraclei on two host plants at the stages of the first instar (A), second instar (B), third instar (C), and fourth instar (D). Data are represented as the mean ± standard error (SE). Different lowercase letters indicate significant differences in the developmental durations of the four aphid clones on different plants based on Tukey’s HSD test (p < 0.05). Statistical significance (t-test, p < 0.05) between two host plants at the same stage is indicated as follows: * p < 0.05, ** p < 0.01. No marks indicate no significant difference (p > 0.05).
Regarding first-instar development, Q1 aphid clones exhibited the shortest duration on both host plants (C. monnieri: F3, 225 = 33.79, p < 0.001; A. graveolens: F3, 209 = 173.63, p < 0.001), followed sequentially by S1, S2, and Q2. At the second instar on C. monnieri, Q1 showed the longest development (F3, 225 = 32.36, p < 0.001), while S1 had the shortest. At the third instar on C. monnieri, Q1 exhibited the shortest development time among all aphid clones (F3, 225 = 39.21, p < 0.001). On A. graveolens, Q2 developed the fastest in comparison to S1 and S2 (F3, 209 = 19.49, p < 0.001). In contrast, S1 took the longest time to reach the fourth instar on both C. monnieri (F3, 225 = 28.01, p < 0.001) and A. graveolens (F3, 209 = 16.44, p < 0.001).
As shown in Figure 3, whenever reared on C. monnieri and A. graveolens, the aphid clone Q1 exhibited the shortest time to last molt (p < 0.001) and to have offspring (p < 0.01) but the longest time from last molt to reproduction (p < 0.001) compared to the other three aphid clones. Furthermore, when reared on A. graveolens, Q1 developed faster than on C. monnieri (p < 0.001), while the opposite trend was observed for aphid clone S1 (p < 0.001). Aphid clone S2 reared on C. monnieri exhibited a longer time from last molt to reproduction (p < 0.05). S. symbiotica prolonged the development time in aphids collected from A. graveolens. Additionally, aphids developed faster when feeding on their natal host plants.
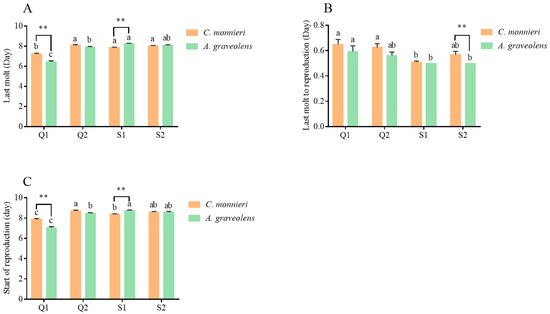
Figure 3.
Development parameters, i.e., last molt (A), last molt to reproduction (B), and start of reproduction (C), of aphid S. heraclei on two host plants. Data are represented as the mean ± standard error (SE). Different lowercase letters indicate significant differences in the three parameters among the four aphid clones based on Tukey’s HSD test (p < 0.05). Statistical significance (t-test, p < 0.05) between two host plants at the same stage is indicated as follows: ** p < 0.01. No marks indicate no significant difference (p > 0.05).
2.3. Performance of Different Aphid Clones on Different Host Plants
The life table parameters, including the intrinsic rate of increase (rm), net reproductive rate (R0), mean generation time (T), doubling time (DT), and finite rate of increase (λ), were significantly affected by the host plant, aphid clone, and their interaction, although the doubling time remained unaffected by the host plant (Table 3). All five parameters varied significantly across aphid clones and host plants (Figure 4). On C. monnieri, rm varied significantly among clones (F3, 16 = 119.44, p < 0.001), ranking as follows: Q2 (0.17) < S1 (0.21) < Q1 (0.26) < S2 (0.29). On A. graveolens, rm also differed significantly (F3, 16 = 28.42, p < 0.001), and that of S1 was lower than that of Q2, S2, and Q1. Moreover, rm was higher on C. monnieri than on A. graveolens for clones S1 and S2 (p < 0.001) but lower for Q2 (p < 0.001). As for the net reproductive rate (R0), on C. monnieri, R0 differed significantly (F3, 16 = 55.99, p < 0.001), ranking as follows: S1 (7.8) < Q2 (8.66) < Q1 (12.36) < S2 (17.2). On A. graveolens, R0 differed significantly (F3, 16 = 44.06, p < 0.001), with that of S1 (6.61) being lower than that of S2 (11.96), Q2 (16.28), and Q1 (17.7). R0 was lower on C. monnieri than on A. graveolens for Q1(t = 7.83, p < 0.001) and Q2 (t = 6.99, p < 0.001), but higher for S2 (t = 4.24, p = 0.003).

Table 3.
The effects of host plants, aphid clones, and their interactions on the performance of aphid S. heraclei following host transfer.
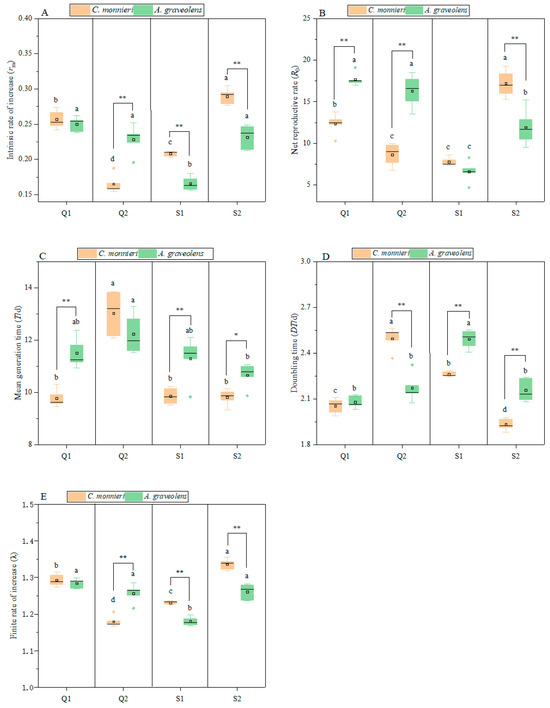
Figure 4.
Life table parameters of four aphid clones on two host plants. Intrinsic rate of increase (A). Net reproductive rate (B). Mean generation time (C). Doubling time (D). Finite rate of increase (E). Data are represented as the mean ± standard error (SE). Different lowercase letters indicate significant differences in the five life table parameters of the four aphid clones on different plants based on Tukey’s HSD test (p < 0.05). * p < 0.05, ** p < 0.01. No marks indicate no significant difference (p > 0.05).
As regards the mean generation time (T), when aphids were reared on C. monnieri, T differed significantly among clones (F3, 16 = 47.96, p < 0.001), with that of Q2 being higher than that of S1, S2, and Q1. On A. graveolens, T differed significantly among clones (F3, 16 = 4.38, p = 0.02), especially between S2 and Q2. T was shorter on C. monnieri than on A. graveolens for S1 (t = 3.43, p = 0.009), S2 (t = 3.31, p = 0.011), and Q1 (t = 5.74, p < 0.001), but not for Q2. On C. monnieri, DT differed significantly (F3, 16 = 116.26, p < 0.001), ranking as follows: S2 < Q1 < S1 < Q2. However, λ showed the opposite (F3, 16 = 117.2, p < 0.001). On A. graveolens, DT differed significantly (F3, 16 = 32.93, p < 0.001), with S1 > Q1, Q2, and S2. λ differed significantly (F3, 16= 27.47, p < 0.001), with S1 < S2, Q1, and Q2. The DT of S1 (t = 7.96, p < 0.001) and S2 (t = 5.82, p < 0.001) reared on C. monnieri was lower than in that reared on A. graveolens, but the opposite was observed for the finite rate of increase (λ) (p < 0.001). Similarly, the DT of Q2 reared on C. monnieri was higher than in that on A. graveolens (t = 5.95, p < 0.001), while λ showed the opposite trend (t = 5.7, p = 0.001).
3. Discussion
3.1. Facultative Endosymbiont Dynamics in Aphid S. heraclei
To our knowledge, this is the first study to investigate the endosymbiont communities and their infection rates in the aphid S. heraclei across three host plants, A. graveolens, C. monnieri, and L. japonica. Interestingly, our results indicated that there were no significant differences in the community structure of facultative endosymbionts among aphids from different host plants.
Notably, four facultative endosymbiont species, S. symbiotica, Spiroplasma spp., Rickettsia spp., and R. insecticola, were detected in aphid S. heraclei. Among these, the infection frequencies of Spiroplasma and Rickettsia varied significantly across host plants, whereas those of S. symbiotica and R. insecticola were host-independent. This contrasts with broader aphid endosymbiont trends [35], where facultative symbiont communities exhibit considerable variation, with S. symbiotica being the most prevalent (47% of 156 aphid species) and Arsenophonus Hertig. (Gammaproteobacteria: Enterobacteriaceae) the least (7% of 131 aphid species). In the present study, S. symbiotica and R. insecticola were ubiquitous in aphid S. heraclei from three host plants (L. japonica, A. graveolens, and C. monnieri), while other facultative symbionts, including Spiroplasma and Rickettsia, exhibited significant variation among different aphid populations. Conversely, cotton aphid A. gossypii exhibits a high prevalence in facultative endosymbionts across China, with six species identified among 34 species of host plants [36]. In particular, frequencies of up to 98% were detected for Arsenophonus in the aphid A. gossypii on Caprifoliaceae, Polygonaceae, and Rosaceae plants [36]. As such, the conserved infection pattern observed here suggests that S. symbiotica and R. insecticola may play particularly stable and essential roles in aphid S. heraclei, possibly reflecting co-adaptive evolution or specific ecological constraints.
The diversity and prevalence of facultative endosymbionts in aphids varied with multiple factors, including the host plant species, geographical location, season, and external temperature. The infection rate of Arsenophonus was higher in aphid A. gossypii feeding on cotton than in aphids on cucumber [31]. Comparative studies revealed a greater diversity of endosymbionts in aphid A. gossypii sampled from Xinjiang than in those from Henan; regional variations are also evident in the infection frequencies, as aphids from Hainan showed higher prevalences of Hamiltonella defensa Moran (Enterobacteriales: Enterobacteriaceae) and S. symbiotica, while those form Xinjiang exhibited a greater incidence of Arsenophonus [37]. Additionally, the facultative endosymbionts in cotton aphid A. gossypii exhibited obvious seasonal dynamics; for example, the Arsenophonus infection rates were lower in summer than in autumn [21]. The infection rates and abundances of endosymbionts in aphids decreased under inappropriate temperatures, with some symbionts being eliminated when exposed to a lower or higher temperature [21,38]. A similar seasonal pattern of Arsenophonus infection has been reported in honeybees, with the prevalence gradually increasing from spring to autumn but being lost during overwintering [39]. In the aphid Adelges tsugae Annand (Hemiptera: Adelgidae), while the overall prevalence of S. symbiotica was higher in Georgia than in New York, no significant differences were observed at finer spatial scales, within individual trees or plots or even across sampling sites within the same state [40]. This suggests that factors other than local selection may shape symbiont distribution. Notably, S. symbiotica has transitioned from a facultative to a co-obligate symbiont in certain aphid lineages [41,42], highlighting its evolving ecological role. Moreover, the infection frequency and distribution of facultative endosymbionts can be influenced by non-selective factors such as the transmission efficiency, host migration, and genetic drift [43]. The generalizability of our findings on the S. heraclei endosymbiont community, particularly the high prevalence of S. symbiotica, is limited by the singular geographical (Jinan) and temporal (April) scope of our sampling. To ascertain whether this pattern is attributable to functional traits, the local climate, or seasonal chance, future work must be expanded to include multi-seasonal and multi-geographic sampling strategies.
3.2. Differentiation of Aphid Host Adaptability
Some characteristics of host plants, including nutrients, secondary metabolites, and morphology, could significantly influence the development and reproduction of herbivorous insects. Previous studies on aphid S. heraclei (originating from L. japonica and Glehniae radix) have demonstrated host-dependent variations in fitness parameters. For example, two populations of S. heraclei exhibited significant differences in the total developmental duration and fecundity when reared on five Apiaceae plants (Angelica dahurica Fisch (Apiaceae), A. graveolens, Bupleurum chinense Franch. (Apiaceae), Glehniae radix (Apiaceae), L. japonica) [15]. Comparable differences were observed across other Apiaceae hosts, including Coriandrum sativum L. (Apiaceae), Daucus carota L. (Apiaceae), and A. graveolens [2]. Consistent with these findings, our study revealed host adaptability differentiation among four populations of S. heraclei reared on two Apiaceae plants (C. monnieri, A. graveolens). Aphids performed better on their natal host plants, exhibiting higher fecundity and shortening nymphal development. Additionally, the presence of S. symbiotica improved the performance of aphids collected from plant C. monnieri. However, it decreased the fecundity of aphids collected from plant A. graveolens only on C. monnieri.
Adaptive differences are likely to be mediated by various plant nutrients and secondary metabolites [21,25,31,44]. The presence of facultative endosymbionts is closely related to the performance of aphids on host plants. For instance, the presence of Aresnophonus improved aphid fitness when feeding on an artificial diet with plant secondary metabolite gossypol, and the population density of Arsenophonus increased as the concentration rose [21]. In addition, plant hormones, such as salicylic acid (SA) and indole acetic acid (IAA), influenced the fitness parameters of aphid S. heraclei when sprayed on host plant C. monnieri [44]. Such aphid–plant interactions might lead to host adaptability differentiation and even specialization for one or more host plant species. This phenomenon has occurred in many species of aphids, including pea aphid A. pisum, A. gossypii, Schizaphis graminum Rondani (Hemiptera: Aphididae), Sitobion avenae Fabricius (Hemiptera: Aphididae), and so on [25,45,46,47]. Commonly, specialized aphid clones exhibit optimal performance on one host plant but perform poorly on alternative hosts. In the present study, the aphids collected from plant C. monnieri exhibited better performance on their natal host C. monnieri than on alternate host A. graveolens and vice versa. Some facultative endosymbionts are related to the host range of aphids, affecting the host’s specialization. Our findings suggest that the role of S. symbiotica in aphid fitness is related to the aphid origin and host plant. Nevertheless, the mechanisms underlying this adaptability differentiation in S. heraclei, and whether host specialization has evolved, require further investigation.
3.3. Multiple Effects of Serratia symbiotica on Aphid Fitness
Infection with facultative endosymbionts confers context-dependent fitness benefits to aphids, varying with the host plant, aphid clone, and symbiont strain. While the predominant literature emphasizes the nutritional and protective roles of S. symbiotica, a notable body of research presents contrasting findings. For instance, studies utilizing cultured S. symbiotica strains have demonstrated that infection reduced development, reproduction, and body weight in the pea aphid A. pisum but increased the susceptibility to certain insecticides [48]. In the case of aphid S. heraclei, our study provides the first investigation of host plant-dependent variations in facultative endosymbiont communities and infection dynamics. Symbionts conferring fitness benefits to their hosts tend to establish persistently high infection rates within host populations, often leading to prevalence [22]. Fitness enhancements, including accelerated development and increased reproductive output, facilitate population expansion. In the present study, an exceptionally high prevalence of S. symbiotica was found in the aphid S. heraclei across aphid populations on diverse host plants. Critically, an increase in the fecundity of the aphid harboring S. symbiotica, together with the acceleration of nymphs, facilitated the population’s expansion, aligning with findings in other aphids [49,50,51] and demonstrating its significant role in plant–insect interactions. Meanwhile, some studies report negative fitness effects for facultative endosymbionts (e.g., H. defensa and R. insecticola) depending on the host/symbiont strains, host plants, and environment [52], or neutral effects of S. symbiotica in the pea aphid A. pisum [34,52]. Our findings suggest that S. symbiotica has broad potential to provide fitness benefits to a wide range of aphid populations due to the widespread context-dependent advantages. S. symbiotica enhances aphid growth by increasing fatty acid synthesis and triacylglycerol storage [50] or by providing essential amino acids [48], as demonstrated in Cinara cedri Mimeur (Hemiptera: Aphididae). Additionally, it can metabolize diverse carbon sources from its host [48]. S. symbiotica enhances host plant feeding by manipulating salivary gland gene expression to inhibit plant defenses [53]. Further investigation is required to elucidate the precise roles of S. symbiotica in aphids and its underlying mechanisms. In particular, the mechanisms through which S. symbiotica modulates the fitness of S. heraclei warrant further investigation.
3.4. Co-Adaptation in Aphid–Plant Associations Mediated by Serratia symbiotica
Strong correlative associations exist between specific facultative endosymbionts and aphid populations adapted to host plants [54]. Our study provides further evidence for this view, demonstrating that the association with S. symbiotica in S. heraclei is not fixed but is a context-dependent relationship influenced by the aphid origin and host plant. This suggests that the functional significance of S. symbiotica is shaped by a complex interplay among the host genotype and ecological factors.
The context-dependent effect appears to be general for facultative endosymbionts. The presence of Arsenophonus alters the aphid’s requirement for amino acids, leading to lower infection rates in aphids feeding on cucumber, with high levels of amino acids [31]. Moreover, Arsenophonus enhances aphid fitness on diets containing the plant secondary metabolite gossypol, with its population density increasing in line with the gossypol concentration [21]. Furthermore, the ability of Arsenophonus to modulate the dietary breadth of A. gossypii by enhancing nutrient synthesis in the obligate symbiont B. aphidicola improved host specialization on cotton [55].
Fitness assessment revealed that S. symbiotica infection did not contribute to the performance of aphids collected from plant A. graveolens on C. monnieri. Conversely, infection with S. symbiotica enhanced the performance of aphids collected from plant C. monnieri on both host plants. These host-specific effects align with previous findings [56]. We speculate that the role of S. symbiotica is not universal but is related to the aphid origin and the quality of host plants. Therefore, the potential influences of aphids’ genetic background and host plant identity on facultative endosymbiont-mediated fitness outcomes cannot be overlooked. This view is supported by early studies employing limited strain diversity, which revealed that S. symbiotica reduced fecundity in only one of three pea aphid clones tested on Lathyrus odoratus L. (Fabales: Fabaceae) and Medicago polymorpha L. (Fabales: Fabaceae) [57], highlighting context-dependent outcomes.
Our findings, which demonstrate a clear contrast in the roles of S. symbiotica between aphids of different origins, reinforce a co-evolutionary arms race. The superior performance of aphids collected from plant C. monnieri when infected with S. symbiotica suggests a co-adapted relationship in their native ecological context. Future work is needed to dissect the molecular and physiological mechanisms underpinning this tripartite interaction.
4. Materials and Methods
4.1. Aphid Samples for Identification of Facultative Symbionts
Aphids were sampled at two fields in Shandong, China: one was at the C. monnieri field at the Experimental and Demonstration Base of Shandong Academy of Agricultural Sciences in Jiyang (117°1′27″ E, 36°56′40″ N); the other was at the experimental base of L. japonica and A. graveolens in Jinan (117°5′12.350″ E, 36°45′22.172″ N). One aphid was sampled every 5 m in one transect, with 10 m between transects. Sampled aphids, whose information is listed in Table 4, were placed in 1.5 mL centrifuge tubes containing 1.0 mL absolute alcohol and then stored at −20 ℃ until DNA extraction. As species-specific primers were shown to be very reliable in detecting the symbiont species in an aphid [58], the symbiont pattern statuses of all aphid strains were periodically characterized by using a multiplex diagnostic PCR method.

Table 4.
Sampling information of aphid S. heraclei.
4.2. Aphid DNA Extraction and Facultative Endosymbiont Detection
A total of 87 aphid samples from C. monnieri, L. japonica, and A. graveolens were used for the identification of facultative endosymbionts. Total aphid genomic DNA was extracted following the methods found in Chang et al. (2022) [21], with minor modification. One aphid was washed with aseptic double-distilled water and ground at 30 Hz for 2 min with two 2 mm zirconia beads using a tissue lyser (TissueLyser II, Retsch GmbH, Hann, Germany) with 50 μL TE buffer (pH = 8.0, Sangon Biotech, Shanghai, China) in an aseptic centrifuge tube. We added 2 μL 20 mg/mL proteinase K (Vazyme, Nanjing, China) and centrifuged the samples at 6000 rpm for 1 min at room temperature using a centrifuge (Eppendorf 5425R, Hamburg, Germany). The homogenate was subsequently incubated at 37 °C for 30 min using an electro-thermostatic water bath (DK-8D-3J, Guangdong, China), followed by 95 °C for 5 min.
All DNA samples were screened for the nine known secondary symbionts using the specific PCR primers listed in Table 5. The 15 μL PCR reaction system contained 7.5 μL 2 × Rapid Taq Master Mix (Vazyme, Nanjing, China), 4 μL ddH2O, 1 μL forward/reverse primer (Sangon Biotech, Shanghai, China), and 1.5 μL sample DNA. The PCR reaction procedures were performed at 94 ℃ for 5 min followed by 35 cycles of 94 ℃ for 15 s, 72 ℃ for 30 s, and 72 ℃ for 10 min for the final extension, being finally held at 4 ℃. Finally, the amplification products were detected by 2% agarose gel electrophoresis and stained with GeneRed nucleic acid dye (RT211, Tiangen, Beijing, China) using the DL 2000 DNA marker (3427A, Takara, Dalian, China). The detection of target amplification products indicated aphid infection with the target facultative symbiont. Conversely, the absence of these specific products confirmed that the aphids were uninfected.

Table 5.
Sequences of specific primers for nine facultative endosymbionts.
Table 5.
Sequences of specific primers for nine facultative endosymbionts.
| Facultative Symbiont | Primer | Sequence 5′-3′ | Tm/°C | Product Size/bp | Reference |
|---|---|---|---|---|---|
| Arsenophonus | fbaAF | GCYGCYAAAGTTCRTTCTCC | 52 | 617 | [59] |
| fbaAR | GGCAAATTAAATTTCTGCGCAACG | [60] | |||
| Wolbachia | wspF | GGGTCCAATAAGTGATGAAGAAAC | 56 | 570 | [61] |
| wspR | TTAAAACGCTACTCCAGCTTCTGC | ||||
| Spiroplasma spp. | 16SA1 | AGAGTTTGATCMTGGCTCAG | 55 | 510 | [62] |
| TKSSsp | TAGCCGTGGCTTTCTGGTAA | [63] | |||
| S. symbiotica | 10F | AGTTTGATCATGGCTCAGATTG | 55 | 480 | [64] |
| R443R | CTTCTGCGAGTAACGTCAATG | [65] | |||
| Rickettsia spp. | 16SA1 | AGAGTTTGATCMTGGCTCAG | 55 | 200 | [62] |
| 16SR | CATCCATCAGCGATAAATCTTTC | [66] | |||
| H. defensa | 10F | AGTTTGATCATGGCTCAGATTG | 50 | 471 | [64] |
| T419R | AAATGGTATTSGCATTTATCG | [65] | |||
| Rickettsiella spp. | P136F | GGGCCTTGCGCTCTAGGT | 55 | 300 | [67] |
| P136R | TGGGTACCGTCACAGTAATCGA | ||||
| R. insecticola | 10F | AGTTTGATCATGGCTCAGATTG | 55 | 200 | [64] |
| U433R | GGTAACGTCAATCGATAAGCA | [65] | |||
| PAXS | PAXSF PAXSR | AGTTTGATCATGGCTCAGATTG | 55 | 500 | [68] |
| GCAACACTCTTTGCATTGCT |
4.3. Aphid Clones and Rearing
The aphid S. heraclei colony was obtained from a laboratory culture reared from the progeny of a single apterous aphid. It was collected from C. monnieri and A. graveolens in the field through the identification of symbionts by PCR; the sampling locations were as described in Section 4.1. Two aphid clones (S1, S2) collected from plant C. monnieri and two clones (Q1, Q2) collected from plant A. graveolens were continuously maintained for more than 20 generations on Petri dishes containing their respective original host plant leaves on 1% agar (Table 6). Aphids were maintained under standardized conditions (25 ± 1 °C, 75% ± 5% RH, 14L:10D photoperiod) [2,69], with fresh leaves replaced triweekly to ensure optimal conditions.

Table 6.
Sampling information and infected facultative endosymbionts of aphid clones.
4.4. Aphid Performance Measurement
The experimental design for fitness assays is summarized in Figure 5. To determine the effects of facultative endosymbiont S. symbiotica on individual performance, a uniform-sized 7-day-old wingless aphid was transferred onto a detached leaf of A. graveolens or C. monnieri on 1% agar in a 30 mm Petri dish. After two hours (post-reproduction onset), all but one newborn nymph were moved to standardize the starting point. Molting processes were recorded at 12 h intervals. Nymphs were tracked using exuviae removed from each Petri dish. For each aphid clone, a minimum of 50 nymphs were tracked to measure their development times and developmental parameters from birth to reproduction. To maintain optimal conditions, the fresh leaves were also replaced every three days.
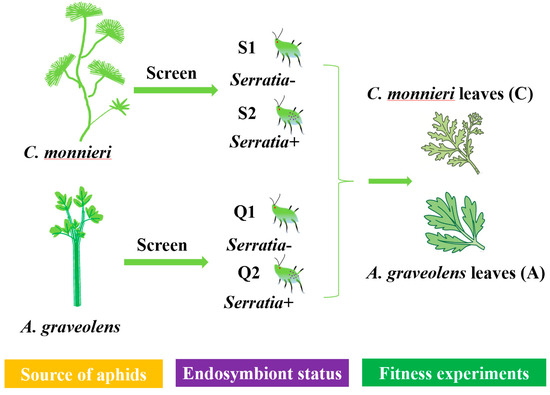
Figure 5.
Experimental design for the performance of S. heraclei on two Apiaceae plants. Note: Two aphid clones (S1—Serratia-, S2—Serratia+) and two aphid clones (Q1—Serratia-, Q2—Serratia+) were, respectively, screened from C. monnieri and A. graveolens through multiplex diagnostic PCR. Then, these four aphid clones were transferred onto the leaves of C. monnieri and A. graveolens for fitness assessment, as described in the main text.
Aphid fitness indices, including the net reproductive rate (R0), mean generation time (T), intrinsic rate of increase (rm), doubling time (DT), and finite rate of increase (λ), were measured by establishing a time-specific life table. Ten 8-day-old adult aphids with a uniform size were transferred onto the leaves of A. graveolens and C. monnieri in 60 mm Petri dishes containing 1% agar. After 6 h, ten newly born aphids were left in the Petri dishes and served as the initial aphid population. Daily monitoring tracked aphid survival and reproduction until all 10 initial aphids died, with newborns counted and removed upon emergence. Five replicates per aphid clone were conducted, with Petri dishes and excised leaves replaced every three days. Aphid fitness indices were calculated following Liu (2016) [70]. The formulae are as follows, where x represents the time; represents the survival rate on a particular day x; and represents the number of offspring produced by each aphid:
4.5. Statistical Analysis
Chi-squared (χ2) tests were used to assess the differences in facultative endosymbiont infection frequencies across different hosts. Two-way ANOVAs were also used to analyze the effects of aphid clones, host plants, and their interactions on the developmental parameters and life table parameters of aphid S. heraclei following host transfer. In addition, the differences in the developmental times of each nymph instar, and the developmental parameters and life table parameters of the aphids among the four aphid clones, were analyzed using one-way analysis of variance (ANOVA) (by using the HSD test at α = 0.05) and an independent T test at α = 0.05. All data were processed in SPSS v.26.0.0 (IBM, New York, NY, USA) and all figures were plotted with Origin 2024 (OriginLab Corporation, Northampton, MA, USA) or Graphpad Prism 9.5.1 (GraphPad Software, San Diego, CA, USA).
5. Conclusions
Our study provides the first investigation of facultative endosymbiont dynamics in aphid S. heraclei across different host plants, with a specific focus on the role of S. symbiotica. Serratia symbiotica was highly prevalent in aphid S. heraclei, suggesting the strong host plant-mediated regulation of this symbiosis. Infection with S. symbiotica was associated with significant fitness advantages for aphids collected from C. monnieri on their native hosts, but with reduced performance in aphids collected from A. graveolens feeding on C. monnieri. The consistent outperformance of aphids on their natal host plants, together with the mediating role of S. symbiotica, suggests that host plant specialization is mediated by co-adaptation between S. symbiotica and the host plant. Furthermore, the superior performance of aphids on their respective natal hosts across all groups points to the evolutionary significance of host–endosymbiont co-adaptation.
Author Contributions
Conceptualization, C.C., Z.L. and F.G.; methodology, C.C.; analysis, C.C.; investigation, C.C., Y.H., K.Y., X.J. and X.Z.; writing—original draft preparation, C.C.; writing—reviewing and editing, C.C.; supervision, Z.L.; project administration, Z.L. and F.G.; funding acquisition, Z.L. and F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Introducing Top Talent Program of Shandong (Grant No. 2023YSYY-006), National Key R&D Program of China (Grant No. 2023YFD1400800), National Natural Science Foundation of China (Grant No. 32202405), State Key Laboratory of Integrated Management of Pest Insects and Rodents (Grant No. IPM2001), and Agricultural Scientific and Technological Innovation Project of Shandong Academy of Agricultural Sciences (Grant No. CXGC2025F05).
Data Availability Statement
All data are contained within the article. The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
Thanks to Luna Grey (University of Otago) for the invaluable grammatical corrections.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, X.B.; Han, S.P.; Liang, C.; Han, H.; Liu, C.X.; He, Y.Z. Functional response of Harmonia axyridis (Pallas) adults to Semiaphis heraclei (Takahashi). China Plant Prot. 2019, 39, 61–63. [Google Scholar] [CrossRef]
- Wang, J.X.; Li, X.J.; Wang, N. Influence of temperature on the development, survival and reproduction of Semiaphis heraclei (Takahashi). Chin. J. Appl. Entomol. 2016, 53, 564–573. [Google Scholar] [CrossRef]
- Csorba, A.B.; Dinescu, S.; Pircalabioru, G.G.; Fora, C.G.; Bálint, J.; Loxdale, H.D.; Balog, A. Aphid adaptation in a changing environment through their bacterial endosymbionts: An overview, including a new major cereal pest (Rhopalosiphum maidis (Fitch) scenario. Symbiosis 2024, 93, 139–152. [Google Scholar] [CrossRef]
- Insausti, P.; Ploschuk, E.L.; Izaguirre, M.M.; Podworny, M. The effect of sunlight interception by sooty mold on chlorophyll content and photosynthesis in orange leaves (Citrus sinensis L.). Eur. J. Plant Pathol. 2015, 143, 559–565. [Google Scholar] [CrossRef]
- Fingu-Mabola, J.C.; Martin, C.; Bawin, T.; Verheggen, F.J.; Francis, F. Does the infections status of aphids influence preference towards healthy, virus-infected and endophytically colonized plants? Insects 2020, 11, 435. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Y.N.; Yang, Y.; Zhang, Y.H.; Li, Y.Y.; Tian, H.G.; Liu, T.X.; Li, Z.F. Plants affect the horizontal transmission of a new densovirus infecting the green peach aphid Myzus persicae by modulating honeydew production. Insect Sci. 2024, 31, 235–254. [Google Scholar] [CrossRef]
- Jayasinghe, W.H.; Akhter, M.S.; Nakahara, K.; Maruthi, M.N. Effect of aphid biology and morphology on plant virus transmission. Pest Manag. Sci. 2021, 78, 416–427. [Google Scholar] [CrossRef]
- Shi, X.; Gao, Y.; Yan, S.; Tang, X.; Zhou, X.G.; Zhang, D.Y.; Liu, Y. Aphid performance changes with plant defense mediated by cucumber mosaic virus titer. Virol. J. 2016, 13, 70. [Google Scholar] [CrossRef]
- Yang, Q.F.; Ouyang, F.; Men, X.Y.; Ge, F. Discovery and utilization of a functional plant, rich in the natural enemies of insect pests, in northern China. Chin. J. Appl. Entomol. 2018, 55, 942–947. [Google Scholar] [CrossRef]
- Han, G.D.; Zhang, X.R.; Cai, Z.P.; Xiao, Y.L.; Ge, F. Flower strips enhance the abundance and biocontrol services of predatory arthropods in a pear orchard. Biol. Control 2025, 200, 105680. [Google Scholar] [CrossRef]
- Cai, Z.P.; Zhang, X.R.; Xiao, Y.L.; Zhang, J.P.; Ge, F. Functional response and predation preference of multicolored Asian lady beetle Harmonia axyridis to two aphids in the micro-landscape of apple and Monnier’s snowparsley Cnidium monnieri. J. Plant Prot. 2024, 51, 96–105. [Google Scholar] [CrossRef]
- Cai, Z.P.; Ouyang, F.; Chen, J.; Yang, Q.F.; Desneux, N.; Xiao, Y.L.; Zhang, J.P.; Ge, F. Biological control of Aphis spiraecola in apples using an insectary plant that attracts and sustains predators. Biol. Control. 2021, 155, 104532. [Google Scholar] [CrossRef]
- Liang, X.Y.; Ouyang, F.; Zhang, X.R.; Sun, Y.C.; Li, Z.; Ge, F. Increasing the proportion of flower strip area in farmland promotes natural enemies to enhance aphid biocontrol and wheat yield. Entomol. Gen. 2024, 44, 1183–1192. [Google Scholar] [CrossRef]
- Zhang, X.R.; Ouyang, F.; Su, J.W.; Li, Z.; Yan, Y.Y.; Sun, Y.C.; Sarkar, S.C.; Xiao, Y.L.; Ge, F. Intercropping flowering plants facilitate conservation, movement and biocontrol performance of predators in insecticide-free apple orchard. Agric. Ecosyst. Environ. 2022, 340, 108157. [Google Scholar] [CrossRef]
- Li, J.P.; An, B.Y.; Lan, Y.Y.; Qin, Q.J.; Wang, D.; He, Y.Z. Comparison of fitness and selectivity behavior of Semiaphis heraclei on different plants. Plant Prot. 2024, 50, 1–9. [Google Scholar] [CrossRef]
- Li, M.X.; Ji, B.R.; Liu, S.; Qiao, H.L.; Wei, H.S.; Guo, K.; Xu, C.Q. Population dynamics and control techniques of aphids on honeysuckle. Mod. Chin. Med. 2024, 26, 830–838. [Google Scholar] [CrossRef]
- Sun, Y.; Xue, M.; Zhang, X.; Zhao, H.P.; Li, Z.X. Population dynamics and control techniques of aphids on honeysuckle. China J. Chin. Mater. Medica 2013, 38, 3676–3680. [Google Scholar] [CrossRef]
- Zhang, X.M.; Xi, Y.M.; Wang, S.; Luo, C.; Zhang, F. Assessment of potential control of Semiaphis heraclei by Harmonia axyridis. Chin. J. Biol. Control. 2015, 31, 317–321. [Google Scholar] [CrossRef]
- Schillewaert, S.; Vantaux, A.; Van den Ende, W.; Wenseleers, T. The effect of host plants on genotype variability in fitness and honeydew composition of Aphis fabae. Insect Sci. 2017, 24, 781–788. [Google Scholar] [CrossRef]
- Liu, D.G.; Dai, P.; Li, S.R.; Ahmed, S.S.; Shang, Z.M.; Shi, X. Life-history responses of insects to water-deficit stress: A case study with the aphid Sitobion avenae. BMC Ecol. 2018, 18, 17. [Google Scholar] [CrossRef]
- Chang, C.Y.; Sun, X.W.; Tian, P.P.; Miao, N.H.; Zhang, Y.L.; Liu, X.D. Plant secondary metabolite and temperature determine the prevalence of Arsenophonus endosymbionts in aphid populations. Environ. Microbiol. 2022, 24, 3764–3776. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, M.Y.; Chang, C.Y.; Chen, F.F.; Hu, Y.; Liu, X.D. The host range of Aphis gossypii is dependent on aphid genetic background and feeding experience. PeerJ 2019, 7, e7774. [Google Scholar] [CrossRef] [PubMed]
- Cardona, J.B.; Grover, S.; Bowman, M.J.; Busta, L.; Kundu, P.; Koch, K.G.; Sarath, G.; Sattler, S.E.; Louis, J. Sugars and culticular waxes impact sugarcane aphid (Melanaphis sacchari) colonization on different developmental stages of sorghum. Plant Sci. 2023, 330, 111646. [Google Scholar] [CrossRef] [PubMed]
- Pons, I.; Renoz, F.; Noël, C.; Hance, T. Circulation of the cultivable symbiont Serratia symbiotica in aphids is mediated by plants. Front. Microbiol. 2019, 10, 764. [Google Scholar] [CrossRef]
- Wang, D.; Zhai, Y.T.; Liu, D.G.; Zhang, N.; Li, C.B.; Shi, X.Q. Identification and genetic differentiation of Sitobion avenae (Hemiptera: Aphididae) biotypes in China. J. Econ. Entomol. 2020, 113, 407–417. [Google Scholar] [CrossRef]
- Leybourne, D.J.; Valentine, T.A.; Bos, J.I.B.; Karley, A.J. Fitness cost resulting from Hamiltonella defensa infection is associated with altered probing and feeding behaviour in Rhopalosiphum padi. J. Exp. Biol. 2020, 223, jeb207936. [Google Scholar] [CrossRef]
- Sochard, C.; Dupont, C.; Simon, J.C.; Outreman, Y. Secondary symbionts affect foraging capacities of plant-specialized genotypes of the pea aphid. Microb. Ecol. 2021, 82, 1009–1019. [Google Scholar] [CrossRef]
- Zhang, B.; Leonard, S.P.; Li, Y.; Moran, N.A. Obligate bacterial endosymbionts limit thermal tolerance of insect host species. Proc. Natl. Acad. Sci. USA 2019, 116, 24712–24718. [Google Scholar] [CrossRef]
- Oliver, K.M.; Degnan, P.H.; Burke, G.R.; Moran, N.A. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu. Rev. Entomol. 2010, 55, 247–266. [Google Scholar] [CrossRef]
- Ayoubi, A.; Talebi, A.A.; Fathipour, Y.; Mehrabadi, M. Coinfection of the secondary symbionts, Hamiltonella defensa and Arsenophonus sp. contribute to the performance of the major aphid pest, Aphis gossypii (Hemiptera: Aphididae). Insect Sci. 2020, 27, 86–98. [Google Scholar] [CrossRef]
- Tian, P.P.; Chang, C.Y.; Miao, N.H.; Li, M.Y.; Liu, X.D. Infections with Arsenophonus facultative endosymbionts alter performance of aphids (Aphis gossypii) on an amino-acid-deficient diet. Appl. Environ. Microbiol. 2019, 85, e01407-19. [Google Scholar] [CrossRef]
- Hopper, K.R.; Kuhn, K.L.; Lanier, K.; Rhoades, J.H.; Oliver, K.M.; White, J.A.; Asplen, M.K.; Heimpel, G.E. The defensive aphid symbiont Hamiltonella defensa affects host quality differently for Aphelinus glycinis versus Aphelinus atriplicis. Biol. Control 2018, 116, 3–9. [Google Scholar] [CrossRef]
- Burke, G.; Fiehn, O.; Moran, N. Effects of facultative symbionts and heat stress on the metabolome of pea aphids. ISME J. 2010, 4, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.W.; Zhang, M.; Cao, H.H.; Guo, S.S.; Liu, F.H.; Liu, T.X. Facultative endosymbiont Serratia symbiotica inhibits the apterization of pea aphid to enhance its spread. Microbiol. Spectr. 2022, 10, e04066-22. [Google Scholar] [CrossRef] [PubMed]
- Zytynska, S.E.; Weisser, W.W. The natural occurrence of secondary bacterial symbionts in aphids. Ecol. Entomol. 2016, 43, 13–25. [Google Scholar] [CrossRef]
- Xu, S.F.; Chen, J.; Qin, M.; Jiang, L.Y.; Qiao, G.X. Geography-dependent symbiont communities in two oligophagous aphid species. FEMS Microbiol. Ecol. 2021, 97, fiab132. [Google Scholar] [CrossRef]
- Zhang, S.; Su, H.H.; Jiang, W.L.; Hu, D.W.; Ali, I.; Jin, T.X.; Yang, Y.Z.; Ma, X.Y. Symbiotic microbial studies in diverse populations of Aphis gossypii, existing on altered host plants in different localities during different times. Ecol. Evol. 2021, 11, 13948–13960. [Google Scholar] [CrossRef]
- Corbin, C.; Heyworth, E.R.; Ferrari, J.; Hurst, G.D.D. Heritable symbionts in a world of varying temperature. Heredity 2017, 118, 10–20. [Google Scholar] [CrossRef]
- Drew, G.C.; Budge, G.E.; Frost, C.L.; Neumann, P.; Siozios, S.; Yanez, O.; Hurst, G.D.D. Transitions in symbiosis: Evidence for environmental acquisition and social transmission within a clade of heritable symbionts. ISME J. 2021, 15, 2956–2968. [Google Scholar] [CrossRef]
- Mech, A.M.; Harper, S.J.; Havill, N.P.; von Dohlen, C.D.; Burke, G.R. Ecological factors influencing the beneficial endosymbionts of the hemlock woolly adelgid (Hemiptera: Adelgidae). Insect Sci. 2017, 26, 97–107. [Google Scholar] [CrossRef]
- Martínez-Díaz, V.; Latorre, A.; Gil, R. Reinventing the wheel and making it round again: Evolutionary convergence in Buchnera-Serratia symbiotic consortia between the distantly related Lachinae aphids Tuberolachnus salignus and Cinara cedri. Biol. Evol. 2016, 8, 1440–1458. [Google Scholar] [CrossRef]
- Ponce-de-leon, M.; Tamarit, D.; Calle-Espinosa, J.; Mori, M.; Latorre, A.; Motero, F.; Pereto, J. Determinism and contingency shape metabolic complementation in an endosymbiotic construction. Front. Micrbiology 2017, 8, 2290. [Google Scholar] [CrossRef]
- Oliver, K.M.; Smith, A.H.; Russell, J.A. Defensive symbiosis in the real world-advancing ecological studies of heritable, protective bacteria in aphids and beyond. Funct. Ecol. 2014, 28, 341–355. [Google Scholar] [CrossRef]
- Jiang, X.S.; Zhang, X.R.; Han, G.D.; Sarkar, S.C.; Ge, F. Natural Enemies Acquire More Prey Aphids from Hormone-Treated Insect-Attracting Plants. Plants 2025, 14, 147. [Google Scholar] [CrossRef]
- Liu, X.D.; Xu, T.T.; Lei, H.X. Refuges and host shift pathways of host-specialized aphids Aphis gossypii. Sci. Rep. 2017, 7, 2008. [Google Scholar] [CrossRef]
- Nouhaud, P.; Peccoud, J.; Mahéo, F.; Mieuzet, L.; Jaquiéry, J.; Simon, J.C. Genomic regions repeatedly involved in divergence among plant-specialized pea aphid biotypes. J. Evol. Biol. 2014, 27, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.Q.; Perumal, A.; Burd, J.D.; Rudd, J.C. Biotypic diversity in greenbug (Hemiptera: Aphididae): Microsatellite-based regional divergence and host-adapted differentiation. J. Econ. Entomol. 2010, 103, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Skaljac, M.; Kirfel, P.; Grotmann, J.; Vilcinskas, A. Fitness costs of infection with Serratia symbiotica are associated with greater susceptibility to insecticides in the pea aphid Acyrthosiphon pisum. Pest Manag. Sci. 2018, 74, 1829–1836. [Google Scholar] [CrossRef]
- Lamelas, A.; Gosalbes, M.J.; Manzano-Marin, A.; Pereto, J.; Moya, A.; Latorre, A. Serratia symbiotica from the aphid Cinara cedri: A missing link from facultative to obligate insect endosymbiont. PLoS Genet. 2011, 7, e1002357. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Zhang, Y.D.; Gong, H.Y.; Lv, Z.Q. Effects of the symbiont Serratia symbiotica on the development and reproduction of the pea aphid, Acyrthosiphon pisum (Hemiptera: Aphididae). Acta Entomol. Sin. 2023, 66, 1311–1318. [Google Scholar] [CrossRef]
- Zhou, X.F.; Ling, X.Y.; Guo, H.J.; Zhu-Salzman, K.; Ge, F.; Sun, Y.C. Serratia symbiotica enhances fatty acid metabolism of pea aphid to promote host development. Int. J. Mol. Sci. 2021, 22, 5951. [Google Scholar] [CrossRef] [PubMed]
- Laughton, A.M.; Fan, M.H.; Gerardo, N.M. The combined effects of bacterial symbionts and aging on life history traits in the pea aphid, Acyrthosiphon pisum. Appl. Environ. Microbiol. 2014, 80, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Y.; Yuan, E.L.; Ling, X.Y.; Zhu-Salzman, K.; Guo, H.J.; Ge, F.; Sun, Y.C. An aphid facultative symbiont suppresses plant defence by manipulating aphid gene expression in salivary glands. Plant Cell Environ. 2020, 43, 2311–2322. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, J.; Scarborough, C.L.; Godfray, H.C.J. Genetic variation in the effect of a facultative symbiont on host-plant use by pea aphids. Oecologia 2007, 153, 323–329. [Google Scholar] [CrossRef]
- Chang, C.Y.; Zhao, Y.N.; Guo, H.F.; Liu, X.D. Food nutrition and facultative endosymbiont modulate dietary breadth of a polyphagous aphid. Insect Sci. 2025, 1–14. [Google Scholar] [CrossRef]
- Mclean, A.H.C.; van Asch, M.; Ferrari, J.; Godfray, H.C.J. Effects of bacterial secondary symbionts on host plant use in pea aphids. Proc. R. Soc. B: Biol. Sci. 2011, 278, 760–766. [Google Scholar] [CrossRef]
- Chen, D.Q.; Montllor, C.B.; Purcell, A.H. Fitness effects of two facultative endosymbiotic bacteria on the pea aphid, Acyrthosiphon pisum, and the blue alfalfa aphid, A. kondoi. Entomol. Exp. Appl. 2000, 95, 315–323. [Google Scholar] [CrossRef]
- Henry, L.M.; Maiden, M.C.; Ferrari, J.; Godfray, H.C. Insect life history and the evolution of bacterial mutualism. Ecol. Lett. 2015, 18, 516–525. [Google Scholar] [CrossRef]
- Duron, Q.; Wilkes, T.E.; Hurst, G.D.D. Interspecific transmission of a male-killing bacterium on an ecological timescale. Ecol. Lett. 2010, 13, 1139–1148. [Google Scholar] [CrossRef]
- Jousselin, E.; d’Acier, A.C.; Vanlerberghe-Masutti, F.; Duron, O. Evolution and diversity of Arsenophonus endosymbionts in aphids. Mol. Ecol. 2013, 22, 260–270. [Google Scholar] [CrossRef]
- Kondo, N.; Nikoh, N.; Ijichi, N.; Fukatsu, T. Genome fragment of Wolbachia endosymbiont transferred to X chromosome of host insect. Proc. Natl. Acad. Sci. USA 2003, 99, 14280–14285. [Google Scholar] [CrossRef] [PubMed]
- Fukatsu, T.; Nikoh, N. Two intracellular symbiotic bacteria from the mulberry psyllid Anomoneura mori (Insecta, Homoptera). Appl. Environ. Microbiol. 1998, 64, 3599–3606. [Google Scholar] [CrossRef] [PubMed]
- Fukatsu, T.; Nikoh, N. Endosymbiotic microbiota of the bamboo pseudococcid Antonina crawii (Insecta, Homoptera). Appl. Environ. Microbiol. 2000, 66, 643–650. [Google Scholar] [CrossRef]
- Sandström, J.P.; Russell, J.A.; White, J.P.; Moran, N.A. Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol. Ecol. 2001, 10, 217–228. [Google Scholar] [CrossRef]
- Ferrari, J.; West, J.A.; Via, S.H.; Charles, J.; Godfray, H.C.J. Population genetic structure and secondary symbionts in host-associated populations of the pea aphid complex. Evolution 2012, 66, 375–390. [Google Scholar] [CrossRef]
- Fukatsu, T.; Tsuchida, T.; Nikoh, N.; Koga, R. Spiroplasma symbiont of the pea aphid, Acyrthosiphon pisum (Insecta: Homoptera). Appl. Environ. Microbiol. 2001, 67, 1284–1291. [Google Scholar] [CrossRef]
- Tsuchida, T.; Koga, R.; Matsumoto, S.; Fukatsu, T. Interspecific symbiont transfection confers a novel ecological trait to the recipient insect. Biol. Lett. 2011, 7, 245–248. [Google Scholar] [CrossRef]
- Guay, J.F.; Boudreault, S.; Michaud, D.; Cloutier, C. Impact of environmental stress on aphid clonal resistance to parasitoids: Role of Hamiltonella defensa bacterial symbiosis in association with a new facultative symbiont of the pea aphid. J. Insect Physiol. 2009, 15, 919–926. [Google Scholar] [CrossRef]
- Wang, J.X.; Li, X.J. Study on life table of population of Semiaphis heraclei (Takahashi) at different temperatures. Liaoning Agric. Sci. 2016, 1–5. [Google Scholar] [CrossRef]
- Liu, X.D. Insect Ecology and Forecast; China Agriculture Press: Beijing, China, 2016; pp. 94–96. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).