Driving Electron Transfer in Photosystem I Using Far-Red Light: Overall Perspectives
Abstract
1. Introduction
1.1. Oxygenic Photosynthesis
1.2. Far-Red Light Photosynthesis
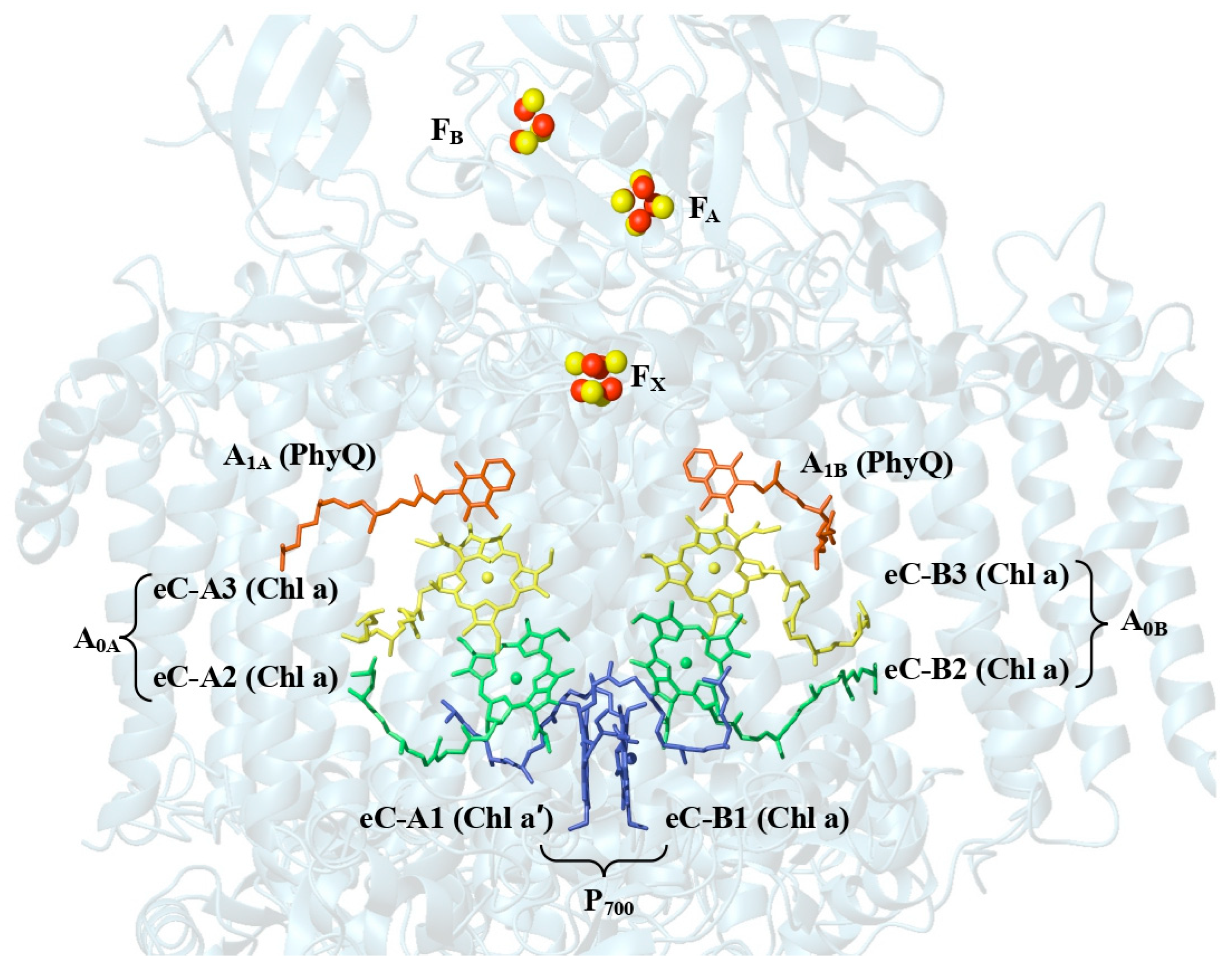
1.3. Structure and Electron Energy Transfer in Far-Red-Light-Adapted Photosystem I
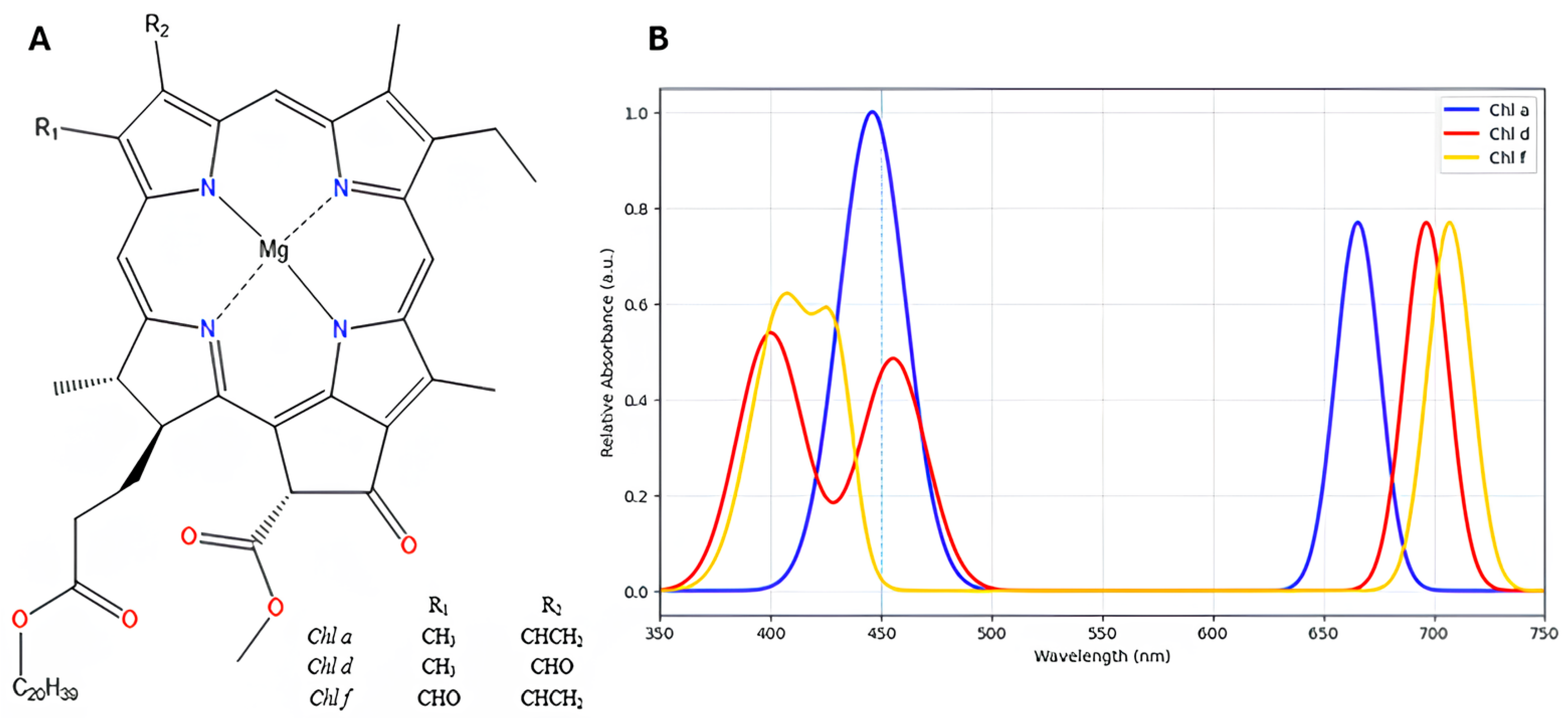
2. Chl d Containing PSI
2.1. Electron Transport Chain (ETC) of Acaryochloris marina PSI
2.2. Absorption Characteristics of P740
2.3. The Midpoint Potential and Electronic Asymmetry of P740
2.4. Electron Transfer Energetics and the Protein Environment
3. Far-Red Light Photoacclimation (FaRLiP)
3.1. Characterization of Chl f-Containing Photosystems
3.2. The Electron Transfer Chain in FRL-PSI from H. hongdechloris and F. thermalis
3.3. Excitation Energy Transfer
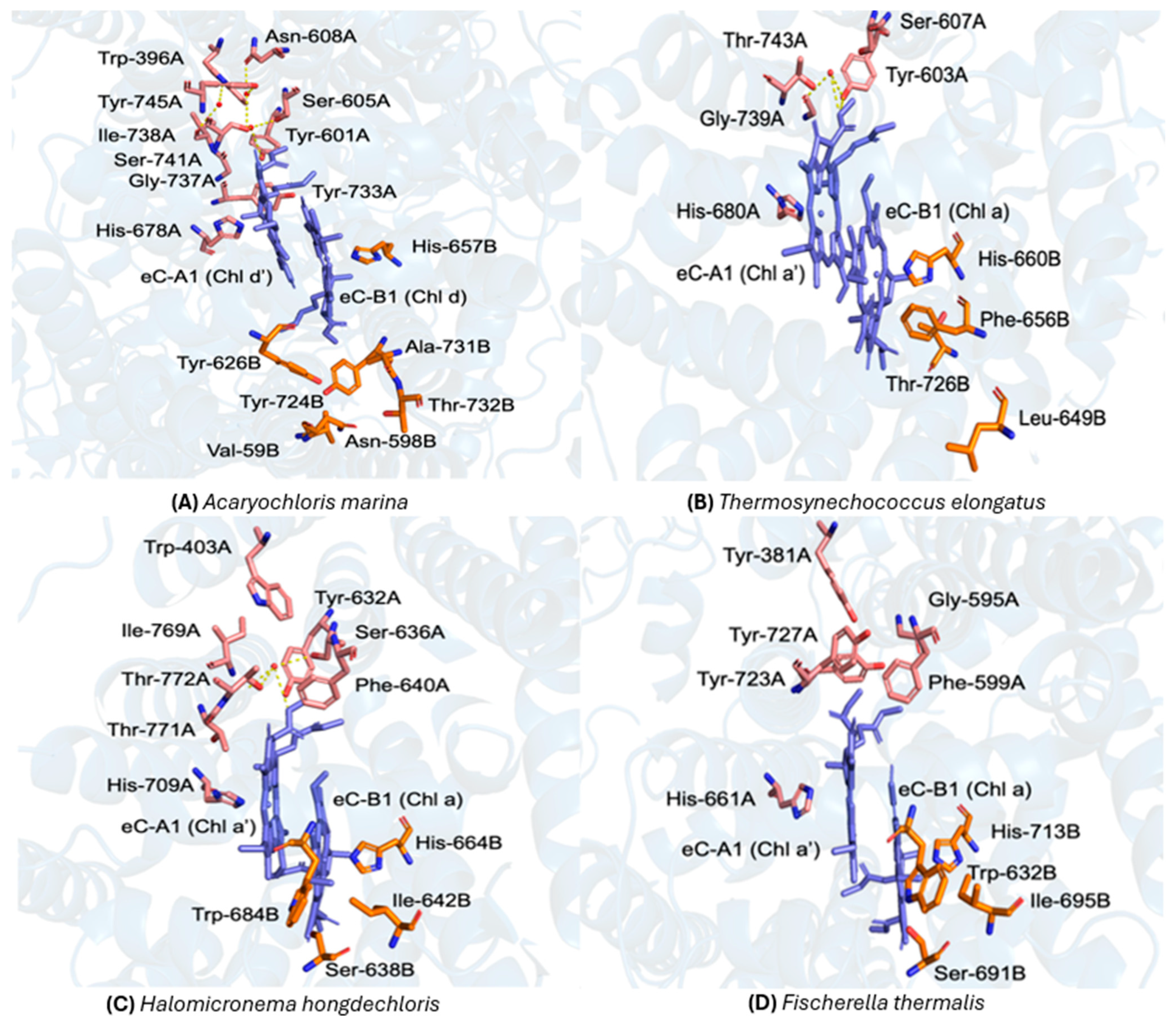
3.4. Comparative Analysis of the Protein Environment Surrounding P700 in H. hongdechloris, F. thermalis and T. elongatus
3.5. Comparison of Water Molecules
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nelson, N.; Junge, W. Structure and Energy Transfer in Photosystems of Oxygenic Photosynthesis. Annu. Rev. Biochem. 2015, 84, 659–683. [Google Scholar] [CrossRef]
- Kaiser, E.; Correa Galvis, V.; Armbruster, U. Efficient Photosynthesis in Dynamic Light Environments: A Chloroplast’s Perspective. Biochem. J. 2019, 476, 2725–2741. [Google Scholar] [CrossRef] [PubMed]
- Gorka, M.; Landry, P.; Gruszecki, E.; Malnati, A.; Kaur, D.; Van Der Est, A.; Golbeck, J.H.; Lakshmi, K.V. Chlorophylls as Primary Electron Acceptors in Reaction Centers. In Photosynthesis; Elsevier: Amsterdam, The Netherlands, 2023; pp. 197–237. [Google Scholar] [CrossRef]
- Hohmann-Marriott, M.F.; Blankenship, R.E. Evolution of Photosynthesis. Annu. Rev. Plant Biol. 2011, 62, 515–548. [Google Scholar] [CrossRef]
- McEvoy, J.P.; Gascon, J.A.; Batista, V.S.; Brudvig, G.W. The Mechanism of Photosynthetic Water Splitting. Photochem. Photobiol. Sci. 2005, 4, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Jordan, P.; Fromme, P.; Witt, H.T.; Klukas, O.; Saenger, W.; Krauß, N. Three-Dimensional Structure of Cyanobacterial Photosystem I at 2.5 Å Resolution. Nature 2001, 411, 909–917. [Google Scholar] [CrossRef]
- Gorka, M.J.; Baldansuren, A.; Malnati, A.; Gruszecki, E.; Golbeck, J.H.; Lakshmi, K.V. Shedding Light on Primary Donors in Photosynthetic Reaction Centers. Biophys. J. 2022, 121, 103a–104a. [Google Scholar] [CrossRef]
- Brudvig, G.W.; Beck, W.F.; Paula, J.C. Mechanism of Photosynthetic Water Oxidation. Annu. Rev. Biophys. Biophys. Chem. 1989, 18, 25–46. [Google Scholar] [CrossRef]
- Gorka, M.; Charles, P.; Kalendra, V.; Baldansuren, A.; Lakshmi, K.V.; Golbeck, J.H. A Dimeric Chlorophyll Electron Acceptor Differentiates Type I from Type II Photosynthetic Reaction Centers. iScience 2021, 24, 102719. [Google Scholar] [CrossRef]
- Blankenship, R.E. Early Evolution of Photosynthesis. Plant Physiol. 2010, 154, 434–438. [Google Scholar] [CrossRef]
- Blankenship, R.E.; Olson, J.M.; Miller, M. Antenna Complexes from Green Photosynthetic Bacteria. In Anoxygenic Photosynthetic Bacteria; Blankenship, R.E., Madigan, M.T., Bauer, C.E., Eds.; Advances in Photosynthesis and Respiration; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; Volume 2, pp. 399–435. [Google Scholar] [CrossRef]
- Sager, R.; Ramanis, Z. Recombination of Nonchromosomal Genes in Chlamydomonas. Proc. Natl. Acad. Sci. USA 1965, 53, 1053–1061. [Google Scholar] [CrossRef]
- Keeling, P.J. Diversity and Evolutionary History of Plastids and Their Hosts. Am. J. Bot. 2004, 91, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- López-Juez, E. Steering the Solar Panel: Plastids Influence Development. New Phytol. 2009, 182, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, H.; Ikemoto, H.; Kurano, N.; Adachi, K.; Chihara, M.; Miyachi, S. Chlorophyll d as a Major Pigment. Nature 1996, 383, 402. [Google Scholar] [CrossRef]
- Alcorta, J.; Vergara-Barros, P.; Antonaru, L.A.; Alcamán-Arias, M.E.; Nürnberg, D.J.; Díez, B. Fischerella Thermalis: A Model Organism to Study Thermophilic Diazotrophy, Photosynthesis and Multicellularity in Cyanobacteria. Extremophiles 2019, 23, 635–647. [Google Scholar] [CrossRef]
- Gan, F.; Bryant, D.A. Adaptive and Acclimative Responses of Cyanobacteria to Far-Red Light. Environ. Microbiol. 2015, 17, 3450–3465. [Google Scholar] [CrossRef]
- Gan, F.; Shen, G.; Bryant, D. Occurrence of Far-Red Light Photoacclimation (FaRLiP) in Diverse Cyanobacteria. Life 2014, 5, 4–24. [Google Scholar] [CrossRef]
- Gisriel, C.J.; Bryant, D.A.; Brudvig, G.W.; Cardona, T. Molecular Diversity and Evolution of Far-Red Light-Acclimated Photosystem I. Front. Plant Sci. 2023, 14, 1289199. [Google Scholar] [CrossRef]
- Gisriel, C.J.; Elias, E.; Shen, G.; Soulier, N.T.; Brudvig, G.W.; Croce, R.; Bryant, D.A. Structural Comparison of Allophycocyanin Variants Reveals the Molecular Basis for Their Spectral Differences. Photosynth. Res. 2024, 162, 157–170. [Google Scholar] [CrossRef]
- Zhou, L.-J.; Höppner, A.; Wang, Y.-Q.; Hou, J.-Y.; Scheer, H.; Zhao, K.-H. Crystallographic and Biochemical Analyses of a Far-Red Allophycocyanin to Address the Mechanism of the Super-Red-Shift. Photosynth. Res. 2024, 162, 171–185. [Google Scholar] [CrossRef]
- Gisriel, C.J.; Brudvig, G.W. Investigations into Cyanobacterial Photoacclimation Processes Address Longstanding Proposals for Improving Crop Yields. Nat. Commun. 2025, 16, 3942. [Google Scholar] [CrossRef]
- Elias, E.; Oliver, T.J.; Croce, R. Oxygenic Photosynthesis in Far-Red Light: Strategies and Mechanisms. Annu. Rev. Phys. Chem. 2024, 75, 231–256. [Google Scholar] [CrossRef]
- Billi, D.; Napoli, A.; Mosca, C.; Fagliarone, C.; De Carolis, R.; Balbi, A.; Scanu, M.; Selinger, V.M.; Antonaru, L.A.; Nürnberg, D.J. Identification of Far-Red Light Acclimation in an Endolithic Chroococcidiopsis Strain and Associated Genomic Features: Implications for Oxygenic Photosynthesis on Exoplanets. Front. Microbiol. 2022, 13, 933404. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Blankenship, R.E. Expanding the Solar Spectrum Used by Photosynthesis. Trends Plant Sci. 2011, 16, 427–431. [Google Scholar] [CrossRef]
- Kato, K.; Shinoda, T.; Nagao, R.; Akimoto, S.; Suzuki, T.; Dohmae, N.; Chen, M.; Allakhverdiev, S.I.; Shen, J.-R.; Akita, F.; et al. Structural Basis for the Adaptation and Function of Chlorophyll f in Photosystem I. Nat. Commun. 2020, 11, 238. [Google Scholar] [CrossRef]
- Ho, M.-Y.; Gan, F.; Shen, G.; Zhao, C.; Bryant, D.A. Far-Red Light Photoacclimation (FaRLiP) in Synechococcus sp. PCC 7335: I. Regulation of FaRLiP Gene Expression. Photosynth. Res. 2017, 131, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, R.; Zhang, X.; Shinoda, T.; Tomo, T.; Ye, S.; Shibata, Y. The Reddest Fluorescence of Photosystem I from Halomicronema hongdechloris Comes from Chlorophyll-f Dimer. J. Phys. Chem. B 2025, 129, 6465–6476. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Scales, N.; Blankenship, R.E.; Willows, R.D.; Chen, M. Extinction Coefficient for Red-Shifted Chlorophylls: Chlorophyll d and Chlorophyll f. Biochim. Biophys. Acta BBA—Bioenerg. 2012, 1817, 1292–1298. [Google Scholar] [CrossRef]
- Montgomery, B.L. Seeing New Light: Recent Insights into the Occurrence and Regulation of Chromatic Acclimation in Cyanobacteria. Curr. Opin. Plant Biol. 2017, 37, 18–23. [Google Scholar] [CrossRef]
- Pinevich, A.V.; Averina, S.G. On the Edge of the Rainbow: Red-Shifted Chlorophylls and Far-Red Light Photoadaptation in Cyanobacteria. Microbiology 2022, 91, 631–648. [Google Scholar] [CrossRef]
- Ho, M.-Y.; Soulier, N.T.; Canniffe, D.P.; Shen, G.; Bryant, D.A. Light Regulation of Pigment and Photosystem Biosynthesis in Cyanobacteria. Curr. Opin. Plant Biol. 2017, 37, 24–33. [Google Scholar] [CrossRef]
- Mullineaux, C.W. How Do Cyanobacteria Sense and Respond to Light? Mol. Microbiol. 2001, 41, 965–971. [Google Scholar] [CrossRef]
- Guergova-Kuras, M.; Boudreaux, B.; Joliot, A.; Joliot, P.; Redding, K. Evidence for Two Active Branches for Electron Transfer in Photosystem I. Proc. Natl. Acad. Sci. USA 2001, 98, 4437–4442. [Google Scholar] [CrossRef]
- Shelaev, I.V.; Gostev, F.E.; Mamedov, M.D.; Sarkisov, O.M.; Nadtochenko, V.A.; Shuvalov, V.A.; Semenov, A.Y. Femtosecond Primary Charge Separation in Synechocystis sp. PCC 6803 Photosystem I. Biochim. Biophys. Acta BBA—Bioenerg. 2010, 1797, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Cherepanov, D.A.; Semenov, A.Y.; Mamedov, M.D.; Aybush, A.V.; Gostev, F.E.; Shelaev, I.V.; Shuvalov, V.A.; Nadtochenko, V.A. Current State of the Primary Charge Separation Mechanism in Photosystem I of Cyanobacteria. Biophys. Rev. 2022, 14, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, N.; Golbeck, J.H. Protein–Cofactor Interactions in Bioenergetic Complexes: The Role of the A1A and A1B Phylloquinones in Photosystem I. Biochim. Biophys. Acta BBA—Bioenerg. 2009, 1787, 1057–1088. [Google Scholar] [CrossRef] [PubMed]
- Heathcote, P.; Jones, M.R.; Fyfe, P.K. Type I Photosynthetic Reaction Centres: Structure and Function. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2003, 358, 231–243. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Kawakami, K.; Shinzawa-Itoh, K.; Inoue-Kashino, N.; Itoh, S.; Ifuku, K.; Yamashita, E.; Maeda, K.; Yonekura, K.; Kashino, Y. Structure of the Far-Red Light Utilizing Photosystem I of Acaryochloris marina. Nat. Commun. 2021, 12, 2333. [Google Scholar] [CrossRef]
- Ruprecht, J.; Iwata, S.; Rothery, R.A.; Weiner, J.H.; Maklashina, E.; Cecchini, G. Perturbation of the Quinone-Binding Site of Complex II Alters the Electronic Properties of the Proximal [3Fe-4S] Iron-Sulfur Cluster. J. Biol. Chem. 2011, 286, 12756–12765. [Google Scholar] [CrossRef]
- Fu, H.-Y.; Picot, D.; Choquet, Y.; Longatte, G.; Sayegh, A.; Delacotte, J.; Guille-Collignon, M.; Lemaître, F.; Rappaport, F.; Wollman, F.-A. Redesigning the QA Binding Site of Photosystem II Allows Reduction of Exogenous Quinones. Nat. Commun. 2017, 8, 15274. [Google Scholar] [CrossRef]
- Xu, C.; Zhu, Q.; Chen, J.; Shen, L.; Yi, X.; Huang, Z.; Wang, W.; Chen, M.; Kuang, T.; Shen, J.; et al. A Unique Photosystem I Reaction Center from a Chlorophyll d-containing Cyanobacterium Acaryochloris marina. J. Integr. Plant Biol. 2021, 63, 1740–1752. [Google Scholar] [CrossRef]
- Miyashita, H.; Adachi, K.; Kurano, N.; Ikemot, H.; Chihara, M.; Miyach, S. Pigment Composition of a Novel Oxygenic Photosynthetic Prokaryote Containing Chlorophyll d as the Major Chlorophyll. Plant Cell Physiol. 1997, 38, 274–281. [Google Scholar] [CrossRef]
- Averina, S.; Velichko, N.; Senatskaya, E.; Pinevich, A. Far-Red Light Photoadaptations in Aquatic Cyanobacteria. Hydrobiologia 2018, 813, 1–17. [Google Scholar] [CrossRef]
- Ulrich, N.J.; Shen, G.; Bryant, D.A.; Miller, S.R. Ecological Diversification of a Cyanobacterium through Divergence of Its Novel Chlorophyll D-Based Light-Harvesting System. Curr. Biol. 2024, 34, 2972–2979.e4. [Google Scholar] [CrossRef] [PubMed]
- Oliver, T.J.; Elias, E.; Croce, R. Acclimation to White Light in a Far-red Light Specialist: Insights from Acaryochloris marina MBIC11017. New Phytol. 2025, 247, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Mielke, S.P.; Kiang, N.Y.; Blankenship, R.E.; Gunner, M.R.; Mauzerall, D. Efficiency of Photosynthesis in a Chl D-Utilizing Cyanobacterium Is Comparable to or Higher than That in Chl a-Utilizing Oxygenic Species. Biochim. Biophys. Acta BBA—Bioenerg. 2011, 1807, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Reassessment of the Redox Potential of P740: The Primary Electron Donor in Photosystem I of the Chlorophyll d Containing Cyanobacterium, Acaryochloris marina. In Photosynthesis. Energy from the Sun; Springer: Dordrecht, The Netherlands, 2008; pp. 219–222. [CrossRef]
- Tomo, T.; Allakhverdiev, S.I.; Mimuro, M. Constitution and Energetics of Photosystem I and Photosystem II in the Chlorophyll D-Dominated Cyanobacterium Acaryochloris marina. J. Photochem. Photobiol. B 2011, 104, 333–340. [Google Scholar] [CrossRef]
- Linnanto, J.; Korppi-Tommola, J. Spectroscopic Properties of Mg-Chlorin, Mg-Porphin and Chlorophylls a, b, C1, C2, C3 and d Studied by Semi-Empirical and Ab Initio MO/CI Methods. Phys. Chem. Chem. Phys. 2000, 2, 4962–4970. [Google Scholar] [CrossRef]
- Fiedor, L.; Kania, A.; Myśliwa-Kurdziel, B.; Orzeł, Ł.; Stochel, G. Understanding Chlorophylls: Central Magnesium Ion and Phytyl as Structural Determinants. Biochim. Biophys. Acta BBA—Bioenerg. 2008, 1777, 1491–1500. [Google Scholar] [CrossRef]
- Bailleul, B.; Johnson, X.; Finazzi, G.; Barber, J.; Rappaport, F.; Telfer, A. The Thermodynamics and Kinetics of Electron Transfer between Cytochrome B6f and Photosystem I in the Chlorophyll D-Dominated Cyanobacterium, Acaryochloris marina. J. Biol. Chem. 2008, 283, 25218–25226. [Google Scholar] [CrossRef]
- Tomo, T.; Kato, Y.; Suzuki, T.; Akimoto, S.; Okubo, T.; Noguchi, T.; Hasegawa, K.; Tsuchiya, T.; Tanaka, K.; Fukuya, M.; et al. Characterization of Highly Purified Photosystem I Complexes from the Chlorophyll D-Dominated Cyanobacterium Acaryochloris marina MBIC 11017. J. Biol. Chem. 2008, 283, 18198–18209. [Google Scholar] [CrossRef]
- Nakamura, A.; Suzawa, T.; Kato, Y.; Watanabe, T. Species Dependence of the Redox Potential of the Primary Electron Donor P700 in Photosystem I of Oxygenic Photosynthetic Organisms Revealed by Spectroelectrochemistry. Plant Cell Physiol. 2011, 52, 815–823. [Google Scholar] [CrossRef]
- Ohashi, S.; Kasahara, M.; Fukuyo, S.; Nakazato, M.; Iwamoto, K.; Shiraiwa, Y.; Kato, Y.; Watanabe, T. Redox Potential of Chlorophyll d. In Photosynthesis. Energy from the Sun; Springer: Dordrecht, The Netherlands, 2008; pp. 105–108. [Google Scholar] [CrossRef]
- Kato, K.; Nagao, R.; Shen, J.R.; Miyazaki, N.; Akita, F. Structure of PSI from H. hongdechloris Grown Under Far-Red Light Condition: 6kmx; Protein Data Bank: Cambridge, UK, 2020. [Google Scholar] [CrossRef]
- Gisriel, C.J.; Wang, J. Quantitative Assessment of Chlorophyll Types in Cryo-EM Maps of Photosystem I Acclimated to Far-Red Light: 7lx0; Protein Data Bank: Cambridge, UK, 2021. [Google Scholar] [CrossRef]
- Wlodawer, A.; Minor, W.; Dauter, Z.; Jaskolski, M. Protein Crystallography for Non-crystallographers, or How to Get the Best (but Not More) from Published Macromolecular Structures. FEBS J. 2008, 275, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Webber, A.N.; Su, H.; Bingham, S.E.; Käss, H.; Krabben, L.; Kuhn, M.; Jordan, R.; Schlodder, E.; Lubitz, W. Site-Directed Mutations Affecting the Spectroscopic Characteristics and Midpoint Potential of the Primary Donor in Photosystem I. Biochemistry 1996, 35, 12857–12863. [Google Scholar] [CrossRef] [PubMed]
- Ivancich, A.; Mattioli, T.A.; Artz, K.; Wang, S.; Allen, J.P.; Williams, J.C. Influence of Asn/His L166 on the Hydrogen-Bonding Pattern and Redox Potential of the Primary Donor of Purple Bacterial Reaction Centers. Biochemistry 1997, 36, 3027–3036. [Google Scholar] [CrossRef]
- Ivancich, A.; Artz, K.; Williams, J.C.; Allen, J.P.; Mattioli, T.A. Effects of Hydrogen Bonds on the Redox Potential and Electronic Structure of the Bacterial Primary Electron Donor. Biochemistry 1998, 37, 11812–11820. [Google Scholar] [CrossRef]
- Saito, K.; Ishikita, H. Cationic State Distribution over the P700 Chlorophyll Pair in Photosystem I. Biophys. J. 2011, 101, 2018–2025. [Google Scholar] [CrossRef]
- Hastings, G.; Wang, R. Vibrational Mode Frequency Calculations of Chlorophyll-d for Assessing (P740+-P740) FTIR Difference Spectra Obtained Using Photosystem I Particles from Acaryochloris marina. Photosynth. Res. 2007, 95, 55–62. [Google Scholar] [CrossRef]
- Mino, H.; Kawamori, A.; Aoyama, D.; Tomo, T.; Iwaki, M.; Itoh, S. Proton ENDOR Study of the Primary Donor P740+, a Special Pair of Chlorophyll d in Photosystem I Reaction Center of Acaryochloris marina. Chem. Phys. Lett. 2005, 411, 262–266. [Google Scholar] [CrossRef]
- Petrova, A.A.; Casazza, A.P.; Shelaev, I.V.; Gostev, F.E.; Aybush, A.V.; Nadtochenko, V.A.; Semenov, A.Y.; Santabarbara, S.; Cherepanov, D.A. Role of Pheophytin a in the Primary Charge Separation of Photosystem I from Acaryochloris marina: Femtosecond Optical Studies of Excitation Energy and Electron Transfer Reactions. Biochim. Biophys. Acta BBA—Bioenerg. 2023, 1864, 148984. [Google Scholar] [CrossRef]
- Cohen, R.O.; Shen, G.; Golbeck, J.H.; Xu, W.; Chitnis, P.R.; Valieva, A.I.; Van Der Est, A.; Pushkar, Y.; Stehlik, D. Evidence for Asymmetric Electron Transfer in Cyanobacterial Photosystem I: Analysis of a Methionine-to-Leucine Mutation of the Ligand to the Primary Electron Acceptor A0. Biochemistry 2004, 43, 4741–4754. [Google Scholar] [CrossRef] [PubMed]
- Dashdorj, N.; Xu, W.; Cohen, R.O.; Golbeck, J.H.; Savikhin, S. Asymmetric Electron Transfer in Cyanobacterial Photosystem I: Charge Separation and Secondary Electron Transfer Dynamics of Mutations Near the Primary Electron Acceptor A0. Biophys. J. 2005, 88, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- McConnell, M.D.; Sun, J.; Siavashi, R.; Webber, A.; Redding, K.E.; Golbeck, J.H.; Van Der Est, A. Species-Dependent Alteration of Electron Transfer in the Early Stages of Charge Stabilization in Photosystem I. Biochim. Biophys. Acta BBA—Bioenerg. 2015, 1847, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Noji, T.; Saito, K.; Ishikita, H. How the Electron-Transfer Cascade Is Maintained in Chlorophyll-d Containing Photosystem I. Biochemistry 2025, 64, 203–212. [Google Scholar] [CrossRef]
- Kato, K.; Hamaguchi, T.; Nagao, R.; Kawakami, K.; Ueno, Y.; Suzuki, T.; Uchida, H.; Murakami, A.; Nakajima, Y.; Yokono, M.; et al. Structural Basis for the Absence of Low-Energy Chlorophylls in a Photosystem I Trimer from Gloeobacter Violaceus. eLife 2022, 11, e73990. [Google Scholar] [CrossRef]
- Chen, M.; Schliep, M.; Willows, R.D.; Cai, Z.-L.; Neilan, B.A.; Scheer, H. A Red-Shifted Chlorophyll. Science 2010, 329, 1318–1319. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Birch, D.; Willows, R.D. A Cyanobacterium That Contains Chlorophyll f—A Red-absorbing Photopigment. FEBS Lett. 2012, 586, 3249–3254. [Google Scholar] [CrossRef]
- Chen, M.; Hernandez-Prieto, M.A.; Loughlin, P.C.; Li, Y.; Willows, R.D. Genome and Proteome of the Chlorophyll F-Producing Cyanobacterium Halomicronema Hongdechloris: Adaptative Proteomic Shifts under Different Light Conditions. BMC Genom. 2019, 20, 207. [Google Scholar] [CrossRef]
- Gan, F.; Zhang, S.; Rockwell, N.C.; Martin, S.S.; Lagarias, J.C.; Bryant, D.A. Extensive Remodeling of a Cyanobacterial Photosynthetic Apparatus in Far-Red Light. Science 2014, 345, 1312–1317. [Google Scholar] [CrossRef]
- Nürnberg, D.J.; Morton, J.; Santabarbara, S.; Telfer, A.; Joliot, P.; Antonaru, L.A.; Ruban, A.V.; Cardona, T.; Krausz, E.; Boussac, A.; et al. Photochemistry beyond the Red Limit in Chlorophyll f–Containing Photosystems. Science 2018, 360, 1210–1213. [Google Scholar] [CrossRef]
- Shen, G.; Canniffe, D.P.; Ho, M.-Y.; Kurashov, V.; Van Der Est, A.; Golbeck, J.H.; Bryant, D.A. Characterization of Chlorophyll f Synthase Heterologously Produced in Synechococcus sp. PCC 7002. Photosynth. Res. 2019, 140, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Gan, F.; Shen, G.; Bryant, D.A. RfpA, RfpB, and RfpC Are the Master Control Elements of Far-Red Light Photoacclimation (FaRLiP). Front. Microbiol. 2015, 6, 1303. [Google Scholar] [CrossRef]
- Gisriel, C.J.; Cardona, T.; Bryant, D.A.; Brudvig, G.W. Molecular Evolution of Far-Red Light-Acclimated Photosystem II. Microorganisms 2022, 10, 1270. [Google Scholar] [CrossRef]
- Hastings, G.; Makita, H.; Agarwala, N.; Rohani, L.; Shen, G.; Bryant, D.A. Fourier Transform Visible and Infrared Difference Spectroscopy for the Study of P700 in Photosystem I from Fischerella Thermalis PCC 7521 Cells Grown under White Light and Far-Red Light: Evidence That the A–1 Cofactor Is Chlorophyll f. Biochim. Biophys. Acta BBA—Bioenerg. 2019, 1860, 452–460. [Google Scholar] [CrossRef]
- Cherepanov, D.A.; Shelaev, I.V.; Gostev, F.E.; Aybush, A.V.; Mamedov, M.D.; Shen, G.; Nadtochenko, V.A.; Bryant, D.A.; Semenov, A.Y.; Golbeck, J.H. Evidence That Chlorophyll f Functions Solely as an Antenna Pigment in Far-Red-Light Photosystem I from Fischerella Thermalis PCC 7521. Biochim. Biophys. Acta BBA—Bioenerg. 2020, 1861, 148184. [Google Scholar] [CrossRef]
- Gisriel, C.; Shen, G.; Kurashov, V.; Ho, M.-Y.; Zhang, S.; Williams, D.; Golbeck, J.H.; Fromme, P.; Bryant, D.A. The Structure of Photosystem I Acclimated to Far-Red Light Illuminates an Ecologically Important Acclimation Process in Photosynthesis. Sci. Adv. 2020, 6, eaay6415. [Google Scholar] [CrossRef]
- Kaucikas, M.; Nürnberg, D.; Dorlhiac, G.; Rutherford, A.W.; Van Thor, J.J. Femtosecond Visible Transient Absorption Spectroscopy of Chlorophyll f-Containing Photosystem I. Biophys. J. 2017, 112, 234–249. [Google Scholar] [CrossRef]
- Zamzam, N.; Kaucikas, M.; Nürnberg, D.J.; Rutherford, A.W.; Van Thor, J.J. Femtosecond Infrared Spectroscopy of Chlorophyll F-Containing Photosystem I. Phys. Chem. Chem. Phys. 2019, 21, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Gisriel, C.J.; Huang, H.-L.; Reiss, K.M.; Flesher, D.A.; Batista, V.S.; Bryant, D.A.; Brudvig, G.W.; Wang, J. Quantitative Assessment of Chlorophyll Types in Cryo-EM Maps of Photosystem I Acclimated to Far-Red Light. BBA Adv. 2021, 1, 100019. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Vella, N.; Chen, M. Characterization of Isolated Photosystem I from Halomicronema Hongdechloris, a Chlorophyll f-Producing Cyanobacterium. Photosynthetica 2018, 56, 306–315. [Google Scholar] [CrossRef]
- Vasil’ev, S.; Shen, J.-R.; Kamiya, N.; Bruce, D. The Orientations of Core Antenna Chlorophylls in Photosystem II Are Optimized to Maximize the Quantum Yield of Photosynthesis. FEBS Lett. 2004, 561, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Käss, H.; Fromme, P.; Witt, H.T.; Lubitz, W. Orientation and Electronic Structure of the Primary Donor Radical Cation in Photosystem I: A Single Crystals EPR and ENDOR Study. J. Phys. Chem. B 2001, 105, 1225–1239. [Google Scholar] [CrossRef]
- Schmitt, F.-J.; Friedrich, T. Adaptation Processes in Halomicronema Hongdechloris, an Example of the Light-Induced Optimization of the Photosynthetic Apparatus on Hierarchical Time Scales. Front. Plant Sci. 2024, 15, 1359195. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, F.-J.; Campbell, Z.Y.; Bui, M.V.; Hüls, A.; Tomo, T.; Chen, M.; Maksimov, E.G.; Allakhverdiev, S.I.; Friedrich, T. Photosynthesis Supported by a Chlorophyll F-Dependent, Entropy-Driven Uphill Energy Transfer in Halomicronema Hongdechloris Cells Adapted to Far-Red Light. Photosynth. Res. 2019, 139, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Gisriel, C.J.; Shen, G.; Kurashov, V.; Ho, M.; Zhang, S.; Williams, D.; Golbeck, J.H.; Fromme, P.; Bryant, D.A. Structure of Photosystem I Acclimated to Far-Red Light: 6pnj; Protein Data Bank: Cambridge, UK, 2020; 6p. [Google Scholar] [CrossRef]
- Jordan, P.; Fromme, P.; Witt, H.T.; Klukas, O.; Saenger, W.; Krauss, N. Crystal Structure of Photosystem I: A Photosynthetic Reaction Center and Core Antenna System from Cyanobacteria: 1jb0; Protein Data Bank: Cambridge, UK, 2001. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, J.; ElMasadef, A.; Karlapudi, A.P.; Etemadi, K.; Lakshmi, K.V.; van der Est, A.; Kaur, D. Driving Electron Transfer in Photosystem I Using Far-Red Light: Overall Perspectives. Plants 2025, 14, 3384. https://doi.org/10.3390/plants14213384
Patel J, ElMasadef A, Karlapudi AP, Etemadi K, Lakshmi KV, van der Est A, Kaur D. Driving Electron Transfer in Photosystem I Using Far-Red Light: Overall Perspectives. Plants. 2025; 14(21):3384. https://doi.org/10.3390/plants14213384
Chicago/Turabian StylePatel, Jimit, Amen ElMasadef, Abraham Peele Karlapudi, Katayoun Etemadi, K. V. Lakshmi, Art van der Est, and Divya Kaur. 2025. "Driving Electron Transfer in Photosystem I Using Far-Red Light: Overall Perspectives" Plants 14, no. 21: 3384. https://doi.org/10.3390/plants14213384
APA StylePatel, J., ElMasadef, A., Karlapudi, A. P., Etemadi, K., Lakshmi, K. V., van der Est, A., & Kaur, D. (2025). Driving Electron Transfer in Photosystem I Using Far-Red Light: Overall Perspectives. Plants, 14(21), 3384. https://doi.org/10.3390/plants14213384




