Fumarylacetoacetate Hydrolase Regulates Seed Dormancy and Germination Through the Gibberellin Pathway in Arabidopsis
Abstract
1. Introduction
2. Results
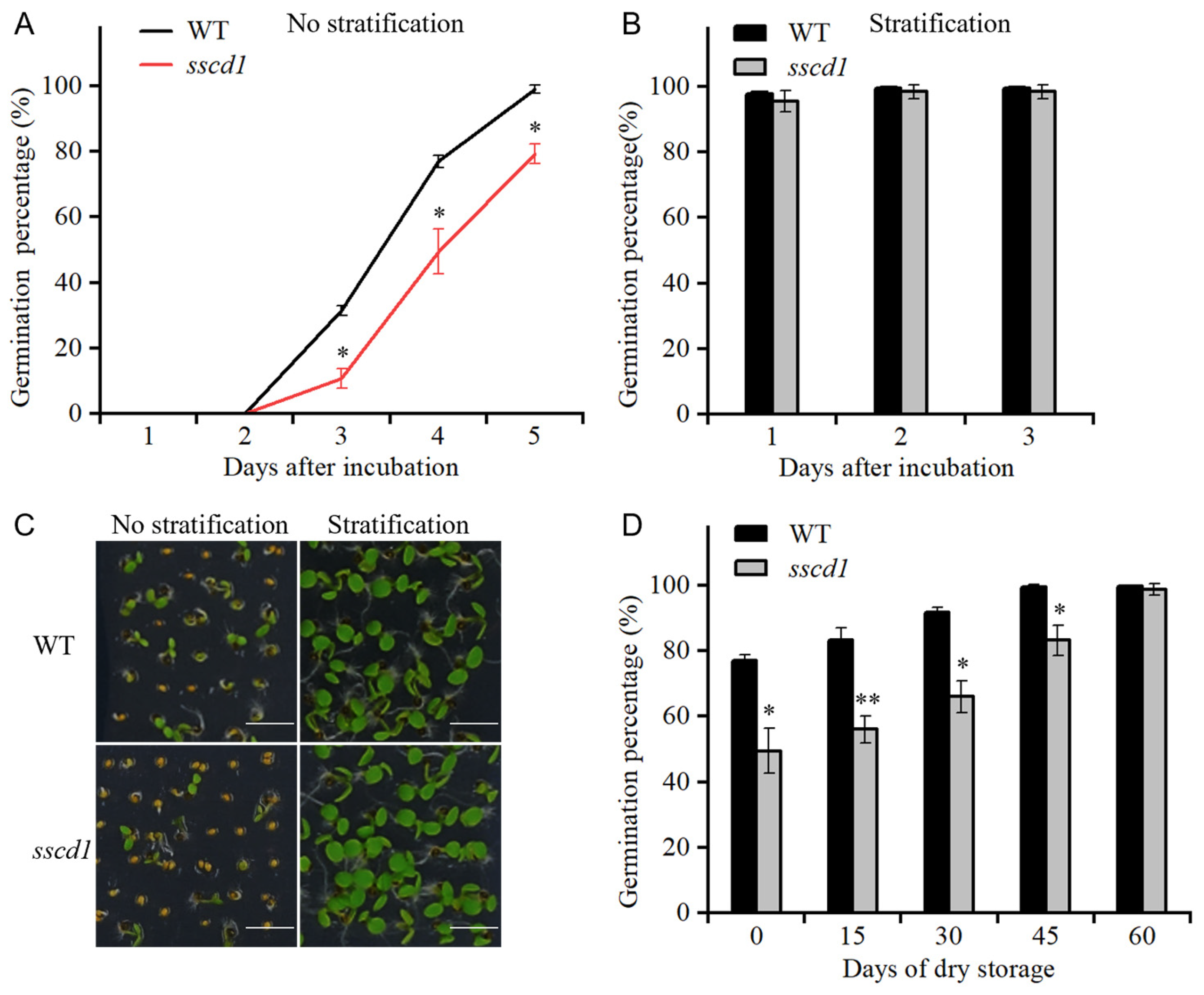
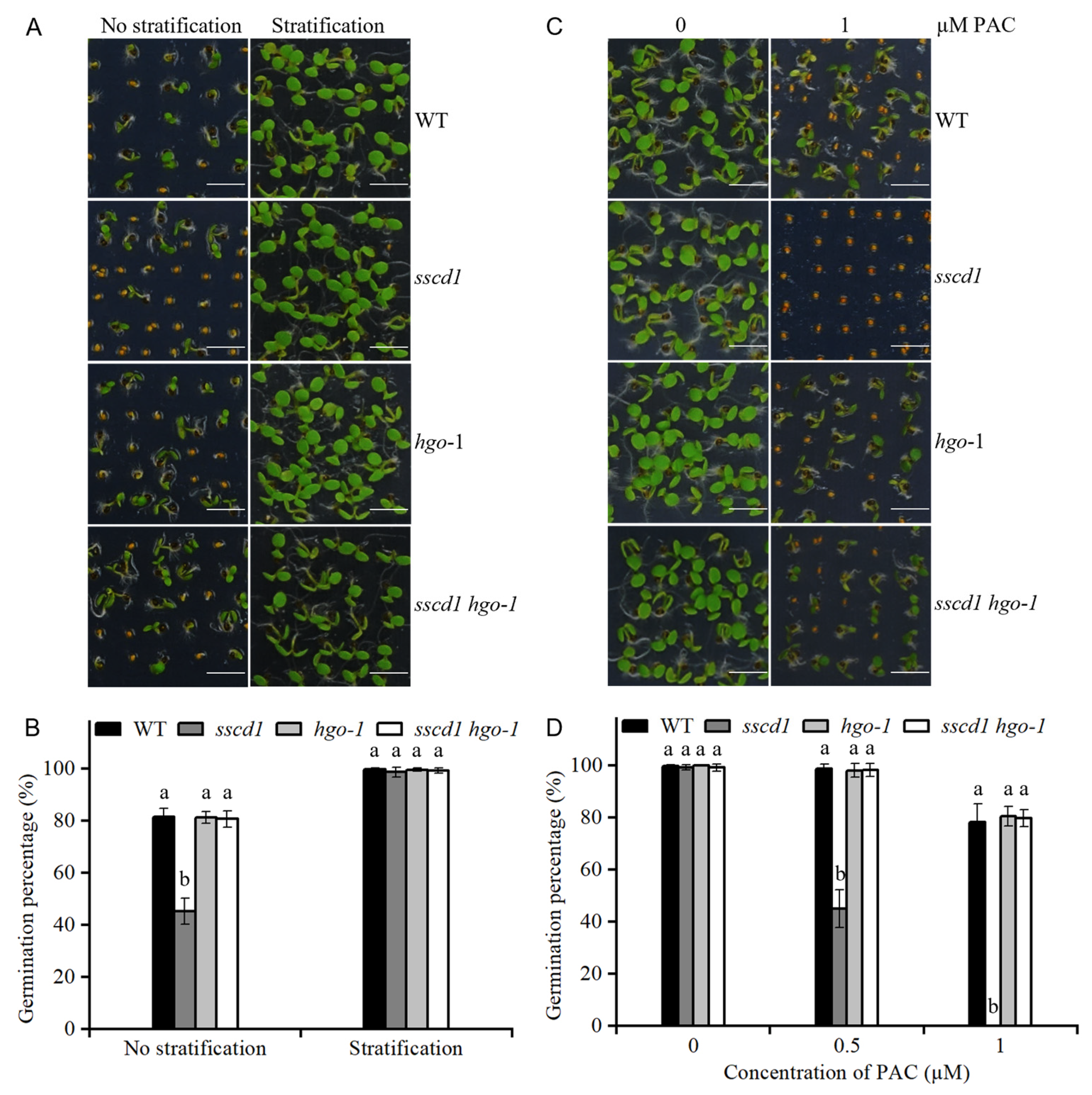
2.1. The sscd1 Mutant Showed Increased Seed Dormancy
2.2. The sscd1 and WT Seed Showed Similar Responses to ABA
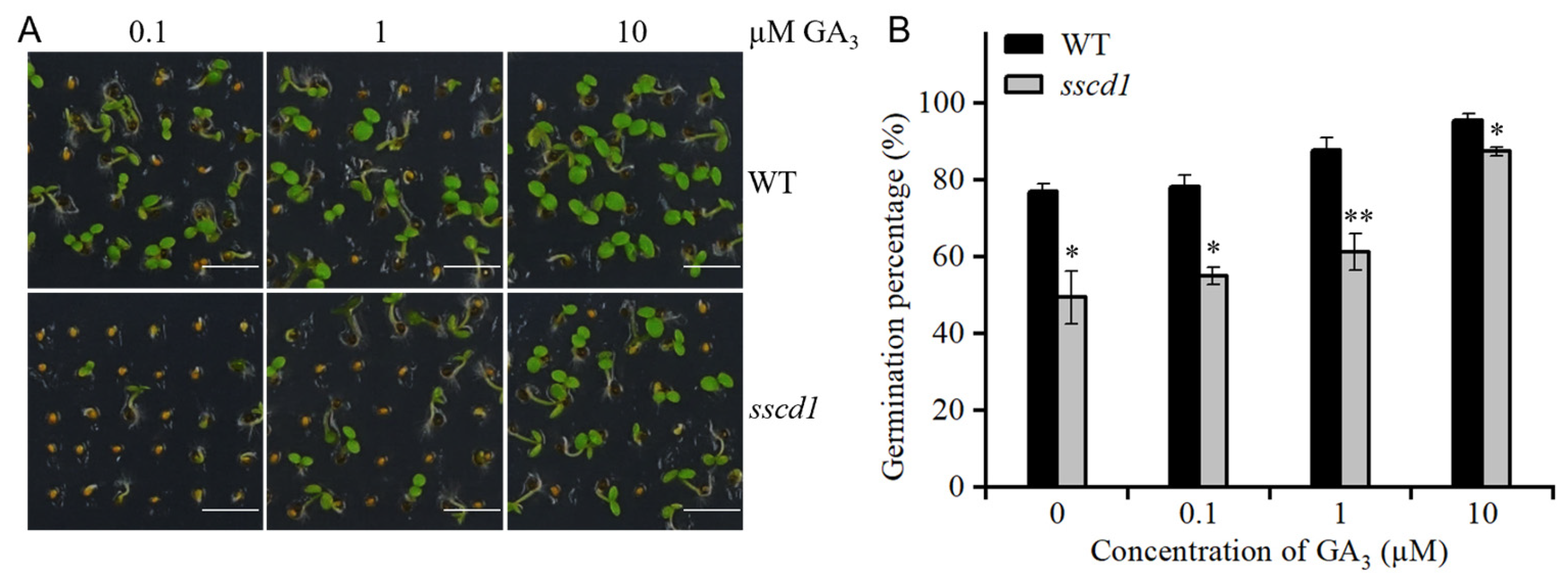
2.3. Dormant sscd1 Seeds Were Less Sensitive to GA
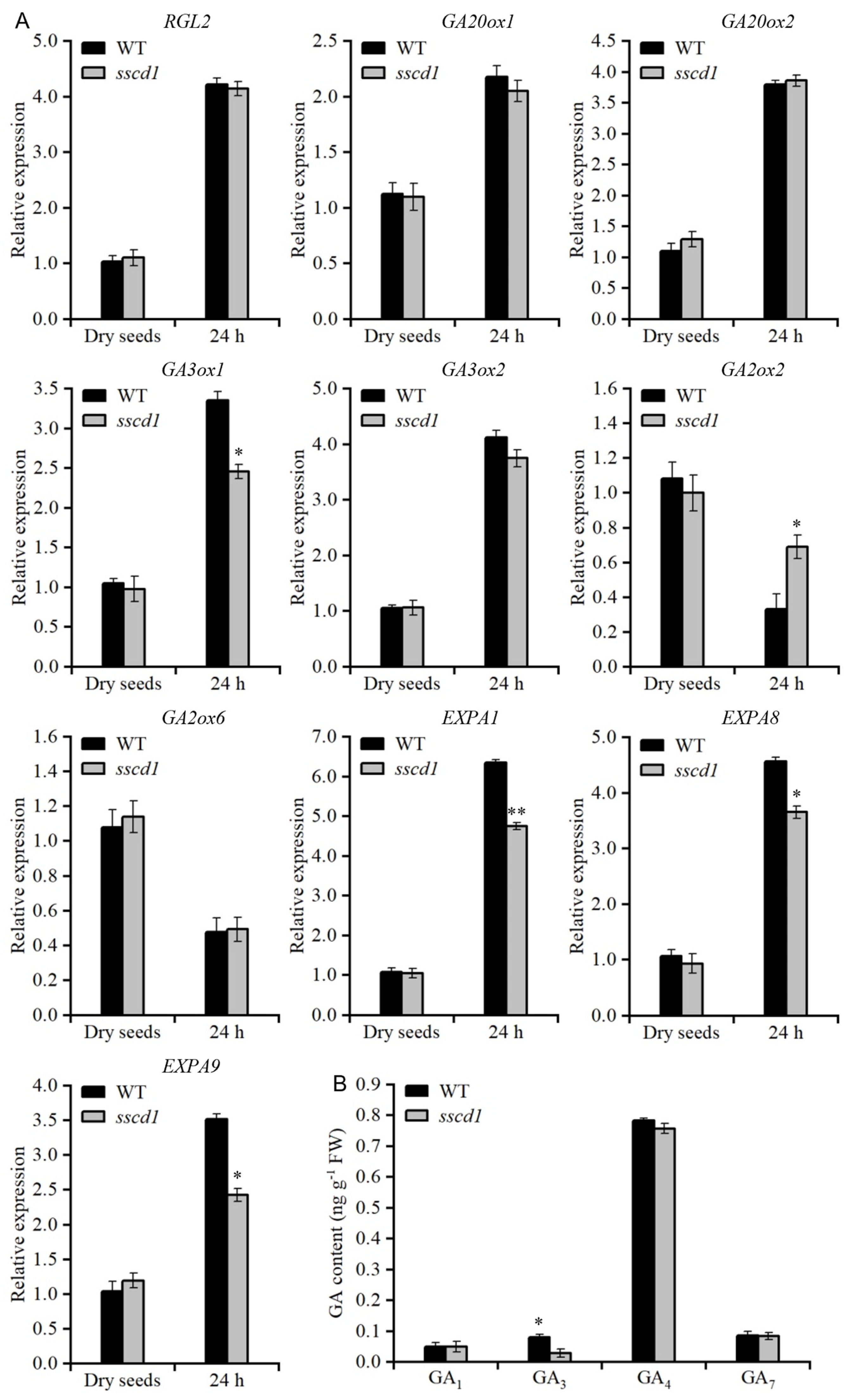
2.4. SSCD1 Mutation Affects GA Biosynthesis
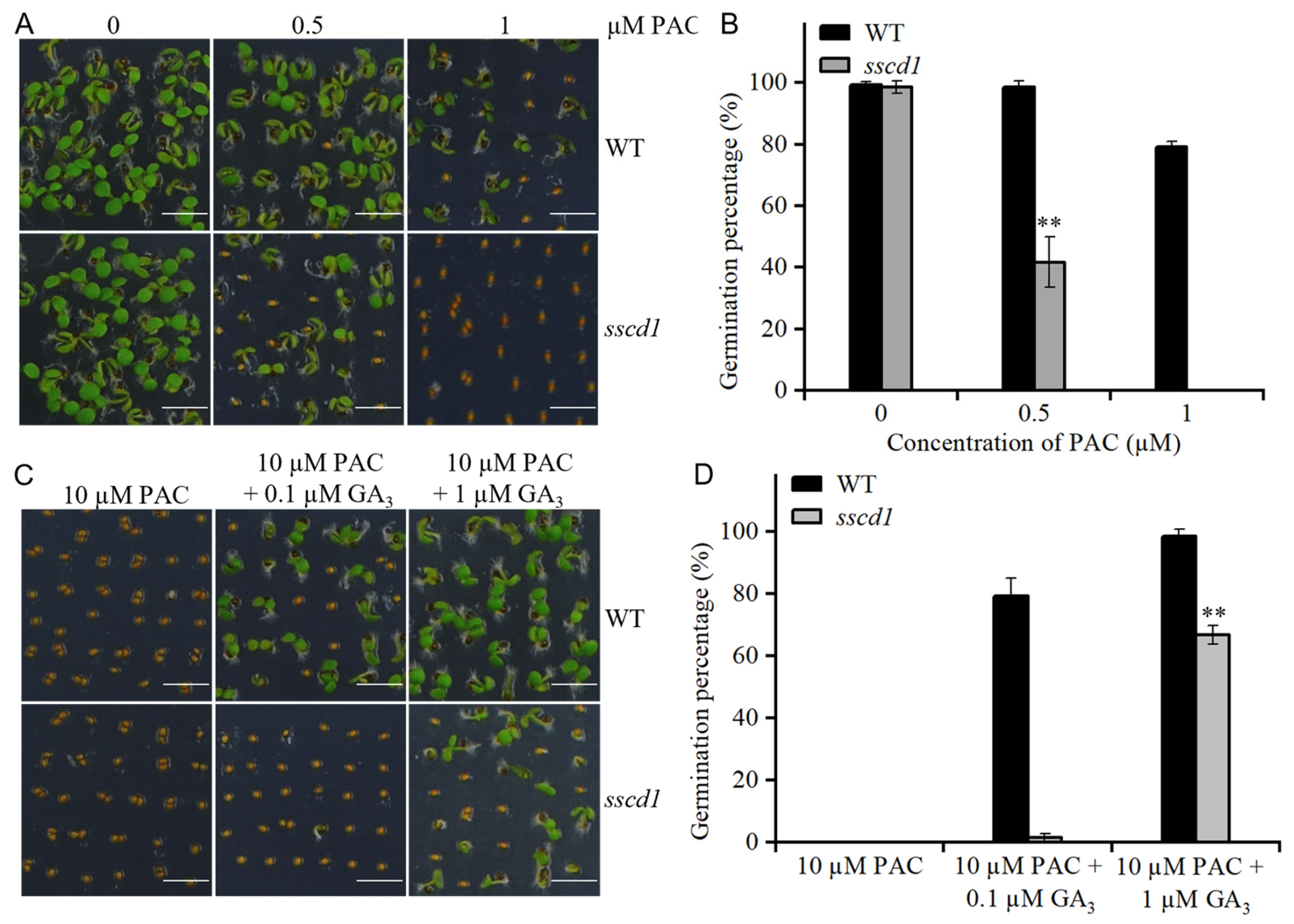
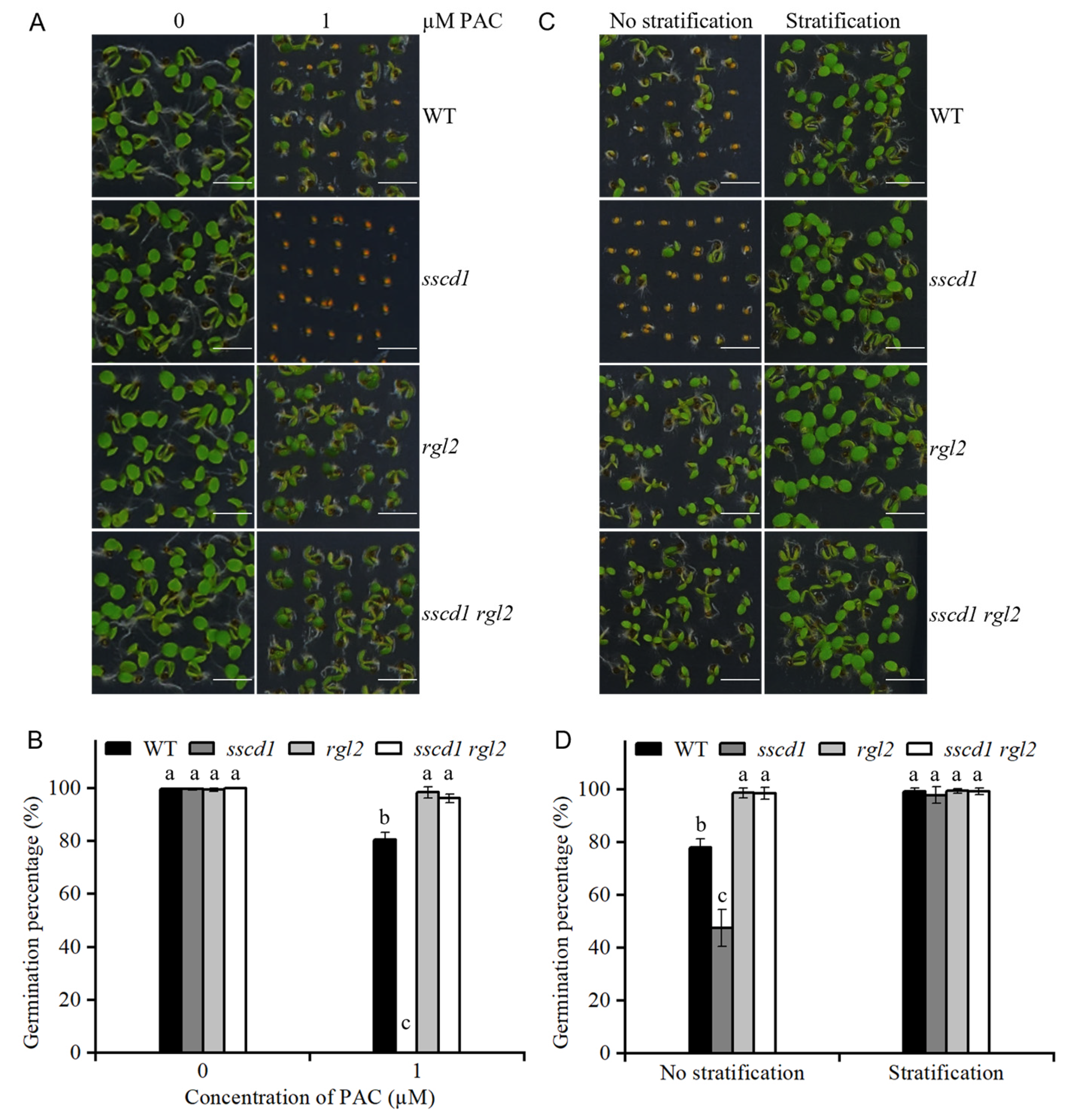
2.5. The sscd1 Seeds Were Hypersensitive to Paclobutrazol
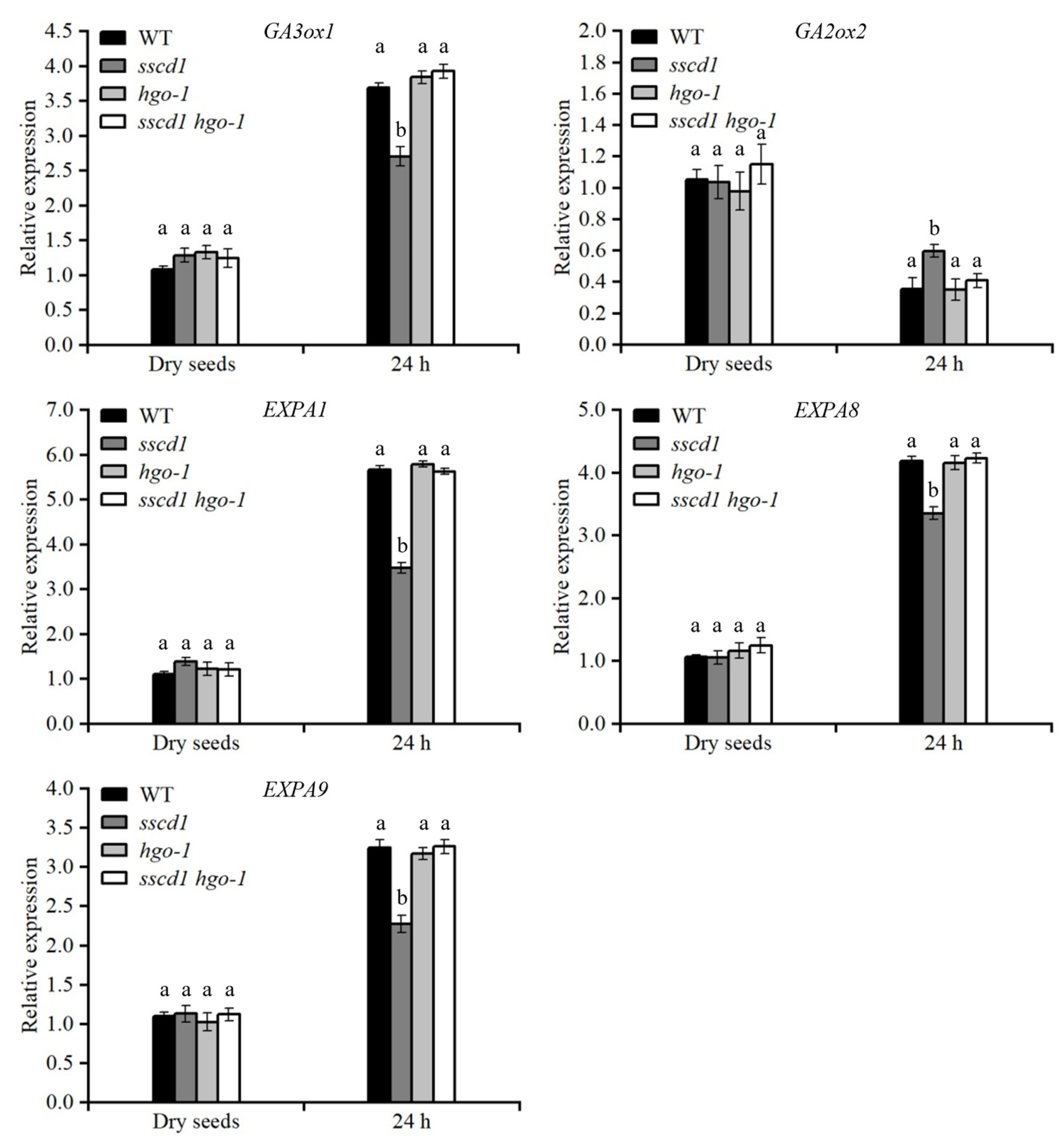
2.6. GA Biosynthesis and Cell Wall Extension Genes Were Modulated in sscd1 Seeds Under PAC Treatment
2.7. SSCD1 Acted Upstream of RGL2
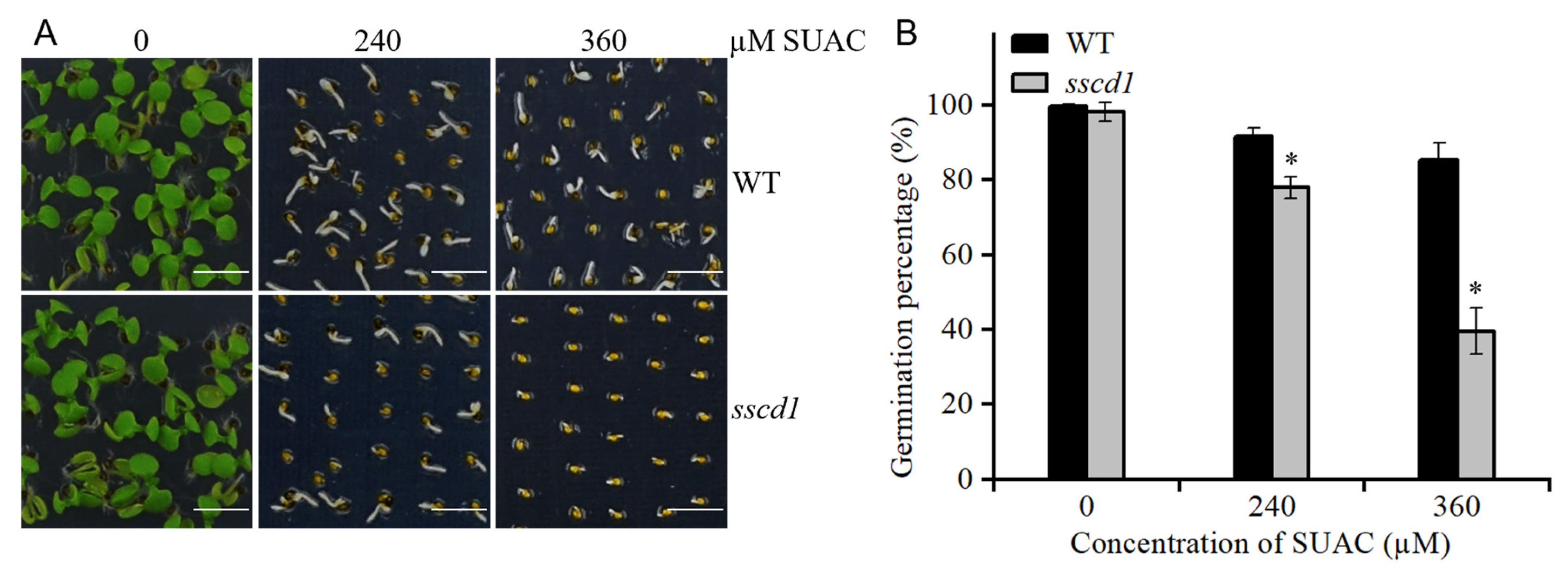
2.8. Tyr Metabolites Were Involved in SSCD1-Regulated Seed Dormancy and Germination
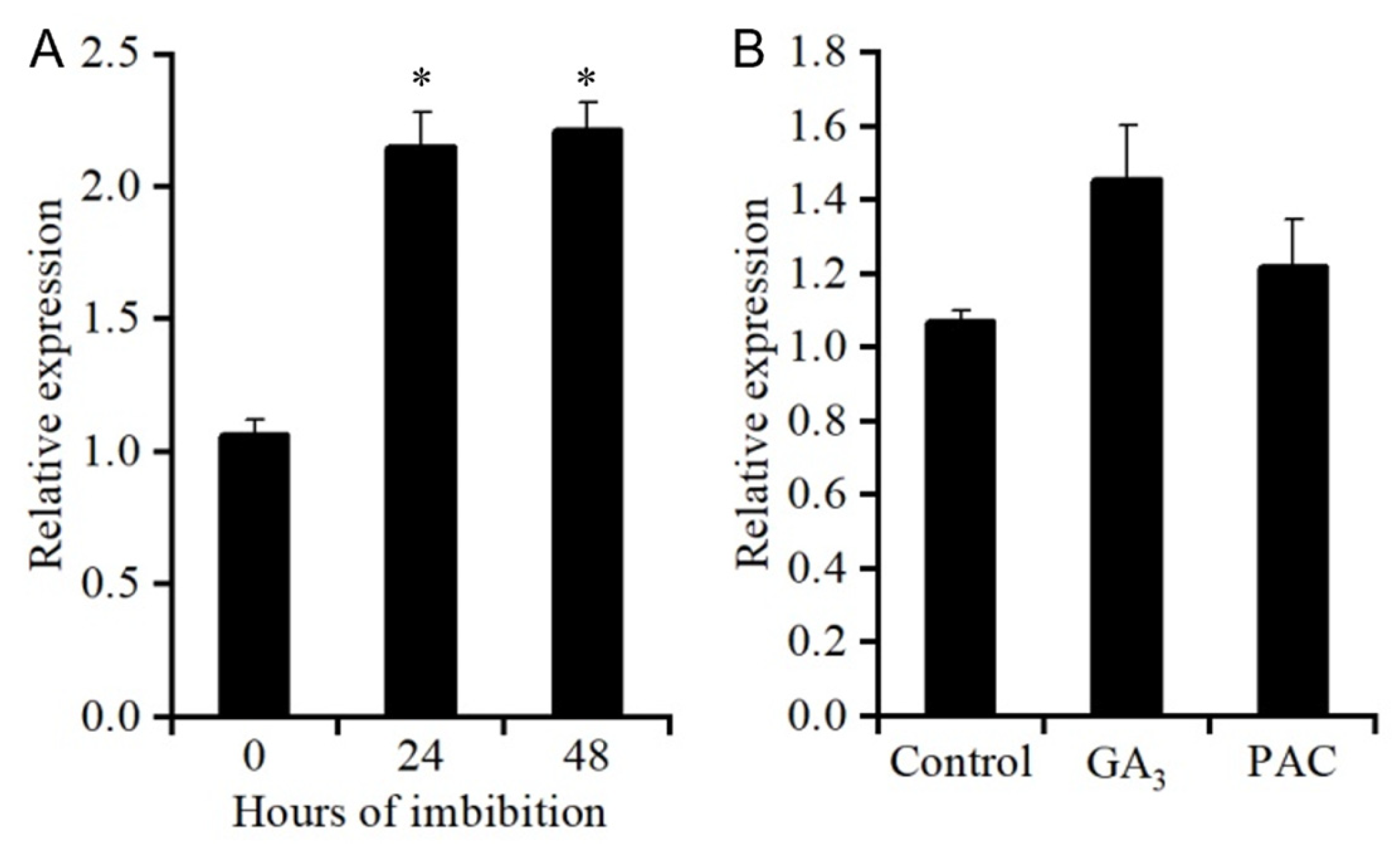
2.9. SSCD1 Expression Was Upregulated During Imbibition of Seeds
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Seed Dormancy and Germination Assays
4.3. Gene Expression Analysis
4.4. ABA and GA Measurements
4.5. Statistical Analysis
4.6. Accession Numbers
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Graeber, K.; Nakabayashi, K.; Miatton, E.; Leubner-Metzger, G.; Soppe, W.J. Molecular mechanisms of seed dormancy. Plant Cell Environ. 2012, 35, 1769–1786. [Google Scholar] [CrossRef]
- Holdsworth, M.J.; Bentsink, L.; Soppe, W.J.J. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol. 2008, 179, 33–54. [Google Scholar] [CrossRef]
- Rueda-Romero, P.; Barrero-Sicilia, C.; Gómez-Cadenas, A.; Carbonero, P.; Oñate-Sánchez, L. Arabidopsis thaliana DOF6 negatively affects germination in non-after-ripened seeds and interacts with TCP14. J. Exp. Bot. 2012, 63, 1937–1949. [Google Scholar] [CrossRef]
- Buijs, G. A perspective on secondary seed dormancy in Arabidopsis thaliana. Plants 2020, 9, 749. [Google Scholar] [CrossRef]
- Lefebvre, V.; North, H.; Frey, A.; Sotta, B.; Seo, M.; Okamoto, M.; Nambara, E.; Marion-Poll, A. Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J. 2006, 45, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Hauser, F.; Waadt, R.; Schroeder, J.I. Evolution of abscisic acid synthesis and signaling mechanisms. Curr. Biol. 2011, 21, R346–R355. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Zhao, Y.; Feng, Z.; Li, Q.; Yang, H.Q.; Luan, S.; Li, J.; He, Z.H. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.; Zhang, H.; Wang, S.; Chen, M.; Wu, Y.; Tang, S.; Liu, C.; Feng, Y.; Cao, X.; Xie, Q. ABI4 regulates primary seed dormancy by regulating the biogenesis of abscisic acid and gibberellins in Arabidopsis. PLoS Genet. 2013, 9, e1003577. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P. The current status of research on gibberellin biosynthesis. Plant Cell Physiol. 2020, 61, 1832–1849. [Google Scholar] [CrossRef]
- Mitchum, M.G.; Yamaguchi, S.; Hanada, A.; Kuwahara, A.; Yoshioka, Y.; Kato, T.; Tabata, S.; Kamiya, Y.; Sun, T.P. Distinct and overlapping roles of two gibberellin 3-oxidases in Arabidopsis development. Plant J. 2006, 45, 804–818. [Google Scholar] [CrossRef]
- Rieu, I.; Ruiz-Rivero, O.; Fernandez-Garcia, N.; Griffiths, J.; Powers, S.J.; Gong, F.; Linhartova, T.; Eriksson, S.; Nilsson, O.; Thomas, S.G.; et al. The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. Plant J. 2008, 53, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Takeda-Kamiya, N.; Hanada, A.; Ogawa, M.; Kuwahara, A.; Seo, M.; Kamiya, Y.; Yamaguchi, S. Contribution of gibberellin deactivation by AtGA2ox2 to the suppression of germination of dark-imbibed Arabidopsis thaliana seeds. Plant Cell Physiol. 2007, 48, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Caruso, L.V.; Downie, A.B.; Perry, S.E. The embryo MADS domain protein AGAMOUS-Like 15 directly regulates expression of a gene encoding an enzyme involved in gibberellin metabolism. Plant Cell 2004, 16, 1206–1219. [Google Scholar] [CrossRef]
- Alabadí, D.; Sun, T.P. Green revolution DELLA proteins, functional analysis and regulatory mechanisms. Annu. Rev. Plant Biol. 2025, 76, 373–400. [Google Scholar] [CrossRef]
- Lee, S.; Cheng, H.; King, K.E.; Wang, W.; He, Y.; Hussain, A.; Lo, J.; Harberd, N.P.; Peng, J. Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev. 2002, 16, 646–658. [Google Scholar] [CrossRef]
- Amir, R.; Galili, G.; Cohen, H. The metabolic roles of free amino acids during seed development. Plant Sci. 2018, 275, 11–18. [Google Scholar] [CrossRef]
- Stamm, P.; Ravindran, P.; Mohanty, B.; Tan, E.L.; Yu, H.; Kumar, P.P. Insights into the molecular mechanism of RGL2-mediated inhibition of seed germination in Arabidopsis thaliana. BMC Plant Biol. 2012, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, B.; Lindstedt, S.; Steen, G. On the enzymic defects in hereditary tyrosinemia. Proc. Natl. Acad. Sci. USA 1977, 74, 4641–4645. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araújo, W.L.; Braun, H.P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef]
- Schenck, C.A.; Maeda, H.A. Tyrosine biosynthesis, metabolism, and catabolism in plants. Phytochemistry 2018, 149, 82–102. [Google Scholar] [CrossRef]
- St-Louis, M.; Tanguay, R.M. Mutations in the fumarylacetoacetate hydrolase gene causing hereditary tyrosinemia type I, overview. Human. Mutat. 1997, 9, 291–299. [Google Scholar] [CrossRef]
- Fisher, A.L.; Page, K.E.; Lithgow, G.J.; Nash, L. The Caenorhabditis elegans K10C2.4 gene encodes a member of the fumarylacetoacetate hydrolase family. J. Biol. Chem. 2008, 283, 9127–9135. [Google Scholar] [CrossRef]
- Gerna, D.; Arc, E.; Holzknecht, M.; Roach, T.; Jansen-Dürr, P.; Weiss, A.K.H.; Kranner, I. AtFAHD1a: A new player influencing seed longevity and dormancy in Arabidopsis? Int. J. Mol. Sci. 2021, 22, 2997. [Google Scholar] [CrossRef]
- Dixon, D.P.; Edwards, R. Enzymes of tyrosine catabolism in Arabidopsis thaliana. Plant Sci. 2006, 171, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Ren, C.; Zhi, T.; Zhou, Z.; Liu, Y.; Chen, F.; Peng, W.; Xie, D. Disruption of fumarylacetoacetate hydrolase causes spontaneous cell death under short-day conditions in Arabidopsis. Plant Physiol. 2013, 162, 1956–1964. [Google Scholar] [CrossRef]
- Zhi, T.; Zhou, Z.; Qiu, B.; Zhu, Q.; Xiong, X.; Ren, C. Loss of fumarylacetoacetate hydrolase causes light-dependent increases in protochlorophyllide and cell death in Arabidopsis. Plant J. 2019, 98, 622–638. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhi, T.; Han, C.; Peng, Z.; Wang, R.; Tong, J.; Zhu, Q.; Ren, C. Cell death resulted from loss of fumarylacetoacetate hydrolase in Arabidopsis is related to phytohormone jasmonate but not salicylic acid. Sci. Rep. 2020, 10, 13714. [Google Scholar] [CrossRef]
- Hu, C.; Huang, L.; Chen, Y.; Liu, J.; Wang, Z.; Gao, B.; Zhu, Q.; Ren, C. Fumarylacetoacetate hydrolase is required for fertility in rice. Planta 2021, 253, 122. [Google Scholar] [CrossRef]
- Olszewski, N.; Sun, T.P.; Gubler, F. Gibberellin signaling, biosynthesis, catabolism, and response pathways. Plant Cell 2002, 14, S61–S80. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, P.; Huang, M.; Tang, Y.; Li, Y.; Li, L.; Hou, X. The NF-YC-RGL2 module integrates GA and ABA signalling to regulate seed germination in Arabidopsis. Nat. Commun. 2016, 7, 12768. [Google Scholar] [CrossRef]
- Cao, D.; Cheng, H.; Wu, W.; Soo, H.M.; Peng, J. Gibberellin mobilizes distinct DELLA-dependent transcriptomes to regulate seed germination and floral development in Arabidopsis. Plant Physiol. 2006, 142, 509–525. [Google Scholar] [CrossRef]
- Voegele, A.; Linkies, A.; Müller, K.; Leubner-Metzger, G. Members of the gibberellin receptor gene family GID1 GIBBERELLIN INSENSITIVE DWARF1, play distinct roles during Lepidium sativum and Arabidopsis thaliana seed germination. J. Exp. Bot. 2011, 62, 5131–5147. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Xu, H.; Ye, S.; Wang, S.; Li, L.; Zhang, S.; Wang, X. Gibberellic acid-stimulated Arabidopsis6 serves as an integrator of gibberellin, abscisic acid, and glucose signaling during seed germination in Arabidopsis. Plant Physiol. 2015, 169, 2288–2303. [Google Scholar] [PubMed]
- Xu, H.; Lantzouni, O.; Bruggink, T.; Benjamins, R.; Lanfermeijer, F.; Denby, K.; Schwechheimer, C.; Bassel, G.W. A molecular signal integration network underpinning Arabidopsis seed germination. Curr. Biol. 2020, 30, 3703–3712. [Google Scholar] [CrossRef]
- Huang, L.; Hu, C.; Cai, W.; Zhu, Q.; Gao, B.; Zhang, X.; Ren, C. Fumarylacetoacetate hydrolase is involved in salt stress response in Arabidopsis. Planta 2018, 248, 499–511. [Google Scholar] [CrossRef]
- Prieto-Alamo, M.J.; Laval, F. Deficient DNA-ligase activity in the metabolic disease tyrosinemia type I. Proc. Natl. Acad. Sci. USA 1998, 95, 12614–12618. [Google Scholar] [CrossRef] [PubMed]
- van Dyk, E.; Pretorius, P.J. Impaired DNA repair and genomic stability in hereditary tyrosinemia type 1. Gene 2012, 495, 56–61. [Google Scholar] [CrossRef]
- Manabe, S.; Sassa, S.; Kappas, A. Hereditary tyrosinemia. Formation of succinylacetone-amino acid adducts. J. Exp. Med. 1985, 162, 1060–1074. [Google Scholar] [CrossRef]
- Taferner, A.; Pircher, H.; Koziel, R.; von Grafenstein, S.; Baraldo, G.; Palikaras, K.; Liedl, K.R.; Tavernarakis, N.; Jansen-Dürr, P. FAH domain containing protein 1 (FAHD-1) is required for mitochondrial function and locomotion activity in C. elegans. PLoS ONE 2015, 10, e0134161. [Google Scholar] [CrossRef]
- Alonso, J.M.; Stepanova, A.N.; Leisse, T.J.; Kim, C.J.; Chen, H.; Shinn, P.; Stevenson, D.K.; Zimmerman, J.; Barajas, P.; Cheuk, R.; et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 2003, 301, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Šimura, J.; Antoniadi, I.; Široká, J.; Tarkowská, D.; Strnad, M.; Ljung, K.; Novák, O. Plant hormonomics, multiple phytohormone profiling by targeted metabolomics. Plant Physiol. 2018, 177, 476–489. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Yang, H.; Zhang, X.; Ren, C.; Huang, L. Fumarylacetoacetate Hydrolase Regulates Seed Dormancy and Germination Through the Gibberellin Pathway in Arabidopsis. Plants 2025, 14, 3342. https://doi.org/10.3390/plants14213342
Hu C, Yang H, Zhang X, Ren C, Huang L. Fumarylacetoacetate Hydrolase Regulates Seed Dormancy and Germination Through the Gibberellin Pathway in Arabidopsis. Plants. 2025; 14(21):3342. https://doi.org/10.3390/plants14213342
Chicago/Turabian StyleHu, Chao, Hua Yang, Xuewen Zhang, Chunmei Ren, and Lihua Huang. 2025. "Fumarylacetoacetate Hydrolase Regulates Seed Dormancy and Germination Through the Gibberellin Pathway in Arabidopsis" Plants 14, no. 21: 3342. https://doi.org/10.3390/plants14213342
APA StyleHu, C., Yang, H., Zhang, X., Ren, C., & Huang, L. (2025). Fumarylacetoacetate Hydrolase Regulates Seed Dormancy and Germination Through the Gibberellin Pathway in Arabidopsis. Plants, 14(21), 3342. https://doi.org/10.3390/plants14213342



