Amplicon Sequencing Reveals Rhizosphere Fungal Dysbiosis Facilitates Goji Berry Root Rot Onset
Abstract
1. Introduction
2. Results
2.1. Sequencing Data Overview
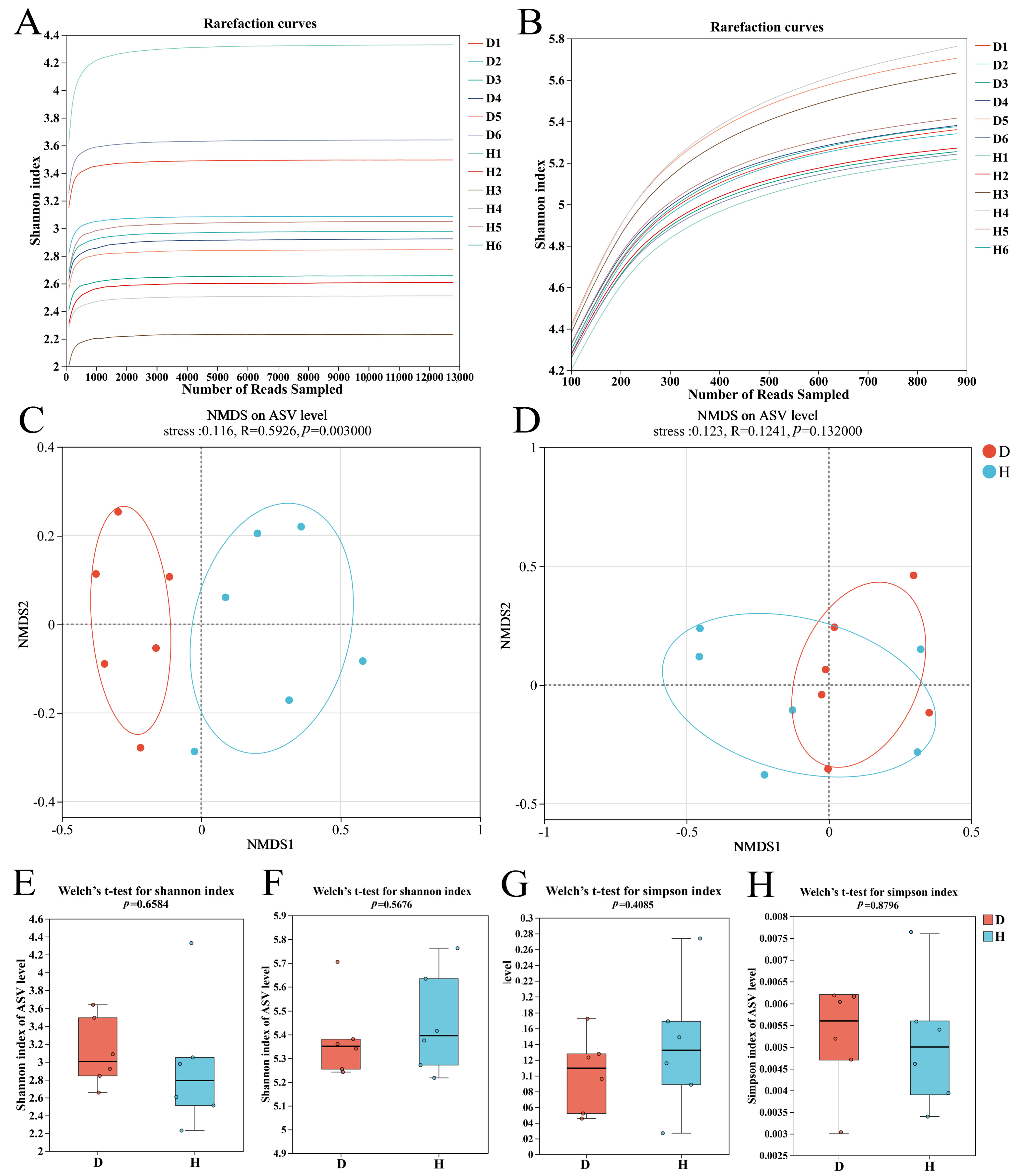
2.2. Root Rot Structurally Alters Microbial Community Diversity
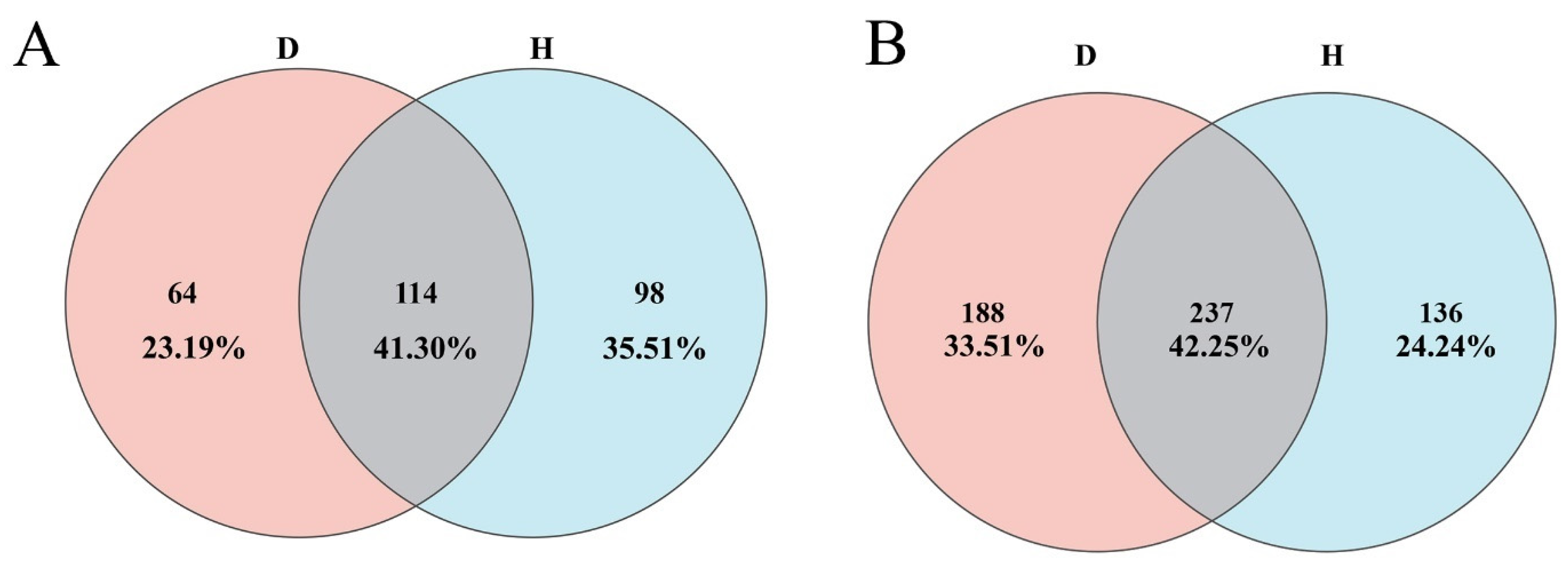
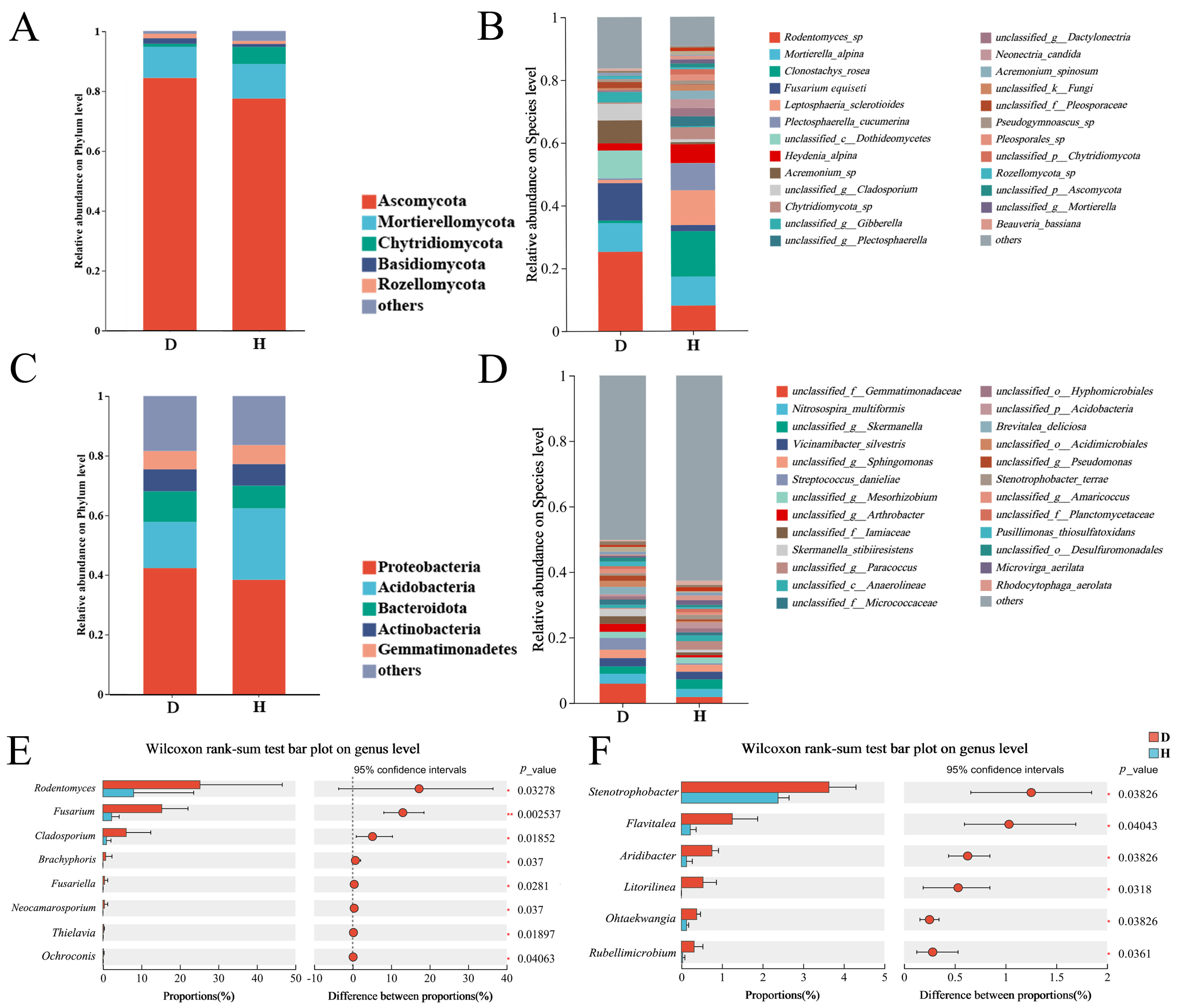
2.3. Community Composition and Key Differential Taxa
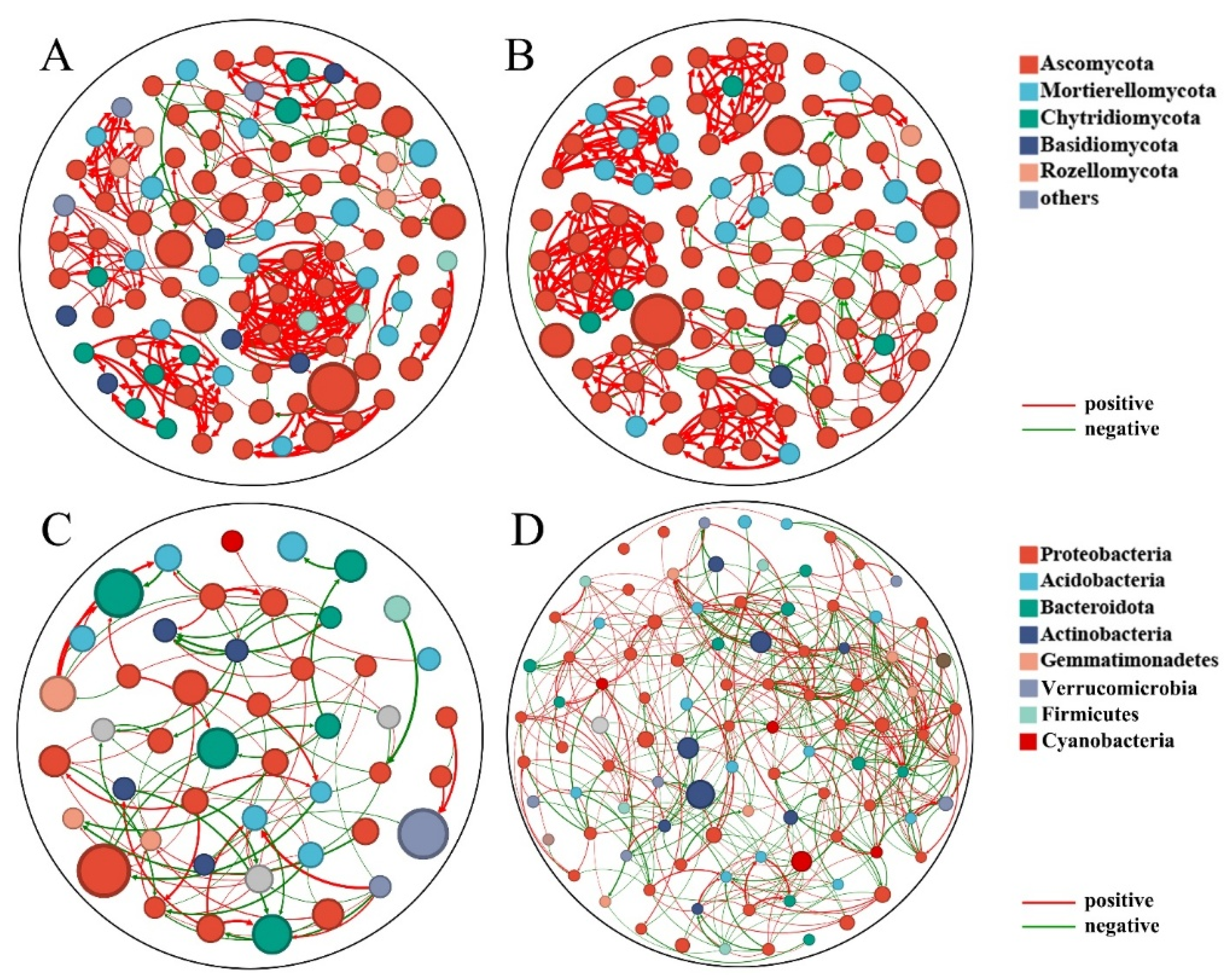
2.4. Co-Occurrence Network Analysis Reveals a Pathogen-Dominated, Destabilized Community
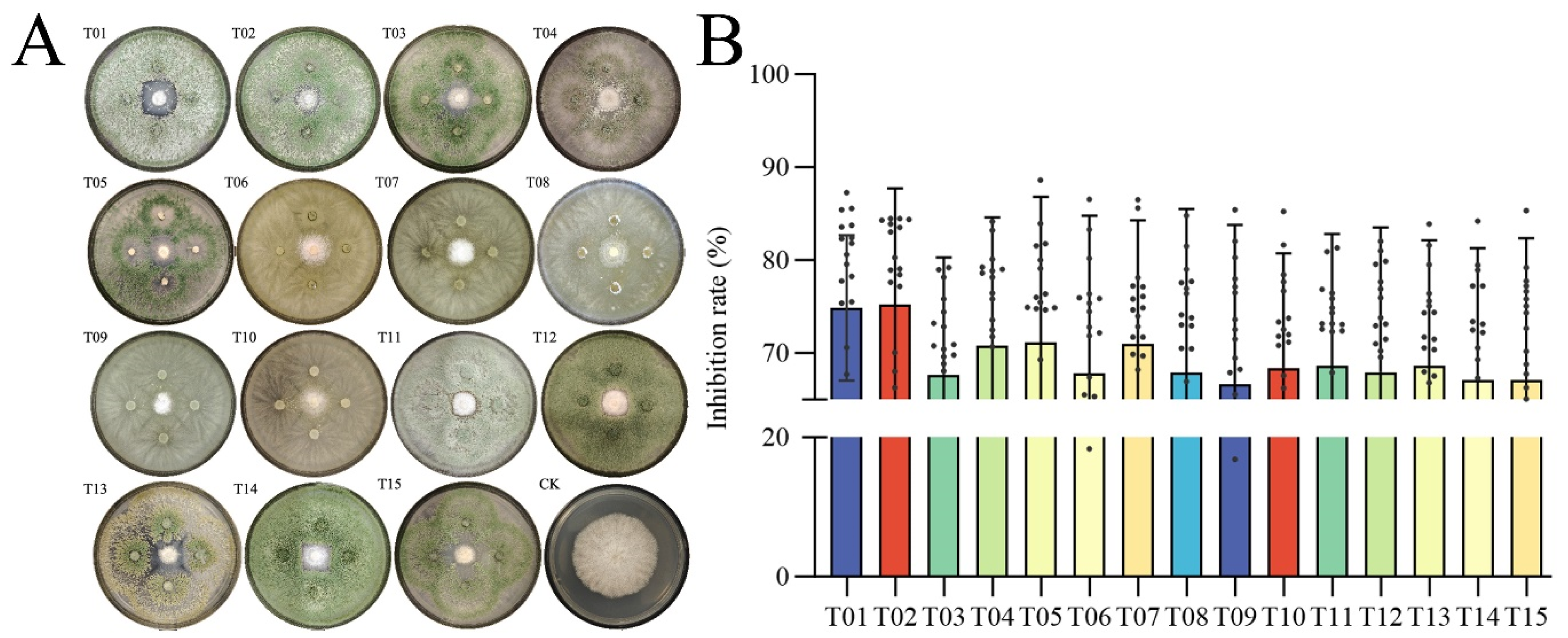
2.5. In Vitro Assays Functionally Validate the Antagonistic Relationship
3. Discussion
4. Materials and Methods
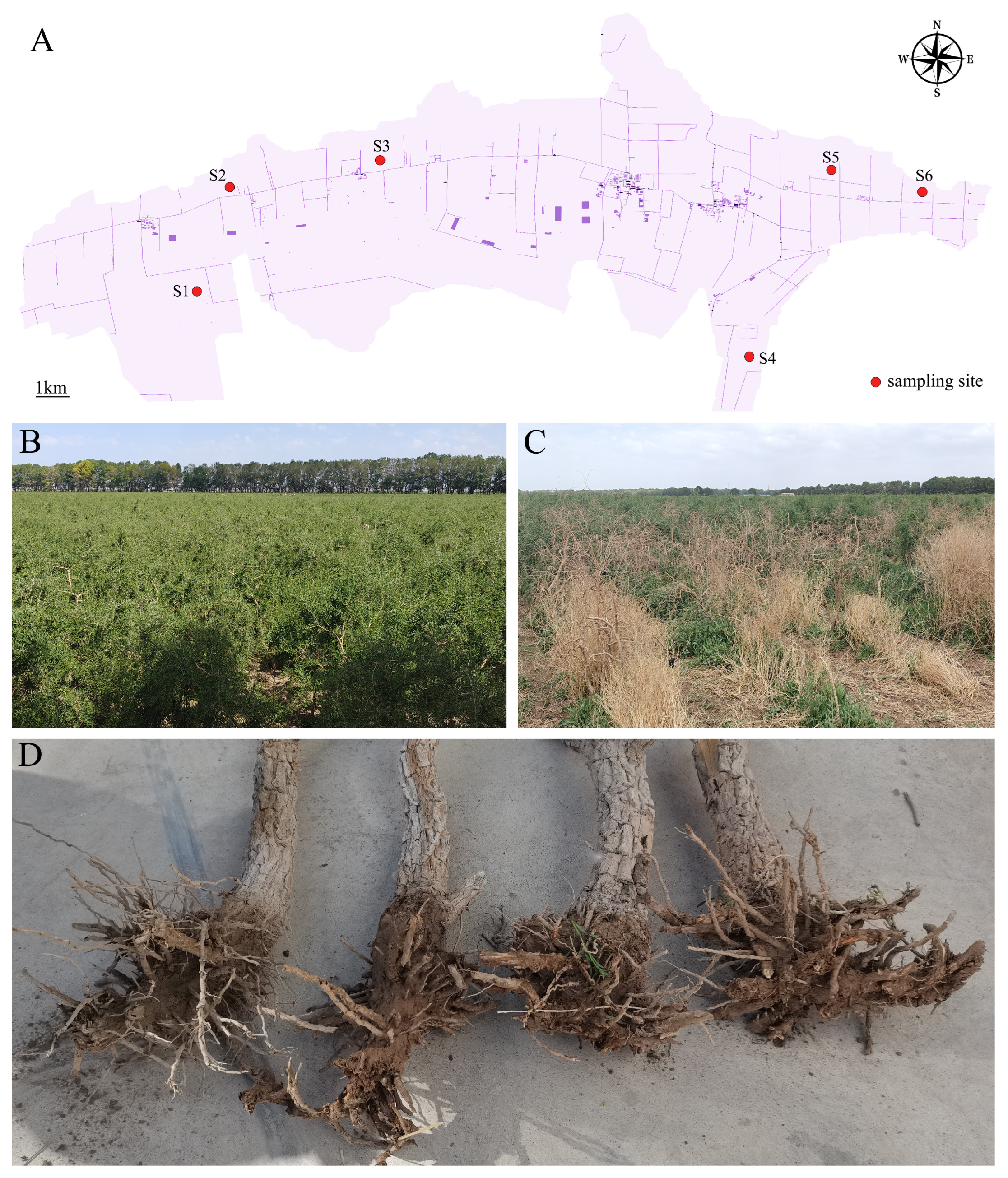
4.1. Sample Collection
4.2. DNA Extraction, PCR Amplification, and PacBio Sequencing
4.3. Bioinformatic and Statistical Analysis
4.4. Differential Abundance and Co-Occurrence Network Analysis
4.5. Fungi Isolation, Identification, and Antagonism Assay
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Tang, W.-M.; Chan, E.; Kwok, C.-Y.; Lee, Y.-K.; Wu, J.-H.; Wan, C.-W.; Chan, R.Y.-K.; Yu, P.H.-F.; Chan, S.-W. A review of the anticancer and immunomodulatory effects of Lycium barbarum fruit. Inflammopharmacology 2012, 20, 307–314. [Google Scholar] [CrossRef]
- Cheng, J.; Zhou, Z.-W.; Sheng, H.-P.; He, L.-J.; Fan, X.-W.; He, Z.-X.; Sun, T.; Zhang, X.; Zhao, R.J.; Gu, L. An evidence-based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides. Drug Des. Dev. Ther. 2014, 9, 33–78. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium barbarum: A traditional Chinese herb and a promising anti-aging agent. Aging Dis. 2017, 8, 778–791. [Google Scholar] [CrossRef]
- Chang, R.C.-C.; So, K.-F. Use of anti-aging herbal medicine, Lycium barbarum, against aging-associated diseases. What do we know so far? Cell. Mol. Neurobiol. 2008, 28, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Cai, Y.; Yan, J.; Sun, M.; Corke, H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004, 76, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Bischin, C.; Hanganu, D.; Gheldiu, A.-M.; Oprean, R.; Silaghi-Dumitrescu, R.; Crișan, G. Polyphenolic content, antioxidant and antimicrobial activities of Lycium barbarum L. and Lycium chinense Mill. leaves. Molecules 2014, 19, 10056–10073. [Google Scholar] [CrossRef] [PubMed]
- Kulczyński, B.; Gramza-Michałowska, A. Goji berry (Lycium barbarum): Composition and health effects—A review. Pol. J. Food Nutr. Sci. 2016, 66, 67–75. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Zhang, X.; Wang, J.; Hou, T.; He, J.; Li, J. Integration of Transcriptome and Metabolome Reveals Wax Serves a Key Role in Preventing Leaf Water Loss in Goji (Lycium barbarum). Int. J. Mol. Sci. 2024, 25, 10939. [Google Scholar] [CrossRef]
- Chen, Y.; Lou, S.; Chen, X.; Yang, S. Effects of brackish water irrigation with different exogenous salt concentrations on the growth and rhizosphere salinity of Lycium barbarum. Sci. Rep. 2024, 14, e21554. [Google Scholar] [CrossRef]
- Sun, C.; Su, J.; Wang, J.; Ding, K.; Chen, C. Lycium barbarum polysaccharide increases thermogenesis and energy metabolism through modulation of the gut microbiota to confer resistance to cold temperatures. FASEB J. 2024, 38, e70010. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, J.; Chen, Y.; Liang, A.; He, W.; Qin, X.; Qin, K.; Mu, Z. Genome-Wide Identification of PYL/RCAR ABA Receptors and Functional Analysis of LbPYL10 in Heat Tolerance in Goji (Lycium barbarum). Plants 2024, 13, 887. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-L.; Li, Y.-l.; Fan, Y.-F.; Li, Z.; Yoshida, K.; Wang, J.-Y.; Ma, X.-K.; Wang, N.; Mitsuda, N.; Kotake, T. Wolfberry genomes and the evolution of Lycium (Solanaceae). Commun. Biol. 2021, 4, e671. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Heinrich, M.; Weckerle, C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J. Ethnopharmacol. 2018, 212, 50–66. [Google Scholar] [CrossRef]
- Xue, J.; Lu, Y.; He, J.; Xie, M.; He, K.; Wang, H. Lycium barbarum studies: A system review on molecular biology, cultivation, and quality characteristics of goji berries. Biochem. Syst. Ecol. 2025, 121, e105020. [Google Scholar] [CrossRef]
- Zhang, Z.; He, K.; Zhang, T.; Tang, D.; Li, R.; Jia, S. Physiological responses of Goji berry (Lycium barbarum L.) to saline-alkaline soil from Qinghai region, China. Sci. Rep. 2019, 9, e12057. [Google Scholar] [CrossRef]
- Feng, Z.; Xiao, Y.; Li, N.; Gao, Q.; Wang, J.; Chen, S.-l.; Xing, R. Effects of root rot on microbial communities associated with goji berry (Lycium barbarum) in the Qaidam Basin, China. Eur. J. Plant Pathol. 2023, 167, 853–866. [Google Scholar] [CrossRef]
- Yin, Y.; Miao, J.; Shao, W.; Liu, X.; Zhao, Y.; Ma, Z. Fungicide resistance: Progress in understanding mechanism, monitoring, and management. Phytopathology 2023, 113, 707–718. [Google Scholar] [CrossRef]
- Naqvi, S.A.H.; Farhan, M.; Ahmad, M.; Kiran, R.; Shahbaz, M.; Abbas, A.; Hakim, F.; Shabbir, M.; Tan, Y.S.; Sathiya Seelan, J.S. Fungicide resistance in Fusarium species: Exploring environmental impacts and sustainable management strategies. Arch. Microbiol. 2025, 207, e31. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; De Bruijn, I.; Dekkers, E.; Van Der Voort, M.; Schneider, J.H.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Zhou, L.; Pei, F.; Pu, W.; Zhang, C.; Chen, F.; Hu, Y.; Chen, J.; Lin, H.; Zhou, X. Diversity Analysis and Biocontrol Potential of Cultivatable Terrestrial Bacterial Streptomyces in Southern China. Agronomy 2023, 13, 2500. [Google Scholar] [CrossRef]
- Wu, Z.; Hao, Z.; Sun, Y.; Guo, L.; Huang, L.; Zeng, Y.; Wang, Y.; Yang, L.; Chen, B. Comparison on the structure and function of the rhizosphere microbial community between healthy and root-rot Panax notoginseng. Appl. Soil Ecol. 2016, 107, 99–107. [Google Scholar] [CrossRef]
- Bi, Y.-M.; Zhang, X.-M.; Jiao, X.-L.; Li, J.-F.; Peng, N.; Tian, G.-L.; Wang, Y.; Gao, W.-W. The relationship between shifts in the rhizosphere microbial community and root rot disease in a continuous cropping American ginseng system. Front. Microbiol. 2023, 14, e1097742. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chen, Y.; Lin, Z.; Tuo, Y.; Li, H.; Wang, Y. Differences in soil microbial community composition between suppressive and root rot-conducive in tobacco fields. Curr. Microbiol. 2021, 78, 624–633. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Wang, P.; Yang, L.; Sun, J.; Yang, Y.; Qu, Y.; Wang, C.; Liu, D.; Huang, L.; Cui, X.; Liu, Y. Structure and function of rhizosphere soil and root endophytic microbial communities associated with root rot of Panax notoginseng. Front. Plant Sci. 2022, 12, e752683. [Google Scholar] [CrossRef]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G. Next-generation sequencing technology: Current trends and advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef]
- Knight, R.; Callewaert, C.; Marotz, C.; Hyde, E.R.; Debelius, J.W.; McDonald, D.; Sogin, M.L. The microbiome and human biology. Annu. Rev. Genom. Hum. Genet. 2017, 18, 65–86. [Google Scholar] [CrossRef]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef]
- Li, P.; Gu, S.; Zhu, Y.; Xu, T.; Yang, Y.; Wang, Z.; Deng, X.; Wang, B.; Li, W.; Mei, W. Soil microbiota plays a key regulatory role in the outbreak of tobacco root rot. Front. Microbiol. 2023, 14, e1214167. [Google Scholar] [CrossRef]
- Gordon, T.R.; Martyn, R.D. The evolutionary biology of Fusarium oxysporum. Annu. Rev. Phytopathol. 1997, 35, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, J.; Cheng, Y.; Zhang, Z.; Wang, X. Study on Control Technology of Green Security of Diseases and Insect Pests about Wolfberry. Hans J. Agric. Sci. 2018, 08, 1183–1191. [Google Scholar] [CrossRef]
- Jackson, E.; Li, J.; Weerasinghe, T.; Li, X. The Ubiquitous Wilt-Inducing Pathogen Fusarium oxysporum-A Review of Genes Studied with Mutant Analysis. Pathogens 2024, 13, 823. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Calabria, J.; Chen, H.-W.; Somssich, M. The Arabidopsis thaliana–Fusarium oxysporum strain 5176 pathosystem: An overview. J. Exp. Bot. 2022, 73, 6052–6067. [Google Scholar] [CrossRef]
- Okungbowa, F.; Shittu, H. Fusarium Wilts: An Overview. Environ. Res. J. 2014, 6, 83–102. [Google Scholar]
- Codello, A.; Hose, G.C.; Chariton, A. Microbial co-occurrence networks as a biomonitoring tool for aquatic environments: A review. Mar. Freshw. Res. 2022, 74, 409–422. [Google Scholar] [CrossRef]
- Wang, B.; Geng, Y.; Lin, Y.; Xia, Q.; Wei, F.; Yang, S.; Huang, X.; Zhang, J.; Cai, Z.; Zhao, J. Root rot destabilizes the Sanqi rhizosphere core fungal microbiome by reducing the negative connectivity of beneficial microbes. Appl. Environ. Microbiol. 2024, 90, e0223723. [Google Scholar] [CrossRef]
- Liu, L.; Jin, Y.; Chen, M.; Lian, H.; Liu, Y.; Yin, Q.; Wang, H. A Study of Soil-Borne Fusarium Wilt in Continuous Cropping Chrysanthemum Cultivar ‘Guangyu’in Henan, China. J. Fungi 2023, 10, 14. [Google Scholar] [CrossRef]
- Awal, M.A.; Abdullah, N.S.; Prismantoro, D.; Dwisandi, R.F.; Safitri, R.; Mohd-Yusuf, Y.; Mohd Suhaimi, N.S.; Doni, F. Mechanisms of action and biocontrol potential of Trichoderma against Fusarium in horticultural crops. Cogent Food Agric. 2024, 10, e2394685. [Google Scholar] [CrossRef]
- Sharma, I.P.; Sharma, A.K. Trichoderma–Fusarium Interactions: A Biocontrol Strategy to Manage Wilt. In Trichoderma: Host Pathogen Interactions and Applications; Sharma, A.K., Sharma, P., Eds.; Springer: Singapore, 2020; pp. 167–185. [Google Scholar] [CrossRef]
- Hiltner, L. Über neuere Erfahrungen und Probleme auf dem Gebiete der Bodenbakteriologie und unter Berücksichtigung der Gründüngung und Brache. Dtsch. Landwirtsch.-Ges. Berl. Arb. Der DLG 1904, 98, 59–78. [Google Scholar]
- Lane, D. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; John Wiley and Sons: Chichester, UK, 1991. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 9780813819198. [Google Scholar]
- Petrini, O.; Kuhls, K.; Lieckfeldt, E.; Kubicek, C. The Hypocrea schweinitzii complex and Trichoderma sect. Longibrachiatum. Stud. Mycol. 1998, 41, 1–54. [Google Scholar]
- Kumar, R.; Swain, D.M.; Yadav, S.K.; Tyagi, I.; Kumar, R.; Das, J.; Ghosh, S.; Jha, G. Bacteria-fungal confrontation and fungal growth prevention assay. Bio-Protocol 2018, 8, e2694. [Google Scholar] [CrossRef]
| Amplicon | Sample Name | Raw Reads | Clean Reads | Base Number | Average Length | ASVs Number |
|---|---|---|---|---|---|---|
| Fungi | H (Mean ± SD) | 27,754.7 ± 49 | 26,965.3 ± 46 | 16,554.8 ± 27 | 616.8 ± 14.2 | 97.7 ± 10.3 |
| D (Mean ± SD) | 36,189.2 ± 12 | 34,625.8 ± 11 | 22,254.2 ± 70 | 637.7 ± 15.1 | 133.0 ± 68.3 | |
| Bacteria | H (Mean ± SD) | 27,562.7 ± 46 | 27,479.5 ± 46 | 41,085.9 ± 68 | 1489.0 ± 2.2 | 359.0 ± 113.8 |
| D (Mean ± SD) | 29,064.0 ± 71 | 28,993.2 ± 71 | 46,249.5 ± 11 | 1491.8 ± 6.0 | 459.5 ± 204.4 |
| Amplicon | Estimators | p-Value | D-Mean | H-Mean |
|---|---|---|---|---|
| Fungi | Shannon | 0.6584 | 2.9509 | 3.1076 |
| Simpson | 0.4085 | 0.1372 | 0.1028 | |
| Chao | 0.2808 | 131.0429 | 96.1250 | |
| Bacteria | Shannon | 0.5676 | 5.3806 | 5.4460 |
| Simpson | 0.8796 | 0.0052 | 0.0051 | |
| Chao | 0.2848 | 342.6116 | 427.9333 |
| Fungus (D) | Fungus (H) | Bacteria (D) | Bacteria (H) | |
|---|---|---|---|---|
| Node | 100 | 100 | 46 | 98 |
| Edge | 289 | 292 | 99 | 449 |
| Positive Correlation Rate | 81.16% | 83.74% | 51.52% | 50.11 |
| Negative Correlation Rate | 18.84% | 16.26% | 48.48% | 49.89 |
| Average Degree | 2.89 | 2.92 | 2.152 | 4.582 |
| Graph Density | 0.021 | 0.029 | 0.048 | 0.047 |
| Modularity | 0.413 | 0.818 | 0.554 | 0.514 |
| Average Clustering Coefficient | 0.347 | 0.379 | 0.236 | 0.242 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Chen, Y.; Yan, M.; Wang, H.; Guo, K.; Zhou, X.; Qi, H.; Zhou, L. Amplicon Sequencing Reveals Rhizosphere Fungal Dysbiosis Facilitates Goji Berry Root Rot Onset. Plants 2025, 14, 3325. https://doi.org/10.3390/plants14213325
Wang T, Chen Y, Yan M, Wang H, Guo K, Zhou X, Qi H, Zhou L. Amplicon Sequencing Reveals Rhizosphere Fungal Dysbiosis Facilitates Goji Berry Root Rot Onset. Plants. 2025; 14(21):3325. https://doi.org/10.3390/plants14213325
Chicago/Turabian StyleWang, Tianyu, Yao Chen, Meng Yan, Haonan Wang, Kai Guo, Xudong Zhou, Hexing Qi, and Lifeng Zhou. 2025. "Amplicon Sequencing Reveals Rhizosphere Fungal Dysbiosis Facilitates Goji Berry Root Rot Onset" Plants 14, no. 21: 3325. https://doi.org/10.3390/plants14213325
APA StyleWang, T., Chen, Y., Yan, M., Wang, H., Guo, K., Zhou, X., Qi, H., & Zhou, L. (2025). Amplicon Sequencing Reveals Rhizosphere Fungal Dysbiosis Facilitates Goji Berry Root Rot Onset. Plants, 14(21), 3325. https://doi.org/10.3390/plants14213325






