Metabolic Responses to the Zinc Stress in the Roots and Leaves of Amaranthus caudatus: The Proteomics View
Abstract
1. Introduction
2. Results
2.1. Suppressing Exposure to Zn Caused Suppression of the Photosystem II Efficiency in the A. caudatus Leaves
2.2. Protein Isolation and Tryptic Digestion
2.3. Identification of Peptides and Annotation of Proteins
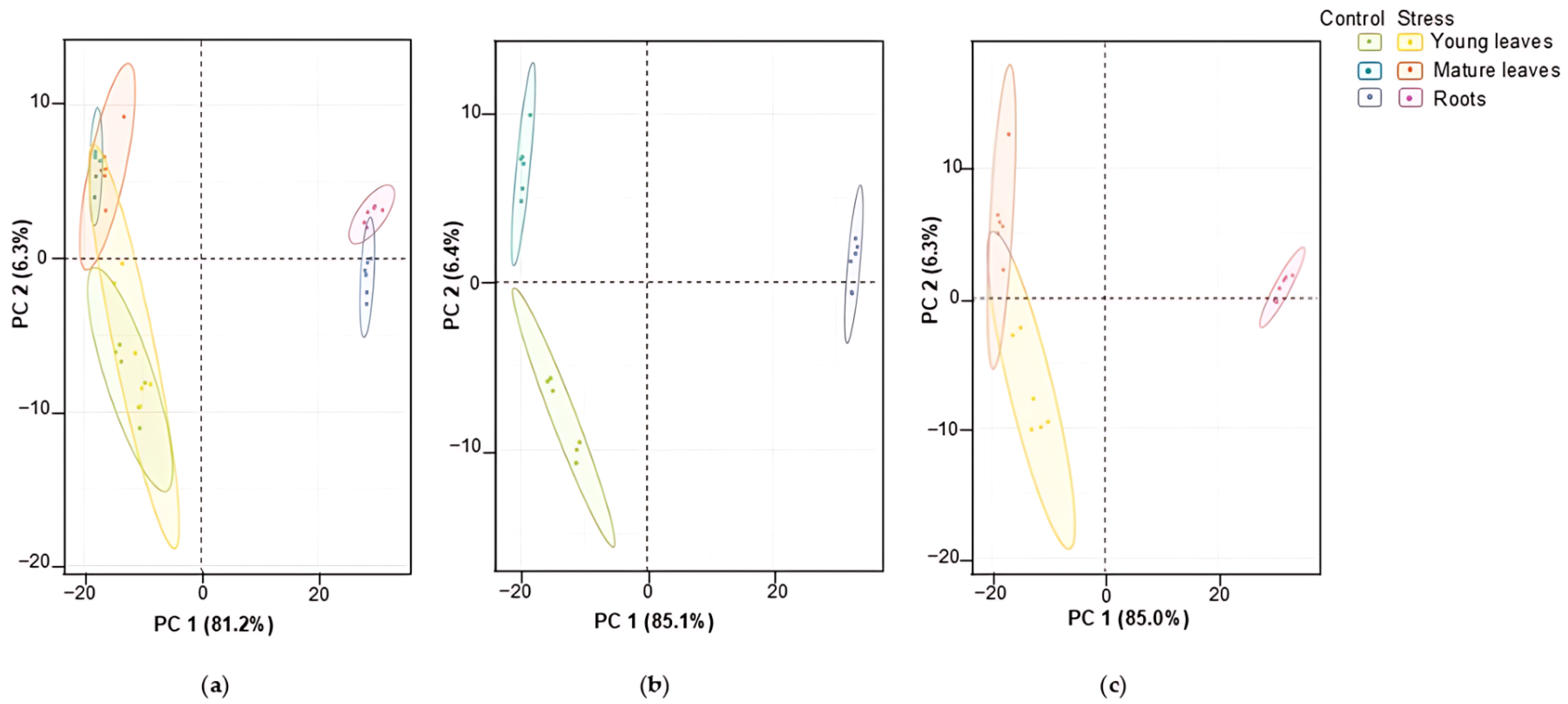
2.4. Label-Free Quantification
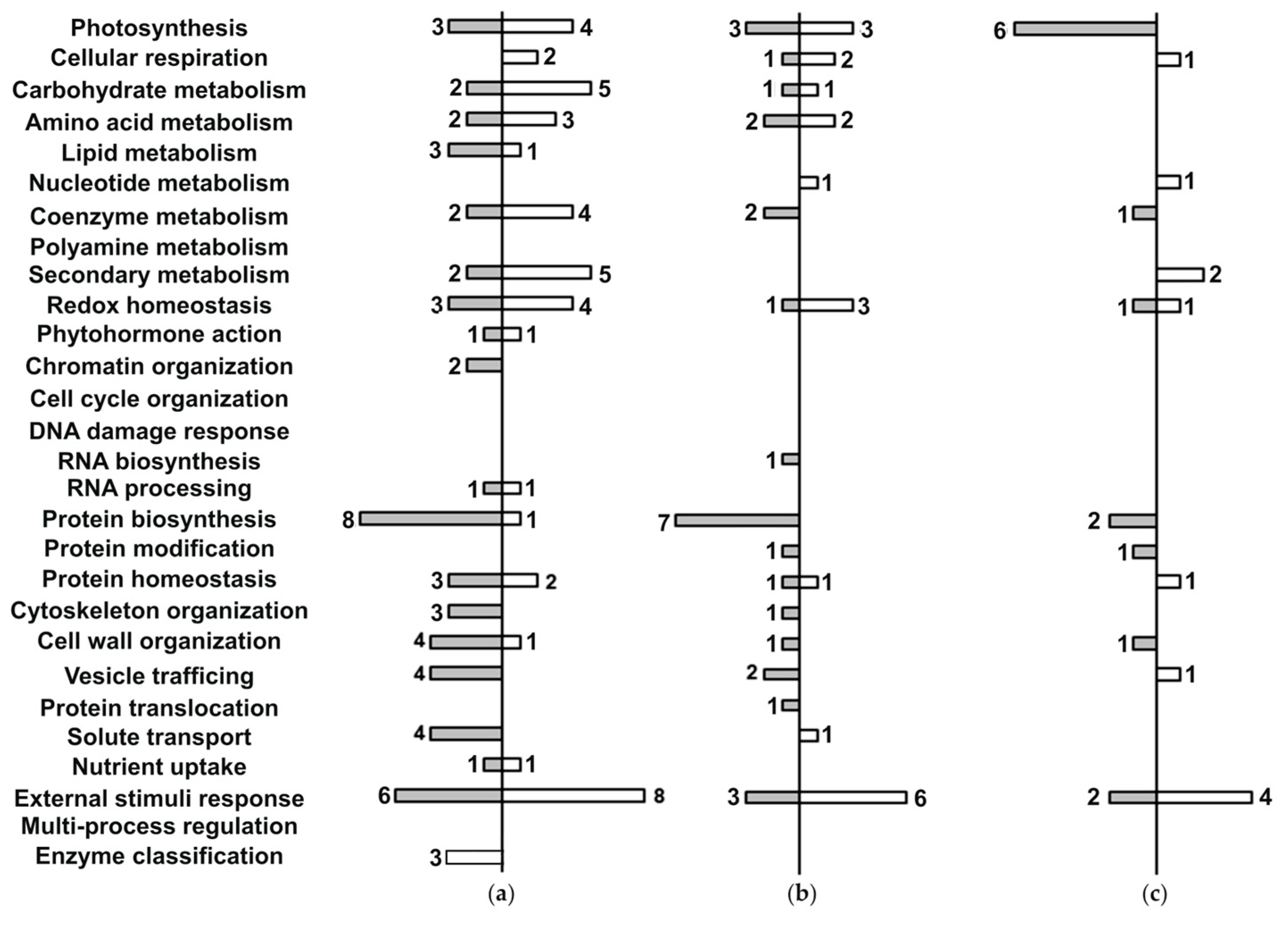
2.5. Functional Annotation of the Differentially Accumulated Proteins
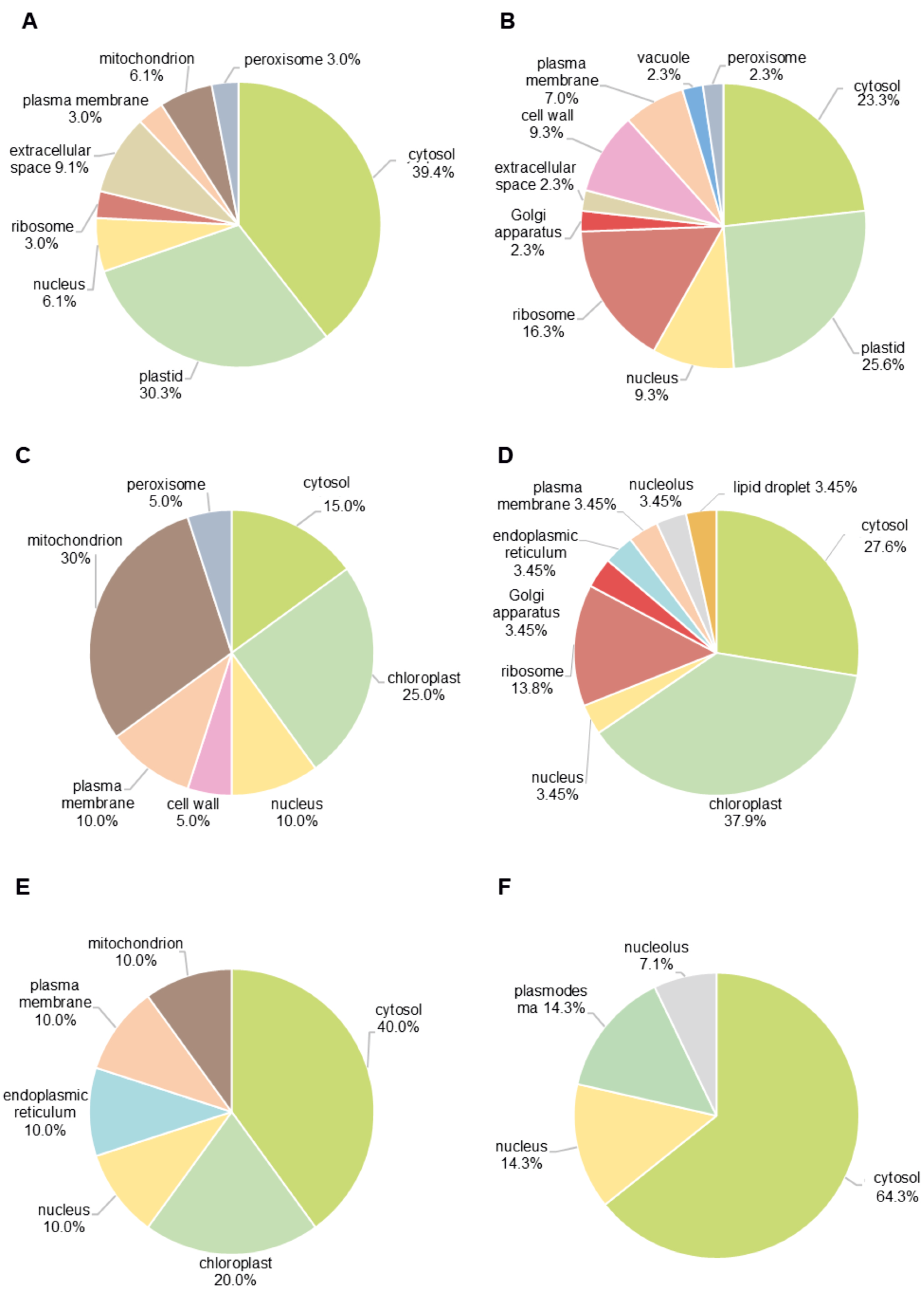
2.6. Prediction of Sub-Cellular Localization of Zn Stress-Exposed Differentially Accumulated Proteins
3. Discussion
3.1. Plant Response to Zn2+ Was Accompanied by Characteristic Changes in Redox Metabolism and Stress-Inducible Proteome
3.2. Exposure to Zn2+ Affects Protein Biosynthesis and Homeostasis
3.3. Zn Stress Results in Expressional Suppression of the Electron Transport Chain Proteins in the Chloroplast
3.4. Zn Stress Results in Rearrangement of Sugar and Energy Metabolism in A. caudatus Roots
3.5. The Excess of the Zn(II) Ions Is Sequestered into the Vacuole and Cell Wall by Means of Several Transport Protein Systems
3.6. Consideration of the Zn-Induced Alterations in A. caudatus Proteome in the Context of the Accompanying Metabolic Adjustments Gives Access to the Mechanisms of Zn Stress Tolerance
4. Materials and Methods
4.1. Plant Growth Conditions and Zn Stress Application
4.2. Protein Isolation and Digestion
4.3. Nano LC-MS/MS Experiments
4.4. Processing, Post-Processing, and Statistical Analysis of the Proteomics Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ali, A.; Bhat, B.A.; Rather, G.A.; Malla, B.A.; Ganie, S.A. Proteomic Studies of Micronutrient Deficiency and Toxicity. In Plant Micronutrients: Deficiency and Toxicity Management; Aftab, T., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 257–284. ISBN 978-3-030-49856-6. [Google Scholar]
- Hamzah Saleem, M.; Usman, K.; Rizwan, M.; Al Jabri, H.; Alsafran, M. Functions and Strategies for Enhancing Zinc Availability in Plants for Sustainable Agriculture. Front. Plant Sci. 2022, 13, 1033092. [Google Scholar] [CrossRef]
- Stanton, C.; Sanders, D.; Krämer, U.; Podar, D. Zinc in Plants: Integrating Homeostasis and Biofortification. Mol. Plant 2022, 15, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in Plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Claus, J.; Bohmann, A.; Chavarría-Krauser, A. Zinc Uptake and Radial Transport in Roots of Arabidopsis Thaliana: A Modelling Approach to Understand Accumulation. Ann. Bot. 2013, 112, 369–380. [Google Scholar] [CrossRef]
- Krämer, U. Changing Paradigms for the Micronutrient Zinc, a Known Protein Cofactor, as a Signal Relaying Also Cellular Redox State. Quant. Plant Biol. 2025, 6, e7. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Physiological Limits to Zinc Biofortification of Edible Crops. Front. Plant Sci. 2011, 2, 80. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular Mechanisms for Heavy Metal Detoxification and Tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Kaur, H.; Garg, N. Zinc Toxicity in Plants: A Review. Planta 2021, 253, 129. [Google Scholar] [CrossRef]
- Oyewo, O.A.; Adeniyi, A.; Bopape, M.F.; Onyango, M.S. Heavy Metal Mobility in Surface Water and Soil, Climate Change, and Soil Interactions. In Climate Change and Soil Interactions; Elsevier: Amsterdam, The Netherlands, 2020; pp. 51–88. ISBN 978-0-12-818032-7. [Google Scholar]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A Review on Heavy Metal Pollution, Toxicity and Remedial Measures: Current Trends and Future Perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Amin, H.; Arain, B.A.; Jahangir, T.M.; Abbasi, A.R.; Abbasi, M.S.; Amin, F. Comparative Zinc Tolerance and Phytoremediation Potential of Four Biofuel Plant Species. Int. J. Phytoremediat. 2023, 25, 1014–1028. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.H.; Wang, P.F.; Hou, J.; Zhang, W.J.; Li, W.; Lin, Z.P. The Effect of Excess Zn on Mineral Nutrition and Antioxidative Response in Rapeseed Seedlings. Chemosphere 2009, 75, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Fontes, R.L.F.; Cox, F.R. Effects of Sulfur Supply on Soybean Plants Exposed to Zinc Toxicity. J. Plant Nutr. 1995, 18, 1893–1906. [Google Scholar] [CrossRef]
- Zemanová, V.; Pavlíková, D.; Novák, M.; Hnilička, F. The Dual Role of Zinc in Spinach Metabolism: Beneficial × Toxic. Plants 2024, 13, 3363. [Google Scholar] [CrossRef]
- Meng, Y.; Xiang, C.; Huo, J.; Shen, S.; Tang, Y.; Wu, L. Toxicity Effects of Zinc Supply on Growth Revealed by Physiological and Transcriptomic Evidences in Sweet Potato (Ipomoea batatas (L.) Lam). Sci. Rep. 2023, 13, 19203. [Google Scholar] [CrossRef]
- Rucińska-Sobkowiak, R. Water Relations in Plants Subjected to Heavy Metal Stresses. Acta Physiol. Plant 2016, 38, 257. [Google Scholar] [CrossRef]
- Balafrej, H.; Bogusz, D.; Triqui, Z.-E.A.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc Hyperaccumulation in Plants: A Review. Plants 2020, 9, 562. [Google Scholar] [CrossRef]
- Barrameda-Medina, Y.; Montesinos-Pereira, D.; Romero, L.; Blasco, B.; Ruiz, J.M. Role of GSH Homeostasis under Zn Toxicity in Plants with Different Zn Tolerance. Plant Sci. 2014, 227, 110–121. [Google Scholar] [CrossRef]
- Szopiński, M.; Sitko, K.; Gieroń, Ż.; Rusinowski, S.; Corso, M.; Hermans, C.; Verbruggen, N.; Małkowski, E. Toxic Effects of Cd and Zn on the Photosynthetic Apparatus of the Arabidopsis halleri and Arabidopsis Arenosa Pseudo-Metallophytes. Front. Plant Sci. 2019, 10, 748. [Google Scholar] [CrossRef]
- DalCorso, G.; Martini, F.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Enhancement of Zn Tolerance and Accumulation in Plants Mediated by the Expression of Saccharomyces Cerevisiae Vacuolar Transporter ZRC1. Planta 2021, 253, 117. [Google Scholar] [CrossRef]
- Sarret, G.; Saumitou-Laprade, P.; Bert, V.; Proux, O.; Hazemann, J.-L.; Traverse, A.; Marcus, M.A.; Manceau, A. Forms of Zinc Accumulated in the Hyperaccumulator Arabidopsis halleri. Plant Physiol. 2002, 130, 1815–1826. [Google Scholar] [CrossRef]
- Zeng, X.-W.; Ma, L.Q.; Qiu, R.-L.; Tang, Y.-T. Effects of Zn on Plant Tolerance and Non-Protein Thiol Accumulation in Zn Hyperaccumulator Arabis paniculata Franch. Environ. Exp. Bot. 2011, 70, 227–232. [Google Scholar] [CrossRef]
- Rastogi, A.; Shukla, S. Amaranth: A New Millennium Crop of Nutraceutical Values. Crit. Rev. Food Sci. Nutr. 2013, 53, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Riggins, C.W.; Barba de la Rosa, A.P.; Blair, M.W.; Espitia-Rangel, E. Editorial: Amaranthus: Naturally Stress-Resistant Resources for Improved Agriculture and Human Health. Front. Plant Sci. 2021, 12, 726875. [Google Scholar] [CrossRef] [PubMed]
- Lukatkin, A.S.; Bashmakov, D.I.; Al Harbawee, W.E.Q.; Teixeira da Silva, J.A. Assessment of Physiological and Biochemical Responses of Amaranthus retroflexus Seedlings to the Accumulation of Heavy Metals with Regards to Phytoremediation Potential. Int. J. Phytoremediat. 2021, 23, 219–230. [Google Scholar] [CrossRef]
- Osmolovskaya, N.; Bilova, T.; Gurina, A.; Orlova, A.; Vu, V.D.; Sukhikh, S.; Zhilkina, T.; Frolova, N.; Tarakhovskaya, E.; Kamionskaya, A.; et al. Metabolic Responses of Amaranthus caudatus Roots and Leaves to Zinc Stress. Plants 2025, 14, 2119. [Google Scholar] [CrossRef]
- Lucini, L.; Bernardo, L. Comparison of Proteome Response to Saline and Zinc Stress in Lettuce. Front. Plant Sci. 2015, 6, 240. [Google Scholar] [CrossRef]
- Šimon, M.; Shen, Z.-J.; Ghoto, K.; Chen, J.; Liu, X.; Gao, G.-F.; Jemec Kokalj, A.; Novak, S.; Drašler, B.; Zhang, J.-Y.; et al. Proteomic Investigation of Zn-Challenged Rice Roots Reveals Adverse Effects and Root Physiological Adaptation. Plant Soil. 2021, 460, 69–88. [Google Scholar] [CrossRef]
- Fukao, Y.; Ferjani, A.; Tomioka, R.; Nagasaki, N.; Kurata, R.; Nishimori, Y.; Fujiwara, M.; Maeshima, M. iTRAQ Analysis Reveals Mechanisms of Growth Defects Due to Excess Zinc in Arabidopsis. Plant Physiol. 2011, 155, 1893–1907. [Google Scholar] [CrossRef]
- Frolov, A.; Blüher, M.; Hoffmann, R. Glycation Sites of Human Plasma Proteins Are Affected to Different Extents by Hyperglycemic Conditions in Type 2 Diabetes Mellitus. Anal. Bioanal. Chem. 2014, 406, 5755–5763. [Google Scholar] [CrossRef]
- Nouri, J.; Khorasani, N.; Lorestani, B.; Karami, M.; Hassani, A.H.; Yousefi, N. Accumulation of Heavy Metals in Soil and Uptake by Plant Species with Phytoremediation Potential. Environ. Earth Sci. 2009, 59, 315–323. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy Metals, Occurrence and Toxicity for Plants: A Review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Subba, P.; Mukhopadhyay, M.; Mahato, S.K.; Bhutia, K.D.; Mondal, T.K.; Ghosh, S.K. Zinc Stress Induces Physiological, Ultra-Structural and Biochemical Changes in Mandarin Orange (Citrus reticulata Blanco) Seedlings. Physiol. Mol. Biol. Plants 2014, 20, 461–473. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Tripathy, B.C.; Oelmüller, R. Reactive Oxygen Species Generation and Signaling in Plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Skórzyńska-Polit, E.; Drążkiewicz, M.; Krupa, Z. Lipid Peroxidation and Antioxidative Response in Arabidopsis Thaliana Exposed to Cadmium and Copper. Acta Physiol. Plant 2010, 32, 169–175. [Google Scholar] [CrossRef]
- Khan, M.; Samrana, S.; Zhang, Y.; Malik, Z.; Khan, M.D.; Zhu, S. Reduced Glutathione Protects Subcellular Compartments From Pb-Induced ROS Injury in Leaves and Roots of Upland Cotton (Gossypium hirsutum L.). Front. Plant Sci. 2020, 11, 412. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Gładysz, O.; Szentner, K.; Goliński, P. Role of Glutathione in Abiotic Stress Tolerance. In Oxidative Damage to Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 149–181. ISBN 978-0-12-799963-0. [Google Scholar]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trivedi, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione and Glutathione Reductase: A Boon in Disguise for Plant Abiotic Stress Defense Operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Xiao, T.; ShangGuan, X.; Wang, Y.; Tian, Z.; Peng, K.; Shen, Z.; Hu, Z.; Xia, Y. The Germin-like Protein OsGLP8-7 Is Involved in Lignin Synthesis for Acclimation to Copper Toxicity in Rice. J. Plant Physiol. 2024, 303, 154335. [Google Scholar] [CrossRef]
- Tamás, L.; Mistrík, I.; Huttová, J.; Halušková, L.; Valentovičová, K.; Zelinová, V. Role of Reactive Oxygen Species-Generating Enzymes and Hydrogen Peroxide during Cadmium, Mercury and Osmotic Stresses in Barley Root Tip. Planta 2010, 231, 221–231. [Google Scholar] [CrossRef]
- Leon, J.; Lawton, M.A.; Raskin, I. Hydrogen Peroxide Stimulates Salicylic Acid Biosynthesis in Tobacco. Plant Physiol. 1995, 108, 1673–1678. [Google Scholar] [CrossRef]
- Qi, X.; Chen, M.; Liang, D.; Xu, Q.; Zhou, F.; Chen, X. Jasmonic Acid, Ethylene and ROS Are Involved in the Response of Cucumber (Cucumis sativus L.) to Aphid Infestation. Sci. Hortic. 2020, 269, 109421. [Google Scholar] [CrossRef]
- León Morcillo, R.J.; Ocampo, J.A.; García Garrido, J.M. Plant 9-Lox Oxylipin Metabolism in Response to Arbuscular Mycorrhiza. Plant Signal. Behav. 2012, 7, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Miersch, O.; Neumerkel, J.; Dippe, M.; Stenzel, I.; Wasternack, C. Hydroxylated Jasmonates Are Commonly Occurring Metabolites of Jasmonic Acid and Contribute to a Partial Switch-off in Jasmonate Signaling. New Phytol. 2008, 177, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, M.; Rasheed, A.; Mahmood, T. Functional Characterization of Germin and Germin-like Protein Genes in Various Plant Species Using Transgenic Approaches. Biotechnol. Lett. 2016, 38, 1405–1421. [Google Scholar] [CrossRef]
- Mao, L.; Ge, L.; Ye, X.; Xu, L.; Si, W.; Ding, T. ZmGLP1, a Germin-like Protein from Maize, Plays an Important Role in the Regulation of Pathogen Resistance. Int. J. Mol. Sci. 2022, 23, 14316. [Google Scholar] [CrossRef]
- Qu, Z.-L.; Zhong, N.-Q.; Wang, H.-Y.; Chen, A.-P.; Jian, G.-L.; Xia, G.-X. Ectopic Expression of the Cotton Non-Symbiotic Hemoglobin Gene GhHbd1 Triggers Defense Responses and Increases Disease Tolerance in Arabidopsis. Plant Cell Physiol. 2006, 47, 1058–1068. [Google Scholar] [CrossRef]
- Oteiza, P.I. Zinc and the Modulation of Redox Homeostasis. Free Radic. Biol. Med. 2012, 53, 1748–1759. [Google Scholar] [CrossRef]
- Dang, P.M.; Lamb, M.C.; Bowen, K.L.; Chen, C.Y. Identification of Expressed R-Genes Associated with Leaf Spot Diseases in Cultivated Peanut. Mol. Biol. Rep. 2019, 46, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xie, Y.; Jiang, Y.; Nadeem, H.; Wang, Y.; Yang, N.; Zhu, H.; Tang, C. GhTLP1, a Thaumatin-like Protein 1, Improves Verticillium Wilt Resistance in Cotton via JA, ABA and MAPK Signaling Pathway-Plant Pathways. Int. J. Biol. Macromol. 2023, 253, 127388. [Google Scholar] [CrossRef] [PubMed]
- Zrenner, R.; Verwaaijen, B.; Genzel, F.; Flemer, B.; Grosch, R. Transcriptional Changes in Potato Sprouts upon Interaction with Rhizoctonia Solani Indicate Pathogen-Induced Interference in the Defence Pathways of Potato. Int. J. Mol. Sci. 2021, 22, 3094. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, M.; Rao, U.; Sreevathsa, R. Novel Biotechnological Strategies to Combat Biotic Stresses: Polygalacturonase Inhibitor (PGIP) Proteins as a Promising Comprehensive Option. Appl. Microbiol. Biotechnol. 2020, 104, 2333–2342. [Google Scholar] [CrossRef]
- Połeć-Pawlak, K.; Ruzik, R.; Lipiec, E.; Ciurzyńska, M.; Gawrońska, H. Investigation of Pb(ii) Binding to Pectin in Arabidopsis Thaliana. J. Anal. At. Spectrom. 2007, 22, 968–972. [Google Scholar] [CrossRef]
- País, S.M.; Téllez-Iñón, M.T.; Capiati, D.A. Serine/Threonine Protein Phosphatases Type 2A and Their Roles in Stress Signaling. Plant Signal. Behav. 2009, 4, 1013–1015. [Google Scholar] [CrossRef]
- Neumann, D.; Lichtenberger, O.; Günther, D.; Tschiersch, K.; Nover, L. Heat-Shock Proteins Induce Heavy-Metal Tolerance in Higher Plants. Planta 1994, 194, 360–367. [Google Scholar] [CrossRef]
- Fitzgerald, T.L.; Waters, D.L.E.; Henry, R.J. Betaine Aldehyde Dehydrogenase in Plants. Plant Biol. 2009, 11, 119–130. [Google Scholar] [CrossRef]
- Konopka-Postupolska, D.; Clark, G. Annexins as Overlooked Regulators of Membrane Trafficking in Plant Cells. Int. J. Mol. Sci. 2017, 18, 863. [Google Scholar] [CrossRef]
- Dufoo-Hurtado, M.D.; Huerta-Ocampo, J.Ã.; Barrera-Pacheco, A.; Barba De La Rosa, A.P.; Mercado-Silva, E.M. Low Temperature Conditioning of Garlic (Allium sativum L.) €œseed†Cloves Induces Alterations in Sprouts Proteome. Front. Plant Sci. 2015, 6, 332. [Google Scholar] [CrossRef]
- Mortimer, J.C.; Laohavisit, A.; Macpherson, N.; Webb, A.; Brownlee, C.; Battey, N.H.; Davies, J.M. Annexins: Multifunctional Components of Growth and Adaptation. J. Exp. Bot. 2008, 59, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Rüffer, M.; Steipe, B.; Zenk, M.H. Evidence against Specific Binding of Salicylic Acid to Plant Catalase. FEBS Lett. 1995, 377, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Liu, F.; Wang, W.; Wang, L.; Wang, Z.; Li, J.; Que, Y.; Xu, L.; Su, Y. The Role of Sugarcane Catalase Gene ScCAT2 in the Defense Response to Pathogen Challenge and Adversity Stress. Int. J. Mol. Sci. 2018, 19, 2686. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; He, G.; Li, J.; Perez-Hormaeche, J.; Becker, T.; Luo, M.; Wallrad, L.; Gao, J.; Li, J.; Pardo, J.M.; et al. A Salt Stress-activated GSO1-SOS2-SOS1 Module Protects the Arabidopsis Root Stem Cell Niche by Enhancing Sodium Ion Extrusion. EMBO J. 2023, 42, e113004. [Google Scholar] [CrossRef]
- Creff, A.; Brocard, L.; Joubès, J.; Taconnat, L.; Doll, N.M.; Marsollier, A.-C.; Pascal, S.; Galletti, R.; Boeuf, S.; Moussu, S.; et al. A Stress-Response-Related Inter-Compartmental Signalling Pathway Regulates Embryonic Cuticle Integrity in Arabidopsis. PLoS Genet. 2019, 15, e1007847. [Google Scholar] [CrossRef]
- Racolta, A.; Bryan, A.C.; Tax, F.E. The Receptor-like Kinases GSO1 and GSO2 Together Regulate Root Growth in Arabidopsis through Control of Cell Division and Cell Fate Specification. Dev. Dyn. 2014, 243, 257–278. [Google Scholar] [CrossRef]
- Zhu, Q.; Feng, Y.; Xue, J.; Chen, P.; Zhang, A.; Yu, Y. Advances in Receptor-like Protein Kinases in Balancing Plant Growth and Stress Responses. Plants 2023, 12, 427. [Google Scholar] [CrossRef]
- Tamás, M.; Sharma, S.; Ibstedt, S.; Jacobson, T.; Christen, P. Heavy Metals and Metalloids As a Cause for Protein Misfolding and Aggregation. Biomolecules 2014, 4, 252–267. [Google Scholar] [CrossRef]
- Stępiński, D. Decoding Plant Ribosomal Proteins: Multitasking Players in Cellular Games. Cells 2025, 14, 473. [Google Scholar] [CrossRef]
- Mazahar, M.; Achala, B.; Anusree, S.; Kirti, P.B. Ribosomal Proteins and Their Extra Ribosomal Functions in Abiotic Stress Tolerance of Plants. Mod. Concepts Dev. Agron. 2019, 4. [Google Scholar] [CrossRef]
- Fakih, Z.; Germain, H. Implication of Ribosomal Protein in Abiotic and Biotic Stress. Planta 2025, 261, 85. [Google Scholar] [CrossRef]
- Grasser, K.D. The Role of RNA Polymerase II Transcript Elongation Factors in Plant Stress Responses. J. Exp. Bot. 2025, 76, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Yu, C.; Li, L.; Wang, M.; Yang, L.; Zhang, Y.; Zhang, Y.; Wang, J.; Li, C.; Reynolds, M.P.; et al. How Many Faces Does the Plant U-Box E3 Ligase Have? Int. J. Mol. Sci. 2022, 23, 2285. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.; Wei, P.; Chen, Q.; An, R.; Chen, J.; Yang, S.; Wang, X. NADK3, a Novel Cytoplasmic Source of NADPH, Is Required under Conditions of Oxidative Stress and Modulates Abscisic Acid Responses in Arabidopsis. Plant J. 2006, 47, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, R.; Corpas, F.J.; Carreras, A.; Gómez-Rodríguez, M.V.; Chaki, M.; Pedrajas, J.R.; Fernández-Ocaña, A.; Del Río, L.A.; Barroso, J.B. The Dehydrogenase-mediated Recycling of NADPH Is a Key Antioxidant System against Salt-induced Oxidative Stress in Olive Plants. Plant Cell Environ. 2006, 29, 1449–1459. [Google Scholar] [CrossRef]
- Moustakas, M.; Dobrikova, A.; Sperdouli, I.; Hanć, A.; Moustaka, J.; Adamakis, I.-D.S.; Apostolova, E. Photosystem II Tolerance to Excess Zinc Exposure and High Light Stress in Salvia sclarea L. Agronomy 2024, 14, 589. Agronomy 2024, 14, 589. [Google Scholar] [CrossRef]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-Mediated Abiotic Stress-Induced Programmed Cell Death in Plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef]
- Zhang, C.; Shuai, J.; Ran, Z.; Zhao, J.; Wu, Z.; Liao, R.; Wu, J.; Ma, W.; Lei, M. Structural Insights into NDH-1 Mediated Cyclic Electron Transfer. Nat. Commun. 2020, 11, 888. [Google Scholar] [CrossRef]
- Shuyskaya, E.; Rakhmankulova, Z.; Prokofieva, M.; Kazantseva, V.; Lunkova, N. Impact of Salinity, Elevated Temperature, and Their Interaction with the Photosynthetic Efficiency of Halophyte Crop Chenopodium quinoa Willd. Agriculture 2023, 13, 1198. [Google Scholar] [CrossRef]
- Huihui, Z.; Xin, L.; Zisong, X.; Yue, W.; Zhiyuan, T.; Meijun, A.; Yuehui, Z.; Wenxu, Z.; Nan, X.; Guangyu, S. Toxic Effects of Heavy Metals Pb and Cd on Mulberry (Morus alba L.) Seedling Leaves: Photosynthetic Function and Reactive Oxygen Species (ROS) Metabolism Responses. Ecotoxicol. Environ. Saf. 2020, 195, 110469. [Google Scholar] [CrossRef]
- Potters, G.; Horemans, N.; Jansen, M.A.K. The Cellular Redox State in Plant Stress Biology—A Charging Concept. Plant Physiol. Biochem. 2010, 48, 292–300. [Google Scholar] [CrossRef]
- Bai, J.; Qin, Y.; Liu, J.; Wang, Y.; Sa, R.; Zhang, N.; Jia, R. Proteomic Response of Oat Leaves to Long-Term Salinity Stress. Environ. Sci. Pollut. Res. 2017, 24, 3387–3399. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, S.; Zhao, C.; Jiang, X.; Gao, D. Responses of Non-Structural Carbohydrates and Biomass in Plant to Heavy Metal Treatment. Sci. Total Environ. 2024, 909, 168559. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.-W.; Huang, W.-X.; Xiong, Z.-T.; Ye, F.-Y.; Ren, C.; Xu, Z.-R.; Liu, C.; Deng, S.-Q.; Zhao, J. Comparative Study of Root Growth and Sucrose-Cleaving Enzymes in Metallicolous and Non-Metallicolous Populations of Rumex Dentatus under Copper Stress. Ecotoxicol. Environ. Saf. 2013, 98, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Y.; Tian, J.; Zhu, Y.; Fan, J. Changes in Sucrose Metabolism in Maize Varieties with Different Cadmium Sensitivities under Cadmium Stress. PLoS ONE 2020, 15, e0243835. [Google Scholar] [CrossRef]
- Varshney, V.; Singh, J.; Salvi, P. Sugar Signaling and Their Interplay in Mitigating Abiotic Stresses in Plant: A Molecular Perspective. In Smart Plant Breeding for Field Crops in Post-Genomics Era; Sharma, D., Singh, S., Sharma, S.K., Singh, R., Eds.; Springer Nature: Singapore, 2023; pp. 369–393. ISBN 978-981-19-8217-0. [Google Scholar]
- German, M.A.; Asher, I.; Petreikov, M.; Dai, N.; Schaffer, A.A.; Granot, D. Cloning, Expression and Characterization of LeFRK3, the Fourth Tomato (Lycopersicon esculentum Mill.) Gene Encoding Fructokinase. Plant Sci. 2004, 166, 285–291. [Google Scholar] [CrossRef]
- Granot, D.; Kelly, G.; Stein, O.; David-Schwartz, R. Substantial Roles of Hexokinase and Fructokinase in the Effects of Sugars on Plant Physiology and Development. J. Exp. Bot. 2014, 65, 809–819. [Google Scholar] [CrossRef]
- Fulda, S.; Mikkat, S.; Stegmann, H.; Horn, R. Physiology and Proteomics of Drought Stress Acclimation in Sunflower (Helianthus annuus L.). Plant Biol. 2011, 13, 632–642. [Google Scholar] [CrossRef]
- Guglielminetti, L.; Morita, A.; Yamaguchi, J.; Loreti, E.; Perata, P.; Alpi, A. Differential Expression of Two Fructokinases in Oryza sativa Seedlings Grown under Aerobic and Anaerobic Conditions. J. Plant Res. 2006, 119, 351–356. [Google Scholar] [CrossRef]
- Bui, L.T.; Novi, G.; Lombardi, L.; Iannuzzi, C.; Rossi, J.; Santaniello, A.; Mensuali, A.; Corbineau, F.; Giuntoli, B.; Perata, P.; et al. Conservation of Ethanol Fermentation and Its Regulation in Land Plants. J. Exp. Bot. 2019, 70, 1815–1827. [Google Scholar] [CrossRef]
- Jardine, K.J.; McDowell, N. Fermentation-mediated Growth, Signaling, and Defense in Plants. New Phytol. 2023, 239, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Wurzinger, B.; Nukarinen, E.; Nägele, T.; Weckwerth, W.; Teige, M. The SnRK1 Kinase as Central Mediator of Energy Signaling between Different Organelles. Plant Physiol. 2018, 176, 1085–1094. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Y. The Critical Roles of Three Sugar-Related Proteins (HXK, SnRK1, TOR) in Regulating Plant Growth and Stress Responses. Hortic. Res. 2024, 11, uhae099. [Google Scholar] [CrossRef]
- Busche, M.; Scarpin, M.R.; Hnasko, R.; Brunkard, J.O. TOR Coordinates Nucleotide Availability with Ribosome Biogenesis in Plants. Plant Cell 2021, 33, 1615–1632. [Google Scholar] [CrossRef]
- Crepin, N.; Rolland, F. SnRK1 Activation, Signaling, and Networking for Energy Homeostasis. Curr. Opin. Plant Biol. 2019, 51, 29–36. [Google Scholar] [CrossRef]
- Tsai, A.Y.-L.; Gazzarrini, S. Trehalose-6-Phosphate and SnRK1 Kinases in Plant Development and Signaling: The Emerging Picture. Front. Plant Sci. 2014, 5, 119. [Google Scholar] [CrossRef]
- Han, C.; Wang, H.; Shi, W.; Bai, M.-Y. The Molecular Associations between the SnRK1 Complex and Carbon/Nitrogen Metabolism in Plants. New Crops 2024, 1, 100008. [Google Scholar] [CrossRef]
- Peixoto, B.; Baena-González, E. Management of Plant Central Metabolism by SnRK1 Protein Kinases. J. Exp. Bot. 2022, 73, 7068–7082. [Google Scholar] [CrossRef]
- Angulo-Bejarano, P.I.; Puente-Rivera, J.; Cruz-Ortega, R. Metal and Metalloid Toxicity in Plants: An Overview on Molecular Aspects. Plants 2021, 10, 635. [Google Scholar] [CrossRef] [PubMed]
- Fukao, Y.; Ferjani, A. V-ATPase Dysfunction under Excess Zinc Inhibits Arabidopsis Cell Expansion. Plant Signal. Behav. 2011, 6, 1253–1255. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, Y.; Yang, Y. The Molecular Mechanism of Plasma Membrane H+-ATPases in Plant Responses to Abiotic Stress. J. Genet. Genom. 2022, 49, 715–725. [Google Scholar] [CrossRef]
- Janicka-Russak, M.; Kabala, K.; Burzynski, M.; Klobus, G. Response of Plasma Membrane H+-ATPase to Heavy Metal Stress in Cucumis sativus Roots. J. Exp. Bot. 2008, 59, 3721–3728. [Google Scholar] [CrossRef] [PubMed]
- Ulhassan, Z.; Sheteiwy, M.S.; Khan, A.R.; Hamid, Y.; Azhar, W.; Hussain, S.; Salam, A.; Zhou, W. Zinc Toxicity in Plants: A Brief Overview on Recent Developments. In Zinc in Plants; Elsevier: Amsterdam, The Netherlands, 2025; pp. 77–93. ISBN 978-0-323-91314-0. [Google Scholar]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular Mechanisms of Metal Hyperaccumulation in Plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef] [PubMed]
- Do, T.H.T.; Martinoia, E.; Lee, Y.; Hwang, J.-U. 2021 Update on ATP-Binding Cassette (ABC) Transporters: How They Meet the Needs of Plants. Plant Physiol. 2021, 187, 1876–1892. [Google Scholar] [CrossRef] [PubMed]
- I. Stolarczyk, E.; J. Reiling, C.; M. Paumi, C. Regulation of ABC Transporter Function Via Phosphorylation by Protein Kinases. CPB 2011, 12, 621–635. [Google Scholar] [CrossRef]
- Yadav, S.; Kalwan, G.; Gill, S.S.; Jain, P.K. The ABC Transporters and Their Epigenetic Regulation under Drought Stress in Chickpea. Plant Physiol. Biochem. 2025, 223, 109903. [Google Scholar] [CrossRef]
- Hashmi, S.S.; Lubna; Bilal, S.; Jan, R.; Asif, S.; Abdelbacki, A.M.M.; Kim, K.-M.; Al-Harrasi, A.; Asaf, S. Exploring the Role of ATP-Binding Cassette Transporters in Tomato (Solanum lycopersicum) under Cadmium Stress through Genome-Wide and Transcriptomic Analysis. Front. Plant Sci. 2025, 16, 1536178. [Google Scholar] [CrossRef]
- Kosakivska, I.V.; Babenko, L.M.; Romanenko, K.O.; Korotka, I.Y.; Potters, G. Molecular Mechanisms of Plant Adaptive Responses to Heavy Metals Stress. Cell Biol. Int. 2021, 45, 258–272. [Google Scholar] [CrossRef]
- Jia, H.; Wang, X.; Wei, T.; Zhou, R.; Muhammad, H.; Hua, L.; Ren, X.; Guo, J.; Ding, Y. Accumulation and Fixation of Cd by Tomato Cell Wall Pectin under Cd Stress. Environ. Exp. Bot. 2019, 167, 103829. [Google Scholar] [CrossRef]
- Bitto, E.; Bingman, C.A.; McCoy, J.G.; Allard, S.T.M.; Wesenberg, G.E.; Phillips, G.N. The Structure at 1.6 Å Resolution of the Protein Product of the At4g34215 Gene from Arabidopsis thaliana. Acta Crystallogr. D Biol. Crystallogr. 2005, 61, 1655–1661. [Google Scholar] [CrossRef]
- Anderson, A.C.; Stangherlin, S.; Pimentel, K.N.; Weadge, J.T.; Clarke, A.J. The SGNH Hydrolase Family: A Template for Carbohydrate Diversity. Glycobiology 2022, 32, 826–848. [Google Scholar] [CrossRef]
- Panikashvili, D.; Shi, J.X.; Schreiber, L.; Aharoni, A. The Arabidopsis DCR Encoding a Soluble BAHD Acyltransferase Is Required for Cutin Polyester Formation and Seed Hydration Properties. Plant Physiol. 2009, 151, 1773–1789. [Google Scholar] [CrossRef]
- Doyama, K.; Yamaji, K.; Haruma, T.; Ishida, A.; Mori, S.; Kurosawa, Y. Zn Tolerance in the Evergreen Shrub, Aucuba Japonica, Naturally Growing at a Mine Site: Cell Wall Immobilization, Aucubin Production, and Zn Adsorption on Fungal Mycelia. PLoS ONE 2021, 16, e0257690. [Google Scholar] [CrossRef]
- Pfaff, J.; Denton, A.K.; Usadel, B.; Pfaff, C. Phosphate Starvation Causes Different Stress Responses in the Lipid Metabolism of Tomato Leaves and Roots. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158763. [Google Scholar] [CrossRef]
- Bouain, N.; Shahzad, Z.; Rouached, A.; Khan, G.A.; Berthomieu, P.; Abdelly, C.; Poirier, Y.; Rouached, H. Phosphate and Zinc Transport and Signalling in Plants: Toward a Better Understanding of Their Homeostasis Interaction. J. Exp. Bot. 2014, 65, 5725–5741. [Google Scholar] [CrossRef]
- Ding, J.; Liu, L.; Wang, C.; Shi, L.; Xu, F.; Cai, H. High Level of Zinc Triggers Phosphorus Starvation by Inhibiting Root-to-Shoot Translocation and Preferential Distribution of Phosphorus in Rice Plants. Environ. Pollut. 2021, 277, 116778. [Google Scholar] [CrossRef] [PubMed]
- Sabet, M.S.; Zamani, K.; Lohrasebi, T.; Malboobi, M.A.; Valizadeh, M. Functional Assessment of an Overexpressed Arabidopsis Purple Acid Phosphatase Gene (Atpap26) in Tobacco Plants. Iran. J. Biotechnol. 2018, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ding, D.; Li, J.; He, L.; Xu, X.; Zhao, Y.; Yan, B.; Li, Z.; Xu, J. Characterisation of Genes Involved in Galactolipids and Sulfolipids Metabolism in Maize and Arabidopsis and Their Differential Responses to Phosphate Deficiency. Funct. Plant Biol. 2020, 47, 279. [Google Scholar] [CrossRef] [PubMed]
- Foroughi, S.; Baker, A.J.M.; Roessner, U.; Johnson, A.A.T.; Bacic, A.; Callahan, D.L. Hyperaccumulation of Zinc by Noccaea caerulescens Results in a Cascade of Stress Responses and Changes in the Elemental Profile. Metallomics 2014, 6, 1671–1682. [Google Scholar] [CrossRef]
- López-Orenes, A.; Martínez-Pérez, A.; Calderón, A.A.; Ferrer, M.A. Pb-Induced Responses in Zygophyllum fabago Plants Are Organ-Dependent and Modulated by Salicylic Acid. Plant Physiol. Biochem. 2014, 84, 57–66. [Google Scholar] [CrossRef]
- Liu, W.; Park, S.-W. 12-Oxo-Phytodienoic Acid: A Fuse and/or Switch of Plant Growth and Defense Responses? Front. Plant Sci. 2021, 12, 724079. [Google Scholar] [CrossRef]
- Kavita, B.; Shukla, S.; Naresh Kumar, G.; Archana, G. Amelioration of Phytotoxic Effects of Cd on Mung Bean Seedlings by Gluconic Acid Secreting Rhizobacterium Enterobacter Asburiae PSI3 and Implication of Role of Organic Acid. World J. Microbiol. Biotechnol. 2008, 24, 2965–2972. [Google Scholar] [CrossRef]
- Kornecki, J.F.; Carballares, D.; Tardioli, P.W.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Alcántara, A.R.; Fernandez-Lafuente, R. Enzyme Production of d -Gluconic Acid and Glucose Oxidase: Successful Tales of Cascade Reactions. Catal. Sci. Technol. 2020, 10, 5740–5771. [Google Scholar] [CrossRef]
- Corkins, M.E.; Wilson, S.; Cocuron, J.-C.; Alonso, A.P.; Bird, A.J. The Gluconate Shunt Is an Alternative Route for Directing Glucose into the Pentose Phosphate Pathway in Fission Yeast. J. Biol. Chem. 2017, 292, 13823–13832. [Google Scholar] [CrossRef]
- Ledesma, A.; De Lacoba, M.; Rial, E. The mitochondrial uncoupling proteins. Genome Biol. 2002, 3, reviews3015.1. [Google Scholar] [CrossRef]
- Bilova, T.; Lukasheva, E.; Brauch, D.; Greifenhagen, U.; Paudel, G.; Tarakhovskaya, E.; Frolova, N.; Mittasch, J.; Balcke, G.U.; Tissier, A.; et al. A Snapshot of the Plant Glycated Proteome. J. Biol. Chem. 2016, 291, 7621–7636. [Google Scholar] [CrossRef]
- Sengupta, D.; Naik, D.; Reddy, A.R. Plant Aldo-Keto Reductases (AKRs) as Multi-Tasking Soldiers Involved in Diverse Plant Metabolic Processes and Stress Defense: A Structure-Function Update. J. Plant Physiol. 2015, 179, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.; Bilova, T.; Paudel, G.; Berger, R.; Balcke, G.U.; Birkemeyer, C.; Wessjohann, L. Early Responses of Mature Arabidopsis Thaliana Plants to Reduced Water Potential in the Agar-Based Polyethylene Glycol Infusion Drought Model. J. Plant Physiol. 2017, 208, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Greifenhagen, U.; Frolov, A.; Blüher, M.; Hoffmann, R. Site-Specific Analysis of Advanced Glycation End Products in Plasma Proteins of Type 2 Diabetes Mellitus Patients. Anal. Bioanal. Chem. 2016, 408, 5557–5566. [Google Scholar] [CrossRef]
- Leonova, T.; Ihling, C.; Saoud, M.; Frolova, N.; Rennert, R.; Wessjohann, L.A.; Frolov, A. Does Filter-Aided Sample Preparation Provide Sufficient Method Linearity for Quantitative Plant Shotgun Proteomics? Front. Plant Sci. 2022, 13, 874761. [Google Scholar] [CrossRef]
- Frolov, A.; Shumilina, J.; Etemadi Afshar, S.; Mashkina, V.; Rhomanovskaya, E.; Lukasheva, E.; Tsarev, A.; Sulima, A.S.; Shtark, O.Y.; Ihling, C.; et al. Responsivity of Two Pea Genotypes to the Symbiosis with Rhizobia and Arbuscular Mycorrhiza Fungi—A Proteomics Aspect of the “Efficiency of Interactions with Beneficial Soil Microorganisms” Trait. Int. J. Mol. Sci. 2025, 26, 463. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bandla, C.; Kundu, D.J.; Kamatchinathan, S.; Bai, J.; Hewapathirana, S.; John, N.S.; Prakash, A.; Walzer, M.; Wang, S.; et al. The PRIDE database at 20 years: 2025 update. Nucleic Acids Res. 2025, 53, D543–D553. [Google Scholar] [CrossRef]



| Protein Name a | KEGG Accession | FC b | p | Bin c | Subcellular Localization |
|---|---|---|---|---|---|
| Proteins displaying a higher abundance under Zn stress in comparison to control | |||||
| probable glutathione S-transferase parC | XP_021729368.1 | 39.1 | 8.6 × 10−6 | 9, 10, 26 | cytoplasm, plastid, nucleus |
| putative 12-oxophytodienoate reductase 11 isoform X1 | XP_021714783.1 | 27.6 | 7.3 × 10−7 | 9 | cytoplasm, peroxisome |
| non-symbiotic hemoglobin 1-like | XP_021731656.1 | 15 | 1.0 × 10−4 | 10 | cytoplasm, nucleus |
| mitochondrial uncoupling protein 4-like | XP_021725393.1 | 13.1 | 1.9 × 10−4 | 2, 26 | mitochondrion |
| glutathione S-transferase-like | XP_021763022.1 | 10.6 | 2.3 × 10−5 | 10, 26 | nucleus, cytoplasm, plasma membrane |
| probable inactive purple acid phosphatase 29 | XP_021726654.1 | 10.5 | 5.0 × 10−5 | 25 | cytoplasm |
| alcohol dehydrogenase 1 | XP_021760951.1 | 10.1 | 1.9 × 10−4 | 3 | cytoplasm |
| probable aldo-keto reductase 4 | XP_021724433.1 | 8.2 | 5.0 × 10−5 | 26 | mitochondrion |
| bifunctional riboflavin biosynthesis protein RIBA 1, chloroplastic-like | XP_021713589.1 | 5.9 | 2.3 × 10−4 | 7 | plastid |
| LOW QUALITY PROTEIN: UPF0603 protein At1g54780, chloroplastic-like | XP_021772984.1 | 4.9 | 7.3 × 10−3 | 1, 19 | plastid |
| Proteins displaying a decreased abundance under Zn stress in comparison to control | |||||
| peroxidase 3-like | XP_021726359.1 | 5 | 3.7 × 10−4 | 21 | cell wall |
| ABC transporter F family member 3-like | XP_021757719.1 | 3.5 | 1.9 × 10−4 | 17 | cytoplasm, nucleus |
| aspartyl protease AED3-like | XP_021752910.1 | 2.9 | 4.0 × 10−4 | 19, 26 | cell wall |
| glutamine synthetase, chloroplastic | XP_021727743.1 | 2.4 | 1.4 × 10−3 | 4, 25 | plastid |
| dihydrolipoyl dehydrogenase 2, chloroplastic-like | XP_021730249.1 | 2.3 | 2.7 × 10−2 | 5 | plastid |
| peroxidase 72-like | XP_021714590.1 | 2.3 | 1.5 × 10−2 | 21 | cell wall |
| vacuolar-processing enzyme-like | XP_021716668.1 | 2.2 | 3.7 × 10−4 | 19 | vacuole |
| apoptosis-inducing factor 2-like | XP_021725188.1 | 2.1 | 4.4 × 10−3 | 35 | cytoplasm |
| 40S ribosomal protein S27-2 | XP_021762421.1 | 2.1 | 2.9 × 10−2 | 17 | ribosome, cytoplasm |
| plasma membrane ATPase 4-like | XP_021738895.1 | 2 | 6.2 × 10−5 | 24, 26 | plasma membrane |
| Protein Name a | KEGG Accession | FC b | p | Bin c | Subcellular Localization |
|---|---|---|---|---|---|
| Proteins displaying a higher abundance under Zn stress in comparison to control | |||||
| receptor-like protein 12 | XP_021726832.1 | 2.7 | 4.1 × 10−3 | 26 | plasma membrane |
| aspartate aminotransferase, cytoplasmic | XP_021723631.1 | 2.2 | 2.3 × 10−2 | 4 | cytoplasm |
| CBS domain-containing protein CBSX3, mitochondrial-like | XP_021763137.1 | 2.2 | 4.4 × 10−4 | 10 | mitochondrion |
| ferredoxin-NADP reductase, chloroplastic-like | XP_021727571.1 | 2.2 | 5.3 × 10−3 | 1 | chloroplast |
| probable aldo-keto reductase 4 | XP_021724433.1 | 2.0 | 3.3 × 10−2 | 26 | mitochondrion |
| polygalacturonase inhibitor-like | XP_021724286.1 | 1.9 | 6.7 × 10−3 | 26 | cell wall |
| plasma membrane ATPase 4-like | XP_021723433.1 | 1.9 | 7.7 × 10−4 | 24, 26 | plasma membrane |
| enolase | XP_021743148.1 | 1.9 | 1.1 × 10−2 | 3 | cytoplasm |
| dihydropyrimidine dehydrogenase (NADP+), chloroplastic-like | XP_021732698.1 | 1.8 | 3.8 × 10−4 | 6 | chloroplast |
| peroxiredoxin-2E-1, chloroplastic-like | XP_021743149.1 | 1.8 | 1.1 × 10−3 | 10 | chloroplast |
| Proteins displaying a decreased abundance under Zn stress in comparison to control | |||||
| magnesium-protoporphyrin IX monomethyl ester [oxidative] cyclase, chloroplastic-like | XP_021745614.1 | 3.1 | 1.7 × 10−3 | 7 | chloroplast |
| PsbC (chloroplast) | YP_009380126.1 | 2.6 | 7.0 × 10−3 | 1 | chloroplast |
| 60S ribosomal protein L4-like | XP_021721538.1 | 2.1 | 6.5 × 10−4 | 17 | ribosome, cytoplasm |
| stearoyl-[acyl-carrier-protein] 9-desaturase, chloroplastic | XP_021751679.1 | 2.0 | 2.5 × 10−2 | 4 | chloroplast |
| porphobilinogen deaminase, chloroplastic-like | XP_021752880.1 | 1.9 | 8.1 × 10−3 | 7 | chloroplast |
| translocase of chloroplast 159, chloroplastic-like | XP_021763183.1 | 1.9 | 4.2 × 10−3 | 23 | chloroplast |
| granule-bound starch synthase 2, chloroplastic/amyloplastic-like | XP_021721398.1 | 1.8 | 1.1 × 10−2 | 3 | chloroplast |
| NdhH (chloroplast) | YP_009380186.1 | 1.7 | 2.3 × 10−2 | 1 | chloroplast |
| 60S ribosomal protein L19-3-like | XP_021724307.1 | 1.7 | 8.4 × 10−3 | 17, 26 | nucleolus |
| 40S ribosomal protein S30 | XP_021774723.1 | 1.7 | 1.5 × 10−2 | 17 | ribosome, cytoplasm |
| Protein Name a | KEGG Accession | FC b | p | Bin c | Subcellular Localization |
|---|---|---|---|---|---|
| Proteins displaying a higher abundance under Zn stress in comparison to control | |||||
| heat shock protein 83 | XP_021726736.1 | 4.2 | 5.1 × 10−6 | 26 | cytoplasm |
| annexin D2-like | XP_021714165.1 | 2.1 | 5.9 × 10−4 | 22, 26 | cytoplasm, plasma membrane |
| probable glutathione S-transferase parC | XP_021729368.1 | 2.1 | 7.2 × 10−6 | 9, 10, 26 | cytoplasm, chloroplast, nucleus |
| uncharacterized protein LOC110692268 | XP_021724961.1 | 2.0 | 1.5 × 10−3 | - | extracellular space |
| low quality protein: urease-like | XP_021744615.1 | 1.8 | 6.1 × 10−3 | 6 | cytoplasm |
| betaine aldehyde dehydrogenase, chloroplastic | XP_021733695.1 | 1.6 | 4.6 × 10−5 | 9, 26 | chloroplast |
| citrate synthase, mitochondrial-like | XP_021731867.1 | 1.5 | 5.6 × 10−4 | 2 | mitochondrion |
| luminal-binding protein-like | XP_021763741.1 | 1.5 | 5.050−3 | 19 | endoplasmic reticulum |
| Proteins displaying a decreased abundance under Zn stress in comparison to control | |||||
| callose synthase 10-like | XP_021720217.1 | 2.6 | 1.0 × 10−2 | 21 | plasma membrane |
| PetA (chloroplast) | YP_009380143.1 | 2.1 | 1.6 × 10−4 | 1 | chloroplast |
| NdhH (chloroplast) | YP_009380186.1 | 1.8 | 1.1 × 10−2 | 1 | chloroplast |
| photosystem II 10 kDa polypeptide, chloroplastic | XP_021748287.1 | 1.8 | 1.9 × 10−3 | 1 | chloroplast |
| magnesium-chelatase subunit ChlI, chloroplastic-like | XP_021749286.1 | 1.7 | 8.9 × 10−3 | 7 | chloroplast |
| ribosomal protein S14 (chloroplast) | YP_009380128.1 | 1.7 | 1.7 × 10−2 | 17 | ribosome, chloroplast |
| LRR receptor-like serine/threonine-protein kinase GSO1 | XP_021775210.1 | 1.7 | 3.1 × 10−3 | 18, 26 | plasma membrane |
| uncharacterized protein LOC110707738 isoform X1 | XP_021741469.1 | 1.7 | 2.1 × 10−2 | - | chloroplast |
| 50S ribosomal protein L9, chloroplastic | XP_021724417.1 | 1.6 | 3.7 × 10−3 | 17 | ribosome, chloroplast |
| cytochrome b6/f complex iron-sulfur subunit, chloroplastic | XP_021726772.1 | 1.6 | 5.7 × 10−3 | 1 | chloroplast |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurina, A.; Bilova, T.; Gorbach, D.; Soboleva, A.; Stepanova, N.; Babich, O.; Ihling, C.; Kamionskaya, A.; Osmolovskaya, N.; Frolov, A. Metabolic Responses to the Zinc Stress in the Roots and Leaves of Amaranthus caudatus: The Proteomics View. Plants 2025, 14, 3315. https://doi.org/10.3390/plants14213315
Gurina A, Bilova T, Gorbach D, Soboleva A, Stepanova N, Babich O, Ihling C, Kamionskaya A, Osmolovskaya N, Frolov A. Metabolic Responses to the Zinc Stress in the Roots and Leaves of Amaranthus caudatus: The Proteomics View. Plants. 2025; 14(21):3315. https://doi.org/10.3390/plants14213315
Chicago/Turabian StyleGurina, Anastasia, Tatiana Bilova, Daria Gorbach, Alena Soboleva, Nataliia Stepanova, Olga Babich, Christian Ihling, Anastasia Kamionskaya, Natalia Osmolovskaya, and Andrej Frolov. 2025. "Metabolic Responses to the Zinc Stress in the Roots and Leaves of Amaranthus caudatus: The Proteomics View" Plants 14, no. 21: 3315. https://doi.org/10.3390/plants14213315
APA StyleGurina, A., Bilova, T., Gorbach, D., Soboleva, A., Stepanova, N., Babich, O., Ihling, C., Kamionskaya, A., Osmolovskaya, N., & Frolov, A. (2025). Metabolic Responses to the Zinc Stress in the Roots and Leaves of Amaranthus caudatus: The Proteomics View. Plants, 14(21), 3315. https://doi.org/10.3390/plants14213315









