Exploring the Biochemical Mechanism Beyond the Cytotoxic Activity of Sesquiterpene Lactones from Sicilian Accession of Laserpitium siler Subsp. siculum (Spreng.) Thell
Abstract
1. Introduction
2. Results and Discussion
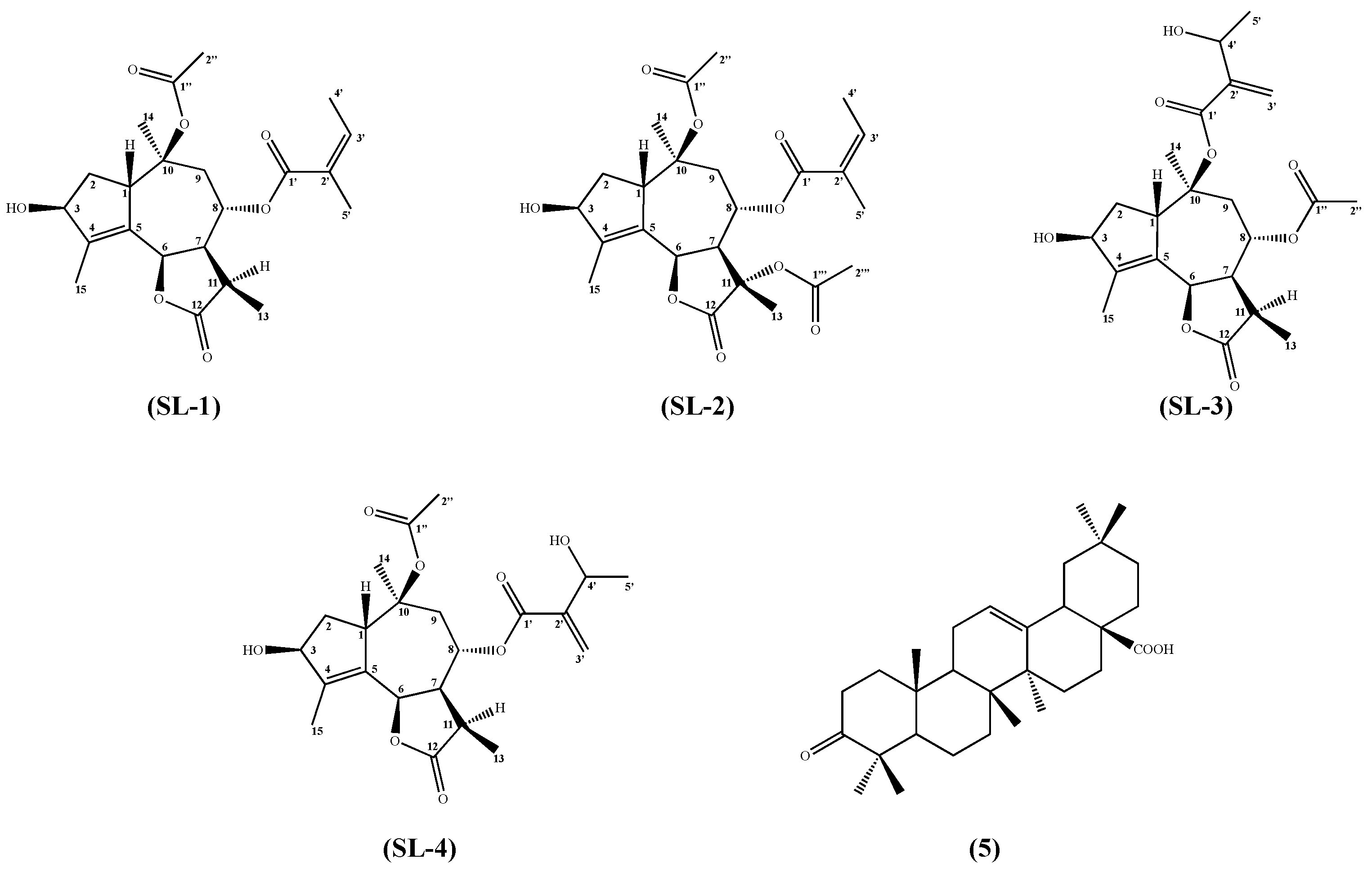
2.1. Metabolomic Analysis
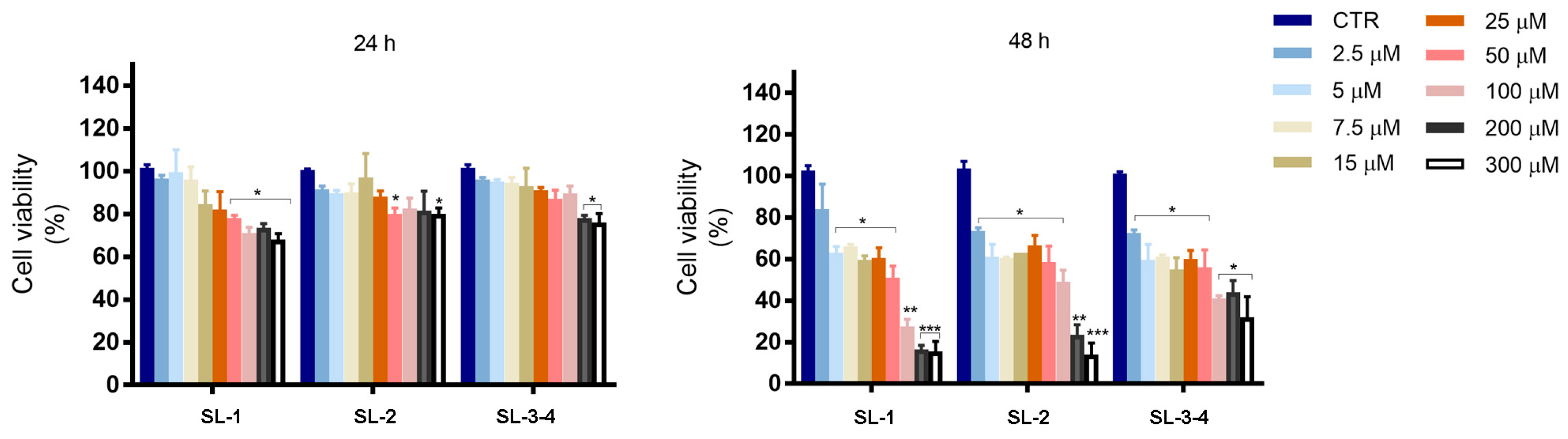
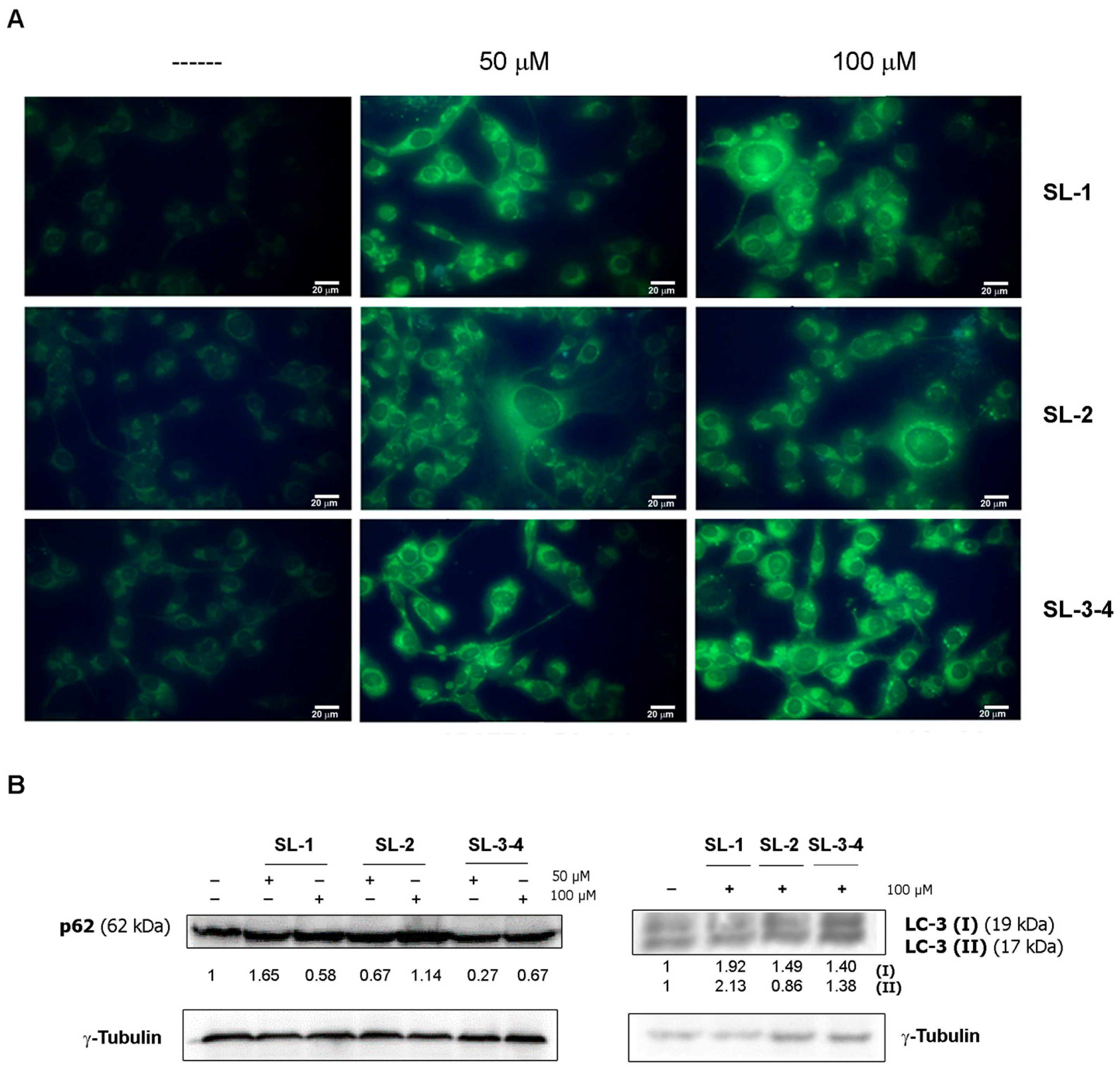
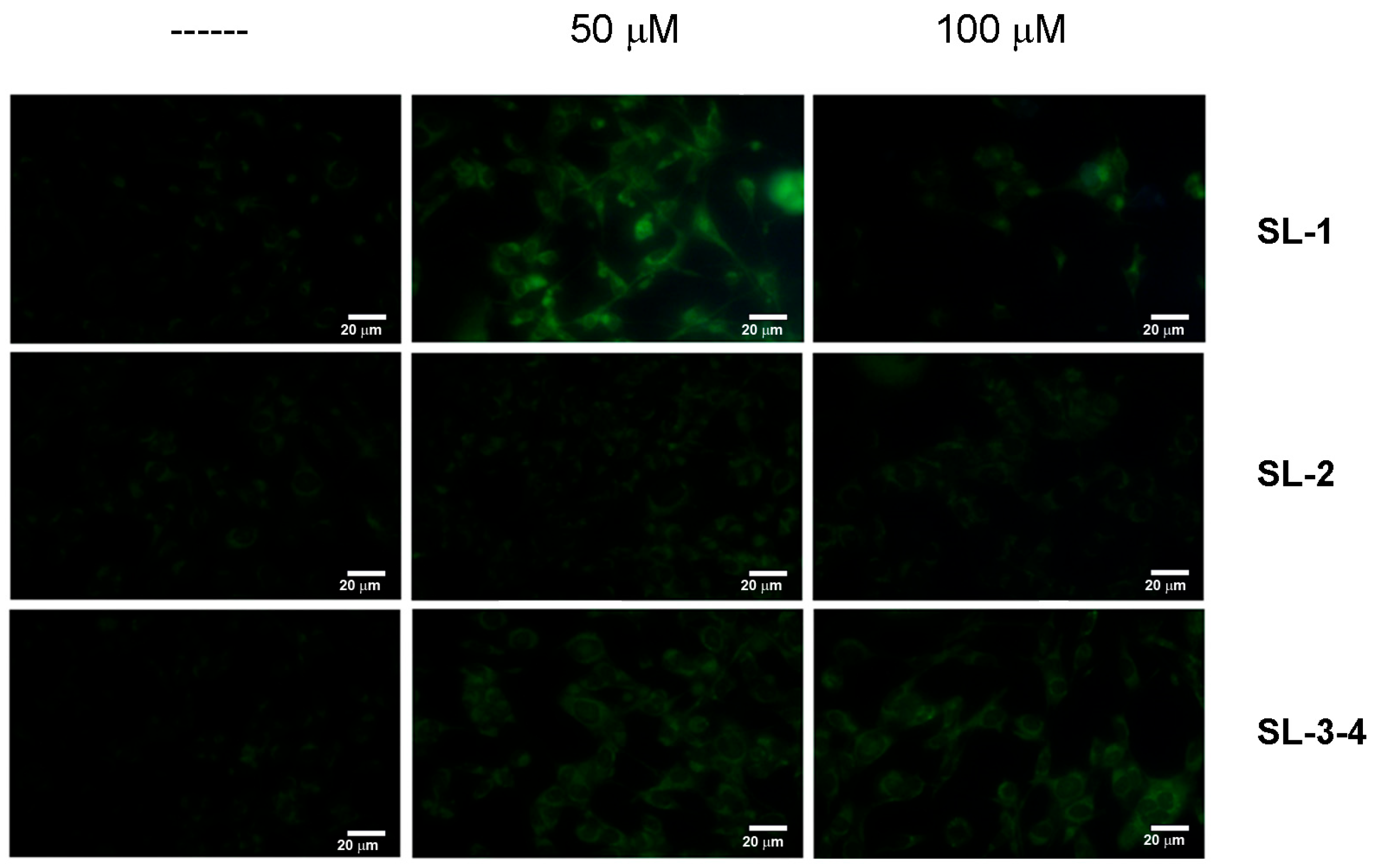
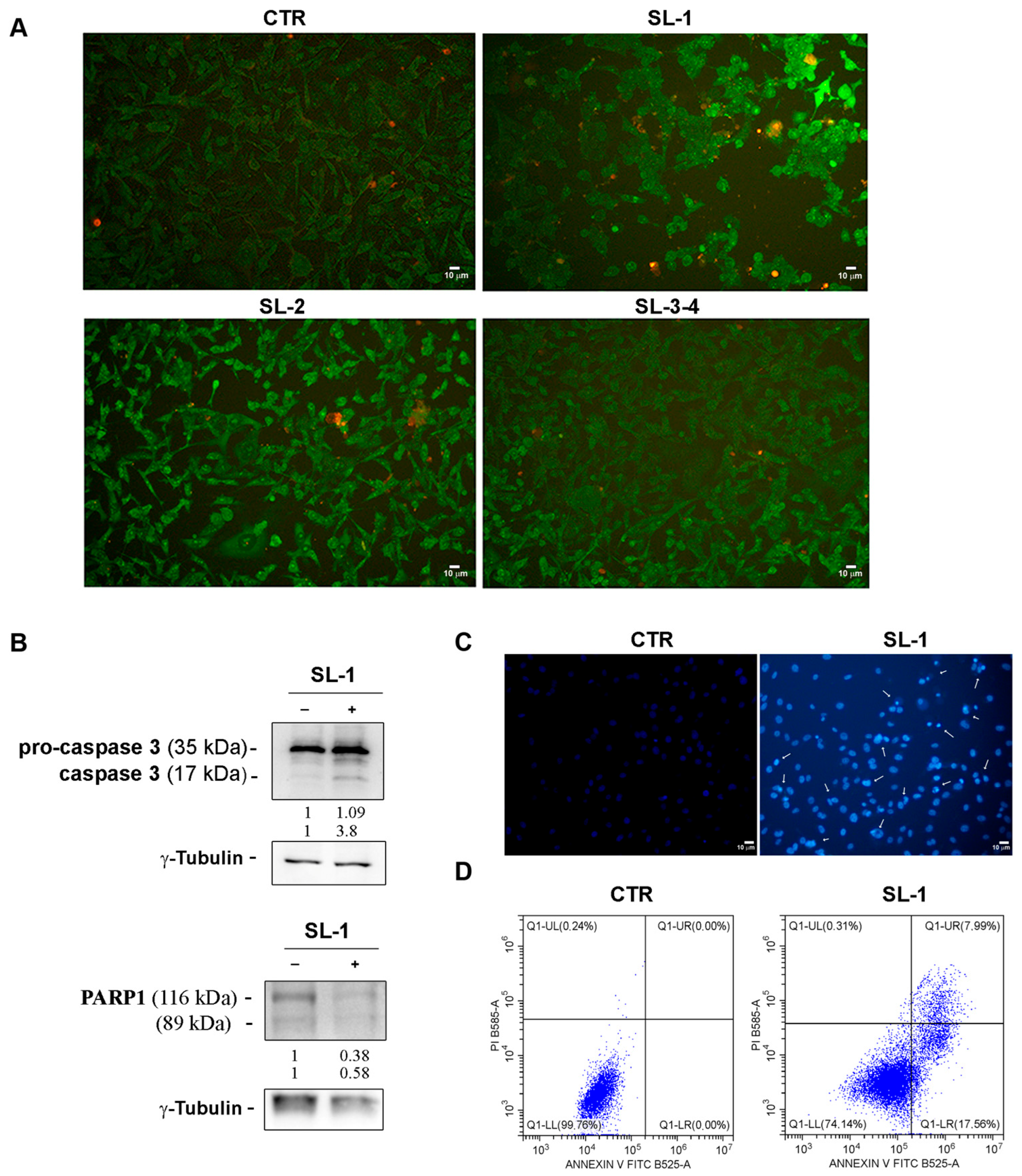
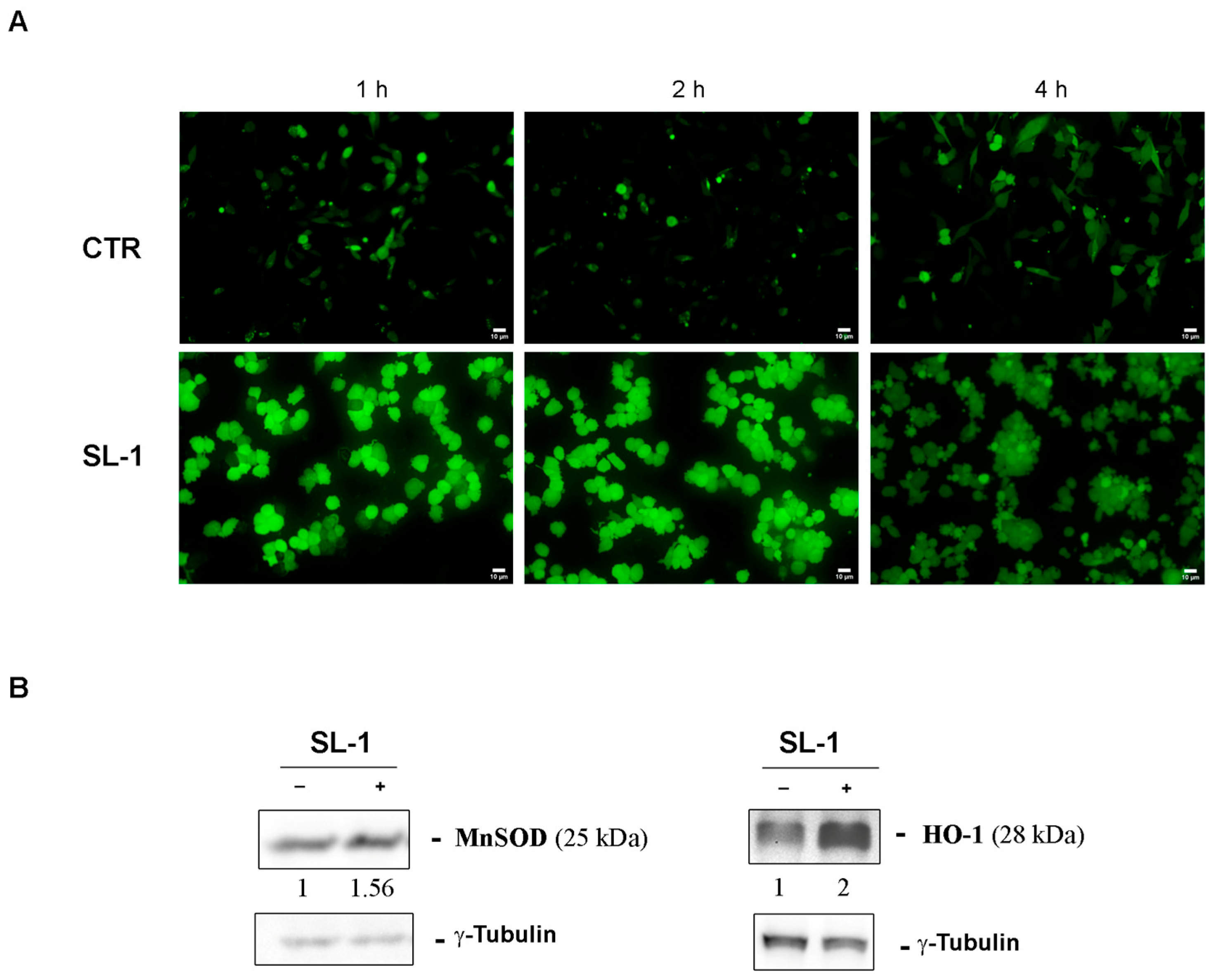
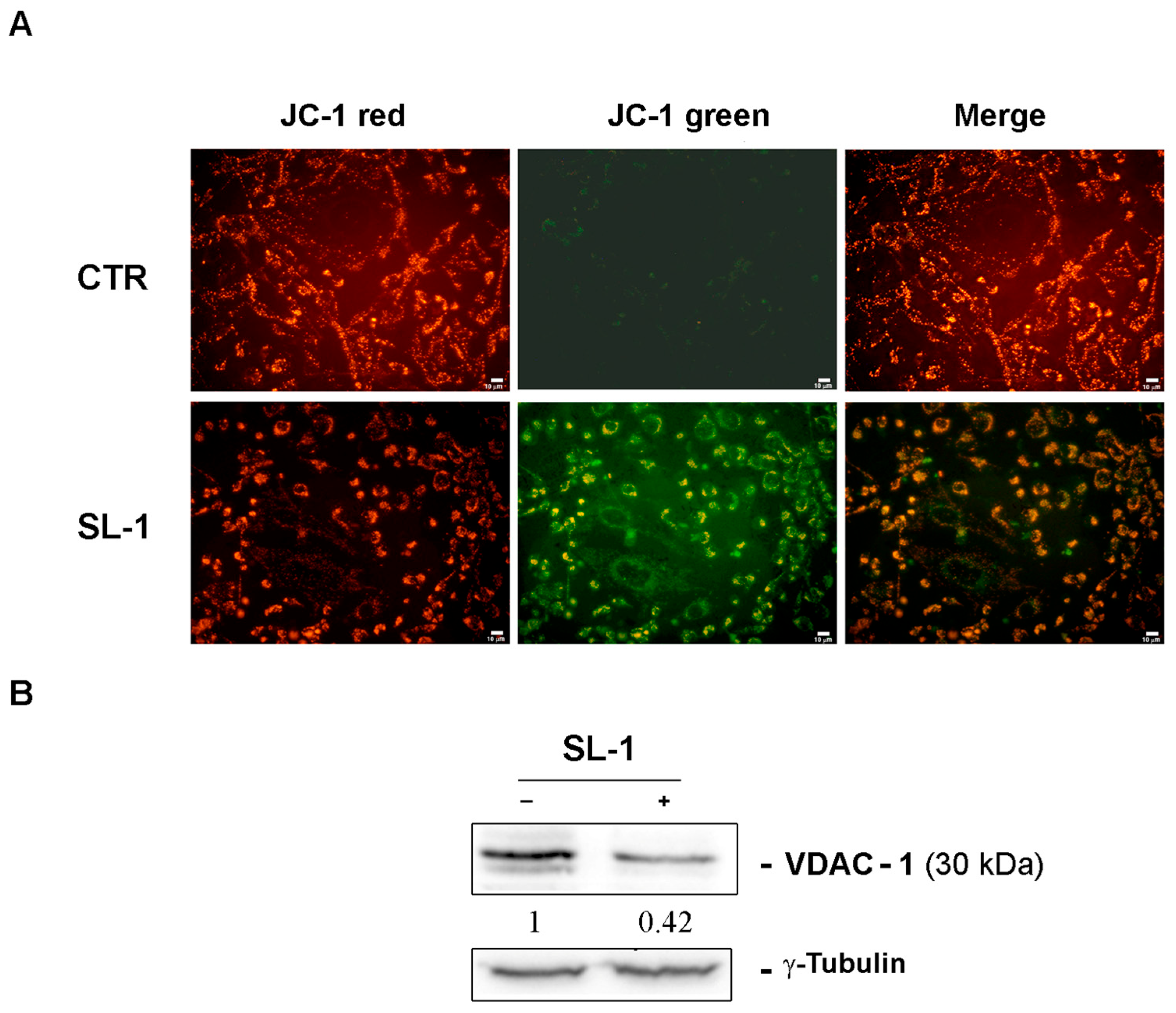
2.2. Bioassays
3. Materials and Methods
3.1. Plant Material
3.2. Extraction and Isolation
3.3. Structural Identification
3.4. Cell Culture and Viability Assay
3.5. Assessment of Cell Viability
3.6. Assessment of Autophagy by Monodansylcadaverine Test
3.7. Apoptotic Cell Demise by Hoechst and Annexin V-FITC/PI Staining
3.8. Western Blotting
3.9. Measurement of Intracellular ROS Content
3.10. Evaluation of the Mitochondrial Membrane Potential
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hartvig, P. Laserpitium L. In Mountain Flora of Greece; Strid, A., Ed.; Cambridge University Press: Cambridge, UK, 1986; Volume 1, pp. 726–733. [Google Scholar]
- Tutin, T.G. Laserpitium L. In Flora Europaea; Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1968; Volume 2, pp. 368–370. [Google Scholar]
- Micevski, K. Laserpitium L. In Flora of Republic of Macedonia; Matevski, V., Ed.; Macedonian Academy of Arts and Sciences: Skopje, North Macedonia, 2005; Volume 1, p. 1647. [Google Scholar]
- Appendino, G.; Cravotto, G.; Nano, G.M. Sesquiterpene lactones from Laserpitium gallicum. Phytochemistry 1993, 33, 883–886. [Google Scholar] [CrossRef]
- Dastan, D.; Salehi, P.; Maroofi, H. Chemical composition, antioxidant and antimicrobial activities on Laserpitium carduchorum Hedge & Lamond essential oil and extracts during various growing stages. Chem. Biodivers. 2016, 13, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Hegi, G. Laserpitium L. In Illustrierte Flora von Mitteleuropa; Beger, H., Ed.; A. Pichler’s Witwe & Sohn: Wien, Austria, 1906; Volume 2, pp. 1472–1501. [Google Scholar]
- Ranđelović, V.; Zlatković, B.; Jušković, M.; Živojinović, L. Endangered species of flora of Mt. Suva planina. In Proceedings of the 6th Symposium on Flora of the Southeastern Serbia and Neighbouring Regions, Sokobanja, Serbia, 4–7 July 2000; pp. 303–322. [Google Scholar]
- Popović, V.; Heyerick, A.; Petrović, S.; Van Calenbergh, S.; Karalić, I.; Niketić, M.; De Force, D. Sesquiterpene lactones from the extracts of two Balkan endemic Laserpitium species and their cytotoxic activity. Phytochemistry 2013, 87, 102–111. [Google Scholar] [CrossRef]
- Chen, Y.L.; Lee, C.Y.; Huang, K.H.; Kuan, Y.H.; Chen, M. Prescription patterns of Chinese herbal products for patients with sleep disorder and major depressive disorder in Taiwan. J. Ethnopharmacol. 2015, 171, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Janačković, P.; Gavrilović, M.; Savić, J.; Marin, P.D.; Dajić Stevanović, Z. Traditional knowledge on plant use from Negotin Krajina (Eastern Serbia): An ethnobotanical study. Indian J. Tradit. Knowl. 2019, 18, 25–33. [Google Scholar]
- Popović, V.; Stojković, D.; Nikolić, M.; Heyerick, A.; Petrović, S.; Soković, M.; Niketić, M. Extracts of three Laserpitium L. species and their principal components laserpitine and sesquiterpene lactones inhibit microbial growth and biofilm formation by oral Candida isolates. Food Funct. 2015, 6, 1205–1211. [Google Scholar] [CrossRef]
- Chizzola, R.; Novak, J.; Franz, C. Fruit of Laserpitium siler grown in France. J. Essen. Oil Res. 1999, 11, 197–198. [Google Scholar] [CrossRef]
- Basile, S.; Badalamenti, N.; Riccobono, O.; Guarino, S.; Ilardi, V.; Bruno, M.; Peri, E. Chemical composition and evaluation of insecticidal activity of Calendula incana subsp. maritima and Laserpitium siler subsp. siculum essential oils against stored products pests. Molecules 2022, 27, 588. [Google Scholar] [CrossRef]
- Mileski, K.S.; Ćirić, A.D.; Milanović, S.; Krivošej, Ž.Đ.; Džamić, A.M. Composition and antibacterial activity of essential oils of Laserpitium latifolium and L. siler (Apiaceae). In Proceedings of the 3rd International Conference on Plant Biology and 22nd SPPS Meeting, Belgrade, Serbia, 9–12 June 2018; pp. 4–14. [Google Scholar]
- Maggi, F.; Bartolucci, F.; Conti, F. Chemical variability in volatile composition between several Italian accessions of Siler montanum (S. montanum subsp. montanum and S. montanum subsp. siculum). Biochem. Syst. Ecol. 2017, 70, 14–21. [Google Scholar] [CrossRef]
- Rimpelová, S.; Jurášek, M.; Peterková, L.; Bejček, J.; Spiwok, V.; Majdl, M.; Jirásko, M.; Buděšínský, M.; Harmatha, J.; Kmoníčková, E.; et al. Archangelolide: A sesquiterpene lactone with immunobiological potential from Laserpitium archangelica. Beilstein J. Org. Chem. 2019, 15, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- Stanković, N.; Mihajilov-Krstev, T.; Zlatković, B.; Stankov-Jovanović, V.; Mitić, V.; Jović, J.; Čomić, L.; Kočić, B.; Bernstein, N. Antibacterial and antioxidant activity of traditional medicinal plants from the Balkan Peninsula. NJAS-Wagen. J. Life Sci. 2016, 78, 21–28. [Google Scholar] [CrossRef]
- Milosavljević, S.; Bulatović, V.; Stefanović, M. Sesquiterpene lactones from the Yugoslavian wild growing plant families Asteraceae and Apiaceae. J. Serb. Chem. Soc. 1999, 64, 397–442. [Google Scholar] [CrossRef]
- Drew, D.P.; Krichau, N.; Reichwald, K.; Simonsen, H.T. Guaianolides in Apiaceae: Perspectives on pharmacology and biosynthesis. Phytochem. Rev. 2009, 8, 581–599. [Google Scholar] [CrossRef]
- Simonsen, H.T.; Weitzel, C.; Christensen, S.B. Guaianolide sesquiterpenoids: Pharmacology and biosynthesis. In Natural Products; Ramawat, K.G., Merillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3069–3098. [Google Scholar]
- Harmatha, J.; Buděšínský, M.; Vokáč, K.; Kostecká, P.; Kmoníčková, E.; Zídek, Z. Trilobolide and related sesquiterpene lactones from Laser trilobum possessing immunobiological properties. Fitoterapia 2013, 89, 157–166. [Google Scholar] [CrossRef]
- Dhyani, P.; Sati, P.; Sharma, E.; Attri, D.C.; Bahukhandi, A.; Tynybekov, B.; Szopa, A.; Sharifi-Rad, J.; Calina, D.; Suleria, H.A.R.; et al. Sesquiterpenoid lactones as potential anti-cancer agents: An update on molecular mechanisms and recent studies. Cancer Cell Int. 2022, 22, 305. [Google Scholar] [CrossRef]
- Won, K.A.; Spruck, C. Triple-negative breast cancer therapy: Current and future perspectives (Review). Int. J. Oncol. 2020, 57, 1245–1261. [Google Scholar] [CrossRef]
- Giaquinto, A.N.; Sung, H.; Newman, L.A.; Freedman, R.A.; Smith, R.A.; Star, J.; Jemal, A.; Siegel, R.L. Breast cancer statistics 2024. CA Cancer J. Clin. 2024, 74, 477–495. [Google Scholar] [CrossRef]
- Sun, X.; Wang, M.; Wang, M.; Yu, X.; Guo, J.; Sun, T.; Li, X.; Yao, L.; Dong, H.; Xu, Y. Metabolic reprogramming in triple-negative breast cancer. Front. Oncol. 2020, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Carlisi, D.; Buttitta, G.; Di Fiore, R.; Scerri, C.; Drago-Ferrante, R.; Vento, R.; Tesoriere, G. Parthenolide and DMAPT exert cytotoxic effects on breast cancer stem-like cells by inducing oxidative stress, mitochondrial dysfunction and necrosis. Cell Death Dis. 2016, 7, e2194. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Cui, Y.; Hu, Z.; Wang, W.; Jiang, W.; Xu, H. Ambrosin sesquiterpene lactone exerts selective and potent anticancer effects in drug-resistant human breast cancer cells (MDA-MB-231) through mitochondrial mediated apoptosis, ROS generation and targeting Akt/β-Catenin signaling pathway. J. Buon 2020, 25, 2221–2227. [Google Scholar] [PubMed]
- Barrero, A.F.; Herrador, M.M.; Arteaga, P. Sesquiterpene lactones and other constituents of Seseli vayredanum. Phytochemistry 1994, 37, 1351–1358. [Google Scholar] [CrossRef]
- Tang, Y.; Tao, J.-Q.; Li, W.; Zhao, Y.-Q. Three New Triterpene Saponins from Bolbostemma paniculatum. Helv. Chim. Acta 2014, 97, 268–277. [Google Scholar] [CrossRef]
- Berezowska, S.; Galván, J.A. Immunohistochemical detection of the autophagy markers LC3 and p62/SQSTM1 in formalin-fixed and paraffin-embedded tissue. Methods Mol. Biol. 2017, 1560, 189–194. [Google Scholar] [PubMed]
- Los, M.; Mozoluk, M.; Ferrari, D.; Stepczynska, A.; Stroh, C.; Renz, A.; Herceg, Z.; Wang, Z.Q.; Schulze-Osthoff, K. Activation and caspase-mediated inhibition of PARP: A molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol. Biol. Cell 2002, 13, 978–988. [Google Scholar] [CrossRef]
- An, X.; Yu, W.; Liu, J.; Tang, D.; Yang, L.; Chen, X. Oxidative cell death in cancer: Mechanisms and therapeutic opportunities. Cell Death Dis. 2024, 15, 556. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2008, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Palma, F.R.; Gantner, B.N.; Sakiyama, M.J.; Kayzuka, C.; Shukla, S.; Lacchini, R.; Cunniff, B.; Bonini, M.G. ROS production by mitochondria: Function or dysfunction? Oncogene 2024, 43, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Venditti, P.; Napolitano, G. Mitochondrial Management of ROS in Physiological and Pathological Conditions. Antioxidants 2025, 14, 43. [Google Scholar] [CrossRef]
- Napolitano, G.; Fasciolo, G.; Venditti, P. Mitochondrial Management of Reactive Oxygen Species. Antioxidants 2021, 10, 1824. [Google Scholar] [CrossRef]
- Camara, A.K.S.; Zhou, Y.; Wen, P.C.; Tajkhorshid, E.; Kwok, W.M. Mitochondrial VDAC1: A Key Gatekeeper as Potential Therapeutic Target. Front. Physiol. 2017, 8, 460. [Google Scholar] [CrossRef]
- Carlisi, D.; D’Anneo, A.; Martinez, R.; Emanuele, S.; Buttitta, G.; Di Fiore, R.; Vento, R.; Tesoriere, G.; Lauricella, M. The oxygen radicals involved in the toxicity induced by parthenolide in MDA-MB-231 cells. Oncol. Rep. 2014, 32, 167–172. [Google Scholar] [CrossRef]
- Liu, K.; Liu, P.C.; Liu, R.; Wu, X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med. Sci. Monit. Basic Res. 2015, 21, 15–20. [Google Scholar] [CrossRef]
- Celesia, A.; Morana, O.; Fiore, T.; Pellerito, C.; D’Anneo, A.; Lauricella, M.; Carlisi, D.; De Blasio, A.; Calvaruso, G.; Giuliano, M.; et al. ROS-dependent ER stress and autophagy mediate the anti-tumor effects of tributyltin (IV) ferulate in colon cancer cells. Int. J. Mol. Sci. 2020, 21, 8135. [Google Scholar] [CrossRef]
- Allegra, M.; D’Anneo, A.; Frazzitta, A.; Restivo, I.; Livrea, M.A.; Attanzio, A.; Tesoriere, L. The phytochemical indicaxanthin synergistically enhances cisplatin-induced apoptosis in hela cells via oxidative stress-dependent p53/p21waf1 axis. Biomolecules 2020, 10, 994. [Google Scholar] [CrossRef]
- Giuliano, M.; Bellavia, G.; Lauricella, M.; D’Anneo, A.; Vassallo, B.; Vento, R.; Tesoriere, G. Staurosporine-induced apoptosis in Chang liver cells is associated with down-regulation of Bcl-2 and Bcl-XL. Int. J. Mol. Med. 2004, 13, 565–571. [Google Scholar] [CrossRef]
- Azizi, K.; Hamedi, A.; Azarpira, N.; Hamedi, A.; Shahini, M.; Pasdaran, A. A new cytotoxic sesquiterpene lactone from Euphorbia microsphaera Boiss against human breast cancer (MCF-7) and human fibrosarcoma (HT1080) cells. Toxicon 2021, 202, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Li, X.W.; Weng, L.; Gao, X.; Zhao, Y.; Pang, F.; Liu, J.H.; Zhang, H.F.; Hu, J.F. Antiproliferative and apoptotic sesquiterpene lactones from Carpesium faberi. Bioorg. Med. Chem. Lett. 2011, 21, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xu, J.; Sun, S.; Lin, W.; Li, Y.; Lu, Q.; Li, F.; Yang, Z.; Lu, Y.; Liu, W. Mecheliolide elicits ROS-mediated ERS driven immunogenic cell death in hepatocellular carcinoma. Redox Biol. 2022, 54, 102351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lu, Y.; Ding, Y.; Zhai, J.; Ji, Q.; Ma, W.; Yang, M.; Fan, H.; Long, J.; Tong, Z.; et al. Guaianolide sesquiterpene lactones, a source to discover agents that selectively inhibit acute myelogenous leukemia stem and progenitor cells. J. Med. Chem. 2012, 55, 8757–8769. [Google Scholar] [CrossRef]
| IC50 (μΜ) | ||
|---|---|---|
| SL-1 | SL-2 | SL-3–4 |
| 50 ± 5 | 90 ± 3 | 250 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaglica, A.; Maggio, A.; Occhipinti, C.; Badalamenti, N.; Lauricella, M.; Bruno, M.; D’Anneo, A. Exploring the Biochemical Mechanism Beyond the Cytotoxic Activity of Sesquiterpene Lactones from Sicilian Accession of Laserpitium siler Subsp. siculum (Spreng.) Thell. Plants 2025, 14, 3289. https://doi.org/10.3390/plants14213289
Vaglica A, Maggio A, Occhipinti C, Badalamenti N, Lauricella M, Bruno M, D’Anneo A. Exploring the Biochemical Mechanism Beyond the Cytotoxic Activity of Sesquiterpene Lactones from Sicilian Accession of Laserpitium siler Subsp. siculum (Spreng.) Thell. Plants. 2025; 14(21):3289. https://doi.org/10.3390/plants14213289
Chicago/Turabian StyleVaglica, Alessandro, Antonella Maggio, Chiara Occhipinti, Natale Badalamenti, Marianna Lauricella, Maurizio Bruno, and Antonella D’Anneo. 2025. "Exploring the Biochemical Mechanism Beyond the Cytotoxic Activity of Sesquiterpene Lactones from Sicilian Accession of Laserpitium siler Subsp. siculum (Spreng.) Thell" Plants 14, no. 21: 3289. https://doi.org/10.3390/plants14213289
APA StyleVaglica, A., Maggio, A., Occhipinti, C., Badalamenti, N., Lauricella, M., Bruno, M., & D’Anneo, A. (2025). Exploring the Biochemical Mechanism Beyond the Cytotoxic Activity of Sesquiterpene Lactones from Sicilian Accession of Laserpitium siler Subsp. siculum (Spreng.) Thell. Plants, 14(21), 3289. https://doi.org/10.3390/plants14213289











