Exogenous Nitric Oxide Promotes the Growth and Cadmium Accumulation of Alfalfa (Medicago sativa) Seedlings Under Cadmium Stress
Abstract
1. Introduction
2. Results
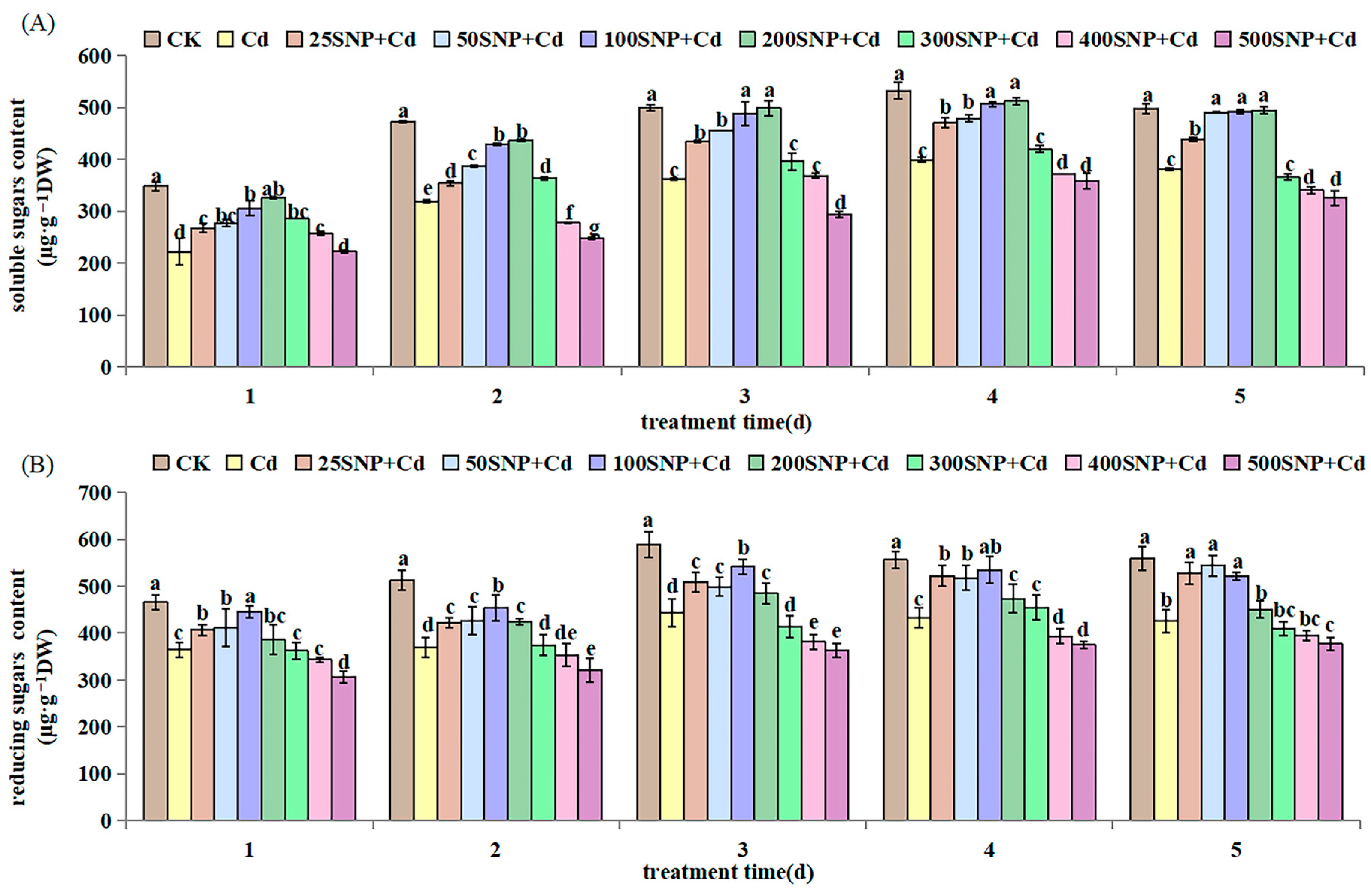
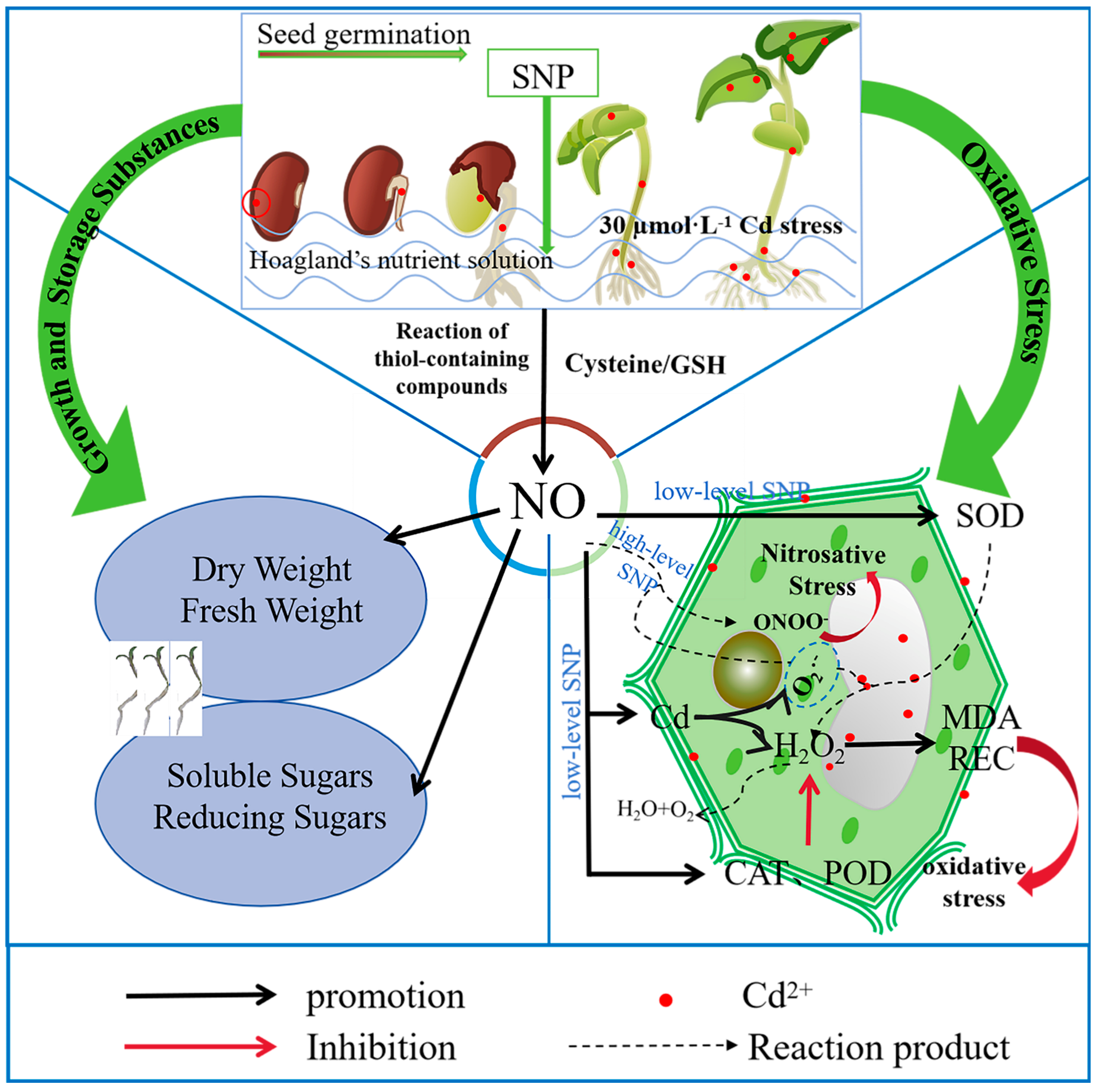
2.1. Seedling Growth
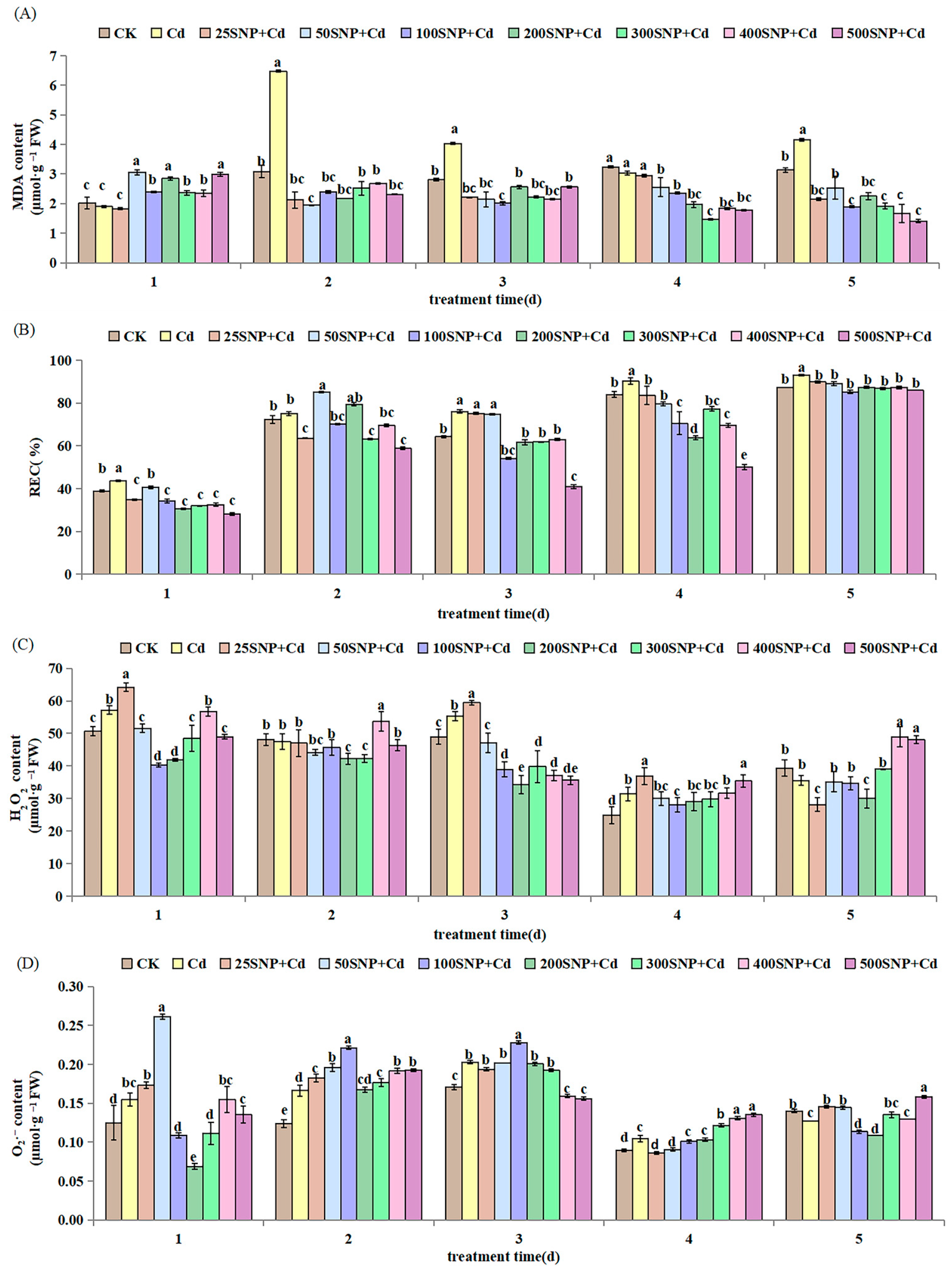
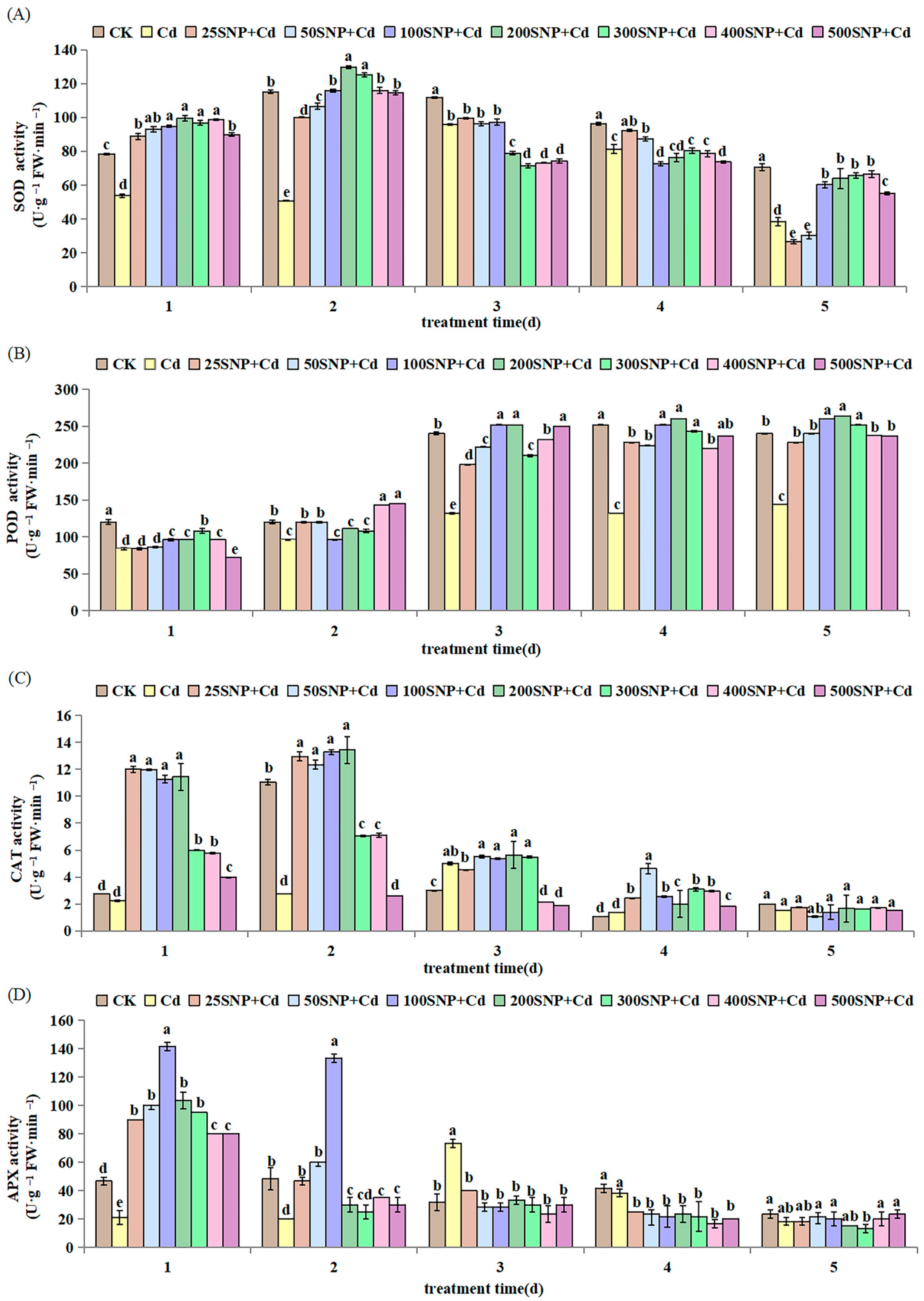
2.2. Antioxidant Metabolism
2.3. Cd Accumulation
2.4. Effect of 200 µM SNP on the Growth and Physiological Metabolism of Alfalfa
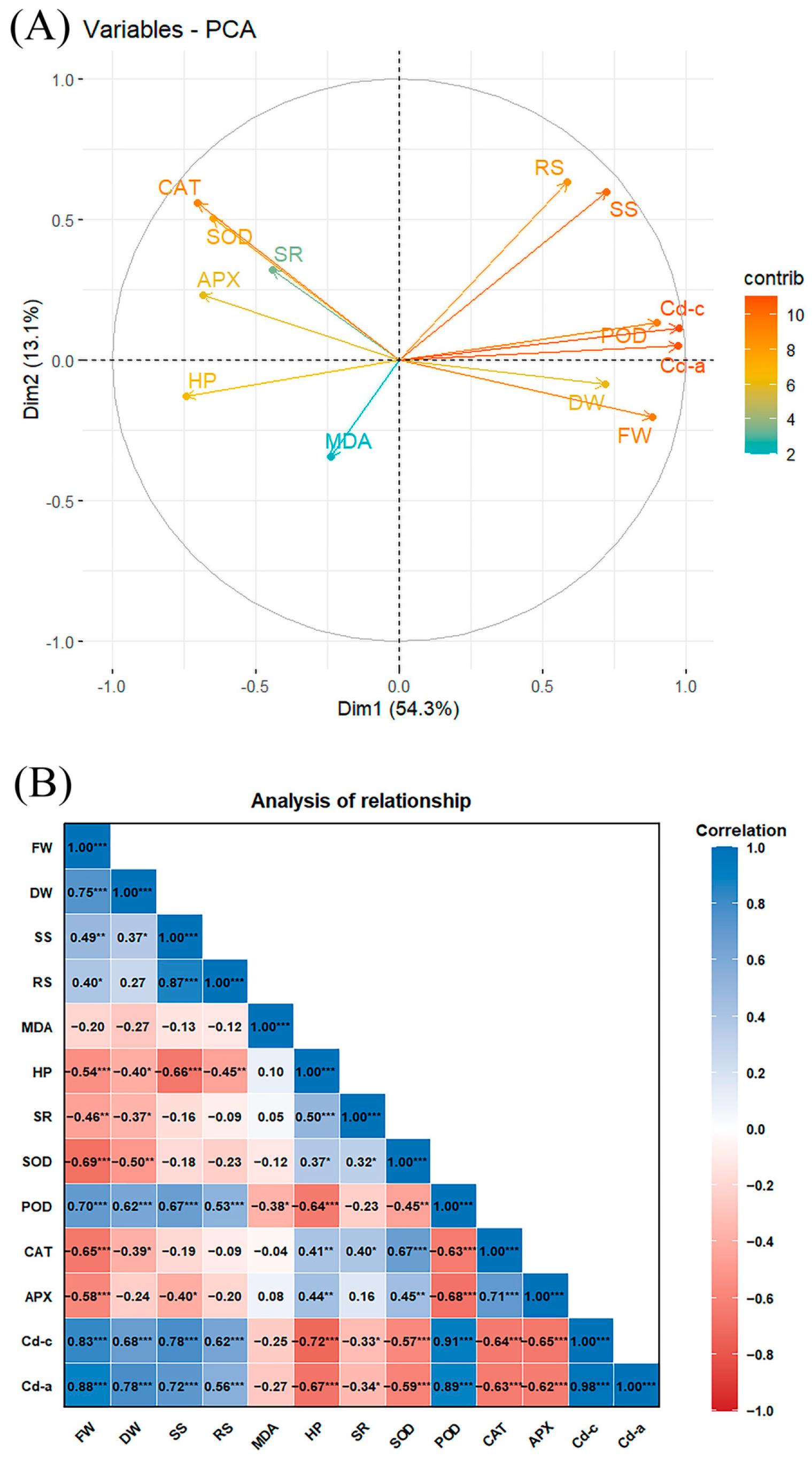
2.5. Principal Component and Pearson Correlation Analyses
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Plant Growth Conditions
4.2. Growth Parameter Determination
4.3. Lipid Peroxidation Determination
4.4. Antioxidant Enzyme Extraction and Assays
4.5. Storage Substances Determination
4.6. Cd Concentration Determination
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kowalska, J.B.; Mazurek, R.; Gasiorek, M.; Zaleski, T. Pollution indices as useful tools for the comprehensive evaluation of the degree of soil contamination—A review. Environ. Geochem. Health. 2018, 40, 2395–2420. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Ecology and Environment. China’s Ecological Environment Status Report; Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2021. [Google Scholar]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.Z.; Ma, W.J.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Sager, S.M.A.; Wijaya, L.; Alyemeni, M.N.; Hatamleh, A.A.; Ahmad, P. Impact of different cadmium concentrations on two Pisum sativum L. genotypes. Pak. J. Bot. 2020, 52, 821–829. [Google Scholar] [CrossRef]
- Arora, N.K.; Chauhan, R. Heavy metal toxicity and sustainable interventions for their decontamination. Environ. Sustain. 2021, 4, 1–3. [Google Scholar]
- Shahzad, A.; Hameed, S.; Qin, M.Z.; Li, H.Y.; Zafar, S.; Siddiqui, S.; Sattar, S.; Mahmood, Z.; Mehwish, S. Cadmium (Cd) detoxification and activation of plant defense enzymes in wheat (Triticum aestivum) through the use of endophytic Bacillus thuringiensis and Salix alba root powder. Environ. Pollut. 2025, 364, 125147. [Google Scholar] [CrossRef]
- Gomes, M.P.; Garcia, Q.S. Reactive oxygen species and seed germination. Biologia 2013, 68, 351–357. [Google Scholar] [CrossRef]
- Müller, K.; Carstens, A.C.; Linkies, A.; Torres, M.A.; Leubner-Metzger, G. The NADPH-oxidase AtrbohB plays a role in Arabidopsis seed after-ripening. New Phytol. 2009, 184, 885–897. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.J.; Zahir, Z.A.; Jamil, A. Effect of cadmium on seed germination and seedling growth of four wheat (Triticum aestivum L.) cultivars. Pak. J. Bot. 2012, 44, 1569–1574. [Google Scholar]
- Ma, J.; Saleem, M.H.; Alsafran, M.; Al Jabri, H.; Mehwish; Rizwan, M.; Nawaz, M.; Ail, S.; Usman, K. Response of cauliflower (Brassica oleracea L.) to nitric oxide application under cadmium stress. Ecotoxicol. Environ. Saf. 2022, 243, 113969. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Zhao, X.Y.; Xuan, W.; Wang, P.; Zhao, F.J. Rice roots avoid asymmetric heavy metal and salinity stress via an RBOH-ROS-auxin signaling cascade. Mol. Plant 2023, 16, 1678–1694. [Google Scholar] [CrossRef]
- Wang, X.; Du, H.; Ma, M.; Rennenberg, H. The dual role of nitric oxide (NO) in plant responses to cadmium exposure. Sci. Total Environ. 2023, 892, 164597. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, X.; Zhao, X.; Shen, R. Potassium affects cadmium resistance in Arabidopsis through facilitating root cell wall Cd retention in a nitric oxide dependent manner. Environ. Exp. Bot. 2020, 178, 104175. [Google Scholar] [CrossRef]
- Zboin´ska, M.; Janeczko, A.; Kabała, K. Involvement of NO in V-ATPase regulation in cucumber roots under control and cadmium stress conditions. Plants 2023, 12, 2884. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol. Plant. 2020, 168, 345–360. [Google Scholar] [CrossRef]
- Wang, Y.; Xian, J.P.; Wang, H.L.; Quan, Y.B.; Liu, L.; Ma, H.L. Effects of exogenous nitric oxide on seed germination and physiological characteristics of Poa pratensis under cadmium stress. J. Nucl. Agric. Sci. 2020, 34, 0169–0176. [Google Scholar]
- Kaya, C.; Polat, T.; Ashraf, M.; Kaushik, P.; Alyemeni, M.N.; Ahmad, P. Endogenous nitric oxide and its potential sources regulate glutathione-induced cadmium stress tolerance in maize plants. Plant Physiol. Biochem. 2021, 167, 723–737. [Google Scholar] [CrossRef]
- Besson-Bard, A.; Gravot, A.; Richaud, P.; Auroy, P.; Duc, C.; Gaymard, F.; Taconnat, L.; Renou, J.P.; Pugin, A.; Wendehenne, D. Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant Physiol. 2009, 149, 1302–1315. [Google Scholar] [CrossRef]
- Liu, X.; Gong, D.; Ke, Q.; Yin, L.; Wang, S.; Gao, T. Meta-Analysis of the effect of nitric oxide application on heavy metal stress tolerance in plants. Plants 2023, 12, 1494. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Y.; Tan, X.; Liu, H.; Zeng, G.; Hu, X.; Jian, H.; Gu, Y. Effect of exogenous nitric oxide on antioxidative system and S-nitrosylation in leaves of Boehmeria nivea (L.) Gaud under cadmium stress. Environ. Sci. Pollut. Res. 2015, 22, 3489–3497. [Google Scholar] [CrossRef] [PubMed]
- Khator, K.; Saxena, I.; Shekhawat, G.S. Nitric oxide induced Cd tolerance and phytoremediation potential of B. juncea by the modulation of antioxidant defense system and ROS detoxification. Biometals 2021, 34, 15–32. [Google Scholar] [CrossRef]
- Xu, S.S.; Lin, S.Z.; Lai, Z.X. Cadmium impairs iron homeostasis in Arabidopsis thaliana by increasing the polysaccharide contents and the iron-binding capacity of root cell walls. Plant Soil 2015, 392, 71–85. [Google Scholar] [CrossRef]
- Wang, T.; Song, J.; Liu, Z.; Liu, Z.; Cui, J. Melatonin alleviates cadmium toxicity by reducing nitric oxide accumulation and IRT1 expression in Chinese cabbage seedlings. Environ. Sci. Pollut. Res. 2021, 28, 15394–15405. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yin, Y.; Yi, H. Decreased endogenous nitric oxide contributes to sulfur dioxide derivative-alleviated cadmium toxicity in foxtail millet roots. Environ. Exp. Bot. 2020, 177, 104144. [Google Scholar] [CrossRef]
- Karimi, N. Comparative phytoremediation of chromium-contaminated soils by alfalfa (Medicago sativa) and Sorghum bicolor (L) Moench. Int. J. Sci. Res. Environ. Sci. 2013, 1, 44–49. [Google Scholar] [CrossRef]
- Wang, F.Q.; Li, Y.J.; Zhang, Q.; Qu, J. Phytoremediation of cadmium, lead and zinc by Medicago sativa L. (alfalfa): A study of different period. Bulg. Chem. Commun. 2015, 47, 167–172. [Google Scholar]
- Calabrese, E.J.; Agathokleous, E. Nitric oxide, hormesis and plant biology. Sci. Total Environ. 2023, 866, 161299. [Google Scholar] [CrossRef]
- Wang, Y.J.; Dong, Y.X.; Wang, J.; Cui, X.M. Alleviating effects of exogenous NO on tomato seedlings under combined Cu and Cd stress. Environ. Sci. Pollut. Res. 2016, 23, 4826–4836. [Google Scholar] [CrossRef]
- Pires, R.M.D.O.; Souza, G.A.D.; Cardoso, A.Á.; Dias, D.C.F.D.S.; Borges, E.E.D.L.E. Action of nitric oxide in sesame seeds (Sesamum indicum L.) submitted to stress by cadmium. J. Seed Sci. 2016, 38, 22–29. [Google Scholar] [CrossRef]
- He, J.; Ren, Y.; Chen, X.; Chen, H. Protective roles of nitric oxide on seed germination and seedling growth of rice (Oryza sativa L.) under cadmium stress. Ecotoxicol. Environ. Saf. 2014, 108, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Rather, B.A.; Mir, I.R.; Sehar, Z.; Anjum, N.A.; Masood, A.; Khan, N.A. The outcomes of the functional interplay of nitric oxide and hydrogen sulfide in metal stress tolerance in plants. Plant Physiol. Biochem. 2020, 155, 523–534. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Rehman, M.Z.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Aziz, H.; Murtaza, G.; Saleem, M.H.; Ali, S.; Rizwan, M.; Riaz, U.; Niaz, A.; Abualreesh, M.H.; Alatawi, A. Alleviation of chlorpyrifos toxicity in maize (Zea mays L.) by reducing its uptake and oxidative stress in response to soil-applied compost and biochar amendments. Plants 2021, 10, 2170. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.A.; Ahmar, S.; Ali, B.; Saleem, M.H.; Khan, M.U.; Zhou, W.; Liu, S. The role of membrane transporters in plant growth and development, and abiotic stress tolerance. Int. J. Mol. Sci. 2021, 22, 12792. [Google Scholar] [CrossRef]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.G.; Yun, B.W. Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ. Exp. Bot. 2019, 161, 120–133. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, L.; Zhu, C. Exogenous nitric oxide mediates alleviation of mercury toxicity by promoting auxin transport in roots or preventing oxidative stress in leaves of rice seedlings. Acta Physiol. Plant. 2015, 37, 1–9. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, X.; Dong, Y.; Xu, L.; Zhang, X.; Hou, J.; Fan, Z. Effects of exogenous nitric oxide on cadmium toxicity, element contents and antioxidative system in perennial ryegrass. Plant Growth Regul. 2013, 69, 11–20. [Google Scholar] [CrossRef]
- Terrón-Camero, L.C.; Peláez-Vico, M.Á.; Del-Val, C.; Sandalio, L.M.; Romero-Puertas, M.C. Role of nitric oxide in plant responses to heavy metal stress: Exogenous application versus endogenous production. J. Exp. Bot. 2019, 70, 4477–4488. [Google Scholar] [CrossRef] [PubMed]
- Molassiotis, A.; Fotopoulos, V. Oxidative and nitrosative signaling in plants: Two branches in the same tree? Plant Signaling Behav. 2011, 6, 210–214. [Google Scholar] [CrossRef]
- Piacentini, D.; Ronzan, M.; Fattorini, L.; Della Rovere, F.; Massimi, L.; Altamura, M.M.; Falasca, G. Nitric oxide alleviates cadmium-but not arsenic-induced damages in rice roots. Plant Physiol. Biochem. 2020, 151, 729–742. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B. Peroxynitrite (ONOO−) is endogenously produced in Arabidopsis peroxisomes and is overproduced under cadmium stress. Ann. Bot. 2014, 113, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Srivasatava, A.; Parida, A.; Mishra, A.K. Nitric oxide: A double-edged sword in photosynthetic stress responses. In Stress Biology in Photosynthetic Organisms: Molecular Insights and Cellular Responses; Mishra, A.K., Ed.; Springer Nature: Singapore, Singapore, 2024; pp. 347–373. [Google Scholar]
- Roy, S. Role of nitric oxide as a double edged sword in root growth and development. In Rhizobiology: Molecular Physiology of Plant Roots; Mukherjee, S., Baluška, F., Eds.; Springer: Berlin, Germany, 2021; pp. 167–193. [Google Scholar]
- Praveen, A.; Gupta, M. Nitric oxide confronts arsenic stimulated oxidative stress and root architecture through distinct gene expression of auxin transporters, nutrient related genes and modulates biochemical responses in Oryza sativa L. Environ. Pollut. 2018, 240, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Dixit, G.; Kumar, A.; Mishra, S.; Kumar, N.; Dixit, S.; Tripathi, R.D. A protective role for nitric oxide and salicylic acid for arsenite phytotoxicity in rice (Oryza sativa L.). Plant Physiol. Biochem. 2017, 115, 163–173. [Google Scholar] [CrossRef]
- Li, L.; Li, N.H.; Jiang, S.M.; Leng, J.Y.; Wang, X.J. Plant Physiology Module Experiment Instructs; Science Publishing: Beijing, China, 2009; pp. 78–86. [Google Scholar]
- Kaur, S.; Singh, H.P.; Batish, D.R.; Negi, A.; Mahajan, P.; Rana, S.; Kohli, R.K. Arsenic (As) inhibits radicle emergence and elongation in Phaseolus aureus by altering starch-metabolizing enzymes vis-à-vis disruption of oxidative metabolism. Biol. Trace Elem. Res. 2012, 146, 360–368. [Google Scholar] [CrossRef]
- Gomes, M.P.; Carneiro, M.M.L.C.; Nogueira, C.O.G.; Soares, A.M.; Garcia, Q.S. The system modulating ROS content in germinating seeds of two Brazilian savanna tree species exposed to As and Zn. Acta Physiol. Plant. 2013, 35, 1011–1022. [Google Scholar] [CrossRef]
- Kong, J.; Dong, Y.; Song, Y.; Bai, X.; Tian, X.; Xu, L.; Liu, S.; He, Z. Role of exogenous nitric oxide in alleviating iron deficiency stress of peanut seedlings (Arachis hypogaea L.). J. Plant Growth Regul. 2016, 35, 31–43. [Google Scholar] [CrossRef]
- Beyer, J.W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Clairborne, A.J.F.C.P. Catalase activity. In Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Chance, B.; Maehly, A.C. Assay of Catalases and Peroxidases; Methods of Biochemical Analysis; Academic Press Ltd-Elsevier Science Ltd.: London, UK, 1955; pp. 764–775. [Google Scholar]
- Gupta, A.S.; Webb, R.P.; Holaday, A.S.; Allen, R.D. Overexpression of superoxide dismutase protects plants from oxidative stress (induction of ascorbate peroxidase in superoxide dismutase-overexpressing plants). Plant Physiol. 1993, 103, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Shu, Q.Y.; Wu, D.X.; Xia, Y.W.; Liu, G.F. Changes of carbohydrate content in the leaves of rice greenable albino mutation line during the greening period. Plant Physiol. Commun. 1996, 32, 120–123. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists, Inc.: Arlington, VA, USA, 1990. [Google Scholar]



| Treatment | FW (g·100 Seedlings−1) | DW (g·100 Seedlings−1) | Germination Percentage (%) | Germination Index | Seedling Vigor Index (cm) | Germination Energy (%) |
|---|---|---|---|---|---|---|
| CK | 1.40 ± 0.07 b | 0.25 ± 0.01 b | 55.68 ± 0.05 b | 177.27 ± 8.55 b | 353.34 ± 18.13 b | 32.89 ± 1.25 b |
| 200SNP | 1.85 ± 0.04 a | 0.36 ± 0.05 a | 75.76 ± 2.22 a | 294.58 ± 38.36 a | 770.75 ± 112.11 a | 54.91 ± 3.82 a |
| Treatment | Root Length (mm) | Shoot Length (mm) | SS (µg·g−1 DW) | RS (µg·g−1 DW) | MDA (µmol·g−1 FW) | REC % |
| CK | 28.22 ± 2.12 b | 18.00 ± 1.73 b | 497.75 ± 10.33 a | 558.82 ± 55.68 b | 3.13 ± 0.09 a | 87.23 ± 0.12 a |
| 200 SNP | 35.84 ± 0.92 a | 24.52 ± 0.31 a | 504.14 ± 16.23 a | 699.18 ± 33.97 a | 1.36 ± 0.12 b | 85.64 ± 5.52 a |
| Treatment | H2O2 (µmol·g−1 FW) | O2·− (µmol·g−1 FW) | POD (U·g−1 FW·min−1) | SOD (U·g−1 FW·min−1) | CAT (U·g−1 FW·min−1) | APX (U·g−1 FW·min−1) |
| CK | 39.37 ± 2.49 a | 0.14 ± 0.01 a | 240.00 ± 0.80 a | 70.53 ± 2.06 a | 1.99 ± 0.02 a | 23.33 ± 2.89 a |
| 200SNP | 12.41 ± 1.78 b | 0.07 ± 0.01 b | 176.69 ± 8.95 b | 59.89 ± 2.29 b | 1.44 ± 0.01 b | 17.95 ± 1.02 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Sun, Y.; Cao, B.; Ngabire, M.; Lu, Y.; Li, Q.; Yuan, Q. Exogenous Nitric Oxide Promotes the Growth and Cadmium Accumulation of Alfalfa (Medicago sativa) Seedlings Under Cadmium Stress. Plants 2025, 14, 3264. https://doi.org/10.3390/plants14213264
Chen Y, Sun Y, Cao B, Ngabire M, Lu Y, Li Q, Yuan Q. Exogenous Nitric Oxide Promotes the Growth and Cadmium Accumulation of Alfalfa (Medicago sativa) Seedlings Under Cadmium Stress. Plants. 2025; 14(21):3264. https://doi.org/10.3390/plants14213264
Chicago/Turabian StyleChen, Yinping, Yong Sun, Bo Cao, Maurice Ngabire, Yuzhi Lu, Qian Li, and Qiaoling Yuan. 2025. "Exogenous Nitric Oxide Promotes the Growth and Cadmium Accumulation of Alfalfa (Medicago sativa) Seedlings Under Cadmium Stress" Plants 14, no. 21: 3264. https://doi.org/10.3390/plants14213264
APA StyleChen, Y., Sun, Y., Cao, B., Ngabire, M., Lu, Y., Li, Q., & Yuan, Q. (2025). Exogenous Nitric Oxide Promotes the Growth and Cadmium Accumulation of Alfalfa (Medicago sativa) Seedlings Under Cadmium Stress. Plants, 14(21), 3264. https://doi.org/10.3390/plants14213264







