A Review of the Regulatory Role of Plant Growth–Promoting Rhizobacteria in Alfalfa Under Stress Conditions
Abstract
1. Introduction
2. Current Status of Alfalfa Research
3. Plant–Microbe Interactions
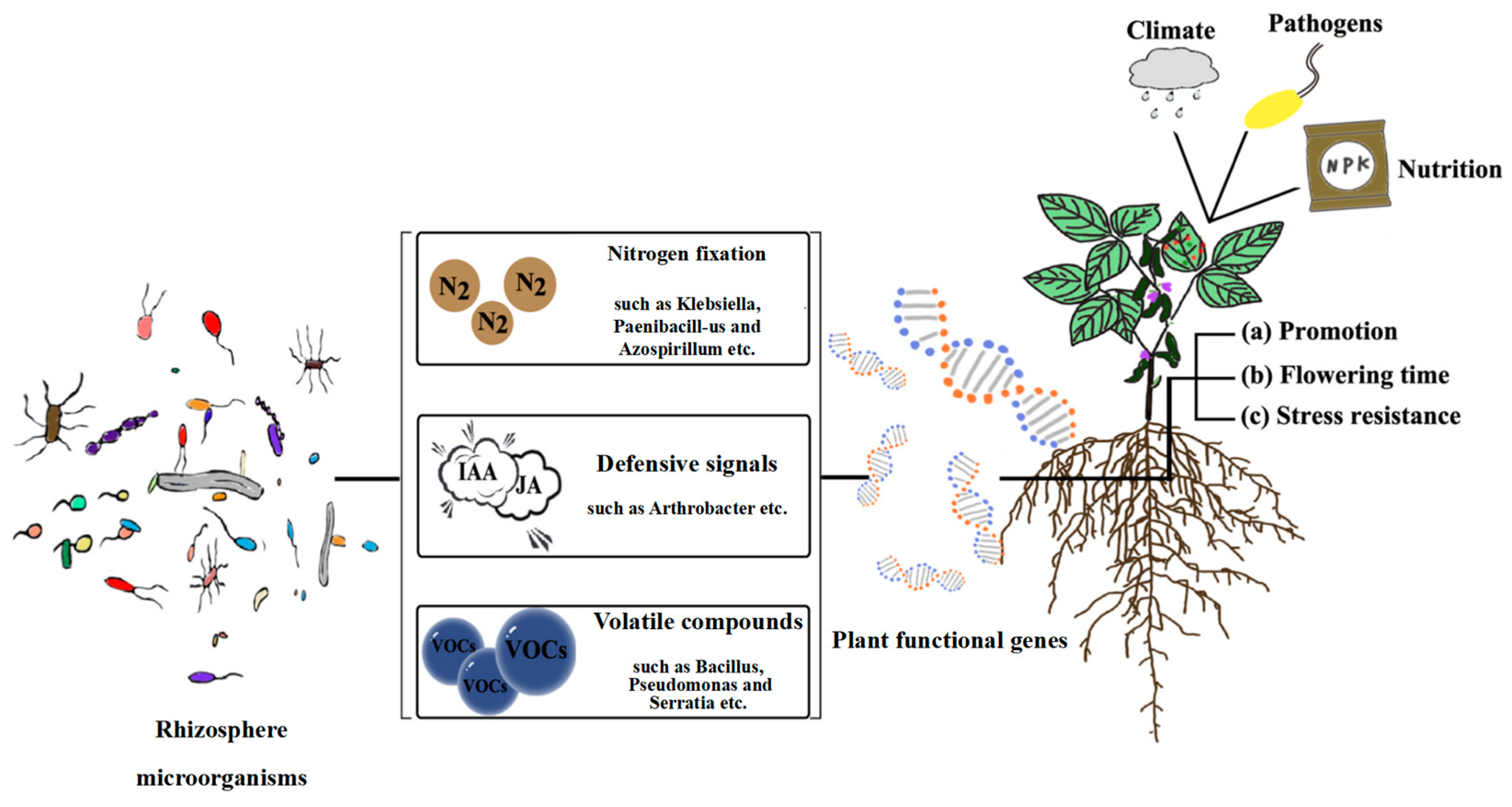
3.1. Importance of Plant–Microbe Interactions
3.2. Alfalfa–Microbe Interaction Mechanism and Its Effects
4. Current Status of PGPR Research
4.1. Current Status of PGPR Genetic Classification Research
4.2. Current Status of PGPR Metabolite Classification Research

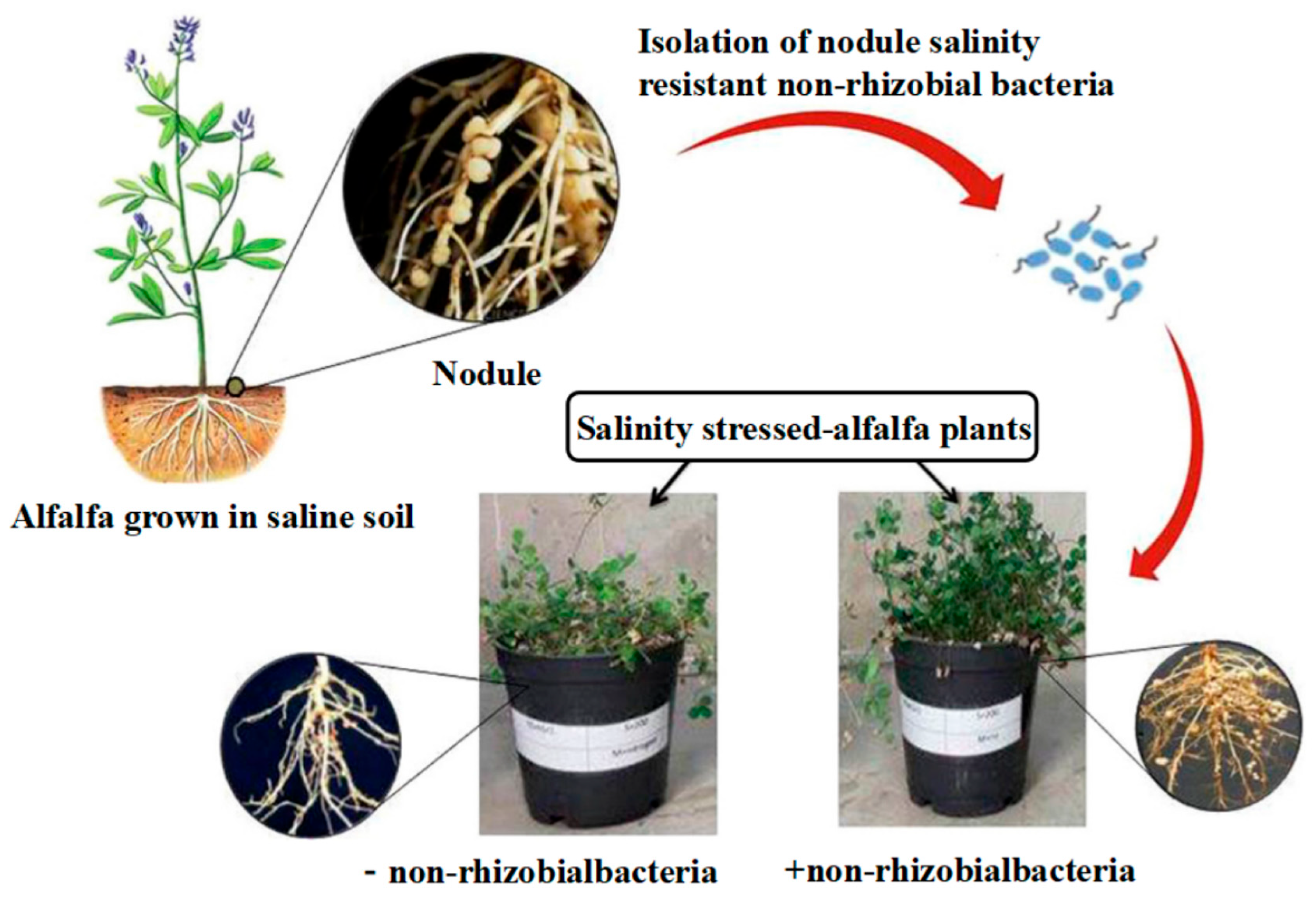
4.3. Current Status of Research on the Sources of PGPR
4.3.1. Research on Inter–Root Endophytes
4.3.2. Research on Inter–Root Soil Bacteria
4.4. Applied Research on PGPR
4.4.1. Application of PGPR in Soil Improvement
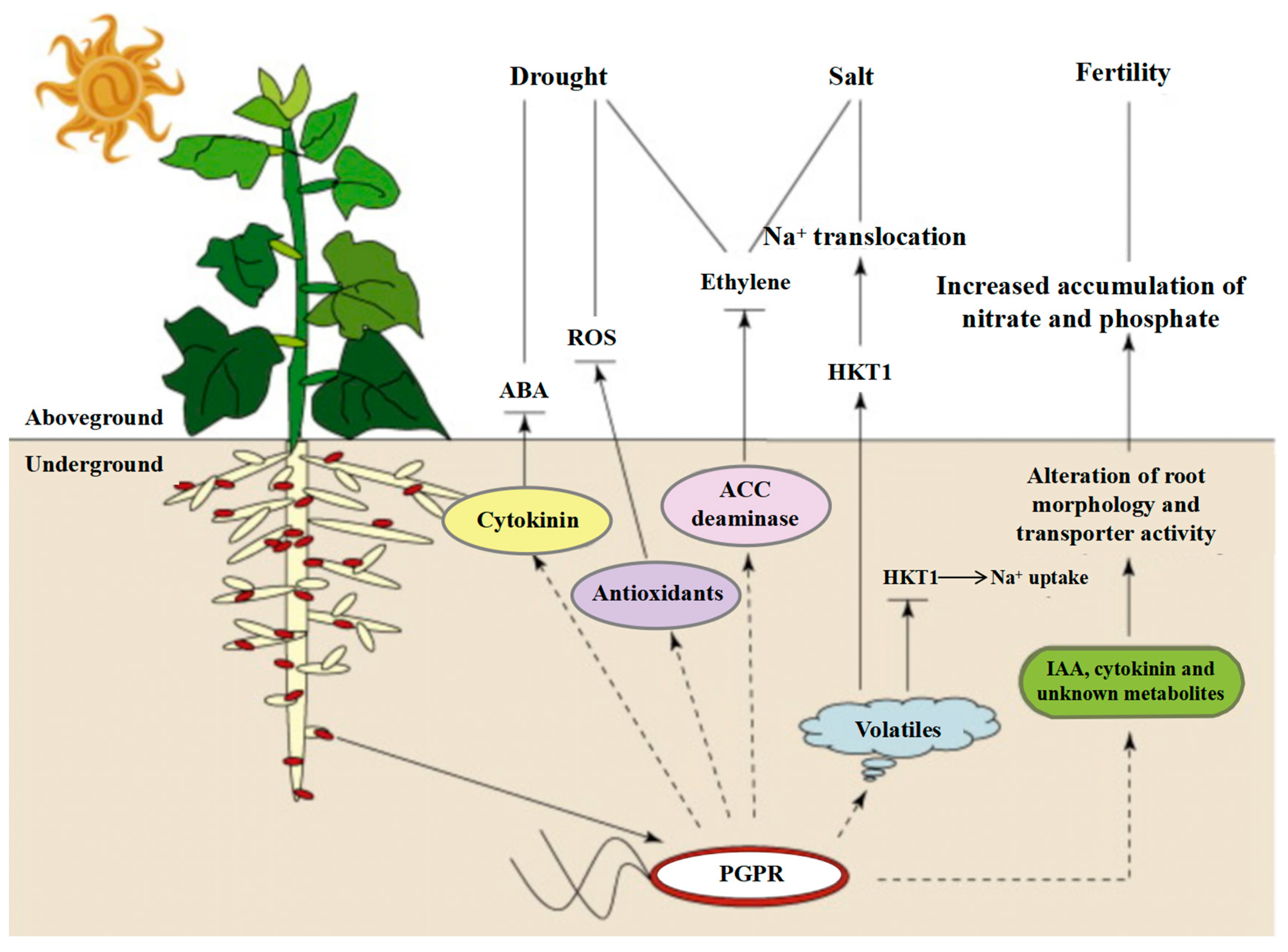
4.4.2. Application of PGPR in the Plant Growth Promotion Mechanism
4.4.3. Application of PGPR in Ecological Environment Remediation
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, W.; Bethel, M.; Mishra, D.R.; Hardy, T. Model selection in Bayesian framework to identify the best WorldView-2 based vegetation index in predicting green biomass of salt marshes in the northern Gulf of Mexico. GISci. Remote Sens. 2018, 55, 880–904. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A. Role of phosphate-solubilizing microorganisms in sustainable agriculture—A review. Agron. Sustain. Dev. 2007, 27, 29–43. [Google Scholar] [CrossRef]
- Chuamnakthong, S.; Nampei, M.; Ueda, A. Characterization of Na+ exclusion mechanism in rice under saline-alkaline stress conditions. Plant Sci. 2019, 287, 110171. [Google Scholar] [CrossRef]
- Zhao, Q.; Suo, J.W.; Chen, S.X.; Jin, Y.; Ma, X.; Yin, Z.; Zhang, Y.; Wang, T.; Luo, J.; Jin, W.; et al. Na2CO3-responsive mechanisms in halophyte Puccinellia tenuiflora roots revealed by physiological and proteomic analyses. Sci. Rep. 2016, 6, 32717. [Google Scholar] [CrossRef]
- Yang, D.H.; Tang, L.; Cui, Y.; Chen, J.; Liu, L.; Guo, C. Saline-alkali stress reduces soil bacterial community diversity and soil enzyme activities. Ecotoxicology 2022, 31, 1356–1368. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, B.; Sun, M.; Li, X. Different Cropping Patterns to Restore Saline-Alkali Soils in Northeast China Affect the Abundance of Functional Genes in the Soil Nitrogen Cycle. Sustainability 2023, 15, 6592. [Google Scholar] [CrossRef]
- Tiwari, S.; Sharma, B.; Bisht, N.; Tewari, L. Role of beneficial microbial gene pool in mitigating salt/nutrient stress of plants in saline soils through underground phytostimulating signalling molecules. Pedosphere 2023, 33, 153–171. [Google Scholar] [CrossRef]
- Abbas, A.; Khan, S.; Hussain, N.; Hanjra, M.A.; Akbar, S. Characterizing soil salinity in irrigated agriculture using a remote sensing approach. Phys. Chem. Earth 2013, 55–57, 43–52. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, P.; Yang, Z.; Lai, Y.; Li, S.; Chai, H.; Xu, Y.; Wu, Y.; Wang, J. Effects of plant density on alfalfa (Medicago sativa L.) seed yield in western Heilongjiang areas. Open Life Sci. 2023, 18, 20220792. [Google Scholar] [CrossRef]
- Wang, H.; Takano, T.; Liu, S.K. Screening and Evaluation of Saline-Alkaline Tolerant Germplasm of Rice (Oryza sativa L.) in Soda Saline-Alkali Soil. Agronomy 2018, 8, 205. [Google Scholar] [CrossRef]
- Li, F.; Guo, Y.; Wang, Z.; Mu, Y. Influence of different phytoremediation on soil microbial diversity and community composition in saline-alkaline land. Int. J. Phytoremediat. 2022, 24, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Wang, C.X.; Chen, S.F. Biofertilization containing Paenibacillus triticisoli BJ-18 alters the composition and interaction of the protistan community in the wheat rhizosphere under field conditions. J. Appl. Microbiol. 2022, 132, 3746–3757. [Google Scholar] [CrossRef]
- Li, Y.Y.; You, X.K.; Tang, Z.; Zhu, T.; Liu, B.; Chen, M.-X.; Xu, Y.; Liu, T.-Y. Isolation and identification of plant growth-promoting rhizobacteria from tall fescue rhizosphere and their functions under salt stress. Physiol. Plant. 2022, 174, e13817. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.S.; Hauggaard-Nielsen, H. How can increased use of biological N2 fixation in agriculture benefit the environment? Plant Soil 2003, 252, 177–186. [Google Scholar] [CrossRef]
- Vocciante, M.; Grifoni, M.; Fusini, D.; Petruzzelli, G.; Franchi, E. The Role of Plant Growth-Promoting rhizobacteria (PGPR) in Mitigating Plant’s Environmental Stresses. Appl. Sci. 2022, 12, 1231. [Google Scholar] [CrossRef]
- Tirry, N.; Kouchou, A.; Laghmari, G.; Lemjereb, M.; Hnadi, H.; Amrani, K.; Bahafid, W.; El Ghachtouli, N. Improved salinity tolerance of Medicago sativa and soil enzyme activities by PGPR. Biocatal. Agric. Biotechnol. 2021, 31, 101914. [Google Scholar] [CrossRef]
- Rengel, Z. Breeding for better symbiosis. Plant Soil 2002, 245, 245–260. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y. Effects of Different Neutral and Alkaline Salinities on Seed Germination and Early Seedling Growth of Maize (Zea mays L.). Afr. J. Agric. Res. 2011, 6, 3515–3521. [Google Scholar]
- Nabti, E.; Schmid, M.; Hartmann, A. Application of Halotolerant Bacteria to Restore Plant Growth Under Salt Stress; Springer International Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Bin, L.; Zhi-Chun, W.; Yuan, C. Resources and sustainable resource exploitation of salinized land in China. Agric. Res. Arid. Areas 2005. [Google Scholar]
- Zhang, L.; Tang, C.; Yang, J.; Yao, R.; Wang, X.; Xie, W.; Ge, A.-H. Salinity-dependent potential soil fungal decomposers under straw amendment. Sci. Total Environ. 2023, 891, 164569. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, H.; Amorim-Silva, V.; Tavares, R.M. Effect of salt on ROS homeostasis, lipid peroxidation and antioxidant mechanisms in Pinus pinaster suspension cells. Ann. For. Sci. 2009, 66, 211. [Google Scholar] [CrossRef]
- Tiwari, V.; Kumar, A.; Singh, P. Effects of Salt Stress on Physiology of Crop Plants. In Physiology of Salt Stress in Plants; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 16–37. [Google Scholar]
- Dzinyela, R.; Alhassan, A.R.; Suglo, P.; Movahedi, A. Advanced study of functional proteins involved in salt stress regulatory pathways in plants. S. Afr. J. Bot. 2023, 159, 425–438. [Google Scholar] [CrossRef]
- Cao, Y.; Song, H.; Zhang, L. New Insight into Plant Saline-Alkali Tolerance Mechanisms and Application to Breeding. Int. J. Mol. Sci. 2022, 23, 16048. [Google Scholar] [CrossRef]
- Jia, X.; Zhu, Y.; Zhang, R.; Zhu, Z.; Zhao, T.; Cheng, L.; Gao, L.; Liu, B.; Zhang, X.; Wang, Y. Ionomic and metabolomic analyses reveal the resistance response mechanism to saline-alkali stress in Malus halliana seedlings. Plant Physiol. Biochem. 2020, 147, 77–90. [Google Scholar] [CrossRef]
- An, Y.; Yang, X.-X.; Zhang, L.; Zhang, J.; Du, B.; Yao, L.; Li, X.-T.; Guo, C. Alfalfa MsCBL4 enhances calcium metabolism but not sodium transport in transgenic tobacco under salt and saline–alkali stress. Plant Cell Rep. 2020, 39, 997–1011. [Google Scholar]
- Yan, L.; Li, G.; Qi, X.; Yuan, J.; Xie, M. Effects of Combined Application of Nitrogen and Organic Fertilizers on Nitrogen Use Efficiency and Soil Nutrients of Dryland Spring Wheat in Central Gansu. Chin. J. Soil Sci. 2023, 54, 1361–1371. [Google Scholar]
- Zhao, X.; Wang, Y.; Wang, J.; Wang, P.; Wang, G.; Zhu, L.; Li, L. Trichoderma Affects Crop Growth and Soil Ecological Environment. J. Agric. Sci. Technol. 2023, 25, 166–172. [Google Scholar]
- Zhou, Q.; Luo, D.; Chai, X.; Wu, Y.; Wang, Y.; Nan, Z.; Yang, Q.; Liu, W.; Liu, Z. Multiple Regulatory Networks Are Activated during Cold Stress in Medicago sativa L. Int. J. Mol. Sci. 2018, 19, 3169. [Google Scholar] [CrossRef]
- Sundaramoorthy, S.; Raguchander, T.; Ragupathi, N.; Samiyappan, R. Combinatorial effect of endophytic and plant growth promoting rhizobacteria against wilt disease of Capsicum annum L. caused by Fusarium solani. Biol. Control. 2012, 60, 59–67. [Google Scholar] [CrossRef]
- Qiu, Y.; Fan, Y.; Chen, Y.; Hao, X.; Li, S.; Kang, S. Response of dry matter and water use efficiency of alfalfa to water and salinity stress in arid and semiarid regions of Northwest China. Agric. Water Manag. 2021, 254, 106934. [Google Scholar] [CrossRef]
- Yang, Q.S.; Peng, J.; Ni, S.M.; Zhang, C. Erosion and deposition significantly affect the microbial diversity, co-occurrence network, and multifunctionality in agricultural soils of Northeast China. J. Soils Sediments 2024, 24, 888–900. [Google Scholar] [CrossRef]
- Sa, D.W.; Lu, Q.; Wang, Z.; Ge, G.; Sun, L.; Jia, Y. The potential and effects of saline-alkali alfalfa microbiota under salt stress on the fermentation quality and microbial. BMC Microbiol. 2021, 21, 149. [Google Scholar] [CrossRef]
- Baral, R.; Lollato, R.P.; Bhandari, K.; Min, D. Yield gap analysis of rainfed alfalfa in the United States. Front. Plant Sci. 2022, 13, 931403. [Google Scholar] [CrossRef]
- Farissi, M.; Bouizgaren, A.; Faghire, M.; Bargaz, A.; Ghoulam, C. Agro-physiological responses of Moroccan alfalfa (Medicago sativa L.) populations to salt stress during germination and early seedling stages. Seed Sci. Technol. 2011, 39, 389–401. [Google Scholar] [CrossRef]
- Noori, F.; Etesami, H.; Najafi Zarini, H.; Khoshkholgh-Sima, N.A.; Salekdeh, G.H.; Alishahi, F. Mining alfalfa (Medicago sativa L.) nodules for salinity tolerant non-rhizobial bacteria to improve growth of alfalfa under salinity stress. Ecotoxicol. Environ. Saf. 2018, 162, 129–138. [Google Scholar] [CrossRef]
- Sun, H.; Peng, Q.; Guo, J.; Zhang, H.; Bai, J.; Mao, H. Effects of short-term soil exposure of different doses of ZnO nanoparticles on the soil environment and the growth and nitrogen fixation of alfalfa. Environ. Pollut. 2022, 309, 119817. [Google Scholar] [CrossRef]
- Oburger, E.; Dell‘mour, M.; Hann, S.; Wieshammer, G.; Puschenreiter, M.; Wenzel, W.W. Evaluation of a novel tool for sampling root exudates from soil-grown plants compared to conventional techniques. Environ. Exp. Bot. 2013, 87, 235–247. [Google Scholar] [CrossRef]
- Pétriacq, P.; Williams, A.; Cotton, A.; McFarlane, A.E.; Rolfe, S.A.; Ton, J. Metabolite profiling of non-sterile rhizosphere soil. Plant J. 2017, 92, 147–162. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef]
- Hacquard, S.; Garrido-Oter, R.; González, A.; Spaepen, S.; Ackermann, G.; Lebeis, S.; McHardy, A.C.; Dangl, J.L.; Knight, R.; Ley, R.; et al. Microbiota and host nutrition across plant and animal kingdoms. Cell Host Microbe 2015, 17, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Zhao, X.; Teng, K.; Tian, M.; Li, S. Plant-Microbial Interaction Mediated by Root Exudates. Plant Health Med. 2022, 1, 11–17. [Google Scholar]
- Hassan, M.K.M.J.A.; Kloepper, J.W. The interactions of rhizodeposits with plant growth-promoting rhizobacteria in the rhizosphere: A review. Agriculture 2019, 9, 142. [Google Scholar] [CrossRef]
- Liu, Q.; Cheng, L.; Nian, H.; Jin, J.; Lian, T. Linking plant functional genes to rhizosphere microbes: A review. Plant Biotechnol. J. 2023, 21, 902–917. [Google Scholar] [CrossRef]
- Panichikkal, J.; Krishnankutty, R.E. Root exudate components induced production of plant beneficial metabolites in rhizospheric Pseudomonas spp. Rhizosphere 2021, 19, 100366. [Google Scholar] [CrossRef]
- Yang, J.; Kloepper, J.W.; Ryu, C.-M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Frey-Klett, P.; Garbaye, J.; Tarkka, M. The mycorrhiza helper bacteria revisited. New Phytol. 2007, 176, 22–36. [Google Scholar] [CrossRef]
- Nanjundappa, A.; Bagyaraj, D.J.; Saxena, A.K.; Kumar, M.; Chakdar, H. Interaction between arbuscular mycorrhizal fungi and Bacillus spp. in soil enhancing growth of crop plants. Fungal Biol. Biotechnol. 2019, 6, 23. [Google Scholar] [CrossRef]
- Rivera-Cruz, M.D.C.; Narcía, A.T.; Ballona, G.C.; Kohler, J.; Caravaca, F.; Roldán, A. Poultry manure and banana waste are effective biofertilizer carriers for promoting plant growth and soil sustainability in banana crops. Soil Biol. Biochem. 2008, 40, 3092–3095. [Google Scholar] [CrossRef]
- Kohler, J.; Caravaca, F.; Roldán, A. Effect of drought on the stability of rhizosphere soil aggregates of Lactuca sativa grown in a degraded soil inoculated with PGPR and AM fungi. Appl. Soil Ecol. 2009, 42, 160–165. [Google Scholar] [CrossRef]
- Singh, N.; Sharma, D.P.; Kumar, V.; Hota, D. Impact of different rootstocks and soil agro-techniques on microbial counts and growth traits on replanted apple under for replant situations. Multilog. Sci. 2017, 7, 88–92. [Google Scholar]
- Vázquez, M.M.; César, S.; Azcón, R.; Barea, J.M. Interactions between arbuscular mycorrhizal fungi and other microbial inoculants (Azospirillum, Pseudomonas, Trichoderma) and their effects on microbial population and enzyme activities in the rhizosphere of maize plants. Appl. Soil Ecol. 2000, 15, 261–272. [Google Scholar] [CrossRef]
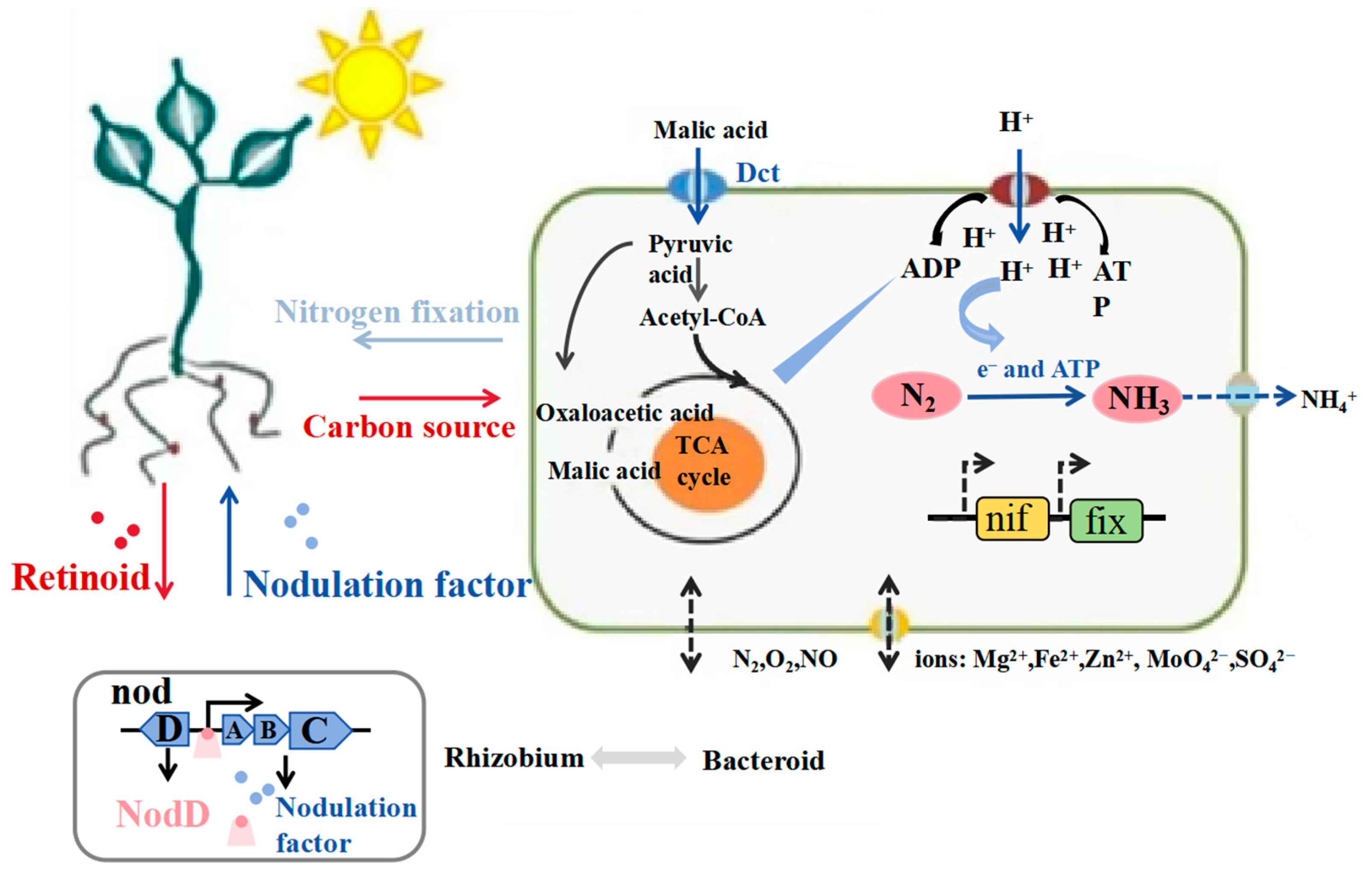
- Udvardi, M.K.; Day, D.A. Metabolite transport across symbiotic membranes of legume nodules. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 493–523. [Google Scholar] [CrossRef]
- Liu, A.; Contador, C.A.; Fan, K.; Lam, H.-M. Interaction and Regulation of Carbon, Nitrogen, and Phosphorus Metabolisms in Root Nodules of Legumes. Front. Plant Sci. 2018, 9, 1860. [Google Scholar] [CrossRef]
- Janczarek, M. Environmental signals and regulatory pathways that influence exopolysaccharide production in rhizobia. Int. J. Mol. Sci. 2011, 12, 7898–7933. [Google Scholar] [CrossRef] [PubMed]
- Zahran, H.H.; Karsisto, M.; Räsänen, L.A.; Lindström, K. Alteration of lipopolysaccharide and protein profiles in SDS-PAGE of rhizobia by osmotic and heat stress. World J. Microbiol. Biotechnol. 1994, 10, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Zahran, H.H. Characterization of root-nodule bacteria indigenous in the salt-affected soils of Egypt by lipopolysaccharide, protein and plasmid profiles. J. Basic Microbiol. 1992, 32, 279–287. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.-X.; Li, S.-Y.; Chen, N.; Bao, F.-X.; Wang, F.; Qu, S.-M.; Liu, G.-F. Effects of Saline-Alkali Tolerant Rhizosphere Growth-Promoting Bacteria on the Growth and Root Physiological Characteristics of alfalfa under Saline-Alkali Stress. Acta Agrestia Sin. 2024, 32, 2323–2329. [Google Scholar]
- Zhou, Y.; Bai, Y.; Yue, T.; Li, Q.; Huang, Y.; Jiang, W.; He, C.; Wang, J. Research progress on the growth-promoting characteristics of plant growth-promoting rhizobacteria. Microbiol. China 2023, 50, 644–666. [Google Scholar]
- Yao, D.; Zhang, J. Effects of Beneficial Soil Bacterium on Nitrogen Fixation Capacity of Medicago sativa under Saline Conditions. J. Green Sci. Technol. 2023, 25, 28–40. (In Chinese) [Google Scholar]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A.; et al. Bacterial Plant Biostimulants: A Sustainable Way Towards Improving Growth, Productivity, and Health of Crops. Sustainability 2021, 13, 2856. [Google Scholar] [CrossRef]
- Ilyas, N.; Mumtaz, K.; Akhtar, N.; Yasmin, H.; Sayyed, R.Z.; Khan, W.; El Enshasy, H.A.; Dailin, D.J.; Elsayed, E.A.; Ali, Z. Exopolysaccharides Producing Bacteria for the Amelioration of Drought Stress in Wheat. Sustainability 2020, 12, 8876. [Google Scholar] [CrossRef]
- Sheikh, T.; Hamid, B.; Baba, Z.; Iqbal, S.; Yatoo, A.; Fatima, S.; Nabi, A.; Kanth, R.; Dar, K.; Hussain, N.; et al. Extracellular polymeric substances in psychrophilic cyanobacteria: A potential bioflocculant and carbon sink to mitigate cold stress. Biocatal. Agric. Biotechnol. 2022, 42, 102375. [Google Scholar] [CrossRef]
- Kumar, A.; Patel, J.S.; Meena, V.S.; Srivastava, R. Recent advances of PGPR based approaches for stress tolerance in plants for sustainable agriculture. Biocatal. Agric. Biotechnol. 2019, 20, 101271. [Google Scholar] [CrossRef]
- Ullah, S.; Ashraf, M.; Asghar, H.N.; Iqbal, Z.; Ali, R. Review: Plant growth promoting rhizobacteria mediated amelioration of drought in crop plants. Soil Environ. 2019, 38, 1–20. [Google Scholar] [CrossRef]
- Bisht, N.; Anshu, A.; Singh, P.C.; Chauhan, P.S. Comprehensive analysis of OsJAZ gene family deciphers rhizobacteria-mediated nutrient stress modulation in rice. Int. J. Biol. Macromol. Struct. Funct. Interact. 2023, 253 Pt 3, 126832. [Google Scholar] [CrossRef]
- Joshi, H.; Mishra, S.K.; Prasad, V.; Chauhan, P.S. Bacillus amyloliquefaciens modulate sugar metabolism to mitigate arsenic toxicity in Oryza sativa L. var Saryu-52. Chemosphere 2023, 311, 137070. [Google Scholar] [CrossRef]
- Shultana, R.; Zuan, A.T.K.; Naher, U.A.; Islam, A.K.M.M.; Rana, M.; Rashid, H.; Irin, I.J.; Islam, S.S.; Rim, A.A.; Hasan, A.K. The PGPR Mechanisms of Salt Stress Adaptation and Plant Growth Promotion. Agronomy 2022, 12, 2266. [Google Scholar] [CrossRef]
- Kumawat, K.C.; Sharma, B.; Nagpal, S.; Kumar, A.; Tiwari, S.; Nair, R.M. Plant growth-promoting rhizobacteria: Salt stress alleviators to improve crop productivity for sustainable agriculture development. Front. Plant Sci. 2023, 13, 1101862. [Google Scholar] [CrossRef]
- Rai, P.K.; Sabharwal, U.; Singh, S.; Yadav, A.N.; Choure, K. Exploration of Drought Tolerant PGPR and Their role in Regulating Antioxidant Enzymes in Maize (Zea mays L.). J. Soil Sci. Plant Nutr. 2024, 24, 4483–4498. [Google Scholar] [CrossRef]
- Bigatton, E.D.; Ayoub, I.; Palmero, F.; Castillejo, M.Á.; Vázquez, C.; Lucini, E.I.; Haro, R.J. Plant-growth promoting rhizobacteria on peanuts: Effects on yield determination, growth rates, and radiation use efficiency in field trials in Argentina. Eur. J. Agron. 2024, 154, 127113. [Google Scholar] [CrossRef]
- Pan, J.; Peng, F.; Xue, X.; You, Q.; Zhang, W.; Wang, T.; Huang, C. The Growth Promotion of Two Salt-Tolerant Plant Groups with PGPR Inoculation: A Meta-Analysis. Sustainability 2019, 11, 378. [Google Scholar] [CrossRef]
- Dutta, A.; Banerjee, S.; Dinda, S.; Chowdhury, I.; Haldar, S.; Bandyopadhyay, S. A critical analysis on the roles of exopolysaccharides and ACC deaminase in salinity stress tolerance in crop plants. Biocatal. Agric. Biotechnol. 2022, 42, 102372. [Google Scholar] [CrossRef]
- Dong, H.; Wang, Y.; Di, Y.; Qiu, Y.; Ji, Z.; Zhou, T.; Shen, S.; Du, N.; Zhang, T.; Dong, X.; et al. Plant growth-promoting rhizobacteria Pseudomonas aeruginosa HG28-5 improves salt tolerance by regulating Na+/K+ homeostasis and ABA signaling pathway in tomato. Microbiol. Res. 2024, 283, 127707. [Google Scholar] [CrossRef]
- Urana, R.; Dahiya, A.; Sharma, P.; Singh, N. Effects of Plant Growth Promoting rhizobacteria on Phytoremediation of Phenanthrene Contaminated Sodic Soil. Polycycl. Aromat. Compd. J. Int. Soc. Polycycl. Aromat. Compd. 2021, 41, 1020–1029. [Google Scholar] [CrossRef]
- Yasmeen, T.; Ahmad, A.; Arif, M.S.; Mubin, M.; Rehman, K.; Shahzad, S.M.; Iqbal, S.; Rizwan, M.; Ali, S.; Alyemeni, M.N.; et al. Biofilm forming rhizobacteria enhance growth and salt tolerance in sunflower plants by stimulating antioxidant enzymes activity. Plant Physiol. Biochem. 2020, 156, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Neshat, M.; Abbasi, A.; Hosseinzadeh, A.; Sarikhani, M.R.; Chavan, D.D.; Rasoulnia, A. Plant growth promoting bacteria (PGPR) induce antioxidant tolerance against salinity stress through biochemical and physiological mechanisms. Physiol. Mol. Biol. Plants 2022, 28, 347–361. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Y.; Yue, T.; Huang, Y.; He, C.; Jiang, W.; Liu, H.; Zeng, H.; Wang, J. Plant growth-promoting rhizobacteria Bacillus velezensis JB0319 promotes lettuce growth under salt stress by modulating plant physiology and changing the rhizosphere bacterial community. Environ. Exp. Bot. 2023, 213, 105451. [Google Scholar] [CrossRef]
- Wang, C.; Pei, J.; Li, H.; Zhu, X.; Zhang, Y.; Wang, Y.; Li, W.; Wang, Z.; Liu, K.; Du, B.; et al. Mechanisms on salt tolerant of Paenibacillus polymyxa SC2 and its growth-promoting effects on maize seedlings under saline conditions. Microbiol. Res. 2024, 282, 127639. [Google Scholar] [CrossRef] [PubMed]
- Ilangumaran, G.; Smith, D.L. Plant Growth Promoting rhizobacteria in Amelioration of Salinity Stress: A Systems Biology Perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef] [PubMed]
- Alishahi, F.; Alikhani, H.A.; Khoshkholgh-Sima, N.A.; Etesami, H. Mining the roots of various species of the halophyte Suaeda for halotolerant nitrogen-fixing endophytic bacteria with the potential for promoting plant growth. Int. Microbiol. 2020, 23, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Stacey, G.; Burris, R.H.; Evans, H.J. Biological Nitrogen Fixation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Zhang, X.; Yang, Z.; Wang, L.; Yue, Y.; Wang, L.; Yang, X. The effects of plant growth-promoting rhizobacteria on plants under temperature stress: A meta-analysis. Rhizosphere 2023, 28, 100788. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, M.; Yuan, M.M.; Wang, E.; Bai, Y.; Crowther, T.W.; Zhou, J.; Ma, Z.; Zhang, L.; Wang, Y.; et al. Root microbiota confers rice resistance to aluminium toxicity and phosphorus deficiency in acidic soils. Nat. Food 2023, 4, 912–924. [Google Scholar] [CrossRef]
- Gou, Y.; Wang, Z.; Zhang, Z.; Wei, H.; Meng, P.; Zeng, Y.; Deng, Z.; Zhou, J. Advance in role mechanisms of plant growth-promoting rhizobacteria. Chin. J. Appl. Environ. Biol. 2023, 29, 495–506. [Google Scholar]
- Lei, S.; Xu, X.; Cheng, Z.; Xiong, J.; Ma, R.; Zhang, L.; Yang, X.; Zhu, Y.; Zhang, B.; Tian, B. Analysis of the community composition and bacterial diversity of the rhizosphere microbiome across different plant taxa. Microbiologyopen 2019, 8, e00762. [Google Scholar] [CrossRef]
- Jones, K.M.; Kobayashi, H.; Davies, B.W.; Taga, M.E.; Walker, G.C. How rhizobial symbionts invade plants: The Sinorhizobium–Medicago model. Nat. Rev. Microbiol. 2007, 5, 619–633. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Halotolerant plant growth–promoting bacteria: Prospects for alleviating salinity stress in plants. Environ. Exp. Bot. 2020, 178, 104124. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, G.; Ma, P.; Lin, Y.; Yang, X.; Cao, C. Isolation and Characterization of a Phosphorus-Solubilizing Bacterium from Rhizosphere Soils and Its Colonization of Chinese Cabbage (Brassica campestris ssp. chinensis). Front. Microbiol. 2017, 8, 1270. [Google Scholar] [CrossRef]
- Sheng, X.F.; Huang, W.Y. Mechanism of potassium release from feldspar affected by the strain NBT of silicate bacterium. Acta Pedol. Sin. 2002, 39, 863–871. [Google Scholar]
- Sabaté Dc, B.C.; Petroselli, G.; Erra-Balsells, R.; Audisio, M.c. Biocontrol of Sclerotinia sclerotiorum (Lib.) de Bary on common bean by native lipopeptide-producer Bacillus strains. Microbiol. Res. 2018, 211, 21–30. [Google Scholar] [CrossRef]
- Cawoy, H.; Debois, D.; Franzil, L.; De Pauw, E.; Thonart, P.; Ongena, M. Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb. Biotechnol. 2014, 8, 281–295. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yang, D.; Yang, L.; Han, S.; Liu, G.; Tang, L.; Chen, J.; Wang, D.; Guo, C. Co-inoculation with sinorhizobium meliloti and Enterobacter ludwigii improves the yield, nodulation, and quality of alfalfa (Medicago sativa L.) under saline-alkali environments. Ind. Crops Prod. 2023, 199, 116818. [Google Scholar] [CrossRef]
- Al-Turki, A.; Murali, M.; Omar, A.F.; Rehan, M.; Sayyed, R.Z. Recent advances in PGPR-mediated resilience toward interactive effects of drought and salt stress in plants. Front. Microbiol. 2023, 14, 1214845. [Google Scholar] [CrossRef]
- Yadav, U.; Anand, V.; Kumar, S.; Verma, I.; Anshu, A.; Pandey, I.A.; Kumar, M.; Behera, S.K.; Srivastava, S.; Singh, P.C. Bacillus subtilis NBRI-W9 simultaneously activates SAR and ISR against Fusarium chlamydosporum NBRI-FOL7 to increase wilt resistance in tomato. J. Appl. Microbiol. 2024, 135, lxae013. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, L.; Wang, Z. Advance and Development Trend of Biological Nitrogen Fixation Research. J. Yunnan Agric. Univ. Nat. Sci. 2015, 30, 810–821. [Google Scholar]
- Santi, C.; Bogusz, D.; Franche, C. Biological nitrogen fixation in non-legume plants. Ann. Bot. 2013, 111, 743–767. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.K.; Mattoo, A.K.; Schmidt, M.A. Rhizobial–Host Interactions and Symbiotic Nitrogen Fixation in Legume Crops Toward Agriculture Sustainability. Front. Microbiol. 2021, 12, 669404. [Google Scholar] [CrossRef]
- Li, X.; Li, Z. What determines symbiotic nitrogen fixation efficiency in rhizobium: Recent insights into Rhizobium leguminosarum. Arch. Microbiol. 2023, 205, 300. [Google Scholar] [CrossRef] [PubMed]
- Kereszt, A.; Mergaert, P.; Montiel, J.; Endre, G.; Kondorosi, É. Impact of plant peptides on symbiotic nodule development and functioning. Front. Plant Sci. 2018, 9, 1026. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Jin, M.; Shi, Z.; Cui, L.; Wei, L.; Zhong, J.; Yang, C. Antagonistic effects of endophytic bacterial strains isolated from alfalfa and identification of strains with excellent antagonistic activities. Grassl. Sci. 2021, 38, 1998–2007. [Google Scholar]
- Zhang, Y.; Liu, Y.; Li, S.; Xie, Y.; Xu, Z.; Yao, T. Study on the distribution characteristics of plant growth promoting rhizobacteria (PGPR) from the rhizosphere of four high-quality herbages. Heilongjiang Anim. Sci. Vet. Med. 2016, 3, 133–136. [Google Scholar]
- Adhikari, L.; Missaoui, A.M. Nodulation response to molybdenum supplementation in alfalfa and its correlation with root and shoot growth in low pH soil. J. Plant Nutr. 2017, 40, 2290–2302. [Google Scholar] [CrossRef]
- Tang, A.; Haruna, A.O.; Majid, N.M.A.; Jalloh, M.B. Potential PGPR Properties of Cellulolytic, Nitrogen-Fixing, Phosphate-Solubilizing Bacteria in Rehabilitated Tropical Forest Soil. Microorganisms 2020, 8, 442. [Google Scholar] [CrossRef]
- Panhwar, Q.A.; Othman, R.; Rahman, Z.A.; Meon, S.; Ismail, M.R. Isolation and characterization of phosphate-solubilizing bacteria from aerobic rice. Afr. J. Biotechnol. 2012, 11, 2711–2719. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Li, Q. Dynamics of the Microbial Flora in the Liriope Rhizosphere and Outrhizosphere During Continuous Cropping Years. Chin. J. Soil Sci. 2006, 37, 563–565. [Google Scholar]
- Chen, C.; Wang, M.; Zhu, J.; Tang, Y.; Zhang, H.; Zhao, Q.; Jing, M.; Chen, Y.; Xu, X.; Jiang, J.; et al. Long-term effect of epigenetic modification in plant-microbe interactions: Modification of DNA methylation induced by plant growth-promoting bacteria mediates promotion process. Microbiome 2022, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Delgado, S.; Núñez, F.; Sánchez, B.; Bermúdez, E.; Rodríguez, J.M. Toxigenic microorganisms in medicinal plants used for ritual protection of infants. Food Res. Int. 2011, 44, 304–309. [Google Scholar] [CrossRef]
- Wei, L.; Xu, T.; Li, Y.; Ai, Z.; Ma, F. The common garden environment and genetic differentiation jointly influence the diversity and community structure of nitrogen-fixing bacteria in the rhizosphere soil of three Caragana species. Biodivers. Sci. 2023, 31, 22477. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, X.J.; Wang, R.H.; Liao, H.J.; Jia, J.P. Isolation and Identification of Azotobacter in the Rhizosphere of Arenaria brevipetala. J. Plateau Agric. 2023, 7, 638–643. [Google Scholar]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. How Plant Root Exudates Shape the Nitrogen Cycle. Trends Plant Sci. 2017, 22, 661–673. [Google Scholar] [CrossRef]
- Li, N.; Wen, J.; Wu, R.; Hu, D.; Zhang, L.; Zhang, W.; Zhang, M. Dual effects of plant growth-promoting rhizobacteria (PGPR) on the Moso bamboo-soil system: Plant growth promotion and microbial community stability. Ind. Crops Prod. 2023, 203, 117151. [Google Scholar] [CrossRef]
- Khoso, M.A.; Wagan, S.; Alam, I.; Hussain, A.; Ali, Q.; Saha, S.; Poudel, T.R.; Manghwar, H.; Liu, F. Impact of plant growth-promoting rhizobacteria (PGPR) on plant nutrition and root characteristics: Current perspective. Plant Stress 2024, 11, 100341. [Google Scholar] [CrossRef]
- Ma, X.; Ji, A.; Zheng, J.; Cao, C.; Gong, Y.; Huang, D.; Wang, B. Research Progress on Growth-promoting Mechanism and Application of Plant Growth-promoting rhizobacteria. J. Agric. Sci. Technol. 2025, 2, 13–23. [Google Scholar]
- Golubev, S.N.; Muratova, A.Y.; Panchenko, L.V.; Shchyogolev, S.Y.; Turkovskaya, O.V. Mycolicibacterium sp. strain PAM1, an alfalfa rhizosphere dweller, catabolizes PAHs and promotes partner-plant growth. Microbiol. Res. 2021, 253, 126885. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Odiyo, J.O.; Popoola, O.E.; Msagati, T.A.M. Determination and Distribution of Polycyclic Aromatic Hydrocarbons in Rivers, Sediments and Wastewater Effluents in Vhembe District, South Africa. Int. J. Environ. Res. Public Health 2016, 13, 387. [Google Scholar]
- Cao, W.; Yin, L.; Zhang, D.; Wang, Y.; Yuan, J.; Zhu, Y.; Dou, J. Contamination, Sources, and Health Risks Associated with Soil PAHs in Rebuilt Land from a Coking Plant, Beijing, China. Int. J. Environ. Res. Public Health 2019, 16, 670. [Google Scholar] [CrossRef]
- Ramesh, A.; Archibong; Hood, D.B.; Guo, Z. Global Environmental Distribution and Human Health Effects of Polycyclic Aromatic Hydrocarbons. In Global Contamination Trends of Persistent Organic Chemicals; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Shamilishvily, G.A.; Abakumov, E.V.E.; Gabov, D.N. Polycyclic aromatic hydrocarbon in urban soils of an Eastern European megalopolis: Distribution, source identification and cancer risk evaluation. Solid Earth Discuss. 2018, 9, 669–682. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Bai, S.H.; Zhang, Y.; Teng, Y.; Xu, Z. Assisted phytoremediation of a co-contaminated soil with biochar amendment: Contaminant removals and bacterial community properties. Geoderma 2019, 348, 115–123. [Google Scholar] [CrossRef]
- Mishra, J.; Singh, R.; Arora, N.K. Alleviation of Heavy Metal Stress in Plants and Remediation of Soil by Rhizosphere Microorganisms. Front. Microbiol. 2017, 8, 1706. [Google Scholar] [CrossRef] [PubMed]
- Kweon, O.; Kim, S.J.; Cerniglia, C.E. An Update on the Genomic View of Mycobacterial High-Molecular-Weight Polycyclic Aromatic Hydrocarbon Degradation. In Aerobic Utilization of Hydrocarbons, Oils, and Lipids; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Mukasheva, T.D.; Omirbekova, A.A.; Sydykbekova, R.S.; Berzhanova, R.Z.; Ignatova, L.V. Biodiversity of Plants Rhizosphere and Rhizoplane Bacteria in the Presence of Petroleum Hydrocarbons. World Appl. Sci. J. 2014, 8, 1647–1652. [Google Scholar]
- Kumar, V.; AlMomin, S.; Al-Aqeel, H.; Al-Salameen, F.; Nair, S. Metagenomic analysis of rhizosphere microflora of oil-contaminated soil planted with barley and alfalfa. PLoS ONE 2018, 13, e0202127. [Google Scholar] [CrossRef]
- Shamseldin, A. Future Outlook of Transferring Biological Nitrogen Fixation (BNF) to Cereals and Challenges to Retard Achieving this Dream. Curr. Microbiol. 2022, 79, 171. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y.; Huang, K.; Wang, F.; Mei, Z. Molecular Mechanism and Agricultural Application of the NifA–NifL System for Nitrogen Fixation. Int. J. Mol. Sci. 2023, 24, 907. [Google Scholar] [CrossRef]
- Wang, Y.J.; Liu, X.J. The Regulating Roles of Root Exudates of Poplars on Soil Nutrients and Microorganisms. J. Shandong Agric. Univ. 2023, 54, 562–569. [Google Scholar]
- Ullah, A.; Bano, A.; Khan, N. Climate Change and Salinity Effects on Crops and Chemical Communication Between Plants and Plant Growth-Promoting Microorganisms Under Stress. Front. Sustain. Food Syst. 2021, 5, 618092. [Google Scholar] [CrossRef]
- Sharma, S.; Kulkarni, J.; Jha, B. Halotolerant rhizobacteria Promote Growth and Enhance Salinity Tolerance in Peanut. Front. Microbiol. 2016, 7, 1600. [Google Scholar] [CrossRef]
- Meena, R.S.; Vijayakumar, V.; Yadav, G.S.; Mitran, T. Response and interaction of Bradyrhizobium japonicum and arbuscular mycorrhizal fungi in the soybean rhizosphere. Plant Growth Regul. 2018, 84, 207–223. [Google Scholar] [CrossRef]
- Takashima, T.; Hikosaka, K.; Hirose, T. Photosynthesis or persistence: Nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ. 2004, 27, 1047–1054. [Google Scholar] [CrossRef]
- Chakraborty, U.; Ramteke, P. Editorial: Plant probiotics: Recent and future prospects. Front. Plant Sci. 2023, 14, 1254184. [Google Scholar] [CrossRef]
- Chowdhury, F.T.; Zaman, N.R.; Islam, M.R.; Khan, H. Anti-fungal secondary metabolites and hydrolytic enzymes from rhizospheric bacteria in crop protection: A review. J. Bangladesh Acad. Sci. 2021, 44, 69–84. [Google Scholar] [CrossRef]
- Gao, T.; Liu, Y.; Yang, D.; Liu, X.; Zuo, M.; He, Y.; Wang, H.; Bao, J.; Shen, Y.; Tai, X.; et al. Inoculation of Exogenous Complex Bacteria to Enhance Resistance in alfalfa and Combined Remediation of Heavy Metal-Contaminated Soil. Curr. Microbiol. Int. J. 2023, 80, 213. [Google Scholar] [CrossRef]
- Oaikhena, E.E.; Makaije, D.B.; Denwe, S.D.; Namadi, M.M.; Haroun, A.A. Bioremediation Potentials of Heavy Metal Tolerant Bacteria Isolated from Petroleum Refinery Effluent. Am. J. Environ. Prot. 2016, 5, 29. [Google Scholar] [CrossRef]
- Din, B.U.; Amna; Rafique, M.; Javed, M.T.; Kamran, M.A.; Mehmood, S.; Khan, M.; Sultan, T.; Munis, M.F.H.; Chaudhary, H.J. Assisted phytoremediation of chromium spiked soils by Sesbania Sesban in association with Bacillus xiamenensis PM14: A biochemical analysis. Plant Physiol. Biochem. 2020, 146, 249–258. [Google Scholar] [CrossRef]
- Tirry, N.; Kouchou, A.; El Omari, B.; Ferioun, M.; El Ghachtouli, N. Improved chromium tolerance of alfalfa by plant growth-promoting rhizobacteria (PGPR). J. Genet. Eng. Biotechnol. 2021, 19, 149. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fan, M.; Shangguan, Z. An Overview of the Research in Soil Nitrogen Cycling in Rhizosphere. Shaanxi For. Sci. Technol. 2022, 50, 116–122. [Google Scholar]
- Li, Z.; Qi, L.; Zhou, W.; Yang, J.; Zhang, X.; Chen, F.; Zhu, Y.; Guan, C.; Yang, S. The effect and mechanism of exogenous P on the efficiency of microbial-assisted alfalfa remediation of Cu-contaminated soil. J. Environ. Chem. Eng. 2025, 13, 115503. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, L.; Cui, Y.; Yang, D.; Gao, H.; Chen, J.; Zheng, Z.; Guo, C. Inoculation of plant growth-promoting rhizobacteria and rhizobia changes the protist community of alfalfa rhizosphere soil under saline-alkali environment. Appl. Soil Ecol. 2025, 206, 105775. [Google Scholar] [CrossRef]
- Jabborova, D.; Kannepalli, A.; Davranov, K.; Narimanov, A.; Enakiev, Y.; Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Wirth, S.; Sayyed, R.Z.; et al. Co-inoculation of rhizobacteria promotes growth, yield, and nutrient contents in soybean and improves soil enzymes and nutrients under drought conditions. Sci. Rep. 2021, 11, 22081. [Google Scholar] [CrossRef] [PubMed]



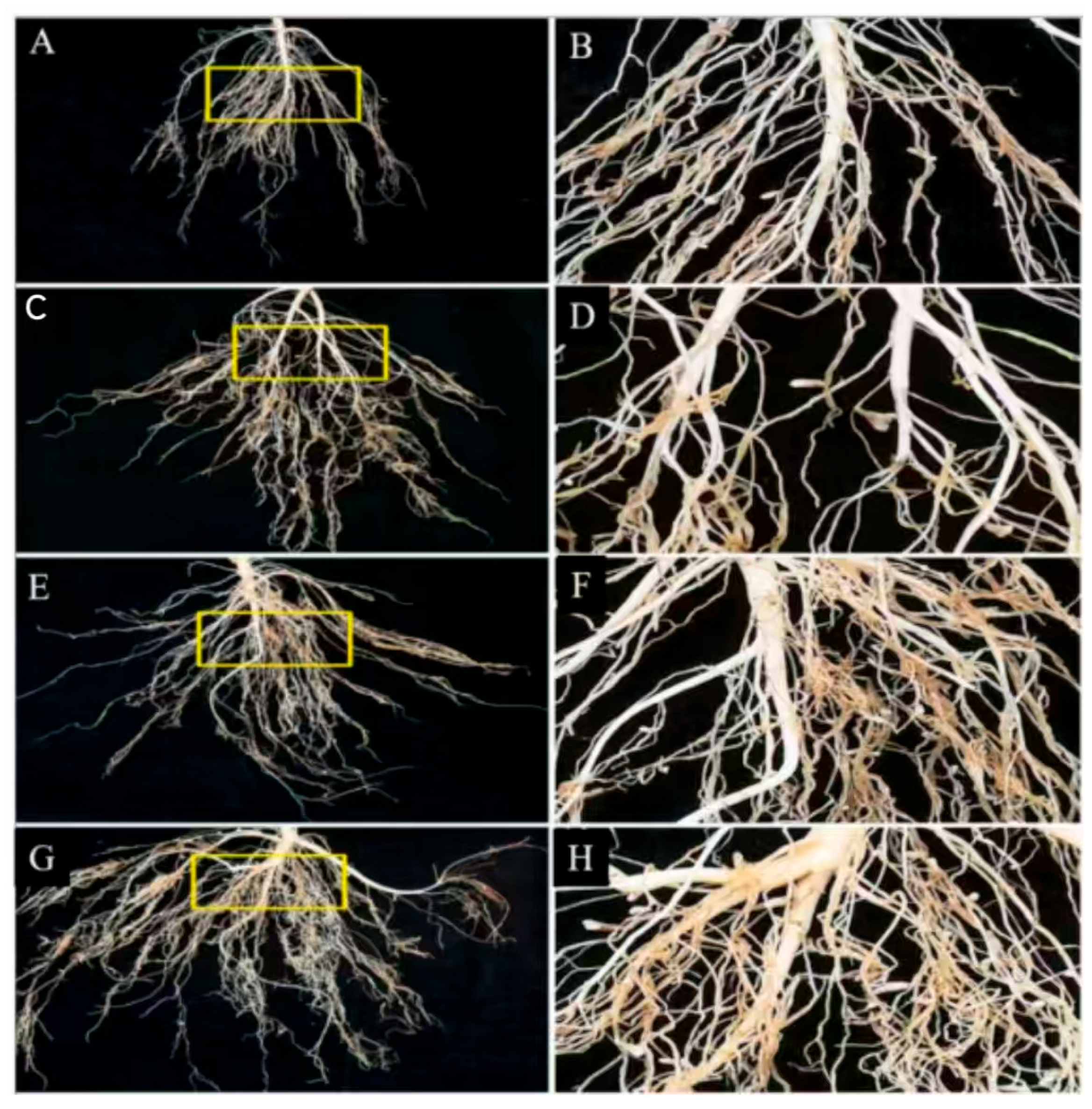
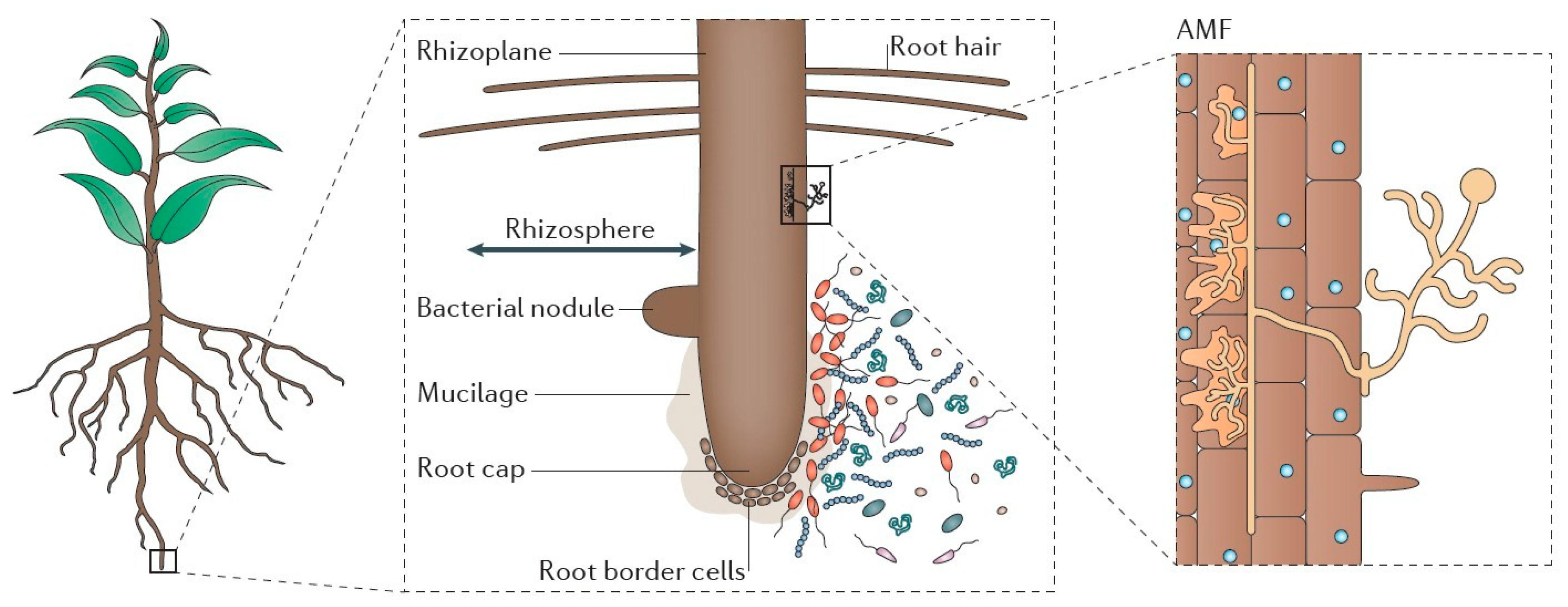



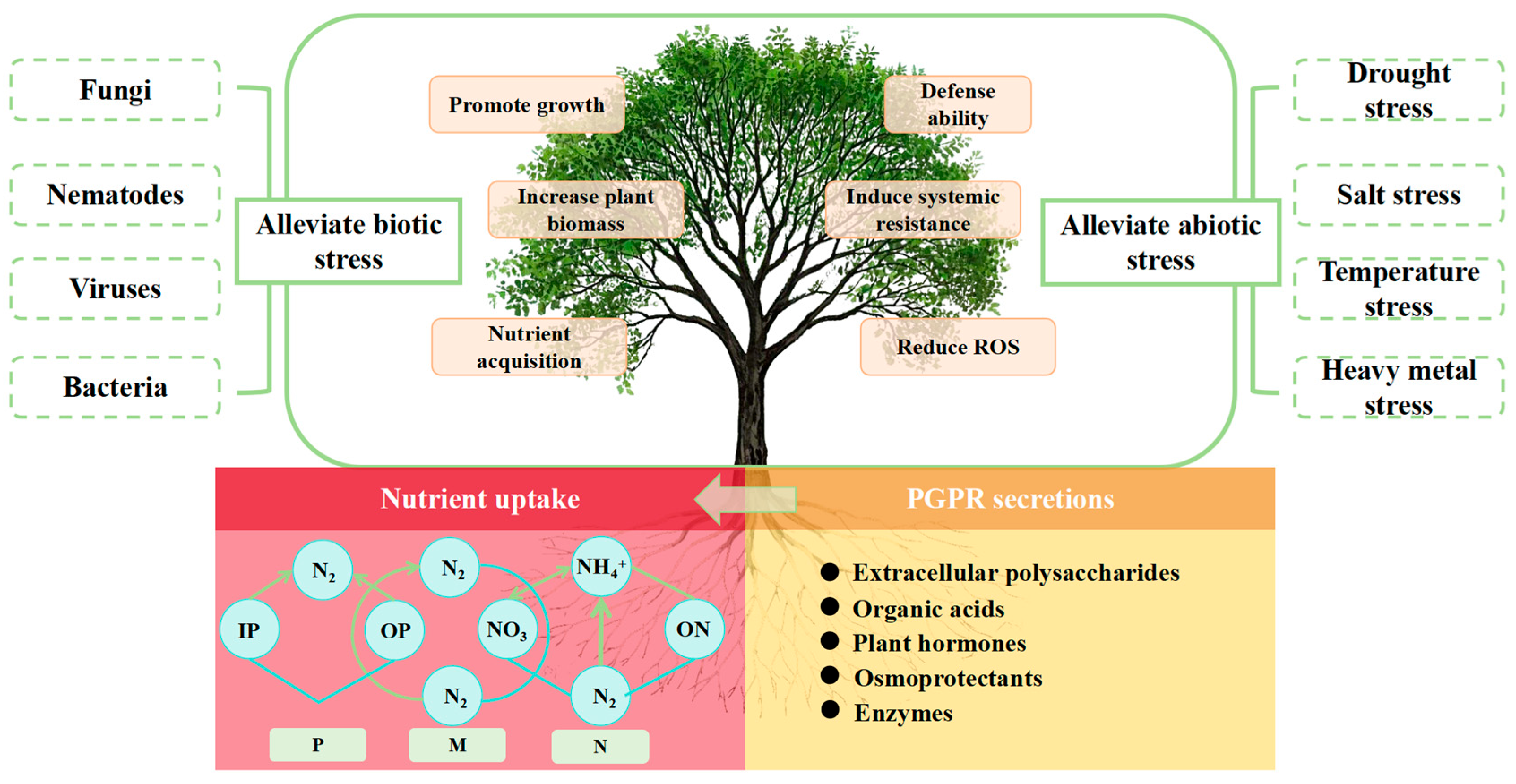
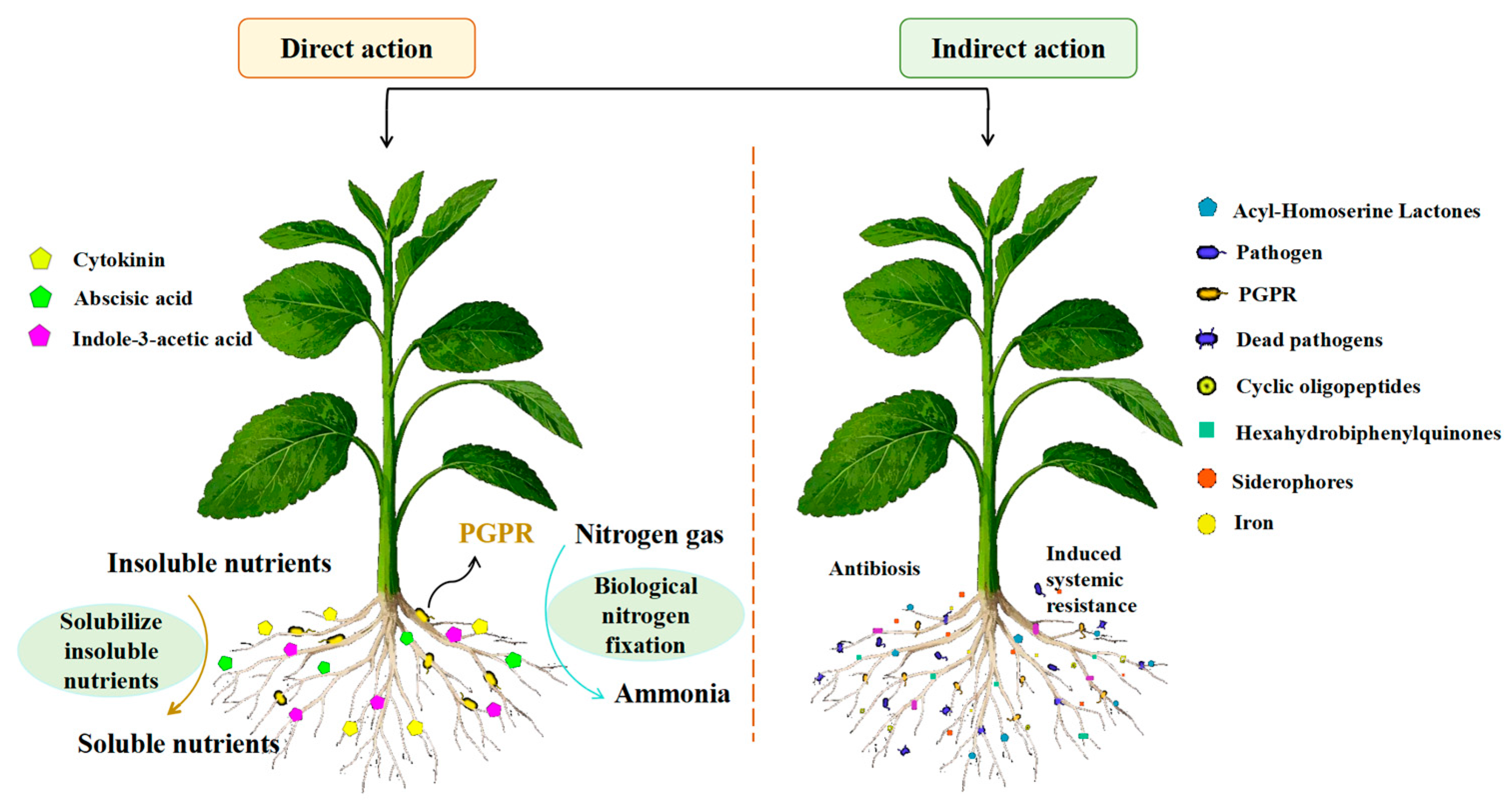
| Biological Nitrogen Fixation System | Types of Nitrogen–Fixing Microorganisms | |
|---|---|---|
| Free living nitrogen fixation microorganisms | Phototrophs | Anabaena, Green sulfur bacteria |
| Chemolithotrophs | Leptospirillum ferrooxidans | |
| Heterotrophs | Aerobic: Azotobacter Facultatively anaerobic: Klebsiella. Some Bacillus spp. Anaerobic: Clostridium, Methanogens | |
| Symbiotic Nitrogen Fixation Microorganisms | Rhizobium-legume symbiosis Rhizobium-Parasponia symbiosis Frankia-dicotyledon (non–legume) symbiosis Diazotrophic cyanobacteria-plant symbiosis | |
| Associative Nitrogen Fixation Microorganisms | Azospirillum, Azotobacter, Some Pseudomonas spp. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-Y.; Liu, J.-L.; Wang, X.; Cao, X.; Liu, K.-H.; Luo, Y.-T.; Chen, J.-Y.; Zhang, J.; Fan, Y.-H. A Review of the Regulatory Role of Plant Growth–Promoting Rhizobacteria in Alfalfa Under Stress Conditions. Plants 2025, 14, 3248. https://doi.org/10.3390/plants14213248
Zhang Y-Y, Liu J-L, Wang X, Cao X, Liu K-H, Luo Y-T, Chen J-Y, Zhang J, Fan Y-H. A Review of the Regulatory Role of Plant Growth–Promoting Rhizobacteria in Alfalfa Under Stress Conditions. Plants. 2025; 14(21):3248. https://doi.org/10.3390/plants14213248
Chicago/Turabian StyleZhang, Yu-Yan, Jin-Lei Liu, Xuan Wang, Xin Cao, Kang-Hui Liu, Yu-Ting Luo, Jia-Yin Chen, Jiang Zhang, and Yong-Hong Fan. 2025. "A Review of the Regulatory Role of Plant Growth–Promoting Rhizobacteria in Alfalfa Under Stress Conditions" Plants 14, no. 21: 3248. https://doi.org/10.3390/plants14213248
APA StyleZhang, Y.-Y., Liu, J.-L., Wang, X., Cao, X., Liu, K.-H., Luo, Y.-T., Chen, J.-Y., Zhang, J., & Fan, Y.-H. (2025). A Review of the Regulatory Role of Plant Growth–Promoting Rhizobacteria in Alfalfa Under Stress Conditions. Plants, 14(21), 3248. https://doi.org/10.3390/plants14213248






