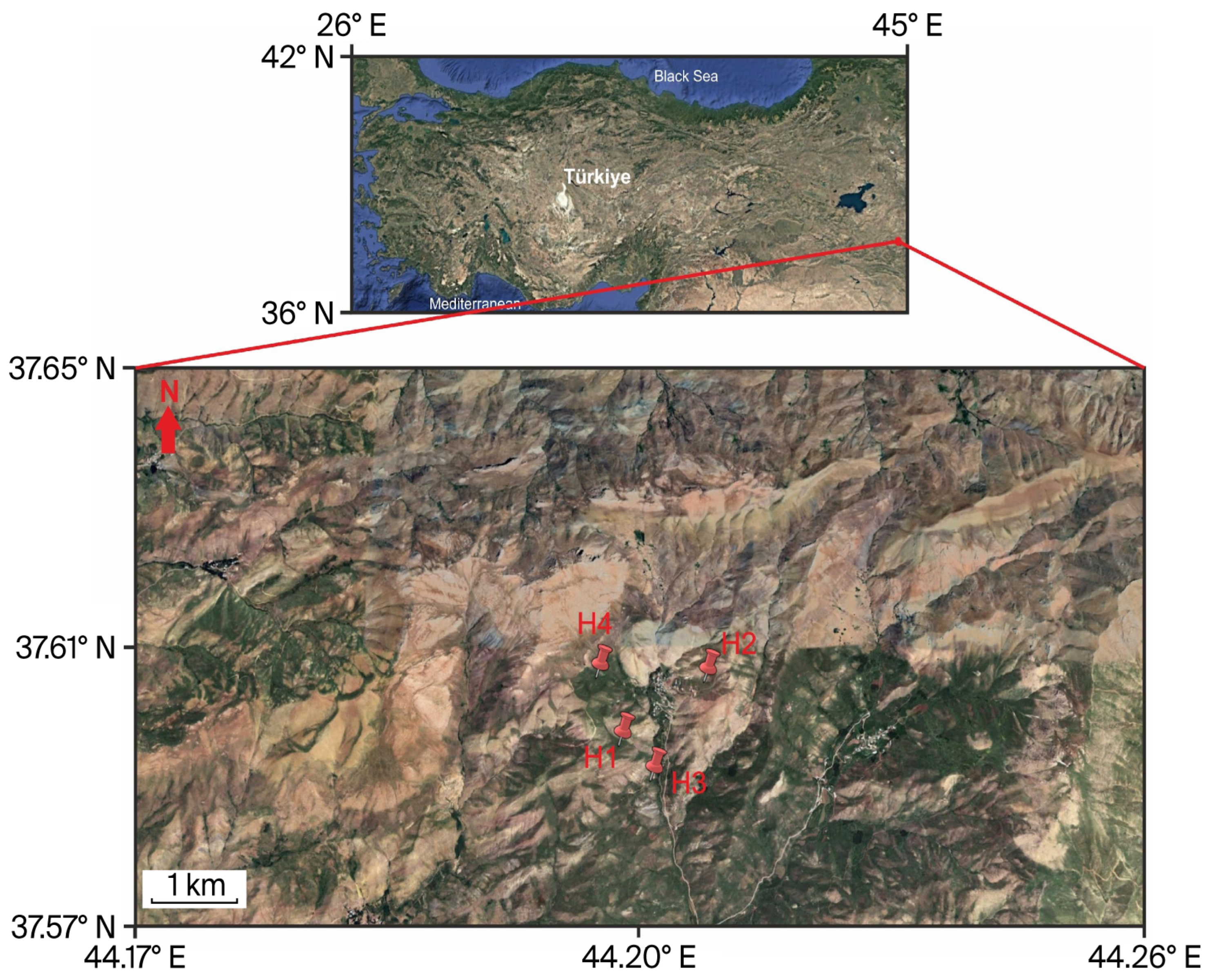
3.1. Levels of Heavy Metals and Essential Minerals in Samples
Inductively coupled plasma optical emission spectrometry (ICP-OES) analysis showed that the four medicinal plants from Hakkari, Türkiye,
Daphne mucronata (H1),
Ferula communis (H2),
Heracleum persicum (H3), and
Tragopogon coloratus (H4), had different amounts of heavy metals (
Table 3). H1 had the highest levels of all the elements, and these levels were often higher than those considered safe according to international standards. H2-H4, on the other hand, was mostly within the safe levels.
There was a large difference in chromium levels (p < 0.001). H1 (2.85 ± 0.20 mg/kg) was above the herbal material limit of 2 mg/kg, but the other species stayed below 0.7 mg/kg. The arsenic level in H1 (0.78 ± 0.05 mg/kg) was also higher than the maximum level of 0.6 mg/kg. The levels in the other taxa, on the other hand, were much lower (0.18–0.22 mg/kg). The amount of lead was about the same: H1 had 14.6 ± 0.6 mg/kg, which is more than the 10 mg/kg limit, and H2–H4 had about 1.2–1.5 mg/kg. H1 had slightly higher levels of copper and zinc than the recommended amounts, which are 34.2 and 31.5 mg/kg, respectively. In all species, Cd, Co, Fe, Ni, and Al stayed within normal or acceptable ranges. In contrast, H1 again exhibited the highest mean values.
These findings indicate that
Daphne mucronata is an efficient accumulator of Pb, As, Cr, Cu, and Zn. Turkish medicinal plants have been shown to have a similar deposition system, which is affected by the location and plant species. For example, Karahan et al. (2020) [
44] found Pb levels as high as 16 mg/kg and Cr levels as high as 42 mg/kg in ethnobotanical species from southern Türkiye; however, most species remained below the WHO/FAO limits. Nationwide surveys indicate that Pb and Cr levels are generally safe on average, but they may increase in areas where mining or heavy traffic is present [
21,
45].
The variability is similar when looked at from a global perspective. Kandić et al. (2023) [
46] found that Serbian medicinal plants had Pb levels ranging from 0.6 to 49 mg/kg and Ni levels up to 12 mg/kg. Some of these levels exceeded the WHO limits, similar to what was found for H1. Market samples of
Heracleum persicum in Iran frequently displayed lead (30–33 mg/kg) and cadmium (5–11 mg/kg) concentrations exceeding permissible limits [
47]. A global review found that a significant number of herbal products still contain Pb, As, and Cd, highlighting the importance of monitoring quality [
20,
48].
The high levels of Pb, As, and Cr in D. mucronata could be attributed to geological enrichment, past mining, atmospheric deposition, and the plant’s ability to move and recycle metals under specific pH and organic matter conditions [
49,
50,
51]. In terms of public health, the high concentrations found in
D. mucronata suggest that raw material from contaminated habitats should not be consumed without prior screening.
Table 3.
Heavy metal concentrations (mg/kg) in four medicinal plants from Hakkari, Türkiye, determined by ICP-OES and compared to worldwide safety standards.
Table 3.
Heavy metal concentrations (mg/kg) in four medicinal plants from Hakkari, Türkiye, determined by ICP-OES and compared to worldwide safety standards.
| Element | Guideline/Limit (mg/kg, Dry) | H1 | H2 | H3 | H4 | p-Value | References |
|---|
| Cr | not exceeding: 2 mg/kg | 2.85 ± 0.20 c | 0.62 ± 0.04 b | 0.45 ± 0.03 a | 0.51 ± 0.04 ab | 0.0007 | [49,52] |
| As | not exceeding: 0.6 mg/kg | 0.78 ± 0.05 c | 0.22 ± 0.02 b | 0.18 ± 0.01 ab | 0.21 ± 0.02 b | 0.012 | [53] |
| Cd | not exceeding: 0.3 mg/kg | 0.24 ± 0.02 c | 0.08 ± 0.01 b | 0.06 ± 0.01 b | 0.07 ± 0.01 b | 0.018 | [53] |
| Co | not exceeding: 0.5 mg/kg | 0.42 ± 0.03 c | 0.19 ± 0.02 b | 0.16 ± 0.02 b | 0.17 ± 0.02 b | 0.001 | [49] |
| Cu | not exceeding: 30 mg/kg | 34.2 ± 1.4 c | 7.3 ± 0.4 a | 9.1 ± 0.5 b | 8.5 ± 0.5 b | 0.0009 | [49,54] |
| Fe | not exceeding: 250 mg/kg | 175.5 ± 5.1 c | 54.3 ± 2.2 a | 61.2 ± 2.5 ab | 58.9 ± 2.4 ab | 0.0013 | [49,54] |
| Pb | not exceeding: 10 mg/kg | 14.6 ± 0.6 c | 1.2 ± 0.1 a | 1.5 ± 0.1 a | 1.3 ± 0.1 a | 0.0001 | [53,55] |
| Zn | not exceeding: 27 mg/kg | 31.5 ± 1.2 c | 10.8 ± 0.4 a | 12.1 ± 0.5 a | 11.5 ± 0.4 a | 0.0003 | [49,54] |
| Ni * | no ML; EFSA TDI 2.8 μg/kg bw/day | 1.8 ± 0.08 c | 0.48 ± 0.03 b | 0.42 ± 0.02 b | 0.45 ± 0.02 b | 0.0004 | [49,56] |
| Al * | no ML; typical background 1–15 mg/kg | 12.5 ± 0.6 c | 4.1 ± 0.3 b | 3.8 ± 0.2 b | 3.6 ± 0.3 b | 0.001 | [49] |
The concentrations of four essential macro-minerals, magnesium (Mg), calcium (Ca), sodium (Na), and potassium (K), were quantified in the leaves of
Daphne mucronata (H1),
Ferula communis (H2),
Heracleum persicum (H3), and
Tragopogon coloratus (H4) using atomic absorption spectroscopy (AAS), as shown in
Table 4.
Magnesium concentrations varied noticeably among the species. The highest level was detected in H2 (147.5 ± 4.8 mg kg−1), whereas H1 showed a slightly lower but still substantial value. H3 (93.2 ± 6.3 mg kg−1) and H4 (86.4 ± 3.9 mg kg−1) contained the least magnesium, and the overall variation was statistically significant (p = 0.0008). Calcium was abundant in all species but especially in H1 (502.5 ± 10.3 mg kg−1), which exceeded the values recorded for H3 and H4 by more than 60%. Even the least-rich sample, H4 (289.4 ± 6.2 mg kg−1), remained within the lower end of internationally recommended limits. Sodium concentrations were comparatively modest, ranging between 28.4 ± 1.7 and 52.0 ± 2.1 mg kg−1. Although the interval was narrow relative to other minerals, the variation among plants was still significant (p = 0.0013). Potassium exhibited the widest amplitude of all measured elements. The leaves of H1 contained an exceptionally high amount (715.2 ± 9.6 mg kg−1), whereas H4 accumulated only 108.4 ± 4.7 mg kg−1, resulting in a more than sixfold difference across species (p = 0.0001).
In general, the macro-mineral profiles of the plants that were analyzed were within or below the ranges that had been reported as acceptable for culinary or medicinal herbs in previous studies. In Türkiye, Özcan (2004) [
57] found that many condiment/herb species had Ca, K, and Mg levels in similar orders of magnitude but often higher values where the fertility of the soil or conditions for growth were favorable. Another Turkish study on wild edible plants found K levels as high as ~2600 mg per 100 g dry weight (≈26,000 mg/kg) for some species. This is significantly higher than what we observe in these medicinal plants, indicating that H1–H4 are moderate in comparison [
58]. Studies conducted outside of Türkiye, such as those on Moroccan medicinal and aromatic plants, found similar broad ranges of Ca, Mg, and K among species, with comparable statistical differences [
59].
Table 4.
Mineral content (mg/kg) of four medicinal plant samples analyzed by AAS (compared with international limits and literature references).
Table 4.
Mineral content (mg/kg) of four medicinal plant samples analyzed by AAS (compared with international limits and literature references).
| Mineral (Limit, mg/kg) | H1 | H2 | H3 | H4 | p-Value | References |
|---|
| Magnesium (1000–5000) | 124.5 ± 5.5 a | 147.5 ± 4.8 b | 93.2 ± 6.3 c | 86.4 ± 3.9 c | 0.0008 | [60] |
| Calcium (2000–10,000) | 502.5 ± 10.3 a | 487.0 ± 9.8 b | 315.5 ± 7.4 c | 289.4 ± 6.2 c | 0.0004 | [54,60] |
| Sodium (100–2000) | 52.0 ± 2.1 a | 44.3 ± 1.9 b | 31.5 ± 2.5 c | 28.4 ± 1.7 c | 0.0013 | [53] |
| Potassium (10,000–30,000) | 715.2 ± 9.6 a | 275.1 ± 8.4 b | 193.8 ± 6.1 c | 108.4 ± 4.7 d | 0.0001 | [54,60] |
3.2. Heavy Metal Related Health Risks from Infused Traditional Medicinal Plants in Hakkâri (Turkey)
Risk estimations were carried out solely for As, Cd, Cr, and Pb, which had approved transfer percentages from plant to infusion (
Table 5 and
Table 6). There was no consistent transfer data for Co, Cu, Fe, Zn, Ni, and Al; hence, the results for these elements are not reported, and more research is recommended.
As shown in
Table 5, the hazard index (HI) for
Daphne mucronata (H1) was 0.64, indicating a combined impact of arsenic (THL = 0.436) and lead (THL = 0.138). Chromium and cadmium contributed modestly (THL = 0.0669 and 0.0027, respectively). Although HI remained below the standard value of one, H1 exhibited the highest potential non-carcinogenic risk of the plants evaluated.
Ferula communis (H2),
Heracleum persicum (H3), and
Tragopogon coloratus (H4) exhibited HI values of 0.15, 0.13, and 0.14, respectively, which were all within commonly accepted ranges.
Table 6 also shows that H1 had the highest lifetime cancer risk (CR) of 4.25 × 10
−3. The majority of the risk (4.18 × 10
−3) was due to arsenic, whereas cadmium (1.62 × 10
−5) and chromium (5.42 × 10
−5) had minor roles. The risks for the other plants were much lower: 1.20 × 10
−3 for H2 (
Ferula communis), 9.78 × 10
−4 for H3 (
Heracleum persicum), and 1.14 × 10
−3 for H4 (
Tragopogon coloratus). All estimates are higher than the U.S. EPA’s standard of 1 × 10
−6, but they are within the highest range found for some herbal infusions in low-exposure situations [
46].
The elevated HI and CR reported for
Daphne mucronata indicate that, under extreme conditions (100% transfer, 100% inorganic As, 11.4 g/day ingestion), this plant may represent a health risk if ingested daily over extended durations. Similar results have been observed in certain teas and medicinal herbs, where arsenic concentrations predominated in the total risk assessment [
60]. Conversely, the remaining three plants exhibited CR values within or slightly beyond the 10
−4 management range, consistent with prior studies on heavy metal exposure via infusions [
61,
62].
Arsenic was the leading cause of cancer risk in all samples, whereas cadmium and chromium were less important. This finding is similar to that of Speer et al. (2023), who identified arsenic as the primary cause of cancer risk in food [
63]. It is essential to note that all non-cancer indices remained far below one, indicating that a moderate consumption of these infusions is unlikely to have negative consequences. Not only that, but the fact that H1 is close to the upper end of reported ranges means that regular consumption should be monitored, especially for groups that are more likely to be affected. Future studies that look into inorganic As speciation and realistic ingestion situations would help us obtain better estimations of the risks and make sure that these plants can be safely used in traditional medicine.
The carcinogenic risk (CR) for arsenic, chromium, and cadmium in four therapeutic plant infusions is shown in
Table 6. The total CR for
Daphne mucronata (H1) is 4.25 × 10
−3, with arsenic alone making up 4.18 × 10
−3 of that. Chromium and cadmium, on the other hand, make up much smaller amounts, 5.42 × 10
−5 and 1.62 × 10
−5, respectively. The total CR values for the other plants are 1.20 × 10
−3 for
Ferula communis (H2), 9.78 × 10
−4 for
Heracleum persicum (H3), and 1.14 × 10
−3 for
Tragopogon coloratus (H4). All of these are higher than the commonly used acceptable level of 1 × 10
−6, and many of them are also higher than the less strict upper limit of 1 × 10
−4 used in risk management situations. Compared to other studies, the CR values for H2 and H4 are high. In a review of 227 tea and herbal infusion studies, Hu et al. (2023) reported that certain samples’ CR values approached 10
−3–10
− 2 for arsenic in teas with high contamination or intake levels [
64]. In research on medicinal plants marketed in Ado Ekiti, Olusola et al. (2021) found elevated arsenic risk indices in some samples, with CR estimations exceeding 10
−3 in the worst cases [
65]. However, reasonable consumption assumptions resulted in lower levels. Many global studies have found substantially lower CR values. For example, black tea samples from Assam and North Bengal revealed a hazard quotient <1 and CR less than 10
−4 under average consumption and transfer rates [
66]. Additionally, a thorough investigation into herbal and traditional teas was conducted by Oliveira et al. (2018) [
67]. The researchers discovered that while certain herbal teas did have total arsenic levels that were higher than the recommended limits, the resulting CR under standard intake rarely went beyond 10
−4 when more practical factors for transfer and speciation were taken into account [
67]. Even though the numbers for H2 and H4 are high, they are just above what has been seen in the literature under similar severe assumptions. These data highlight the fact that arsenic remains the primary contributor to carcinogenic risk in herbal infusions under worst-case situations.
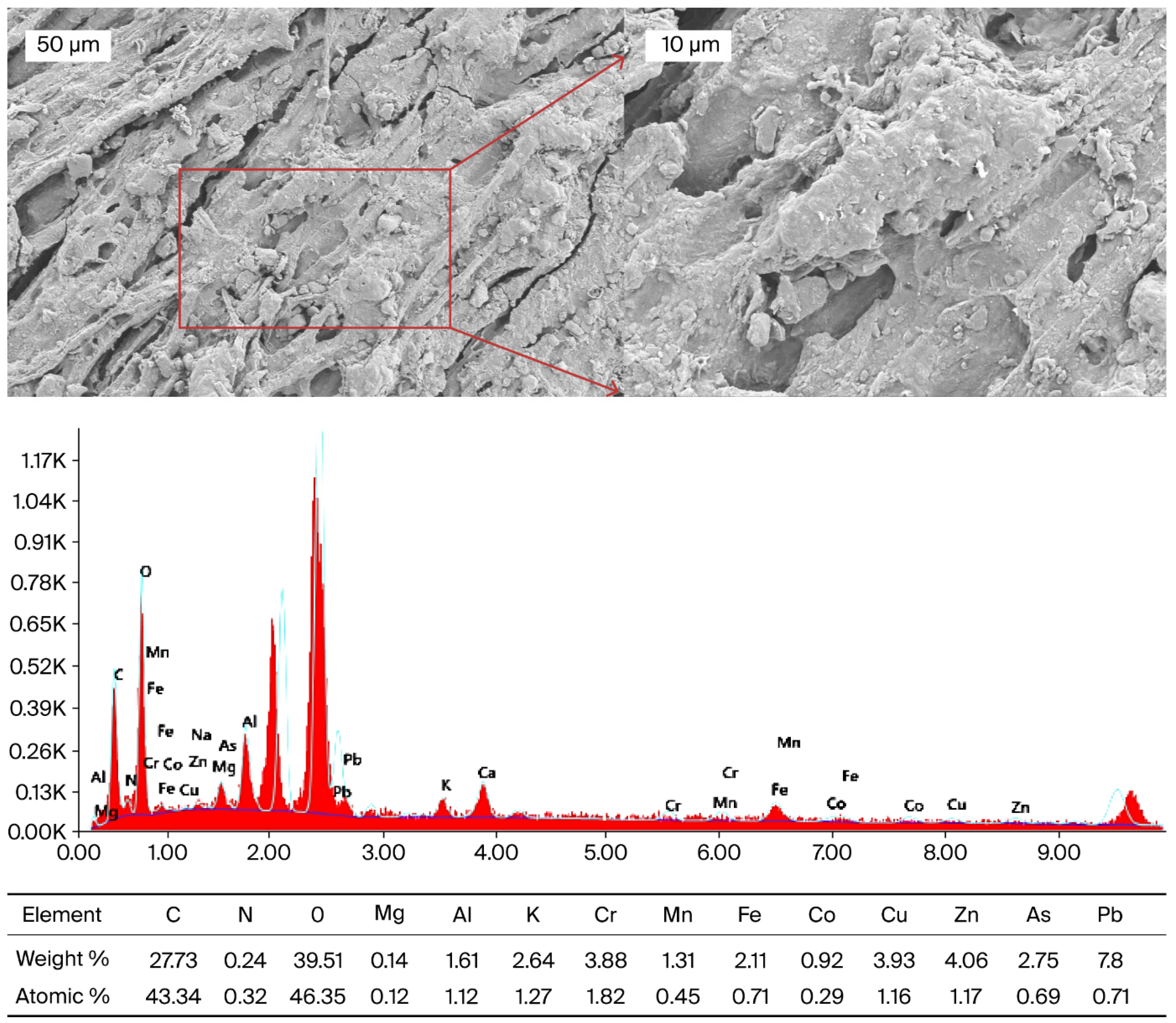
3.3. Scanning Electron Microscopy with Energy-Dispersive X-Ray Spectroscopy (SEM-EDX) of H1 Sample
In this study, SEM-EDX was applied to morphologically demonstrate the distribution of heavy metals in the dried root sample of
Daphne mucronata (H1) (
Figure 5) and to support ICP-OES data. ICP-OES analysis revealed high levels of Pb, Cr, Cu, Zn and As in
Daphne mucronata (H1) dried leaf samples. However, the entry and accumulation sites of heavy metals in plants are predominantly root tissues. Therefore, SEM-EDX analysis was performed on the roots to show the starting points of metal uptake and the possible sources of high metal content in the leaves. Similarly, in the literature, SEM-EDX analyses performed on root surfaces have been reported to successfully reveal the retention regions of elements such as cadmium, arsenic, and uranium [
68,
69,
70]. An irregular and fibrous surface structure was observed in the SEM image of the root tissue. Small voids are considered to be active regions where metal ions can bind. EDX spectra showed that the surface consists mainly of O (39.51%), C (27.73%) and N (0.24%), while Mg, Al, K, Cr, Mn, Fe, Co, Cu, Zn, As and Pb elements are present in trace or higher proportions. The particularly high levels of Pb (7.80%) and As (2.75%) indicate that these elements form a layer on the root surface together with Cu (3.93%), Zn (4.06%), and Cr (3.88%). These results are consistent with ICP-OES data and support the notion that the high levels of Pb, Cr, Cu, Zn, and As in the leaves are related to accumulation in the roots.
The findings indicate that the root tissue of
Daphne mucronata plays a critical role in heavy metal uptake. Phenolic, hydroxyl, and carboxyl functional groups in the lignocellulosic structure may facilitate binding to the root surface by chelating Pb
2+ and As oxo-anions [
71]. Similarly, previous studies have indicated that Pb and As are predominantly retained in the root systems of plants growing in arid and semi-arid regions [
72]. He et al. (2024) also emphasised that SEM-EDX is a powerful technique that can show where metals accumulate and how they are distributed in plant tissues at high resolution [
73]. Future research should include comparative SEM-EDX mapping of root, stem, and leaf tissues, supported by Micro X-ray Fluorescence (µXRF) and Laser Ablation-Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) methods, which could reveal in greater detail how metals are distributed within the plant tissues and in which regions they are concentrated.
The four plant species examined in this study have been traditionally used for medicinal purposes by local communities in southeastern Türkiye, particularly in the Hakkâri region, for centuries. This research goes beyond a scientific investigation, aiming to bridge traditional ethnobotanical knowledge with modern pharmacological science. Considering their bioactive potential, identifying toxic element accumulation is an important step to ensure the safe use of these species in both traditional and modern medicine. The results of this study may help evaluate local plants as potential natural sources for future drug development and support further research on regional plant diversity and pharmaceutical safety.
3.4. Phenolic Acid Results (Via HPLC) and Antioxidant Capacities of Samples
High-pressure liquid chromatography (HPLC) is presently the most prevalent and dependable technique for the identification and quantification of flavonoid and phenolic compounds. This approach allows for the utilization of various columns and mobile phases according to the polarity and chemical characteristics of the analytes. Isocratic and gradient elution methods can be utilized to get optimum compound separation. In this study, isocratic elution was applied because it provides higher reproducibility, stable baseline conditions, and consistent peak resolution for flavonoid and phenolic compounds under fixed mobile phase composition. Since the phenolic composition of the plant extracts was not highly complex, an isocratic system allowed sufficient separation efficiency without the need for a gradient program, minimizing solvent variation and improving quantification accuracy. Similar methodological approaches have been reported in HPLC analyses of plant phenolics, where isocratic elution was preferred for stable peak resolution and reliable baseline separation of simple phenolic profiles [
74,
75,
76].
The high extraction efficiency obtained in this study can be attributed to the use of 80% acidified ethanol (EtOH 80.0%, HCl 0.1%, H
2O 19.9%), which facilitates the hydrolysis of ester and glycosidic bonds and enhances the release of bound phenolic acids and flavonoid glycosides. These results are consistent with previous reports, where acidified ethanol systems effectively improved phenolic and flavonoid extraction from various plant matrices, including leafy vegetables, seeds, and edible flowers [
76,
77,
78]. The influence of solvent polarity and extraction parameters on phenolic recovery has also been highlighted by Azwanida [
79], supporting the selection of this solvent system in our work.
HPLC-DAD can detect compounds in plant extracts by comparing their retention periods and UV-visible absorption spectra to established standards. All phenolic compounds have significant absorption in the ultraviolet range, with each class of phenolics exhibiting distinct absorption peaks. For example, ellagitannins typically absorb at roughly 250 nm, while hydroxybenzoic acids, isoflavones, flavanones, and catechins absorb at 280 nm. In contrast, hydroxycinnamic acids, stilbenes, and flavones exhibit peak absorption near 320 nm.
In this study, flavonoid and phenolic compounds were identified by comparing their retention periods and UV spectra to those of reference standards. Quantification was executed utilizing the external standard approach, predicated on calibration curves developed for each standard chemical. The concentrations of different phenolics and flavonoid (rutin) were reported as milligrams per 100 g of dry weight (mg/100 g DW) of the extracts.
Table 7 shows that the four species (
Daphne mucronata,
Ferula communis,
Heracleum persicum,
and Tragopogon coloratus) have quite different flavonoid (rutin) and phenolic acid profiles.
Daphne mucronata (H1) has the most gallic acid (5.20 mg/g), followed by
Heracleum persicum (H3).
Ferula communis (H2) and
Tragopogon coloratus (H4) had much lower amounts. Chlorogenic acid was most plentiful in H4, with a concentration of 7.80 mg/g. H2 and H3 had high quantities of p-coumaric and ferulic acids, while H3 had the most rutin, and H4 had the least. The order of protocatechuic and vanillic acids was H3 > H1 > H2 > H4. These differences are statistically significant (ANOVA + Tukey,
p < 0.05) and match the letters that are above the numbers in the table. The highest levels of gallic acid were found in H1 (
Daphne mucronata), matching HPLC studies that found gallic acid and rutin among flavonoids in
D. mucronata leaves [
2]. As previously observed [
80], H2 (
Ferula communis) had significant amounts of p–coumaric, o–coumaric and ferulic acids, as well as chlorogenic acid. H3 (
Heracleum persicum) contained high levels of vanillic, ferulic, and chlorogenic acids, which were identified as important compounds across multiple growth stages in a phenological study [
81]. H4 (
Tragopogon coloratus) was dominated by chlorogenic acid, which was found in all analyzed samples, typically at the highest levels [
82], consistent with previous research.
Although only a limited number of flavonoid and phenolic compounds were identified in this study, the selection was intentional and based on their pharmacological relevance. Compounds such as gallic acid, rutin, chlorogenic acid, ferulic acid, and p-coumaric acid were targeted due to their well-documented biological activities in related plant species. For example, gallic acid and rutin from
Daphne mucronata have demonstrated antioxidant, antibacterial, hepatoprotective, and nephroprotective properties [
1,
2];
Ferula communis extracts rich in rutin and gallic acid have shown cytotoxic, estrogenic, and anti-inflammatory effects [
5,
6]; and phenolic acids such as ferulic acid, p-coumaric acid, and vanillic acid in
Heracleum persicum are associated with antidiabetic and analgesic activities [
8,
9,
10]. Similarly, Tragopogon species have been reported to contain chlorogenic acid and rutin, which contribute to their antioxidant, antimicrobial, and anticancer potential [
12,
14,
15].
The presence of these bioactive compounds may partially explain the traditional therapeutic use of plants in the region. Indeed, the flavonoid and phenolics analysed in this study are not only analytically detectable by HPLC-DAD, but also represent the key flavonoid and phenolic compounds previously associated with therapeutic effects in these plants. Therefore, priority was given to these compounds to establish a meaningful link between traditional use and phytochemical content. Although more advanced analytical techniques such as LC–MS/MS could provide more detailed metabolomic profiles, HPLC-DAD remains a widely used and accessible method for phenolic profiling, particularly when the goal is targeted quantification of known bioactive compounds [
2,
9,
12]. In this study, it allowed for precise detection and steady chromatographic response for target flavonoid and phenolic acids, and was selected based on both scientific suitability and laboratory feasibility.
While this study provides valuable insight into the elemental composition and phenolic content of the selected plants, it does not encompass a full phytochemical characterization. Our analytical focus was guided by the public health relevance of heavy metals and selected antioxidant-related phenolics traditionally associated with these species. Naturally, many other secondary metabolites—such as flavonoids, furanocoumarins, and others—remain to be explored. This should be considered a limitation of the current scope, and future studies may expand on these findings using broader metabolite profiling techniques. More comprehensive phytochemical analyses using advanced techniques such as LC–MS/MS and NMR in the future are likely to reveal additional components. Nevertheless, the findings obtained in this study are intended to provide a scientific basis for future research that will address the metabolomic profiles, pharmacological effects, and safety assessments of the relevant plants in greater detail.
The four medicinal plants (H1–H4) were tested for their antioxidant levels using the DPPH, ABTS, and CUPRAC assays. The results are shown in
Table 8. All of the tests showed that the plant extracts were statistically different (
p < 0.05).
Tragopogon coloratus (H4) had the most antioxidant activity in all tests, with 88.1% for DPPH, 85.6% for ABTS, and 56.8 µmol TE/g for CUPRAC. This shows that
Tragopogon coloratus is the most powerful antioxidant source among the studied species, owing to its probable strong radical-removal and electron-transfer capacity. The high antioxidant power of H4 probably comes from its very high amounts of p-coumaric acid (3.40 µg/g) and chlorogenic acid (7.80 µg/g), both of which are known to be powerful antioxidants [
83]. Although none of the extracts exceeded the synthetic antioxidant BHT (92.4%),
Tragopoon coloratus reached nearly 95% of its radical scavenging capacity, indicating probable potential for natural antioxidant use. The dominance of hydroxycinnamic acids such as p-coumaric and chlorogenic acids likely favored electron-transfer (ET) reactions, explaining the strong CUPRAC response [
83].
Heracleum persicum (H3) ranked second in antioxidant activity (DPPH: 82.4%; ABTS: 79.2%; CUPRAC: 49.7 µmol TE/g). This plant also contained high levels of gallic acid (4.10 µg/g) and rutin (0.83 µg/g), both probably contributing to its antioxidant strength [
84]. Rutin, a flavonoid glycoside, can neutralize free radicals and chelate metal ions, in alignment with the observed CUPRAC findings. The combined presence of chlorogenic, p-coumaric, and rutin suggests probable synergistic effects, since these compounds can reinforce each other’s radical-scavenging mechanisms through hydrogen atom transfer (HAT) and single-electron transfer (SET) pathways [
84].
Ferula communis (H2) also performed well as an antioxidant, particularly in CUPRAC (46.1 µmol TE/g). This is probably due to its high ferulic acid (3.40 µg/g) and p-coumaric acid (5.90 µg/g) contents. Structural studies [
84] have shown that hydroxycinnamic acids stabilize radicals through resonance and electron delocalization, enhancing their redox efficiency.
Daphne mucronata (H1), on the other hand, had the lowest antioxidant activity (DPPH: 66.3%; ABTS: 61.8%; CUPRAC: 33.9 µmol TE/g). Although this species exhibited the highest gallic acid concentration (5.20 µg/g), its radical scavenging potential may have been limited by low chlorogenic (1.10 µg/g) and ferulic acid (0.80 µg/g) levels. In complex plant matrices, probable interactions among phenolic compounds can significantly alter overall antioxidant outcomes; a lack of multiple active phenolics may reduce the total scavenging performance [
85]. These findings indicate that the antioxidant capacity of these species is strongly dependent on their specific flavonoid and phenolic compositions. In particular, chlorogenic acid, rutin, and p-coumaric acid appear to be the probable dominant contributors that improve all three antioxidant assays. A positive relationship is typically observed between these hydroxycinnamic acids and radical scavenging parameters, confirming their probable role in both hydrogen-donating and electron-transfer systems. Notably, previous research from the same study found that
Daphne mucronata accumulated significant quantities of heavy metals, particularly arsenic. When plants are exposed to arsenic, it can induce oxidative stress by generating reactive oxygen species (ROS), which interfere with the biosynthesis of phenolic compounds and related enzymes. Such probable metal-induced oxidative stress can impair antioxidant defense mechanisms, explaining the comparatively weak antioxidant performance of
Daphne mucronata despite its high gallic acid content [
86]. Overall, these results emphasize that phenolic acid diversity—not just total content—probably determines the antioxidant potential of medicinal plants. The observed differences are consistent with redox mechanisms and structure–activity relationships documented for hydroxycinnamic and flavonoid compounds, supporting their use as probable natural antioxidants in nutraceutical and pharmaceutical formulations.