Ubiquitin-Conjugating Enzyme Positively Regulates Salicylic Acid and Jasmonic Acid Biosynthesis to Confer Broad-Spectrum Antiviral Resistance in Nicotiana benthamiana
Abstract
1. Introduction
2. Results
2.1. TuMV-GFP Infection Enhanced UBC mRNA Expression
2.2. UBC Silencing Promoted TuMV-GFP Infection in N. benthamiana
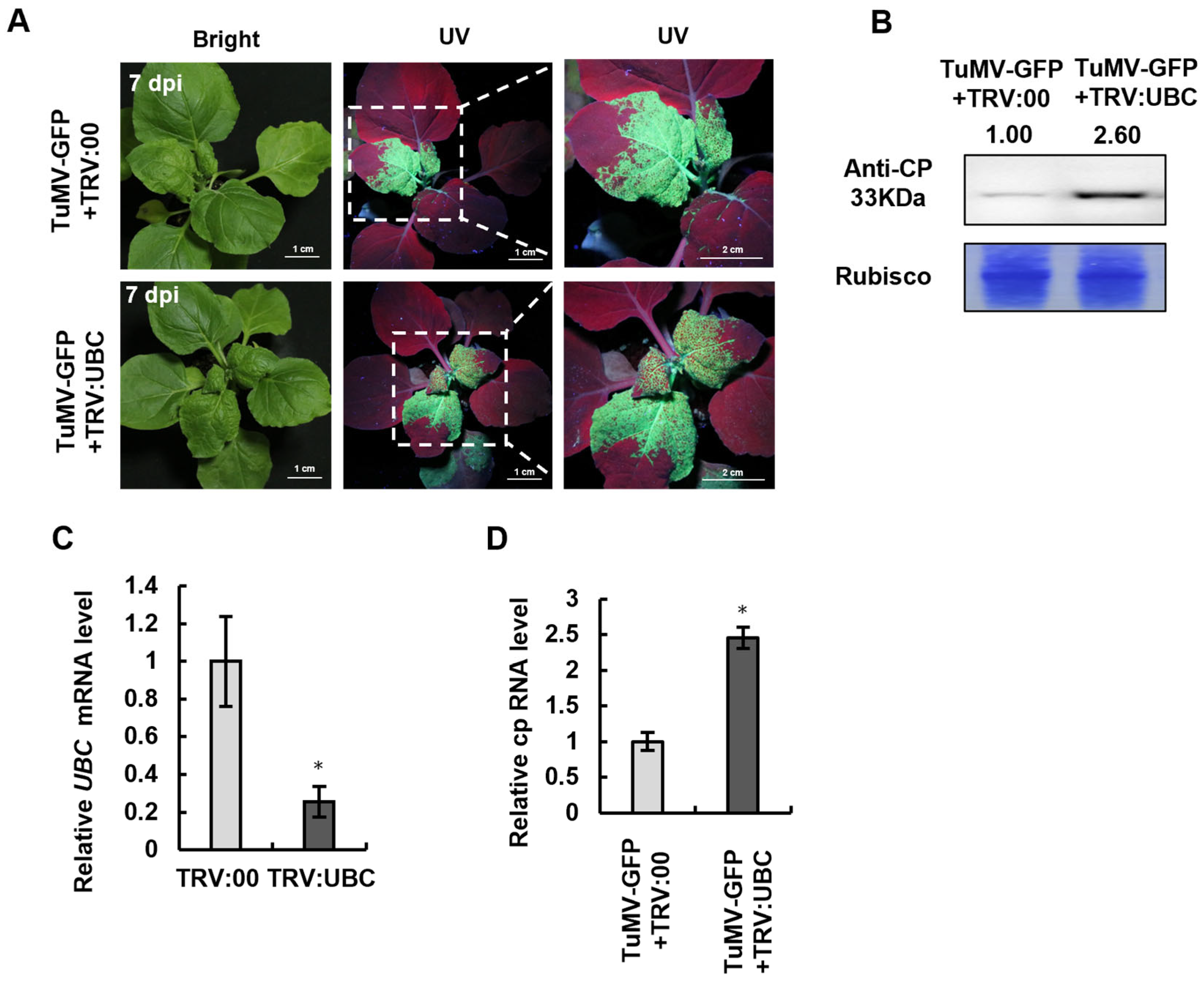
2.3. UBC Overexpression Inhibits TuMV-GFP Accumulation in N. benthamiana
2.4. The Accumulation of Endogenous SA Decreased in UBC-Silenced N. benthamiana

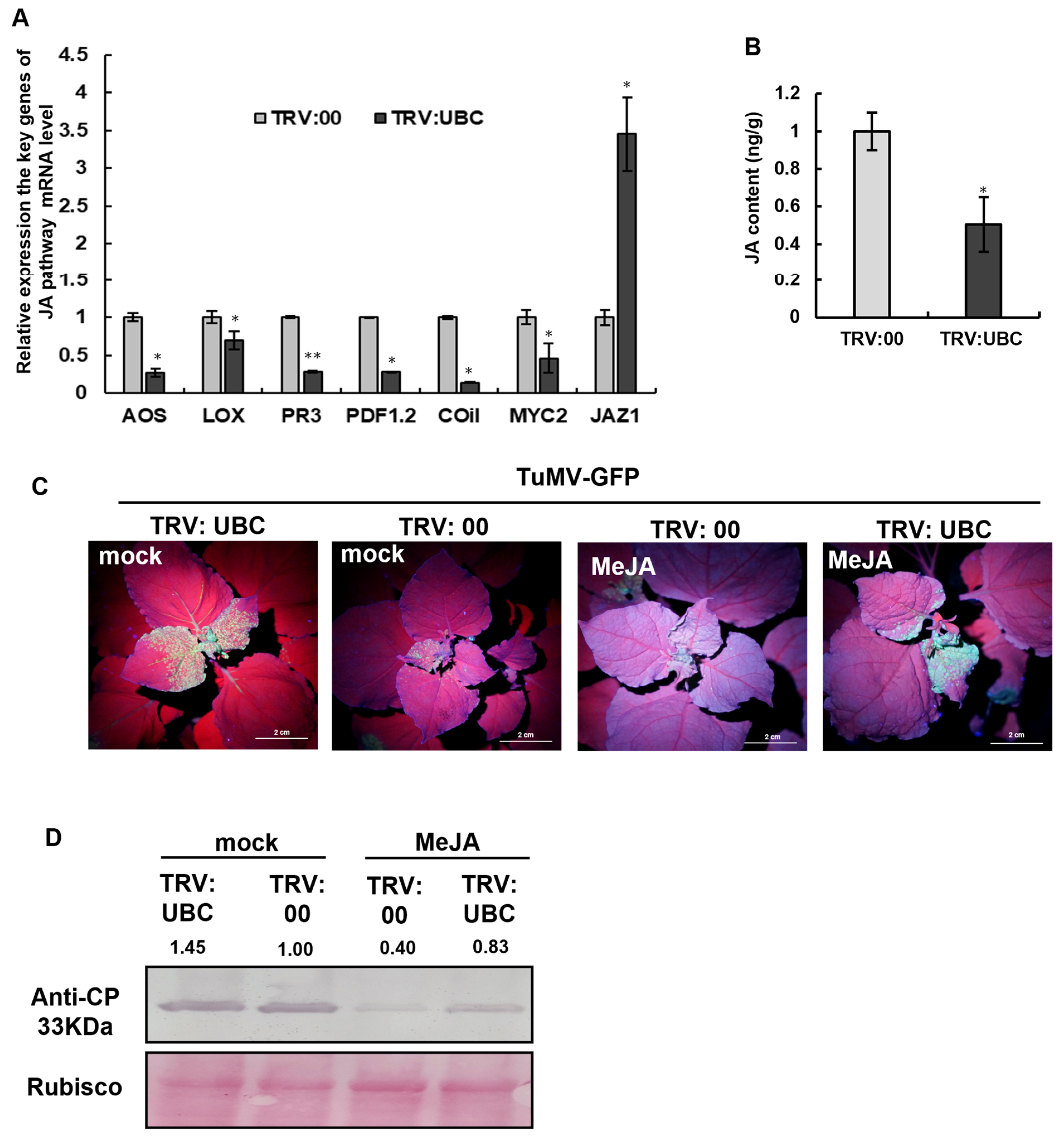
2.5. The Accumulation of Phytohormone JA Was Decreased in UBC-Silenced N. benthamiana
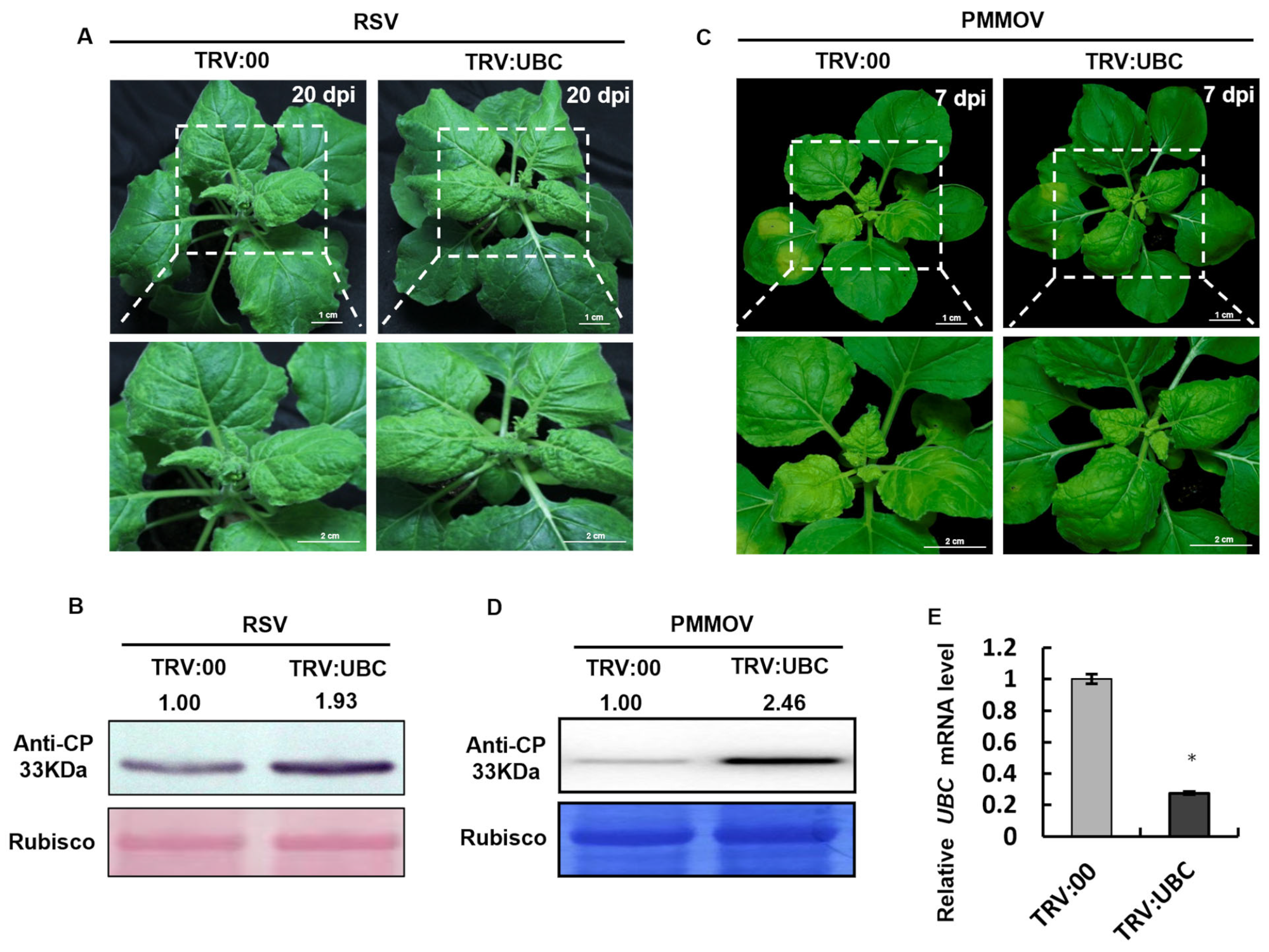
2.6. The Accumulation of RSV and PMMoV Was Increased in UBC-Silenced Plants
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Agrobacterium Infiltration
4.2. Virus-Mediated Gene Silencing (VIGS) and Plasmid Constructs
4.3. RNA Extraction and RT-qPCR
4.4. Plant Hormone Treatment
4.5. Virus Inoculation
4.6. Protein Analysis
4.7. Detection of Hormone Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicolas, O.; Laliberté, J.F. The complete nucleotide sequence of turnip mosaic potyvirus RNA. J. Gen. Virol. 1992, 73, 2785–2793. [Google Scholar] [CrossRef]
- Fang, X.; Chen, J.; Yan, F.; Wu, G. Subcellular Colocalization Assay of Host Factors with Viral Replication Complex in the dsRNA Reporter Nicotiana benthamiana. Methods Mol. Biol. 2024, 2771, 39–45. [Google Scholar] [PubMed]
- Wang, S.; Cui, W.; Wu, X.; Yuan, Q.; Zhao, J.; Zheng, H.; Lu, Y.; Peng, J.; Lin, L.; Chen, J.; et al. Suppression of nbe-miR166h-p5 attenuates leaf yellowing symptoms of potato virus X on Nicotiana benthamiana and reduces virus accumulation. Mol. Plant Pathol. 2018, 19, 2384–2396. [Google Scholar] [CrossRef] [PubMed]
- Moreira, X.; Abdala-Roberts, L.; Castagneyrol, B. Interactions between plant defence signalling pathways: Evidence from bioassays with insect herbivores and plant pathogens. J. Ecol. 2018, 106, 2353–2364. [Google Scholar] [CrossRef]
- Pec, M.; Mendoza, P.S.; Magalhães, D.M.; Delalibera, I.; Bento, J.M.S. The endophytic entomopathogenic fungus Metarhizium robertsii alters sugarcane plant defenses, reducing Diatraea saccharalis oviposition and enhancing the attraction of Cotesia flavipes. Crop Prot. 2024, 184, 106872. [Google Scholar] [CrossRef]
- Wasternack, C.; Song, S. Jasmonates: Biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J. Exp. Bot. 2017, 68, 1303–1321. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, Y.; Li, Y.; Wang, Y.; Rodamilans, B.; Ji, W.; Wu, X.; García, J.A.; Wu, X.; Cheng, X. Plant viruses convergently target NPR1 with various strategies to suppress salicylic acid-mediated antiviral immunity. J. Integr. Plant Biol. 2025, 67, 1395–1412. [Google Scholar] [CrossRef]
- Wu, D.; Qi, T.; Li, W.X.; Tian, H.; Gao, H.; Wang, J.; Ge, J.; Yao, R.; Ren, C.; Wang, X.-B.; et al. Viral effector protein manipulates host hormone signaling to attract insect vectors. Cell Res. 2017, 27, 402–415. [Google Scholar] [CrossRef]
- Li, R.; Weldegergis, B.T.; Li, J.; Jung, C.; Qu, J.; Sun, Y.; Qian, H.; Tee, C.; van Loon, J.J.; Dicke, M.; et al. Virulence factors of geminivirus interact with MYC2 to subvert plant resistance and promote vector performance. Plant Cell 2014, 26, 4991–5008. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Q.; Li, J.; Wang, X.; Li, C.; Bin, Y.; Song, Z. The coat protein of citrus yellow vein clearing virus directly targets the ascorbate peroxidase 1 in lemon (ClAPX1) to facilitate virus accumulation. Front. Plant Sci. 2023, 14, 1306580–1306589. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, C.; Gao, C.; Yan, W.; Song, W.; Hu, X.; Li, L.; Wei, Z.; Li, Y.; Chen, J.; et al. Evolutionary-Distinct Viral Proteins Subvert Rice Broad-Spectrum Antiviral Immunity Mediated by the RAV15-MYC2 Module. Adv. Sci. 2025, 12, e2412835. [Google Scholar] [CrossRef]
- Wang, G.; Lu, M.; He, Q.; Du, J.; Song, W.; Li, L.; Zhang, H.; Wei, Z.; Lu, Y.; Chen, J.; et al. Low-dose of oligosaccharins boosts antiviral immunity through induction of multiple defense pathways in rice. Phytopathol. Res. 2024, 6, 47–54. [Google Scholar] [CrossRef]
- Li, T.; Huang, Y.; Xu, Z.S.; Wang, F.; Xiong, A.S. Salicylic acid-induced differential resistance to the Tomato yellow leaf curl virus among resistant and susceptible tomato cultivars. BMC Plant Biol. 2019, 19, 173. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, A.; Wang, Q.; Song, Y.; Zhang, M.; Ding, X.; Li, Y.; Geng, Q.; Zhu, C. Ultrahigh-activity immune inducer from Endophytic Fungi induces tobacco resistance to virus by SA pathway and RNA silencing. BMC Plant Biol. 2020, 20, 169. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.H.; Ding, S.W. The suppressor of transgene RNA silencing encoded by Cucumber mosaic virus interferes with salicylic acid-mediated virus resistance. Mol. Plant-Microbe Interact. 2001, 14, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.S.; Kim, J.B.; Kim, D.Y.; Seo, Y.W.; Hong, M.J. Wheat CHIP E3 ubiquitin ligase, TaCEU, forms a complex with TaCHSP70 to regulate targeted proteins, enhancing thermotolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2025, 221, 109615. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.R. E3 Ubiquitin Ligases: Key Regulators of Hormone Signaling in Plants. Mol. Cell. Proteom. 2018, 17, 1047–1054. [Google Scholar] [CrossRef]
- Sanchez, J.A.G.; Bonnet, E.; Loubatier, C.; Doye, A.; Paillier, G.; Segui, F.; Larbret, F.; Chaintreuil, P.; Batistic, L.; Torre, C.; et al. Evolutionary conserved regulation of TFEB stability by the E3 ubiquitin ligase WWP2 modulates response to stress in vivo. iScience 2025, 28, 111838–111848. [Google Scholar] [CrossRef]
- Tang, H.; Wei, C. DYNLL1 ubiquitinated and degraded by E3 ligase PRKN regulates cell cycle arrest and apoptosis in lung adenocarcinoma cells. Discov. Oncol. 2024, 15, 806–816. [Google Scholar] [CrossRef]
- Li, C.; Lei, C.; Huang, Y.; Zheng, Y.; Wang, K. PpWRKY22 physically interacts with PpHOS1/PpTGA1 and positively regulates several SA-responsive PR genes to modulate disease resistance in BABA-primed peach fruit. Sci. Hortic. 2021, 290, 110479–110489. [Google Scholar] [CrossRef]
- Wan, S.; Xin, X.-F. Regulation and integration of plant jasmonate signaling: A comparative view of monocot and dicot. J. Genet. Genom. 2022, 49, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wang, Y.; Yu, B.; Gan, Y.; Lei, J.; Chen, C.; Zhu, Z.; Qiu, Z.; Cao, B. A putative E3 ubiquitin ligase substrate receptor degrades transcription factor SmNAC to enhance bacterial wilt resistance in eggplant. Hortic. Res. 2024, 11, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, M.; Zhang, X.; Zhao, T.; Huang, C.; Tang, Y.; Li, Y.; Zhang, C. The ubiquitin ligase VviPUB19 negatively regulates grape cold tolerance by affecting the stability of ICEs and CBFs. Hortic. Res. 2025, 12, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nat. Int. Wkly. J. Sci. 2007, 448, 661–665. [Google Scholar] [CrossRef]
- Hu, J.; Huang, J.; Xu, H.; Wang, Y.; Li, C.; Wen, P.; You, X.; Zhang, X.; Pan, G.; Li, Q.; et al. Rice stripe virus suppresses jasmonic acid-mediated resistance by hijacking brassinosteroid signaling pathway in rice. PLoS Pathog. 2020, 16, e1008801. [Google Scholar] [CrossRef]
- He, Y.; Zhang, H.; Sun, Z.; Li, J.; Hong, G.; Zhu, Q.; Zhou, X.; MacFarlane, S.; Yan, F.; Chen, J. Jasmonic acid-mediated defense suppresses brassinosteroid-mediated susceptibility to Rice black streaked dwarf virus infection in rice. New Phytol. 2017, 214, 388–399. [Google Scholar] [CrossRef]
- Zhu, F.; Xi, D.H.; Yuan, S.; Xu, F.; Zhang, D.W.; Lin, H.H. Salicylic acid and Jasmonic acid are essential for systemic resistance against tobacco mosaic virus in Nicotiana benthamiana. Mol. Plant-Microbe Interact. 2014, 27, 567–577. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Y.; Yang, J.; Yao, S.; Zhao, K.; Wang, D.; Qin, Q.; Bian, Z.; Li, Y.; Lan, Y.; et al. Jasmonate Signaling Enhances RNA Silencing and Antiviral Defense in Rice. Cell Host Microbe 2020, 28, 89–103. [Google Scholar] [CrossRef]
- Wu, X.; Ye, J. Manipulation of Jasmonate Signaling by Plant Viruses and Their Insect Vectors. Viruses 2020, 12, 148. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, Z.; Wu, K.; Yang, L.; Li, Y.; Yang, Z.; Shi, S.; Liu, X.; Zhao, S.; Yang, Z.; et al. Suppression of Jasmonic Acid-Mediated Defense by Viral-Inducible MicroRNA319 Facilitates Virus Infection in Rice. Mol. Plant 2016, 9, 1302–1314. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Duran, R.; Rosas-Díaz, T.; Gusmaroli, G.; Luna, A.P.; Taconnat, L.; Deng, X.W.; Bejarano, E.R. Geminiviruses subvert ubiquitination by altering CSN-mediated derubylation of SCF E3 ligase complexes and inhibit jasmonate signaling in Arabidopsis thaliana. Plant Cell 2011, 23, 1014–1032. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Strickler, S.R.; Richards, E.J. Loss of CRWN nuclear proteins induces cell death and salicylic acid defense signaling. Plant Physiol. 2019, 179, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Loake, G.; Grant, M. Salicylic acid in plant defence—the players and protagonists. Curr. Opin. Plant Biol. 2007, 10, 466–472. [Google Scholar] [CrossRef]
- Desaki, Y.; Takahashi, S.; Sato, K.; Maeda, K.; Matsui, S.; Yoshimi, I.; Miura, T.; Jumonji, J.-I.; Takeda, J.; Yashima, K.; et al. PUB4, a CERK1-Interacting Ubiquitin Ligase, Positively Regulates MAMP-Triggered Immunity in Arabidopsis. Plant Cell Physiol. 2019, 60, 2573–2583. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, Y.; Su, Y.; Jia, Z.; Jiang, L.; Lu, Y.; Zheng, H.; Peng, J.; Rao, S.; Wu, G.; et al. eIF4A, a target of siRNA derived from rice stripe virus, negatively regulates antiviral autophagy by interacting with ATG5 in Nicotiana benthamiana. PLoS Pathog. 2021, 17, e1009963. [Google Scholar] [CrossRef]
- Liu, Y.; Schiff, M.; Dinesh-Kumar, S.P. Virus-induced gene silencing in tomato. Plant J. 2002, 31, 777–786. [Google Scholar] [CrossRef]
- Xu, Y.; Fu, S.; Tao, X.; Zhou, X. Rice stripe virus: Exploring Molecular Weapons in the Arsenal of a Negative-Sense RNA Virus. Annu. Rev. Phytopathol. 2021, 59, 351–371. [Google Scholar] [CrossRef]
- Zhang, X.; Wan, Q.; Rui, P.; Lu, Y.; Sun, Z.; Chen, J.; Wang, Y.; Yan, F. Rice stripe virus p2 protein interacts with ATG5 and is targeted for degradation by autophagy. Front. Microbiol. 2023, 14, 1191403. [Google Scholar] [CrossRef]
- Forcat, S.; Bennett, M.H.; Mansfield, J.W.; Grant, M.R. A rapid and robust method for simultaneously measuring changes in the phytohormones ABA, JA and SA in plants following biotic and abiotic stress. Plant Methods 2008, 4, 16. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Chen, Z.; Jiang, S.; Xie, L.; Fan, J.; Hu, N.; Zhang, X. Ubiquitin-Conjugating Enzyme Positively Regulates Salicylic Acid and Jasmonic Acid Biosynthesis to Confer Broad-Spectrum Antiviral Resistance in Nicotiana benthamiana. Plants 2025, 14, 3234. https://doi.org/10.3390/plants14203234
Zhang X, Chen Z, Jiang S, Xie L, Fan J, Hu N, Zhang X. Ubiquitin-Conjugating Enzyme Positively Regulates Salicylic Acid and Jasmonic Acid Biosynthesis to Confer Broad-Spectrum Antiviral Resistance in Nicotiana benthamiana. Plants. 2025; 14(20):3234. https://doi.org/10.3390/plants14203234
Chicago/Turabian StyleZhang, Xianglong, Zihao Chen, Shijie Jiang, Lin Xie, Jingjing Fan, Nengbing Hu, and Xiangxiang Zhang. 2025. "Ubiquitin-Conjugating Enzyme Positively Regulates Salicylic Acid and Jasmonic Acid Biosynthesis to Confer Broad-Spectrum Antiviral Resistance in Nicotiana benthamiana" Plants 14, no. 20: 3234. https://doi.org/10.3390/plants14203234
APA StyleZhang, X., Chen, Z., Jiang, S., Xie, L., Fan, J., Hu, N., & Zhang, X. (2025). Ubiquitin-Conjugating Enzyme Positively Regulates Salicylic Acid and Jasmonic Acid Biosynthesis to Confer Broad-Spectrum Antiviral Resistance in Nicotiana benthamiana. Plants, 14(20), 3234. https://doi.org/10.3390/plants14203234




