The Phytohormone Signaling Pathway and Immunity Responses to BYDV Infection in Resistant and Susceptible Oat Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Aphid Reproduction and Inoculation
2.3. Oat Plant Preparation and Treatment
2.4. Detection of Phytohormone
2.5. Transcriptomic Analysis
2.5.1. RNA Extraction, cDNA Library Construction, and RNA-Seq
2.5.2. Quality Control
2.5.3. De Novo Transcriptome Assembly
2.5.4. Unigene Functional Annotation and Classification
2.5.5. Identification and Analysis of Differentially Expressed Genes (DEGs)
2.6. Statistical Analysis
3. Results
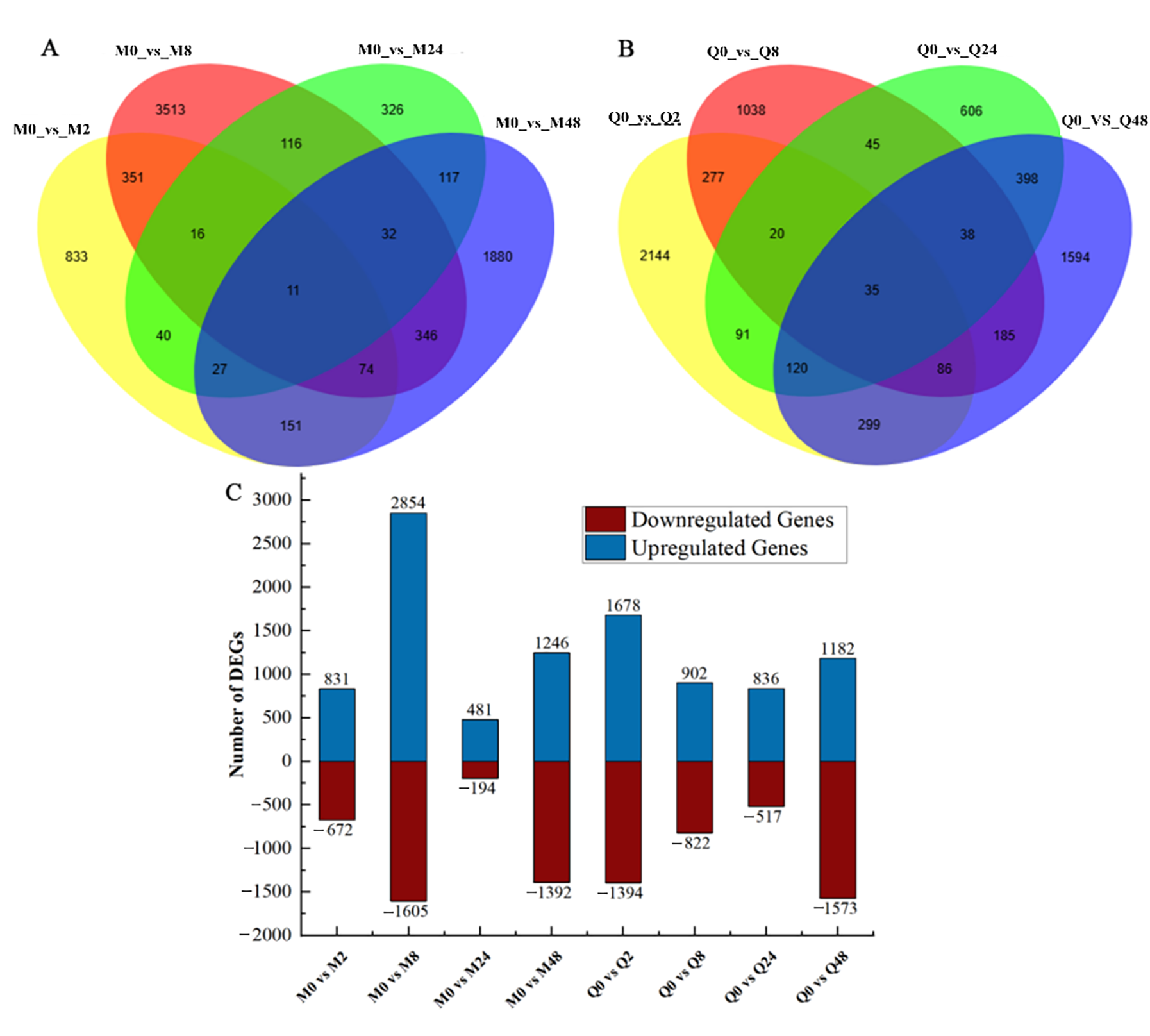
3.1. Screening of Different Expressed Genes (DEGs)
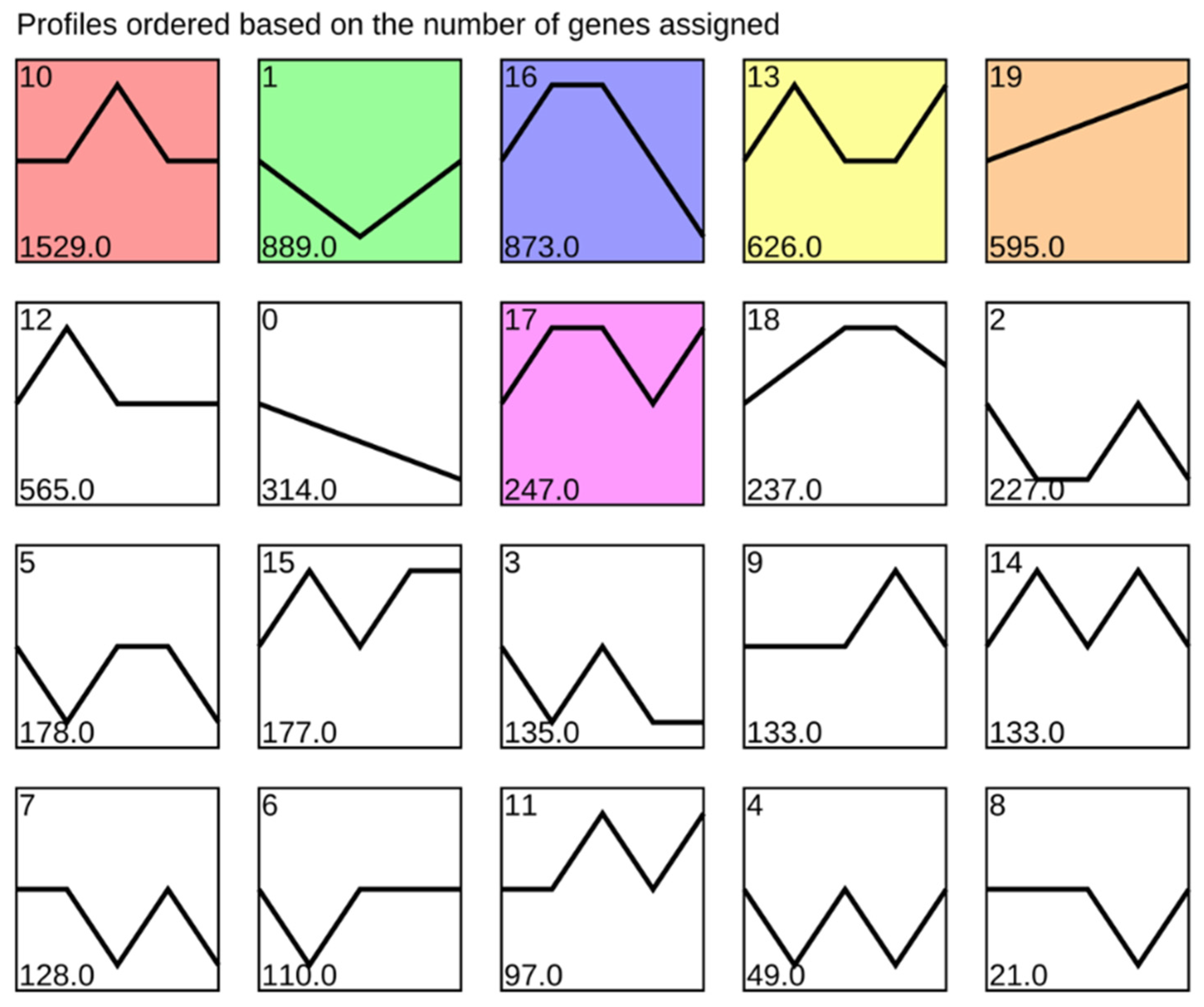
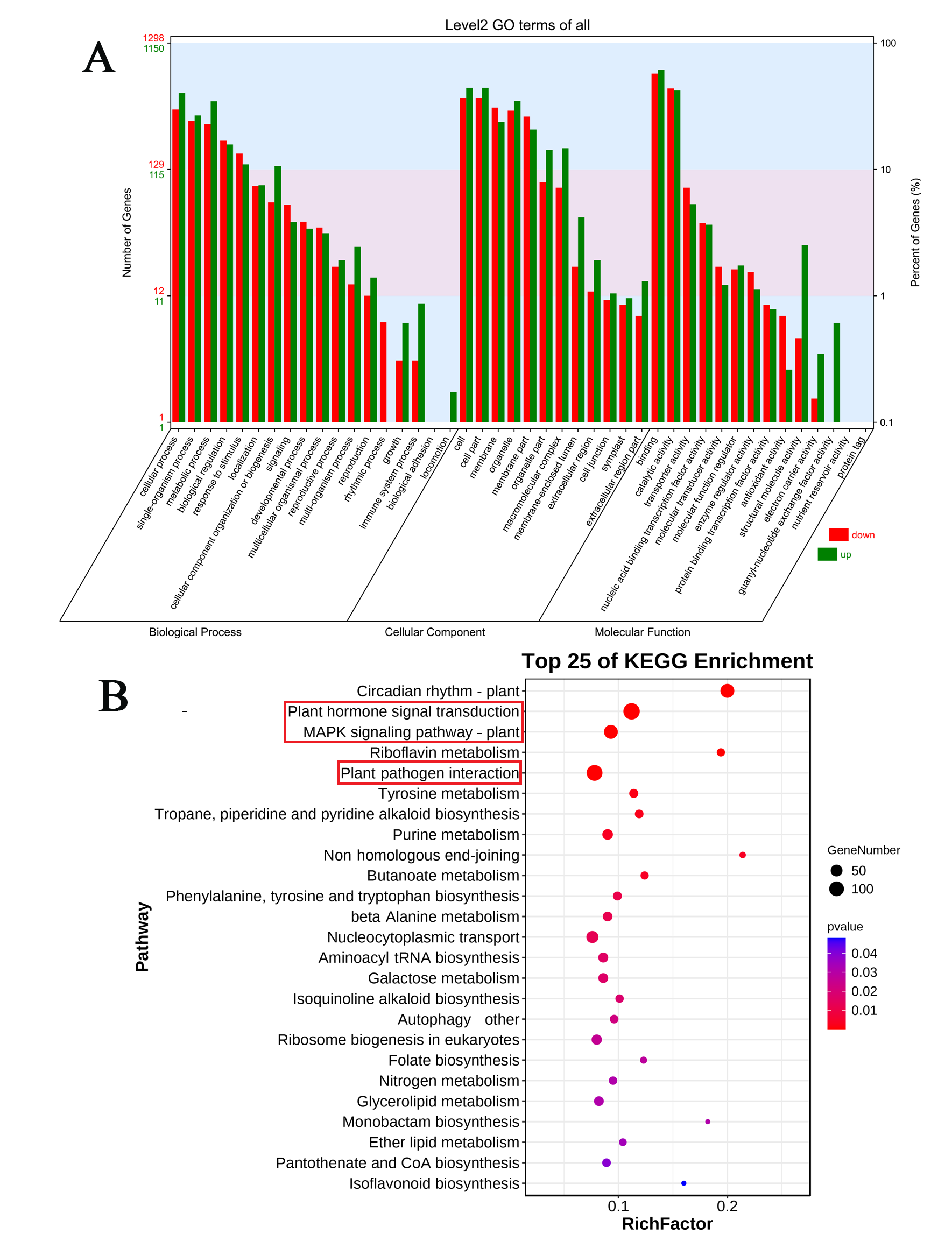
3.2. STEM Analysis and GO and KEGG Enrichment of DEGs
3.3. DEGs Related to Plant–Pathogen Interaction
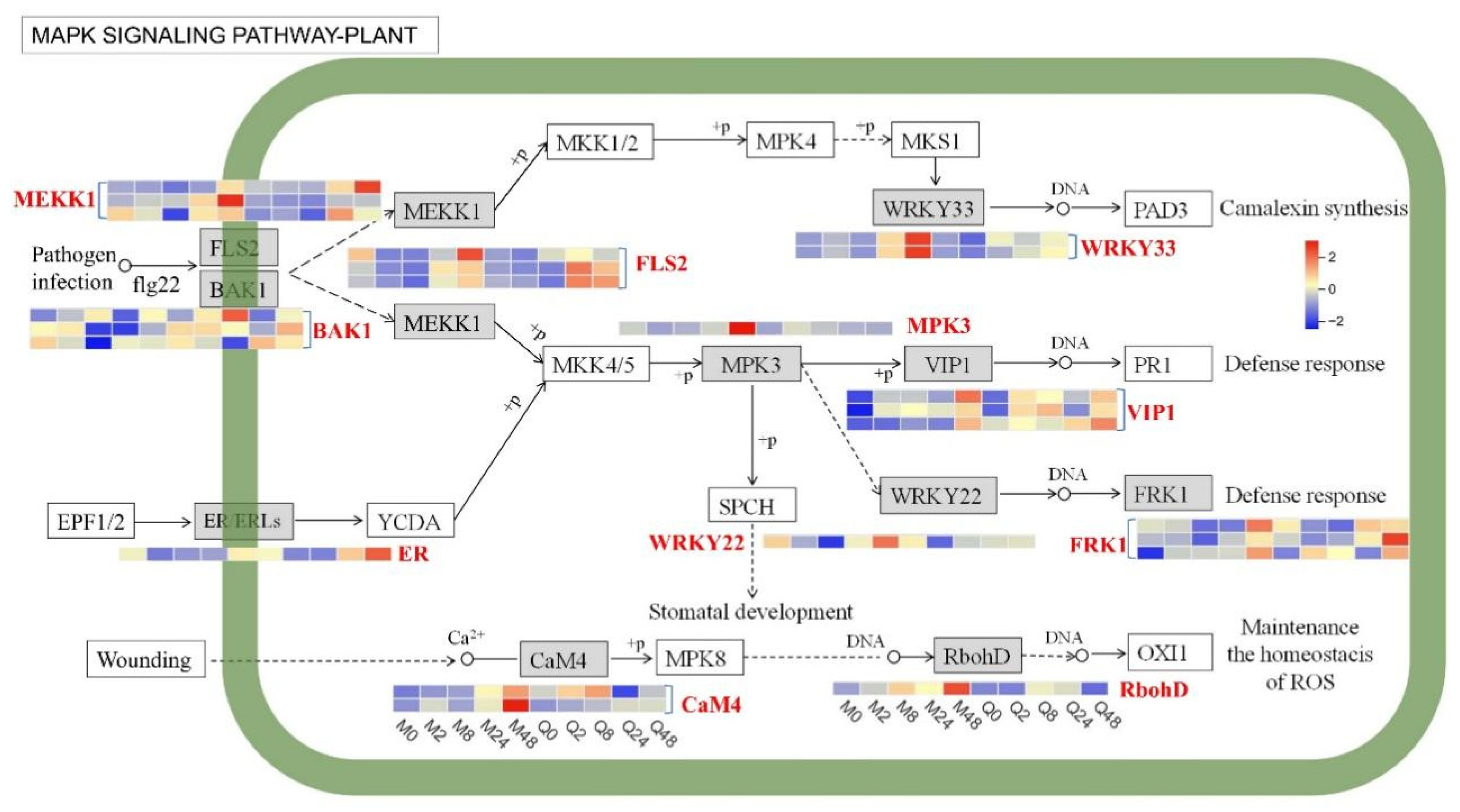
3.4. DEGs Involved in MAPK Signaling Pathway
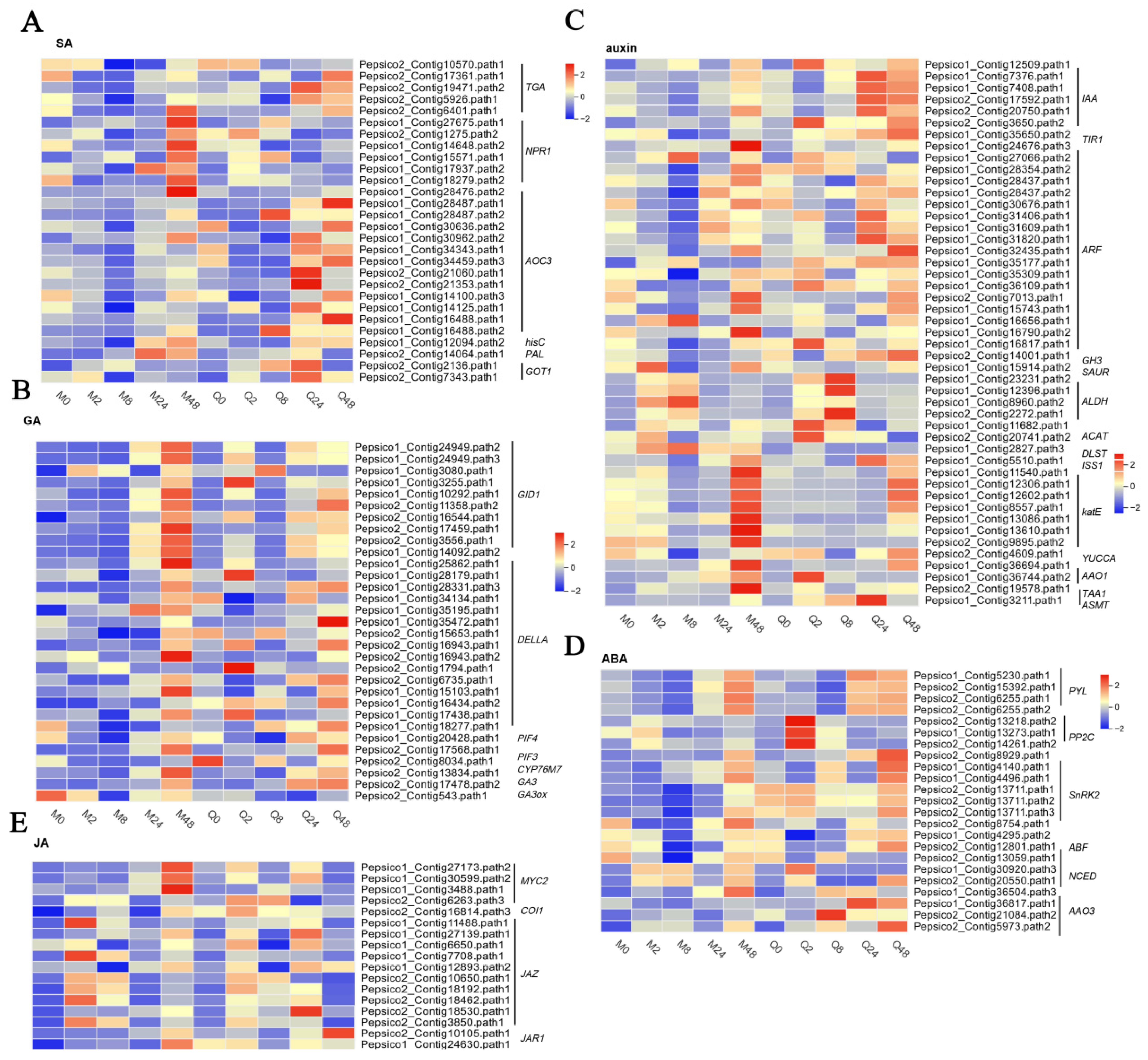
3.5. DEGs Involved in Phytohormone Signal Transduction
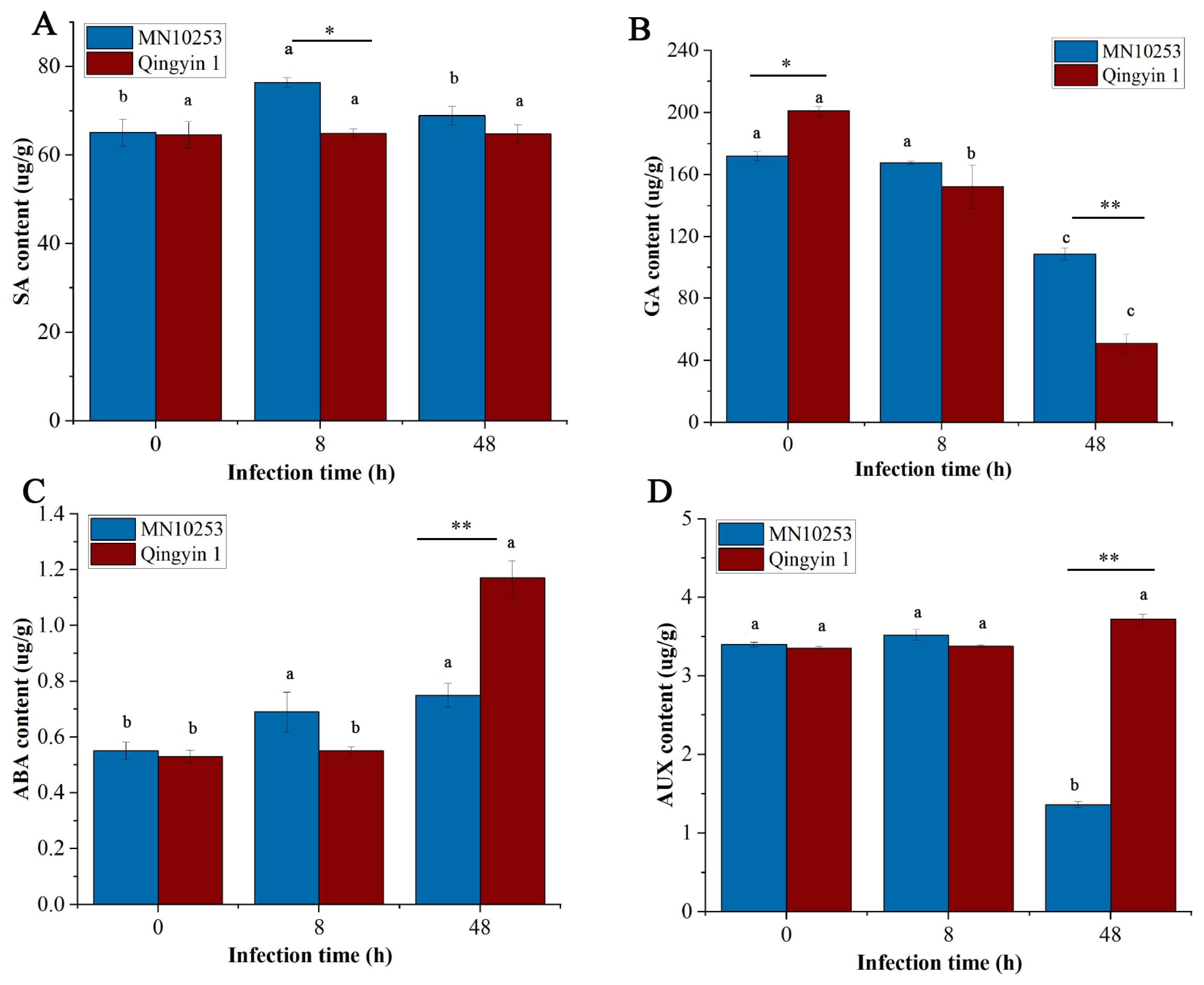
3.6. Effect of BYDV Infection on Phytohormone Content
4. Discussion
4.1. BYDV Triggers Immunity Response in Oat
4.2. MAPK Signaling Pathway Activation in Response to BYDV in Oat
4.3. Differential Effects on Phytohormone Levels and Signaling Pathways
4.3.1. Salicylic Acid
4.3.2. Gibberellin
4.3.3. Auxin
4.3.4. Abscisic Acid
4.4. Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, Y.; Hu, Y.; Ren, C.; Guo, L.; Wang, C.; Jiang, Y.; Wang, X.; Phendukani, H.; Zeng, Z. Effects of nitrogen application on chlorophyll fluorescence parameters and leaf gas exchange in naked oat. J. Integr. Agric. 2013, 12, 2164–2171. [Google Scholar] [CrossRef]
- Lu, P.; Yang, T.; Li, L. Response of oat morphologies, root exudates, and rhizosphere fungal communities to amendments in a saline-alkaline environment. PLoS ONE 2020, 15, e0243301. [Google Scholar] [CrossRef]
- Gong, W.; Ju, Z.; Chai, J. Physiological and transcription analyses reveal the regulatory mechanism in oat (Avena sativa) seedlings with different drought resistance under PEG-induced drought stress. Agronomy 2022, 12, 1005. [Google Scholar] [CrossRef]
- Yu, W.; Bosquée, E.; Fan, J.; Liu, Y.; Bragard, C.; Francis, F.; Chen, J. Proteomic and transcriptomic analysis for identification of endosymbiotic bacteria associated with BYDV transmission efficiency by Sitobion miscanthi. Plants 2022, 11, 3352. [Google Scholar] [CrossRef]
- Riedel, C.; Habekuss, A.; Schliephake, E. Pyramiding of Ryd2 and Ryd3 conferring tolerance to a German isolate of Barley yellow dwarf virus-PAV (BYDV-PAV-ASL-1) leads to quantitative resistance against this isolate. Theor. Appl. Genet. 2011, 123, 69–76. [Google Scholar] [CrossRef]
- Choudhury, S.; Hu, H.; Larkin, P. Agronomical, biochemical and histological response of resistant and susceptible wheat and barley under BYDV stress. PeerJ 2018, 6, e4833. [Google Scholar] [CrossRef]
- Jones, J.; Dang, J. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Tang, D.; Wang, G.; Zhou, J. Receptor kinases in plant-pathogen interactions: More than pattern recognition. Plant Cell 2017, 29, 618–637. [Google Scholar] [CrossRef]
- Huang, W.; Joosten, M. Immune signaling: Receptor-like proteins make the difference. Trends Plant Sci. 2024, 1, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Zhou, J. Phytopathogen effectors subverting host immunity: Different foes, similar battleground. Cell Host Microbe 2012, 12, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Lüdke, D.; Pai, H.; Toghani, A.; Kamoun, S. NLR receptors in plant immunity: Making sense of the alphabet soup. EMBO Rep. 2023, 24, e57495. [Google Scholar] [CrossRef]
- Zhai, K.; Liang, D.; Li, H.; Jiao, F.; Yan, B.; Liu, J.; Lei, Z.; Huang, L.; Gong, X.; Wang, X.; et al. NLRs guard metabolism to coordinate pattern-and effector-triggered immunity. Nature 2022, 601, 245–251. [Google Scholar] [CrossRef]
- Vlot, A.; Dempsey, D.; Klessig, D. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef]
- Ullah, C.; Chen, Y.; Ortega, M.; Tsai, C. The diversity of salicylic acid biosynthesis and defense signaling in plants: Knowledge gaps and future opportunities. Curr. Opin. Plant Biol. 2023, 72, 102349. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, T.; Sun, Y. Diverse roles of the salicylic acid receptors NPR1 and NPR3/NPR4 in plant immunity. Plant Cell 2020, 32, 4002–4016. [Google Scholar] [CrossRef]
- Murphy, A.; Zhou, T.; Carr, J. An update on salicylic acid biosynthesis, its induction and potential exploitation by plant viruses. Curr. Opin. Virol. 2020, 42, 8–17. [Google Scholar] [CrossRef]
- Sharma, S.; Prasad, M. Diverse roles of phytohormonal signaling in modulating plant-virus interaction. J. Exp. Bot. 2024, 76, 1921–1940. [Google Scholar] [CrossRef] [PubMed]
- de Torres Zabala, M.; Bennett, M.; Truman, W.; Grant, M. Antagonism between salicylic and abscisic acid reflects early host-pathogen conflict and moulds plant defense responses. Plant J. 2009, 59, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Dommel, M.; Mou, Z. Abscisic acid promotes proteasome-mediated degradation of the transcription coactivator NPR 1 in Arabidopsis thaliana. Plant J. 2016, 86, 20–34. [Google Scholar] [CrossRef]
- Tian, H.; Xu, L.; Li, X. Salicylic acid: The roles in plant immunity and crosstalk with other hormones. J. Integr. Plant Biol. 2024, 67, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Sarsaiya, S.; Singh, R.; Gong, Q.; Wu, Q.; Shi, J. Omics approaches in understanding the benefits of plant-microbe interactions. Front. Microbiol. 2024, 15, 1391059. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, G.; Chai, J.; Guo, J.; Jiao, R. Evaluation of resistance to aphids and barley yellow dwarf virus in 33 oat germplasms. Pratacultural Sci. 2019, 36, 3155–3165. [Google Scholar]
- Liu, Y.; Guo, J.; Yuan, W.; Zhang, X.; He, C.; Zhao, G.; Zhang, Z. Resistance of MN hulled oats Lines to biotype E of the greenbug (Schizaphis graminum). J. Triticeae Crops 2018, 38, 1199–1206. [Google Scholar]
- Guo, J.; Guo, M.; Guo, C.; Wei, H.; Liu, Y.; He, S.; Zhao, G. Identification of the resistance of oats to aphid and barley yellow dwarf virus and evaluation of its utilization. Sci. Agric. Sin. 2012, 45, 575–583. [Google Scholar]
- Zhou, X.; Wang, M.; Yang, L. Comparative physiological and transcriptomic analyses of oat (Avena sativa) seedlings under salt stress reveal salt tolerance mechanisms. Plants 2024, 13, 2238. [Google Scholar] [CrossRef]
- Lu, R.; Li, P. Transcriptome analysis of Thinopyrum triticum under salt stress. Acta Agrestia Sin. 2020, 28, 31–44. [Google Scholar]
- Romeis, T.; Herde, M. From local to global: CDPKs in systemic defense signaling upon microbial and herbivore attack. Curr. Opin. Plant Biol. 2014, 20, 1–10. [Google Scholar] [CrossRef]
- Wang, C.; Luan, S. Calcium homeostasis and signaling in plant immunity. Curr. Opin. Plant Biol. 2024, 77, 102485. [Google Scholar] [CrossRef]
- Wang, Y.; Loake, G.; Chu, C. Cross-talk of nitric oxide and reactive oxygen species in plant programed cell death. Front. Plant Sci. 2013, 4, 314. [Google Scholar] [CrossRef]
- Bernoux, M.; Timmers, T.; Jauneau, A.; Briere, C.; de Wit, P.; Marco, Y.; Deslandes, L. RD19, an Arabidopsis cysteine protease required for RRS1-R–mediated resistance, is relocalized to the nucleus by the Ralstonia solanacearum PopP2 effector. Plant Cell 2008, 20, 2252–2264. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shao, J.; Luo, M.; Chen, D.; Tang, D.; Shi, H. BRASSINOSTEROID-SIGNALING KINASE1 associates with and is required for cysteine protease RESPONSE TO DEHYDRATION 19-mediated disease resistance in Arabidopsis. Plant Sci. 2024, 342, 112033. [Google Scholar] [CrossRef]
- Li, Z.; Huang, J.; Wang, Z.; Meng, F.; Zhang, S.; Wu, X.; Gao, Z. Overexpression of Arabidopsis nucleotide-binding and leucine-rich repeat genes RPS2 and RPM1 (D505V) confers broad-spectrum disease resistance in rice. Front. Plant Sci. 2019, 10, 417. [Google Scholar] [CrossRef]
- Pottinger, S.; Innes, R. RPS5-mediated disease resistance: Fundamental insights and translational applications. Annu. Rev. Phytopathol. 2020, 58, 139–160. [Google Scholar] [CrossRef]
- Park, C.; Seo, Y. Heat shock proteins: A review of the molecular chaperones for plant immunity. Plant Pathol. J. 2015, 31, 323. [Google Scholar] [CrossRef] [PubMed]
- Manna, M.; Rengasamy, B.; Sinha, A. Revisiting the role of MAPK signaling pathway in plants and its manipulation for crop improvement. Plant Cell Environ. 2023, 46, 2277–2295. [Google Scholar] [CrossRef]
- Garcia, A.; Charrier, A.; Schikora, A.; Bigeard, J.; Pateyron, S.; de Tauzia-Moreau, M.; Hirt, H. Salmonella enterica flagellin is recognized via FLS2 and activates PAMP-triggered immunity in Arabidopsis thaliana. Mol. Plant 2014, 7, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Qamar, S.; Chen, Z.; Mengiste, T. Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 2006, 48, 592–605. [Google Scholar] [CrossRef]
- Patil, V.; Nandi, A. Role of MAPK cascade in local and systemic immunity of plants. In Protein Kinases and Stress Signaling in Plants; Wiley: Hoboken, NJ, USA, 2020; pp. 422–444. [Google Scholar]
- Kadota, Y.; Shirasu, K.; Zipfel, C. Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiol. 2015, 56, 1472–1480. [Google Scholar] [CrossRef]
- Lai, Y.; Renna, L.; Yarema, J.; Ruberti, C.; He, S.; Brandizzi, F. Salicylic acid-independent role of NPR1 is required for protection from proteotoxic stress in the plant endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 2018, 115, E5203–E5212. [Google Scholar] [CrossRef]
- Zhou, X.; Hoang, N.; Tao, F.; Fu, T.; Guo, S.; Guo, C.; Zhou, C.; Thanh, T.; Buensanteai, K. Transcriptomics and phytohormone metabolomics provide comprehensive insights into the response mechanism of tea against blister blight disease. Sci. Hortic. 2024, 324, 112611. [Google Scholar] [CrossRef]
- Mauch-Mani, B.; Slusarenko, A. Production of salicylic acid precursors is a major function of phenylalanine ammonia-lyase in the resistance of Arabidopsis to Peronospora parasitica. Plant Cell 1996, 8, 203–212. [Google Scholar] [CrossRef]
- Kumar, D. Salicylic acid signaling in disease resistance. Plant Sci. 2014, 228, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X. Salicylic acid: Biosynthesis, perception, and contributions to plant immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Chu, C. Gibberellin metabolism and signaling: Targets for improving agronomic performance of crops. Plant Cell Physiol. 2020, 61, 1902–1911. [Google Scholar] [CrossRef]
- Yimer, H.; Nahar, K.; Kyndt, T.; Haeck, A.; Van Meulebroek, L.; Vanhaecke, L.; Gheysen, G. Gibberellin antagonizes jasmonate-induced defense against Meloidogyne graminicola in rice. New Phytol. 2018, 218, 646–660. [Google Scholar] [CrossRef]
- Yoshida, H.; Hideki, M.; Miyako Ueguchi-Tanaka, M. Regulatory Networks Acted Upon by the GID1-DELLA System After Perceiving Gibberellin. In The Enzymes; Academic Press: Cambridge, MA, USA, 2014; pp. 1–25. [Google Scholar]
- De Vleesschauwer, D.; Seifi, H.; Filipe, O.; Haeck, A.; Huu, S.; Demeestere, K.; Höfte, M. The DELLA protein SLR1 integrates and amplifies salicylic acid-and jasmonic acid-dependent innate immunity in rice. Plant Physiol. 2016, 170, 1831–1847. [Google Scholar] [CrossRef]
- Yang, D.; Li, Q.; Deng, Y.; Lou, Y.; Wang, M.; Zhou, G.; He, Z. Altered disease development in the eui mutants and Eui overexpressors indicates that gibberellins negatively regulate rice basal disease resistance. Mol. Plant 2008, 1, 528–537. [Google Scholar] [CrossRef]
- Qin, X.; Liu, J.; Zhao, W.; Chen, X.; Guo, Z.; Peng, Y. Gibberellin 20-oxidase gene OsGA20ox3 regulates plant stature and disease development in rice. Mol. Plant-Microbe Interact. 2013, 26, 227–239. [Google Scholar] [CrossRef]
- Naseem, M.; Kaltdorf, M.; Dandekar, T. The nexus between growth and defence signalling: Auxin and cytokinin modulate plant immune response pathways. Exp. Bot. 2015, 66, 4885–4896. [Google Scholar] [CrossRef]
- Mutka, A.; Fawley, S.; Tsao, T.; Kunkel, B. Auxin promotes susceptibility to Pseudomonas syringae via a mechanism independent of suppression of salicylic acid-mediated defenses. Plant J. 2013, 74, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, F.; Gallei, M.; Kornienko, A.; Saado, I.; Khan, M.; Chia, K.; Djamei, A. TOPLESS promotes plant immunity by repressing auxin signaling and is targeted by the fungal effector Naked1. Plant Commun. 2022, 3, 100269. [Google Scholar] [CrossRef]
- Kazan, K.; Manners, J. Linking development to defense: Auxin in plant-pathogen interactions. Trends Plant Sci. 2009, 14, 373–382. [Google Scholar] [CrossRef]
- Wang, D.; Pajerowska-Mukhtar, K.; Culler, A.; Dong, X. Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr. Biol. 2007, 17, 1784–1790. [Google Scholar] [CrossRef]
- Navarro, L.; Dunoyer, P.; Jay, F.; Arnold, B.; Dharmasiri, N.; Estelle, M.; Jones, J. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 2006, 312, 436–439. [Google Scholar] [CrossRef]
- Boba, A.; Kostyn, K.; Kozak, B.; Wojtasik, W.; Preisner, M.; Prescha, A.; Kulma, A. Fusarium oxysporum infection activates the plastidial branch of the terpenoid biosynthesis pathway in flax, leading to increased ABA synthesis. Planta 2020, 251, 50. [Google Scholar] [CrossRef] [PubMed]
- Dunn, R.; Hedden, P.; Bailey, J. A physiologically-induced resistance of Phaseolus vulgaris to a compatible race of Colletotrichum lindemuthianum is associated with increases in ABA content. Physiol. Mol. Plant Pathol. 1990, 36, 339–349. [Google Scholar] [CrossRef]
- Alazem, M.; Lin, N. Roles of plant hormones in the regulation of host-virus interactions. Mol. Plant Pathol. 2015, 16, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Bharath, P.; Gahir, S.; Raghavendra, A. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Asselbergh, B.; De Vleesschauwer, D.; Höfte, M. Global switches and fine-tuning-ABA modulates plant pathogen defense. Mol. Plant-Microbe Interact. 2008, 21, 709–719. [Google Scholar] [CrossRef]
- Ton, J.; Flors, V.; Mauch-Mani, B. The multifaceted role of ABA in disease resistance. Trends Plant Sci. 2009, 14, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Hill, L.; Crooks, C.; Doerner, P.; Lamb, C. Abscisic acid has a key role in modulating diverse plant-pathogen interactions. Plant Physiol. 2009, 150, 1750–1761. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, C.; Ding, Z.; Zhao, Y.; Dai, J.; Li, J.; Tao, X. A plant NLR receptor employs ABA central regulator PP2C-SnRK2 to activate antiviral immunity. Nat. Commun. 2024, 15, 3205. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, J.; Niu, K.; Huang, P.; Gong, W.; Zhang, Y.; Ju, Z.; Zhao, G. The Phytohormone Signaling Pathway and Immunity Responses to BYDV Infection in Resistant and Susceptible Oat Cultivars. Plants 2025, 14, 3229. https://doi.org/10.3390/plants14203229
Chai J, Niu K, Huang P, Gong W, Zhang Y, Ju Z, Zhao G. The Phytohormone Signaling Pathway and Immunity Responses to BYDV Infection in Resistant and Susceptible Oat Cultivars. Plants. 2025; 14(20):3229. https://doi.org/10.3390/plants14203229
Chicago/Turabian StyleChai, Jikuan, Kuiju Niu, Panpan Huang, Wenlong Gong, Yuehua Zhang, Zeliang Ju, and Guiqin Zhao. 2025. "The Phytohormone Signaling Pathway and Immunity Responses to BYDV Infection in Resistant and Susceptible Oat Cultivars" Plants 14, no. 20: 3229. https://doi.org/10.3390/plants14203229
APA StyleChai, J., Niu, K., Huang, P., Gong, W., Zhang, Y., Ju, Z., & Zhao, G. (2025). The Phytohormone Signaling Pathway and Immunity Responses to BYDV Infection in Resistant and Susceptible Oat Cultivars. Plants, 14(20), 3229. https://doi.org/10.3390/plants14203229





