Characterization and Biological Characteristics of Alternaria, Botryosphaeria, Pestalotiopsis, and Trichothecium Species Associated with Postharvest Loquat Fruit Rot in Yunnan, China
Abstract
1. Introduction
2. Results
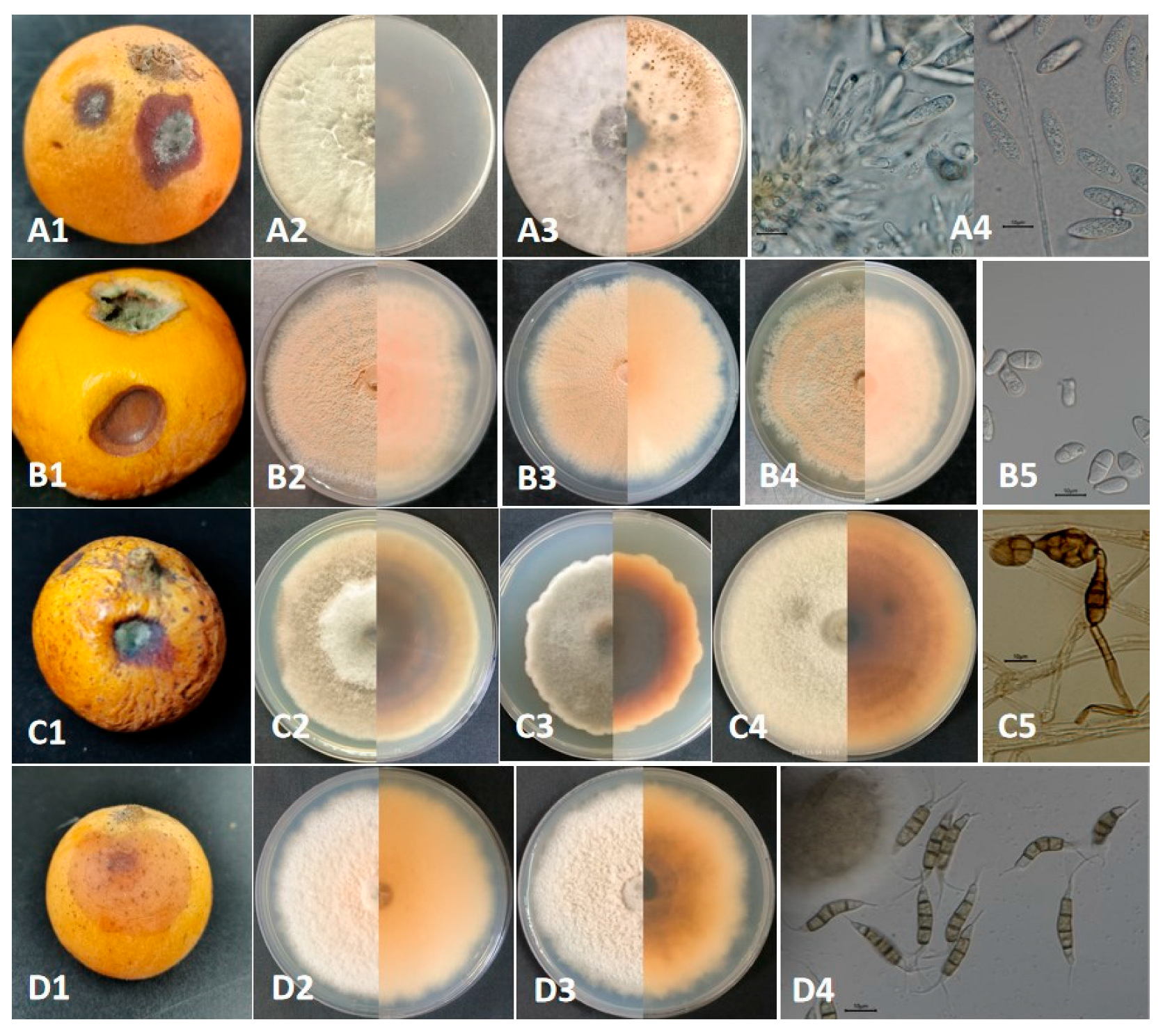
2.1. Natural Symptoms, Fungal Isolation
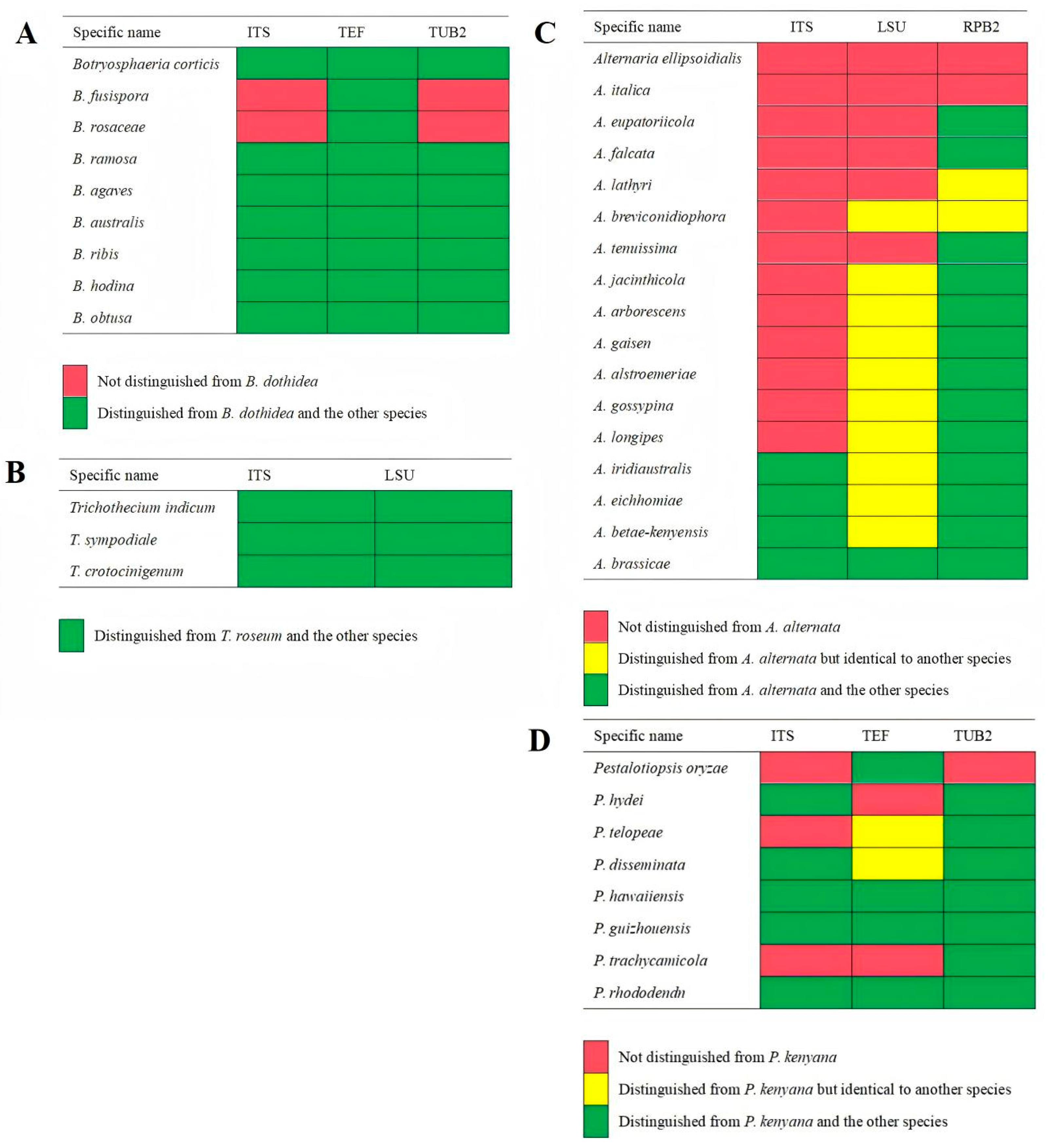
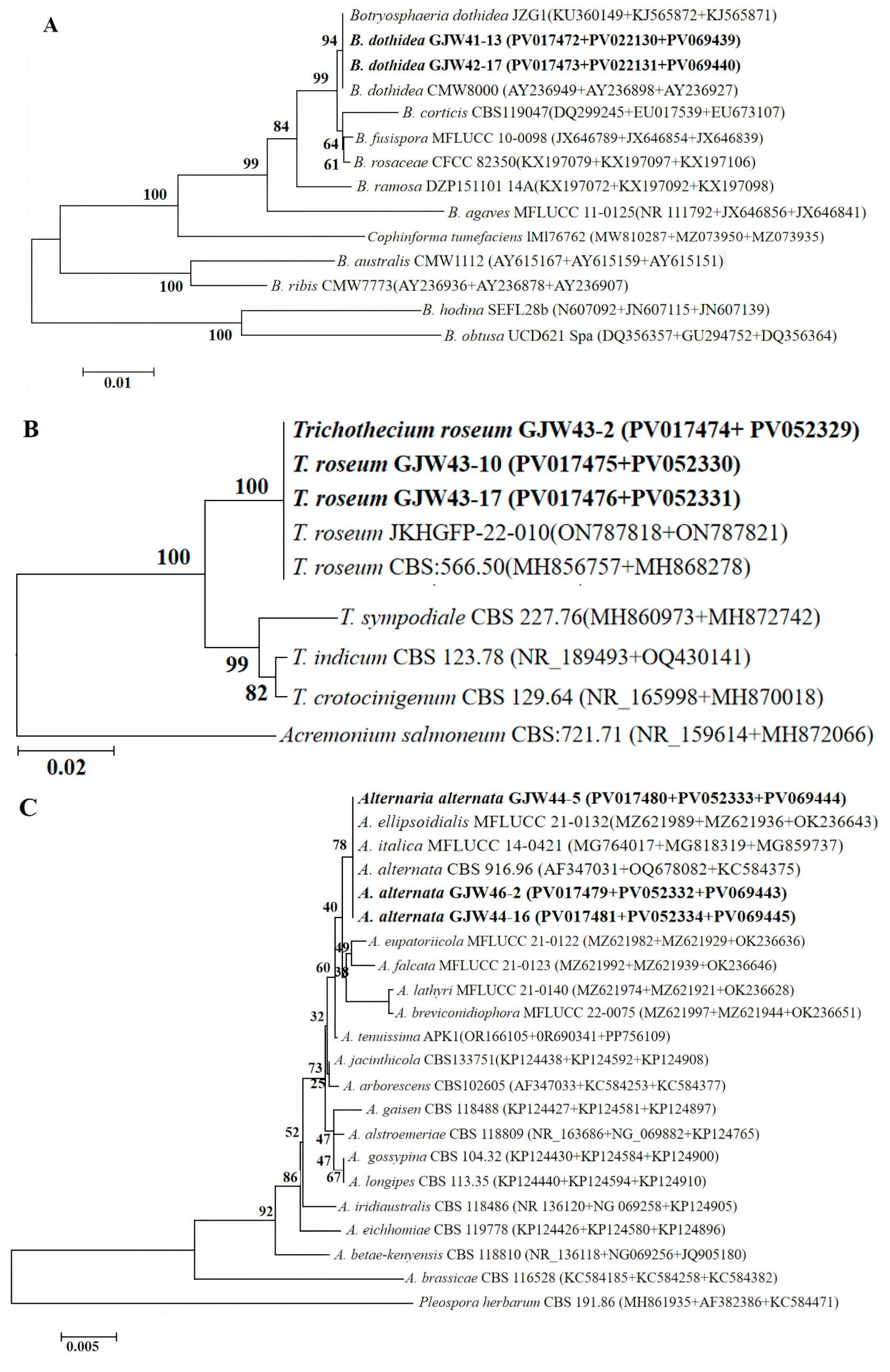
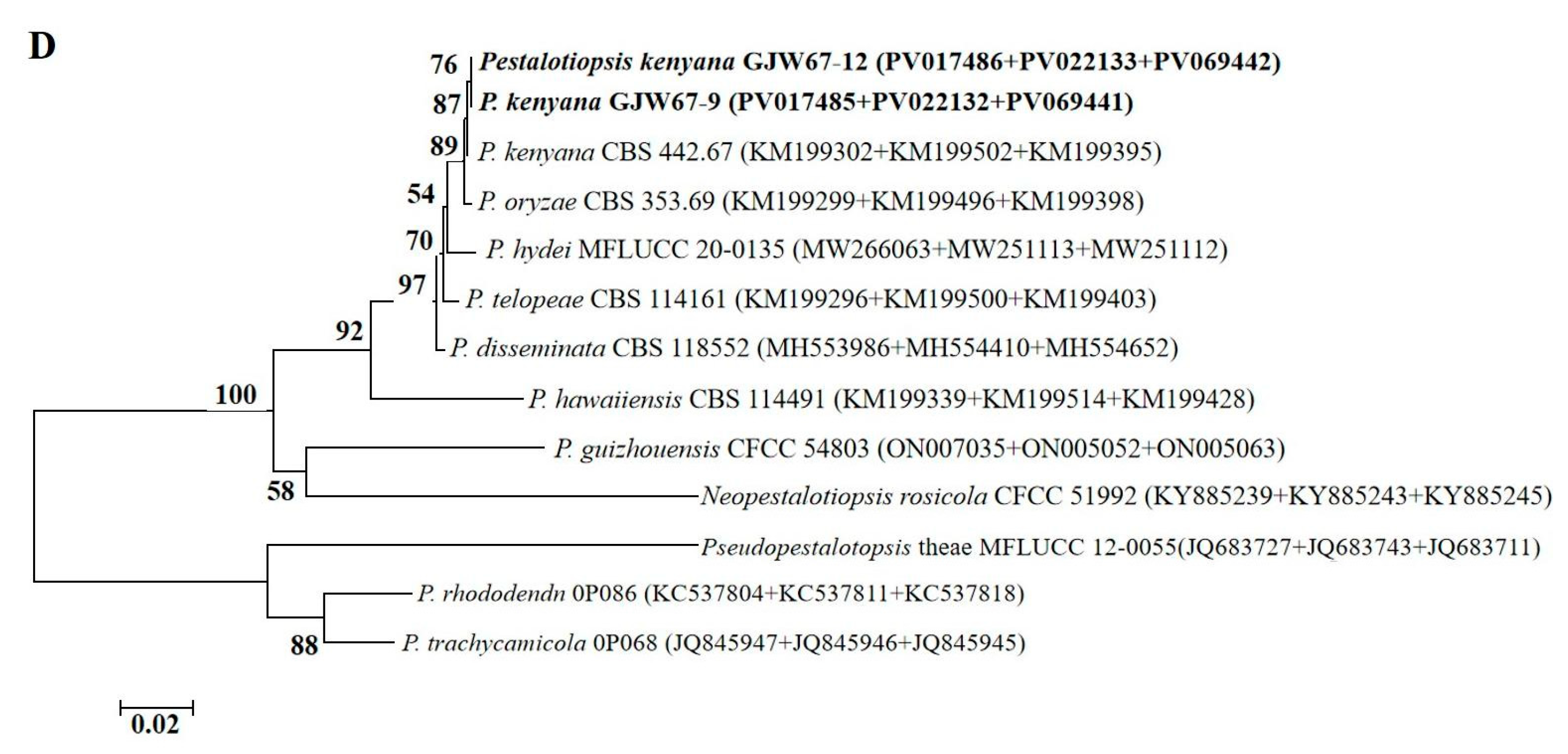
2.2. Morphological and Molecular Identification of Potential Pathogenic Fungi
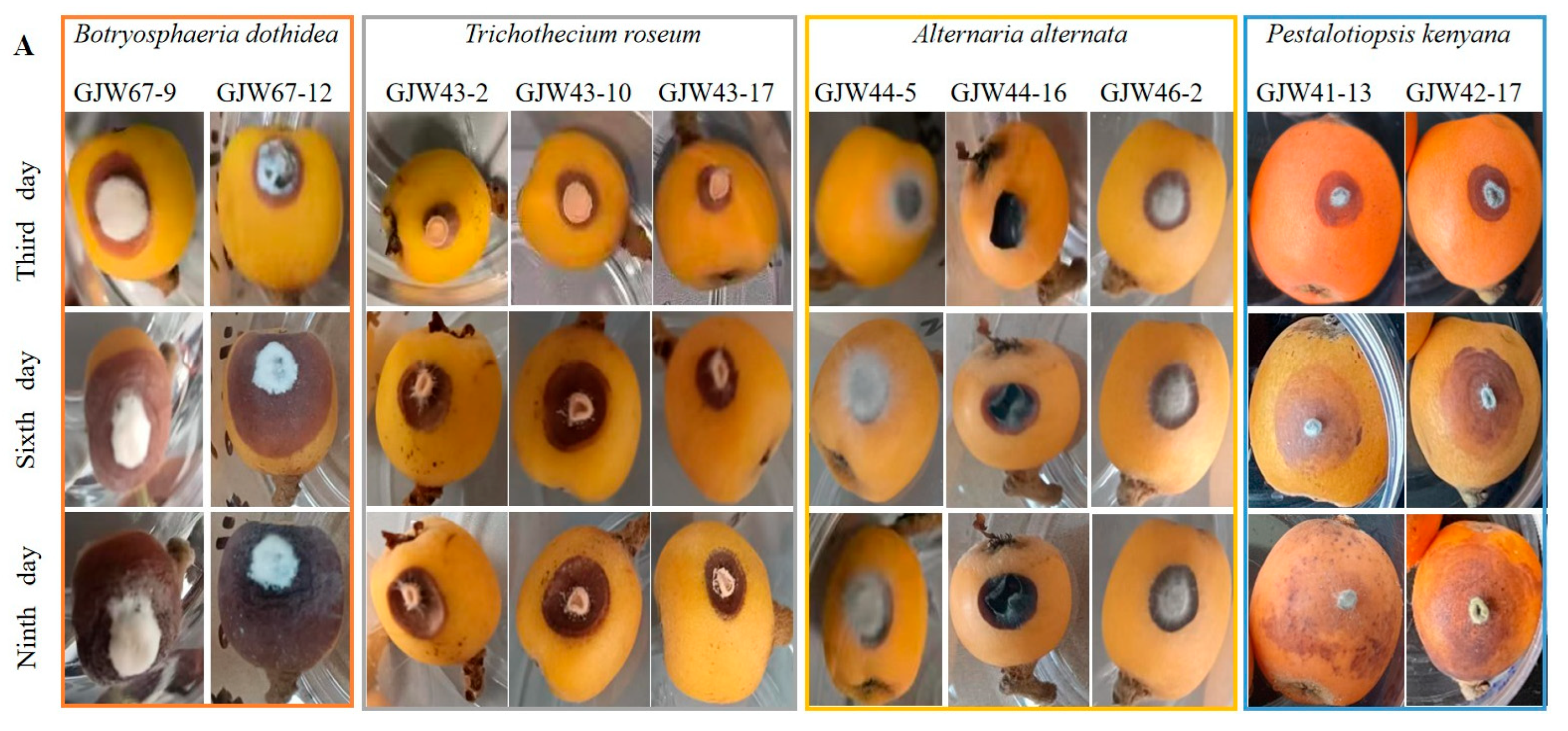
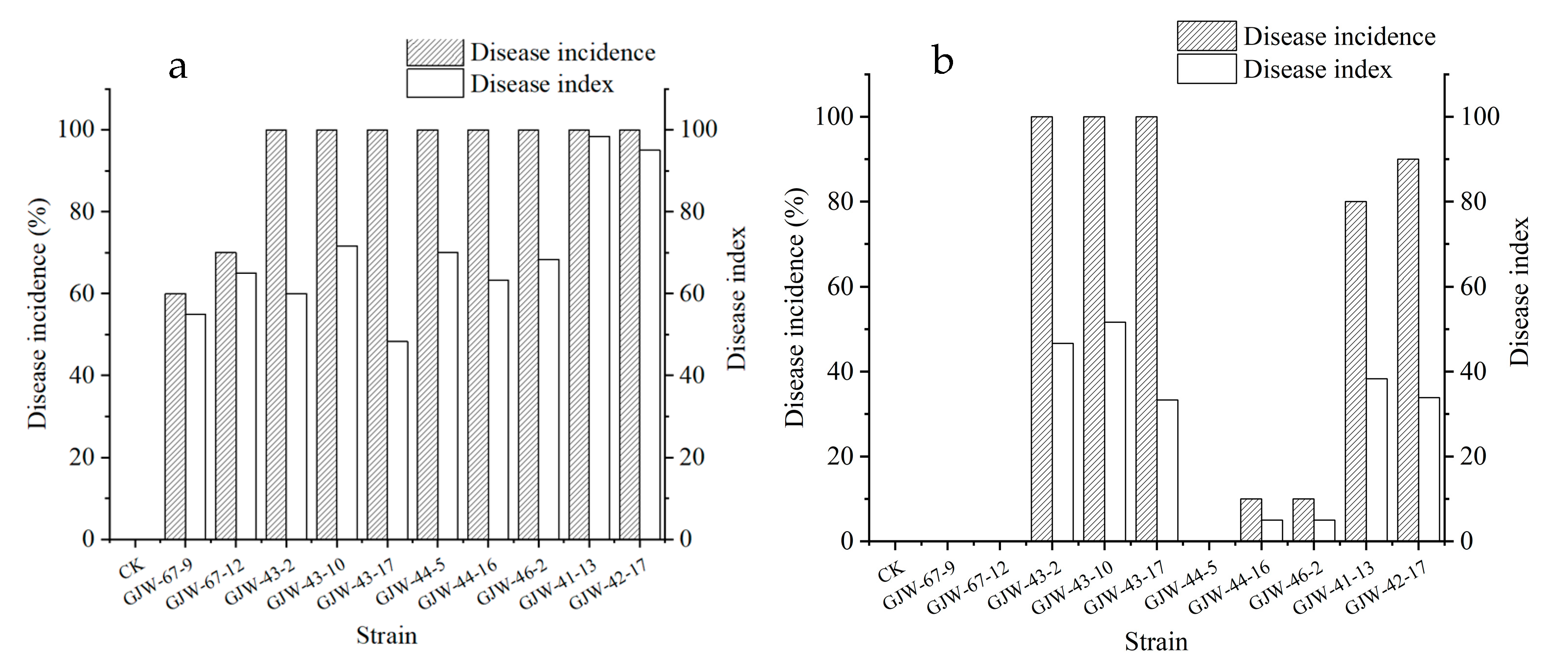
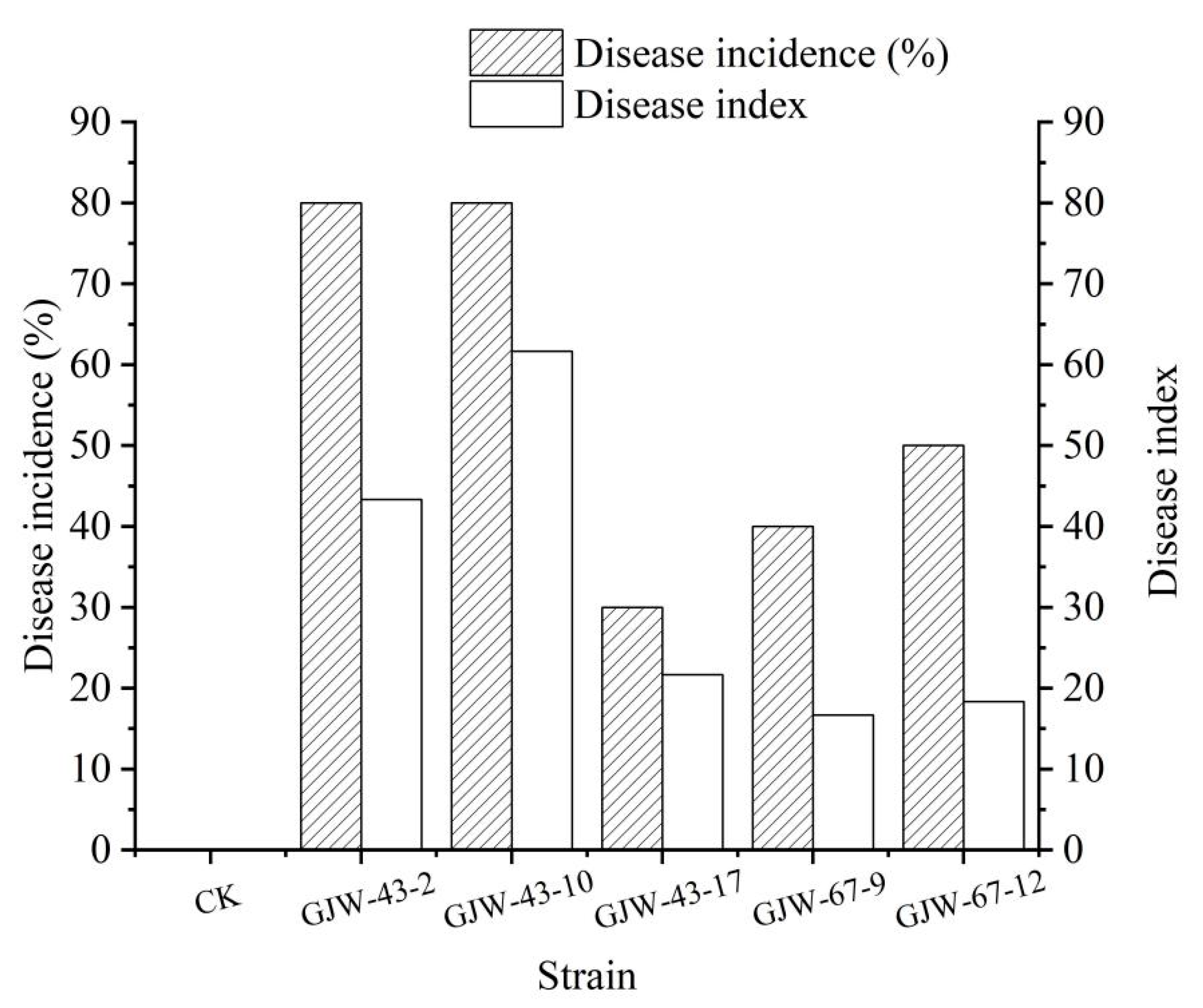
2.3. Pathogenicity on Loquat Fruits
2.4. Effect of Temperature and Humidity on Pathogenicity of Alternaria alternata on Loquat Leaves
3. Discussion
4. Materials and Methods
4.1. Field Sampling and Isolation
4.2. Pathogenicity Test
4.3. Morphological Identification and Characterization
4.4. Genomic DNA Extraction, Sequencing, and Phylogenetic Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González-Domínguez, E.; Alves, A.; León, M.; Armengol, J. Characterization of Botryosphaeriaceae species associated with diseased loquat (Eriobotrya japonica) in Spain. Plant Pathol. 2017, 66, 77–89. [Google Scholar] [CrossRef]
- He, C.; Wang, L.; Li, Y.; Zhou, K.; Zhao, K.; Chen, D.; Li, J.; Song, H.; Tu, M. Loquat (Eriobotrya japonica) is a new natural host of tomato mosaic virus and citrus exocortis viroid. Plants 2024, 13, 1965. [Google Scholar] [CrossRef]
- Cai, J.; Chen, T.; Zhang, Z.; Li, B.; Qin, G.; Tian, S. Metabolic dynamics during loquat fruit ripening and postharvest technologies. Front. Plant Sci. 2019, 10, 619. [Google Scholar] [CrossRef]
- Luo, J.Q.; Zhang, Y.J.; Jing, L.H.; Wu, A.P.; Xia, M. Application of nutritional and functional value of Eriobotrya japonica. Farm Prod. Process. 2021, 4, 83–87. [Google Scholar]
- Palou, L.; Sánchez-Torres, P.; Montesinos-Herrero, C.; Taberner, V. Incidence and etiology of postharvest fungal diseases of loquat fruit (Eriobotrya japonica (Thunb.) Lindl. cv. ‘Algerie’) in Alacant province (Spain). Eur. J. Plant Pathol. 2016, 146, 847–860. [Google Scholar] [CrossRef]
- Takata, Y.; Komine, M.; Uchikawa, K.; Nozawa, S.; Watanabe, K. Pathogenicity of Colletotrichum species isolated from rotten fruit and asymptomatic flowers of loquat in Nagasaki Prefecture, Japan and characterization of C. nagasakiense Takata & Kyoko Watan. sp. nov. J. Gen. Plant Pathol. 2024, 90, 69–81. [Google Scholar] [CrossRef]
- Gu, H.; Liu, A.Y.; Chen, W.X.; Feng, S.J.; Shi, J.Y. Development and control of postharvest diseases of loquat fruit. Acta Hortic. 2007, 750, 437–443. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y. Effect of 1-methylcyclopropene on anthracnose rot caused by Colletotrichum acutatum and disease resistance in loquat fruit. J. Sci. Food Agr. 2010, 90, 2289–2294. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Fang, L.; Xie, Y.; Wang, L. First report of Colletotrichum scovillei causing anthracnose fruit rot on postharvested Eriobotrya japonica in Zhejiang Province, China. Plant Dis. 2022, 106, 2752. [Google Scholar] [CrossRef]
- Wu, W.X.; Liu, Y.; Huang, X.Q.; Zhang, L. First report of anthracnose caused by Colletotrichum nymphaeae on loquat fruit in China. Plant Dis. 2018, 102, 243. [Google Scholar] [CrossRef]
- Nozawa, S.; Uchikawa, K.; Suga, Y.; Watanabe, K. Infection sources of Pestalotiopsis sensu lato related to loquat fruit rot in Nagasaki Prefecture, Japan. J. Gen. Plant Pathol. 2020, 86, 173–179. [Google Scholar] [CrossRef]
- Abbas, M.F.; Batool, S.; Khan, T.; Rashid, M. First report of Neopestalotiopsis clavispora causing postharvest fruit rot of loquat in Pakistan. J. Plant Pathol. 2022, 104, 459. [Google Scholar] [CrossRef]
- Palou, L.; Montesinos-Herreros, C.; Guardado, A.; Taberner, V. First report of Pestalotiopsis clavispora causing postharvest fruit rot of loquat in Spain. J. Plant Pathol. 2013, 95, S4-69. [Google Scholar]
- Bibi, H.; Haroon, U.; Farhana; Akbar, A.K.M.; Anar, M.; Batool, S.S.; Bilal, A.; Jabeen, H.; Ahmed, J.; Chaudhary, H.J.; et al. Impact of bacterial synthesized nanoparticles on quality attributes and postharvest disease control efficacy of apricot and loquat. J. Food Sci. 2023, 88, 16695. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.F.; Irshad, G.; Gondal, A.S.; Sajid, M.N.; Naz, F.; Karamat, M.Z.; Bashir, A.; Hyder, S.; Ahmed, R. First report of Rhizopus stolonifer causing postharvest fruit rot of loquat (Eriobotrya japonica) from Pakistan. Plant Dis. 2019, 103, 1410. [Google Scholar] [CrossRef]
- Li, S.N.; Jiang, S.C.; Zhang, W.M. First report of postharvest fruit rot of loquat (Rhaphiolepis loquata) caused by Ceratobasidium sp. in China. Plant Dis. 2024, 108, 533. [Google Scholar] [CrossRef]
- Hafeez, R.; Akhtar, N.; Shoaib, A.; Bashir, U.; Haider, M.S.; Awan, Z.A. First report of Geotrichum candidum from Pakistan causing postharvest sour rot in loquat (Eriobotrya japonica). J. Anim. Plant Sci. 2015, 25, 1737–1740. [Google Scholar]
- Gariglio, N.; Fonfría, M.A. Effect of fruit thinning on the mineral composition of loquat (“Eriobotrya japonica” Lindl.) fruit and its connection with purple spot. Span. J. Agric. Res. 2005, 4, 439–446. [Google Scholar] [CrossRef]
- Naz, F.; Abbas, M.F.; Rauf, C.A.; Tariq, A.; Mumtaz, F.A. First report of Colletotrichum gloeosporioides causing anthracnose on loquat in Pakistan. Plant Dis. 2017, 101, 1550. [Google Scholar] [CrossRef]
- Juárez-Vázquez, S.B.; Silva-Rojas, H.V.; Rebollar-Alviter, A.; Maidana-Ojeda, M.; Osnaya-González, M.; Fuentes-Aragón, D. Phylogenetic and morphological identification of Colletotrichum godetiae, a novel pathogen causing anthracnose on loquat fruits (Eriobotrya japonica). J. Plant Dis. Prot. 2019, 126, 593–598. [Google Scholar] [CrossRef]
- Damm, U.; Sun, Y.-C.; Huang, C.-J. Colletotrichum eriobotryae sp. nov. and C. nymphaeae, the anthracnose pathogens of loquat fruit in central Taiwan, and their sensitivity to azoxystrobin. Mycol. Prog. 2020, 19, 367–380. [Google Scholar] [CrossRef]
- Poti, T.; Kisaki, G.; Arita, K.; Akimitsu, K. Colletotrichum species associated with loquat anthracnose in Kagawa and Tokushima prefectures, Japan. J. Gen. Plant Pathol. 2024, 90, 241–253. [Google Scholar] [CrossRef]
- Perelló, A.E.; Larran, S. First report of Pestalotiopsis guepini on loquat in Argentina. Plant Dis. 1999, 83, 695. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, W.-X.; Zhang, A.-F.; Yang, X.; Xu, Y.-L. First Report of Pestalotiopsis theae on loquat (Eriobotrya japonica) in Anhui Province of China. Plant Dis. 2013, 97, 558. [Google Scholar] [CrossRef]
- Tziros, G.T. Alternaria alternata causes leaf spot and fruit rot on loquat (Eriobotrya japonica) in Greece. Australas. Plant Dis. 2013, 8, 123–124. [Google Scholar] [CrossRef]
- Batta, Y. Control of Alternaria alternata on loquat (Eriobotrya japonica Lindl.) using detached fruits and leaf-disk assay. An-Najah Univ. J. Res. 2005, 19, 69–82. [Google Scholar] [CrossRef]
- Ko, Y.; Liu, C.W.; Chen, S.S.; Chen, C.Y.; Yao, K.S.; Maruthasalam, S.; Lin, C.H. First report of fruit rot of loquat caused by an Alternaria sp. in Taiwan. Plant Dis. 2010, 94, 481. [Google Scholar] [CrossRef]
- González-Domínguez, E.; Hinarejos, R.; Tuset, J.J. Identification and characterization of Fusicladium eriobotryae: Fungal pathogen causing mediterranean loquat scab. Acta Hortic. 2007, 750, 343–347. [Google Scholar] [CrossRef]
- Palou, L.; Taberner, V.; Montesinos-Herrero, C. First report of Diplodia seriata causing loquat fruit rot in Spain. Plant Dis. 2013, 97, 421. [Google Scholar] [CrossRef]
- Abbas, M.F.; Naz, F. First report of Diplodia seriata causing fruit rot of loquat in Pakistan. J. Plant Pathol. 2018, 100, 325. [Google Scholar] [CrossRef]
- Niazi, F.; Ali, M.; Haroon, U.; Farhana; Kamal, A.; Rashid, T.; Anwar, F.; Nawab, R.; Chaudhary, H.J.; Munis, M.F.H. Effect of green Fe2O3 nanoparticles in controlling Fusarium fruit rot disease of loquat in Pakistan. Brazil. J. Microb. 2023, 54, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.F.; Naz, F.; Rauf, C.A.; Mehmood, N.; Shah, P.M.A.; Zhang, X.; Rosli, B.H.; Gleason, M.L. First report of Fusarium solani causing fruit rot of loquat (Eriobotrya japonica) in Pakistan. Plant Dis. 2017, 101, 839. [Google Scholar] [CrossRef]
- Zhai, L.; Zhang, M. First report of Neofusicoccum parvum causing fruit rot on Eriobotrya japonica in China. Plant Dis. 2019, 103, 2125. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, S.; Du, J.; Wang, X.; Xu, W.; Luo, C. Monilinia fructicola on loquat: An old pathogen invading a new host. J. Integ. Agri. 2021, 20, 2009–2014. [Google Scholar] [CrossRef]
- Nwe, Z.M.; Htut, K.N.; Aung, S.L.L.; Gou, Y.-N.; Huang, C.-X.; Deng, J.-X. Two novel species and a new host record of Alternaria (Pleosporales, Pleosporaceae) from sunflower (Compositae) in Myanmar. MycoKeys 2024, 105, 337–354. [Google Scholar] [CrossRef]
- Hyde, K.D.; Saleh, A.; Aumentado, H.D.R.; Boekhout, T.; Bera, I.; Khyaju, S.; Bhunjun, C.S.; Chethana, K.W.T.; Phukhamsakda, C.; Doilom, M.; et al. Fungal numbers: Global needs for a realistic assessment. Fungal Divers. 2024, 128, 191–225. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Rathnayake, A.R.; Marasinghe, D.S.; Calabon, M.S.; Gentekaki, E.; Lee, H.B.; Hurdeal, V.G.; Pem, D.; Dissanayake, L.S.; Wijesinche, S.N.; et al. Morphological approaches in studying fungi: Collection, examination, isolation, sporulation and preservation. Mycosphere 2020, 11, 2678–2754. [Google Scholar] [CrossRef]
- Li, J.F.; Jiang, H.B.; Jeewon, R.; Hongsanan, S.; Bhat, D.J.; Tang, S.M.; Lumyong, S.; Mortimer, P.E.; Xu, J.-C.; Camporesi, E.; et al. Alternaria: Update on species limits, evolution, multi-locus phylogeny, and classification. Stud. Fungi 2023, 8, 1. [Google Scholar] [CrossRef]
- Summerbell, R.C.; Gueidan, C.; Schroers, H.-J.; de Hoog, G.S.; Starink, M.; Rosete, Y.A.; Guarro, J.; Scott, J.A. Acremonium phylogenetic overview and revision of Gliomastix, Sarocladium, and Trichothecium. Stud. Mycol. 2011, 68, 139–162. [Google Scholar] [CrossRef]
- Woudenberg, J.H.C.; Groenewald, J.Z.; Binder, M.; Crous, P.W. Alternaria redefined. Stud. Mycol. 2013, 75, 171–212. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Hyde, K.D.; Groenewald, J.Z.; Xu, J.; Crous, P.W. Pestalotiopsis revisited. Stud. Mycol. 2014, 79, 121–186. [Google Scholar] [CrossRef] [PubMed]
- Batista, E.; Lopes, A.; Alves, A. Botryosphaeriaceae species on forest trees in Portugal: Diversity, distribution and pathogenicity. Eur. J. Plant Pathol. 2020, 158, 693–720. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Dissanayake, A.J.; Liu, Z.-Y.; Liu, J.-K. Additions to karst fungi 4: Botryosphaeria spp. associated with woody hosts in Guizhou province, China including B. guttulata sp. nov. Phytotaxa 2020, 454, 186–202. [Google Scholar] [CrossRef]
- Götz, M.; Karbowy-Thongbai, B. First detection of Trichothecium roseum causing leaf spots on tomato in Germany. Plant Dis. 2023, 107, 1233. [Google Scholar] [CrossRef]
- Fonseca-Guerra, I.R.; Beltrán Pineda, M.E.; Benavides Rozo, M.E. Characterization of Alternaria alternata and Alternaria scrophulariae Brown Spot in Colombian quinoa (Chenopodium quinoa). J. Fungi 2023, 9, 947. [Google Scholar] [CrossRef]
- Li, W.-L.; Dissanayake, A.J.; Zhang, T.; Maharachchikumbura, S.S.N.; Liu, J.-K. Identification and pathogenicity of pestalotiod fungi associated with woody oil plants in Sichuan Province, China. J. Fungi 2022, 8, 1175. [Google Scholar] [CrossRef]
- Ni, Y.Q.Z.; Li, Y.; Liu, S. List of common fungal diseases of crops and their pathogens in China-the main grain and oil crops. J. Fung. Res. 2023, 21, 247–274. [Google Scholar]
- Wang, K.; Liu, F.; Cai, L. A name list of common agricultural phytopathogenic fungi in China. Mycosystema 2022, 41, 361–386. [Google Scholar]
- Han, S.L.; Wang, M.M.; Ma, Z.Y.; Raza, M.; Zhao, P.; Liang, J.M.; Wang, J.W.; Cai, L. Fusarium diversity associated with diseased cereals in China, with an updated phylogenomic assessment of the genus. Stud. Mycol. 2023, 104, 87–148. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Chen, X.Y.; Guo, L.Y. Population structure of brown rot fungi on stone fruits in China. Plant Dis. 2011, 95, 1284–1291. [Google Scholar] [CrossRef]
- Lai, D.; Wang, D.; Shao, X.; Qin, J.; Zhuang, Q.; Xiao, W. First report of Botryosphaeria dothidea causing fruit rot on Chinese olive (Canarium album) in Guangdong Province of China. Plant Dis. 2024, 108, 1109. [Google Scholar] [CrossRef]
- Yuan, X.; Qi, Y.; Wu, Z.; Yang, C.; Cui, C. First report of Botryosphaeria dothidea causing postharvest fruit rot of plum in China. Plant Dis. 2024, 108, 1111. [Google Scholar] [CrossRef]
- Gu, C.-Y.; Yang, X.; Al-Attala, M.N.; Abid, M.; Phyo, S.S.M.; Zang, H.-Y.; Pan, R.; Chen, Y. First report of pomegranate fruit rot caused by Botryosphaeria dothidea in Anhui Province of China. Plant Dis. 2020, 104, 2736. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.X.; Han, X.L.; Zhang, Y.L.; Liang, Q.; Li, S.K.; Yang, K.Q. First report of Botryosphaeria dothidea causing fruit rot of rellowhorn (Xanthoceras sorbifolium) in China. Plant Dis. 2018, 102, 1662. [Google Scholar] [CrossRef]
- Ran, L.X.; Zhang, M.; Shen, H.M. First report of red rot of jujube fruit caused by Botryosphaeria dothidea in China. Plant Dis. 2018, 102, 1458. [Google Scholar] [CrossRef]
- Yu, C.; Diao, Y.; Lu, Q.; Zhao, J.; Cui, S.; Peng, C.; He, B.; Liang, Y.; Liu, H. Genome assembly and annotation of Botryosphaeria dothidea sdau11-99, a latent pathogen of apple fruit ring rot in China. Plant Dis. 2021, 105, 1555–1557. [Google Scholar] [CrossRef]
- Li, C.; Zhao, J.; Wang, J.; Wang, X.; Xiang, W.; Zhao, J. First report of Trichothecium roseum causing postharvest fruit rot on purple passion fruit in China. Plant Dis. 2022, 106, 3212. [Google Scholar] [CrossRef]
- Zhu, M.; Duan, X.; Cai, P.; Qiu, Z.; Li, Z. Genome sequence resource of Trichothecium roseum (ZM-Tr2021), the causal agent of postharvest pink rot. Plant Dis. 2023, 107, 205–209. [Google Scholar] [CrossRef]
- Liu, C.; Luo, F.; Zhu, T.; Han, S.; Li, S. Leaf spot disease caused by Pestalotiopsis kenyana on Zanthoxylum schinifolium in Sichuan Province, China. Plant Dis. 2021, 105, 3747. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Li, H.; Lin, J.; Zuo, Y.; Peng, L.; Ding, H. Pestalotiopsis kenyana causes leaf spot disease on Rhododendron agastum in China. Crop Protect. 2024, 184, 106859. [Google Scholar] [CrossRef]
- Xun, W.; Wu, C.; Wu, X.; Bai, Q.; Sun, Y.; Shi, J.; Xie, D.; Jin, L. First report of bayberry leaf blight caused by Pestalotiopsis kenyana in Zhejiang Province, China. Plant Dis. 2023, 107, 2860. [Google Scholar] [CrossRef]
- Tan, Q.; Schnabel, G.; Chaisiri, C.; Yin, L.F.; Yin, W.X.; Luo, C.X. Colletotrichum species associated with peaches in China. J. Fungi 2022, 8, 313. [Google Scholar] [CrossRef]
- Díaz, G.A.; Valdez, A.; Halleen, F.; Ferrada, E.; Lolas, M.; Latorre, B. Characterization and pathogenicity of Diplodia, Lasiodiplodia, and Neofusicoccum species causing Botryosphaeria canker and dieback of apple trees in Central Chile. Plant Dis. 2022, 106, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Imathiu, S.M.; Ray, R.V.; Back, M.; Hare, M.C.; Edwards, S.G. Fusarium langsethiae pathogenicity and aggressiveness towards oats and wheat in wounded and unwounded in vitro detached leaf assays. Eur. J. Plant Pathol. 2009, 124, 117–126. [Google Scholar] [CrossRef]
- Santos, M.C.; Viteri, L.O.; Araujo, S.H.; Mourão, D.C.; Câmara, M.P.; Amaral, A.G.; Oliveira, E.E.; Santos, G.R.d. Molecular characterization and pathogenicity of Colletotrichum on banana fruits: Wound effects on virulence and cross-infection. Microbiol. Res. 2025, 16, 4. [Google Scholar] [CrossRef]
- Davydenko, K.; Nowakowska, J.; Kaluski, T.; Gawlak, M.; Sadowska, K.; García, J.; Diez, J.; Okorski, A.; Oszako, T.A. Comparative study of the pathogenicity of Fusarium circinatum and other Fusarium species in Polish provenances of P. sylvestris L. Forests 2018, 9, 560. [Google Scholar] [CrossRef]
- López-Moral, A.; Lovera, M.; Antón-Domínguez, B.; Gámiz, A.M.; Michailides, T.J.; Arquero, O.; Trapero, A.; Agustí-Brisach, C. Effects of cultivar susceptibility, branch age, and temperature on infection by Botryosphaeriaceae and Diaporthe fungi on English walnut (Juglans regia). Plant Dis. 2022, 106, 2920–2926. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, Z.; Xie, J.; Lan, B.; Shen, Z.; Shi, H.; Lin, F.; Shen, X.; Kou, Y. Dual impact of ambient humidity on the virulence of Magnaporthe oryzae and basal resistance in rice. Plant Cell Environ. 2022, 42, 3399–3411. [Google Scholar] [CrossRef]
- Guo, J.-W.; Yang, L.-F.; Liu, Y.-H.; Yang, J.; Wang, H.-F.; Liu, Y.-H.; Li, W.J. First report of pseudostem black spot caused by Pestalotiopsis microspora on tsao-ko in Yunnan, China. Plant Dis. 2016, 100, 1021. [Google Scholar] [CrossRef]
- Xue, L.; Liu, Y.; Zhou, S.; White, J.F.; Li, C. Characterization of Pyrenophora species causing brown leaf spot on Italian ryegrass (Lolium multiflorum) in Southwestern China. Plant Dis. 2020, 104, 1900–1907. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Jiang, N.; Fan, X.L.; Tian, C.M. Identification and characterization of leaf inhabiting fungi from Castanea plantations in China. J. Fungi 2021, 7, 64. [Google Scholar] [CrossRef]
- Doilom, M.; Guo, J.-W.; Phookamsak, R.; Mortimer, P.E.; Karunarathna, S.C.; Dong, W.; Liao, C.-F.; Yan, K.; Pem, D.; Suwannarach, N.; et al. Screening of phosphate-solubilizing fungi from air and soil in Yunnan, China: Four novel species in Aspergillus, Gongronella, Penicillium, and Talaromyces. Front. Microbiol. 2020, 11, 585215. [Google Scholar] [CrossRef]
- Udayanga, D.; Castlebury, L.A.; Rossman, A.Y.; Hyde, K.D. Species limits in Diaporthe: Molecular reassessment of D. citri, D. cytosporella, D. foeniculina and D. rudis. Persoonia 2014, 32, 83–101. [Google Scholar] [CrossRef]

| No. | Pathogens | Period | Location | Authors and References |
|---|---|---|---|---|
| 1 | Colletotrichum gloeosporioides | Before-harvest | Taxila and Wah Cantt, Pakistan | Naz, F. [19] |
| Postharvest | Alacant Province, Spain | Palou, L. [5] | ||
| Postharvest | Nagasaki Prefecture, Japan | Takata, Y. [6] | ||
| 2 | C. godetiae | Before-harvest | Tetela, Morelos State, Mexico | Juárez-Vázquez, S.B. [20] |
| 3 | C. acutatum | Postharvest | Fujian Province, China | Gu, H. [7] |
| Postharvest | Jiangsu Province, China | Cao, S. [8] | ||
| 4 | C. fioriniae | Postharvest | Nagasaki Prefecture, Japan | Takata, Y. [6] |
| Before-harvest | Nagasaki Prefecture, Japan | Poti, T. [22] | ||
| 5 | C. scovillei | Postharvest | Zhejiang Province, China | Wu, J. [9] |
| 6 | C. eriobotryae | Before-harvest | Taiwan Province, China | Damm, U. [21] |
| 7 | C. nymphaeae | Postharvest | Nagasaki Prefecture, Japan | Takata, Y. [6] |
| Before-harvest | Nagasaki Prefecture, Japan | Poti, T. [22] | ||
| Before-harvest | Taiwan Province, China | Damm, U. [21] | ||
| Postharvest | Sichuan Province, China | Wu, W.X. [10] | ||
| 8 | Pestalotiopsis eriobotryfolia | Postharvest | Fujian Province, China | Gu, H. [7] |
| 9 | P. sensu | Postharvest | Alacant Province, Spain | Nozawa, S. [11] |
| 10 | P. guepini | Before-harvest | Buenos Aires, Argentina | Perelló A.E. [23] |
| P. theae | Before-harvest | Anhui Province, China | Chen, Y. [24] | |
| 11 | Neopestalotiopsis clavispora | Postharvest | Alacant Province, Spain | Palou, L. [5] |
| Postharvest | Pakistan | Abbas, M.F. [12] | ||
| Postharvest | Spain | Palou, L. [13] | ||
| 12 | Alternaria alternata | Before-harvest | Thessaloniki, Greece | Tziros, G.T. [25] |
| Before-harvest | Palestine | Batta, Y. [26] | ||
| Postharvest | Islamabad, Pakistan | Bibi, H. [14] | ||
| 13 | A. tenuissima | Postharvest | Fujian Province, China | Gu, H. [7] |
| 14 | Alternaria sp. | Before-harvest | Taiwan Province, China | Ko, Y. [27] |
| 15 | Fusicladium eriobotryae | Before-harvest | Spain | González-Domínguez, E. [28] |
| 16 | Botrytis cinerea | Postharvest | Alacant Province, Spain | Palou, L. [5] |
| 17 | Diplodia seriata | Postharvest | Alacant Province, Spain | Palou, L. [5] |
| Before-harvest | Alicante Province, Spain | Palou, L. [29] | ||
| Before-harvest | Punjab and Khyber Paktoon Khawa, Pakistan | Abbas, M.F. [30] | ||
| 18 | Penicillium expansum | Postharvest | Alacant Province, Spain | Palou, L. [5] |
| 19 | Rhizopus stolonifer | Postharvest | Alacant Province, Spain | Palou, L. [5] |
| Postharvest | Rawalpindi and Swat, Pakistan | Aslam, M.F. [15] | ||
| 20 | Ceratobasidium sp. | Postharvest | Guangdong Province, China | Li, S.N. [16] |
| 21 | Fusarium oxysporum | Before-harvest | Islamabad, Pakistan | Niazi, F. [31] |
| 22 | F. solani | Before-harvest | Punjab Province, Pakistan | Abbas, M.F [32] |
| 23 | Geotrichum candidum | Postharvest | Lahore, Pakistan | Hafeez, R. [17] |
| 24 | Neofusicoccum parvum | Before-harvest | Chongqing, China | Zhai, L. [33] |
| 25 | Diplocarpon mespili | Before and Postharvest | Spain | Gariglio, N. [18] |
| 26 | Monilinia fructicola | Before-harvest | Wuhan, Hubei Province, China | Yin, L. [34] |
| Morphotype Group | Strain Name | Colony Character on PDA | Conidia | Similar Species |
|---|---|---|---|---|
| Group Ⅰ Botryosphaeria dothidea | GJW41-13 GJW42-17 | Circular and initially white colonies gradually turned gray-green, with a dark green color on the back, and featured short and thick aerial hyphae with an irregular colony margin. Colony diameters were 8.0 cm and 9.0 cm after 3 d at 28 °C in the light, respectively. | Hyaline and subcylindrical, aseptate, 17.5–24.1 × 5.7 to 7.2 μm, L/W = 2.7–4.1 (mean 21.0 × 6.6 μm, average L/W = 3.2, n = 30) | Botryosphaeria dothidea [(20-)23-27(-30) × 4-5(-6) (mean 26.2 × 5.4 μm)] |
| Group Ⅱ Trichothecium roseum | GJW43-2 GJW43-10 GJW43-17 | Circular and initially white colonies gradually produced dense, pink, and circular structures (conidiophores and conidia) with a rough colony margin. Colony diameters were 2.7 cm, 2.7 cm, and 2.8 cm after 3 d at 28 °C in the light, respectively. | Produced in clusters, smooth, hyaline, thick-walled, 1-septa, ellipsoid to pyriform, 9.1–16.3 × 4.5–8.8 μm, L/W = 1.4–2.6 (mean 12.8 × 6.3 μm, average L/W = 2.1, n = 30) | Trichothecium roseum (10 to 18 × 7 to 9.5 μm) |
| Group Ⅲ Alternaria alternata | GJW44-5 GJW44-16 GJW46-2 | Initially a white colony and turned olive green to black 7 days post-incubation. Featured pale brown, thick, and cottony aerial hyphae, with a reserve of black surrounded by a light-brown circle. Colony diameters were 6.7 cm, 5.6 cm, and 7.2 cm after 3 d at 28 °C in the light, respectively | Produce chained conidia singly, separated, and pale brown conidiophores. Conidia yellow-brown or black-brown, obclavate, subglobose, ellipsoid, with 1–5 transverse septa and 1–3 longitudinal septa, 13.4–42.9 × 7.7–22.0 µm, L/W = 1.5–4.6 (mean 27.6 × 13.5 µm, average L/W = 2.8, n = 30) | Alternaria alternata [26–30 × 5–9 µm with 4–7 transverse septa and a few or no longisepta] |
| Group Ⅳ Pestalotiopsis kenyana | GJW67-9 GJW67-12 | Smooth-edged, dense, whitish, with sparse aerial mycelium on the surface, produced a yellowish or black oil-drop mass (conidiophores and conidia) and a yellowish reserve with an irregular margin—colony diameter was 3.3 cm after 4 d at 28 °C in the light. | Fusoid, ellipsoid, straight or slightly curved, 4-septate, 20.9–27.6 × 4.0–5.4 μm, L/W = 4.5–6.9 (mean 24.3 × 4.6 μm, average L/W = 5.5, n = 20); three median cells were darker than other cells; apical cell with 2–3 tubular appendages (mainly three) ranging from 6.6 to 16.3 μm, basal cell with one tubular appendage ranging from 4.1 to 7.6 μm. | Pestalotiopsis kenyana [17.5–22 × 6–7 µm (mean 20 × 6.5 µm)] |
| Temperature/℃ | Alternaria alternata GJW44-5 | A. alternata GJW44-16 | A. alternata GJW46-2 | |||
|---|---|---|---|---|---|---|
| Disease Incidence/% | Disease Index | Disease Incidence/% | Disease Index | Disease Incidence/% | Disease Index | |
| 15 | 100 | 32 | 100 | 46 | 100 | 62 |
| 25 | 100 | 42 | 100 | 62 | 100 | 70 |
| 30 | 100 | 68 | 100 | 70 | 100 | 82 |
| Humidity/% | Alternaria alternata GJW44-5 | A. alternata GJW44-16 | A. alternata GJW46-2 | |||
|---|---|---|---|---|---|---|
| Disease Incidence/% | Disease Index | Disease Incidence/% | Disease Index | Disease Incidence/% | Disease Index | |
| 45 | 90 | 32 | 90 | 32 | 90 | 36 |
| 55 | 100 | 34 | 100 | 40 | 90 | 36 |
| 65 | 100 | 40 | 100 | 40 | 100 | 38 |
| 75 | 100 | 44 | 100 | 40 | 100 | 38 |
| 85 | 100 | 44 | 100 | 44 | 100 | 44 |
| 95 | 100 | 44 | 100 | 42 | 90 | 38 |
| Locus | Primer Names and Primer Sequences | PCR Protocols | References |
|---|---|---|---|
| ITS | ITS1: TCC GTA GGT GAA CCT GCG G ITS4: TCC TCC GCT TAT TGA TAT GC | (95 °C 30 s, 55 °C 50 s, 72 °C 1 min) × 39 cycles | White, T.J. [71] Udayanga, D. [75] |
| LSU | LROR: ACC CGC TGA ACT TAA GC LR5: ATC CTG AGG GAA ACT TC | (95 °C 1 min, 55 °C 2 min, 72 °C 90 s) × 35 cycles | Vilgalys, R. [72] Doilom, M. [74] |
| TEF | EF1-728F: CAT CGA GAA GTT CGA GAA GG EF1-986R: TAC TTG AAG GAA CCC TTA CC | (95 °C 30 s, 58 °C 50 s, 72 °C 1 min) × 39 cycles | Vilgalys, R. [72] Udayanga, D. [75] |
| TUB | T1: AAC ATG CGT GAG ATT GTA AGT Bt2b: ACC CTC AGT GTA GTG ACC CTT GGC | (95 °C 30 s, 58 °C 50 s, 72 °C 1 min) × 39 cycles | Jiang, N. [73] Udayanga, D. [75] |
| RPB2 | RPB2-5F2: GGG GWG AYC AGA AGA AGG C fRPB2-7CR: CCC ATR GCT TGY TTR CCC AT | (95 °C 1 min, 52 °C 2 min, 72 °C 90 s) × 35 cycles | Jiang, N. [73] Doilom, M. [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.-W.; Yang, C.-L.; Dong, B.-Z.; Tian, R.-C.; Yang, M.; Li, L.; Gao, P.; Zhou, S.-Y.; Muhammad, M.; Bu, Y.; et al. Characterization and Biological Characteristics of Alternaria, Botryosphaeria, Pestalotiopsis, and Trichothecium Species Associated with Postharvest Loquat Fruit Rot in Yunnan, China. Plants 2025, 14, 3201. https://doi.org/10.3390/plants14203201
Guo J-W, Yang C-L, Dong B-Z, Tian R-C, Yang M, Li L, Gao P, Zhou S-Y, Muhammad M, Bu Y, et al. Characterization and Biological Characteristics of Alternaria, Botryosphaeria, Pestalotiopsis, and Trichothecium Species Associated with Postharvest Loquat Fruit Rot in Yunnan, China. Plants. 2025; 14(20):3201. https://doi.org/10.3390/plants14203201
Chicago/Turabian StyleGuo, Jian-Wei, Chun-Lian Yang, Beng-Zha Dong, Rong-Chuan Tian, Min Yang, Lifang Li, Penghua Gao, Su-Yue Zhou, Murad Muhammad, Yu Bu, and et al. 2025. "Characterization and Biological Characteristics of Alternaria, Botryosphaeria, Pestalotiopsis, and Trichothecium Species Associated with Postharvest Loquat Fruit Rot in Yunnan, China" Plants 14, no. 20: 3201. https://doi.org/10.3390/plants14203201
APA StyleGuo, J.-W., Yang, C.-L., Dong, B.-Z., Tian, R.-C., Yang, M., Li, L., Gao, P., Zhou, S.-Y., Muhammad, M., Bu, Y., Zhang, J., Kong, C.-S., & Yu, L. (2025). Characterization and Biological Characteristics of Alternaria, Botryosphaeria, Pestalotiopsis, and Trichothecium Species Associated with Postharvest Loquat Fruit Rot in Yunnan, China. Plants, 14(20), 3201. https://doi.org/10.3390/plants14203201







