Unraveling the Effects of Climate Change and Human Activity on Potential Habitat Range Shifts in Four Symplocos Species in China
Abstract
1. Introduction
2. Materials and Methods
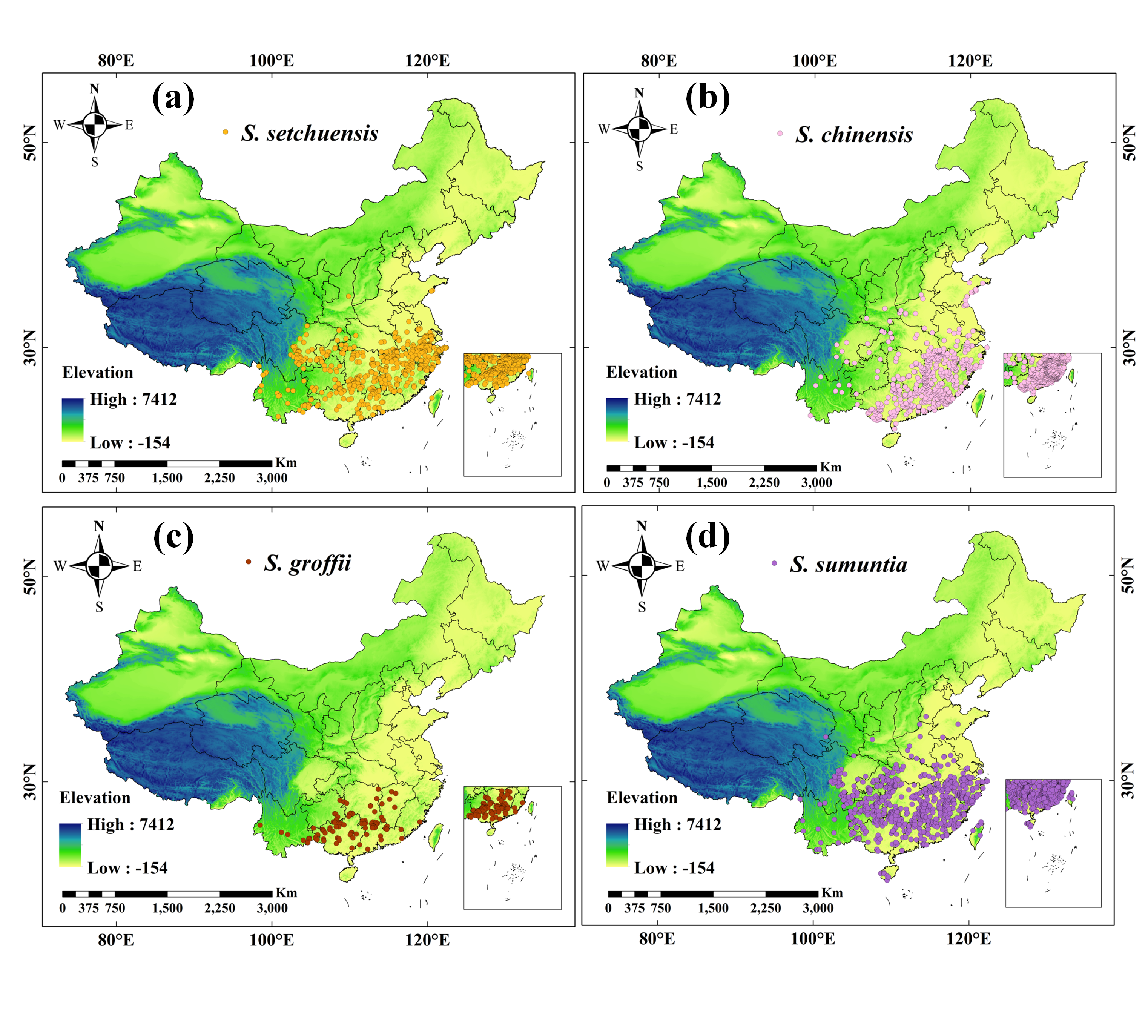
2.1. Species Distribution Data Pre-Processing
2.2. Environmental and Anthropogenic Parameters
2.3. Model Optimization and Evaluation
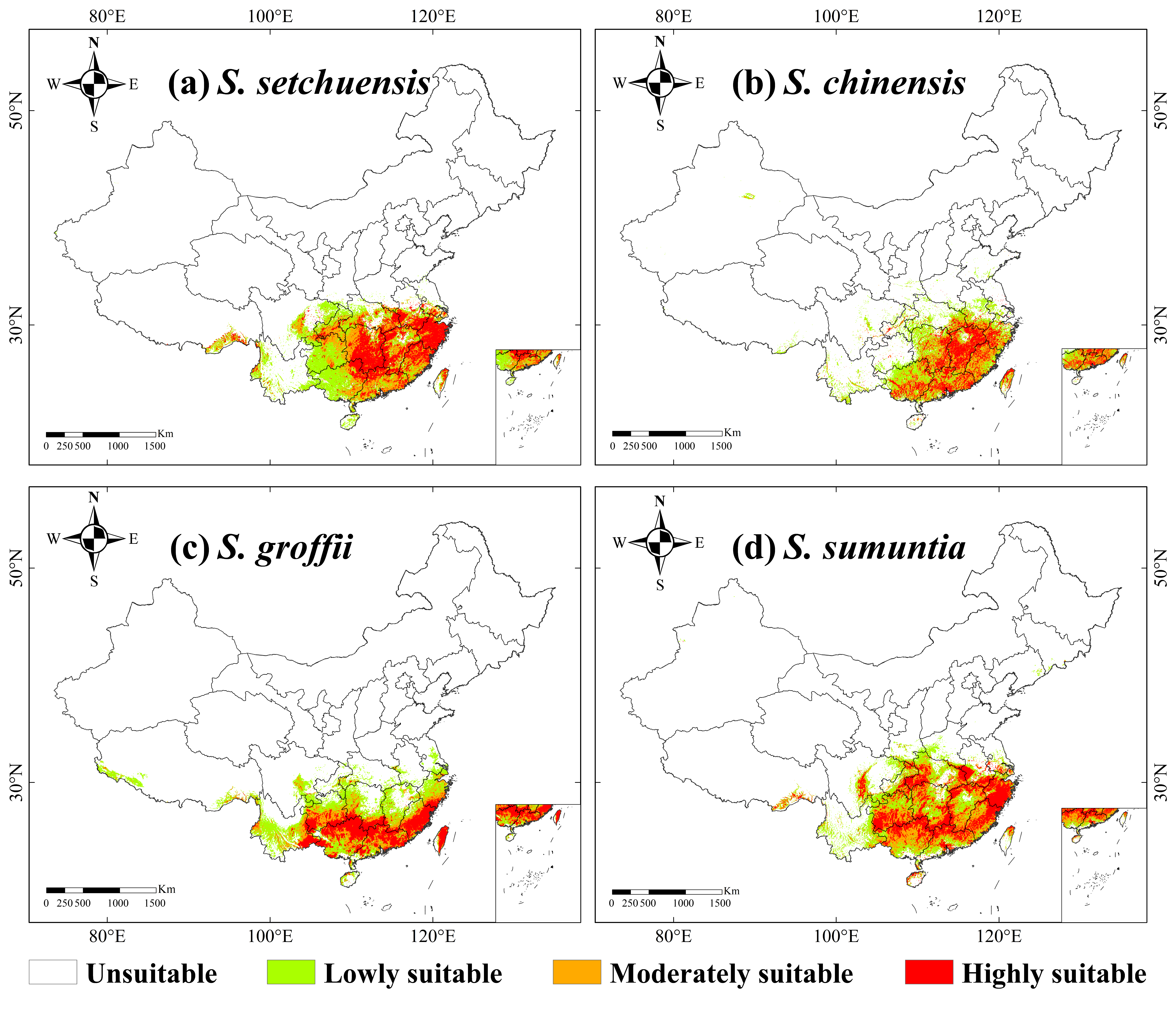
2.4. Classification of Suitable Habitat
3. Results
3.1. The Optimal Model and Its Accuracy
3.2. Current Suitable Habitat Distribution of the Four Symplocos Species
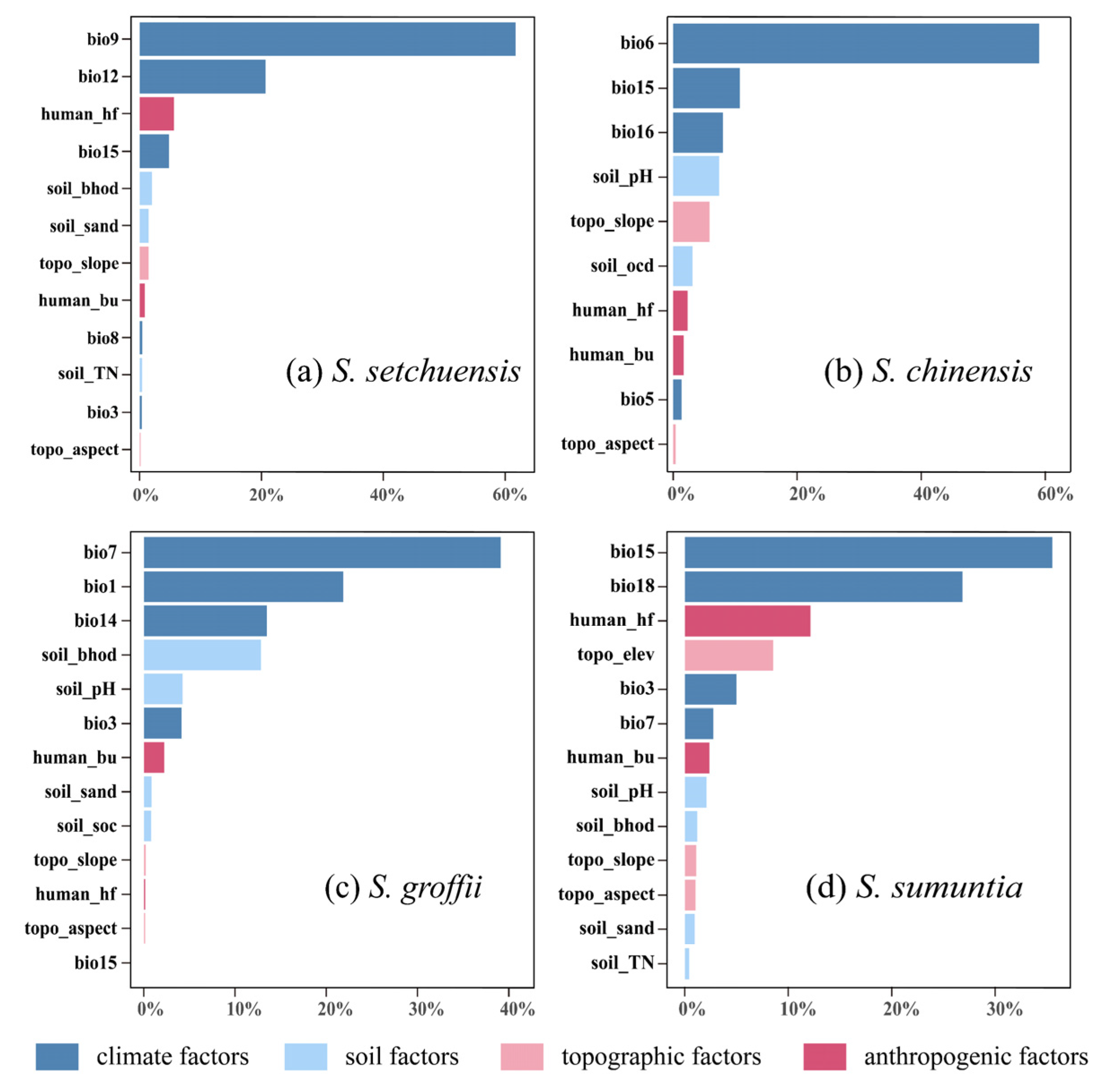
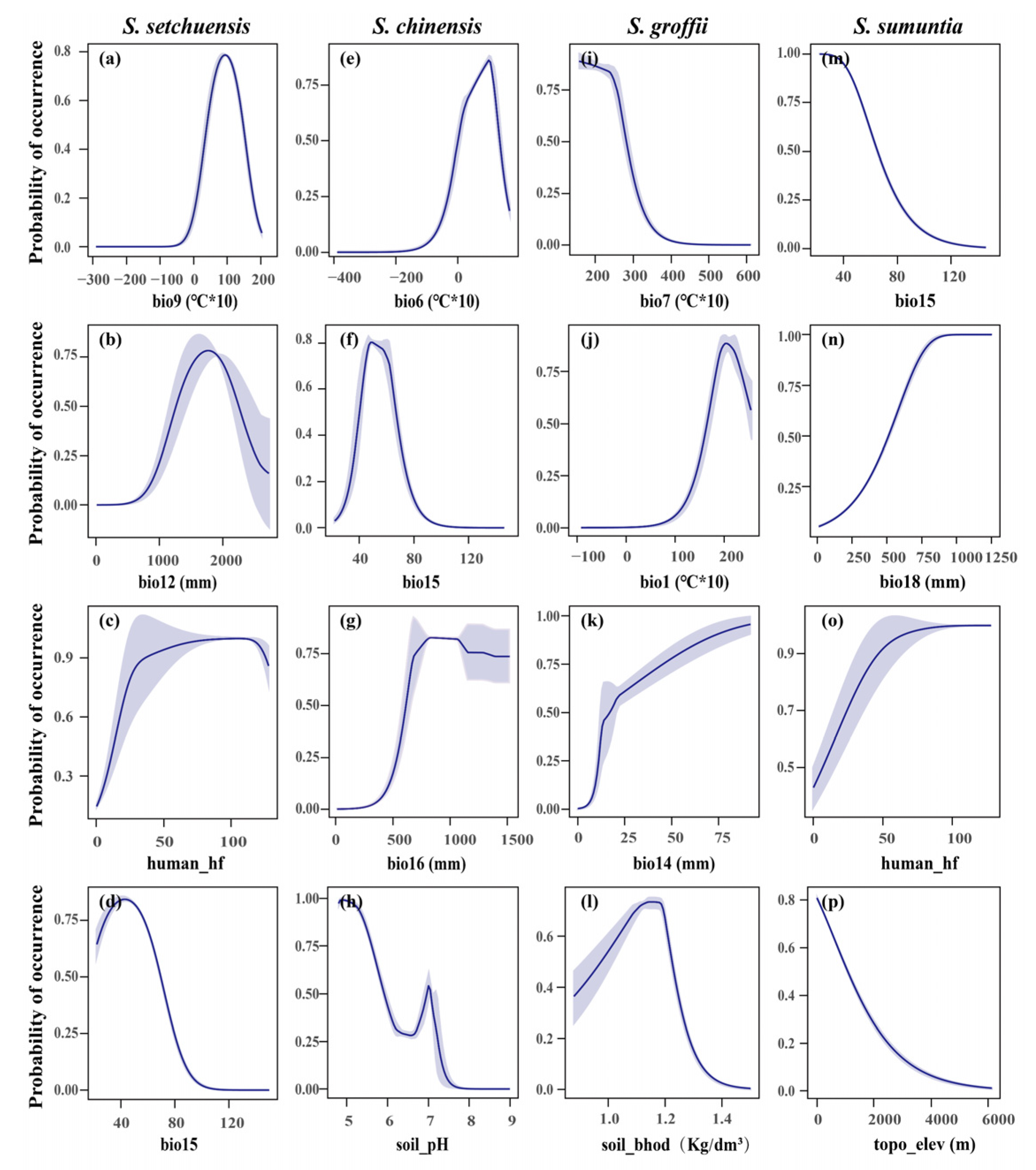
3.3. Important Variables Affecting the Habitat Ranges
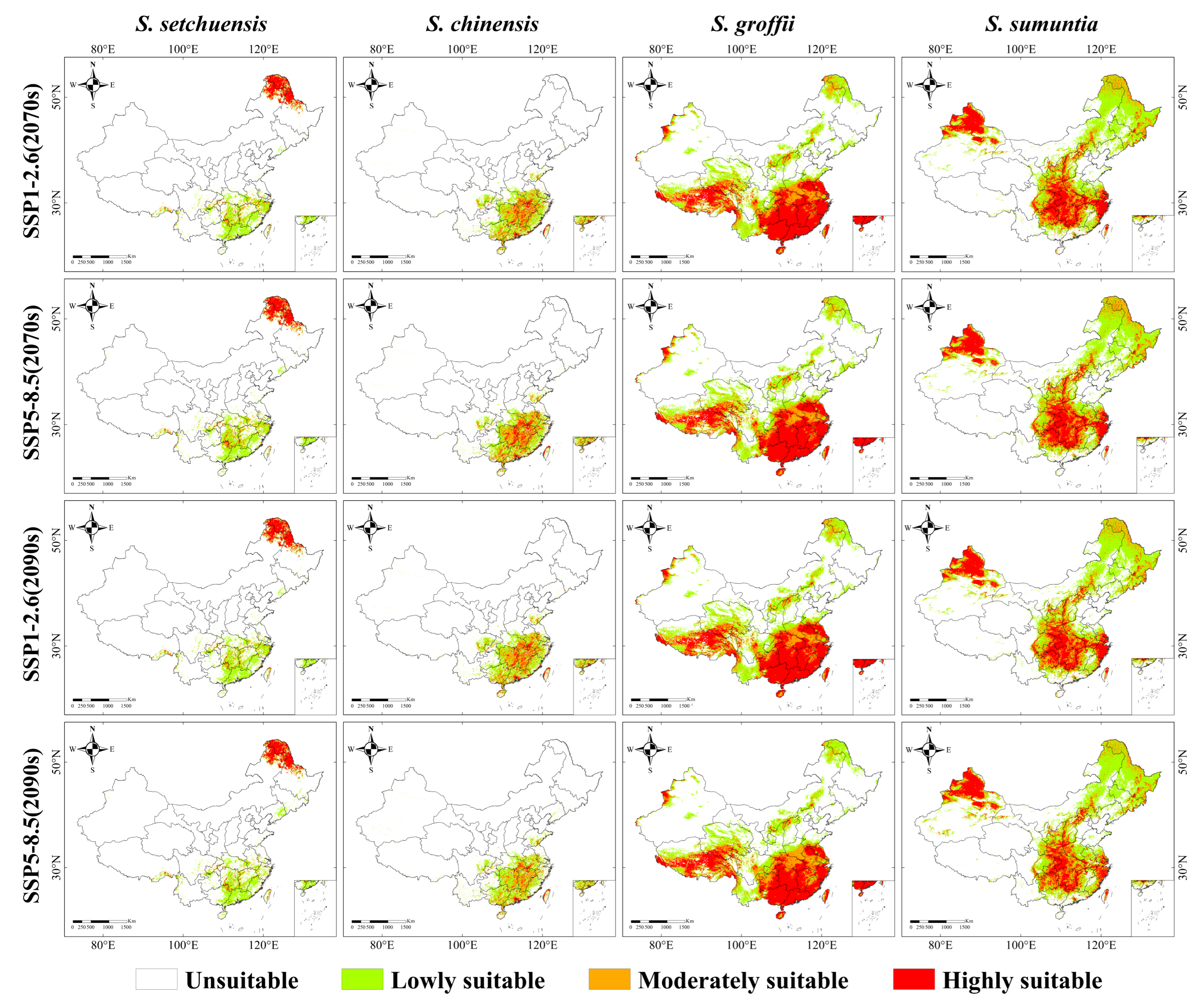
3.4. Potential Suitable Habitat Ranges in the Future
4. Discussion
4.1. Model Predictive Performance
4.2. Habitat Distribution and Key Predictors Under the Current Environment
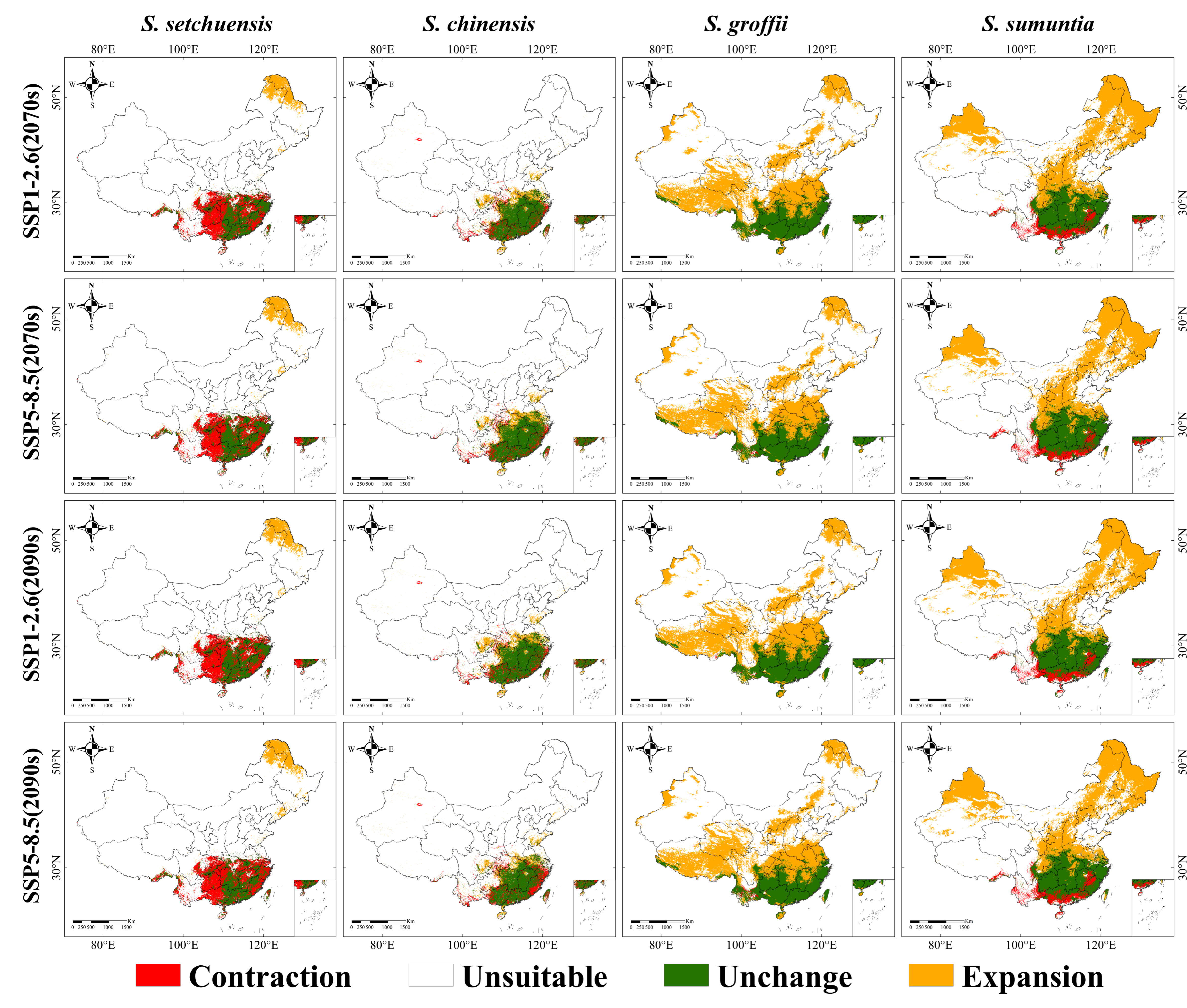
4.3. Changes in the Distribution Habitats of Symplocos Species Under Future Climatic Scenarios
4.4. Limitations and Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Fritsch, P.W.; Shi, S.; Almeda, F.; Kelly, C.L.M. Phylogeny and infrageneric classification of Symplocos (Symplocaceae) inferred from DNA sequence data. Am. J. Bot. 2004, 91, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Badoni, R.; Semwal, D.K.; Kothiyal, S.K.; Rawat, U. Chemical constituents and biological applications of the genus Symplocos. J. Asian Nat. Prod. Res. 2010, 12, 1069–1080. [Google Scholar] [CrossRef]
- Pan, L.-Q.; Yang, T.; Liao, N.-Y. Medicinal value and biological prospect prediction of Symplocos. J. Anhui Agric. Sci. 2018, 46, 8–11. [Google Scholar] [CrossRef]
- Fritsch, P.W.; Kelly, L.M.; Wang, Y.; Almeda, F.; Kriebel, R. Revised infrafamilial classification of Symplocaceae based on phylogenetic data from DNA sequences and morphology. Taxon 2008, 57, 823–852. [Google Scholar] [CrossRef]
- Huong, T.T.; Tram, L.H.; Minh, T.T.; Thong, N.V.; Giang, D.H.; Dang, N.H.; Dat, N.T. Investigation of anti-inflammatory lignans from the leaves of Symplocos sumuntia Buch-Ham ex D Don (Symplocaceae). Trop. J. Pharm. Res. 2017, 16, 2191–2196. [Google Scholar] [CrossRef]
- Ishida, J.; Wang, H.K.; Oyama, M.; Cosentino, M.L.; Hu, C.Q.; Lee, K.H. Anti-AIDS agents. 46. Anti-HIV activity of harman, an anti-HIV principle from Symplocos setchuensis, and its derivatives. J. Nat. Prod. 2001, 64, 958–960. [Google Scholar] [CrossRef]
- Li, X.H.; Shen, D.D.; Li, N.; Yu, S.S. Bioactive triterpenoids from Symplocos chinensis. J. Asian Nat. Prod. Res. 2003, 5, 49–56. [Google Scholar] [CrossRef]
- Lim, J.S.; Bae, J.; Lee, S.; Lee, D.Y.; Yao, L.; Cho, N.; Bach, T.T.; Yun, N.; Park, S.-J.; Cho, Y.-C. In Vitro Anti-Inflammatory Effects of Symplocos sumuntia Buch.-Ham. Ex D. Don Extract via Blockage of the NF-κB/JNK Signaling Pathways in LPS-Activated Microglial Cells. Plants 2022, 11, 3095. [Google Scholar] [CrossRef]
- Wang, K.W.; Yan, S.N. Chemical constituents of Symplocos setchuensis. Chem. Nat. Compd. 2018, 54, 1012–1014. [Google Scholar] [CrossRef]
- Burrows, M.T.; Schoeman, D.S.; Buckley, L.B.; Moore, P.; Poloczanska, E.S.; Brander, K.M.; Brown, C.; Bruno, J.F.; Duarte, C.M.; Halpern, B.S. The pace of shifting climate in marine and terrestrial ecosystems. Science 2011, 334, 652–655. [Google Scholar] [CrossRef]
- Roman-Palacios, C.; Wiens, J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 4211–4217. [Google Scholar] [CrossRef]
- Dawson, T.P.; Jackson, S.T.; House, J.I.; Prentice, I.C.; Mace, G.M. Beyond predictions: Biodiversity conservation in a changing climate. Science 2011, 332, 53–58. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, L.; Nobis, M.; Wu, X.; Pan, K.; Wang, K.; Dakhil, M.A.; Du, M.; Xiong, Q.; Pandey, B.; et al. Climate change jointly with migration ability affect future range shifts of dominant fir species in Southwest China. Divers. Distrib. 2020, 26, 352–367. [Google Scholar] [CrossRef]
- Freeman, B.G.; Lee-Yaw, J.A.; Sunday, J.M.; Hargreaves, A.L. Expanding, shifting and shrinking: The impact of global warming on species’ elevational distributions. Glob. Ecol. Biogeogr. 2018, 27, 1268–1276. [Google Scholar] [CrossRef]
- Xu, W.B.; Svenning, J.C.; Chen, G.K.; Zhang, M.G.; Huang, J.H.; Chen, B.; Ordonez, A.; Ma, K.P. Human activities have opposing effects on distributions of narrow-ranged and widespread plant species in China. Proc. Natl. Acad. Sci. USA 2019, 116, 26674–26681. [Google Scholar] [CrossRef]
- Humphreys, A.M.; Govaerts, R.; Ficinski, S.Z.; Lughadha, E.N.; Vorontsova, M.S. Global dataset shows geography and life form predict modern plant extinction and rediscovery. Nat. Ecol. Evol. 2019, 3, 1043–1047. [Google Scholar] [CrossRef]
- Theobald, D.M.; Kennedy, C.; Chen, B.; Oakleaf, J.; Baruch-Mordo, S.; Kiesecker, J. Earth transformed: Detailed mapping of global human modification from 1990 to 2017. Earth Syst. Sci. Data 2020, 12, 1953–1972. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Borger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, M.; Santini, L. Human pressures predict species’ geographic range size better than biological traits. Global Change Biol. 2015, 21, 2169–2178. [Google Scholar] [CrossRef]
- Laanisto, L.; Sammul, M.; Kull, T.; Macek, P.; Hutchings, M.J. Trait-based analysis of decline in plant species ranges during the 20th century: A regional comparison between the UK and Estonia. Global Change Biol. 2015, 21, 2726–2738. [Google Scholar] [CrossRef] [PubMed]
- Brummitt, N.A.; Bachman, S.P.; Griffiths-Lee, J.; Lutz, M.; Moat, J.F.; Farjon, A.; Donaldson, J.S.; Hilton-Taylor, C.; Meagher, T.R.; Albuquerque, S.; et al. Green plants in the red: A baseline global assessment for the IUCN sampled red list index for plants. PLoS ONE 2015, 10, e0135152. [Google Scholar] [CrossRef]
- Santos, B.A.; Peres, C.A.; Oliveira, M.A.; Grillo, A.; Alves-Costa, C.P.; Tabarelli, M. Drastic erosion in functional attributes of tree assemblages in Atlantic forest fragments of northeastern Brazil. Biol. Conserv. 2008, 141, 249–260. [Google Scholar] [CrossRef]
- Lôbo, D.; Leão, T.; Melo, F.P.; Santos, A.M.; Tabarelli, M. Forest fragmentation drives Atlantic forest of northeastern Brazil to biotic homogenization. Divers. Distrib. 2011, 17, 287–296. [Google Scholar] [CrossRef]
- Laurance, W.F.; Hem, N.; Laurance, S.G.; Andrade, A.C.; Fearnside, P.M.; Jel, R.; Capretz, R.L. Rain forest fragmentation and the proliferation of successional trees. Ecology 2006, 87, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-P.; Hansen, M.C.; Stehman, S.V.; Potapov, P.V.; Tyukavina, A.; Vermote, E.F.; Townshend, J.R. Global land change from 1982 to 2016. Nature 2018, 560, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Chen, L.; Yu, X. Impact of China’s Grain for Green Project on the landscape of vulnerable arid and semi-arid agricultural regions: A case study in northern Shaanxi Province. J. Appl. Ecol. 2009, 46, 536–543. [Google Scholar] [CrossRef]
- Hua, F.; Wang, X.; Zheng, X.; Fisher, B.; Wang, L.; Zhu, J.; Tang, Y.; Yu, D.W.; Wilcove, D.S. Opportunities for biodiversity gains under the world’s largest reforestation programme. Nat. Commun. 2016, 7, 12717. [Google Scholar] [CrossRef]
- Huang, L.; Jin, C.; Pan, Y.; Zhou, L.; Hu, S.; Guo, Y.; Meng, Y.; Song, K.; Pang, M.; Li, H. Human activities and species biological traits drive the long-term persistence of old trees in human-dominated landscapes. Nat. Plants 2023, 9, 898–907. [Google Scholar] [CrossRef]
- Jin, C.; Hu, S.; Zhou, L.; Huang, L.; Pan, Y.; Jim, C.; Song, K.; Minor, J.; Yang, Y. Religious uses shape the selection and distribution of Chinese Buddhist tree species. People Nat. 2025, 7, 2094–2105. [Google Scholar] [CrossRef]
- Zhang, G.; Zhu, A.; Windels, S.K.; Qin, C. Modelling species habitat suitability from presence-only data using kernel density estimation. Ecol. Indic. 2018, 93, 387–396. [Google Scholar] [CrossRef]
- Wang, G.; Wang, C.; Guo, Z.; Dai, L.; Xue, F. Integrating Maxent model and landscape ecology theory for studying spatiotemporal dynamics of habitat: Suggestions for conservation of endangered Red-crowned crane. Ecol. Indic. 2020, 116, 106472. [Google Scholar] [CrossRef]
- Ma, B.; Sun, J. Predicting the distribution of Stipa purpurea across the Tibetan Plateau via the MaxEnt model. BMC Ecol. 2018, 18, 10. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Yi, Y.J.; Zhou, Y.; Cai, Y.P.; Yang, W.; Li, Z.W.; Zhao, X. The influence of climate change on an endangered riparian plant species: The root of riparian Homonoia. Ecol. Indic. 2018, 92, 40–50. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Hutchinson, M.F. Biochim: The first species distribution modelling package, its early applications and relevance to most current MaxEnt studies. Divers. Distrib. 2013, 20, 1–9. [Google Scholar] [CrossRef]
- Carpenter, G.; Gillison, A.N.; Winter, J. Domain—A flexible modeling procedure for mapping potential distributions of plants and animals. Biodivers. Conserv. 1993, 2, 667–680. [Google Scholar] [CrossRef]
- Yee, T.W.; Mitchell, N.D. Generalized additive models in plant ecology. J. Veg. Sci. 1991, 2, 587–602. [Google Scholar] [CrossRef]
- Lehmann, A.; Overton, J.M.; Leathwick, J.R. GRASP: Generalized regression analysis and spatial prediction. Ecol. Model. 2002, 157, 189–207. [Google Scholar] [CrossRef]
- Hirzel, A.; Guisan, A. Which is the optimal sampling strategy for habitat suitability modelling. Ecol. Model. 2002, 157, 331–341. [Google Scholar] [CrossRef]
- Yi, Y.J.; Cheng, X.; Wieprecht, S.; Tang, C.H. Comparison of habitat suitability models using different habitat suitability evaluation methods. Ecol. Eng. 2014, 71, 335–345. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.; De Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef]
- Peterson, A.T. Predicting the geography of species’ invasions via ecological niche modeling. Q. Rev. Biol. 2004, 78, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Beerling, D.J.; Huntley, B.; Bailey, J.P. Climate and the distribution of Fallopia japonica: Use of an introduced species to test the predictive capacity of response surfaces. J. Veg. Sci. 1995, 6, 269–282. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Alsamadisi, A.G.; Tran, L.T.; Papes, M. Employing inferences across scales: Integrating spatial data with different resolutions to enhance Maxent models. Ecol. Model. 2020, 415, 108857. [Google Scholar] [CrossRef]
- Li, J.; Fan, G.; He, Y. Predicting the current and future distribution of three Coptis herbs in China under climate change conditions, using the MaxEnt model and chemical analysis. Sci. Total Environ. 2020, 698, 134141–134148. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wei, H.; Lu, C.; Gao, B.; Gu, W. Predictions of potential geographical distribution and quality of Schisandra sphenanthera under climate change. PeerJ 2016, 4, e2554. [Google Scholar] [CrossRef]
- Chinese Virtual Herbarium (CVH). Available online: http://www.cvh.ac.cn/ (accessed on 25 February 2025).
- Global Biodiversity Information Facility (GBIF). (http://www.gbif.org/, accessed on 25 February 2025). Available online: https://doi.org/10.15468/dl.qx3exa (accessed on 25 February 2025).
- Map Location Website. Available online: https://maplocation.sjfkai.com/ (accessed on 25 February 2025).
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- Bi, D.; Dix, M.; Marsland, S.; O’farrell, S.; Sullivan, A.; Bodman, R.; Law, R.; Harman, I.; Srbinovsky, J.; Rashid, H.A. Configuration and spin-up of ACCESS-CM2, the new generation Australian community climate and earth system simulator coupled model. J. South. Hemisph. Earth Syst. Sci. 2020, 70, 225–251. [Google Scholar] [CrossRef]
- Wu, T.; Lu, Y.; Fang, Y.; Xin, X.; Li, L.; Li, W.; Jie, W.; Zhang, J.; Liu, Y.; Zhang, L. The Beijing climate center climate system model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Lovato, T.; Peano, D.; Butenschön, M.; Materia, S.; Iovino, D.; Scoccimarro, E.; Fogli, P.; Cherchi, A.; Bellucci, A.; Gualdi, S. CMIP6 simulations with the CMCC Earth system model (CMCC-ESM2). J. Adv. Model. Earth Syst. 2022, 14, e2021MS002814. [Google Scholar] [CrossRef]
- Kelley, M.; Schmidt, G.A.; Nazarenko, L.S.; Bauer, S.E.; Ruedy, R.; Russell, G.L.; Ackerman, A.S.; Aleinov, I.; Bauer, M.; Bleck, R. GISS-E2.1: Configurations and climatology. J. Adv. Model. Earth Syst. 2020, 12, e2019MS002025. [Google Scholar] [CrossRef]
- Boucher, O.; Servonnat, J.; Albright, A.L.; Aumont, O.; Balkanski, Y.; Bastrikov, V.; Bekki, S.; Bonnet, R.; Bony, S.; Bopp, L. Presentation and evaluation of the IPSL-CM6A-LR climate model. J. Adv. Model. Earth Syst. 2020, 12, e2019MS002010. [Google Scholar] [CrossRef]
- Tatebe, H.; Ogura, T.; Nitta, T.; Komuro, Y.; Ogochi, K.; Takemura, T.; Sudo, K.; Sekiguchi, M.; Abe, M.; Saito, F. Description and basic evaluation of simulated mean state, internal variability, and climate sensitivity in MIROC6. Geosci. Model Dev. 2019, 12, 2727–2765. [Google Scholar] [CrossRef]
- Müller, W.A.; Jungclaus, J.H.; Mauritsen, T.; Baehr, J.; Bittner, M.; Budich, R.; Bunzel, F.; Esch, M.; Ghosh, R.; Haak, H. A higher-resolution version of the max planck institute earth system model (MPI-ESM1.2-HR). J. Adv. Model. Earth Syst. 2018, 10, 1383–1413. [Google Scholar] [CrossRef]
- Yukimoto, S.; Kawai, H.; Koshiro, T.; Oshima, N.; Yoshida, K.; Urakawa, S.; Tsujino, H.; Deushi, M.; Tanaka, T.; Hosaka, M. The Meteorological Research Institute Earth System Model version 2.0, MRI-ESM2.0: Description and basic evaluation of the physical component. J. Meteorol. Soc. Japan Ser. II 2019, 97, 931–965. [Google Scholar] [CrossRef]
- Riahi, K.; Van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O. The Shared Socioeconomic Pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Global Environ. Change 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Geospatial Data Cloud. Available online: http://www.gscloud.cn (accessed on 25 February 2025).
- Hengl, T.; Mendes de Jesus, J.; Heuvelink, G.B.; Ruiperez Gonzalez, M.; Kilibarda, M.; Blagotić, A.; Shangguan, W.; Wright, M.N.; Geng, X.; Bauer-Marschallinger, B. SoilGrids250m: Global gridded soil information based on machine learning. PLoS ONE 2017, 12, e0169748. [Google Scholar] [CrossRef] [PubMed]
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.R.; Beher, J.; Jones, K.R.; Possingham, H.P.; Laurance, W.F.; Wood, P.; Fekete, B.M. Global terrestrial Human Footprint maps for 1993 and 2009. Sci. Data 2016, 3, 160067. [Google Scholar] [CrossRef]
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Y.; Bi, Y.; Liu, X.; Li, X.; Sun, T.; Chen, H.; Li, J. Identification of potential distribution area for Hippophae rhamnoides subsp. sinensis by the MaxEnt model. Acta Ecol. Sin. 2022, 42, 1420–1428. [Google Scholar] [CrossRef]
- Yan, X.; Wang, S.; Duan, Y.; Han, J.; Huang, D.; Zhou, J. Current and future distribution of the deciduous shrub Hydrangea macrophylla in China estimated by MaxEnt. Ecol. Evol. 2021, 11, 16099–16112. [Google Scholar] [CrossRef]
- Wang, A.; Melton, A.E.; Soltis, D.E.; Soltis, P.S. Potential distributional shifts in North America of allelopathic invasive plant species under climate change models. Plant Divers. 2022, 44, 9. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Arnold, R.J.; Chen, S.; Xie, Y.; He, S.; Liu, X.; Zhang, W. Prediction of the suitable distribution of Eucalyptus grandis in China and its responses to climate change. New For. 2021, 53, 81–99. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Global Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Li, A.; Wang, J.; Wang, R.; Yang, H.; Yang, W.; Yang, C.; Jin, Z.J.E. MaxEnt modeling to predict current and future distributions of Batocera lineolata (Coleoptera: Cerambycidae) under climate change in China. Ecoscience 2020, 27, 23–31. [Google Scholar] [CrossRef]
- Zhang, X.L.; Li, X.F.; Feng, Y.C.; Liu, Z.J. The use of ROC and AUC in the validation of objective image fusion evaluation metrics. Signal Process. 2015, 115, 38–48. [Google Scholar] [CrossRef]
- Shabani, F.; Kumar, L.; Ahmadi, M. Assessing accuracy methods of species distribution models: AUC, specificity, sensitivity and the true skill statistic. Global J. Hum. Soc. Sci. 2018, 18, 6–18. [Google Scholar] [CrossRef]
- Xiao, J.; Eziz, A.; Zhang, H.; Wang, Z.; Tang, Z.; Fang, J. Responses of four dominant dryland plant species to climate change in the Junggar Basin, northwest China. Ecol. Evol. 2019, 9, 13596–13607. [Google Scholar] [CrossRef]
- Yang, X.Q.; Kushwaha, S.P.S.; Saran, S.; Xu, J.C.; Roy, P.S. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda L. in Lesser Himalayan foothills. Ecol. Eng. 2013, 51, 83–87. [Google Scholar] [CrossRef]
- Pagel, J.; Schurr, F.M. Forecasting species ranges by statistical estimation of ecological niches and spatial population dynamics. Global Ecol. Biogeogr. 2012, 21, 293–304. [Google Scholar] [CrossRef]
- Li, W.B.; Teng, Y.; Zhang, M.Y.; Shen, Y.; Liu, J.W.; Qi, J.W.; Wang, X.C.; Wu, R.F.; Li, J.H.; Garber, P.A. Human activity and climate change accelerate the extinction risk to non-human primates in China. Global Change Biol. 2024, 30, e17114. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.B.; Fedrigo, M.; Kasel, S.; Roxburgh, S.H.; Choden, K.; Tenzin, K.; Allen, K.; Nitschke, C.R. Predicting plant species distributions using climate-based model ensembles with corresponding measures of congruence and uncertainty. Divers. Distrib. 2022, 28, 1105–1122. [Google Scholar] [CrossRef]
- Schelhaas, M.J.; Nabuurs, G.J.; Hengeveld, G.; Reyer, C.; Cullmann, D. Adaptive forest management to account for climate change-induced productivity and species suitability changes in Europe. Reg. Envir. Change 2015, 15, 1581–1594. [Google Scholar] [CrossRef]
- Deb, J.C.; Phinn, S.; Butt, N.; McAlpine, C.A. Climatic-induced shifts in the distribution of teak (Tectona grandis) in tropical Asia: Implications for forest management and planning. Environ. Manage. 2017, 60, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Sillero, N.; Arenas-Castro, S.; Enriquez-Urzelai, U.; Vale, C.G.; Sousa-Guedes, D.; Martínez-Freiría, F.; Real, R.; Barbosa, A.M. Want to model a species niche? A step-by-step guideline on correlative ecological niche modelling. Ecol. Model. 2021, 456, 109671. [Google Scholar] [CrossRef]
- Brun, P.; Thuiller, W.; Chauvier, Y.; Pellissier, L.; Wüest, R.O.; Wang, Z.; Zimmermann, N.E. Model complexity affects species distribution projections under climate change. J. Biogeogr. 2020, 47, 130–142. [Google Scholar] [CrossRef]
- Li, X.Y.; Xu, D.P.; Jin, Y.W.; Zhuo, Z.H.; Yang, H.J.; Hu, J.M.; Wang, R.L. Predicting the current and future distributions of Brontispa longissima (Coleoptera: Chrysomelidae) under climate change in China. Glob. Ecol. Conserv. 2021, 25, e01444. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P.; McPherson, J. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Jin, Z.N.; Yu, W.T.; Zhao, H.X.; Xian, X.Q.; Jing, K.T.; Yang, N.W.; Lu, X.M.; Liu, W.X. Potential global distribution of invasive alien species, Anthonomus grandis Boheman, under current and future climate using optimal MaxEnt Model. Agric.-Basel 2022, 12, 1759. [Google Scholar] [CrossRef]
- Yang, X.; Yan, H.; Li, B.; Han, Y.; Song, B. Spatial distribution patterns of Symplocos congeners in a subtropical evergreen broad-leaf forest of southern China. J. For. Res. 2018, 29, 773–784. [Google Scholar] [CrossRef]
- Ouyang, W.; Qiu, H.; Chen, Z.; Wu, Y.; Li, J. Changes in the potential habitat distribution of typical fire-resistant forest species under climate change in the subtropical regions of china. Forests 2023, 14, 1897. [Google Scholar] [CrossRef]
- Bai, Y.; Li, J.; Lei, Q.; Long, C.; Liu, B. Potential ornamental plants in Symplocaceae from China. Acta Hortic. 2017, 1167, 23–30. [Google Scholar] [CrossRef]
- Yue, Y.; Zhang, P.; Shang, Y. The potential global distribution and dynamics of wheat under multiple climate change scenarios. Sci. Total Environ. 2019, 688, 1308–1318. [Google Scholar] [CrossRef]
- Wang, D.; Shi, C.; Alamgir, K.; Kwon, S.M.; Pan, L.; Zhu, Y.; Yang, X. Global assessment of the distribution and conservation status of a key medicinal plant (Artemisia annua L.): The roles of climate and anthropogenic activities. Sci. Total Environ. 2022, 821, 153378. [Google Scholar] [CrossRef]
- Naudiyal, N.; Wang, J.; Ning, W.; Gaire, N.P.; Peili, S.; Yanqiang, W.; Jiali, H.; Ning, S. Potential distribution of Abies, Picea, and Juniperus species in the sub-alpine forest of Minjiang headwater region under current and future climate scenarios and its implications on ecosystem services supply. Ecol. Indic. 2021, 121, 107131. [Google Scholar] [CrossRef]
- Zelazowski, P. Changes in the potential distribution of humid tropical forests on a warmer planet. Philos. Trans. R. Soc. A 2011, 369, 137–160. [Google Scholar] [CrossRef]
- Wanga, V.O.; Kngarega, B.; Oulo, M.A.; Mkala, E.M.; Ngumbau, V.M.; Onjalalaina, G.E.; Odago, W.O.; Nanjala, C.; Ochieng, C.O.; Gichua, M.K. Projected impacts of climate change on the habitat of Xerophyta species in Africa. Plant Divers. 2024, 46, 91–100. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Don, L.S.; Yong, C.H.; Chan, P.Y.; Jae, H.D.; Kyong, L.J.; Seong, J.C. The rice RING finger E3 ligase, OsHCI1, drives nuclear export of multiple substrate proteins and its heterogeneous overexpression enhances acquired thermotolerance. J. Exp. Bot. 2013, 64, 2899–2914. [Google Scholar] [CrossRef]
- Zu, K.; Wang, Z.; Zhu, X.; Lenoir, J.; Zhou, J. Upward shift and elevational range contractions of subtropical mountain plants in response to climate change. Sci. Total Environ. 2021, 783, 146896. [Google Scholar] [CrossRef]
- Schmitt, C.B.; Senbeta, F.; Woldemariam, T.; Rudner, M.; Denich, M. Importance of regional climates for plant species distribution patterns in moist Afromontane forest. J. Veg. Sci. 2013, 24, 553–568. [Google Scholar] [CrossRef]
- Zhang, K.L.; Zhang, Y.; Zhou, C.; Meng, J.S.; Sun, J.; Zhou, T.H.; Tao, J. Impact of climate factors on future distributions of Paeonia ostii across China estimated by MaxEnt. Ecol. Inform. 2019, 50, 62–67. [Google Scholar] [CrossRef]
- Petrie, M.D.; Wildeman, A.M.; Bradford, J.B.; Hubbard, R.M.; Lauenroth, W.K. A review of precipitation and temperature control on seedling emergence and establishment for ponderosa and lodgepole pine forest regeneration. For. Ecol. Manag. 2016, 361, 328–338. [Google Scholar] [CrossRef]
- Liu, D.T.; Chen, J.Y.; Sun, W.B. Distributional responses to climate change of two maple species in southern China. Ecol. Evol. 2023, 13, e10490. [Google Scholar] [CrossRef]
- Hereford, J.; Schmitt, J.; Ackerly, D.D.; Satake, A. The seasonal climate niche predicts phenology and distribution of an ephemeral annual plant, Mollugo verticillata. J. Ecol. 2017, 105, 1323–1334. [Google Scholar] [CrossRef]
- Zhang, J.T.; Dong, Y. Factors affecting species diversity of plant communities and the restoration process in the loess area of China. Ecol. Eng. 2010, 36, 345–350. [Google Scholar] [CrossRef]
- Moeslund, J.E.; Arge, L.; Bøcher, P.K.; Dalgaard, T.; Svenning, J.C. Topography as a driver of local terrestrial vascular plant diversity patterns. Nord. J. Bot. 2013, 31, 129–144. [Google Scholar] [CrossRef]
- Rodríguez-Ramírez, E.C.; Martínez-Mejía, M.d.R.; Ghimire, B.; Luna-Vega, I. Response of leaf morphological traits of relict-endemic Symplocos species (S. coccinea and S. speciosa) to elevation and abiotic fluctuations. Plant Ecol. 2021, 222, 693–704. [Google Scholar] [CrossRef]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Kirkegaard, J.A. The effect of compaction on the growth of pigeonpea on clay soils. I. Mechanisms of crop response and seasonal effects on a vertisol in a sub-humid environment. Soil Tillage Res. 1990, 24, 129–147. [Google Scholar] [CrossRef]
- Håkansson, I.; Voorhees, W.B.; Riley, H. Vehicle and wheel factors influencing soil compaction and crop response in different traffic regimes. Soil Tillage Res. 1988, 11, 239–282. [Google Scholar] [CrossRef]
- Wright, S.J. Seasonal drought, soil fertility and the species density of tropical forest plant communities. Trends Ecol. Evol. 1992, 7, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Wedin, D.; Tilman, D. Competition among grasses along a nitrogen gradient—Initial conditions and mechanisms of competition. Ecol. Monogr. 1993, 63, 199–229. [Google Scholar] [CrossRef]
- Khuroo, A.A.; Hamid, M.; Malik, A.H. Elevation and aspect determine the differences in soil properties and plant species diversity on Himalayan mountain summits. Ecol. Res. 2021, 36, 340–352. [Google Scholar] [CrossRef]
- Di Marco, M.; Santini, L. Human pressures predict species’ geographic range size better than biological traits. Global Change Biol. 2015, 21, 2169–2178. [Google Scholar] [CrossRef]
- Zwiener, V.; Padial, A.; Marques, M.; Faleiro, F.; Loyola, R.; Peterson, A.T. Planning for conservation and restoration under climate and land use change in the Brazilian Atlantic Forest. Divers. Distrib. 2017, 23, 955–966. [Google Scholar] [CrossRef]
- Naves, R.P.; Grotan, V.; Prado, P.I.; Vidal, E.; Ferreira Batista, J.L. Tropical forest management altered abundances of individual tree species but not diversity. For. Ecol. Manag. 2020, 475, 118399. [Google Scholar] [CrossRef]
- Luo, W.; Sun, C.; Yang, S.; Chen, W.; Sun, Y.; Li, Z.; Liu, J.; Tao, W.; Tao, J. Contrasting range changes and drivers of four forest foundation species under future climate change in China. Sci. Total Environ. 2024, 942, 173784. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Contu, S.; Hill, S.L.L.; Beck, J.; Liu, Y.; Meyer, C.; Phillips, H.R.P.; Scharlemann, J.P.W.; Purvis, A. Widespread winners and narrow-ranged losers: Land use homogenizes biodiversity in local assemblages worldwide. PLoS Biol. 2018, 16, e2006841. [Google Scholar] [CrossRef]
- Köster, N.; Kreft, H.; Nieder, J.; Barthlott, W.; Jetz, W. Range size and climatic niche correlate with the vulnerability of epiphytes to human land use in the tropics. J. Biogeogr. 2013, 40, 963–976. [Google Scholar] [CrossRef]
- Wan, J.Z.; Wang, C.J. Contribution of environmental factors toward distribution of ten most dangerous weed species globally. Appl. Ecol. Environ. Res. 2019, 17, 14835–14846. [Google Scholar] [CrossRef]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Song, H.; Zhang, X.; Wang, X.; Wang, Y.; Li, S.; Xu, Y. Not the expected poleward migration: Impact of climate change scenarios on the distribution of two endemic evergreen broad-leaved Quercus species in China. Sci. Total Environ. 2023, 889, 164273. [Google Scholar] [CrossRef]
- Cheuk, M.L.; Fischer, G.A. The impact of climate change on the distribution of Castanopsis (Fagaceae) species in South China and Indo-China region. Glob. Ecol. Conserv. 2020, 26, e01388. [Google Scholar] [CrossRef]
- Thomas, C.D. Climate, climate change and range boundaries. Divers. Distrib. 2010, 16, 488–495. [Google Scholar] [CrossRef]
- Daws, M.I.; Bolton, S.; Burslem, D.F.R.P.; Garwood, N.C.; Mullins, C.E. Loss of desiccation tolerance during germination in neo-tropical pioneer seeds: Implications for seed mortality and germination characteristics. Seed Sci. Res. 2007, 17, 273–281. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate change threats to plant diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef]
- Sun, J.J.; Jiao, W.X.; Wang, Q.; Wang, T.L.; Yang, H.Q.; Jin, J.X.; Feng, H.L.; Guo, J.H.; Feng, L.; Xu, X.; et al. Potential habitat and productivity loss of Populus deltoides industrial forest plantations due to global warming. For. Ecol. Manag. 2021, 496, 119474. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Paz, S.; Frelich, L.E.; Jagodzinski, A.M. How much does climate change threaten European forest tree species distributions? Global Change Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Brecka, A.F.J.; Shahi, C.; Chen, H.Y.H. Climate change impacts on boreal forest timber supply. Forest Policy Econ. 2018, 92, 11–21. [Google Scholar] [CrossRef]
- Jiguet, F.; Julliard, R.; Thomas, C.D.; Dehorter, O.; Newson, S.E.; Couvet, D. Thermal range predicts bird population resilience to extreme high temperatures. Ecol. Lett. 2006, 9, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Calosi, P.; Bilton, D.T.; Spicer, J.I. Thermal tolerance, acclimatory capacity and vulnerability to global climate change. Biol. Lett. 2008, 4, 99–102. [Google Scholar] [CrossRef]
- Araujo, M.B.; Rahbek, C. How does climate change affect biodiversity? Science 2006, 313, 1396–1397. [Google Scholar] [CrossRef]
- Jiang, C.; Tan, K.; Ren, M.-X. Effects of monsoon on distribution patterns of tropical plants in Asia. Chin. J. Plant Ecol. 2017, 41, 1103–1112. [Google Scholar]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Change 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Lenoir, J.; Gegout, J.C.; Marquet, P.A.; de Ruffray, P.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef]
- Fei, S.; Desprez, J.M.; Potter, K.M.; Jo, I.; Knott, J.A.; Oswalt, C.M. Divergence of species responses to climate change. Sci. Adv. Mater. 2017, 3, e1603055. [Google Scholar] [CrossRef] [PubMed]
- Pintor, A.F.; Schwarzkopf, L.; Krockenberger, A.K. Rapoport’s Rule: Do climatic variability gradients shape range extent? Ecol. Monogr. 2015, 85, 643–659. [Google Scholar] [CrossRef]
- Gaston, K.J. Geographic range limits: Achieving synthesis. Proc. R. Soc. B 2009, 276, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Wisz, M.S.; Pottier, J.; Kissling, W.D.; Pellissier, L.; Lenoir, J.; Damgaard, C.F.; Dormann, C.F.; Forchhammer, M.C.; Grytnes, J.A.; Guisan, A.; et al. The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling. Biol. Rev. Camb. Philos. Soc. 2013, 88, 15–30. [Google Scholar] [CrossRef]
- Grytnes, J.A.; Kapfer, J.; Jurasinski, G.; Birks, H.H.; Henriksen, H.; Klanderud, K.; Odland, A.; Ohlson, M.; Wipf, S.; Birks, H.J.B. Identifying the driving factors behind observed elevational range shifts on European mountains. Global Ecol. Biogeogr. 2014, 23, 876–884. [Google Scholar] [CrossRef]
- Ribes, A.; Qasmi, S.; Gillett, N.P. Making climate projections conditional on historical observations. Sci. Adv. 2021, 7, eabc0671. [Google Scholar] [CrossRef]
- Tang, J.; Cheng, Y.; Luo, L.; Zhang, L.; Jiang, X. Maxent-based prediction of overwintering areas of Loxostege sticticalis in China under different climate change scenarios. Acta Ecol. Sin. 2017, 37, 4852–4863. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants FACE the future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef] [PubMed]
- Gamage, D.; Thompson, M.; Sutherland, M.; Hirotsu, N.; Makino, A.; Seneweera, S. New insights into the cellular mechanisms of plant growth at elevated atmospheric carbon dioxide. Plant Cell Environ. 2017, 41, 1233–1246. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Elith, J.; Graham, C.H.; Lehmann, A.; Leathwick, J.; Ferrier, S. Sample selection bias and presence-only distribution models: Implications for background and pseudo-absence data. Ecol. Appl. 2009, 19, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papes, M.; Soberón, J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol. Model. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef] [PubMed]
| Species | FC | RM | AUC | TSS | Kappa | OR10 |
|---|---|---|---|---|---|---|
| S. setchuensis | LH | 3.00 | 0.94 | 0.80 | 0.58 | 0.13 |
| S. chinensis | LH | 3.00 | 0.94 | 0.78 | 0.58 | 0.12 |
| S. groffii | LQPH | 3.00 | 0.95 | 0.86 | 0.45 | 0.19 |
| S. sumuntia | LH | 3.00 | 0.93 | 0.78 | 0.62 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Sun, Y.; Chen, W.; Sun, C.; Tao, W.; Tao, J.; Luo, W.; Liu, J. Unraveling the Effects of Climate Change and Human Activity on Potential Habitat Range Shifts in Four Symplocos Species in China. Plants 2025, 14, 3200. https://doi.org/10.3390/plants14203200
Li Z, Sun Y, Chen W, Sun C, Tao W, Tao J, Luo W, Liu J. Unraveling the Effects of Climate Change and Human Activity on Potential Habitat Range Shifts in Four Symplocos Species in China. Plants. 2025; 14(20):3200. https://doi.org/10.3390/plants14203200
Chicago/Turabian StyleLi, Zongfeng, Yuhong Sun, Wenke Chen, Chengxiang Sun, Wenjing Tao, Jianping Tao, Weixue Luo, and Jinchun Liu. 2025. "Unraveling the Effects of Climate Change and Human Activity on Potential Habitat Range Shifts in Four Symplocos Species in China" Plants 14, no. 20: 3200. https://doi.org/10.3390/plants14203200
APA StyleLi, Z., Sun, Y., Chen, W., Sun, C., Tao, W., Tao, J., Luo, W., & Liu, J. (2025). Unraveling the Effects of Climate Change and Human Activity on Potential Habitat Range Shifts in Four Symplocos Species in China. Plants, 14(20), 3200. https://doi.org/10.3390/plants14203200





