Antibiotic and Copper Sensitivity in Erwinia amylovora Isolates from Northern Saudi Arabia, and the Induction of Fire Blight Suppression by Salicylic Acid
Abstract
1. Introduction
2. Results
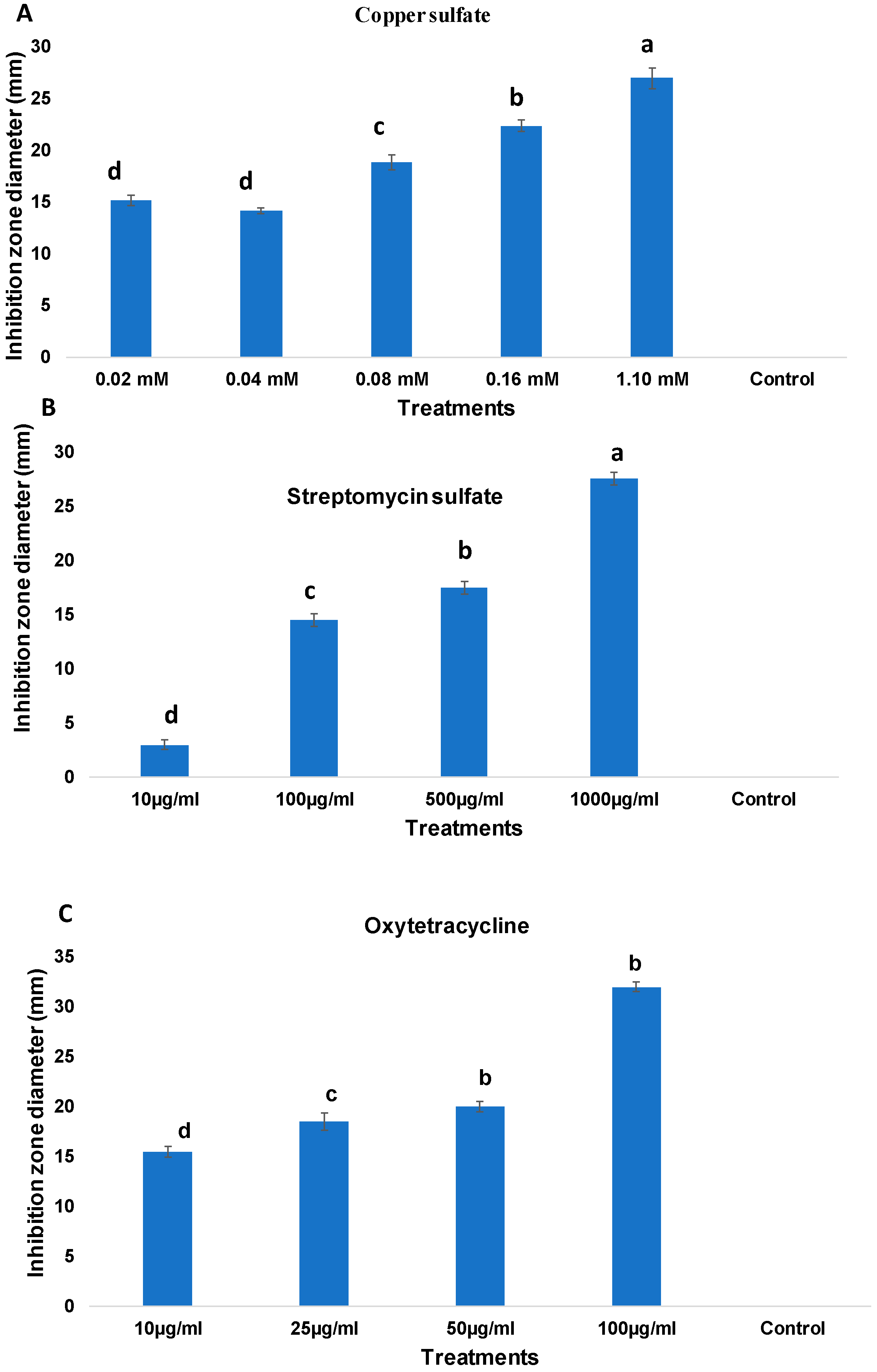
2.1. Antimicrobial Sensitivity of Erwinia amylovora Isolates from Northern Saudi Arabia
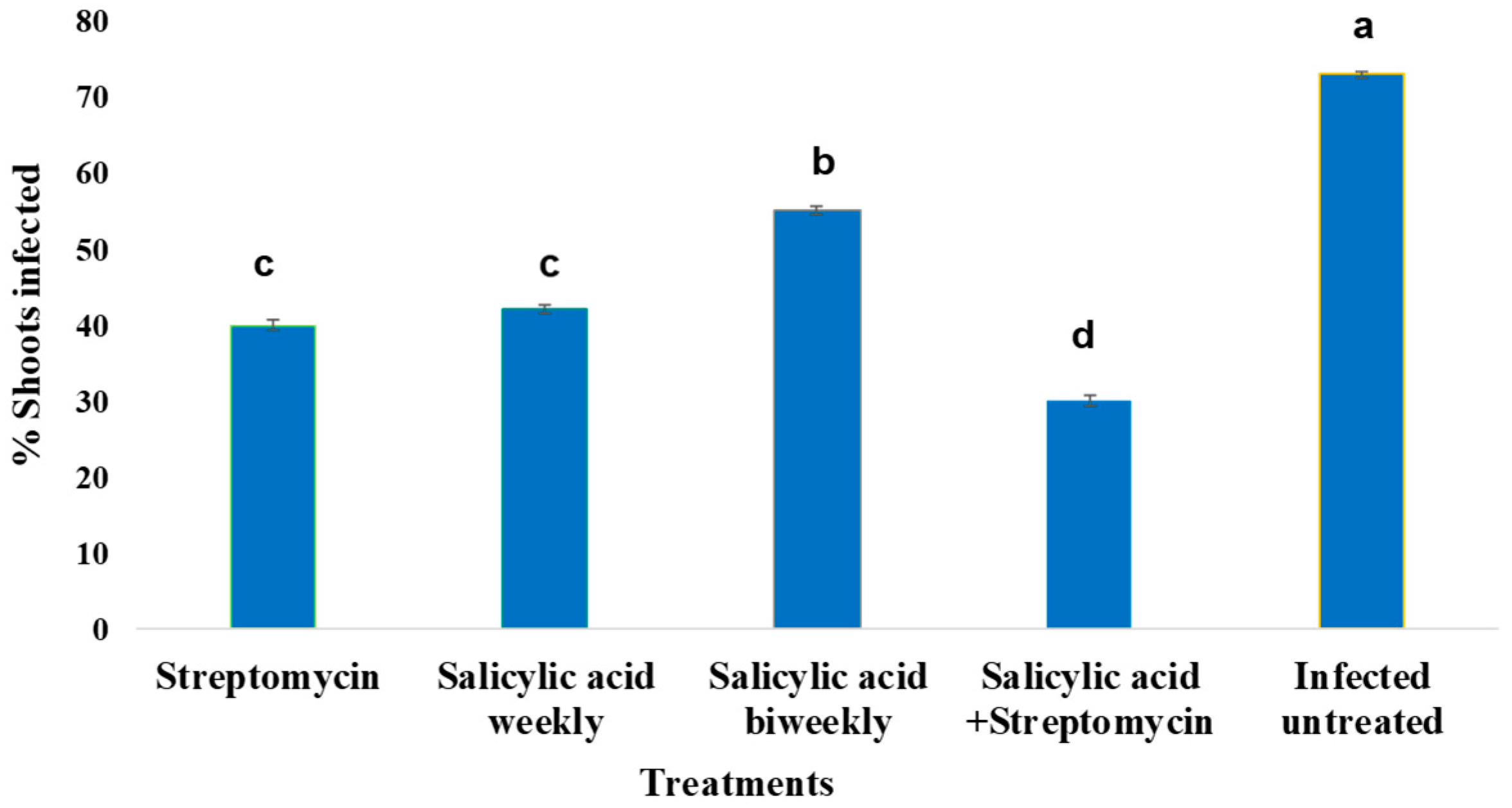
2.2. Effect of Treatments on Shoot Infection by Erwinia amylovora
2.3. Effect of Treatments on Canker Development
2.4. Salicylic Acid Induces Defense-Related Enzyme Activities in Apple
2.4.1. Peroxidase Activity
2.4.2. Polyphenol Oxidase Activity
2.4.3. Hydrogen Peroxide Accumulation
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates Collection and Identification
4.2. Antibiotic Sensitivity Testing
4.3. Copper Sensitivity Testing
4.4. Greenhouse Evaluation of SA and Streptomycin
4.4.1. Apple Seedlings
4.4.2. Inoculum Preparation
4.4.3. Greenhouse Experiments
4.5. Defense Enzymes and Hydrogen Peroxide Assays
4.5.1. Peroxidase (POX) Assay
4.5.2. Polyphenol Oxidase (PPO) Assay
4.5.3. Hydrogen Peroxide (H2O2) Assay
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Burrill, T.J. New species of Micrococcus. Am. Nat. 1883, 17, 319–320. [Google Scholar]
- Winslow, C.E.; Broadhurst, J.; Buchanan, R.E.; Krumwiede, C., Jr.; Rogers, L.A.; Smith, G.H. The families and genera of the bacteria final report of the committee of the society of American bacteriologists on characterization and classification of bacterial types. J. Bacteriol. 1920, 5, 191–229. [Google Scholar] [CrossRef]
- Sundin, G.W.; Wang, N. Antibiotic resistance in plant-pathogenic bacteria. Annu. Rev. Phytopathol. 2018, 56, 161–180. [Google Scholar] [CrossRef] [PubMed]
- EPPO. PM 7/20 (3) Erwinia amylovora. EPPO Bull. 2022, 52, 198–224. [Google Scholar] [CrossRef]
- Beer, S.V. Fire blight. In Compendium of Apple and Pear Diseases; Jones, A.L., Aldwinckle, H.S., Eds.; American Phyto-Pathological Society: St. Paul, MN, USA, 1997; pp. 61–63. [Google Scholar]
- Cooley, D.R.; Autio, W.R.; Clements, J.M.; Cowgill, W.P.; Spitko, R. Annual Fire Blight Management Programs for Apples. University of Massachusetts Extension. 2008. Available online: http://www.umass.edu/fruitadvisor/factsheets/F-133.pdf (accessed on 8 July 2025).
- Ibrahim, Y.E.; Rafique, A.M.; Al-Masrahi, A.A.; Al-Saleh, M.A. Characterization of Erwinia amylovora isolates from the northern region of Saudi Arabia, including CRISPR genotyping. Plant Pathol. 2024, 73, 2193–2210. [Google Scholar] [CrossRef]
- Norelli, J.L.; Holleran, H.T.; Johnson, W.C.; Robinson, T.L.; Aldwinckle, H.S. Resistance of Geneva and other apple rootstocks to Erwinia amylovora. Plant Dis. 2003, 87, 26–32. [Google Scholar] [CrossRef]
- Van der Zwet, T.; Orolaza-Halbrendt, N.; Zeller, W. Fire Blight: History, Biology, and Management; APS Press: St. Paul, MN, USA, 2012. [Google Scholar]
- EFSA Panel on Plant Health (PLH). Scientific Opinion on the pest categorisation of Erwinia amylovora (Burr.). EFSA J. 2014, 12, 3922. [Google Scholar] [CrossRef]
- Russo, N.L.; Burr, T.J.; Breth, D.I.; Aldwinckle, H.S. Isolation of streptomycin-resistant isolates of Erwinia amylovora in New York. Plant Dis. 2008, 92, 714–718. [Google Scholar] [CrossRef]
- Sundin, G.W.; Werner, N.A.; Yoder, K.S.; Aldwinckle, H.S. Field evaluation of biological control of fire blight in the eastern United States. Plant Dis. 2009, 93, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Moller, W.J.; Beutel, J.A.; Reil, W.O.; Zoller, B.G. Fire blight resistance to streptomycin in California. In Phytopathology; AMER Phytopathological Society: St. Paul, MN, USA, 1972; Volume 62, p. 779. [Google Scholar]
- Coyier, D.L.; Covey, R.P. Tolerance of Erwinia amylovora to streptomycin sulfate in Oregon and Washington. Plant Dis. Rep. 1975, 59, 849–852. [Google Scholar]
- El-Goorani, M.A.; El-Kasheir, H.M.A.; Shoeib, A.A.; Hassanein, F.M. Distribution of streptomycin resistant strains of Erwinia amylovora in Egypt during 1988. J. Phytopathol. 1989, 127, 69–74. [Google Scholar] [CrossRef]
- Saad, A.T.; Hanna, L.; Choueiri, E. Evaluation of streptomycin and oxytetracycline resistance of Erwinia amylovora populations in Lebanon. Phytopathology 2000, 90, S68. [Google Scholar]
- McGhee, G.C.; Guasco, J.; Bellomo, L.M.; Blumer-Schuette, S.E.; Shane, W.W.; Irish-Brown, A.; Sundin, G.W. Genetic analysis of streptomycin-resistant (SmR) strains of Erwinia amylovora suggests that dissemination of two genotypes is responsible for the current distribution of SmR E. amylovora in Michigan. Phytopathology 2011, 101, 182–191. [Google Scholar] [CrossRef]
- de León Door, A.P.; Romo Chacón, A.; Acosta Muñiz, C. Detection of streptomycin resistance in Erwinia amylovora strains isolated from apple orchards in Chihuahua, Mexico. Eur. J. Plant Pathol. 2013, 137, 223–229. [Google Scholar] [CrossRef]
- Thomson, S.V.; Gouk, S.C.; Vanneste, J.L.; Hale, C.N.; Clark, R.G. The presence of streptomycin resistant strains of Erwinia amylovora in New Zealand. In Proceedings of the VI International Workshop on Fire Blight, Athens, Greece, 20–23 October 1992; ISHS: Wageningen, The Netherlands, 1993; pp. 223–230. [Google Scholar]
- Stockwell, V.O.; Temple, T.N.; Johnson, K.B.; Loper, J.E. Integrated control of fire blight with antagonists and oxytetracycline. In Proceedings of the XI International Workshop on Fire Blight, Portland, OR, USA, 12–17 August 2007; pp. 383–390. [Google Scholar]
- Yoder, K.S.; Cochran, A.E.; Royston, W.S., Jr.; Kilmer, S.W.; Kowalski, A.L. Suppression of fire blight blossom blight on Idared apple. Plant Dis. Manag. Rep. 2017, 11, PF023. [Google Scholar]
- Jamar, L.; Lateur, M. Strategies to reduce copper use in organic apple production. In Proceedings of the I International Symposium on Organic Apple and Pear, Wolfville, NS, Canada, 28 February 2006; pp. 113–120. [Google Scholar]
- Johnson, K.B. Fire blight of apple and pear. Plant Health Instr. 2000, 1, 1. [Google Scholar] [CrossRef]
- Jones, A.L.; Schnabel, E.L. The development of streptomycin-resistant strains of Erwinia amylovora. In Fire Blight: The Disease and Its Causative Agent, Erwinia amylovora; Vanneste, J.L., Ed.; CAB International: London, UK, 2000; pp. 235–252. [Google Scholar]
- Johnson, K.B.; Temple, T.N.; Kc, A.; Elkins, R.B. Refinement of nonantibiotic spray programs for fire blight control in organic pome fruit. Plant Dis. 2022, 106, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Dagher, F.; Olishevska, S.; Philion, V.; Zheng, J.; Déziel, E. Development of a novel biological control agent targeting the phytopathogen Erwinia amylovora. Heliyon 2020, 6, e05222. [Google Scholar] [CrossRef]
- Zurn, J.D.; Norelli, J.L.; Montanari, S.; Bell, R.; Bassil, N. V Dissecting genetic resistance to fire blight in three pear populations. Phytopathology 2020, 110, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Q.; Dong, X. Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef] [PubMed]
- Kessmann, H.; Staub, T.; Ligon, J.I.M.; Oostendorp, M.; Ryals, J. Activation of systemic acquired disease resistance in plants. Eur. J. Plant Pathol. 1994, 100, 359–369. [Google Scholar] [CrossRef]
- Kuc, J.; Richmond, S. Aspects of the protection of cucumber against Colletotrichum lagenarium. Phytopathology 1977, 67, 533–536. [Google Scholar] [CrossRef]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef]
- van Loon, L.C.; Geraats, B.P.J.; Linthorst, H.J.M. Ethylene as a modulator of disease resistance in plants. Trends Plant Sci. 2006, 11, 184–191. [Google Scholar] [CrossRef]
- Aćimović, S.G.; Zeng, Q.; McGhee, G.C.; Sundin, G.W.; Wise, J.C. Control of fire blight (Erwinia amylovora) on apple trees with trunk-injected plant resistance inducers and antibiotics and assessment of induction of pathogenesis-related protein genes. Front. Plant Sci. 2015, 6, 16. [Google Scholar]
- Yuan, W.; Ruan, S.; Qi, G.; Wang, R.; Zhao, X. Plant growth-promoting and antibacterial activities of cultivable bacteria alive in tobacco field against Ralstonia solanacearum. Environ. Microbiol. 2022, 24, 1411–1429. [Google Scholar] [CrossRef]
- McManus, P.S.; Stockwell, V.O.; Sundin, G.W.; Jones, A.L. Antibiotic use in plant agriculture. Annu. Rev. Phytopathol. 2002, 40, 443–465. [Google Scholar] [CrossRef]
- Billing, E.; Baker, L.A.E.; Crosse, J.E.; Garrett, C.M.E. Characteristics of English isolates of Erwinia amylovora (Burrill) Winslow et al. J. Appl. Microbiol. 1961, 24, 195–211. [Google Scholar] [CrossRef]
- Burr, T.J.; Norelli, J.L.; Katz, B.; Wilcox, W.F.; Hoying, S.A. Streptomycin resistance of Pseudomonas syringae pv. papulans in apple orchards and its association with a conjugative plasmid. Phytopathology 1988, 78, 410–413. [Google Scholar] [CrossRef]
- Loper, J.E. Evaluation of streptomycin, oxytetracycline, and copper resistance of Erwinia amylovora isolated from pear orchards in Washington State. Plant Dis. 1991, 75, 287–290. [Google Scholar] [CrossRef]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar] [PubMed]
- Norelli, J.L.; Gilpatrick, J.D. Techniques for screening chemicals for fire blight control. Plant Dis. 1982, 66, 1162–1165. [Google Scholar] [CrossRef]
- Stefanovits-Bányai, É.; Sárdi, É.; Lakatos, S.; Zayan, M.; Velich, I. Drought stress, peroxidase activity and formaldehyde metabolism in bean plants. Acta Biol. Hung. 1998, 49, 309–316. [Google Scholar] [CrossRef]
- Ramanathan, A.; Vidhyasekaran, P.; Samiyappan, R. Two pathogenesis-related peroxidases in greengram (Vigna radiata (L.) wilczek) leaves and cultured cells induced by Macrophomina phaseolina (Tassi) Goid. and its elicitor. Microbiol. Res. 2001, 156, 139–144. [Google Scholar] [CrossRef]
- Kocaqaliskan, I.; Demir, Y.; Kabar, K. A study on polyphenol oxidase activity during seed germination. Phyton 1995, 35, 37. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Chiou, C.-S.; Jones, A.L. The analysis of plasmid-mediated streptomycin resistance in Erwinia amylovora. Phytopathology 1991, 81, 710–714. [Google Scholar] [CrossRef]
- McGhee, G.C.; Sundin, G.W. Evaluation of kasugamycin for fire blight management, effect on nontarget bacteria, and assessment of kasugamycin resistance potential in Erwinia amylovora. Phytopathology 2011, 101, 192–204. [Google Scholar] [CrossRef]
- Ordax, M.; Marco-Noales, E.; López, M.M.; Biosca, E.G. Exopolysaccharides favor the survival of Erwinia amylovora under copper stress through different strategies. Res. Microbiol. 2010, 161, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Águila-Clares, B.; Castiblanco, L.F.; Quesada, J.M.; Penyalver, R.; Carbonell, J.; López, M.M.; Marco-Noales, E.; Sundin, G.W. Transcriptional response of Erwinia amylovora to copper shock: In vivo role of the copA gene. Mol. Plant Pathol. 2018, 19, 169–179. [Google Scholar] [CrossRef]
- Lagonenko, L.; Lagonenko, A.; Evtushenkov, A. Impact of salicylic acid on biofilm formation by plant pathogenic bacteria. J. Biol. Earth Sci. 2013, 3, 176–181. [Google Scholar]
- Lu, H.; Lema, A.S.; Planas-Marquès, M.; Alonso-Díaz, A.; Valls, M.; Coll, N.S. Type III secretion–dependent and–independent phenotypes caused by Ralstonia solanacearum in Arabidopsis roots. Mol. Plant-Microbe Interact. 2018, 31, 175–184. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Mou, Z. Salicylic acid and its function in plant immunity F. J. Integr. Plant Biol. 2011, 53, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Klessig, D.F.; Durner, J.; Noad, R.; Navarre, D.A.; Wendehenne, D.; Kumar, D.; Zhou, J.M.; Shah, J.; Zhang, S.; Kachroo, P. Nitric oxide and salicylic acid signaling in plant defense. Proc. Natl. Acad. Sci. USA 2000, 97, 8849–8855. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, V.O.; Duffy, B. Use of antibiotics in plant agriculture. Rev. Sci. Tech. Int. Des Epizoot. 2012, 31, 199–210. [Google Scholar] [CrossRef]
- Atanasova, I.; Moncheva, P.; Kabadjova, P.; Tishkov, S.; Stefanova, K.; Dimitrov, Z.; Bogatzevska, N. Phenotypic diversity of Erwinia amylovora in Bulgaria. Z. Fur Naturforschung Sect. C-A J. Biosci. 2007, 62, 857–868. [Google Scholar] [CrossRef]



| Isolates | Streptomycin (µg/mL) | Oxytetracycline (µg/mL) | Copper Sulfate (mM) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.0 | 10 | 100 | 500 | 1000 | 10 | 25 | 50 | 100 | 0.00 | 0.02 | 0.04 | 0.08 | 0.16 | 1.10 | |
| Ea01_Tabuk | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea02_Tabuk | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea03_Tabuk | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea04_Tabuk | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea05_Tabuk | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea06_Tabuk | - | + | (+) | (+) | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea07_Tabuk | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea08_Tabuk | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea09_Tabuk | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea10_Al-Jouf | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea11_Al-Jouf | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea12_Al-Jouf | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea13_Al-Jouf | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea14_Al-Jouf | - | + | - | - | - | - | (+) | (+) | - | - | + | + | + | (+) | - |
| Ea15_Al-Jouf | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea16_Al-Jouf | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea17_Hail | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea18_Hail | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea19_Hail | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea20_Hail | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea21_Hail | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea22_Hail | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea23_Al-Jouf | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea24_Al-Jouf | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea25_Al-Jouf | - | + | - | - | - | - | - | - | - | - | + | + | + | (+) | - |
| Ea26_Al-Jouf | - | + | (+) | (+) | - | - | - | - | - | - | + | + | + | (+) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Masrahi, A.A.; Rafique, A.M.; Al Hashel, A.F.; Al Saleh, M.A.; Ibrahim, Y.E. Antibiotic and Copper Sensitivity in Erwinia amylovora Isolates from Northern Saudi Arabia, and the Induction of Fire Blight Suppression by Salicylic Acid. Plants 2025, 14, 3192. https://doi.org/10.3390/plants14203192
Al Masrahi AA, Rafique AM, Al Hashel AF, Al Saleh MA, Ibrahim YE. Antibiotic and Copper Sensitivity in Erwinia amylovora Isolates from Northern Saudi Arabia, and the Induction of Fire Blight Suppression by Salicylic Acid. Plants. 2025; 14(20):3192. https://doi.org/10.3390/plants14203192
Chicago/Turabian StyleAl Masrahi, Ali A., Abdurrehman M. Rafique, Abdullah F. Al Hashel, Mohammed A. Al Saleh, and Yasser E. Ibrahim. 2025. "Antibiotic and Copper Sensitivity in Erwinia amylovora Isolates from Northern Saudi Arabia, and the Induction of Fire Blight Suppression by Salicylic Acid" Plants 14, no. 20: 3192. https://doi.org/10.3390/plants14203192
APA StyleAl Masrahi, A. A., Rafique, A. M., Al Hashel, A. F., Al Saleh, M. A., & Ibrahim, Y. E. (2025). Antibiotic and Copper Sensitivity in Erwinia amylovora Isolates from Northern Saudi Arabia, and the Induction of Fire Blight Suppression by Salicylic Acid. Plants, 14(20), 3192. https://doi.org/10.3390/plants14203192






