Effect of Pollen Storage Duration on Stainability, Fruit Set, and Physical Traits in Date Palm (Phoenix dactylifera L.) Cultivar ‘Mejhoul’
Abstract
1. Introduction
2. Results and Discussion
2.1. Pollen Stainability Results
2.2. Fruit Set Percentages
2.3. Fruit and Seed Characteristics
3. Materials and Methods
3.1. Experimental Area Characterization
3.2. Phenotypic Characterization of Male Trees and Pollen Extraction
3.3. Treatments and Experimental Design
3.4. Pollination
3.5. Pollen Stainability Assessment Method
3.6. Fruit Set Percentage
3.7. Measurement of Physical Properties
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MJL | Mejhoul |
| KDY | Khadrawy |
| DGN | Deglet Nour |
| ZHI | Zahidi |
References
- Zaid, A.; Arias-Jimenez, E.J. Date Palm Cultivation; Food and Agricultural Organization of the United Nations: Rome, Italy, 2002. [Google Scholar]
- Anushma, P.L.; Vincent, L.; Rajesekharan, P.E.; Ganeshan, S. Pollen storage studies in date palm (Phoenix dactylifera L.). Int. J. Chem. Stud. 2018, 6, 2640–2642. [Google Scholar]
- Salomón-Torres, R.; Krueger, R.; García-Vázquez, J.P.; Villa-Angulo, R.; Villa-Angulo, C.; Ortiz-Uribe, N.; Sol-Uribe, J.A.; Samaniego-Sandoval, L. Date palm pollen: Features, production, extraction and pollination methods. Agronomy 2021, 11, 504. [Google Scholar] [CrossRef]
- Mesnoua, M.; Roumani, M.; Salem, A. The effect of pollen storage temperatures on pollen viability, fruit set and fruit quality of six date palm cultivars. Sci. Hortic. 2018, 236, 279–283. [Google Scholar] [CrossRef]
- Shivanna, K.R.; Johri, B.M. The Angiosperm Pollen: Structure and Function, 1st ed.; Wiley Eastern Limited: California, CA, USA, 1985. [Google Scholar]
- Maryam; Jaskani, M.J.; Naqvi, S.A. Storage and Viability Assessment of Date Palm Pollen. In Date Palm Biotechnology Protocols Volume II: Germplasm Conservation and Molecular Breeding; Springer: New York, NY, USA, 2017; pp. 3–13. [Google Scholar]
- Araújo de Oliveira, A.C.; da Silva Lédo, A.; Polek, M.; Krueger, R.; Shepherd, A.; Volk, G.M. Optimization of in vitro germination and cryopreservation conditions for preserving date palm pollen in the USDA National Plant Germplasm System. Plant Cell Tissue Organ Cult. 2021, 144, 223–232. [Google Scholar] [CrossRef]
- Karim, K.; Awad, M.A.; Manar, A.; Monia, J.; Karim, A.; Mohammed, E. Effect of flowering stage and storage conditions on pollen quality of six male date palm genotypes. Saudi J. Biol. Sci. 2022, 29, 2564–2572. [Google Scholar] [CrossRef]
- Al-Najm, A.; Brauer, S.; Trethowan, R.; Merchant, A.; Ahmad, N. Optimization of in vitro pollen germination and viability testing of some Australian selections of date palm (Phoenix dactylifera L.) and their xenic and metaxenic effects on the tissue culture–derived female cultivar “Barhee. ” Vitr. Cell. Dev. Biol. Plant 2021, 57, 771–785. [Google Scholar] [CrossRef]
- Shafique, M.; Khan, A.S.; Malik, A.U.; Shahid, M.; Rajwana, I.A.; Saleem, B.A.; Amin, M. Ahmad I Influence of pollen source and pollination frequency on fruit drop, yield and quality of date palm (Phoenix dactylifera L.) cv. Dhakki. Pak. J. Bot. 2011, 43, 831–839. [Google Scholar]
- Elsabagh, A.S.; Farag, K.M.; Elashry, H.A. Fruit Characteristics of “Zaghloul” Date Palm in Relation to Metaxenic Influences of Used Pollinator. J. Agric. Environ. Sci. 2012, 12, 842–855. [Google Scholar]
- Nixon, R.W. Metaxenia in Dates. Proc. Am. Soc. Hort. Sci. 1956, 32, 221–226. [Google Scholar]
- Nixon, R.W. The direcy effect of pollen on the fruit on the date palm. J. Agric. Res. 1928, 36, 97–128. [Google Scholar]
- Babůrek, I.; Kiesselbach, T.A. The Structure and Reproduction of Corn. 50th Anniversary Edition. Biol. Plant. 2001, 44, 238. [Google Scholar] [CrossRef]
- Stout, A.B. Seedlessness in Grapes; New York, NY, USA, 1936. Available online: https://www.mavo.biz/Reprints/Stout1936.pdf (accessed on 21 August 2025).
- Olfati, J.A.; Sheykhtaher, Z.; Qamgosar, R.; Khasmakhi-Sabet, A.; Peyvast, G.; Samizadeh, H.; Rabiee, B. Xenia and Metaxenia on Cucumber Fruit and Seed Characteristics. Int. J. Veg. Sci. 2010, 16, 243–252. [Google Scholar] [CrossRef]
- Salomon-Torres, R.; Ortiz-Uribe, N.; Villa-Angulo, R.; Villa-Angulo, C.; Norzagaray-Plasencia, S.; García-Verdugo, C.D. Effect of Pollenizers on Production and Fruit Characteristics of Date Palm (Phoenix dactylifera L.) Cultivar Medjool in Mexico. Turk. J. Agric. For. 2017, 41, 338–347. [Google Scholar] [CrossRef]
- Salomón-Torres, R.; Ortiz-Uribe, N.; Sol-Uribe, J.A.; Villa-Angulo, C.; Villa-Angulo, R.; Valdez-Salas, B.; García-González, C.; Iñiguez Monroy, C.G.; Norzagaray-Plasencia, S. Influence of different sources of pollen on the chemical composition of date (Phoenix dactylifera L.) cultivar Medjool in México. Aust. J. Crop Sci. 2018, 5, 30–40. [Google Scholar] [CrossRef]
- Kadri, K.; Elsafy, M.; Makhlouf, S.; Awad, M.A. Effect of pollination time, the hour of daytime, pollen storage temperature and duration on pollen viability, germinability, and fruit set of date palm (Phoenix dactylifera L.) cv “Deglet Nour”. Saudi J. Biol. Sci. 2022, 29, 1085–1091. [Google Scholar] [CrossRef]
- Alyafei, M.A.S.; Al Dakheel, A.; Almoosa, M.; Ahmed, Z.F.R. Innovative and Effective Spray Method for Artificial Pollination of Date Palm Using Drone. HortScience 2022, 57, 1298–1305. [Google Scholar] [CrossRef]
- Sharma, K.M.; Baidiayavadra, D.A.; Muralidharan, C.M.; Panchal, C.N.; Verma, P. Effect of Storage Temperature and Containers on Date Palm (Phoenix dactylifera L.) Pollen Viability and Post-storage Pollination. Sugar Tech 2024, 26, 1516–1521. [Google Scholar] [CrossRef]
- Ordoñez, B. Brochure: Pollen Viability Assessment; International Potato Center (CIP): Lima, Peru, 2014. [Google Scholar]
- Dafni, A.; Firmage, D. Pollen viability and longevity: Practical, ecological and evolutionary implications. Plant Syst. Evol. 2000, 222, 113–132. [Google Scholar] [CrossRef]
- Salomón-Torres, R.; Ortiz-Uribe, N.; Krueger, R.; García-Vázquez, J.P.; Cohen, Y.; Wright, G.C.; Samaniego-Sandoval, L. Evaluation of Pollen Production of Common Male Date Palms Grown in the Mexicali Valley, Mexico. Agriculture 2022, 12, 1248. [Google Scholar] [CrossRef]
- Swingle, W.T. Metaxenia in the Date Palm: Possibly a hormone action by the embryo or endosperm. J. Hered. 1928, 19, 257–268. [Google Scholar] [CrossRef]
- Shaheen, M.A. Evaluation of date palm males using pollen viability and ultrastructure. Acta Hortic. 2004, 632, 37–43. [Google Scholar] [CrossRef]
- Jafar Jaskani, M.; Fatima, B.; Salman Haider, M.; Abbas Naqvi, S.; Nafees, M.; Ahmad, R.; Ahmad Khan, I. Evaluation of Pollen Viability in Date Palm Cultivars Under Different Storage Temperatures. Pak. J. Bot. 2015, 47, 377–381. [Google Scholar]
- Nixon, R.W.; Carpenter, J.B. Growing Dates in the United States. In Agriculture Information Bulletin Number 207; United States. In Agriculture Information Bulletin Number 207; United States Department of Agriculture: Washington, DC, USA, 1978. [Google Scholar]
- Bedjaoui, H.; Benbouza, H. Assessment of phenotypic diversity of local Algerian date palm (Phoenix dactylifera L.) cultivars. J. Saudi Soc. Agric. Sci. 2020, 19, 65–75. [Google Scholar] [CrossRef]
- Khalilia, W.M.; Abuamsha, R.; Alqaddi, N.; Omari, A. Phenotypic Characterization of Local Date Palm Cultivars at Jericho in Palestinian Jordan Valley District. Indian J. Sci. Technol. 2022, 15, 989–1000. [Google Scholar] [CrossRef]
- Elsafy, M.; Garkava-Gustavsson, L.; Mujaju, C. Phenotypic Diversity of Date Palm Cultivars (Phoenix dactylifera L.) from Sudan Estimated by Vegetative and Fruit Characteristics. Int. J. Biodivers. 2015, 2015, 610391. [Google Scholar] [CrossRef]
- Peña-Yam, L.P.; Muñoz-Ramírez, L.S.; Avilés-Viñas, S.A.; Canto-Flick, A.; Guzmán-Antonio, A.; Santana-Buzzy, N. Floral Biology Studies in Habanero pepper (Capsicum chinense Jacq.) to Implement in a Cross-Breeding Program. Agriculture 2019, 9, 249. [Google Scholar] [CrossRef]
- Mir, B.A.; Koul, S.; Soodan, A.S. Reproductive biology of Withania ashwagandha sp. novo (Solanaceae). Ind. Crops Prod. 2013, 45, 442–446. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics; McGraw Hill Book Co., Inc.: New York, NY, USA, 1980. [Google Scholar]
- Kabacoff, R.I. R in Action. In Data Analysis and Graphics with R, 2nd ed.; Manning Publications: Shelter Island, NY, USA, 2011. [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2019. [Google Scholar]

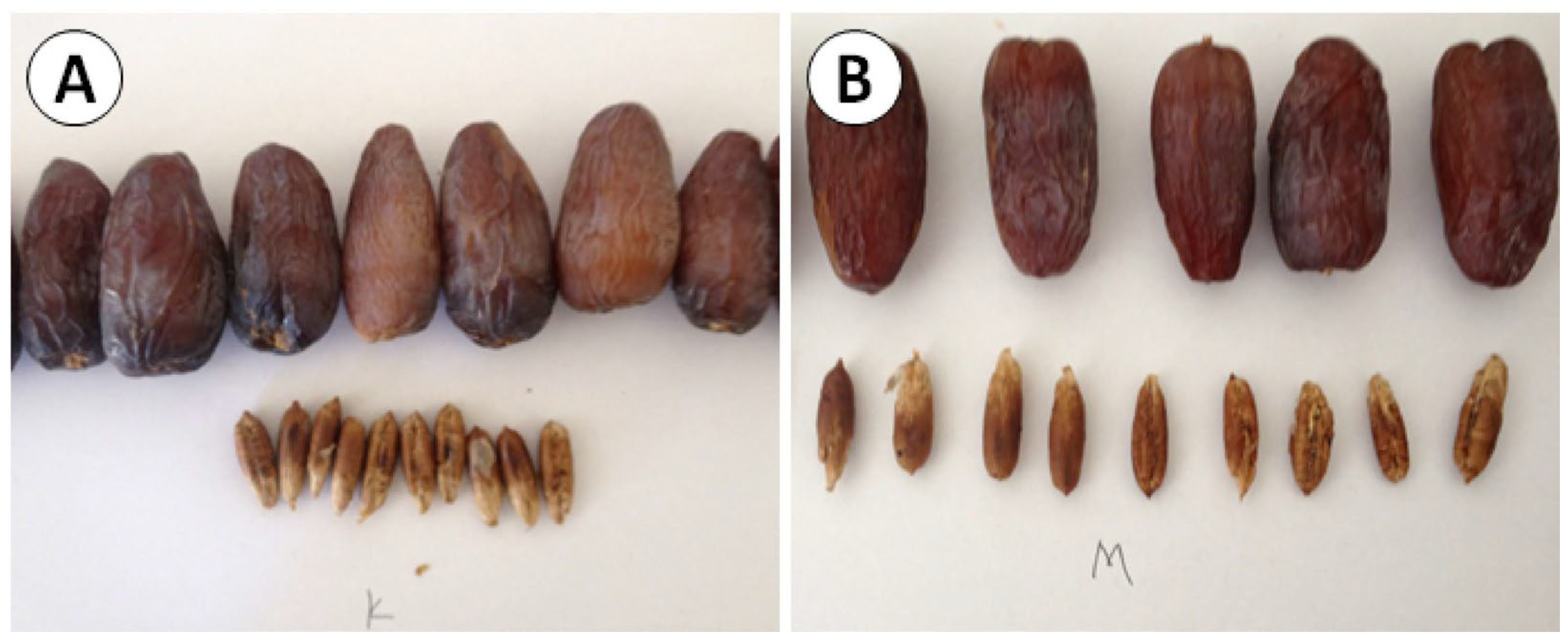

| Season | Pollen Donor | Fresh (2022/2023) | One-Year Stored (2021/2022) | Two-Year Stored (2020/2021) |
|---|---|---|---|---|
| 2022 | MJL | 95.6 ± 1.9 a | 68.3 ± 2.4 ab | 45.4 ± 1.2 ab |
| KDY | 94.2 ± 2.0 a | 63.1 ± 1.6 b | 41.0 ± 1.4 b | |
| DGN | 93.8 ± 1.8 a | 66.0 ± 2.3 a | 44.2 ± 2.1 a | |
| ZHI | 91.2 ± 2.1 b | 59.4 ± 1.5 c | 38.8 ± 2.3 c | |
| 2023 | MJL | 95.0 ± 1.8 a | 67.0 ± 1.3 ab | 46.0 ± 2.1 ab |
| KDY | 94.0 ± 1.9 a | 62.0 ± 2.5 b | 40.0 ± 1.3 b | |
| DGN | 95.0 ± 1.7 a | 68.0 ± 2.2 a | 45.0 ± 2.1 a | |
| ZHI | 92.0 ± 2.0 b | 60.0 ± 1.6 c | 39.0 ± 1.7 c |
| Pollen Donor | Fresh | One-Year Stored | Two-Year Stored | |||
|---|---|---|---|---|---|---|
| 2022 | 2023 | 2021 | 2022 | 2020 | 2021 | |
| MJL | 68.87 ± 11.14 bc | 69.41 ± 7.64 b | 58.22 ± 3.21 b | 55.44 ± 3.98 b | 45.20 ± 3.69 c | 46.44 ± 3.98 ab |
| KDY | 72.08 ± 13.23 b | 64.71 ± 11.43 c | 57.99 ± 5.79 b | 54.52 ± 4.25 bc | 50.53 ± 4.89 a | 49.52 ± 4.25 a |
| DGN | 81.71 ± 3.72 a | 74.92 ± 10.93 a | 64.50 ± 6.23 a | 62.72 ± 4.32 a | 52.02 ± 3.78 a | 50.72 ± 4.32 a |
| ZHI | 63.80 ± 12.74 c | 65.15 ± 7.59 c | 54.29 ± 4.59 c | 53.60 ± 5.06 c | 49.97 ± 5.98 b | 49.60 ± 5.06 a |
| Pollen Donor | One-Year Stored (%) | Two-Year Stored (%) | ||||
|---|---|---|---|---|---|---|
| 2021 | 2022 | Average | 2020 | 2021 | Average | |
| MJL | 18.29 | 25.20 | 21.74 | 52.37 | 49.46 | 50.91 |
| KDY | 24.29 | 18.69 | 21.49 | 42.64 | 30.67 | 36.65 |
| DGN | 26.68 | 19.46 | 23.07 | 57.11 | 47.71 | 52.41 |
| ZHI | 17.52 | 21.55 | 19.53 | 57.67 | 31.35 | 44.51 |
| Measuring Parameter | Pollen Donor | Fresh | One-Year Stored | Two-Year Stored | |||
|---|---|---|---|---|---|---|---|
| 2022 | 2023 | 2021 | 2022 | 2020 | 2021 | ||
| Weight (g) | MJL | 18.05 ± 4.48 b | 21.74 ± 2.80 ab | 19.00 ± 2.91 a | 20.96 ± 3.49 ab | 20.35 ± 3.95 a | 19.60 ± 2.89 a |
| KDY | 19.70 ± 1.9 ab | 22.70 ± 3.21 a | 20.49 ± 1.25 a | 19.57 ± 2.66 b | 20.35 ± 3.70 a | 21.17 ± 2.94 a | |
| DGN | 21.18 ± 4.49 a | 17.70 ± 3.64 c | 20.64 ± 2.68 a | 22.47 ± 2.72 a | 18.47 ± 3.37 a | 20.93 ± 3.09 a | |
| ZHI | 20.10 ± 2.33 ab | 20.12 ± 2.60 b | 19.45 ± 3.11 a | 21.88 ± 2.30 a | 20.01 ± 3.14 a | 21.32 ± 4.4 a | |
| Length (cm) | MJL | 4.10 ± 0.53 c | 4.58 ± 0.26 a | 4.28 ± 0.33 a | 4.42 ± 0.34 b | 4.46 ± 0.39 a | 5.00 ± 0.22 b |
| KDY | 4.27 ± 0.47 bc | 4.55 ± 0.31 a | 4.41 ± 0.39 a | 4.26 ± 0.24 bc | 4.46 ± 0.21 a | 5.02 ± 0.22 ab | |
| DGN | 4.49 ± 0.20 ab | 4.16 ± 0.20 b | 4.48 ± 0.30 a | 4.77 ± 0.29 a | 4.31 ± 0.28 a | 5.17 ± 0.30 a | |
| ZHI | 4.56 ± 0.28 a | 4.69 ± 0.27 a | 4.37 ± 0.42 a | 4.19 ± 0.47 c | 4.49 ± 0.36 a | 4.97 ± 0.26 b | |
| Diameter (cm) | MJL | 2.42 ± 0.32 b | 2.57 ± 0.12 a | 2.58 ± 0.31 a | 2.44 ± 0.24 b | 2.63 ± 0.23 a | 2.40 ± 0.19 a |
| KDY | 2.56 ± 0.24 ab | 2.58 ± 0.24 a | 2.51 ± 0.21 a | 2.65 ± 0.28 a | 2.51 ± 0.12 ab | 2.55 ± 0.24 a | |
| DGN | 2.62 ± 0.10 a | 2.30 ± 0.25 b | 2.67 ± 0.23 a | 2.65 ± 0.22 a | 2.59 ± 0.23 b | 2.51 ± 0.20 a | |
| ZHI | 2.69 ± 0.22 a | 2.59 ± 0.13 a | 2.62 ± 0.25 a | 2.53 ± 0.30 ab | 2.43 ± 0.27 a | 2.56 ± 0.38 a | |
| Measuring Parameter | Pollen Donor | Fresh | One-Year Stored | Two-Year Stored | |||
|---|---|---|---|---|---|---|---|
| 2022 | 2023 | 2021 | 2022 | 2020 | 2021 | ||
| Weight (g) | MJL | 1.47 ± 0.14 a | 1.30 ± 0.24 a | 1.43 ± 0.15 a | 1.42 ± 0.22 a | 1.27 ± 0.16 b | 1.15 ± 0.14 bc |
| KDY | 1.29 ± 0.12 bc | 1.18 ± 0.11 a | 1.41 ± 0.23 a | 1.33 ± 0.16 a | 1.35 ± 0.16 ab | 1.24 ± 0.17 ab | |
| DGN | 1.25 ± 0.21 c | 1.30 ± 0.17 a | 1.40 ± 0.12 a | 1.36 ± 0.15 a | 1.48 ± 0.20 a | 1.37 ± 0.21 a | |
| ZHI | 1.43 ± 0.11 ab | 1.32 ± 0.14 a | 1.30 ± 0.14 a | 1.37 ± 0.15 a | 1.41 ± 0.19 ab | 1.05 ± 0.16 c | |
| Length (cm) | MJL | 2.69 ± 0.13 a | 2.70 ± 0.15 a | 2.77 ± 0.12 a | 2.66 ± 0.15 a | 2.55 ± 0.21 bc | 2.44 ± 0.11 ab |
| KDY | 2.53 ± 0.16 b | 2.40 ± 0.13 c | 2.68 ± 0.15 ab | 2.71 ± 0.15 a | 2.51 ± 0.20 c | 2.50 ± 0.17 a | |
| DGN | 2.60 ± 0.13 ab | 2.52 ± 0.15 bc | 2.64 ± 0.13 ab | 2.66 ± 0.15 a | 2.73 ± 0.22 a | 2.59 ± 0.20 a | |
| ZHI | 2.66 ± 0.17 ab | 2.54 ± 0.14 b | 2.60 ± 0.20 b | 2.70 ± 0.13 a | 2.71 ± 0.11 ab | 2.30 ± 0.19 b | |
| Diameter (cm) | MJL | 0.88 ± 0.04 ab | 0.82 ± 0.05 a | 0.90 ± 0.06 a | 0.89 ± 0.05 a | 0.88 ± 0.04 a | 0.79 ± 0.03 b |
| KDY | 0.81 ± 0.04 c | 0.82 ± 0.07 a | 0.85 ± 0.07 a | 0.91 ± 0.05 a | 0.91 ± 0.04 a | 0.83 ± 0.04 ab | |
| DGN | 0.83 ± 0.08 bc | 0.85 ± 0.06 a | 0.88 ± 0.03 a | 0.89 ± 0.06 a | 0.91 ± 0.04 a | 0.85 ± 0.06 a | |
| ZHI | 0.93 ± 0.04 a | 0.87 ± 0.03 a | 0.87 ± 0.04 a | 0.93 ± 0.07 a | 0.91 ± 0.06 a | 0.81 ± 0.05 ab | |
| Cultivar | Leaf Length (cm) | Leaf Width (cm) | Middle Leaflet Length (cm) | Middle Leaflet Width (cm) | Middle Spine Length (cm) | Middle Spine Width (cm) | Spined Portion Length (cm) | Leaflet Number | Spine Number |
|---|---|---|---|---|---|---|---|---|---|
| MJL | 304–376 | 65–82 | 50–64 | 2.8–3.4 | 11–16.5 | 0.5–0.8 | 57–88 | 143–156 | 20–30 |
| DGN | 327–400 | 63–74 | 49–53 | 2.9–3.7 | 8.1–13.5 | 0.6–0.8 | 103–122 | 117–182 | 36–44 |
| KDY | 316–329 | 71–89 | 47–57 | 3.6–4.5 | 9.8–13.2 | 0.5–0.6 | 57–64 | 130–143 | 20–22 |
| ZHI | 443–468 | 57–64 | 46–52 | 2.7–3.7 | 5.2–7.6 | 0.6–0.7 | 90–120 | 117–133 | 28–32 |
| Palm ID | Phenotypic Similarity | Leaf Length (cm) | Leaf Width (cm) | Middle Leaflet Length (cm) | Middle Leaflet Width (cm) | Middle Spine Length (cm) | Middle Spine Width (cm) | Spined Portion Length (cm) | Leaflet Number | Spine Number |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | MJL | 308 | 72 | 52 | 3.4 | 13.7 | 0.7 | 61 | 147 | 24 |
| P2 | MJL | 348 | 71 | 55 | 3.2 | 12.4 | 0.5 | 73 | 156 | 26 |
| P3 | MJL | 354 | 80 | 63 | 3.2 | 12.3 | 0.5 | 66 | 152 | 21 |
| P4 | MJL | 337 | 76 | 57 | 3.3 | 12.8 | 0.6 | 69 | 150 | 29 |
| P5 | DGN | 330 | 66 | 49 | 3.1 | 10.3 | 0.6 | 104 | 169 | 42 |
| P6 | DGN | 397 | 69 | 50 | 3.1 | 9.1 | 0.7 | 105 | 180 | 37 |
| P7 | DGN | 385 | 71 | 53 | 3.5 | 12.1 | 0.7 | 112 | 155 | 38 |
| P8 | KDY | 323 | 82 | 51 | 4.0 | 11.2 | 0.5 | 62 | 137 | 21 |
| P9 | KDY | 321 | 79 | 56 | 3.7 | 11.9 | 0.6 | 60 | 140 | 20 |
| P10 | KDU | 326 | 87 | 47 | 4.2 | 10.2 | 0.5 | 63 | 130 | 22 |
| P11 | ZHI | 450 | 61 | 48 | 3.1 | 6.7 | 0.6 | 108 | 120 | 30 |
| P12 | ZHI | 447 | 60 | 46 | 2.9 | 7.5 | 0.6 | 115 | 127 | 32 |
| Treatments | Replications | Pollen Donor | Pollen Age | Extraction Year | Pollination Season |
|---|---|---|---|---|---|
| T1–T4 | R1–R3 | MJL, DGN, KDY, ZHI | Fresh | 2022 2023 | 2022 2023 |
| T5–T8 | R1–R3 | MJL, DGN, KDY, ZHI | one-year-stored | 2021 2022 | 2022 2023 |
| T9–T12 | R1–R3 | MJL, DGN, KDY, ZHI | Two-years stored | 2020 2021 | 2022 2023 |
| Female Palms | Treatments | Replications | Pollen Age |
|---|---|---|---|
| P1–P3 | T1–T4 | R1–R3 | Fresh |
| P4–P6 | T5–T8 | R1–R3 | One-year-stored |
| P7–P9 | T9–T12 | R1–R3 | Two years stored |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salomón-Torres, R.; Elhoumaizi, M.A.; Zambrano-Reyes, C.; Zaid, A.A.; Ruisanchez-Ortega, Y.; Peña-Yam, L.P.; Gutiérrez-Pacheco, M.M. Effect of Pollen Storage Duration on Stainability, Fruit Set, and Physical Traits in Date Palm (Phoenix dactylifera L.) Cultivar ‘Mejhoul’. Plants 2025, 14, 3189. https://doi.org/10.3390/plants14203189
Salomón-Torres R, Elhoumaizi MA, Zambrano-Reyes C, Zaid AA, Ruisanchez-Ortega Y, Peña-Yam LP, Gutiérrez-Pacheco MM. Effect of Pollen Storage Duration on Stainability, Fruit Set, and Physical Traits in Date Palm (Phoenix dactylifera L.) Cultivar ‘Mejhoul’. Plants. 2025; 14(20):3189. https://doi.org/10.3390/plants14203189
Chicago/Turabian StyleSalomón-Torres, Ricardo, Mohammed Aziz Elhoumaizi, Carlos Zambrano-Reyes, Abdelouahhab Alboukhari Zaid, Yohandri Ruisanchez-Ortega, Laura Patricia Peña-Yam, and María Melissa Gutiérrez-Pacheco. 2025. "Effect of Pollen Storage Duration on Stainability, Fruit Set, and Physical Traits in Date Palm (Phoenix dactylifera L.) Cultivar ‘Mejhoul’" Plants 14, no. 20: 3189. https://doi.org/10.3390/plants14203189
APA StyleSalomón-Torres, R., Elhoumaizi, M. A., Zambrano-Reyes, C., Zaid, A. A., Ruisanchez-Ortega, Y., Peña-Yam, L. P., & Gutiérrez-Pacheco, M. M. (2025). Effect of Pollen Storage Duration on Stainability, Fruit Set, and Physical Traits in Date Palm (Phoenix dactylifera L.) Cultivar ‘Mejhoul’. Plants, 14(20), 3189. https://doi.org/10.3390/plants14203189








