Optimizing Conditions for Bacillus subtilis Ectopic Gene Expression and Delivery via Seed Treatment
Abstract
1. Introduction
2. Results
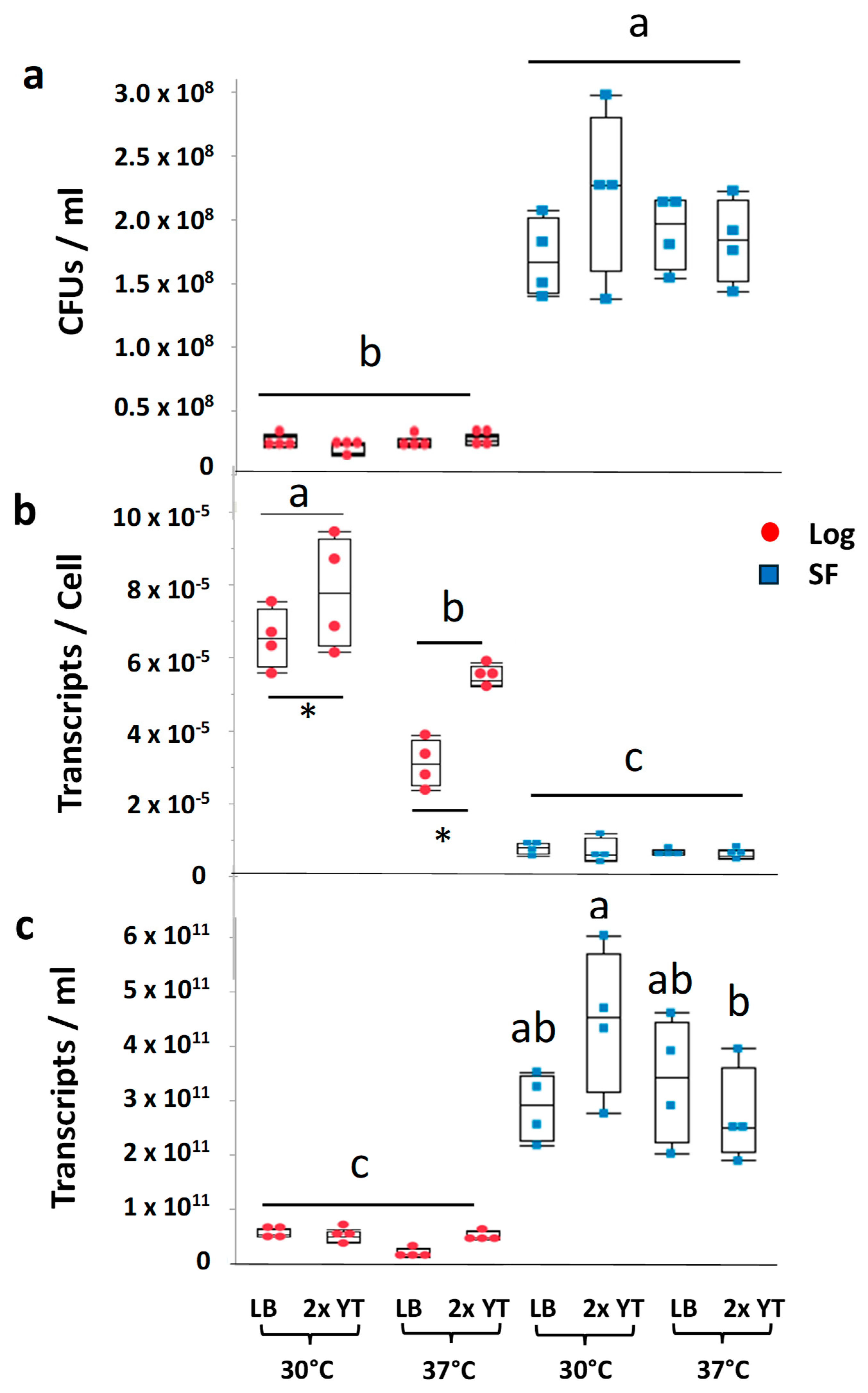
2.1. Influence of Growth Phase and Growth Conditions on the Number of Viable B. subtilis Cells Ectopic Transcripts per Cell
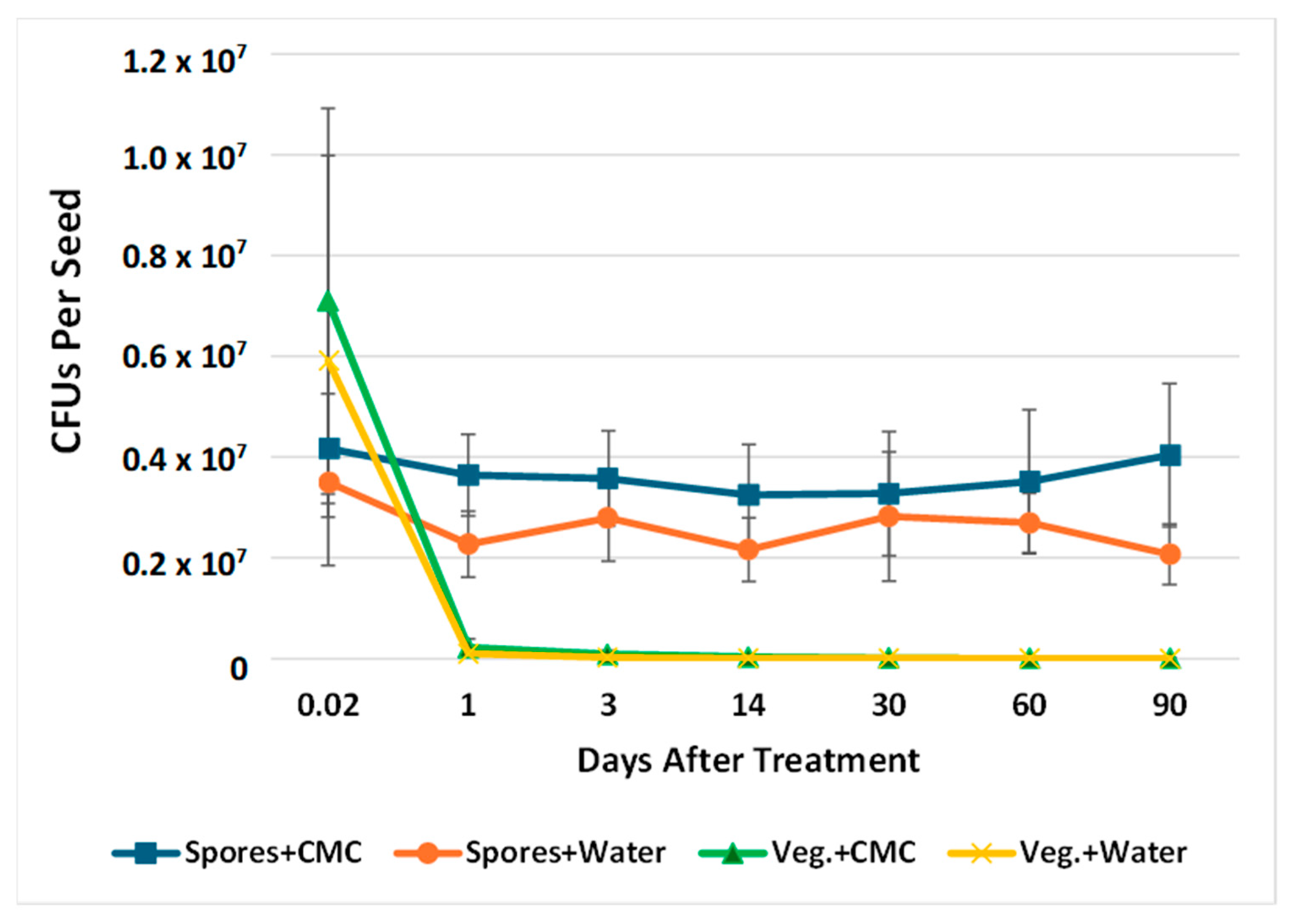
2.2. Retention of B. subtilis Endospores and Vegetative Cells with and Without Carboxymethylcellulose on Soybean Seeds
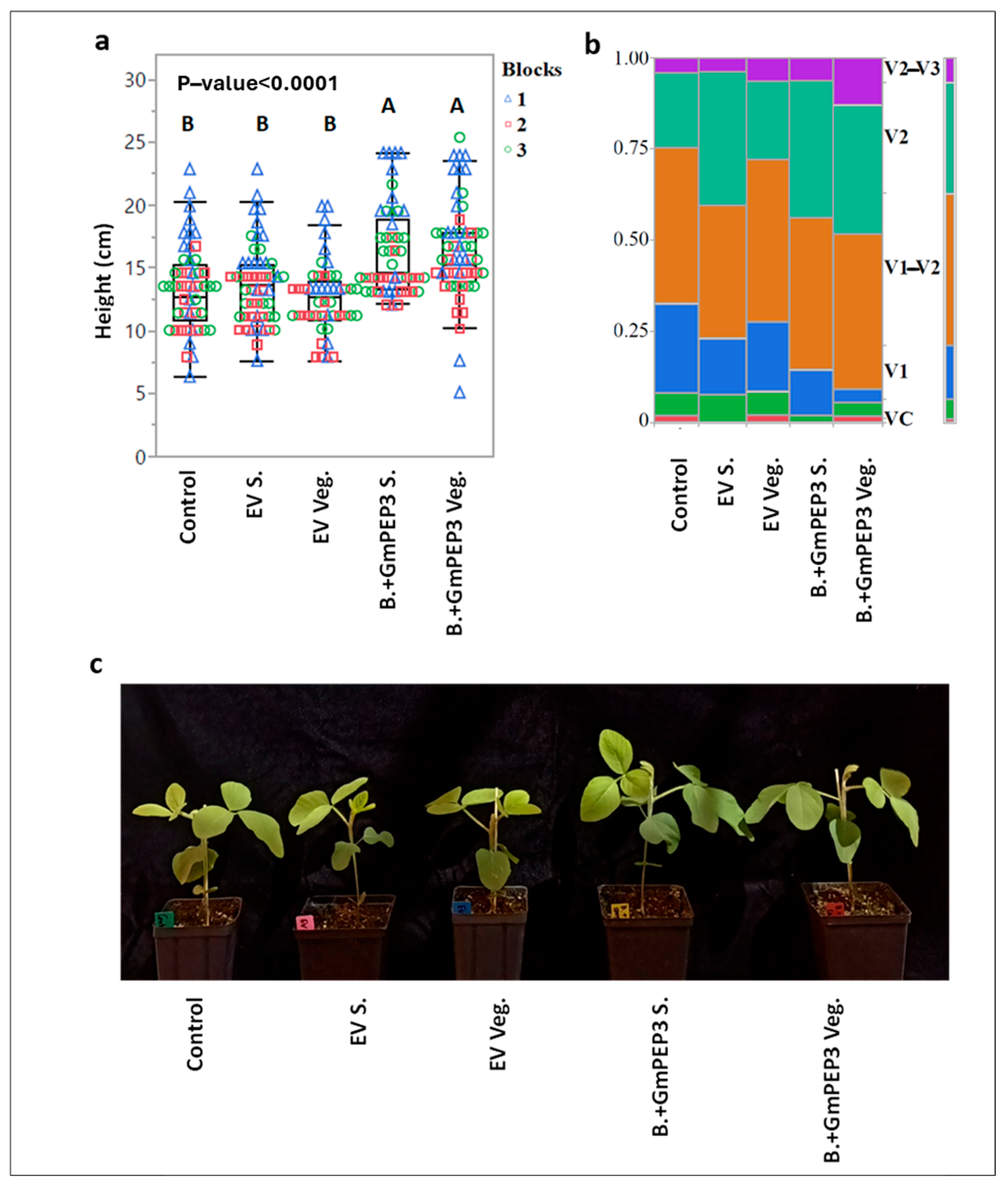
2.3. Influence of Seed Treatments with B. subtilis Endospores or Vegetative Cells on Soybean Seedling Growth
3. Discussion
4. Materials and Methods
4.1. Biological Materials
4.2. Selection of Time Points for Collection of the Log and Stationary Growth Phases of B. subtilis
4.3. Absolute Quantification of Expression
4.4. Preparation of Endospores
4.5. Bacterial Seed Treatments
4.6. Measurement of Retention of Viable Bacteria on Treated Seeds
4.7. Measurement of Seedling Growth After Bacterial Seed Treatments
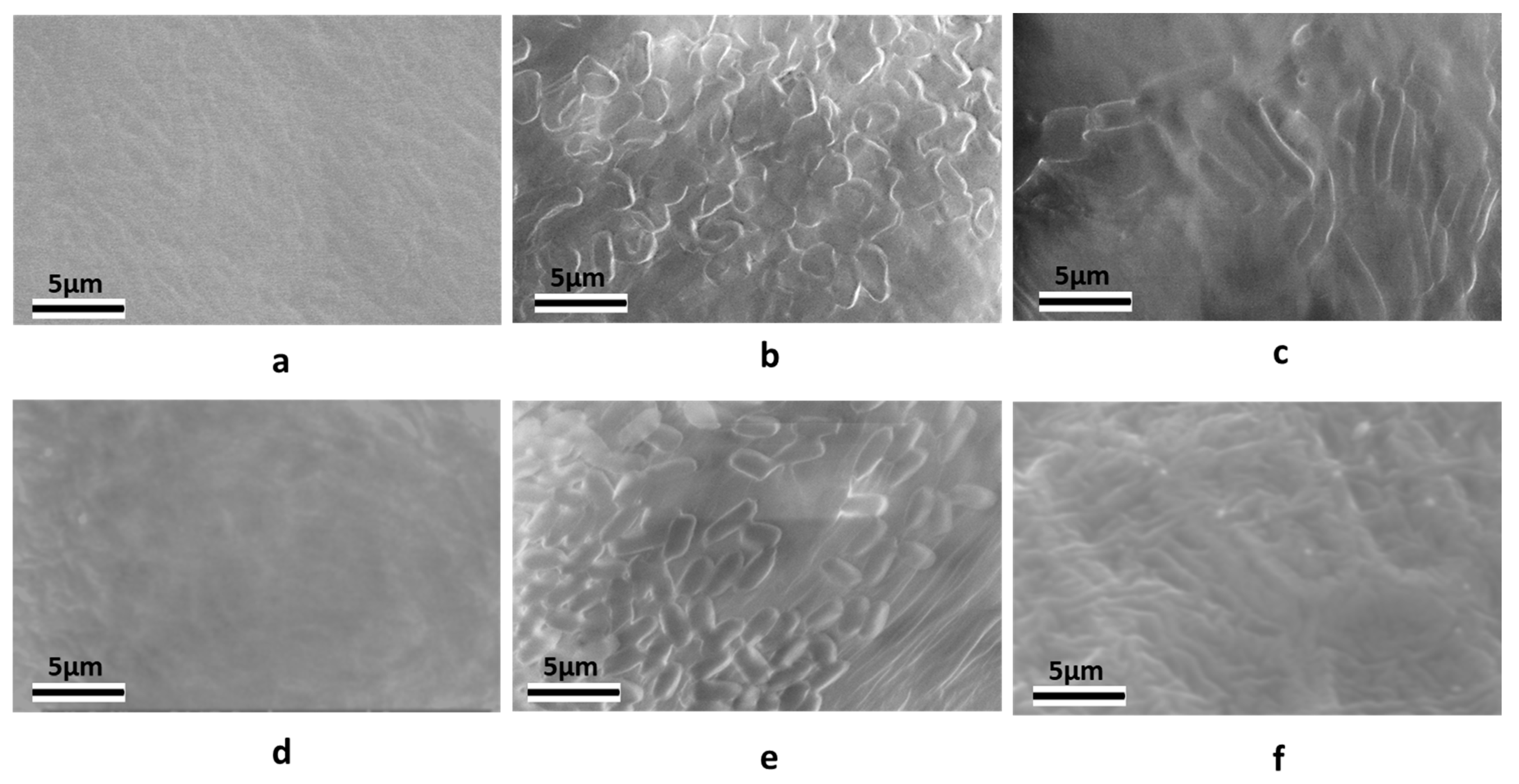
4.8. Micrographs of B. subtilis Spores and Vegetative Cells on the Soybean Seed Surface
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2x YT | Yeast Extract Tryptone media |
| LB | Luria–Bertani (LB) media |
| CFU | Colony forming units |
| CMC | Carboxymethylcellulose |
References
- Earl, A.M.; Losick, R.; Kolter, R. Ecology and genomics of Bacillus subtilis. Trends Microbiol. 2008, 16, 269–275. [Google Scholar] [CrossRef]
- Moharrami, M.; Mojgani, N.; Bagheri, M.; Toutiaee, S. Role of honey bee gut microbiota in the control of American foulbrood and European foulbrood diseases. Arch. Razi Inst. 2022, 77, 1331. [Google Scholar]
- Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Bacillus: A biological tool for crop improvement through bio-molecular changes in adverse environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef]
- Cutting, S.M. Bacillus probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus subtilis: A universal cell factory for industry, agriculture, biomaterials and medicine. Microb. Cell Factories 2020, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-M.; Ye, D.-X.; Liu, Y.; Zhang, X.-Y.; Zhou, Y.-L.; Zhang, L.; Yang, X.-L. Peptides, new tools for plant protection in eco-agriculture. Adv. Agrochem 2023, 2, 58–78. [Google Scholar] [CrossRef]
- Jeong, D.-E.; Park, S.-H.; Pan, J.-G.; Kim, E.-J.; Choi, S.-K. Genome engineering using a synthetic gene circuit in Bacillus subtilis. Nucleic Acids Res. 2015, 43, e42. [Google Scholar] [CrossRef]
- Yan, X.; Yu, H.-J.; Hong, Q.; Li, S.-P. Cre/lox System and PCR-Based Genome Engineering in Bacillus subtilis. Appl. Environ. Microbiol. 2008, 74, 5556–5562. [Google Scholar] [CrossRef] [PubMed]
- Altenbuchner, J. Editing of the Bacillus subtilis Genome by the CRISPR-Cas9 System. Appl. Environ. Microbiol. 2016, 82, 5421–5427. [Google Scholar] [CrossRef]
- Zweers, J.C.; Barák, I.; Becher, D.; Driessen, A.J.; Hecker, M.; Kontinen, V.P.; Saller, M.J.; Vavrová, L.; Van Dijl, J.M. Towards the development of Bacillus subtilis as a cell factory for membrane proteins and protein complexes. Microb. Cell Factories 2008, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Vlamakis, H.; Chai, Y.; Beauregard, P.; Losick, R.; Kolter, R. Sticking together: Building a biofilm the Bacillus subtilis way. Nat. Rev. Microbiol. 2013, 11, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Patko, D.; Engelhardt, I.; George, T.S.; Stanley-Wall, N.R.; Ladmiral, V.; Ameduri, B.; Daniell, T.J.; Holden, N.; MacDonald, M.P.; et al. Plant–environment microscopy tracks interactions of Bacillus subtilis with plant roots across the entire rhizosphere. Proc. Natl. Acad. Sci. USA 2021, 118, e2109176118. [Google Scholar] [CrossRef]
- Blake, C.; Christensen, M.N.; Kovács, Á.T. Molecular Aspects of Plant Growth Promotion and Protection by Bacillus subtilis. Mol. Plant-Microbe Interact. 2021, 34, 15–25. [Google Scholar] [CrossRef]
- Alnasrawi, A.; Sanadhya, P.; Zhang, L.; Gleason, C.; Minor, K.; Crippen, D.; Goggin, F. The effects of Bacillus subtilis expressing a plant elicitor peptide on nematode infection on soybean. Phytopathology 2024, 114, 2143–2150. [Google Scholar] [CrossRef]
- Bartels, S.; Boller, T. Quo vadis, Pep? Plant elicitor peptides at the crossroads of immunity, stress, and development. J. Exp. Bot. 2015, 66, 5183–5193. [Google Scholar] [CrossRef]
- Zhang, L.; Gleason, C. Enhancing potato resistance against root-knot nematodes using a plant-defence elicitor delivered by bacteria. Nat. Plants 2020, 6, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Ramos, K.R.M.; Valdehuesa, K.N.G.; Cabulong, R.B.; Moron, L.S.; Nisola, G.M.; Hong, S.-K.; Lee, W.-K.; Chung, W.-J. Overexpression and secretion of AgaA7 from Pseudoalteromonas hodoensis sp. nov in Bacillus subtilis for the depolymerization of agarose. Enzyme Microb. Technol. 2016, 90, 19–25. [Google Scholar] [CrossRef]
- Gimenez, G.G.; Costa, H.; De Lima Neto, Q.A.; Fernandez, M.A.; Ferrarotti, S.A.; Matioli, G. Sequencing, cloning, and heterologous expression of cyclomaltodextrin glucanotransferase of Bacillus firmus strain 37 in Bacillus subtilis WB800. Bioprocess Biosyst. Eng. 2019, 42, 621–629. [Google Scholar] [CrossRef]
- Jemli, S.; Ben Messaoud, E.; Ben Mabrouk, S.; Bejar, S. The Cyclodextrin Glycosyltransferase of Paenibacillus pabuli US132 Strain: Molecular Characterization and Overproduction of the Recombinant Enzyme. BioMed Res. Int. 2008, 2008, 692573. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, J.K.; Kim, H.K.; Sohn, C.B.; Oh, T.K. Overexpression of cyclodextrin glycosyltransferase gene from Brevibacillus brevis in Escherichia coli by control of temperature and mannitol concentration. Biotechnol. Tech. 1999, 13, 765–770. [Google Scholar] [CrossRef]
- Terpe, K. Overview of bacterial expression systems for heterologous protein production: From molecular and biochemical fundamentals to commercial systems. Appl. Microbiol. Biotechnol. 2006, 72, 211–222. [Google Scholar] [CrossRef]
- Paravar, A.; Piri, R.; Balouchi, H.; Ma, Y. Microbial seed coating: An attractive tool for sustainable agriculture. Biotechnol. Rep. 2023, 37, e00781. [Google Scholar] [CrossRef]
- Pedrini, S.; Merritt, D.J.; Stevens, J.; Dixon, K. Seed coating: Science or marketing spin? Trends Plant Sci. 2017, 22, 106–116. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, M. Microbial inoculation of seed for improved crop performance: Issues and opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef] [PubMed]
- Köhl, J.; Butterbach, P.; Ehlers, R.-U.; Gaildry, T.; Groenenboom-de Haas, L.; Groot, S.P.; van der Heijden, L.; Houwers, I.; de Lange, E.; Lopez, G. Screening criteria for microbial bioprotectants for seed coating to protect seeds and seedlings from diseases. Biol. Control 2024, 190, 105450. [Google Scholar] [CrossRef]
- Williams, N.; Weir, T.L. Spore-based probiotic Bacillus subtilis: Current applications in humans and future perspectives. Fermentation 2024, 10, 78. [Google Scholar] [CrossRef]
- Yánez-Mendizabal, V.; Viñas, I.; Usall, J.; Cañamás, T.; Teixidó, N. Endospore production allows using spray-drying as a possible formulation system of the biocontrol agent Bacillus subtilis CPA-8. Biotechnol. Lett. 2012, 34, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Mattei, V.; Motta, A.; Saracchi, M.; Kunova, A.; Cortesi, P.; Pizzatti, C.; Pasquali, M. Wheat seed coating with Streptomyces sp. strain DEF39 spores protects against Fusarium head blight. Microorganisms 2022, 10, 1536. [Google Scholar] [CrossRef]
- Pimentel, M.F.; Arnao, E.; Warner, A.J.; Rocha, L.F.; Subedi, A.; Elsharif, N.; Chilvers, M.I.; Matthiesen, R.; Robertson, A.E.; Bradley, C.A.; et al. Reduction of Pythium Damping-Off in Soybean by Biocontrol Seed Treatment. Plant Dis. 2022, 106, 2403–2414. [Google Scholar] [CrossRef]
- Jan, J.; Valle, F.; Bolivar, F.; Merino, E. Characterization of the 5′ subtilisin (aprE) regulatory region from Bacillus subtilis. FEMS Microbiol. Lett. 2000, 183, 9–14. [Google Scholar] [CrossRef]
- Xu, Y.; Xuan, X.; Gao, R.; Xie, G. Increased expression levels of thermophilic serine protease TTHA0724 through signal peptide screening in Bacillus subtilis and applications of the enzyme. Int. J. Mol. Sci. 2023, 24, 15950. [Google Scholar] [CrossRef] [PubMed]
- Bergkessel, M. Bacterial transcription during growth arrest. Transcription 2021, 12, 232–249. [Google Scholar] [CrossRef]
- Cross, W.F.; Hood, J.M.; Benstead, J.P.; Huryn, A.D.; Nelson, D. Interactions between temperature and nutrients across levels of ecological organization. Glob. Change Biol. 2015, 21, 1025–1040. [Google Scholar] [CrossRef]
- Young, C.S.; Lethbridge, G.; Shaw, L.J.; Burns, R.G. Survival of inoculated Bacillus cereus spores and vegetative cells in non-planted and rhizosphere soil. Soil Biol. Biochem. 1995, 27, 1017–1026. [Google Scholar] [CrossRef]
- Setlow, P. Germination of Spores of Bacillus Species: What We Know and Do Not Know. J. Bacteriol. 2014, 196, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Xue, A.G.; Tambong, J.T. Evaluation of Seed and Soil Treatments with Novel Bacillus subtilis Strains for Control of Soybean Root Rot Caused by Fusarium oxysporum and F. graminearum. Plant Dis. 2009, 93, 1317–1323. [Google Scholar] [CrossRef]
- Dawar, S.; Wahab, S.; Tariq, M.; Zaki, M.J. Application of Bacillus species in the control of root rot diseases of crop plants. Arch. Phytopathol. Plant Prot. 2010, 43, 412–418. [Google Scholar] [CrossRef]
- Chai, Y.N.; Futrell, S.; Schachtman, D.P. Assessment of bacterial inoculant delivery methods for cereal crops. Front. Microbiol. 2022, 13, 791110. [Google Scholar] [CrossRef]
- Hashmi, I.; Paul, C.; Al-Dourobi, A.; Sandoz, F.; Deschamps, P.; Junier, T.; Junier, P.; Bindschedler, S. Comparison of the plant growth-promotion performance of a consortium of Bacilli inoculated as endospores or as vegetative cells. FEMS Microbiol. Ecol. 2019, 95, fiz147. [Google Scholar] [CrossRef]
- Nihorimbere, G.; Korangi Alleluya, V.; Nimbeshaho, F.; Nihorimbere, V.; Legrève, A.; Ongena, M. Bacillus-based biocontrol beyond chemical control in central Africa: The challenge of turning myth into reality. Front. Plant Sci. 2024, 15, 1349357. [Google Scholar] [CrossRef] [PubMed]
- Haskett, T.L.; Tkacz, A.; Poole, P.S. Engineering rhizobacteria for sustainable agriculture. ISME J. 2021, 15, 949–964. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Potential use of Bacillus spp. as an effective biostimulant against abiotic stresses in crops—A review. Curr. Res. Biotechnol. 2023, 5, 100128. [Google Scholar] [CrossRef]
- Jensen, C.N.G.; Pang, J.K.Y.; Gottardi, M.; Kračun, S.K.; Svendsen, B.A.; Nielsen, K.F.; Kovács, Á.T.; Moelbak, L.; Fimognari, L.; Husted, S.; et al. Bacillus subtilis promotes plant phosphorus (P) acquisition through P solubilization and stimulation of root and root hair growth. Physiol. Plant. 2024, 176, e14338. [Google Scholar] [CrossRef] [PubMed]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Rogers, A.T.; Bullard, K.R.; Dod, A.C.; Wang, Y. Bacterial Growth Curve Measurements with a Multimode Microplate Reader. Bio-Protocol 2022, 12, e4410. [Google Scholar] [CrossRef]
- Harwood, C.R.; Cutting, S.M. Molecular Biological Methods for Bacillus; Wiley: Hoboken, NJ, USA, 1990; Volume 35, Available online: https://library.wur.nl/WebQuery/titel/972501 (accessed on 30 June 2025).
- Leighton, T.J.; Doi, R.H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J. Biol. Chem. 1971, 246, 3189–3195. [Google Scholar] [CrossRef]
- Bartholomew, J.W.; Mittwer, T. A Simplified Bacterial Spore Stain. Stain Technol. 1950, 25, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Daroodi, Z.; Taheri, P.; Tarighi, S. Effect of tomato seed coating with spores of the endophytic fungus Acrophialophora jodhpurensis on plant growth and control of crown and root rot caused by Rhizoctonia solani. Biol. Control. Pests Plant Dis. 2022, 11, 95–113. [Google Scholar] [CrossRef]
- Nleya, T.; Sexton, P.; Gustafson, K.; Miller, J.M. Soybean growth stages. In iGrow Soybean: Best Management Practices for Soybean Production; SDSU Extension-iGrow: Brookings, SD, USA, 2013; Available online: https://openprairie.sdstate.edu/cgi/viewcontent.cgi?filename=2&article=1001&context=plant_book&type=additional (accessed on 30 June 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnasrawi, A.; Li, J.; Sanadhya, P.; Rojas, J.A.; Goggin, F.L. Optimizing Conditions for Bacillus subtilis Ectopic Gene Expression and Delivery via Seed Treatment. Plants 2025, 14, 3184. https://doi.org/10.3390/plants14203184
Alnasrawi A, Li J, Sanadhya P, Rojas JA, Goggin FL. Optimizing Conditions for Bacillus subtilis Ectopic Gene Expression and Delivery via Seed Treatment. Plants. 2025; 14(20):3184. https://doi.org/10.3390/plants14203184
Chicago/Turabian StyleAlnasrawi, Abeer, Jiamei Li, Payal Sanadhya, J. Alejandro Rojas, and Fiona L. Goggin. 2025. "Optimizing Conditions for Bacillus subtilis Ectopic Gene Expression and Delivery via Seed Treatment" Plants 14, no. 20: 3184. https://doi.org/10.3390/plants14203184
APA StyleAlnasrawi, A., Li, J., Sanadhya, P., Rojas, J. A., & Goggin, F. L. (2025). Optimizing Conditions for Bacillus subtilis Ectopic Gene Expression and Delivery via Seed Treatment. Plants, 14(20), 3184. https://doi.org/10.3390/plants14203184







