How a Green Roof Becomes Biodiverse: Vegetation Analysis on a Green Roof with no Maintenance in Rome (Italy)
Abstract
1. Introduction
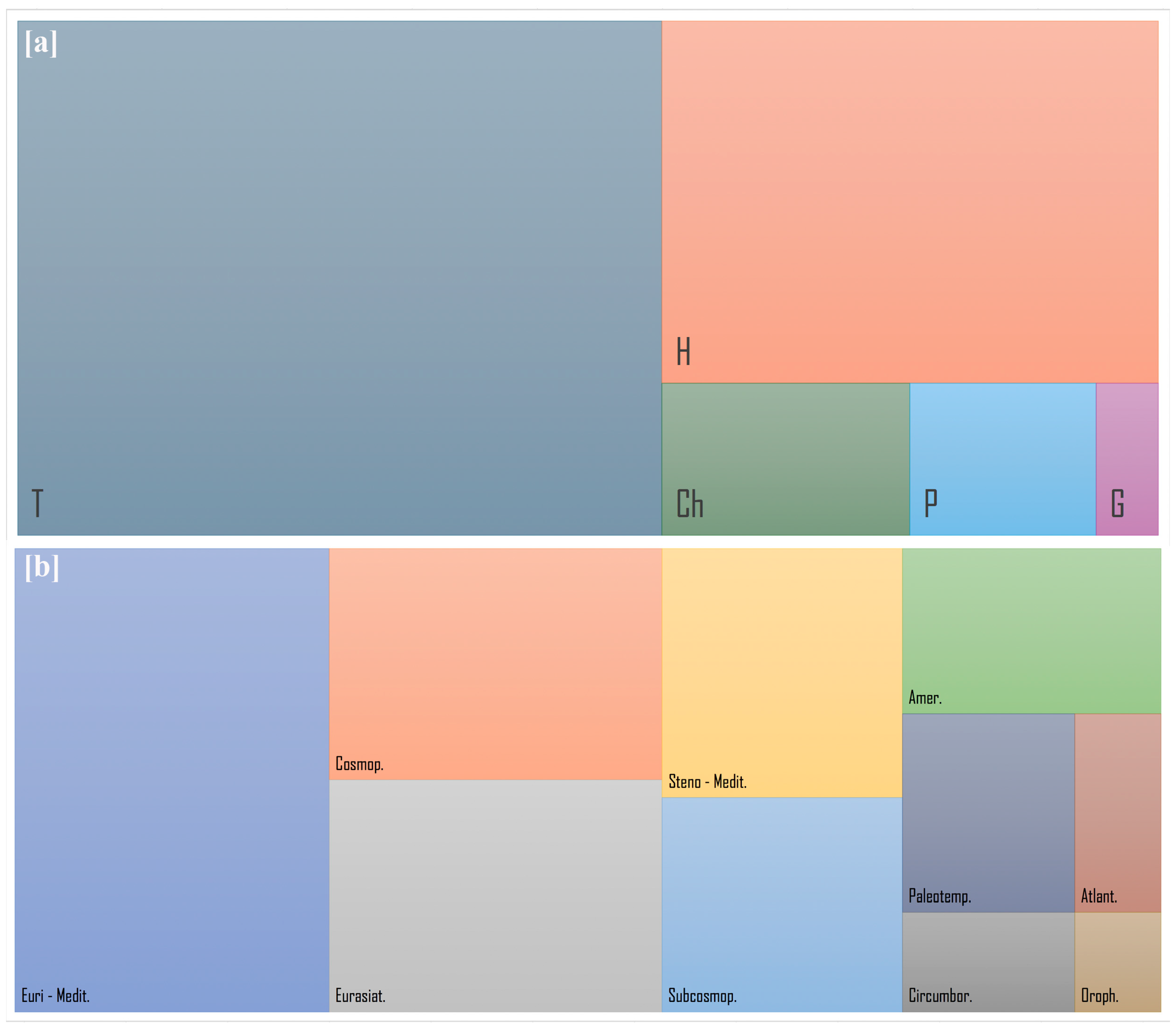
2. Results
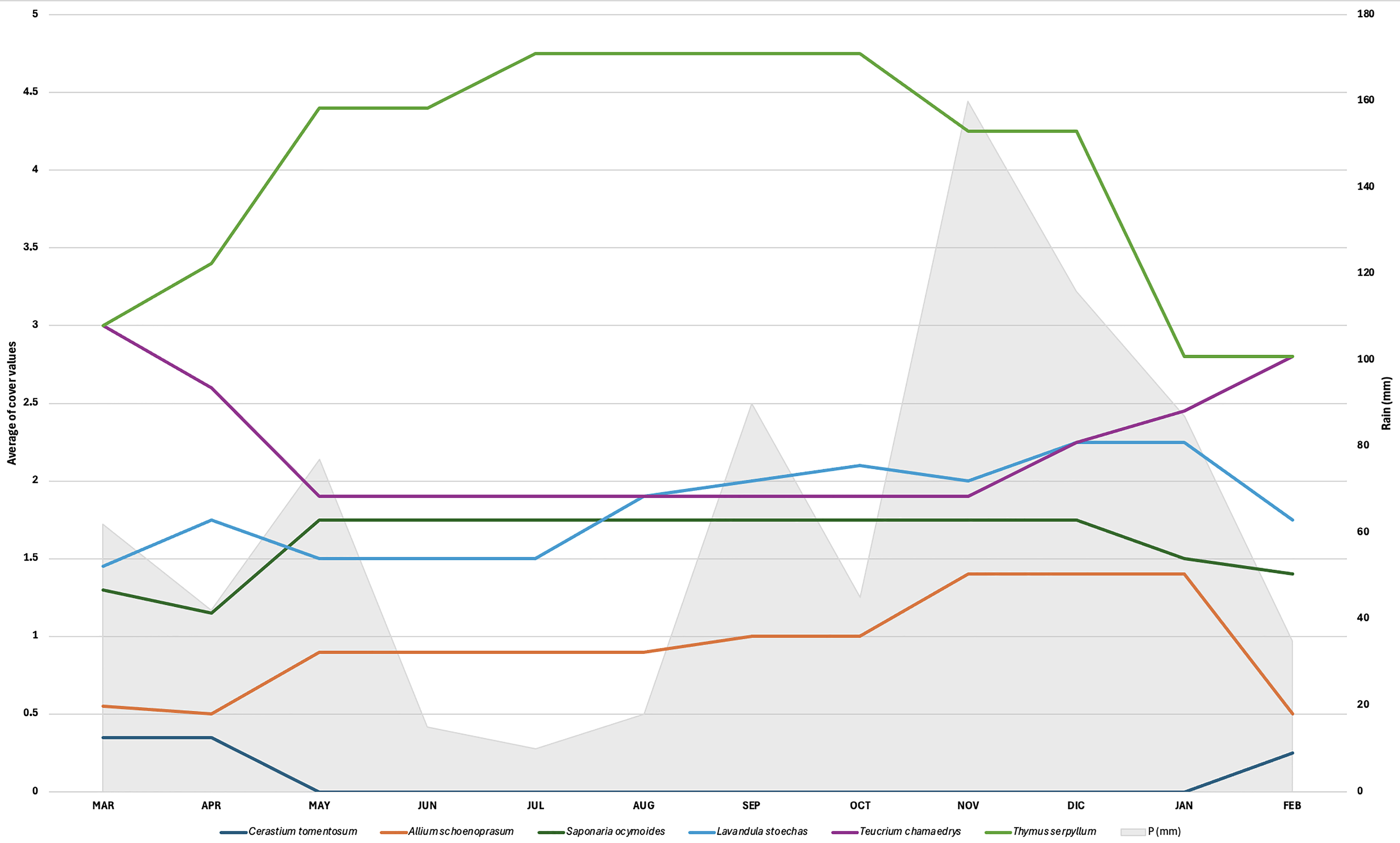
2.1. Evaluation of the Survival Rates of Planted Species Without Irrigation
2.2. Evaluation of the Natural Recolonization Dynamics
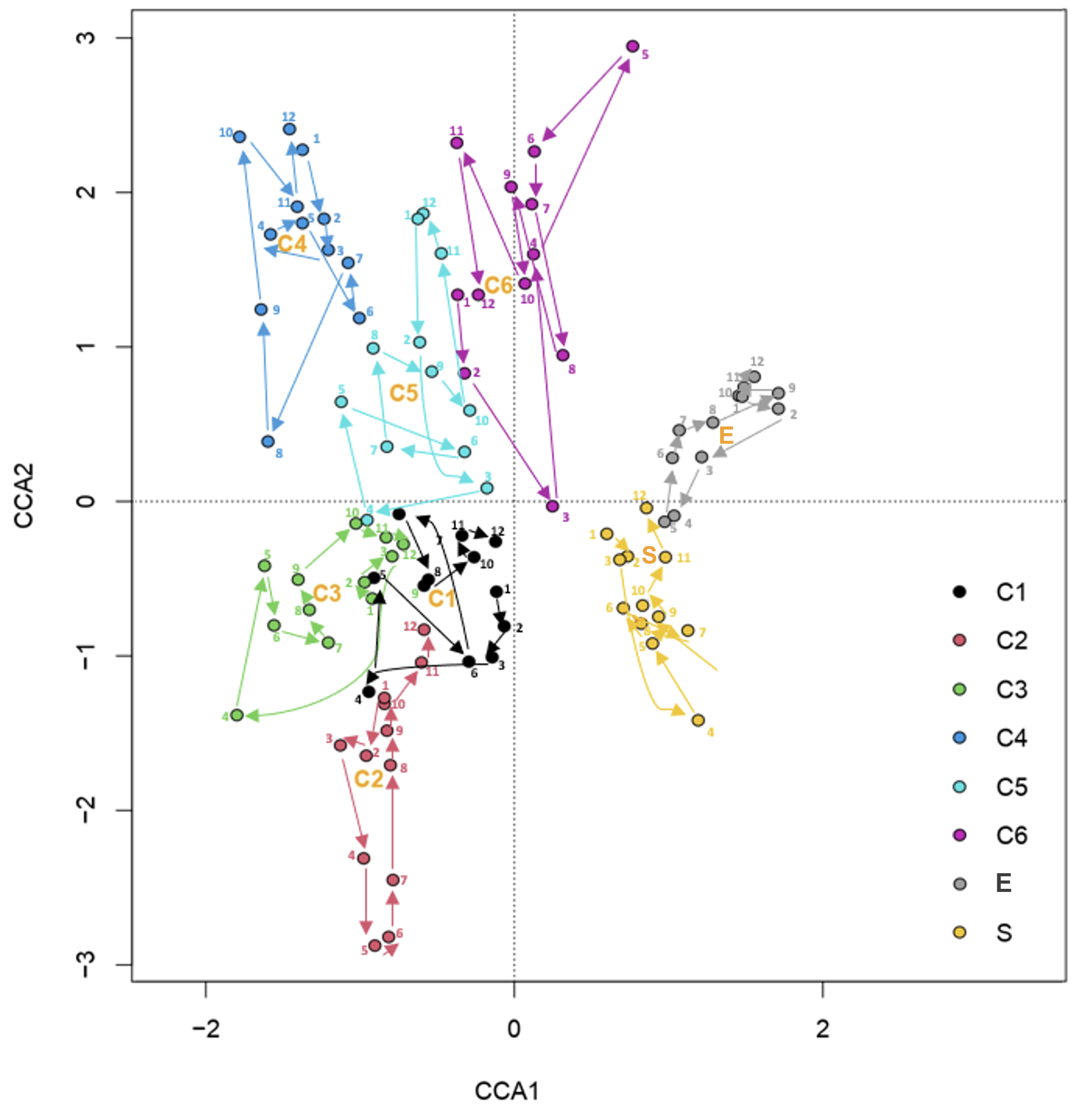
3. Discussion
4. Materials and Methods
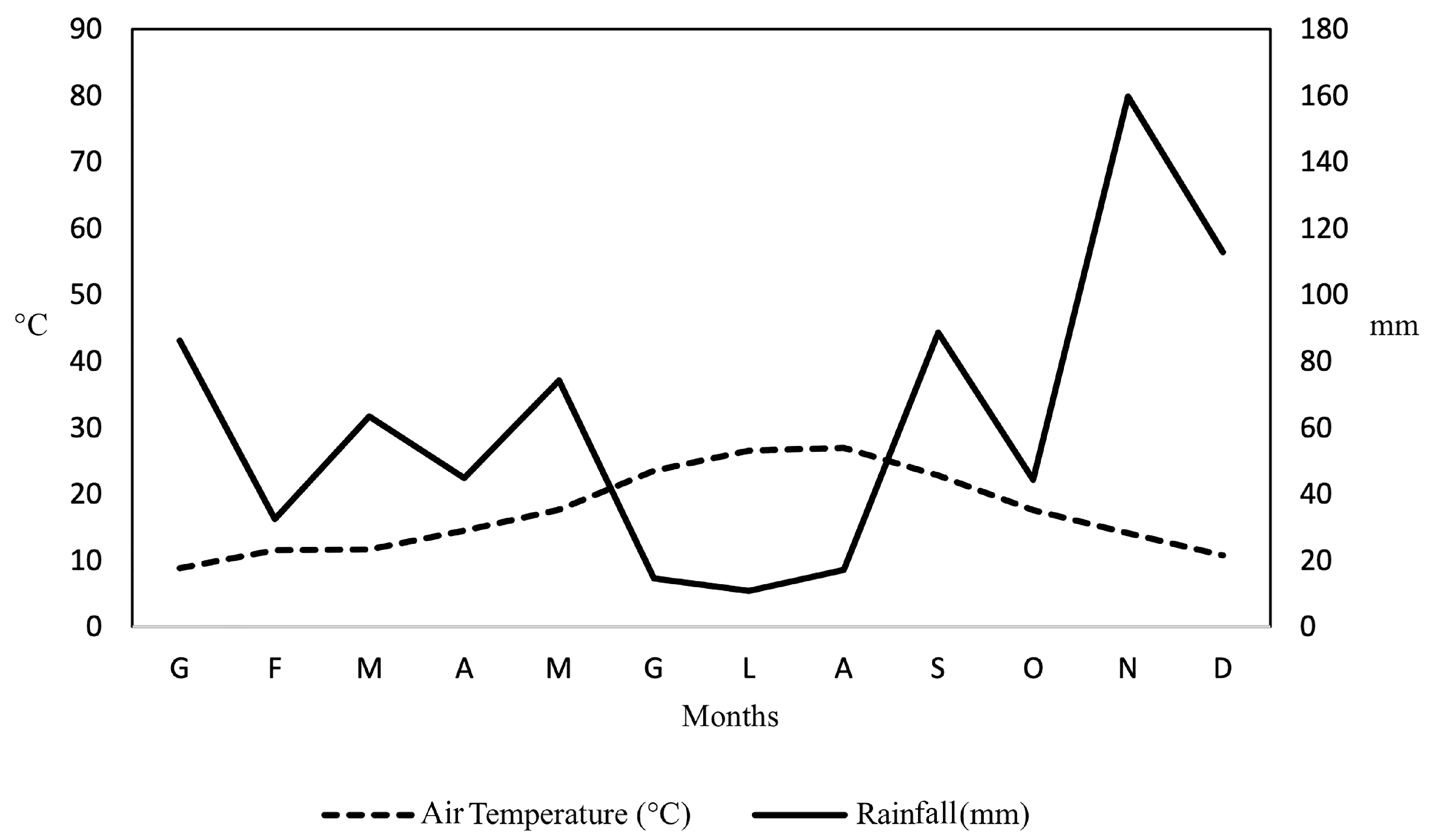
4.1. Climatic Conditions
4.2. The Green Roof Structure and Characteristics
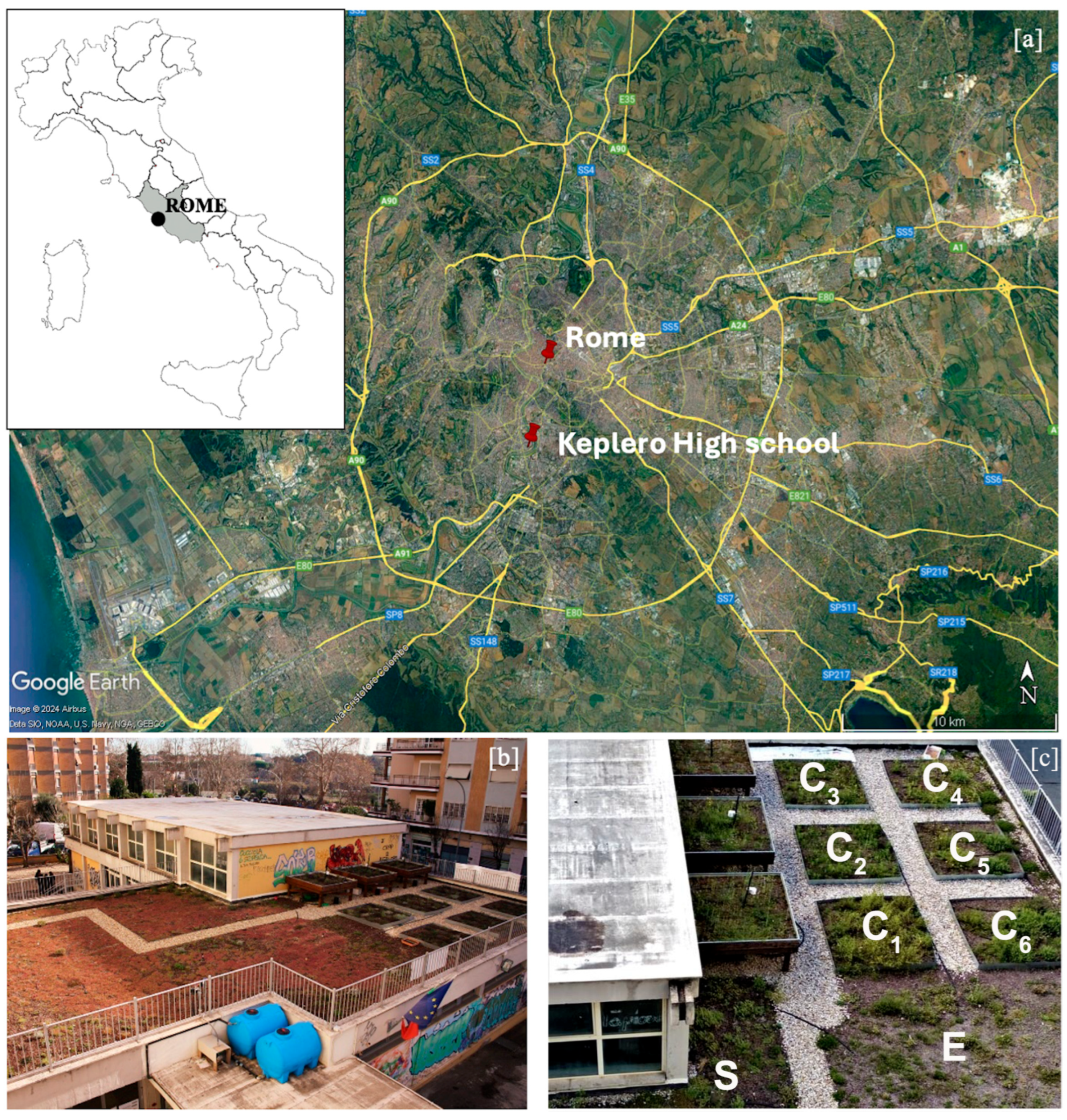
4.3. Experimental Design
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elmqvist, T.; Fragkias, M.; Goodness, J.; Güneralp, B.; Marcotullio, P.J.; McDonald, R.I.; Parnell, S.; Schewenius, M.; Sendstad, M.; Seto, K.C.; et al. Urbanization, Biodiversity and Ecosystem Services: Challenges and Opportunities; Springer: Dordrecht, The Netherlands, 2013; 755p. [Google Scholar]
- Oberndorfer, E.; Lundholm, J.; Bass, B.; Coffman, R.R.; Doshi, H.; Dunnett, N.; Gaffin, S.; Köhler, M.; Liu, K.K.; Rowe, B. Green roofs as urban ecosystems: Ecological structures, functions, and services. BioScience 2007, 57, 823–833. [Google Scholar] [CrossRef]
- Veerkamp, C.J.; Schipper, A.M.; Hedlund, K.; Lazarova, T.; Nordin, A.; Hanson, H.I. A review of studies assessing ecosystem services provided by urban green and blue infrastructure. Ecosyst. Serv. 2021, 52, 101367. [Google Scholar] [CrossRef]
- Bellini, A.; Bartoli, F.; Caneva, G. Extensive Green Roofs (EGRs) and the Five Ws: A Quantitative Analysis on the Origin and Evolution, Aims, Approaches, and Botanical Views. Sustainability 2024, 16, 1033. [Google Scholar] [CrossRef]
- Berardi, U.; GhaffarianHoseini, A. State-of-the-art analysis of the environmental benefits of green roofs. Appl. Energy 2014, 115, 411–428. [Google Scholar] [CrossRef]
- Vijayaraghavan, K. Green roofs: A critical review on the role of components, benefits, limitations and trends. Renew. Sustain. Energy Rev. 2016, 57, 740–752. [Google Scholar] [CrossRef]
- Mentens, J.; Raes, D.; Hermy, M. Green roofs as a tool for solving the rainwater runoff problem in the urbanized 21st century? Landsc. Urban Plan. 2006, 77, 217–226. [Google Scholar] [CrossRef]
- Yang, J.; Yu, Q.; Gong, P. Quantifying air pollution removal by green roofs in Chicago. Atmos. Environ. 2008, 42, 7266–7273. [Google Scholar] [CrossRef]
- Van Renterghem, T.; Botteldooren, D. In-situ measurements of sound propagating over extensive green roofs. Build. Environ. 2011, 46, 729–738. [Google Scholar] [CrossRef]
- Santamouris, M. Cooling the cities—A review of reflective and green roof mitigation technologies to fight heat island and improve comfort in urban environments. Sol. Energy 2014, 103, 682–703. [Google Scholar] [CrossRef]
- Akther, M.; He, J.; Chu, A.; Huang, J.; Van Duin, B. A review of green roof applications for managing urban stormwater in different climatic zones. Sustainability 2018, 10, 2864. [Google Scholar] [CrossRef]
- Kadas, G. Rare invertebrates colonizing green roofs in London. Urban Habitats 2006, 4, 66–86. [Google Scholar]
- Dvorak, B.; Volder, A. Green roof vegetation for North American ecoregions: A literature review. Landsc. Urban Plan. 2010, 96, 197–213. [Google Scholar] [CrossRef]
- Fernández Cañero, R.; González Redondo, P. Green roofs as a habitat for birds: A review. J. Anim. Vet. Adv. 2010, 9, 2041–2052. [Google Scholar]
- Cook-Patton, S.C.; Bauerle, T.L. Potential benefits of plant diversity on vegetated roofs: A literature review. J. Environ. Manag. 2012, 106, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Bretzel, F.; Vannucchi, F.; Benvenuti, S.; Rumble, H. Biodiversity of flora and fauna. In Rooftop Urban Agriculture; Orsini, F., Dubbeling, M., de Zeeuw, H., Gianquinto, G., Eds.; Springer: Cham, Switzerland, 2017; pp. 235–252. [Google Scholar]
- MacIvor, J.S.; Sookhan, N.; Arnillas, C.A.; Bhatt, A.; Das, S.; Yasui, S.L.E.; Xie, G.; Cadotte, M.W. Manipulating plant phylogenetic diversity for green roof ecosystem service delivery. Evol. Appl. 2018, 11, 2014–2024. [Google Scholar] [CrossRef] [PubMed]
- Lundholm, J.; MacIvor, J.S.; MacDougall, Z.; Ranalli, M. Plant species and functional group combinations affect green roof ecosystem functions. PLoS ONE 2010, 5, 9677. [Google Scholar] [CrossRef] [PubMed]
- Vera, S.; Viecco, M.; Jorquera, H. Effects of biodiversity in green roofs and walls on the capture of fine particulate matter. Urban For. Urban Green. 2021, 63, 127229. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Wang, Y.; Che, Y.; Ge, Z.; Mao, L. The relationship between green roofs and urban biodiversity: A systematic review. Biodivers. Conserv. 2022, 31, 1771–1796. [Google Scholar] [CrossRef]
- Lee, K.E.; Williams, K.J.; Sargent, L.D.; Farrell, C.; Williams, N.S. Living roof preference is influenced by plant characteristics and diversity. Landsc. Urban Plan. 2014, 122, 152–159. [Google Scholar] [CrossRef]
- MacIvor, J.S.; Lundholm, J. Performance evaluation of native plants suited to extensive green roof conditions in a maritime climate. Ecol. Eng. 2011, 37, 407–417. [Google Scholar] [CrossRef]
- Shafique, M.; Kim, R.; Rafiq, M. Green roof benefits, opportunities and challenges—A review. Renew. Sustain. Energy Rev. 2018, 90, 757–773. [Google Scholar] [CrossRef]
- Lundholm, J.T.; Walker, E.A. Evaluating the habitat template approach applied to green roofs. Urban Nat. 2018, 1, 39–51. [Google Scholar]
- Van Mechelen, C.; Dutoit, T.; Hermy, M. Mediterranean open habitat vegetation offers great potential for extensive green roof design. Landsc. Urban Plan. 2014, 121, 81–91. [Google Scholar] [CrossRef]
- Caneva, G.; Kumbaric, A.; Savo, V.; Casalini, R. Ecological approach in selecting extensive green roof plants: A data-set of Mediterranean plants. Plant Biosyst. 2015, 149, 374–383. [Google Scholar] [CrossRef]
- Heim, A.; Biermann, B.; Hicks, T.; Buffam, I.; Lundholm, J. More than sedum: Colonizing weedy species can provide equivalent green roof ecosystem services. Nat.-Based Solut. 2024, 5, 100101. [Google Scholar] [CrossRef]
- Dunnett, N. Ruderal green roofs. In Green Roof Ecosystems; Sutton, R., Ed.; Springer: Cham, Switzerland, 2015; pp. 233–255. [Google Scholar]
- Carlisle, S.; Piana, M. Green roof plant assemblage and dynamics. In Green Roof Ecosystems; Sutton, R., Ed.; Springer: Cham, Switzerland, 2015; pp. 285–310. [Google Scholar]
- Madre, F.; Vergnes, A.; Machon, N.; Clergeau, P. Green roofs as habitats for wild plant species in urban landscapes: First insights from a large-scale sampling. Landsc. Urban Plan. 2014, 122, 100–107. [Google Scholar] [CrossRef]
- Deng, H.; Jim, C.Y. Spontaneous plant colonization and bird visits of tropical extensive green roof. Urban Ecosyst. 2017, 20, 337–352. [Google Scholar] [CrossRef]
- Aloisio, J.M.; Palmer, M.I.; Tuininga, A.R.; Lewis, J.D. Plant colonization of green roofs is affected by composition of established native plant communities. Front. Ecol. Evol. 2019, 6, 238. [Google Scholar] [CrossRef]
- Bates, A.J.; Sadler, J.P.; Mackay, R. Vegetation development over four years on two green roofs in the UK. Urban For. Urban Green. 2013, 12, 98–108. [Google Scholar] [CrossRef]
- Van Mechelen, C.; Dutoit, T.; Kattge, J.; Hermy, M. Plant trait analysis delivers an extensive list of potential green roof species for Mediterranean France. Ecol. Eng. 2014, 67, 48–59. [Google Scholar] [CrossRef]
- Vannucchi, F.; Buoncristiano, A.; Scatena, M.; Caudai, C.; Bretzel, F. Low productivity substrate leads to functional diversification of green roof plant assemblage. Ecol. Eng. 2022, 176, 106547. [Google Scholar] [CrossRef]
- Casalini, R.; Bartoli, F.; Caneva, G. Investigation of seed germination of twelve Mediterranean wildflowers for evaluating their potential use on extensive green roofs. Acta Hortic. 2017, 1189, 263–266. [Google Scholar] [CrossRef]
- Bretzel, F.; Vannucchi, F.; Buoncristiano, A.; Caneva, G. Phytosociological approach to implement a Sedum-dominated plant community in extensive Mediterranean green roofs in different N enriched substrates. Acta Hortic. 2022, 1345, 267–274. [Google Scholar] [CrossRef]
- Ndayambaje, P.; MacIvor, J.S.; Cadotte, M.W. Plant diversity on green roofs: A review of the ecological benefits, challenges, and best management practices. Nat.-Based Solut. 2024, 6, 100162. [Google Scholar] [CrossRef]
- Paço, T.A.; Cruz de Carvalho, R.; Arsénio, P.; Martins, D. Green roof design techniques to improve water use under Mediterranean conditions. Urban Sci. 2019, 3, 14. [Google Scholar] [CrossRef]
- Esfahani, R.E.; Paco, T.A.; Martins, D.; Arsenio, P. Increasing the resistance of Mediterranean extensive green roofs by using native plants from old roofs and walls. Ecol. Eng. 2022, 178, 106576. [Google Scholar] [CrossRef]
- Leotta, L.; Toscano, S.; Romano, D. Which plant species for green roofs in the Mediterranean environment? Plants 2023, 12, 3985. [Google Scholar] [CrossRef]
- Dirks, I.; Raviv, B.; Shelef, O.; Hill, A.; Eppel, A.; Aidoo, M.K.; Hoefgen, B.; Rapaport, T.; Gil, H.; Geta, E.; et al. Green roofs: What can we learn from desert plants? Isr. J. Ecol. Evol. 2016, 62, 58–67. [Google Scholar] [CrossRef]
- Fioretti, R.; Palla, A.; Lanza, L.G.; Principi, P. Green roof energy and water related performance in the Mediterranean climate. Build. Environ. 2010, 45, 1890–1904. [Google Scholar] [CrossRef]
- Lynn, B.H.; Lynn, I.M. The impact of cool and green roofs on summertime temperatures in the cities of Jerusalem and Tel Aviv. Sci. Total Environ. 2020, 743, 140568. [Google Scholar] [CrossRef]
- Silva, J.; Paço, T.A.; Sousa, V.; Silva, C.M. Hydrological performance of green roofs in mediterranean climates: A review and evaluation of patterns. Water 2021, 13, 2600. [Google Scholar] [CrossRef]
- De Cristo, E.; Evangelisti, L.; Barbaro, L.; De Lieto Vollaro, R.; Asdrubali, F. A Systematic Review of Green Roofs’ Thermal and Energy Performance in the Mediterranean Region. Energies 2025, 18, 2517. [Google Scholar] [CrossRef]
- Vasl, A.; Schindler, B.Y.; Kadas, G.J.; Blaustein, L. Fine-scale substrate heterogeneity in green roof plant communities: The constraint of size. Ecol. Evol. 2019, 9, 11557–11568. [Google Scholar] [CrossRef] [PubMed]
- Varela-Stasinopoulou, D.S.; Nektarios, P.A.; Ntoulas, N.; Trigas, P.; Roukounakis, G.I. Sustainable growth of medicinal and aromatic Mediterranean plants growing as communities in shallow substrate urban green roof systems. Sustainability 2023, 15, 5940. [Google Scholar] [CrossRef]
- Benvenuti, S.; Bacci, D. Initial agronomic performances of Mediterranean xerophytes in simulated dry green roofs. Urban Ecosyst. 2010, 13, 349–363. [Google Scholar] [CrossRef]
- Kotsiris, G.; Nektarios, P.A.; Ntoulas, N.; Kargas, G. An adaptive approach to intensive green roofs in the Mediterranean climatic region. Urban For. Urban Green. 2013, 12, 380–392. [Google Scholar] [CrossRef]
- Raimondo, F.; Trifilò, P.; Lo Gullo, M.A.; Andri, S.; Savi, T.; Nardini, A. Plant performance on Mediterranean green roofs: Interaction of species-specific hydraulic strategies and substrate water relations. AoB Plants 2015, 7, 007. [Google Scholar] [CrossRef] [PubMed]
- Brandão, C.; do Rosário Cameira, M.; Valente, F.; de Carvalho, R.C.; Paço, T.A. Wet season hydrological performance of green roofs using native species under Mediterranean climate. Ecol. Eng. 2017, 102, 596–611. [Google Scholar] [CrossRef]
- Diffenbaugh, N.S.; Pal, J.S.; Giorgi, F.; Gao, X. Heat stress intensification in the Mediterranean climate change hotspot. Geophys. Res. Lett. 2007, 34. [Google Scholar] [CrossRef]
- Tramblay, Y.; Koutroulis, A.; Samaniego, L.; Vicente-Serrano, S.M.; Volaire, F.; Boone, A.; Le Page, M.; Llasat, M.C.; Albergel, C.; Burak, S.; et al. Challenges for drought assessment in the Mediterranean region under future climate scenarios. Earth-Sci. Rev. 2020, 210, 103348. [Google Scholar] [CrossRef]
- Trenta, M.; Quadri, A.; Sambuco, B.; Perez Garcia, C.A.; Barbaresi, A.; Tassinari, P.; Torreggiani, D. Green Roof Management in Mediterranean Climates: Evaluating the Performance of Native Herbaceous Plant Species and Green Manure to Increase Sustainability. Buildings 2025, 15, 640. [Google Scholar] [CrossRef]
- Lundholm, J.T. Green roof plant species diversity improves ecosystem multifunctionality. J. Appl. Ecol. 2015, 52, 726–734. [Google Scholar] [CrossRef]
- Filazzola, A.; Shrestha, N.; MacIvor, J.S. The contribution of constructed green infrastructure to urban biodiversity: A synthesis and meta-analysis. J. Appl. Ecol. 2019, 56, 2131–2143. [Google Scholar]
- Perrelet, K.; Moretti, M.; Dietzel, A.; Altermatt, F.; Cook, L.M. Engineering blue-green infrastructure for and with biodiversity in cities. NPJ Urban Sustain. 2024, 4, 27. [Google Scholar]
- Rohlf, F.J. Classification of Aedes by numerical taxonomic methods (Diptera: Culicidae). Ann. Entomol. Soc. Am. 1963, 56, 798–804. [Google Scholar] [CrossRef]
- Bellini, A.; Bartoli, F.; Kumbaric, A.; Casalini, R.; Caneva, G. Evaluation of Mediterranean perennials for extensive green roofs in water-limited regions: A two-year experiment. Ecol. Eng. 2024, 209, 107399. [Google Scholar]
- Prodromo Della Vegetazione d’Italia. Available online: https://www.prodromo-vegetazione-italia.org/ (accessed on 10 January 2024).
- Zhang, H.; Lu, S.; Fan, X.; Wu, J.; Jiang, Y.; Ren, L.; Wu, J.; Zhao, H. Is sustainable extensive green roof realizable without irrigation in a temperate monsoonal climate? A case study in Beijing. Sci. Total Environ. 2021, 753, 142067. [Google Scholar] [CrossRef] [PubMed]
- Oschmann, C.; Kobayashi, N.; Perkuhn, C.; Gruneberg, H.; Wissemeier, A.H. Study to expand the range of wild plants for extensive roof greening systems using superabsorbent polymers (SAP). In Proceedings of the VI International Symposium on New Floricultural Crops, Funchal, Portugal, 11–15 June 2007. [Google Scholar]
- Esfahani, R. Applying Ellenberg’s Indicator Values to the Study of Green Roofs Installed with Native Plants. Doctoral Dissertation, Universidade de Lisboa, Lisbon, Portugal, 2020. [Google Scholar]
- Tamarro, F. Taxonomic and vegetational observations on Cerastium tomentosum L. (Caryophyllaceae), an Apennine endemic orophyte (Italy). Fitosociologia 1994, 26, 133–144. [Google Scholar]
- Piro, P.; Porti, M.; Veltri, S.; Lupo, E.; Moroni, M. Hyperspectral monitoring of green roof vegetation health state in sub-mediterranean climate: Preliminary results. Sensors 2017, 17, 662. [Google Scholar] [CrossRef] [PubMed]
- Nagase, A.; Dunnett, N.; Choi, M.S. Investigation of weed phenology in an establishing semi-extensive green roof. Ecol. Eng. 2013, 58, 156–164. [Google Scholar] [CrossRef]
- Vanstockem, J.; Bastiaens, A.; Helsen, K.; Somers, B.; Hermy, M. Community assembly on extensive green roofs: Effects of dispersal-, abiotic- and biotic filtering on the spontaneous species-and functional diversity. J. Veg. Sci. 2019, 30, 1078–1088. [Google Scholar] [CrossRef]
- Heim, A.; Lundholm, J. Changes in plant community composition and functional plant traits over a four-year period on an extensive green roof. J. Environ. Manag. 2022, 304, 114154. [Google Scholar] [CrossRef]
- Catalano, C.; Marcenò, C.; Laudicina, V.A.; Guarino, R. Thirty years unmanaged green roofs: Ecological research and design implications. Landsc. Urban Plan. 2016, 149, 11–19. [Google Scholar] [CrossRef]
- Ksiazek-Mikenas, K.; Köhler, M. Traits for stress-tolerance are associated with long-term plant survival on green roofs. J. Urban Ecol. 2018, 4, 16. [Google Scholar] [CrossRef]
- Howe, H.F.; Smallwood, J. Ecology of seed dispersal. Annu. Rev. Ecol. Syst. 1982, 13, 201–228. [Google Scholar] [CrossRef]
- Knapp, S.; Kühn, I.; Wittig, R.; Ozinga, W.A.; Poschlod, P.; Klotz, S. Urbanization causes shifts of species’ trait state frequencies. Preslia 2008, 80, 375–388. [Google Scholar]
- Williams, N.S.; Hahs, A.K.; Vesk, P.A. Urbanisation, plant traits and the composition of urban floras. Perspect. Plant Ecol. Evol. Syst. 2015, 17, 78–86. [Google Scholar] [CrossRef]
- Getter, K.L.; Rowe, D.B. Substrate depth influences Sedum plant community on a green roof. HortScience 2009, 44, 401–407. [Google Scholar] [CrossRef]
- Thuring, C.E.; Dunnett, N. Vegetation composition of old extensive green roofs (from 1980s Germany). Ecol. Process. 2014, 3, 4. [Google Scholar] [CrossRef]
- Rumble, H.; Gange, A.C. Soil microarthropod community dynamics in extensive green roofs. Ecol. Eng. 2013, 57, 197–204. [Google Scholar] [CrossRef]
- Getter, K.L.; Rowe, D.B.; Cregg, B.M. Solar radiation intensity influences extensive green roof plant communities. Urban For. Urban Green. 2009, 8, 269–281. [Google Scholar] [CrossRef]
- Köhler, M.; Poll, P.H. Long-term performance of selected old Berlin greenroofs in comparison to younger extensive greenroofs in Berlin. Ecol. Eng. 2010, 36, 722–729. [Google Scholar] [CrossRef]
- Savi, T.; Dal Borgo, A.; Love, V.L.; Andri, S.; Tretiach, M.; Nardini, A. Drought versus heat: What’s the major constraint on Mediterranean green roof plants? Sci. Total Environ. 2016, 566, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Lönnqvist, J.; Hanslin, H.M.; Johannessen, B.G.; Muthanna, T.M.; Viklander, M.; Blecken, G. Temperatures and precipitation affect vegetation dynamics on Scandinavian extensive green roofs. Int. J. Biometeorol. 2021, 65, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, R.J.; Arico, S.; Aronson, J.; Baron, J.S.; Bridgewater, P.; Cramer, V.A.; Epstein, P.R.; Ewel, J.J.; Klink, C.A.; Lugo, A.E.; et al. Novel ecosystems: Theoretical and management aspects of the new ecological world order. Glob. Ecol. Biogeogr. 2006, 15, 1–7. [Google Scholar] [CrossRef]
- Van Mechelen, C.; Van Meerbeek, K.; Dutoit, T.; Hermy, M. Functional diversity as a framework for novel ecosystem design: The example of extensive green roofs. Landsc. Urban Plan. 2015, 136, 165–173. [Google Scholar] [CrossRef]
- Heim, A.; Bradbury, C.; Xie, G.; Lundholm, J. Green Roof Plant Traits: Influence of Functional Diversity on Ecosystem Services and Coexistence. Nat.-Based Solut. 2023, 4, 100091. [Google Scholar] [CrossRef]
- Muratet, A.; Barra, M.; Hardion, L.; Chiron, F. Origins and drivers of roof plant assemblages: Designing green roofs for biodiversity conservation. Urban For. Urban Green. 2024, 94, 128247. [Google Scholar] [CrossRef]
- Gao, Z.; Pan, Y.; Song, K.; Yang, Y.; Zhuge, M.; Wu, T.; Hu, Y.; Da, L.; Cieraad, E. Response and sensitivity of urban plants with different seed dispersal modes. Nat. Cities 2025, 2, 28–37. [Google Scholar] [CrossRef]
- Köhler, M. Long-term vegetation research on two extensive green roofs in Berlin. Urban Habitats 2006, 4, 3–26. [Google Scholar]
- Dunnett, N.; Nagase, A.; Hallam, A. The dynamics of planted and colonising species on a green roof over six growing seasons 2001–2006: Influence of substrate depth. Urban Ecosyst. 2008, 11, 373–384. [Google Scholar] [CrossRef]
- Rowe, D.B.; Getter, K.L.; Durhman, A.K. Effect of green roof media depth on Crassulacean plant succession over seven years. Landsc. Urban Plan. 2012, 104, 310–319. [Google Scholar] [CrossRef]
- Xie, G.; Lundholm, J.T.; MacIvor, J.S. Phylogenetic diversity and plant trait composition predict multiple ecosystem functions in green roofs. Sci. Total Environ. 2018, 628, 1017–1026. [Google Scholar] [CrossRef]
- Guattari, C.; Evangelisti, L.; Balaras, C.A. On the assessment of urban heat island phenomenon and its effects on building energy performance: A case study of Rome (Italy). Energy Build. 2018, 158, 605–615. [Google Scholar] [CrossRef]
- ISO 12958; Geotextiles and Geotextile-Related Products — Determination of Water Flow Capacity in Their Plane. International Standard Published: Vernier, Switzerland, 2020. Available online: https://www.iso.org/obp/ui/en/#iso:std:74632:en (accessed on 12 October 2025).
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, 2nd ed.; Edagricole: Milano, Italy, 2017; Volume 2. [Google Scholar]
- Index Plantarum Flora Italicae IPFI. Available online: https://www.actaplantarum.org/flora/flora.php (accessed on 8 October 2023).
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge der Vegetationskunde; Springer: Vienna, Austria, 1964. [Google Scholar]
- Biondi, E.; Allegrezza, M.; Casavecchia, S.; Galdenzi, D.; Gasparri, R.; Pesaresi, S.; Soriano, P.; Tesei, G.; Blasi, C. New insight on Mediterranean and sub-Mediterranean syntaxa included in the Vegetation Prodrome of Italy. Flora Mediterr. 2015, 25, 77–102. [Google Scholar] [CrossRef]
- Biondi, E.; Allegrezza, M.; Casavecchia, S.; Galdenzi, D.; Gasparri, R.; Pesaresi, S.; Poldini, L.; Sburlino, G.; Vagge, I.; Venanzoni, R. New syntaxonomic contribution to the Vegetation Prodrome of Italy. Plant Biosyst. 2015, 149, 603–615. [Google Scholar] [CrossRef]
- Database of European Vegetation, Habitats and Flora. Available online: https://floraveg.eu/ (accessed on 8 October 2023).
- Van der Maarel, E. Transformation of cover-abundance values in phytosociology and its effects on community similarity. Vegetatio 1979, 39, 97–114. [Google Scholar]
- McCune, B.; Mefford, M.J. PC-ORD. Multivariate Analysis of Ecological Data. Version 4; MjM Software Design: Gleneden Beach, OR, USA, 1999. [Google Scholar]
- Sokal, R.R.; Michener, C.D. A statistical method for evaluating systematic relationships. Univ. Kans. Sci. Bull. 1958, 38, 1409–1438. https://sid.ir/paper/549615/en.
- Kurita, T. Principal component analysis (PCA). In Computer Vision: A Reference Guide; Ikeuchi, K., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1013–1016. [Google Scholar]
- Uurtio, V.; Monteiro, J.M.; Kandola, J.; Shawe-Taylor, J.; Fernandez-Reyes, D.; Rousu, J. A tutorial on canonical correlation methods. ACM Comput. Surv. (CSUR) 2017, 50, 1–33. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002; 300p. [Google Scholar]
- R Programming Language. Available online: https://www.r-project.org (accessed on 2 February 2024).

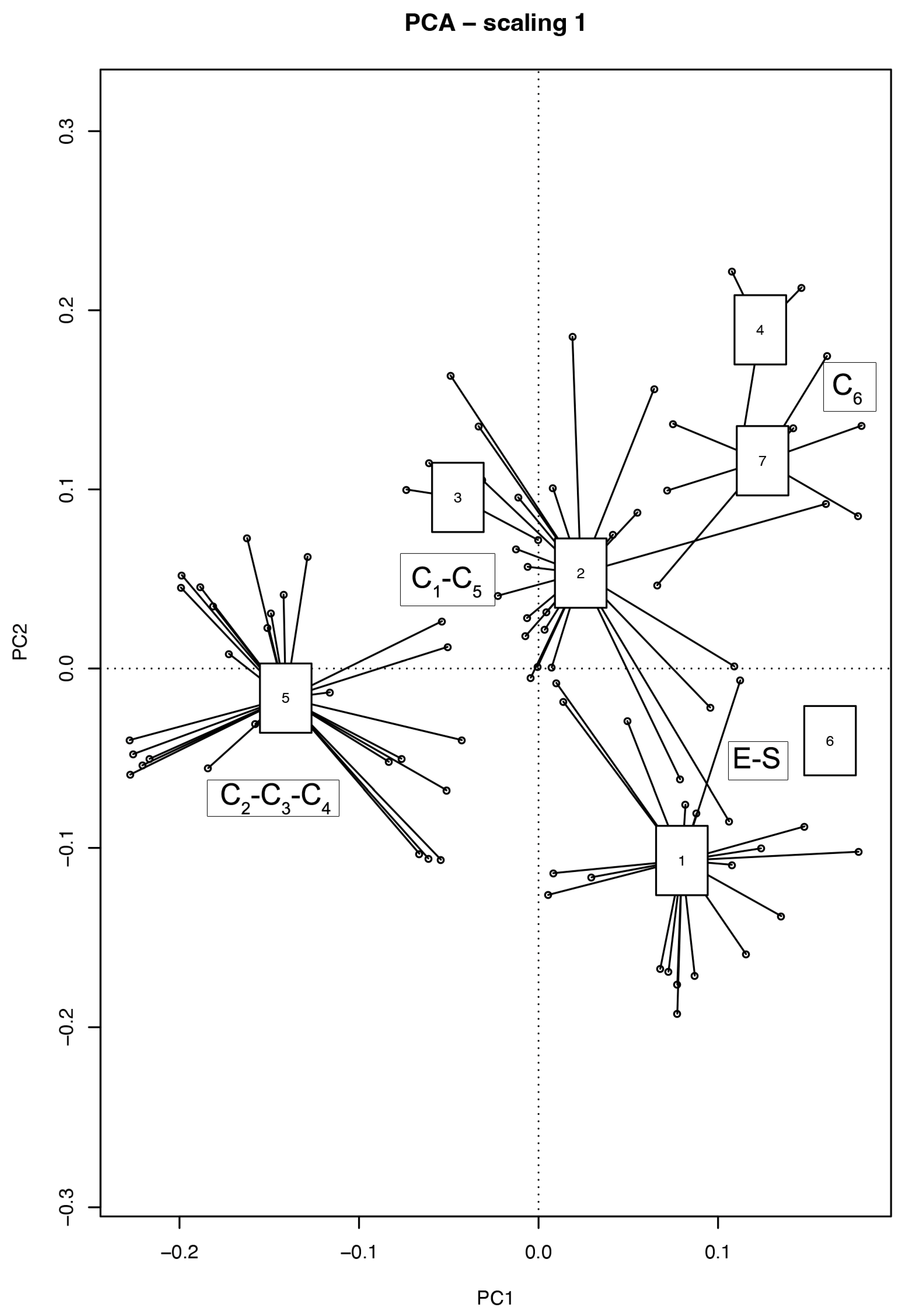
| Group 1: (E and S) | A | B | IndVal | p. Value |
| Veronica persica | 0.9623 | 1 | 0.981 | 0.001 |
| Erigeron canadensis | 1 | 0.9167 | 0.957 | 0.001 |
| Geranium rotundifolium | 0.5917 | 1 | 0.769 | 0.001 |
| Festuca bromoides | 0.9219 | 0.5833 | 0.733 | 0.005 |
| Sonchus asper | 0.5124 | 1 | 0.716 | 0.001 |
| Group 2: C1, C2, C3, C4, C5, C6 | A | B | IndVal | p. value |
| Helminthotheca echioides | 0.9091 | 1 | 0.953 | 0.001 |
| Hypochaeris achyrophorus | 0.5732 | 0.92 | 0.726 | 0.001 |
| Erigeron sumatrensis | 0.5209 | 1 | 0.722 | 0.001 |
| Medicago sativa | 0.4367 | 1 | 0.661 | 0.009 |
| Erigeron karviskianus | 0.4008 | 1 | 0.633 | 0.001 |
| Species | Chorotype | Life Form | Family |
|---|---|---|---|
| Teucrium chamaedrys L. | Steno-Medit | Chamaephytes | Lamiaceae |
| Lavandula stoechas L. | Steno-Medit. | Nanophanerophytes | Lamiaceae |
| Cerastium tomentosum L. | Endem. Ital. | Chamaephytes | Caryophyllaceae |
| Thymus serpyllum L. | S-Europ | Chamaephytes | Lamiaceae |
| Saponaria ocymoides L. | Oroph. S-Europ/Oroph. SW-Europ. | Hemicryptophytes | Caryophyllaceae |
| Allium schoenoprasum L. | Eurosiber-Circumbor. | Geophytes | Amaryllidaceae |
| Braun–Blanquet Scale | Range of Cover (%) | Van der Maarel’s Ordinal Scale |
|---|---|---|
| V | 75–100 | 9 |
| IV | 50–75 | 8 |
| III | 25–50 | 7 |
| II | 5–25 | 5 |
| I | 1–5 | 3 |
| + | <1 | 2 |
| - | absent | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellini, A.; Savo, V.; Caneva, G.; D’Amico, E.; Casalini, R.; Bartoli, F. How a Green Roof Becomes Biodiverse: Vegetation Analysis on a Green Roof with no Maintenance in Rome (Italy). Plants 2025, 14, 3180. https://doi.org/10.3390/plants14203180
Bellini A, Savo V, Caneva G, D’Amico E, Casalini R, Bartoli F. How a Green Roof Becomes Biodiverse: Vegetation Analysis on a Green Roof with no Maintenance in Rome (Italy). Plants. 2025; 14(20):3180. https://doi.org/10.3390/plants14203180
Chicago/Turabian StyleBellini, Amii, Valentina Savo, Giulia Caneva, Elettra D’Amico, Roberto Casalini, and Flavia Bartoli. 2025. "How a Green Roof Becomes Biodiverse: Vegetation Analysis on a Green Roof with no Maintenance in Rome (Italy)" Plants 14, no. 20: 3180. https://doi.org/10.3390/plants14203180
APA StyleBellini, A., Savo, V., Caneva, G., D’Amico, E., Casalini, R., & Bartoli, F. (2025). How a Green Roof Becomes Biodiverse: Vegetation Analysis on a Green Roof with no Maintenance in Rome (Italy). Plants, 14(20), 3180. https://doi.org/10.3390/plants14203180










