Environmental Influences on Growth and Secondary Metabolite Accumulation in Eleutherococcus sessiliflorus Across Korean Cultivation Sites
Abstract
1. Introduction
2. Results
2.1. Growth Characteristics
2.2. Soil and Meteorological Characteristics
2.3. Validation of Analytical Method and Quantification of Major Compounds
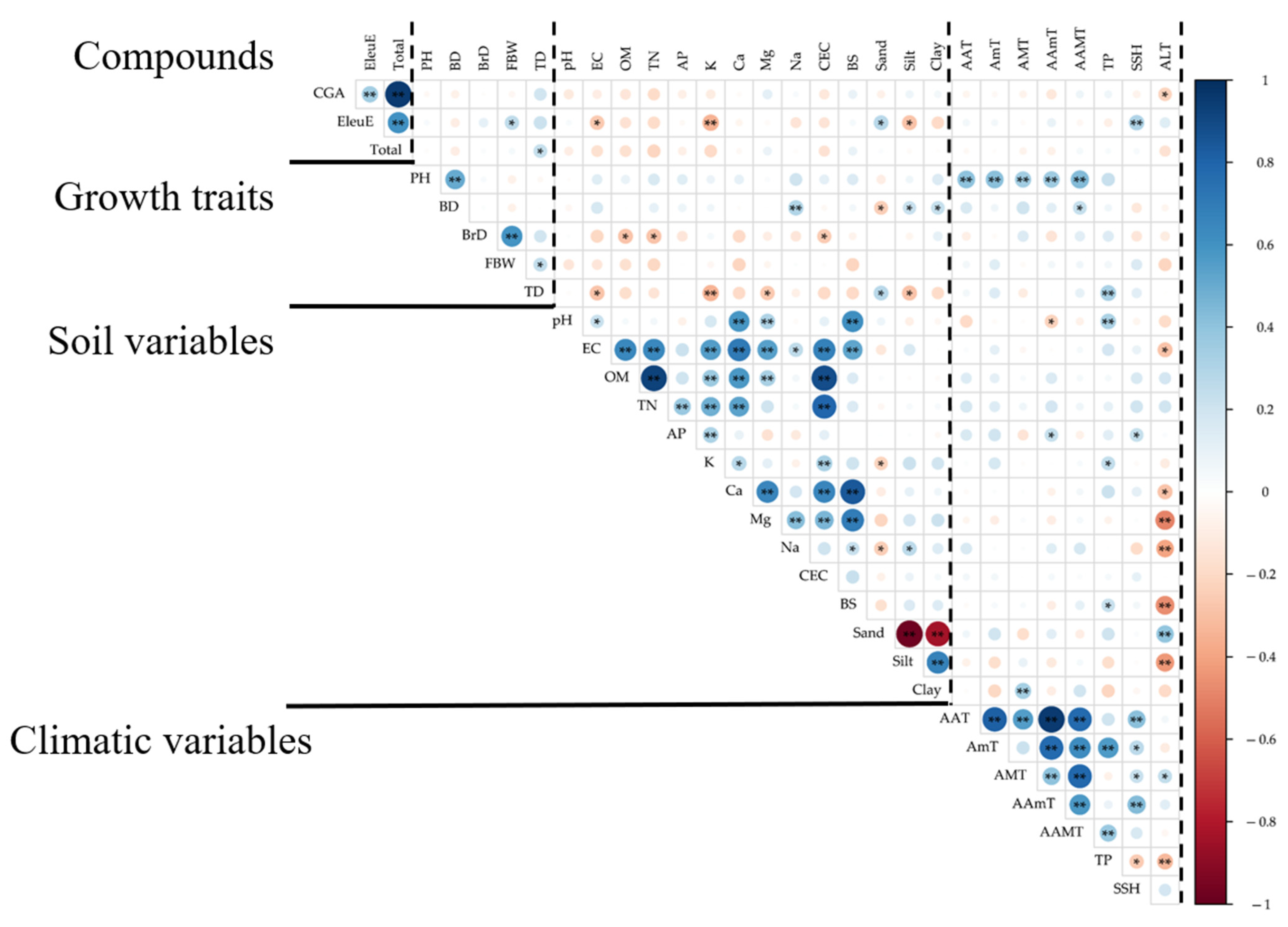
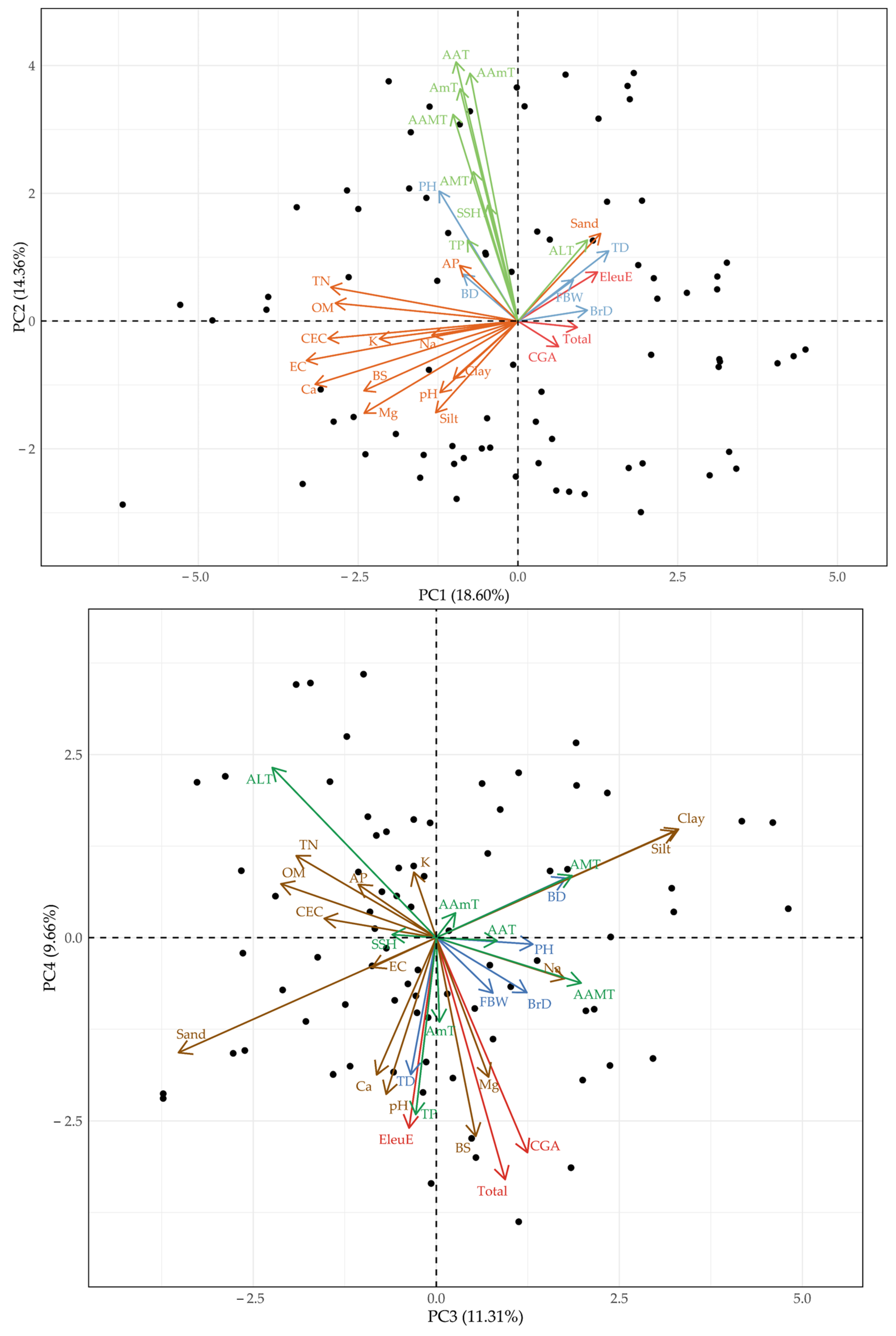
2.4. Correlation Analysis
3. Discussion
4. Materials & Methods
4.1. Sampling of Plant and Material
4.2. Sample Preparation
4.3. UPLC Conditions and Validation
4.4. Soil Sample and Meteorological Data
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Korea National Arboretum. Checklist of Vascular Plants in Korea; Korean Plant Names Index Committee: Pocheon, Republic of Korea, 2025; Available online: http://www.nature.go.kr/kpni/index.do (accessed on 6 September 2025).
- Flora of China Editorial Committee. Flora of China; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2007; Volume 13, pp. 466–472. [Google Scholar]
- Ministry of Food and Drug Safety. The Korean Pharmacopoeia, 12th ed.; Ministry of Food and Drug Safety: Cheongju, Republic of Korea, 2020; pp. 2321–2322.
- Pharmacopoeia Commission of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China, 2020 ed.; China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Huang, L.; Zhao, H.; Huang, B.; Zheng, C. The Traditional Uses, Secondary Metabolites, and Pharmacology of Eleutherococcus Species. Diversity 2021, 13, 462. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, X.; Cai, E.B.; Gao, Y.G.; He, Z.M.; Zhu, H.Y.; Liu, S.L.; Yang, H.; Zhang, L.X. Research Progress on Chemical Constituents and Pharmacological Effects of Acanthopanax sessiliflorus. Shanghai J. Tradit. Chin. Med. 2016, 50, 98–101. [Google Scholar]
- Sun, H.; Feng, J.; Sun, Y.; Sun, S.; Li, L.; Zhu, J.; Zang, H. Phytochemistry and Pharmacology of Eleutherococcus sessiliflorus (Rupr. & Maxim.) S.Y. Hu: A Review. Molecules 2023, 28, 6564. [Google Scholar]
- Kim, H.; Park, J.; Kim, J.; Lee, S.; Lee, C.K.; Oh, S.R.; Ahn, K.S. Anti-Inflammatory Effects of Fermented Bark of Acanthopanax sessiliflorus. Evid. Based Complement. Alternat. Med. 2020, 2020, 4581276. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Kim, M.J.; Ha, E.; Uhm, Y.K.; Kim, H.K.; Lee, H.Y. Antihypertensive Effect of Ethanolic Extract from Acanthopanax sessiliflorus. Oxid. Med. Cell. Longev. 2018, 2018, 6102458. [Google Scholar] [CrossRef]
- Wang, W.; Xu, J.; Huang, Q.; Huang, X.J.; Zhao, C.; Xu, W.; Xu, J.K.; Ye, W.C. Cytotoxic and Anti-Tumor Effects of 3,4-Seco-Lupane Triterpenoids from the Leaves of Eleutherococcus sessiliflorus against Hepatocellular Carcinoma. Nat. Prod. Res. 2020, 34, 1683–1689. [Google Scholar] [CrossRef]
- Park, H.R.; Lee, J.M.; Lee, J.S.; Kim, M.J.; Chang, S.M.; Lee, J.H.; Kim, H.S.; Hwang, B.Y.; Kim, S.Y. Acanthopanax sessiliflorus Stem Confers Increased Resistance to Environmental Stresses and Lifespan Extension in Caenorhabditis elegans. Nutr. Res. Pract. 2014, 8, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, H.G.; Han, J.M.; Kim, D.W.; Kim, M.C.; Lee, C.H.; Son, C.G. Neuroprotective Effect of Acanthopanax sessiliflorus Stem Bark on Ischemia-Induced Neuronal Death in the Rat Hippocampus. Biol. Pharm. Bull. 2005, 28, 1816–1820. [Google Scholar]
- Ma, Y.; Zhang, D.; Jiang, M. Chemical Constituents of Eleutherococcus sessiliflorus (Rupr. & Maxim.). Nat. Prod. Commun. 2020, 15, 1934578X20905760. [Google Scholar] [CrossRef]
- He, C.; Chen, X.; Zhao, C.; Qie, Y.; Yan, Z.; Zhu, X. Eleutheroside E ameliorates arthritis severity in collagen-induced arthritis mice model by suppressing inflammatory cytokine release. Inflammation 2014, 37, 1533–1543. [Google Scholar] [CrossRef]
- Ahn, J.; Um, M.Y.; Lee, H.; Jung, C.H.; Heo, S.H.; Ha, T.Y. Eleutheroside E, an active component of Eleutherococcus senticosus, ameliorates insulin resistance in type 2 diabetic db/db mice. Evid. Based Complement. Alternat. Med. 2013, 2013, 934183. [Google Scholar] [CrossRef]
- Zeng, D.; Xiong, Y.; Yin, Y.; Shan, S.; Duan, F.; Gao, X.; Song, C.; Liu, M.; Zhang, Y.; Lu, W. Identification of targets and mechanisms for eleutheroside E in the treatment of cancer. J. Future Foods 2022, 2, 69–81. [Google Scholar] [CrossRef]
- Xu, S.; Cui, B.; Zhao, G.; Zhang, Y.; Ying, J.; Qiu, D.; Yang, Y.; Bao, L.; Liu, Z.; Liu, J.; et al. Simultaneous Determination of Eleutheroside E, Isothiazine, Chlorogenic Acid, Protocatechuic Acid and Oleanolic Acid in Acanthopanax by LC-MS/MS. Bot. Res. 2017, 6, 201–210. (In Chinese) [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, Y.H.; Lee, S.; Kim, J.H.; Park, H. Optimization of Conditions for High Concentration of Eleutheroside E and Chlorogenic Acid Components of Acanthopanax koreanum Stem Extract. Appl. Biol. Chem. 2020, 63, 47. [Google Scholar]
- Bączek, K.; Kosakowska, O.; Przybył, J.L.; Kuźma, P.; Głowniak, K.; Węglarz, Z. Phytoconstituents and Nutritional Properties of the Fruits of Eleutherococcus divaricatus and Eleutherococcus sessiliflorus: A Study of Non-European Species. Oxid. Med. Cell. Longev. 2017, 2017, 8374295. [Google Scholar]
- Kil, Y.S.; Park, J.; Seo, E.K. Utilization of Circular Dichroism Experiment to Distinguish Acanthoside D and Eleutheroside E. J. Nat. Med. 2015, 69, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Cieśla, Ł.; Załuski, D.; Smolarz, H.D.; Hajnos, M.; Waksmundzka-Hajnos, M. HPTLC-Densitometric Method for Determination of Eleutherosides B, E, and E1 in Different Eleutherococcus Species. J. Chromatogr. Sci. 2011, 49, 182–188. [Google Scholar] [CrossRef]
- Murthy, H.N.; Kim, Y.S.; Georgiev, M.I.; Paek, K.Y. Biotechnological production of eleutherosides: Current state and perspectives. Appl. Microbiol. Biotechnol. 2014, 98, 7319–7329. [Google Scholar] [CrossRef] [PubMed]
- Załuski, D.; Smolarz, H.D.; Szewczyk, A.; Komsta, Ł. Bioactive compounds and antioxidative, antileukemic and anti-MMPs activity of Eleutherococcus species cultivated in Poland. Nat. Prod. Commun. 2012, 7, 1659–1662. [Google Scholar] [CrossRef]
- Załuski, D.; Smolarz, H.D.; Chomicki, A. TLC screening for eleutherosides B, E, and E1, and isofraxidin in the roots of six Eleutherococcus species cultivated in Poland. Acta. Chromatogr. 2010, 22, 581–589. [Google Scholar] [CrossRef]
- Załuski, D.; Smolarz, H.D.; Szpilewska, M. Eleutherosides in aerial parts of Eleutherococcus species cultivated in Poland. J. AOAC Int. 2011, 94, 1422–1426. [Google Scholar] [CrossRef]
- Xu, M.; Wang, H. Changes in growth and photosynthetic parameters and medicinal compounds in Eleutherococcus senticosus Harms under drought stress. Ind. Crops Prod. 2019, 141, 111776. [Google Scholar] [CrossRef]
- Xu, S.; Wu, K.; Liu, Y.; Liu, J.; Tang, Z. Effects of light intensity on the growth, photosynthetic characteristics, and secondary metabolites of Eleutherococcus senticosus. Photosynthetica 2020, 58, 881–889. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kim, Y.S.; Moon, H.K.; Hwang, S.J.; Choi, Y.E. Effects of LED on growth, morphogenesis and eleutheroside contents of in vitro cultured plantlets of Eleutherococcus senticosus Maxim. Korean J. Med. Crop Sci. 2009, 17, 39–45. [Google Scholar]
- Antonio, A.S.; Wiedemann, L.S.M.; Veiga Junior, V.F. The genus Capsicum: A phytochemical review of bioactive secondary metabolites. RSC Adv. 2018, 8, 25767–25784. [Google Scholar] [CrossRef]
- Mossi, A.J.; Cansian, R.L.; Carvalho, A.Z.; Dariva, C.; Oliveira, J.V.; Mazutti, M.A.; Vladimir Oliveira, D.; Echeverrigaray, S. Morphological characterisation and agronomical parameters of different species of Salvia sp. (Lamiaceae). Braz. J. Biol. 2011, 71, 121–127. [Google Scholar] [CrossRef]
- Lee, D.H.; Son, Y.; Jang, J.H.; Jeong, D.H.; Kim, H.J.; Kim, J.A. Environmental effects on Atractylodes macrocephala rhizome growth and compounds. Agriculture 2025, 15, 1950. [Google Scholar] [CrossRef]
- National Institute of Forest Science (NIFoS). Status of Forest Soil Acidification in Korea; Research Data No. 1107; National Institute of Forest Science: Seoul, Republic of Korea, 2023; pp. 19–24. [Google Scholar]
- Rural Development Administration (RDA). The New Resource Plants. Agricultural Technology Guide No. 122, 4th ed.; Rural Development Administration: Jeonju, Republic of Korea, 2018; pp. 12–26. [Google Scholar]
- Tang, C. Soil Fertility, Plant Nutrition, and Nutrient Management. Plants 2025, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Messaoud, Y.; Charrier, G.; Wortemann, R.; Obojes, N.; Peters, R.; Lingua, E.; Carrer, M. Tree Growth in Relation to Climate Change: Understanding Past, Present, and Future Trends. Forests 2024, 15, 1601. [Google Scholar] [CrossRef]
- Li, Y.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Son, Y.; Jang, J.H.; Lee, S.Y.; Kim, H.J. The growth characteristics and the active compounds of Cudrania tricuspidata fruits in different cultivation environments in South Korea. Plants 2023, 12, 2107. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Li, Z.; Li, C. Soil texture’s hidden influence: Decoding plant diversity patterns in arid ecosystems. Soil Syst. 2025, 9, 84. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Z.; Zhang, J.; Zhou, B.; Lv, T.; Li, W. Effects of soil texture on soil leaching and cotton (Gossypium hirsutum L.) growth under combined irrigation and drainage. Water 2021, 13, 3614. [Google Scholar] [CrossRef]
- Sprugel, D.G. When branch autonomy fails: Milton’s law of resource availability and allocation. Tree Physiology 2002, 22, 1119–1124. [Google Scholar] [CrossRef]
- King, D.A. Branch growth and biomass allocation in Abies amabilis saplings in contrasting light environments. Tree Physiolo 1997, 17, 251–258. [Google Scholar] [CrossRef]
- Yang, A.; Qi, X.; Wang, Q.M.; Wang, H.; Wang, Y.; Li, L.; Liu, W.; Qiao, Y. The branch-thorn occurrence of Lycium ruthenicum is associated with leaf DNA hypermethylation in response to soil water content. Mol. Biol. Rep. 2022, 49, 1925–1934. [Google Scholar] [CrossRef]
- Ninkuu, V.; Aluko, O.O.; Yan, J.; Zeng, H.; Liu, G.; Zhao, J.; Li, H.; Chen, S.; Dakora, F.D. Phenylpropanoids metabolism: Recent insight into stress tolerance and plant development cues. Front. Plant Sci. 2025, 16, 1571825. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.X.; Liu, J.; Liu, Y.; Guo, X.R.; Mu, L.Q.; Hu, X.H.; Tang, Z.H. A comparative metabolomics analysis reveals the tissue-specific phenolic profiling in two Acanthopanax species. Molecules 2018, 23, 2078. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Food and Drug Safety (MFDS). Establishment of Marker Compounds and Content Criteria for Herbal Medicines; Final Report of Research Center on Standardization of Herbal Medicines; Chungnam National University: Daejeon, Republic of Korea, 2014; pp. 562–568.
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, M.K.; Kumar, P.; Dogra, V.; Kumar, S. Prickle morphogenesis in rose is coupled with secondary metabolite accumulation and governed by canonical MBW transcriptional complex. Plant Direct 2021, 5, e00325. [Google Scholar] [CrossRef] [PubMed]
- ICH Guideline. Validation of Analytical Procedures Q2 (R2); ICH: Geneva, Switzerland, 2022. [Google Scholar]
- United States Department of Agriculture. Soil Survey Manual; United States Department of Agriculture: Washington, DC, USA, 2017; pp. 120–125.
- Rural Development Administration (RDA). Comprehensive Testing Laboratory Analysis Manual (2023); Rural Development Administration: Jeonju, Republic of Korea, 2023; pp. 59–85. (In Korean) [Google Scholar]

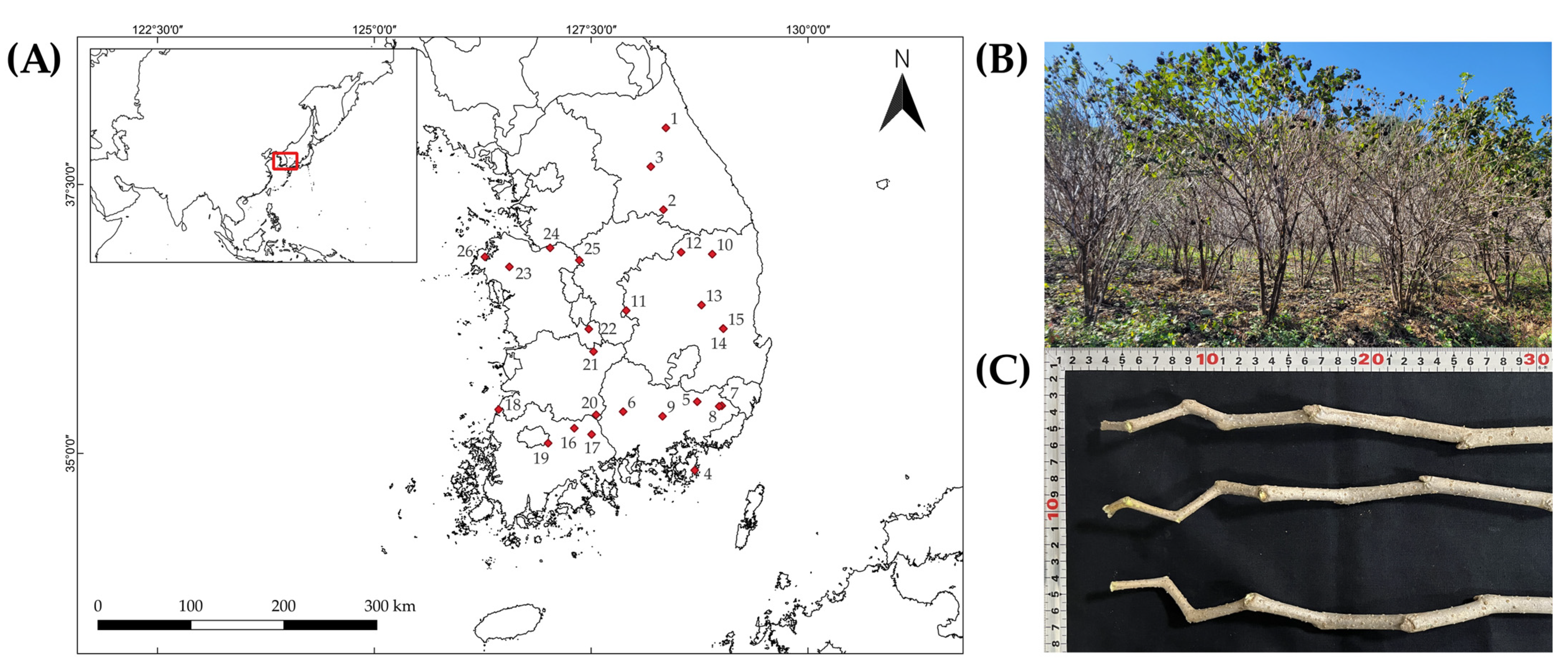
Cultivation Sites | PH | BD | BrD | FBW | TD |
|---|---|---|---|---|---|
| (m) | (cm) | (mm) | (g) | (ea/cm2) | |
| 1 | 2.38 ± 0.16 defg | 3.37 ± 0.82 bcd | 6.40 ± 0.71 b | 19.24 ± 1.06 de | 0.00 ± 0.00 b |
| 2 | 2.10 ± 0.04 defgh | 1.80 ± 0.06 cd | 8.65 ± 0.44 ab | 62.24 ± 6.03 b | 1.00 ± 0.58 ab |
| 3 | 2.09 ± 0.21 defgh | 3.47 ± 0.13 bcd | 8.45 ± 0.59 ab | 34.52 ± 6.23 bcde | 0.00 ± 0.00 b |
| 4 | 3.00 ± 0.10 abcdef | 2.77 ± 0.03 bcd | 7.21 ± 0.98 ab | 46.59 ± 9.03 bcde | 1.33 ± 0.33 ab |
| 5 | 3.30 ± 0.70 abcd | 2.23 ± 0.48 bcd | 7.43 ± 1.08 ab | 40.89 ± 13.09 bcde | 0.33 ± 0.33 ab |
| 6 | 3.77 ± 0.20 abc | 2.57 ± 0.24 bcd | 7.41 ± 0.55 ab | 55.66 ± 7.18 bc | 2.67 ± 1.20 a |
| 7 | 3.97 ± 0.15 ab | 2.63 ± 0.12 bcd | 6.84 ± 0.13 ab | 29.12 ± 2.91 cde | 1.33 ± 0.67 ab |
| 8 | 2.13 ± 0.18 defgh | 2.93 ± 0.59 bcd | 7.10 ± 0.55 ab | 30.71 ± 4.91 bcde | 0.33 ± 0.33 ab |
| 9 | 3.80 ± 0.12 abc | 8.97 ± 0.87 a | 7.05 ± 0.72 ab | 49.82 ± 9.96 bcd | 1.00 ± 0.58 ab |
| 10 | 1.83 ± 0.31 fgh | 2.33 ± 0.43 bcd | 6.56 ± 0.04 b | 42.64 ± 3.64 bcde | 0.67 ± 0.33 ab |
| 11 | 2.00 ± 0.15 efgh | 2.03 ± 0.09 cd | 7.46 ± 0.32 ab | 43.97 ± 5.22 bcde | 2.67 ± 0.67 a |
| 12 | 2.27 ± 0.13 defgh | 3.40 ± 0.06 bcd | 5.48 ± 0.31 b | 29.08 ± 3.50 cde | 2.00 ± 0.00 ab |
| 13 | 2.07 ± 0.15 defgh | 2.40 ± 0.21 bcd | 8.80 ± 1.00 ab | 41.71 ± 7.65 bcde | 0.67 ± 0.67 ab |
| 14 | 2.73 ± 0.24 bcdef | 4.53 ± 0.98 bc | 6.33 ± 0.34 b | 49.20 ± 2.18 bcd | 1.33 ± 0.33 ab |
| 15 | 2.60 ± 0.12 cdefg | 2.93 ± 0.43 bcd | 6.27 ± 0.23 b | 51.23 ± 1.74 bcd | 0.67 ± 0.33 ab |
| 16 | 2.37 ± 0.09 defg | 5.53 ± 1.91 b | 7.36 ± 0.85 ab | 23.91 ± 6.77 cde | 2.33 ± 0.33 ab |
| 17 | 1.33 ± 0.09 gh | 1.77 ± 0.03 cd | 10.08 ± 0.17 a | 103.94 ± 4.31 a | 1.67 ± 0.67 ab |
| 18 | 2.65 ± 0.51 cdef | 4.57 ± 1.19 bc | 6.84 ± 1.04 ab | 45.02 ± 6.90 bcde | 0.67 ± 0.33 ab |
| 19 | 4.20 ± 0.06 a | 4.93 ± 0.18 bc | 8.15 ± 0.84 ab | 45.32 ± 1.64 bcde | 1.33 ± 0.33 ab |
| 20 | 2.70 ± 0.21 bcdef | 3.07 ± 0.44 bcd | 5.72 ± 0.25 b | 13.85 ± 0.80 e | 0.00 ± 0.00 b |
| 21 | 1.06 ± 0.06 h | 1.06 ± 0.07 d | 7.42 ± 0.20 ab | 44.71 ± 5.42 bcde | 2.67 ± 0.33 a |
| 22 | 2.60 ± 0.06 cdefg | 2.43 ± 0.35 bcd | 7.89 ± 0.23 ab | 41.09 ± 3.18 bcde | 1.67 ± 0.33 ab |
| 23 | 1.97 ± 0.17 fgh | 2.07 ± 0.24 cd | 6.25 ± 0.53 b | 36.50 ± 4.28 bcde | 0.00 ± 0.00 b |
| 24 | 2.27 ± 0.09 defgh | 2.70 ± 0.23 bcd | 5.89 ± 0.63 b | 33.84 ± 2.31 bcde | 0.00 ± 0.00 b |
| 25 | 1.73 ± 0.03 fgh | 1.80 ± 0.06 cd | 5.68 ± 0.27 b | 35.71 ± 3.01 bcde | 0.67 ± 0.67 ab |
| 26 | 3.27 ± 0.34 abcde | 3.73 ± 0.78 bcd | 7.71 ± 0.73 ab | 47.31 ± 10.26 bcd | 0.67 ± 0.33 ab |
| No. | pH [1:5] | EC | OM | TN | AP | Exchangeable Cation | CEC | BS | Soil Texture | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K+ | Ca2+ | Mg2+ | Na+ | Sand | Silt | Clay | ||||||||
| (dS/m) | (%) | (mg/kg) | (cmol+/kg) | (%) | (%) | |||||||||
| 1 | 5.92 ± 0.07 defg | 0.24 ± 0.01 abc | 3.17 ± 0.36 cd | 0.16 ± 0.02 bcdefg | 689.90 ± 190.12 bcde | 0.45 ± 0.16 abc | 4.13 ± 0.80 bcdefgh | 0.68 ± 0.15 hi | 0.03 ± 0.01 c | 15.90 ± 1.34 bcdefg | 33.63 ± 5.93 defgh | 76.06 ± 1.67 abcd | 9.69 ± 0.67 fg | 14.25 ± 2.03 cd |
| 2 | 6.93 ± 0.08 ab | 0.32 ± 0.04 abc | 2.48 ± 0.17 cd | 0.11 ± 0.00 defg | 201.14 ± 32.72 de | 0.36 ± 0.02 abc | 11.82 ± 0.75 ab | 2.30 ± 0.37 cdef | 0.02 ± 0.01 c | 17.38 ± 0.80 bcdef | 83.51 ± 0.73 ab | 69.72 ± 0.92 cdefg | 14.28 ± 0.54 bcdefg | 15.99 ± 1.19 bcd |
| 3 | 6.43 ± 0.04 bcde | 0.13 ± 0.01 c | 0.93 ± 0.13 d | 0.07 ± 0.00 g | 38.27 ± 4.51 e | 0.13 ± 0.01 bc | 2.41 ± 0.34 efgh | 0.93 ± 0.05 hi | 0.03 ± 0.01 c | 11.55 ± 0.52 fgh | 30.22 ± 2.25 efgh | 64.74 ± 0.45 g | 18.76 ± 0.97 b | 16.50 ± 1.42 abcd |
| 4 | 6.43 ± 0.04 bcde | 0.35 ± 0.01 abc | 5.14 ± 0.28 abc | 0.23 ± 0.01 ab | 1250.26 ± 36.73 ab | 0.69 ± 0.14 abc | 10.95 ± 0.14 ab | 1.70 ± 0.13 cdefgh | 0.09 ± 0.01 abc | 20.01 ± 0.61 abc | 67.35 ± 3.60 abcd | 72.17 ± 0.83 abcdefg | 15.28 ± 1.01 bcdefg | 12.56 ± 1.71 d |
| 5 | 6.49 ± 0.06 bcde | 0.36 ± 0.02 abc | 2.25 ± 0.07 cd | 0.11 ± 0.00 defg | 135.59 ± 1.61 de | 0.47 ± 0.07 abc | 9.81 ± 0.54 abcd | 3.75 ± 0.38 b | 0.04 ± 0.01 c | 15.61 ± 0.32 bcdefg | 90.25 ± 2.36 a | 75.20 ± 0.85 abcde | 10.19 ± 0.94 efg | 14.62 ± 1.57 cd |
| 6 | 6.35 ± 0.11 cde | 0.21 ± 0.02 bc | 3.98 ± 0.64 abcd | 0.15 ± 0.02 bcdefg | 40.05 ± 26.23 e | 0.27 ± 0.05 abc | 4.38 ± 0.56 cdefgh | 0.67 ± 0.05 hi | 0.06 ± 0.02 abc | 18.37 ± 0.55 bcde | 29.27 ± 2.95 efgh | 69.83 ± 0.54 bcdefg | 14.16 ± 2.37 bcdefg | 16.01 ± 1.84 bcd |
| 7 | 6.04 ± 0.13 defg | 0.29 ± 0.05 abc | 2.54 ± 0.58 cd | 0.13 ± 0.03 bcdefg | 792.25 ± 260.08 bcd | 0.27 ± 0.07 abc | 8.87 ± 1.17 abcdef | 1.26 ± 0.13 efghi | 0.04 ± 0.00 c | 14.59 ± 1.30 cdefgh | 73.59 ± 14.52 abc | 65.62 ± 0.76 g | 17.06 ± 0.76 bcd | 17.32 ± 1.06 abcd |
| 8 | 6.74 ± 0.02 bcde | 0.31 ± 0.03 abc | 3.55 ± 0.29 bcd | 0.17 ± 0.01 bcdefg | 25.20 ± 2.27 e | 0.76 ± 0.03 ab | 6.28 ± 0.39 abcdefgh | 0.97 ± 0.07 hi | 0.05 ± 0.01 bc | 16.10 ± 0.34 bcdefg | 50.25 ± 3.78 bcdefg | 76.24 ± 0.43 abcd | 10.39 ± 0.35 efg | 13.37 ± 0.34 cd |
| 9 | 6.12 ± 0.05 defg | 0.44 ± 0.11 ab | 3.54 ± 0.22 bcd | 0.19 ± 0.00 abcde | 584.05 ± 97.26 bcde | 0.56 ± 0.08 abc | 9.01 ± 0.61 abcd | 2.36 ± 0.18 cde | 0.16 ± 0.07 ab | 18.74 ± 0.95 bcd | 64.44 ± 1.11 abcd | 43.69 ± 0.54 h | 34.52 ± 1.09 a | 21.79 ± 1.52 ab |
| 10 | 6.43 ± 0.04 bcde | 0.33 ± 0.05 abc | 2.47 ± 0.25 cd | 0.13 ± 0.00 bcdefg | 582.29 ± 160.28 bcde | 0.56 ± 0.12 abc | 7.43 ± 0.36 abcdefgh | 1.34 ± 0.08 efghi | 0.01 ± 0.00 c | 15.54 ± 1.31 bcdefg | 60.87 ± 5.25 abcde | 71.69 ± 0.87 abcdefg | 15.90 ± 0.47 bcde | 12.41 ± 0.91 d |
| 11 | 5.73 ± 0.07 fgh | 0.29 ± 0.09 abc | 4.23 ± 1.53 abcd | 0.18 ± 0.06 bcdefg | 378.72 ± 178.37 cde | 0.15 ± 0.02 bc | 7.88 ± 2.12 abcdefg | 1.17 ± 0.05 fghi | 0.05 ± 0.01 bc | 17.77 ± 3.17 bcdef | 50.93 ± 3.48 bcdefg | 73.60 ± 3.42 abcdef | 13.44 ± 2.26 bcdefg | 12.96 ± 1.19 d |
| 12 | 7.13 ± 0.04 a | 0.44 ± 0.02 ab | 4.42 ± 0.75 abcd | 0.23 ± 0.03 ab | 1068.95 ± 326.60 abc | 0.62 ± 0.09 abc | 11.96 ± 1.12 a | 1.63 ± 0.34 cdefgh | 0.02 ± 0.00 c | 17.72 ± 0.57 bcdef | 80.53 ± 8.84 abc | 76.02 ± 1.05 abcd | 10.71 ± 1.22 efg | 13.27 ± 1.65 cd |
| 13 | 6.18 ± 0.09 cdef | 0.30 ± 0.03 abc | 3.92 ± 0.40 abcd | 0.19 ± 0.02 abcde | 579.00 ± 104.48 bcde | 0.72 ± 0.36 abc | 8.57 ± 0.29 abcdefg | 2.26 ± 0.11 cdefg | 0.04 ± 0.00 c | 16.72 ± 0.28 bcdefg | 69.46 ± 2.59 abc | 43.39 ± 1.60 h | 33.20 ± 0.79 a | 23.41 ± 1.51 a |
| 14 | 5.85 ± 0.07 efg | 0.20 ± 0.03 bc | 4.57 ± 0.43 abcd | 0.20 ± 0.00 abcde | 101.96 ± 8.73 de | 0.13 ± 0.01 bc | 3.88 ± 0.56 defgh | 0.76 ± 0.06 hi | 0.04 ± 0.01 c | 16.63 ± 0.46 bcdefg | 28.81 ± 2.98 efgh | 69.04 ± 0.72 defg | 16.16 ± 1.09 bcde | 14.80 ± 0.38 bcd |
| 15 | 5.27 ± 0.18 h | 0.25 ± 0.02 abc | 3.20 ± 0.48 cd | 0.16 ± 0.02 bcdefg | 1531.34 ± 358.98 a | 0.43 ± 0.15 abc | 1.11 ± 0.25 h | 0.25 ± 0.07 i | 0.02 ± 0.01 c | 14.48 ± 0.23 cdefgh | 12.50 ± 2.45 h | 75.09 ± 0.99 abcde | 10.94 ± 0.35 defg | 13.97 ± 0.74 cd |
| 16 | 6.21 ± 0.12 cdef | 0.26 ± 0.02 abc | 1.46 ± 0.16 d | 0.09 ± 0.01 efg | 265.19 ± 85.00 de | 0.26 ± 0.05 abc | 3.93 ± 0.29 defgh | 0.99 ± 0.11 hi | 0.01 ± 0.00 c | 8.99 ± 1.06 h | 59.26 ± 6.80 abcde | 77.53 ± 0.56 ab | 10.40 ± 1.09 efg | 12.07 ± 1.39 d |
| 17 | 5.58 ± 0.04 gh | 0.29 ± 0.02 abc | 2.44 ± 0.18 cd | 0.11 ± 0.01 defg | 287.36 ± 59.18 de | 0.48 ± 0.12 abc | 2.15 ± 0.11 gh | 0.57 ± 0.02 hi | 0.03 ± 0.00 c | 16.38 ± 0.46 bcdefg | 19.66 ± 1.15 gh | 76.27 ± 0.39 abcd | 11.78 ± 0.14 cdefg | 11.95 ± 0.30 d |
| 18 | 6.06 ± 0.12 defg | 0.25 ± 0.03 abc | 1.16 ± 0.47 d | 0.08 ± 0.02 fg | 110.42 ± 78.17 de | 0.23 ± 0.03 abc | 5.29 ± 1.45 bcdefg | 2.74 ± 0.23 bc | 0.17 ± 0.01 a | 12.06 ± 0.14 efgh | 69.67 ± 12.79 abc | 68.09 ± 1.27 efg | 15.11 ± 0.93 bcdef | 16.80 ± 0.77 abcd |
| 19 | 5.59 ± 0.04 gh | 0.30 ± 0.02 abc | 3.66 ± 0.10 abcd | 0.18 ± 0.00 abcdef | 494.81 ± 130.61 cde | 0.70 ± 0.21 abc | 2.31 ± 0.46 fgh | 0.58 ± 0.10 hi | 0.02 ± 0.00 c | 16.45 ± 0.74 bcdefg | 21.72 ± 3.14 fgh | 70.83 ± 3.07 abcdefg | 13.24 ± 1.70 bcdefg | 15.93 ± 1.37 bcd |
| 20 | 6.03 ± 0.25 defg | 0.41 ± 0.07 ab | 6.91 ± 1.01 ab | 0.29 ± 0.02 a | 244.83 ± 106.90 de | 0.87 ± 0.17 a | 10.51 ± 3.21 abc | 1.14 ± 0.20 ghi | 0.02 ± 0.00 c | 21.79 ± 2.74 ab | 55.40 ± 11.62 bcdef | 74.40 ± 0.41 abcdef | 10.06 ± 0.93 efg | 15.55 ± 1.06 bcd |
| 21 | 5.99 ± 0.09 defg | 0.14 ± 0.01 c | 1.15 ± 0.03 d | 0.08 ± 0.00 fg | 299.99 ± 6.37 de | 0.25 ± 0.03 abc | 0.99 ± 0.08 h | 0.20 ± 0.01 i | 0.02 ± 0.01 c | 10.40 ± 0.15 gh | 14.05 ± 0.60 h | 76.89 ± 0.69 abc | 9.48 ± 0.61 fg | 13.63 ± 1.25 cd |
| 22 | 6.14 ± 0.01 defg | 0.15 ± 0.00 c | 0.95 ± 0.08 d | 0.07 ± 0.00 g | 59.70 ± 20.78 de | 0.06 ± 0.01 c | 6.37 ± 0.40 abcdefgh | 1.11 ± 0.13 hi | 0.09 ± 0.04 abc | 12.29 ± 0.32 defgh | 62.07 ± 2.63 abcde | 77.86 ± 0.88 a | 8.90 ± 0.48 g | 13.25 ± 1.28 cd |
| 23 | 6.11 ± 0.05 defg | 0.33 ± 0.08 abc | 2.86 ± 0.40 cd | 0.14 ± 0.02 bcdefg | 636.18 ± 45.70 bcde | 0.80 ± 0.26 ab | 6.44 ± 1.03 abcdefgh | 1.58 ± 0.32 defgh | 0.04 ± 0.02 c | 15.32 ± 0.16 bcdefgh | 57.66 ± 9.47 abcde | 46.05 ± 0.27 h | 33.71 ± 0.78 a | 20.24 ± 1.04 abc |
| 24 | 5.74 ± 0.10 fgh | 0.30 ± 0.02 abc | 2.74 ± 0.16 cd | 0.12 ± 0.01 cdefg | 46.51 ± 6.10 e | 0.39 ± 0.02 abc | 6.16 ± 1.16 abcdefgh | 1.43 ± 0.08 defgh | 0.04 ± 0.02 c | 16.30 ± 0.55 bcdefg | 48.94 ± 6.13 cdefg | 48.72 ± 0.87 h | 32.85 ± 0.88 a | 18.43 ± 0.27 abcd |
| 25 | 6.01 ± 0.27 defgh | 0.49 ± 0.09 a | 7.28 ± 2.20 a | 0.23 ± 0.03 abc | 39.77 ± 6.97 e | 0.36 ± 0.09 abc | 12.10 ± 3.40 a | 5.06 ± 0.58 a | 0.13 ± 0.06 abc | 25.78 ± 2.66 a | 66.94 ± 7.83 abcd | 70.52 ± 3.29 abcdefg | 14.76 ± 2.31 bcdefg | 14.72 ± 1.17 cd |
| 26 | 6.40 ± 0.08 bcde | 0.45 ± 0.07 ab | 3.92 ± 0.70 abcd | 0.20 ± 0.03 abcde | 476.49 ± 44.54 cde | 0.68 ± 0.11 abc | 8.93 ± 0.45 abcde | 2.56 ± 0.22 cd | 0.08 ± 0.01 abc | 18.70 ± 0.81 bcd | 65.58 ± 1.62 abcd | 67.12 ± 1.76 fg | 17.95 ± 0.34 bc | 14.93 ± 2.00 bcd |
Cultivation Sites (n = 3) | AAT | AmT | AMT | AAmT | AAMT | TP | SSH | ALT |
|---|---|---|---|---|---|---|---|---|
| (°C) | (°C) | (°C) | (°C) | (°C) | (mm) | (h) | (m) | |
| 1 | 11.8 | −20.8 | 35.2 | 6.5 | 18.1 | 1225.6 | 1945.3 | 419.0 |
| 2 | 12.4 | −18.9 | 36.5 | 7.0 | 19.0 | 1507.5 | 2264.7 | 280.0 |
| 3 | 12.1 | −20.9 | 36.8 | 6.9 | 18.5 | 1392.4 | 1989.4 | 427.5 |
| 4 | 15.3 | −10.4 | 34.5 | 11.4 | 19.6 | 2585.5 | 2114.9 | 43.2 |
| 5 | 15.0 | −12.4 | 37.8 | 9.6 | 21.4 | 1874.0 | 2327.7 | 40.9 |
| 6 | 13.9 | −13.5 | 36.4 | 8.5 | 20.3 | 1938.2 | 2124.3 | 315.3 |
| 7 | 16.0 | −10.6 | 37.4 | 11.2 | 21.7 | 2172.0 | 2163.7 | 264.4 |
| 8 | 16.0 | −10.6 | 37.4 | 11.2 | 21.7 | 2172.0 | 2163.7 | 343.6 |
| 9 | 14.5 | −15.9 | 37.6 | 8.6 | 21.6 | 1939.9 | 2146.7 | 23.3 |
| 10 | 11.6 | −15.5 | 33.1 | 6.1 | 17.9 | 1828.7 | 2205.5 | 254.0 |
| 11 | 13.9 | −16.8 | 36.0 | 8.7 | 19.8 | 1670.6 | 2331.0 | 268.1 |
| 12 | 12.4 | −17.7 | 34.8 | 6.9 | 18.3 | 2216.1 | 2271.7 | 243.0 |
| 13 | 12.9 | −19.2 | 37.1 | 6.7 | 20.3 | 1575.4 | 2224.3 | 209.0 |
| 14 | 15.7 | −13.9 | 36.7 | 12.0 | 19.8 | 1112.2 | 2466.9 | 549.0 |
| 15 | 15.7 | −13.9 | 36.7 | 12.0 | 19.8 | 1112.2 | 2466.9 | 520.0 |
| 16 | 13.5 | −14.2 | 35.6 | 8.2 | 19.8 | 2139.5 | 2099.7 | 202.0 |
| 17 | 13.5 | −14.2 | 35.6 | 8.2 | 19.8 | 2139.5 | 2099.7 | 124.9 |
| 18 | 14.1 | −16.7 | 35.3 | 9.5 | 19.4 | 1496.1 | 2038.8 | 43.2 |
| 19 | 13.5 | −14.2 | 35.6 | 8.2 | 19.8 | 2139.5 | 2099.7 | 391.5 |
| 20 | 13.8 | −16.3 | 37.0 | 8.6 | 19.9 | 1805.7 | 2143.6 | 562.4 |
| 21 | 13.0 | −17.8 | 35.2 | 7.4 | 19.3 | 1800.9 | 2170.2 | 233.3 |
| 22 | 13.0 | −17.8 | 35.2 | 7.4 | 19.3 | 1800.9 | 2170.2 | 269.5 |
| 23 | 13.3 | −15.5 | 35.2 | 8.6 | 18.5 | 1529.7 | 2220.9 | 46.9 |
| 24 | 13.0 | −18.0 | 35.0 | 7.7 | 19.0 | 1502.5 | 2134.1 | 41.2 |
| 25 | 13.0 | −18.0 | 35.0 | 7.7 | 19.0 | 1502.5 | 2134.1 | 201.6 |
| 26 | 13.3 | −15.5 | 35.2 | 8.6 | 18.5 | 1529.7 | 2220.9 | 58.8 |
| Cultivation Sites | Chlorogenic Acid | Eleutheroside E | Total |
|---|---|---|---|
| (mg/g) | |||
| 1 | 0.534 ± 0.041 a | 0.129 ± 0.019 bcdef | 0.663 ± 0.058 ab |
| 2 | 0.292 ± 0.022 a | 0.157 ± 0.004 abcde | 0.449 ± 0.025 b |
| 3 | 0.345 ± 0.019 a | 0.117 ± 0.003 cdef | 0.462 ± 0.022 b |
| 4 | 0.437 ± 0.078 a | 0.116 ± 0.008 cdef | 0.553 ± 0.071 ab |
| 5 | 0.629 ± 0.089 a | 0.191 ± 0.014 abc | 0.820 ± 0.090 ab |
| 6 | 0.497 ± 0.142 a | 0.154 ± 0.019 bcde | 0.651 ± 0.160 ab |
| 7 | 0.549 ± 0.044 a | 0.147 ± 0.012 bcde | 0.696 ± 0.048 ab |
| 8 | 0.317 ± 0.099 a | 0.062 ± 0.015 ef | 0.379 ± 0.114 b |
| 9 | 0.427 ± 0.093 a | 0.058 ± 0.008 ef | 0.485 ± 0.100 ab |
| 10 | 0.431 ± 0.085 a | 0.130 ± 0.018 bcdef | 0.561 ± 0.101 ab |
| 11 | 0.504 ± 0.034 a | 0.134 ± 0.009 bcdef | 0.638 ± 0.025 ab |
| 12 | 0.449 ± 0.058 a | 0.185 ± 0.020 abcd | 0.635 ± 0.039 ab |
| 13 | 0.673 ± 0.068 a | 0.131 ± 0.037 bcdef | 0.804 ± 0.039 ab |
| 14 | 0.465 ± 0.008 a | 0.264 ± 0.029 a | 0.729 ± 0.035 ab |
| 15 | 0.322 ± 0.017 a | 0.131 ± 0.020 bcdef | 0.453 ± 0.015 b |
| 16 | 0.526 ± 0.110 a | 0.134 ± 0.012 bcdef | 0.660 ± 0.109 ab |
| 17 | 0.534 ± 0.017 a | 0.181 ± 0.017 abcd | 0.715 ± 0.033 ab |
| 18 | 0.551 ± 0.106 a | 0.152 ± 0.011 bcde | 0.704 ± 0.101 ab |
| 19 | 0.499 ± 0.025 a | 0.136 ± 0.024 bcdef | 0.635 ± 0.027 ab |
| 20 | 0.317 ± 0.036 a | 0.038 ± 0.003 f | 0.355 ± 0.033 b |
| 21 | 0.522 ± 0.028 a | 0.078 ± 0.015 def | 0.600 ± 0.042 ab |
| 22 | 0.708 ± 0.086 a | 0.231 ± 0.047 ab | 0.939 ± 0.072 a |
| 23 | 0.628 ± 0.185 a | 0.118 ± 0.002 cdef | 0.746 ± 0.183 ab |
| 24 | 0.427 ± 0.062 a | 0.063 ± 0.021 ef | 0.491 ± 0.083 ab |
| 25 | 0.514 ± 0.144 a | 0.123 ± 0.027 cdef | 0.637 ± 0.171 ab |
| 26 | 0.303 ± 0.049 a | 0.060 ± 0.018 ef | 0.363 ± 0.064 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, Y.; Lee, D.H.; Jang, J.H.; Kim, H.-J.; Kim, J.A. Environmental Influences on Growth and Secondary Metabolite Accumulation in Eleutherococcus sessiliflorus Across Korean Cultivation Sites. Plants 2025, 14, 3175. https://doi.org/10.3390/plants14203175
Son Y, Lee DH, Jang JH, Kim H-J, Kim JA. Environmental Influences on Growth and Secondary Metabolite Accumulation in Eleutherococcus sessiliflorus Across Korean Cultivation Sites. Plants. 2025; 14(20):3175. https://doi.org/10.3390/plants14203175
Chicago/Turabian StyleSon, Yonghwan, Dong Hwan Lee, Jun Hyuk Jang, Hyun-Jun Kim, and Ji Ah Kim. 2025. "Environmental Influences on Growth and Secondary Metabolite Accumulation in Eleutherococcus sessiliflorus Across Korean Cultivation Sites" Plants 14, no. 20: 3175. https://doi.org/10.3390/plants14203175
APA StyleSon, Y., Lee, D. H., Jang, J. H., Kim, H.-J., & Kim, J. A. (2025). Environmental Influences on Growth and Secondary Metabolite Accumulation in Eleutherococcus sessiliflorus Across Korean Cultivation Sites. Plants, 14(20), 3175. https://doi.org/10.3390/plants14203175






