Priming Effect of Seeds with Niobium (Nb) on the Performance of Maize Plants Under Water Deficit Conditions
Abstract
1. Introduction
2. Results
2.1. Germination Speed Index
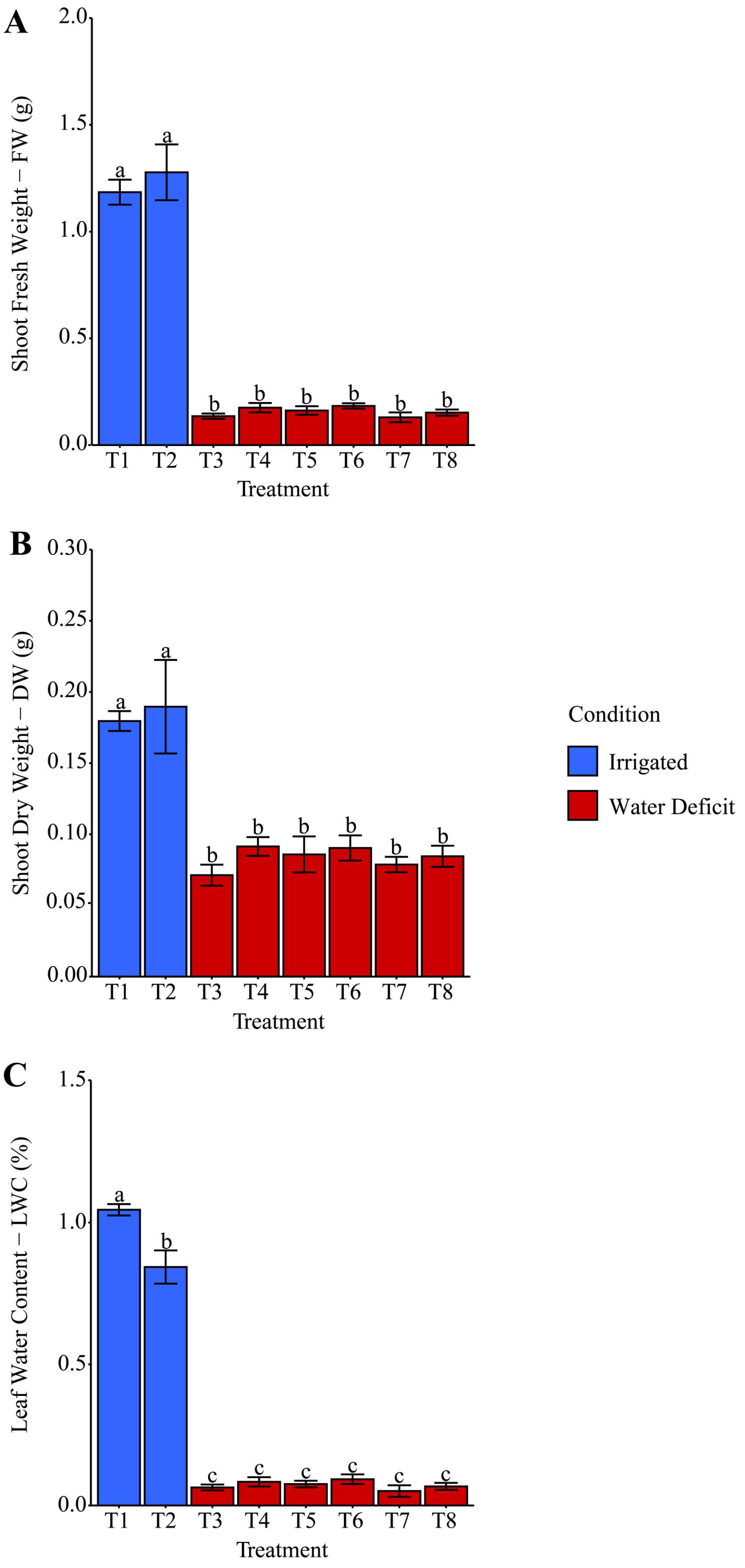
2.2. Biomass
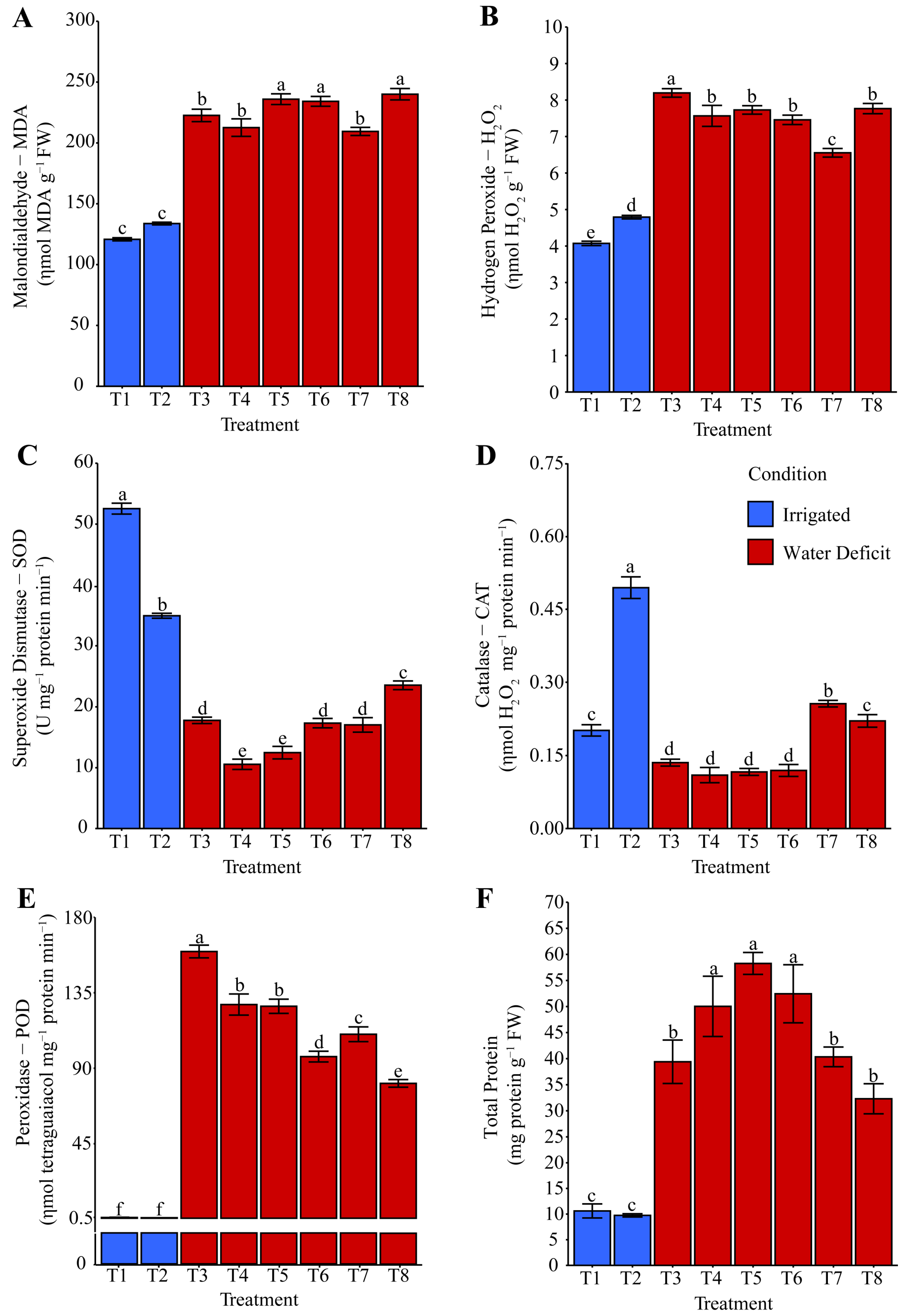
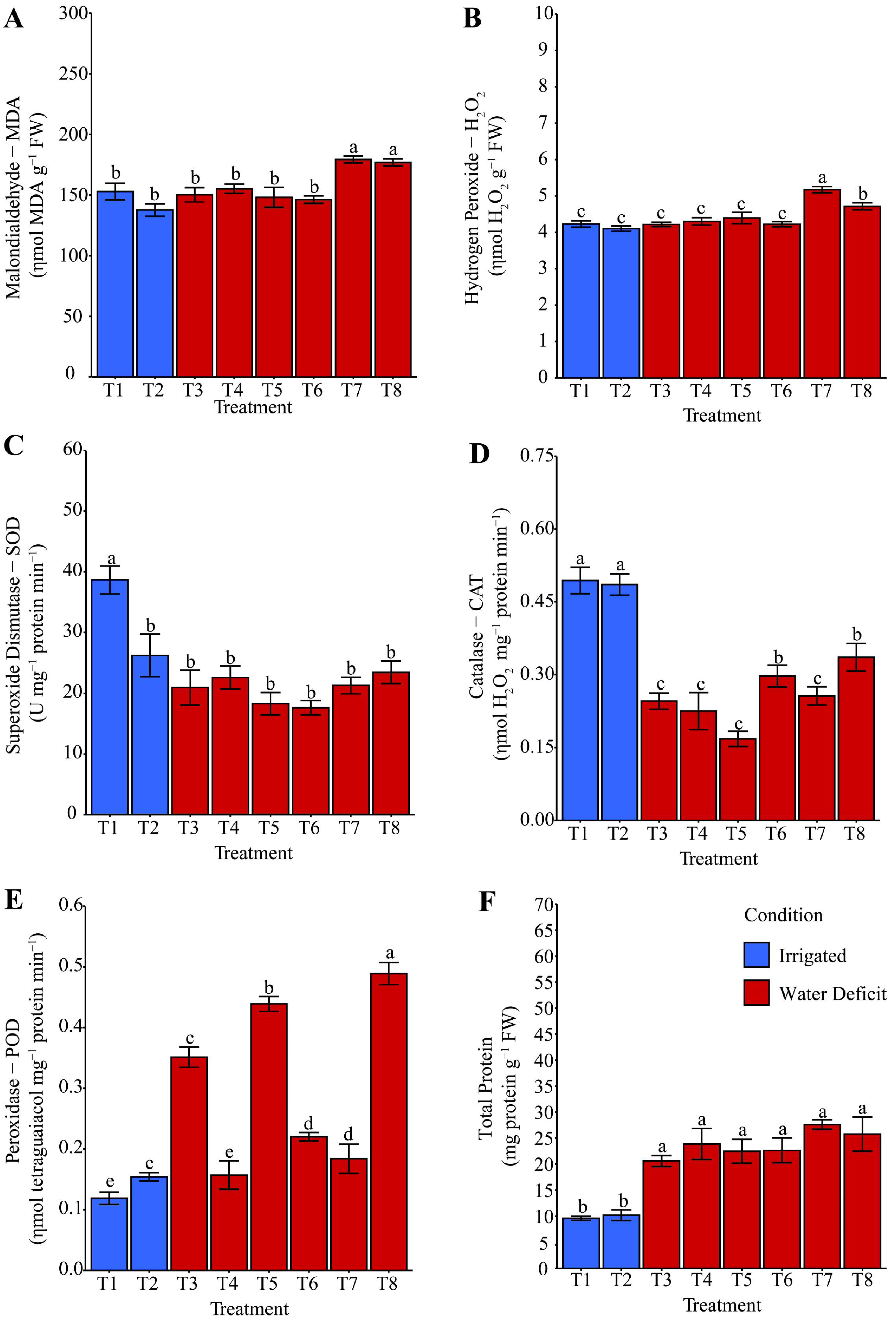
2.3. Oxidative Stress and Antioxidant System Activity
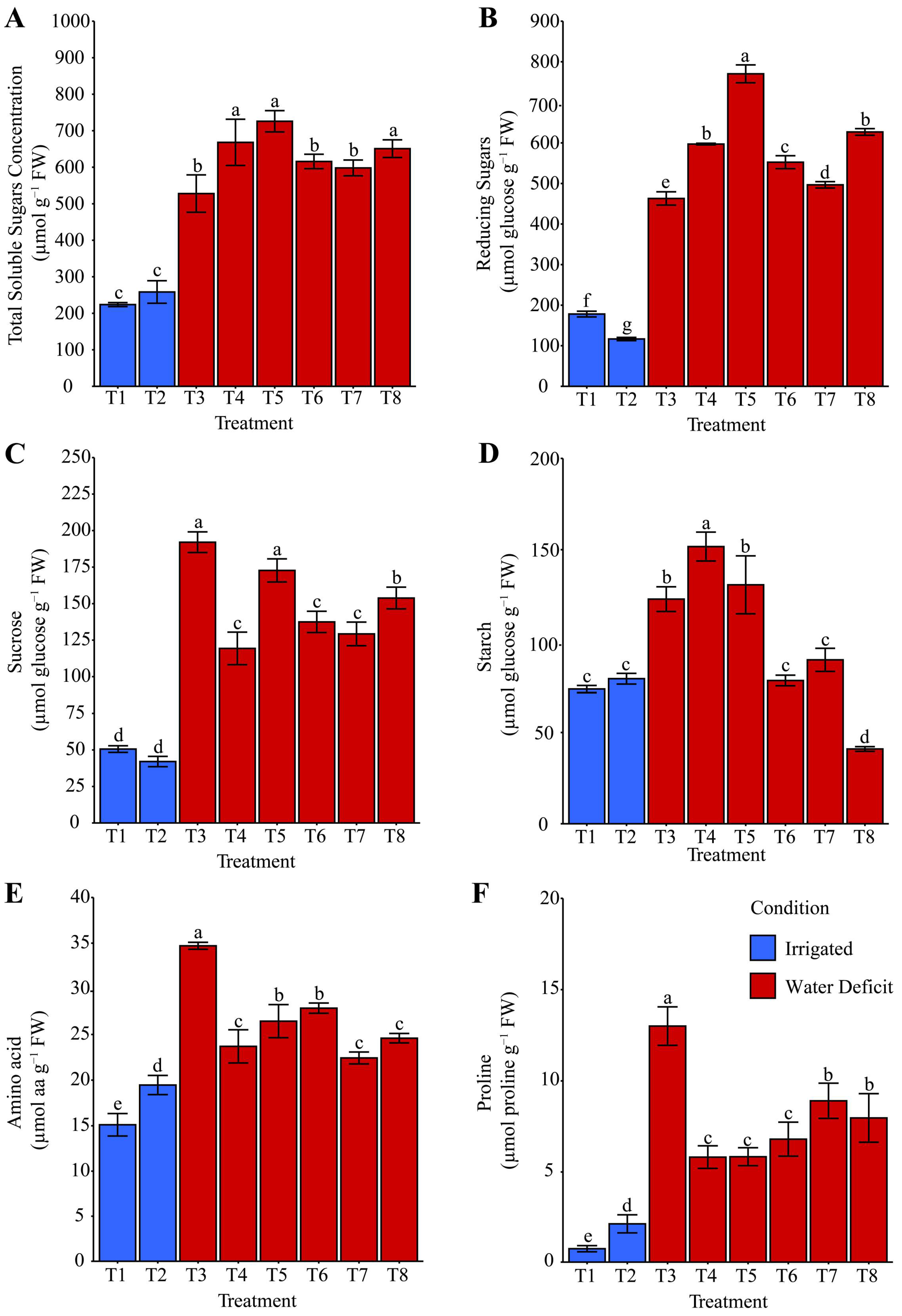
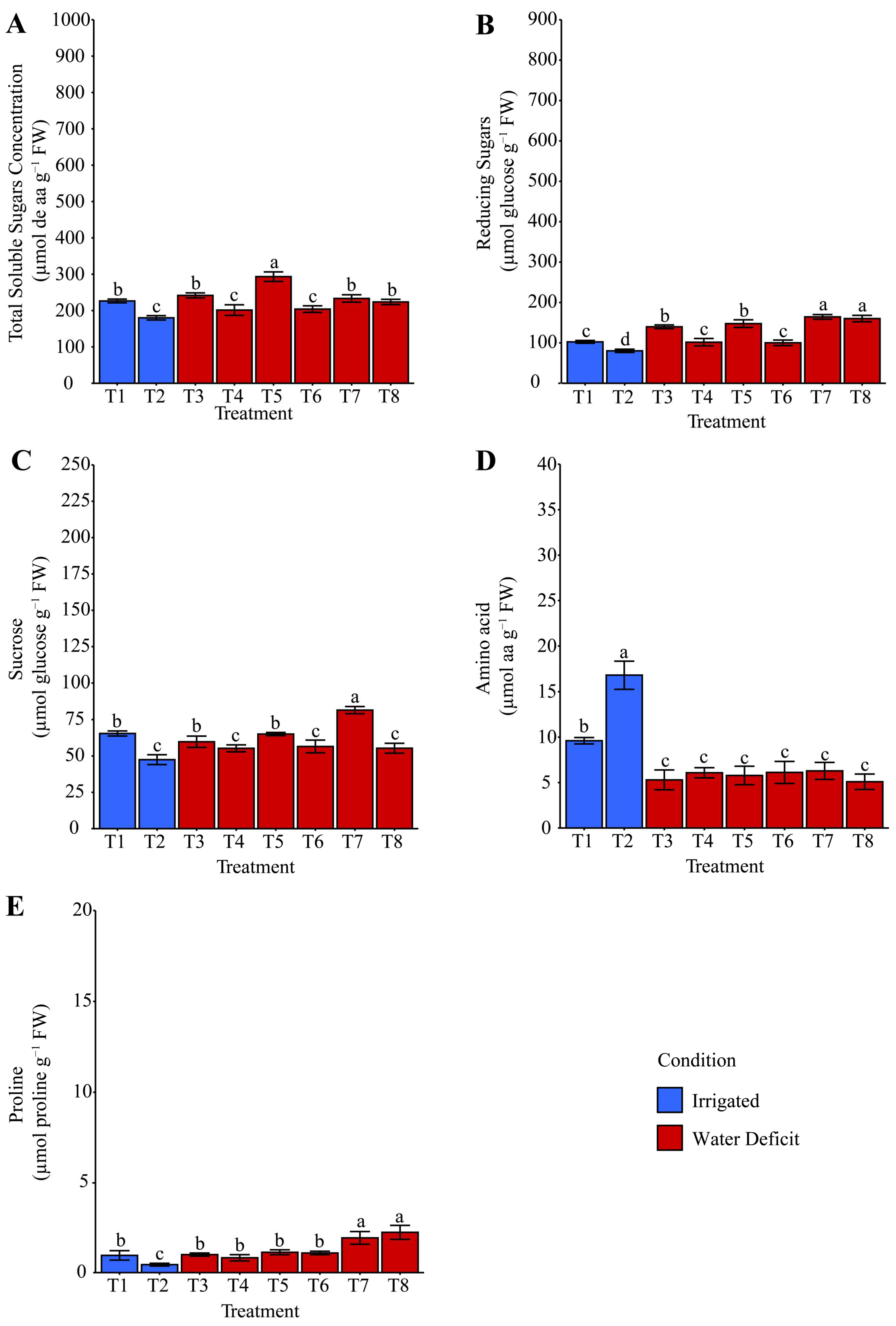
2.4. Starch and Compatible Osmolytes
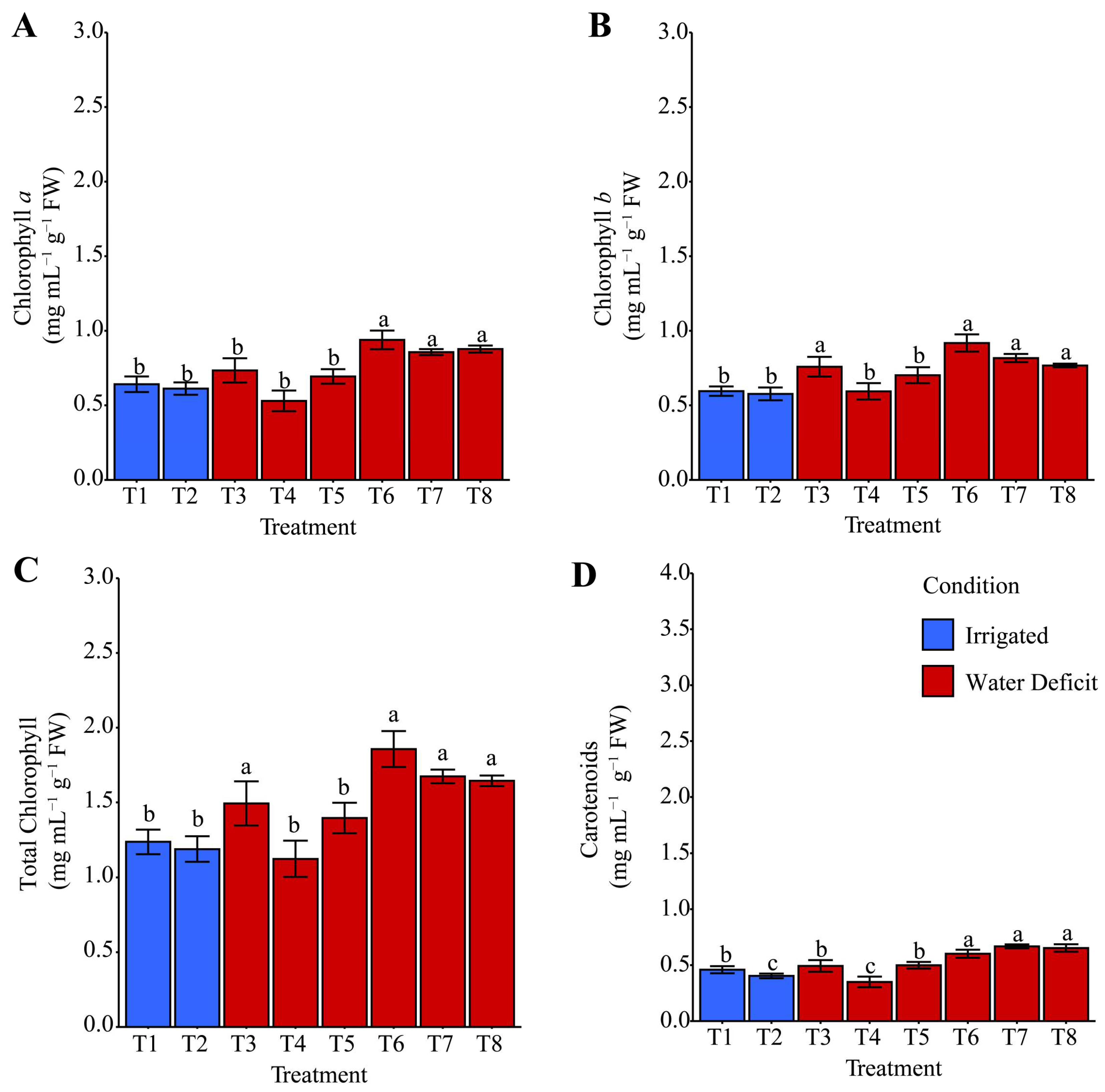
2.5. Chlorophyll and Carotenoid Contents
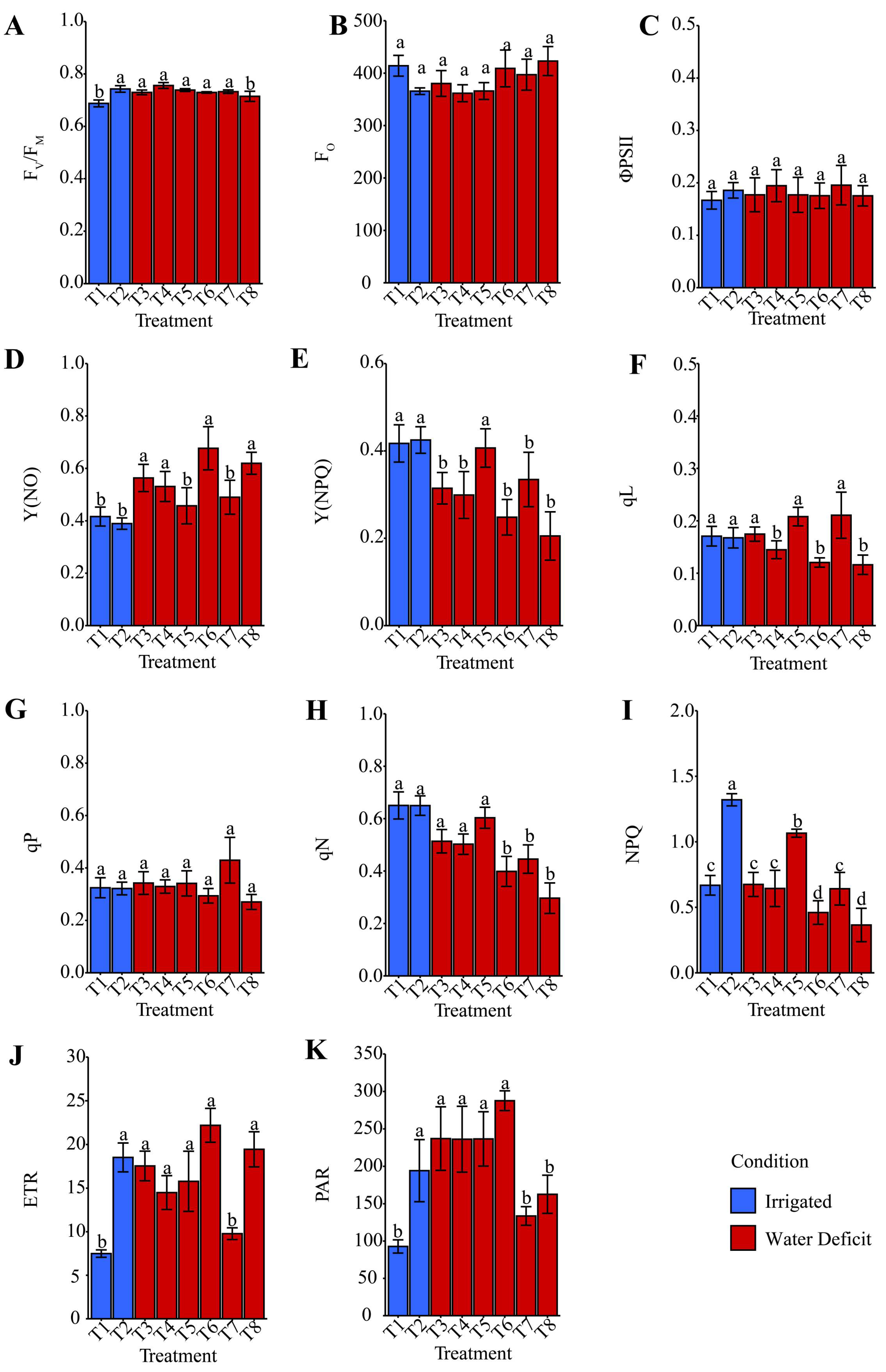
2.6. MINI-PAM Analysis
2.7. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Condition
4.2. Experimental Design and Treatments
4.3. MINI-PAM Analysis and Leaf Sample Collection
4.4. Biomass
4.5. Chlorophyll, Macromolecules, H2O2 Content, and MDA
4.6. Antioxidant Activities
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sangoi, L.; Silva, L.M.M.d.; Mota, M.R.; Panison, F.; Schmitt, A.; Souza, N.M.d.; Giordani, W.; Schenatto, D.E. Maize Agronomic Performance as Affected by Seed Treatment with Azospirillum sp. and Mineral Nitrogen Rates. Rev. Bras. Cienc. Solo 2015, 39, 1141–1150. [Google Scholar] [CrossRef]
- Acosta-Bayona, J.; León-Martínez, G.; Vielle-Calzada, J.-P. Origin and Diversification of Maize: Two Teosintes but Different Contributions. Mol. Plant 2024, 17, 233–235. [Google Scholar] [CrossRef]
- Rouf Shah, T.; Prasad, K.; Kumar, P. Maize—A Potential Source of Human Nutrition and Health: A Review. Cogent Food Agric. 2016, 2, 1166995. [Google Scholar] [CrossRef]
- Maitra, S.; Singh, V. Invited Review on ‘Maize in the 21st Century’ Emerging Trends of Maize Biorefineries in the 21st Century: Scientific and Technological Advancements in Biofuel and Bio-Sustainable Market. J. Cereal Sci. 2021, 101, 103272. [Google Scholar] [CrossRef]
- Nayyar, H.; Gupta, D. Differential Sensitivity of C3 and C4 Plants to Water Deficit Stress: Association with Oxidative Stress and Antioxidants. Environ. Exp. Bot. 2006, 58, 106–113. [Google Scholar] [CrossRef]
- Santana, L.R.; da Silva, L.N.; Tavares, G.G.; Batista, P.F.; Cabral, J.S.R.; Souchie, E.L. Arbuscular Mycorrhizal Fungi Associated with Maize Plants during Hydric Deficit. Sci. Rep. 2023, 13, 1519. [Google Scholar] [CrossRef]
- CONAB. Acompanhamento Da Safra Brasileira de Grãos—Safra 2024/25; Companhia Nacional de Abastecimento: Brasília, Brazil, 2025.
- Zabel, F.; Müller, C.; Elliott, J.; Minoli, S.; Jägermeyr, J.; Schneider, J.M.; Franke, J.A.; Moyer, E.; Dury, M.; Francois, L.; et al. Large Potential for Crop Production Adaptation Depends on Available Future Varieties. Glob. Change Biol. 2021, 27, 3870–3882. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Rayamajhi, M. Different Aspects of Weed Management in Maize (Zea mays L.): A Brief Review. Adv. Agric. 2022, 2022, 7960175. [Google Scholar] [CrossRef]
- Andrade, F.H. Analysis of Growth and Yield of Maize, Sunflower and Soybean Grown at Balcarce, Argentina. Field Crops Res. 1995, 41, 1–12. [Google Scholar] [CrossRef]
- Luna-Flores, W.; Estrada-Medina, H.; Morales-Maldonado, E.; Álvarez-Rivera, O. Estrés Por Déficit Hídrico En Plantas: Una Revisión. Chil. J. Agric. Anim. Sci. 2015, 30, 61–69. [Google Scholar]
- Rane, J.; Singh, A.K.; Kumar, M.; Boraiah, K.M.; Meena, K.K.; Pradhan, A.; Prasad, P.V.V. The Adaptation and Tolerance of Major Cereals and Legumes to Important Abiotic Stresses. Int. J. Mol. Sci. 2021, 22, 12970. [Google Scholar] [CrossRef]
- Multsch, S.; Krol, M.S.; Pahlow, M.; Assunção, A.L.C.; Barretto, A.G.O.P.; de Jong van Lier, Q.; Breuer, L. Assessment of Potential Implications of Agricultural Irrigation Policy on Surface Water Scarcity in Brazil. Hydrol. Earth Syst. Sci. 2020, 24, 307–324. [Google Scholar] [CrossRef]
- Heydecker, W.; Higgins, J.; Gulliver, R.L. Accelerated Germination by Osmotic Seed Treatment. Nature 1973, 246, 42–44. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Breaking Seed Dormancy during Dry Storage: A Useful Tool or Major Problem for Successful Restoration via Direct Seeding? Plants 2020, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Esper Neto, M.; Britt, D.W.; Lara, L.M.; Cartwright, A.; dos Santos, R.F.; Inoue, T.T.; Batista, M.A. Initial Development of Corn Seedlings after Seed Priming with Nanoscale Synthetic Zinc Oxide. Agronomy 2020, 10, 307. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9780429192036. [Google Scholar]
- Chizenga, A.P.; Blanco, G.; Almeida, J. A Categoria de Lugar e Sua Relevância Para as Ciências Sociais: Uma Reflexão a Partir de Conflitos Ambientais Em Moatize (Moçambique) e Araxá (Brasil). Horiz. Antropológicos 2022, 28, 359–395. [Google Scholar] [CrossRef]
- Alves, A.R.; Coutinho, A. dos R. Life Cycle Assessment of Niobium: A Mining and Production Case Study in Brazil. Miner. Eng. 2019, 132, 275–283. [Google Scholar] [CrossRef]
- Gómez, M.; Li, J.; Zeng, X. Niobium: The Unseen Element—A Comprehensive Examination of Its Evolution, Global Dynamics, and Outlook. Resour. Conserv. Recycl. 2024, 209, 107744. [Google Scholar] [CrossRef]
- Alves, A.R.; Coutinho, A. dos R. The Evolution of the Niobium Production in Brazil. Mater. Res. 2015, 18, 106–112. [Google Scholar] [CrossRef]
- Satya Prasad, V.V.; Baligidad, R.G.; Gokhale, A.A. Niobium and Other High Temperature Refractory Metals for Aerospace Applications. In Aerospace Materials and Material Technologies; Springer: Singapore, 2017; pp. 267–288. [Google Scholar]
- de Oliveira, C.R.S.; da Silva Júnior, A.H.; Mulinari, J.; Ferreira, A.J.S.; da Silva, A. Fibrous Microplastics Released from Textiles: Occurrence, Fate, and Remediation Strategies. J. Contam. Hydrol. 2023, 256, 104169. [Google Scholar] [CrossRef]
- CBMM (Companhia Brasileira de Metalurgia e Mineração): Araxá, Brasil. 2025. O que é Nióbio (Nb)?. Available online: https://cbmm.com/pt/inove-com-niobio/o-que-%C3%A9-o-ni%C3%B3bio (accessed on 2 May 2025).
- Ray, R.; Dutta, B.; Mandal, S.K.; González, A.G.; Pokrovsky, O.S.; Jana, T.K. Bioaccumulation of Vanadium (V), Niobium (Nb) and Tantalum (Ta) in Diverse Mangroves of the Indian Sundarbans. Plant Soil. 2020, 448, 553–564. [Google Scholar] [CrossRef]
- Reimann, C.; de Caritat, P. Chemical Elements in the Environment; Springer: Berlin/Heidelberg, Germany, 1998; ISBN 978-3-642-72018-5. [Google Scholar]
- Schulz, K.J.; Piatak, N.M.; Papp, J.F. Niobium and Tantalum; Schulz, K.J., DeYoung, J.H., Seal, R.R., Bradley, D.C., Eds.; Professional Paper; USGS Numbered Series; U.S. Geological Survey: Reston, VA, USA, 2017. [CrossRef]
- Sahoo, L.; Swain, B.; Yadav, D. A Review on Different Priming Strategies to Mitigate Abiotic Stress in Plants. Discov. Appl. Sci. 2025, 7, 618. [Google Scholar] [CrossRef]
- Van Nguyen, D.; Nguyen, H.M.; Le, N.T.; Nguyen, K.H.; Nguyen, H.T.; Le, H.M.; Nguyen, A.T.; Dinh, N.T.T.; Hoang, S.A.; Van Ha, C. Copper Nanoparticle Application Enhances Plant Growth and Grain Yield in Maize Under Drought Stress Conditions. J. Plant Growth Regul. 2022, 41, 364–375. [Google Scholar] [CrossRef]
- Ding, Y.; Tang, Y.; Chen, Y.; Wang, Q.; Rui, Y. Nano-Priming with MnFe2O4 NMs Enhances Resistance to Drought Stress and Salt Stress in Maize. Russ. J. Plant Physiol. 2025, 72, 16. [Google Scholar] [CrossRef]
- Lima, J.d.S.; Andrade, O.V.S.; Morais, E.G.d.; Machado, G.G.L.; Santos, L.C.d.; Andrade, E.S.d.; Benevenute, P.A.N.; Martins, G.S.; Nascimento, V.L.; Marchiori, P.E.R.; et al. KI Increases Tomato Fruit Quality and Water Deficit Tolerance by Improving Antioxidant Enzyme Activity and Amino Acid Accumulation: A Priming Effect or Relief during Stress? Plants 2023, 12, 4023. [Google Scholar] [CrossRef]
- Quispe, A.P.V.; Morais, E.G.d.; Benevenute, P.A.N.; Lima, J.d.S.; dos Santos, L.C.; Silva, M.A.; Chalfun-Júnior, A.; Marchiori, P.E.R.; Guilherme, L.R.G. Priming Effect with Selenium and Iodine on Broccoli Seedlings: Activation of Biochemical Mechanisms to Mitigate Cold Damages. Plant Physiol. Biochem. 2025, 223, 109876. [Google Scholar] [CrossRef]
- Sousa, G.F.d.; Silva, M.A.; Carvalho, M.R.D.; Morais, E.G.D.; Benevenute, P.A.N.; Van Opbergen, G.A.Z.; Van Opbergen, G.G.A.Z.; Guilherme, L.R.G. Foliar Selenium Application to Reduce the Induced-Drought Stress Effects in Coffee Seedlings: Induced Priming or Alleviation Effect? Plants 2023, 12, 3026. [Google Scholar] [CrossRef]
- Ahmad, P. (Ed.) Plant Metal Interaction: Emerging Remediation Rechniques; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128031582. [Google Scholar]
- Singh, H.; Jassal, R.K.; Kang, J.S.; Sandhu, S.S.; Kang, H.; Grewal, K. Seed Priming Techniques in Field Crops—A Review. Agric. Rev. 2015, 36. [Google Scholar] [CrossRef]
- Zhang, H.; Zang, J.; Huo, Y.; Zhang, Z.; Chen, H.; Chen, X.; Liu, J. Identification of the Potential Genes Regulating Seed Germination Speed in Maize. Plants 2022, 11, 556. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-32713-4. [Google Scholar]
- Chandrasekar, S.; A, N.; Thiruppathi, G.; Sundararaj, P.; C, S.; J, H.; I, P. Multiprocessing Substrates Enhanced Ta2O5/NiCo2O4 Spinel Nanocomposites for Effective Electro-/Photocatalytic and Toxicological Effects via the Caenorhabditis elegans Model. Langmuir 2024, 40, 6077–6093. [Google Scholar] [CrossRef]
- Coryell, C.D.; Sugarman, N. The Acceptance of New Official Names for the Elements. J. Chem. Educ. 1950, 27, 460. [Google Scholar] [CrossRef]
- Alcântara, B.K.; Rizzi, V.; Gaziola, S.A.; Azevedo, R.A. Soluble Amino Acid Profile, Mineral Nutrient and Carbohydrate Content of Maize Kernels Harvested from Plants Submitted to Ascorbic Acid Seed Priming. An. Acad. Bras. Cienc. 2017, 89, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.M. The Role of Cell Wall Adjustments in Plant Resistance to Water Deficits. Crop Sci. 1995, 35, 1258–1266. [Google Scholar] [CrossRef]
- Abdelhameed, R.E.; Soliman, E.R.S.; Gahin, H.; Metwally, R.A. Enhancing Drought Tolerance in Malva Parviflora Plants through Metabolic and Genetic Modulation Using Beauveria Bassiana Inoculation. BMC Plant Biol. 2024, 24, 662. [Google Scholar] [CrossRef]
- Ferreira, C.M.H.; Pinto, I.S.S.; Soares, E.V.; Soares, H.M.V.M. (Un)Suitability of the Use of PH Buffers in Biological, Biochemical and Environmental Studies and Their Interaction with Metal Ions—A Review. RSC Adv. 2015, 5, 30989–31003. [Google Scholar] [CrossRef]
- Vieira, V.M.N.C.S.; Lopes, I.E.; Creed, J.C. The Biomass–Density Relationship in Seagrasses and Its Use as an Ecological Indicator. BMC Ecol. 2018, 18, 44. [Google Scholar] [CrossRef]
- Poorter, H.; Nagel, O. The Role of Biomass Allocation in the Growth Response of Plants to Different Levels of Light, CO2, Nutrients and Water: A Quantitative Review. Funct. Plant Biol. 2000, 27, 1191. [Google Scholar] [CrossRef]
- Bergamaschi, H.; Dalmago, G.A.; Comiran, F.; Bergonci, J.I.; Müller, A.G.; França, S.; Santos, A.O.; Radin, B.; Bianchi, C.A.M.; Pereira, P.G. Deficit Hídrico e Produtividade Na Cultura Do Milho. Pesqui. Agropecu. Bras. 2006, 41, 243–249. [Google Scholar] [CrossRef]
- Hendges, F.B.; Rambo, C.R.; Alcassa, L.P.; Liebl, J.; Vendruscolo, E.C.G.; Costa, A.C.T. da Avaliação Enzimática e Fisiológica de Plântulas de Milho Submetidas à Seca. Rev. Bras. Energ. Renov. 2015, 4, 52–63. [Google Scholar] [CrossRef]
- Leitenmaier, B.; Küpper, H. Compartmentation and Complexation of Metals in Hyperaccumulator Plants. Front. Plant Sci. 2013, 4, 374. [Google Scholar] [CrossRef] [PubMed]
- Kakar, H.A.; Ullah, S.; Shah, W.; Ali, B.; Satti, S.Z.; Ullah, R.; Muhammad, Z.; Eldin, S.M.; Ali, I.; Alwahibi, M.S.; et al. Seed Priming Modulates Physiological and Agronomic Attributes of Maize (Zea mays L.) under Induced Polyethylene Glycol Osmotic Stress. ACS Omega 2023, 8, 22788–22808. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.d.; Oliveira, G.P.d. Tratamento de Sementes Com Micronutrientes Na Cultura Do Milho (Zea mays L.). Rev. Bras. Multidiscip. 2021, 24, 130–135. [Google Scholar] [CrossRef]
- Maia Júnior, S.D.O.; Andrade, J.R.D.E.; Dutra, A.F.; Santos, A.F.D.S.; Barros, J.M.T.D.M.; Silva, A.C.S.D. Respostas Da Aplicação Foliar de Glicina Betaína Em Cana-de-Açúcar Submetida a Estresse Hídrico e Reidratação. Rev. Ciências Agroveterinárias 2021, 20, 128–133. [Google Scholar] [CrossRef]
- Moterle, L.M.; Lopes, P.d.C.; Braccini, A.d.L.e.; Scapim, C.A. Germination of Seeds and Seedling Growth of Popcorn Cultivars under Water and Salinity Stress. Rev. Bras. Sementes 2006, 28, 169–176. [Google Scholar] [CrossRef]
- Anjum, N.A.; Sharma, P.; Gill, S.S.; Hasanuzzaman, M.; Khan, E.A.; Kachhap, K.; Mohamed, A.A.; Thangavel, P.; Devi, G.D.; Vasudhevan, P.; et al. Catalase and Ascorbate Peroxidase—Representative H2O2-Detoxifying Heme Enzymes in Plants. Environ. Sci. Pollut. Res. 2016, 23, 19002–19029. [Google Scholar] [CrossRef]
- Xu, L.; Han, L.; Huang, B. Antioxidant Enzyme Activities and Gene Expression Patterns in Leaves of Kentucky Bluegrass in Response to Drought and Post-Drought Recovery. J. Am. Soc. Hortic. Sci. 2011, 136, 247–255. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as Key Players in Plant Stress Signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Saharan, B.S.; Brar, B.; Duhan, J.S.; Kumar, R.; Marwaha, S.; Rajput, V.D.; Minkina, T. Molecular and Physiological Mechanisms to Mitigate Abiotic Stress Conditions in Plants. Life 2022, 12, 1634. [Google Scholar] [CrossRef]
- Redillas, M.C.F.R.; Park, S.-H.; Lee, J.W.; Kim, Y.S.; Jeong, J.S.; Jung, H.; Bang, S.W.; Hahn, T.-R.; Kim, J.-K. Accumulation of Trehalose Increases Soluble Sugar Contents in Rice Plants Conferring Tolerance to Drought and Salt Stress. Plant Biotechnol. Rep. 2012, 6, 89–96. [Google Scholar] [CrossRef]
- Chaves Filho, J.T.; Stacciarini-Seraphin, E. Alteração No Potencial Osmótico e Teor de Carboidratos Solúveis Em Plantas Jovens de Lobeira (Solanum lycocarpum St.-Hil.) Em Resposta Ao Estresse Hídrico. Rev. Bras. Botânica 2001, 24, 199–204. [Google Scholar] [CrossRef]
- Timpa, J.D.; Burke, J.J.; Quisenberry, J.E.; Wendt, C.W. Effects of Water Stress on the Organic Acid and Carbohydrate Compositions of Cotton Plants. Plant Physiol. 1986, 82, 724–728. [Google Scholar] [CrossRef]
- Peloso, A.F.; Tatagiba, S.; dos Reis, E.; Pezzopane, J.E.M.; do Amaral, J.F.T. Photosynthetic Limitations in Leaves of Arabic Coffee Promoted by the Water Deficit. Coffee Sci. 2017, 389–399. [Google Scholar] [CrossRef]
- Krause, G.H.; Weis, E. Chlorophyll Fluorescence and Photosynthesis: The Basics. Annu. Rev. Plant Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, H.; Kaur, H.; Srivastava, S. The Beneficial Roles of Trace and Ultratrace Elements in Plants. Plant Growth Regul. 2023, 100, 219–236. [Google Scholar] [CrossRef]
- Nciizah, A.D.; Rapetsoa, M.C.; Wakindiki, I.I.C.; Zerizghy, M.G. Micronutrient Seed Priming Improves Maize (Zea mays) Early Seedling Growth in a Micronutrient Deficient Soil. Heliyon 2020, 6, e04766. [Google Scholar] [CrossRef]
- BRAZIL. Rules for Seed Analysis, Ministry of Agriculture, Livestock and Supply; Ministry of Agriculture, Livestock and Supply: Brasília, Brazil, 2009.
- Ávila, M.R.; Braccini, A.d.L.e.; Scapim, C.A.; Martorelli, D.T.; Albrecht, L.P. Testes de Laboratório Em Sementes de Canola e a Correlação Com a Emergência Das Plântulas Em Campo. Rev. Bras. Sementes 2005, 27, 62–70. [Google Scholar] [CrossRef]
- Maguire, J.D. Speed of Germination—Aid in Selection and Evaluation for Seedling Emergence and Vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Barrs, H.; Weatherley, P. A Re-Examination of the Relative Turgidity Technique for Estimating Water Deficits in Leaves. Aust. J. Biol. Sci. 1962, 15, 413. [Google Scholar] [CrossRef]
- WALZ (Ed.) WALZ MINI-PAM Portable Chlorophyll Fluorometer: Handbook of Operation; Heinz Walz GmbH: Effeltrich, Germany, 1999. [Google Scholar]
- López-Hidalgo, C.; Meijón, M.; Lamelas, L.; Valledor, L. The Rainbow Protocol: A Sequential Method for Quantifying Pigments, Sugars, Free Amino Acids, Phenolics, Flavonoids and MDA from a Small Amount of Sample. Plant Cell Environ. 2021, 44, 1977–1986. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Chow, P.S.; Landhausser, S.M. A Method for Routine Measurements of Total Sugar and Starch Content in Woody Plant Tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The Effect of Drought and Ultraviolet Radiation on Growth and Stress Markers in Pea and Wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Biemelt, S.; Keetman, U.; Albrecht, G. Re-Aeration Following Hypoxia or Anoxia Leads to Activation of the Antioxidative Defense System in Roots of Wheat Seedlings. Plant Physiol. 1998, 116, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Havir, E.A.; McHale, N.A. Biochemical and Developmental Characterization of Multiple Forms of Catalase in Tobacco Leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 1, 309–314. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing 2025; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Wickham, H.; Bryan, J. Readxl: Read Excel Files, Version 1.3.1; The R Foundation: Vienna, Austria, 2019; Available online: https://CRAN.R-project.org/package=readxl (accessed on 15 September 2025).
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A. ExpDes.Pt: Pacote Experimental Designs (Portugues) 2022. Available online: https://cran.r-project.org/web/packages/ExpDes.pt/ExpDes.pt.pdf (accessed on 15 September 2025).
- Lemon, J. Plotrix: Various Plotting Functions, Version 3.8-2; The R Foundation: Vienna, Austria, 2021; Available online: https://CRAN.R-project.org/package=plotrix (accessed on 15 September 2025).
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix 2017, Version 0.84; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses 2020, R Package Version 1.0.7.; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Shimizu, G.D.; Marubayashi, R.Y.P.; Gonçalves, L.S.A. AgroR: An R Package and a Shiny Interface for Agricultural Experiment Analysis. Acta Sci. Agron. 2025, 47, e73889. [Google Scholar] [CrossRef]
- Davis, T.L. ggpattern: Pattern geoms for ggplot2 (Version 1.0.0) [Software]. The R Foundation: Vienna, Austria, 2020. Available online: https://CRAN.R-project.org/package=ggpattern (accessed on 15 September 2025).





| Treatment | Hydropriming | Composition | Stress Condition | pH | Electrical Conductivity |
|---|---|---|---|---|---|
| T1 | Distilled Water | H2O | Irrigation | 5.142 | 72.0 µS cm−1 |
| T2 | Phosphate Buffer | 0.5 mL K2HPO4 (1 M) + 0.5 mL KH2PO4 (1 M) | Irrigation | 7.046 | 6599.3 µS cm−1 |
| T3 | Distilled Water | H2O | Water Deficit | 5.142 | 72.0 µS cm−1 |
| T4 | Phosphate Buffer | 0.5 mL K2HPO4 (1 M) + 0.5 mL KH2PO4 (1 M) | Water Deficit | 7.046 | 6599.3 µS cm−1 |
| T5 | Ammonium Oxalate | 13.45 mL (NH4)2C2O4·H2O (0.2 M), equivalent to 250 mg kg−1 ammonium niobate (V) oxalate (C4H4NNbO9) | Water Deficit | 5.444 | 16,004.9 µS cm−1 |
| T6 | Ammonium Oxalate + Phosphate Buffer | 13.45 mL of (NH4)2C2O4·H2O (0.2 M), in the same proportion as the concentration of 250 mg kg−1 of ammonium niobate (V) oxalate (C4H4NNbO9) + 0.5 mL of K2HPO4 (1 M) + 0.5 mL of KH2PO4 (1 M) | Water Deficit | 6.593 | 18,079.9 µS cm−1 |
| T7 | Ammonium Niobate (V) Oxalate | 250 mg kg−1 of ammonium niobate (V) oxalate (C4H4NNbO9), 1.35 mL of C4H4NNbO9 (1 M) | Water Deficit | 3.571 | 1860.9 µS cm−1 |
| T8 | Ammonium Niobate (V) Oxalate + Phosphate Buffer | 250 mg kg−1 of ammonium niobate (V) oxalate (C4H4NNbO9), 1.35 mL of C4H4NNbO9 (1 M) + 0.5 mL of K2HPO4 (1 M) + 0.5 mL of KH2PO4 (1 M) | Water Deficit | 6.247 | 70,641.1 µS cm−1 |
| Parameter | Description | Formula |
|---|---|---|
| PAR | Photosynthetically active radiation. Value measured directly by MINI-PAM and used in the calculation of ETR | - |
| FO | Minimum Chl a fluorescence yield in the dark-adapted state | - |
| FM | Maximum Chl a fluorescence yield in the dark-adapted state | - |
| FV/FM | A quantity related to the maximum quantum yield of PSII photochemistry | |
| FM′ | Maximum fluorescence (measured during a saturation pulse under growth light conditions) | - |
| F | Fluorescence before the saturation pulse | - |
| Yield PSII (ΦPSII) | Effective quantum yield of PSII | |
| Y(NO) | Quantum yield of non-regulated energy dissipation | |
| Y(NPQ) | Quantum yield of regulated energy dissipation via NPQ-dependent mechanisms | Y(NPQ) = 1 − (YIELD − YNO) |
| FO′ | Minimum fluorescence under light | - |
| qP | Photochemical quenching coefficient based on the ratio between open and closed centers | |
| qL | Photochemical quenching coefficient based on the fraction of open PSII reaction centers | |
| qN | Non-photochemical quenching coefficient | |
| NPQ | Stern–Volmer non-photochemical quenching | |
| ETR | Electron transport rate | ETR = YIELD × PAR × 0.5 × ETR factor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evangelista, M.N.L.; Benevenute, P.A.N.; Lima, J.d.S.; dos Santos, L.C.; Morais, E.G.d.; Nascimento, V.L.; Lopes, G.; Guilherme, L.R.G. Priming Effect of Seeds with Niobium (Nb) on the Performance of Maize Plants Under Water Deficit Conditions. Plants 2025, 14, 3173. https://doi.org/10.3390/plants14203173
Evangelista MNL, Benevenute PAN, Lima JdS, dos Santos LC, Morais EGd, Nascimento VL, Lopes G, Guilherme LRG. Priming Effect of Seeds with Niobium (Nb) on the Performance of Maize Plants Under Water Deficit Conditions. Plants. 2025; 14(20):3173. https://doi.org/10.3390/plants14203173
Chicago/Turabian StyleEvangelista, Maisa Natália Leite, Pedro Antônio Namorato Benevenute, Jucelino de Sousa Lima, Leônidas Canuto dos Santos, Everton Geraldo de Morais, Vitor L. Nascimento, Guilherme Lopes, and Luiz Roberto Guimarães Guilherme. 2025. "Priming Effect of Seeds with Niobium (Nb) on the Performance of Maize Plants Under Water Deficit Conditions" Plants 14, no. 20: 3173. https://doi.org/10.3390/plants14203173
APA StyleEvangelista, M. N. L., Benevenute, P. A. N., Lima, J. d. S., dos Santos, L. C., Morais, E. G. d., Nascimento, V. L., Lopes, G., & Guilherme, L. R. G. (2025). Priming Effect of Seeds with Niobium (Nb) on the Performance of Maize Plants Under Water Deficit Conditions. Plants, 14(20), 3173. https://doi.org/10.3390/plants14203173








