Diversity Patterns of Spontaneous Plants and Their Multi-Scale Driving Mechanisms in Cold Regions: A Case of 14 Cities in Heilongjiang Province, China
Abstract
1. Introduction
2. Results
2.1. Spontaneous Plant Composition
2.2. Classification of Community Types and Their Distribution Characteristics
Clustering Results and Community Type Classification
2.3. Distribution Patterns of α and β Diversity Indices of Spontaneous Plants Across Multiple Scales and Dimensions
2.3.1. α-Diversity Index
Influence of Climate on the Distribution of α-Diversity Index
Influence of Habitat Types on the Distribution of α-Diversity Index
2.3.2. β-Diversity Index
Differences in Community β-Diversity Index Across Climatic Subzones
- (1)
- S-Type Communities
- (2)
- SAB-Type Communities
- (3)
- SP-Type Communities
Analysis of β-Diversity Differences Across Habitat Types
- (1)
- S-Type Communities
- (2)
- SAB-Type Communities
- (3)
- SP-Type Communities
3. Discussion
3.1. Characteristics of Species Composition and Potential Adaptation-Related Interpretations of Spontaneous Plants in Heilongjiang Province
3.2. Multi-Scale Driving Mechanisms of Spontaneous Diversity Distribution Patterns: Synergistic Effects of Climatic Gradient and Habitat Heterogeneity
3.2.1. Driving Factors of α-Diversity Index
3.2.2. Driving Factors of β-Diversity Index
3.3. Implications and Recommendations for the Maintenance and Management of Spontaneous Plant Diversity in Cold Regions
3.3.1. Conservation and Management of Spontaneous Plant Diversity in Different Habitat Types
- Lawns (LA): Annual/biennial plants in this habitat exhibit significant differences compared to other habitats. It is recommended to prioritize the retention of stress-tolerant native herbaceous species and reduce trimming frequency to minimize disturbance. The “land-sharing” model of Berlin community gardens [53] can be adopted, where reducing localized impervious surfaces and promoting the coexistence of cultivated and spontaneous plants can maximize the biodiversity value of lawns.
- Shrub-Grassland Gaps (SG): This habitat generally exhibits optimal α-diversity. It is advised to maintain the existing shrub-herb structure, reduce maintenance intensity to avoid over-clearing, and utilize canopy heterogeneity to create diverse microhabitats that facilitate the dispersal of native species [54]. This approach provides direct reference value for managing SG habitats.
- Forest Gaps (FG): These habitats show the greatest variability in community composition, and ferns are exclusively found here. To reduce competition from invasive species, shade-tolerant native plants can be moderately reintroduced to enhance forest layer complexity. Additionally, protecting the litter layer and recognizing the positive role of fallen log microhabitats are recommended, especially in the I A1 subzone (Greater Khingan Range area). Fallen logs reduce surface radiative cooling, decreasing soil freezing depth by 20–30% and extending the growing season by approximately 1–2 weeks, thereby providing “microclimatic refugia” for cold-tolerant species [29].
- Soil-Type Abandoned Land (SA) and Gravel-Type Abandoned Land (GA): Both are degraded habitats resulting from human disturbance. The core restoration logic involves using native species to rapidly occupy ecological niches [23]. SA has the highest overall species richness, dominated by annual/biennial plants, and relatively intact soil substrate. It is recommended to prioritize the introduction of native pioneer species of the same life form to synergistically compete with existing vegetation and reduce invasive species’ survival space. GA, characterized by a gravel substrate, has stable communities but all non-native plants are invasive species. Restoration efforts should focus on drought tolerance and soil improvement functions, selecting cold- and drought-tolerant native species. Plant roots can enhance water and nutrient retention in the substrate, creating more stable growth conditions that feedback to promote plant prosperity, thereby forming a synergistic progression between roots and substrate.
3.3.2. Regulation Strategies for Spontaneous Plant Diversity in Different Climatic Subzones
- Subzones I A1 and II A1: Both are located in the Greater and Lesser Khingan Mountains regions, with perennial plants as the core of their communities. Subzone I A1 has the highest proportion of perennial herbs but the lowest species richness, with cold tolerance being its key community characteristic. The principle of “protection priority” should be adopted to minimize human disturbance to natural patches at urban edges and maintain habitat integrity. Subzone II A1 exhibits the highest Shannon index for perennial plants and strong community stability. The focus should be on “enhancing connectivity” by preserving existing habitat heterogeneity, constructing ecological corridors to link fragmented green spaces, providing migration pathways for native species, and promoting seed dispersal to enhance community resilience [27,55].
- Subzones II A2 and II B2: Both are situated in mid-temperate zones and host larger cities in Heilongjiang Province. Subzone II A2 has high species diversity and relatively balanced life form distribution. It is recommended to delineate ecological conservation redlines, establish multi-layered vegetation structures (tree-shrub-herb) [56,57], strengthen natural maintenance capacity of communities, and prevent habitat fragmentation. Subzone IIB2 has the richest species diversity but the greatest internal community variability and faces significant invasion pressure. The strategy should focus on “connectivity and prevention,” controlling urban warming, curbing the spread of non-native species, and implementing quarterly invasive species coverage surveys [25,36,58].
- Subzone II C1: This subzone shows the highest variability in perennial plants and a high proportion of invasive species. It is recommended for plant drought-tolerant native species to improve soil quality, reduce exposed habitats, and enhance community resistance. Additionally, vertical habitats such as building gaps and walls should be utilized to retain drought- and cold-tolerant spontaneous plants, creating green spaces in vertical dimensions [59]. The “low-maintenance spontaneous vegetation landscapes” model can be applied to synergistically enhance ecological functions and landscape value [5,23].
4. Materials and Methods
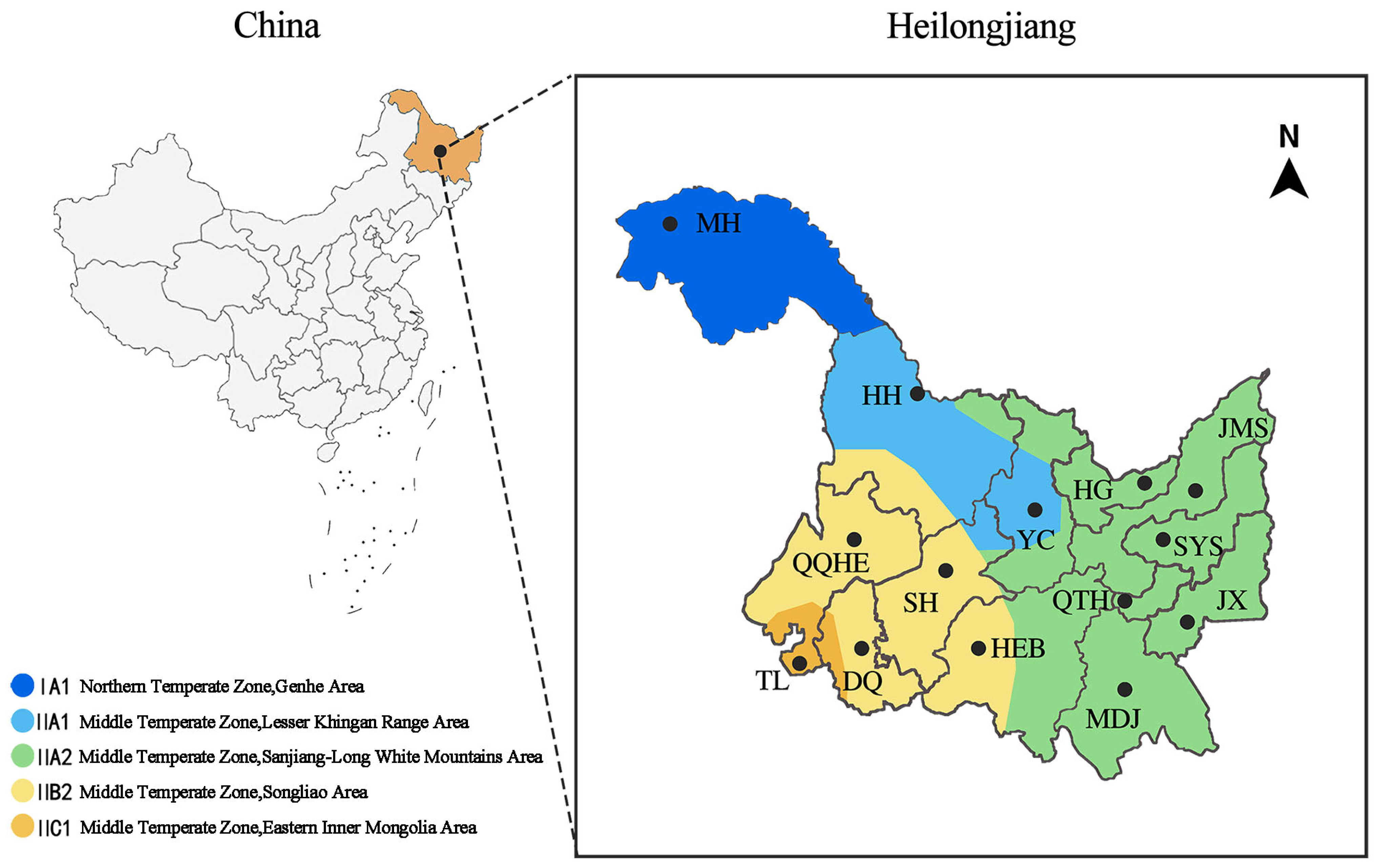
4.1. Study Area
4.2. Survey Methods for Spontaneous Plants
4.3. Data Processing
4.3.1. Classification of Community Types
4.3.2. Calculation Methods of Diversity Indices
- (1)
- Species Dominance
- (2)
- α-diversity
- (3)
- β-diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Anderson, E.C.; Minor, E.S. Assessing social and biophysical drivers of spontaneous plant diversity and structure in urban vacant lots. Sci. Total Environ. 2019, 653, 1272–1281. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, S.; Li, X.; Li, K.; Xing, X.; Hao, P.; Dong, L. How urban riparian corridors affect the diversity of spontaneous herbaceous plants as pollination and dispersal routes—A case of the Wenyu River-North Canal in Beijing, China. Ecol. Indic. 2023, 146, 110027. [Google Scholar] [CrossRef]
- Alston, K.P.; Richardson, D.M. The roles of habitat features, disturbance, and distance from putative source populations in structuring alien plant invasions at the urban/wildland interface on the Cape Peninsula, South Africa. Biol. Conserv. 2006, 132, 183–198. [Google Scholar] [CrossRef]
- Kühn, N. Intentions for the Unintentional: Spontaneous Vegetation as the Basis for Innovative Planting Design in Urban Areas. J. Landsc. Archit. 2006, 1, 46–53. [Google Scholar] [CrossRef]
- Del Tredici, P. Spontaneous Urban Vegetation: Reflections of Change in a Globalized World. Nat. Cult. 2010, 5, 299–315. [Google Scholar] [CrossRef]
- Cervelli, E.W.; Lundholm, J.T.; Du, X. Spontaneous Urban Vegetation and Habitat Heterogeneity in Xi’an, China. Landsc. Urban Plan. 2013, 120, 25–33. [Google Scholar] [CrossRef]
- Alberti, M.; Marzluff, J.M.; Hunt, V.M. Urban driven phenotypic changes: Empirical observations and theoretical implications for eco-evolutionary feedback. Philos. Trans. R. Soc. B 2017, 372, 20160029. [Google Scholar] [CrossRef]
- Ling, Q.; Ling, Z.; Chen, P.; Wang, J.; Fan, J.; Tian, G. Is urban spontaneous vegetation rich in species and has potential for exploitation?—A case study in Baoji, China. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2019, 155, 42–53. [Google Scholar] [CrossRef]
- Sjöman, H.; Nielsen, A.B. Selecting Trees for Urban Paved Sites in Scandinavia—A Review of Information on Stress Tolerance and Its Relation to the Requirements of Tree Planners. Urban For. Urban Green. 2010, 9, 281–293. [Google Scholar] [CrossRef]
- Kowarik, I. Novel Urban Ecosystems, Biodiversity, and Conservation. Environ. Pollut. 2011, 159, 1974–1983. [Google Scholar] [CrossRef]
- Sjöman, H.; Östberg, J.; Bühler, O. Diversity and distribution of the urban tree population in ten major Nordic cities. J. Urban For. Urban Green. 2012, 11, 31–39. [Google Scholar] [CrossRef]
- Lönnqvist, J.; Hanslin, H.M.; Johannessen, B.G.; Muthanna, T.M.; Viklander, M.; Blecken, G. Temperatures and Precipitation Affect Vegetation Dynamics on Scandinavian Extensive Green Roofs. Int. J. Biometeorol. 2020, 64, 1645–1657. [Google Scholar] [CrossRef]
- Dean, S.; Joel, L.; Tobias, B.G.; Williams, W.N.S.; Fleming, C. Socio-Ecological Dimensions of Spontaneous Plants on Green Roofs. Front. Sustain. Cities 2021, 3, 777128. [Google Scholar] [CrossRef]
- Ávila-Rodríguez, J.; Pérez-Alvarez, A.; Fernández-Jiménez, J.C.; Torres-Montes, M.; García-Álvarez, M.N. Evidence supporting the value of spontaneous vegetation for phytomanagement of soil ecosystem functions in abandoned metal(loid) mine tailings. Catena 2021, 201, 105877. [Google Scholar] [CrossRef]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global Change and the Ecology of Cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef] [PubMed]
- von der Lippe, M.; Kowarik, I. Do cities export biodiversity? Traffic as dispersal vector across urban–rural gradients. Divers. Distrib. 2008, 14, 18–25. [Google Scholar] [CrossRef]
- Prach, K.; Bartha, S.; Joyce, C.B.; Pyšek, P.; van Diggelen, R.; Wiegleb, G. The Role of Spontaneous Vegetation Succession in Ecosystem Restoration: A Perspective. Appl. Veg. Sci. 2001, 4, 111–114. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Zhang, M.; Luo, Q.; Li, Y.; Dong, L. Urban park attributes as predictors for the diversity and composition of spontaneous plants−A case in Beijing, China. Urban For. Urban Green. 2024, 91, 128185. [Google Scholar] [CrossRef]
- Zhang, M.; Li, K.; Xing, X.; Fan, S.; Xu, Y.; Hao, P.; Dong, L. Response of spontaneous plant diversity to urbanization in the Wenyu River-North Canal ecological corridor, Beijing. Acta Ecol. Sin. 2022, 42, 2582–2592. [Google Scholar] [CrossRef]
- Chen, C. Overlooked urban habitats: Investigation and analysis of spontaneous plants on walls in Chongqing. Acta Ecol. Sin. 2020, 40, 473–483. [Google Scholar] [CrossRef]
- Chen, X.; Liang, H.; Song, K.; Da, L. Species diversity of weeds and their distribution in heterogeneous habitats in the central urban area of Harbin. Chin. J. Ecol. 2014, 33, 946–952. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, Y.; Da, L. Distribution patterns and driving factors of weed communities in different urban habitats in Hangzhou. J. East China Norm. Univ. (Nat. Sci. Ed.) 2021, 2021, 120–131. [Google Scholar] [CrossRef]
- Yao, X.; Li, H.; Jin, Y.; Shi, Y.; Bao, Z. Diversity and community composition of spontaneous herbaceous plants in the herb layer of Xixi National Wetland Park, Hangzhou. Chin. Landsc. Archit. 2021, 37, 123–128. [Google Scholar] [CrossRef]
- Tian, Z. Spatial Patterns of Weed Community Diversity and Their Driving Factors in Urban-Rural Terrestrial Ecosystems of Shanghai. Ph.D. Thesis, East China Normal University, Shanghai, China, 2011. [Google Scholar]
- Gao, Z. Patterns of Spontaneous Plant Diversity and Their Influencing Factors in Nine Cities of Yunnan Province. Ph.D. Thesis, Yunnan University, Kunming, China, 2021. [Google Scholar]
- Zhu, H.; Zhao, C.; Li, F.; Shen, P.; Liu, L.; Hu, Y. Distribution Patterns of Urban Spontaneous Vegetation Diversity and Their Response to Habitat Heterogeneity: A Case Study of Five Cities in Heilongjiang Province, China. Plants 2024, 13, 2982. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Ren, S.; Liang, X.; Hu, Y. Diversity and distribution characteristics of spontaneous herbaceous plants in urban river corridors of Harbin. Landsc. Archit. 2024, 31, 28–36. [Google Scholar] [CrossRef]
- GB/T 17297-2021; Climate Regionalization of China. Standard Press of China: Beijing, China, 2021.
- Zhang, M.; Li, X.; Fan, S.; Li, K.; Xing, X.; Xu, Y.; Hao, P.; Dong, L. Response of spontaneous plant communities to microhabitats in a riparian corridor in Beijing, China. Sci. Rep. 2024, 14, 17642. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, S.; Gao, J.; Kong, W.; Du, C.; Liu, Y.; Feng, Y.; Kong, D. Distribution patterns of plant diversity and their influencing factors in the built-up area of Zhengzhou. J. Henan Agric. Univ. 2025, 59, 422–433. [Google Scholar] [CrossRef]
- Su, Y.; Masulya, M.; Li, Y.; Wei, Q.; Wang, H.; Li, W. Species diversity and distribution patterns of threatened vascular plants in Kyrgyzstan. Arid Zone Res. 2024, 41, 1405–1412. [Google Scholar]
- Lu, Q.; Wu, S.; Zhao, D. Changes in alpine grassland coverage on the Qinghai-Tibet Plateau from 1982 to 2013 and their relationship with climate. Sci. Geogr. Sin. 2017, 37, 292–300. [Google Scholar] [CrossRef]
- Géron, C.; Lembrechts, J.J.; Hamdi, R.; Berckmans, J.; Nijs, I.; Monty, A. Phenotypic Variation Along Urban-to-Rural Gradients: An Attempt to Disentangle the Mechanisms at Play Using the Alien Species Matricaria discoidea (Asteraceae). Plant Ecol. 2022, 223, 1219–1231. [Google Scholar] [CrossRef]
- Vigeland, M.D.; Spannagl, M.; Asp, T.; Paina, C.; Rudi, H.; Rognli, O.A.; Fjellheim, S.; Sandve, S.R. Evidence for Adaptive Evolution of Low-Temperature Stress Response Genes in a Pooideae Grass Ancestor. New Phytol. 2013, 199, 1060–1068. [Google Scholar] [CrossRef]
- Li, Z.; Dao, Z.; Zhao, G.; Chen, C.; Zhang, S.; Zhang, C.; Li, S.; Wen, H.; Li, T.; Chen, Y. Composition and diversity patterns of plant communities along an elevation gradient in the Yuanjiang dry-hot valley. Guihaia 2024, 44, 2141–2151. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W.; Liang, H.; Liu, X.; Da, L. Dynamics of ruderal species diversity under rapid urbanization over the past half century in Harbin, Northeast China. Urban Ecosyst. 2014, 17, 455–472. [Google Scholar] [CrossRef]
- Schatz, J.; Kucharik, C.J. Urban Heat Island Effects on Growing Seasons and Heating and Cooling Degree Days in Madison, Wisconsin, USA. Int. J. Climatol. 2016, 36, 4873–4884. [Google Scholar] [CrossRef]
- Yang, S. Physiological Responses of Different Gramineous Forage Species to Drought and Low-Temperature Stress. Master’s Thesis, Northwest A&F University, Yangling, China, 2008. [Google Scholar]
- Liu, Y. Morphological and Physiological Adaptability of Different Trifolium Repens Varieties Under Low-Temperature Stress. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2008. [Google Scholar]
- Körner, C. Plant adaptation to cold climates. F1000Research 2016, 5, 9107. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, X.; Wang, Z.; Fang, W. Responses of vegetation phenology to climate change in Northeast China. Chin. J. Ecol. 2010, 29, 578–585. [Google Scholar] [CrossRef]
- Chen, X. Distribution Patterns of Weed Communities and Their Responses to Habitat Heterogeneity in Urban Areas of Harbin. Ph.D. Thesis, East China Normal University, Shanghai, China, 2014. [Google Scholar] [CrossRef]
- Funk, J.L. Differences in Plasticity between Invasive and Native Plants from a Low Resource Environment. J. Ecol. 2008, 96, 1162–1173. [Google Scholar] [CrossRef]
- Feng, J.; Dong, X.; Xu, C. Spatial distribution patterns of alien invasive plant species diversity in China and their relationship with native plants. J. Southwest Univ. (Nat. Sci. Ed.) 2010, 32, 50–57. [Google Scholar] [CrossRef]
- Han, Y.; Chen, G.; Zhou, G.; Sun, J.; Li, J. Responses of individual plant traits of alpine grassland to grazing in the Qinghai Lake region. J. Grad. Sch. Chin. Acad. Sci. 2006, 23, 118–124. [Google Scholar] [CrossRef]
- Wang, Y. Application of Niche Models in Risk Assessment of Alien Invasive Species. Ph.D. Thesis, Hunan Agricultural University, Changsha, China, 2007. [Google Scholar]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Kattge, J.; Niinemets, P.; Peñuelas, J.R.; Minden, V.; Hallik, L.; Aakala, T.; Rönkäs, T.; Beierkuhnlein, C.; Virtanen, R.; Atkin, O.K.; et al. Consistent trait–environment relationships within and across tundra plant communities. Nat. Ecol. Evol. 2021, 5, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ryu, Y. Seasonal changes in vertical canopy structure in a temperate broadleaved forest in Korea. Ecol. Res. 2015, 30, 821–831. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C.; Chester, E.T.; Vicentini, A. The evolutionary responses of life-history strategies to climatic variability in flowering plants. New Phytol. 2023, 240, 1587–1600. [Google Scholar] [CrossRef]
- Wang, Y.C.; Zhang, X.N.; Yang, J.F.; Tian, J.Y.; Song, D.H.; Li, X.H.; Zhou, S.F. Spatial heterogeneity of soil factors enhances intraspecific variation in plant functional traits in a desert ecosystem. Front. Plant Sci. 2024, 15, 1504238. [Google Scholar] [CrossRef] [PubMed]
- Fahrig, L. Ecological Responses to Habitat Fragmentation Per Se. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 1–23. [Google Scholar] [CrossRef]
- Seitz, B.; Buchholz, S.; Kowarik, I.; Herrmann, J.; Neuerburg, L.; Wendler, J.; Winker, L.; Egerer, M. Land sharing between cultivated and wild plants: Urban gardens as hotspots for plant diversity in cities. Urban Ecosyst. 2022, 25, 927–939. [Google Scholar] [CrossRef]
- Yang, W. Study on Spontaneous Plant Diversity and Maintenance Management Strategies in Xiongan Urban Forests. Master’s Thesis, Beijing Forestry University, Beijing, China, 2022. [Google Scholar]
- Czárán, K.R.; Szerdahelyi, A.M.; Juhász, D.B. Improving the Biodiversity in Urban Green Spaces: A Nature-Based Approach. Ecol. Eng. 2021, 173, 106398. [Google Scholar] [CrossRef]
- Jia, B. Plant Diversity and Community Types of Urban Green Spaces along the Urban-Rural Gradient in Harbin. Master’s Thesis, East China Normal University, Shanghai, China, 2021. [Google Scholar]
- Charoenlertthanakit, N.; Inta, A.; Shannon, D.P.; Boonsuk, B.; Tiansawat, P. Greens in the Gaps: Diversity and the Ecological Potential of Urban Spontaneous Vegetation in Sidewalk Ecosystems. Plants 2025, 14, 2542. [Google Scholar] [CrossRef]
- Ilie, D.; Cosmulescu, S. Spontaneous Plant Diversity in Urban Contexts: A Review of Its Impact and Importance. Diversity 2023, 15, 277. [Google Scholar] [CrossRef]
- Chen, C.; Mao, L.; Qian, Y.; Chen, J.; Wang, Y. Walls offer potential to improve urban biodiversity. Sci. Rep. 2020, 10, 9905. [Google Scholar] [CrossRef]
- Patrick, R. A Proposed Biological Measure of Stream Conditions, Based on a Survey of the Conestoga Basin, Lancaster County, Pennsylvania. Proc. Acad. Nat. Sci. Phila. 1949, 101, 277–341. [Google Scholar]
- Magurran, A.E. Ecological Diversity and Its Measurement, 2nd ed.; Springer: Dordrecht, The Netherlands, 2004; pp. 1–200. ISBN 978-1-4020-2613-0. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. ACM SIGMOBILE Mob. Comput. Commun. Rev. 2001, 5, 3–55. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Jaccard, P. The distribution of the flora in the alpine zone. New Phytol. 1912, 11, 37–50. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Zhao, C.; Zhu, H.; Yang, X.; Hu, Y. Diversity Patterns of Spontaneous Plants and Their Multi-Scale Driving Mechanisms in Cold Regions: A Case of 14 Cities in Heilongjiang Province, China. Plants 2025, 14, 3145. https://doi.org/10.3390/plants14203145
Li F, Zhao C, Zhu H, Yang X, Hu Y. Diversity Patterns of Spontaneous Plants and Their Multi-Scale Driving Mechanisms in Cold Regions: A Case of 14 Cities in Heilongjiang Province, China. Plants. 2025; 14(20):3145. https://doi.org/10.3390/plants14203145
Chicago/Turabian StyleLi, Feinuo, Congcong Zhao, Haiyan Zhu, Xueting Yang, and Yuandong Hu. 2025. "Diversity Patterns of Spontaneous Plants and Their Multi-Scale Driving Mechanisms in Cold Regions: A Case of 14 Cities in Heilongjiang Province, China" Plants 14, no. 20: 3145. https://doi.org/10.3390/plants14203145
APA StyleLi, F., Zhao, C., Zhu, H., Yang, X., & Hu, Y. (2025). Diversity Patterns of Spontaneous Plants and Their Multi-Scale Driving Mechanisms in Cold Regions: A Case of 14 Cities in Heilongjiang Province, China. Plants, 14(20), 3145. https://doi.org/10.3390/plants14203145





