Bryophytes of the Loess Cliffs in the Pannonian Area of Austria
Abstract
1. Introduction
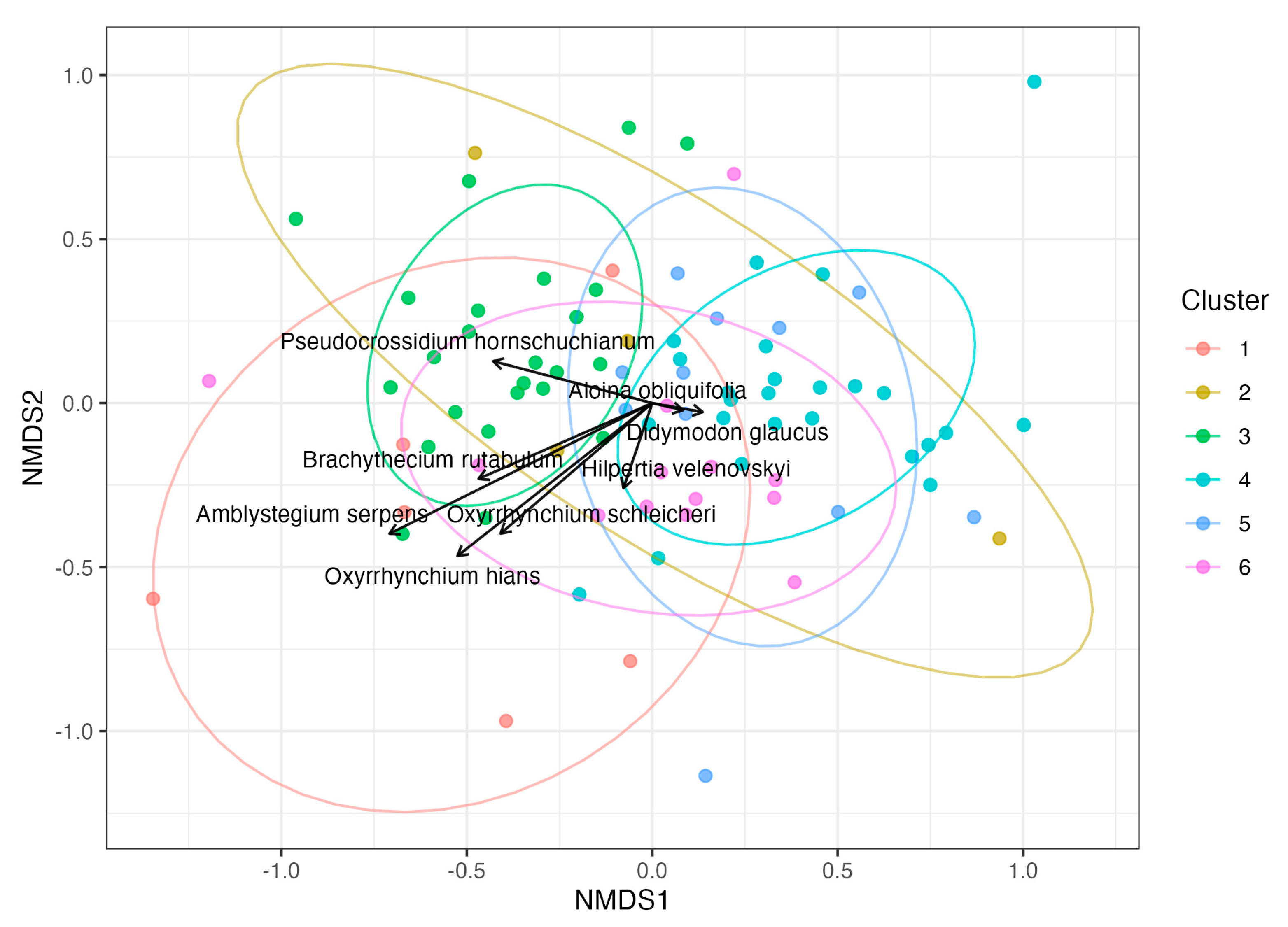
2. Results
2.1. Bryophyte Species
2.2. Site Characteristics
2.3. Syntaxonomic Classification
3. Discussion
3.1. Discussion on Species and Communities
- Psoretea decipientis Matt. Ex Follm. 1974
- Barbulatalia unguiculatae v. Hübschmann 1960
- Grimmaldion fragrantis Šm. et. Had. 1944
- Didymodontetum glauci Ahrens ex Marst. 2015 didymodontetosum cordati subass. nov.
- Pterygoneuro-Acaulonetum triquetri ass. nov.
- Aloinetum rigidae Stod. 1937
- Aloinetum rigidae Stod. 1937 aloinetosum obliquifoliae subass. nov.
- Hilpertio velenovskyi-Pterygoneuretum compacti Kürschner & Pócs 2002
- Eurhynchietum schleicheri Waldh. 1944 didymodontetosum cordati subass. nov.
3.2. Conservation Context
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | RL status |
|---|---|
| Abietinella abietina (Hedw.) M.Fleisch. var. abietina | LC |
| Acaulon muticum (Hedw.) Müll.Hal. | EN |
| Acaulon triquetrum (Spruce) Müll.Hal. | VU |
| Aloina aloides (Koch ex Schultz) Kindb. | * CR |
| Aloina ambigua (Bruch & Schimp.) Limpr. | EN |
| Aloina brevirostris (Hook. & Grev.) Kindb. | RE |
| Aloina obliquifolia (Müll. Hal.) Broth. | * EN |
| Aloina rigida (Hedw.) Limpr. | VU |
| Amblystegium serpens (L. ex Hedw.) Schimp. | LC |
| Barbula convoluta Hedw. | LC |
| Barbula unguiculata Hedw. | LC |
| Brachytheciastrum velutinum (L. ex Hedw.) Ignatov & Huttunen | LC |
| Brachythecium albicans (Neck. ex Hedw.) Schimp. | LC |
| Brachythecium campestre (Müll.Hal.) Schimp. | NT |
| Brachythecium glareosum (Bruch ex Spruce) Schimp. var. glareosum | LC |
| Brachythecium rutabulum (L. ex Hedw.) Schimp. | LC |
| Bryum argenteum Hedw. | LC |
| Bryum dichotomum | LC |
| Bryum gemmiferum R.Wilczek & Demaret | VU-R |
| Bryum klinggraeffii Schimp. | NT |
| Bryum radiculosum Brid. | VU |
| Bryum violaceum Crundw. & Nyholm | NT |
| Campyliadelphus chrysophyllus (Brid.) R.S.Chopra | LC |
| Ceratodon purpureus (Hedw.) Brid. | LC |
| Dicranella howei Renauld & Cardot | VU |
| Didymodon acutus (Brid.) K.Saito | LC |
| Didymodon cordatus Jur. | NT |
| Didymodon fallax (Hedw.) R.H.Zander | LC |
| Didymodon ferrugineus (Schimp. ex Besch.) M.O.Hill | LC |
| Didymodon glaucus Ryan | NT |
| Didymodon insulanus (De Not.) M.O.Hill | NT |
| Didymodon rigidulus Hedw. | LC |
| Didymodon vinealis (Brid.) R.H.Zander | EN |
| Ditrichum pusillum (Hedw.) Hampe | NT |
| Encalypta streptocarpa Hedw. | LC |
| Encalypta vulgaris Hedw. | LC |
| Entodon concinnus (De Not.) Paris | LC |
| Fossombronia wondraczekii (Corda) Lindb. | NT |
| Funaria hygrometrica Hedw. | LC |
| Grimmia montana Bruch & Schimp. | VU |
| Grimmia pulvinata (Timm. ex Hedw.) Sm. | LC |
| Grimmia tergestina var. tergestinoides Culm. | LC |
| Hilpertia velenovskyi (Schiffn.) R.H.Zander | * CR |
| Homalothecium lutescens (Hedw.) H.Rob. | LC |
| Microbryum curvicollum (Ehrh. ex Hedw.) R.H.Zander | VU |
| Microbryum floerkeanum (F.Weber & D.Mohr) Schimp. | EN |
| Microbryum rectum (With.) R.H.Zander | * CR |
| Microbryum starckeanum (Hedw.) R.H.Zander | EN |
| Orthotrichum anomalum Hedw. | LC |
| Oxyrrhynchium hians (Hedw.) Loeske var. hians | LC |
| Oxyrrhynchium schleicheri (R.Hedw.) Röll | LC |
| Plagiomnium cuspidatum (Hedw.) T.J.Kop. | LC |
| Plagiomnium undulatum (Hedw.) T.J.Kop. | LC |
| Pseudocrossidium hornschuchianum (Schultz) R.H.Zander | LC |
| Pseudocrossidium revolutum (Brid.) R.H.Zander | EN |
| Pterygoneurum compactum M.J.Cano, J.Guerra & Ros | CR |
| Pterygoneurum crossidioides W.Frey, Herrnst. & Kürschner | CR |
| Pterygoneurum lamellatum (Lindb.) Jur. | EN |
| Pterygoneurum ovatum (Hedw.) Dixon | VU |
| Pterygoneurum squamosum Segarra & Kürschner | CR |
| Pterygoneurum subsessile (Brid.) Jur. | EN |
| Ptycostomum capillare (Hedw.) Holyoak & N.Pedersen | LC |
| Ptychostomum creberrimum (Taylor) J.R.Spence & H.P.Ramsay | LC |
| Ptychostomum imbricatulum (Müll.Hal.) Holyoak & N.Pedersen | LC |
| Syntrichia calcicola J.J.Amann | NT |
| Syntrichia ruraliformis (Besch.) Cardot | VU |
| Syntrichia ruralis (Hedw.) F.Weber & D.Mohr | LC |
| Tortella squarrosa (Brid.) Limpr. | VU |
| Tortula acaulon (With.) R.H.Zander var. acaulon | LC |
| Tortula acaulon var. pilifera (Lindb.) R.H.Zander | NT |
| Tortula brevissima Schiffn. | *CR |
| Tortula caucasica Broth. | VU |
| Tortula lindbergii Broth. | VU |
| Tortula muralis Hedw. subsp. muralis var. muralis | LC |
| Tortula protobryoides R.H.Zander | VU |
| Tortula truncata (Hedw.) Mitt. | LC |
| Weissia condensa (Voit) Lindb. | VU |
| Weissia controversa Hedw. | LC |
| Weissia longifolia Mitt. | VU |
| Species/Cluster | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| constancy | ||||||
| Character and differential species | ||||||
| Oxyrrhynchium schleicheri | 83 | 0 | 0 | 0 | 0 | 0 |
| Oxyrrhynchium hians | 67 | 0 | 8.3 | 0 | 0 | 0 |
| Brachythecium rutabulum | 67 | 0 | 4.2 | 0 | 0 | 0 |
| Amblystegium serpens | 67 | 0 | 0 | 0 | 0 | 0 |
| Didymodon glaucus | 0 | 100 | 0 | 0 | 0 | 0 |
| Acaulon triquetrum | 0 | 25 | 54 | 3.8 | 18 | 14 |
| Bryum dichotomum | 0 | 0 | 46 | 3.8 | 9.1 | 0 |
| Didymodon acutus | 0 | 0 | 33 | 0 | 0 | 0 |
| Tortula brevissima | 0 | 0 | 25 | 3.8 | 0 | 7.1 |
| Homalothecium lutescens | 0 | 0 | 25 | 0 | 0 | 0 |
| Aloina rigida | 17 | 25 | 33 | 88 | 100 | 14 |
| Aloina obliquifolia | 0 | 0 | 4.2 | 0 | 100 | 21 |
| Hilpertia velenovskyi | 0 | 0 | 0 | 0 | 0 | 50 |
| Pterygoneurum compactum | 0 | 0 | 0 | 0 | 0 | 50 |
| Didymodon vinealis | 0 | 0 | 8.3 | 0 | 0 | 29 |
| Pterygoneurm squamosum | 0 | 0 | 0 | 0 | 0 | 14 |
| Didymodon cordatus | 83 | 100 | 96 | 100 | 100 | 93 |
| Grimmaldion | ||||||
| Tortula lindbergii | 50 | 75 | 79 | 46 | 64 | 43 |
| Aloina ambigua | 50 | 75 | 79 | 12 | 18 | 71 |
| Streblotrichum convolutum var. convolutum | 50 | 50 | 79 | 23 | 18 | 7.1 |
| Pterygoneurum lamellatum | 25 | 54 | 27 | 45 | 57 | |
| Pterygoneurum subsessile | 0 | 50 | 29 | 23 | 27 | 21 |
| Tortula acaulon var. pilifera | 0 | 50 | 29 | 7.7 | 18 | 29 |
| Pseudocrossidium hornschuchianum | 0 | 75 | 29 | 3.8 | 9.1 | 7.1 |
| Aloina brevirostris | 0 | 0 | 13 | 12 | 9.1 | 21 |
| Pterygoneurum crossidioides | 0 | 0 | 17 | 3.8 | 0 | 14 |
| Tortula protobryoides | 0 | 0 | 8.3 | 3.8 | 0 | 0 |
| Barbuletalia | ||||||
| Pterygoneurum ovatum | 50 | 50 | 88 | 65 | 73 | 93 |
| Barbula unguiculata | 83 | 50 | 67 | 12 | 36 | 7.1 |
| Abietinella abietina var. abietina | 33 | 25 | 17 | 0 | 9.1 | 0 |
| Ptychostomum imbricatulum | 17 | 25 | 46 | 15 | 0 | 29 |
| Tortula acaulon var. acaulon | 33 | 0 | 42 | 0 | 9.1 | 0 |
| Syntrichia ruralis | 17 | 25 | 17 | 0 | 0 | 0 |
| Ptychostomum capillare | 17 | 0 | 13 | 3.8 | 0 | 0 |
| Didymodon fallax | 50 | 0 | 21 | 0 | 0 | 0 |
| Others | ||||||
| Bryum argenteum | 50 | 75 | 92 | 35 | 73 | 64 |
| Didymodon rigidulus | 50 | 25 | 46 | 35 | 36 | 21 |
| Tortula muralis subsp. muralis var. muralis | 17 | 50 | 38 | 15 | 36 | 7.1 |
| Bryum violaceum | 0 | 25 | 8.3 | 0 | 0 | 7.1 |
| Bryum radiculosum | 0 | 25 | 8.3 | 0 | 0 | 7.1 |
| Grimmia pulvinata | 0 | 25 | 13 | 0 | 0 | 0 |
| Tortula caucasica | 0 | 25 | 13 | 0 | 0 | 0 |
| Dicranella howei | 0 | 25 | 4.2 | 0 | 0 | 0 |
| Funaria hygrometrica | 17 | 0 | 4.2 | 0 | 0 | 0 |
| Syntrichia ruraliformis | 0 | 0 | 4.2 | 3.8 | 0 | 0 |
| Entodon concinnus | 0 | 0 | 4.2 | 0 | 9.1 | 0 |
| Campyliadelphus chrysophyllus | 17 | 25 | 0 | 0 | 0 | 0 |
References
- Pye, K. The Nature, Origin and Accumulation of Loess. Quat. Sci. Rev. 1995, 14, 653–667. [Google Scholar] [CrossRef]
- Pécsi, M. Loess is not just the accumulation of dust. Quat. Int. 1990, 7–8, 1–21. [Google Scholar] [CrossRef]
- Sprafke, T.; Obreht, I. Loess: Rock, sediment or soil?–What is missing for its definition? Quat. Int. 2016, 399, 198–207. [Google Scholar] [CrossRef]
- Brandtner, F. Jungpleistozäner Löß und fossile Böden in Niederösterreich. E&G Quat. Sci. J. 1954, 4, 49–82. [Google Scholar] [CrossRef]
- Smalley, I.J.; Jefferson, I.F.; Dijkstra, T.A.; Derbyshire, E. Some major events in the development of the scientific study of loess. Earth-Sci. Rev. 2001, 54, 5–18. [Google Scholar] [CrossRef]
- Rabeder, J.; Wimmer-Frey, I.; Reitner, H.; Filzmoser, P.; Mert, M.C.; Reitner, J.M.; Heinrich, M.; Hobiger, G.; Benold, C. Baurohstoffvorsorge Lösse und Lösslehme; Endbericht; Geologische Bundesanstalt: Vienna, Austria, 2019; p. 317. [Google Scholar]
- Lehmkuhl, F.; Nett, J.J.; Potter, S.; Schulte, P.; Sprafke, T.; Jary, Z.; Antoine, P.; Wacha, L.; Wolf, D.; Zerboni, A.; et al. Loess landscapes of Europe–Mapping, geomorphology, and zonal differentiation. Earth-Sci. Rev. 2021, 215, 103496. [Google Scholar] [CrossRef]
- Hofer, I. Sedimentologische und Elementaranalytische Untersuchungen an Löss-/Paläobodensequenzen in der Umgebung von Krems/Niederösterreich; University of Vienna: Vienna, Austria, 2010; p. 205. [Google Scholar]
- Lais, R. Über den jüngeren Löß in Niederösterreich, Mähren und Böhmen. Berichte Der Naturforschenden Ges. Zu Freibg. Im Breisgau 1951, 41, 119–178. [Google Scholar]
- Smalley, I.J. The properties of glacial loess and the formation of loess deposits. J. Sediment. Petrol. 1966, 36, 669–676. [Google Scholar] [CrossRef]
- Konstantinov, E.A.; Zakharov, A.L.; Sychev, N.V.; Mazneva, E.A.; Kurbanov, R.N.; Morozova, P.A. Loess Accumulation in the Southern Part of European Russia at the End of the Quaternary Period. Herald. Russ. Acad. Sci. 2022, 92, 342–351. [Google Scholar] [CrossRef]
- Krenmayer, H.G.; Schnabel, W. Quartär–Ober-Pliozän. In Legende und Kurze Erläuterung zur Geologischen Karte von Niederösterreich; Schnabel, W., Ed.; Geologische Bundesanstalt: Vienna, Austria, 2002; pp. 20–23. [Google Scholar]
- Lehmkuhl, F.; Bösken, J.; Hošek, J.; Sprafke, T.; Marković, S.B.; Obreht, I.; Hambach, U.; Sümegi, P.; Thiemann, A.; Steffens, S.; et al. Loess distribution and related Quaternary sediments in the Carpathian Basin. J. Maps 2018, 14, 661–670. [Google Scholar] [CrossRef]
- Rungaldier, R. Der Löß in Niederösterreich, seine Bedeutung und Verbreitung. Jahrb. Für Landeskd. Von Niederösterreich 1968, 34, 20–35. [Google Scholar]
- Pócs, T. Studies on the cryptogamic vegetation of loess cliffs, I. Orographic desert in the Carpathian Basin. Kitaibelia 1999, 4, 143–156. [Google Scholar]
- Wiesbauer, H. Löss- und Lehmwände–einige Bemerkungen aus naturschutzfachlicher Sicht. Berichte Geol. B.-A. 2009, 80, 24–34. [Google Scholar]
- Frey, W.; Herrnstadt, I.; Kürschner, H. Verbreitung und Soziologie terrestrischer Bryophytengesellschaften in der Judäischen Wüste. Phytocoenologia 1990, 19, 233–265. [Google Scholar] [CrossRef]
- Frey, W.; Kürschner, H. Lebensstrategien von terrestrischen Bryophyten in der Judäischen Wüste. Life strategies of terrestrial bryophytes in the Judean Desert. Bot. Acta 1991, 104, 172–182. [Google Scholar] [CrossRef]
- Sabovljević, M. Life strategies of bryophytes on loess cliffs in Vójvodina (Serbia). Arch. Biol. Sci. Belgrade 2004, 56, 127–130. [Google Scholar] [CrossRef]
- Kürschner, H.; Wagner, D. Phytosociology and life strategies of a new loess slope bryophyte community from N China (Gansu), including Crossidium laxefilamentosum new to China. Nova Hedwig. 2005, 81, 229–246. [Google Scholar] [CrossRef]
- Poćs, T. Bryophyte communities at the edge of Tunisian Sahara, with the description of Gymnostomum viridulum Brid. subsp saharae, subsp. nov. (Pottiaceae, Bryophyta). Nova Hedwig. 2007, 84, 101–120. [Google Scholar]
- Van der Linden, J.; Farrar, D.R.; Churchill, S.P. Bryophytes of the Loess Hills of Western Iowa. Proc. Iowa Acad. Sci. 1985, 92, 193–195. [Google Scholar]
- Kürschner, H.; Pócs, T. Bryophyte communities of the loess cliffs of the Pannonian basin and adjacent areas, with the description of Hilpertio velenovskyi-Pterygoneuretum compacti ass. nov. Nova Hedwig. 2002, 75, 101–119. [Google Scholar] [CrossRef]
- Kürschner, H. Life strategies of Pannonian loess cliff bryophyte communities. Nova Hedwig. 2002, 78, 307–318. [Google Scholar] [CrossRef]
- Van Zanten, B.O. Studies on the cryptogamic vegetation of loess cliffs, II. The genus Bryum Hedw. on loess cliffs in the Pannonian Basin, including Bryum gemmiferum Wilz. & Demar. and Bryum violaceum Crundw. & Nyh. new to Hungary. Kitabelia 1999, 4, 143–148. [Google Scholar]
- Poćs, T.; Sabovljević, M.; Puche, F.; Segarra, J.G.; Moragues, S.; Gimeno, C.; Kürschner, H. Crossidium laxefilamentosum Frey & Kürschner (Bryopsida: Pottiaceae), new to Europe and to North Africa. J. Bryol. 2004, 26, 113–124. [Google Scholar] [CrossRef]
- Poćs, T.; Goia, I.; Kis, G.; Orbán, S.; Sass-Gyarmati, A.; Van Zanten, B.O. Hilpertia velenovskyi (Schiffn.) Zander and other Pottioid mosses (bryophyta) new to Romania. Studies on the cryptogamic vegetation of loess cliffs, IX. Contrib. Bot. 2002, 37, 13–22. [Google Scholar]
- Sabovljević, M.; Frahm, J.P.; Schaumann, F. The origin of German populations of Hilpertia velenovskyi (Pottiaceae, Bryopsida): Interferences from variation in the nuclear ITS region. Cryptogam. Bryol. 2006, 27, 357–365. [Google Scholar]
- Németh, C. Two new occurrences of Hilpertia velenovskyi (Schiffn.) Zander in Hungary. Acta Biol. Plant. Agriensis 2023, 11, 314–322. [Google Scholar] [CrossRef]
- Karczmarz, K. The bryological characteristics of the Polish loess area. Ann. Univ. Mariae Curie-Skłodowska 1960, 15, 186–209. [Google Scholar]
- Natcheva, R.; Ganeva, A. Bryophytes on loess cliffs in Bulgaria–a preliminary study. Phytol. Balc. Sofia 2006, 12, 47–50. [Google Scholar]
- Marstaller, R. Mossgesellschaften Mitteldeutschlands und angrenzender Gebiete. Hausknechtia 2023, Beiheft 21, 1–928. [Google Scholar]
- Zechmeister, H.G.; Kropik, M. Tortula brevissima Schiffn. and Microbryum rectum (With.) R.H. Zander new to Austria. Herzogia 2024, 37, 394–399. [Google Scholar]
- Zechmeister, H.G.; Kropik, M.; Hagel, H. Neufunde und andere bemerkenswerte Funde von Moosen (Bryophyta) in Niederösterreich. Stapfia 2017, 107, 131–145. [Google Scholar]
- Zechmeister, H.G.; Hagel, H.; Gendo, A.; Osvaldik, V.; Patek, M.; Prinz, M.; Schröck, C.; Köckinger, H. Die Rote Liste der Moose Niederösterreichs. Wiss. Mitt. Niederösterr. Landesmus 2013, 24, 7–126. [Google Scholar]
- Hodgetts, N.; Lockhart, N. Checklist and Country Status of European Bryophytes–Update 2020; National Parks and Wildlife Service: Dublin, Ireland, 2020; p. 213.
- Ignatova, E.; Ignatov, M. Didymodon glaucus Ryan (Pottiaceae, Musci)–The first record from Siberia. Arctoa 2007, 16, 139–143. [Google Scholar]
- Hill, M.O. TWINSPAN a FORTRAN Program for Arranging Multivariate Data in an Ordered Two-Way Table by Classification of the Individuals and Attributes; Cornell University: Ithaca, NY, USA, 1979. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie. Grundzüge der Vegetationskunde; Springer: Vienna, Austria, 1951; p. 631. [Google Scholar]
- Theurillat, J.P.; Willner, W.; Fernández-González, F.; Bültmann, H.; Čarni, A.; Gigante, D.; Mucina, L.; Weber, H. International Code of Phytosociological Nomenclature. 4th edition. Appl. Veg. Sci. 2020, 24, e12491. [Google Scholar] [CrossRef]
- Meinunger, L.; Schröder, W. Verbreitungsatlas der Moose Deutschlands. Band 2.; Regensburgische Botanische Gesellschaft: Regensburg, Germany, 2007; p. 699. [Google Scholar]
- Meier, M.K.; Roloff, F. Moosflora der Schweiz. Didymodon glaucus Ryan. 2017. Available online: www.swissbryophytes.ch (accessed on 5 September 2025).
- Ahrens, M. Die Moosgesellschaften des Nördlichen Bodenseegebietes; Universität Karlsruhe: Berlin/Stuttgart, Germany, 1992; p. 681. [Google Scholar]
- Köckinger, H. Didymodon. In Moosflora von Österreich. Teil 1; Berg, C., Köckinger, H., Kropik, M., Pöltl, M., Schröck, C., Zechmeister, H.G., Eds.; Verlag des Naturwissenschaftlichen Vereins für Kärnten: Klagenfurt am Wörthersee, Austria, 2025; pp. 592–608. [Google Scholar]
- Dierschke, H. Zur Benennung zentraler Syntaxa ohne eigene Kenn- und Trennarten. Tuexenia 1988, 8, 381–382. [Google Scholar]
- Ros, R.M.; Guerra, M. Vegetacion briofitica terri cola de la Region de Murcia (sureste de Espana). Phytocoenologia 1987, 15, 505–567. [Google Scholar]
- Drehwald, U.; Preising, E. Die Pflanzengesellschaften Niedersachsens–Bestandsentwicklung, Gefährdung und Schutzprobleme–Moosgesellschaften. Naturschutz Landschaftspfl. Niedersachs. 2009, 20, 1–202. [Google Scholar]
- Marstaller, R. Syntaxonomischer Konspekt der Moosgesellschaften Europas und angrenzender Gebiete. Hausknechtia 2006, Beiheft 13, 3–192. [Google Scholar]
- Müller, F. Das Laubmoos Hilpertia velenovskyi (Schiffn.) Zander (Pottiaceae)–eine für die Flora Deutschlands neue Moosart. Limprichtia 2000, 14, 49–58. [Google Scholar]
- Sprafke, T. Löss in Niederosterreich-Archiv Quartärer Klima- und Landschaftsveränderungen. Ph.D. Thesis, University of Würzburg, Würzburg, Germany, 2016; p. 253. [Google Scholar]
- Müller, F.; Otte, V. Verzeichnis und Rote Liste der Moos- und Flechtengesellschaften Sachsens; Sächsisches Landesamt für Umwelt und Geologie: Dresden, Germany, 2008; p. 133. [Google Scholar]
- Hodgetts, N.; Cálix, M.; Englefield, E.; Fettes, N.; Criado García, M.; Patin, L.; Nieto, A.; Bergamini, A.; Bisang, I.; Baisheva, E.; et al. A Miniature World in Decline: European Red List of Mosses, Liverworts and Hornworts. 2019. Available online: https://portals.iucn.org/library/node/48520 (accessed on 24 June 2023).
- Grill, R. Erdgeschichte des Donaugebietes in Österreich. Nat. Und Land 1959, 11–14, 170–176. [Google Scholar]
- Gruber, B. Die Geschichte der Donau. Oberösterr. GEO-Nachr. 1993, 8, 23–30. [Google Scholar]
- Hodgetts, N.G.; Söderström, L.; Blockeel, T.L.; Caspari, S.; Ignatov, M.; Konstantinova, N.A.; Lockhart, N.; Papp, B.; Schröck, C.; Sim-Sim, M.; et al. An annotated checklist of bryophytes of Europe, Macaronesia and Cyprus. J. Bryol. 2020, 42, 1–116. [Google Scholar] [CrossRef]
- Fink, J. Zur Korrelation der Terrassen und Lösse in Österreich. Eiszeitalt. Ggw. 1956, 7, 49–77. [Google Scholar] [CrossRef]
- Hermann, P. Das Quartär. In Erläuterungen zur Geologischen Karte des Burgenlandes 1:200.00; Schönlaub, H.P., Ed.; Geologische Bundesanstalt: Vienna, Austria, 2000; pp. 12–14. [Google Scholar]
- Kümel, F. Der Löß des Laaerberges bei Wien. Verhandlungen Der Geol. Bundesanst. 1935, 8–9, 132–135. [Google Scholar]
- Fink, J. Quartärprobleme des Wiener Raumes. Geomorphol. Stud. 1957, 262, 199–206. [Google Scholar]
- Fink, J. Exkursion Durch den Österreichischen Teil des Nördlichen Alpenvorlandes und den Donauraum Zwischen Krems und der Wiener Pforte; Mitteilungen der Kommission für Quartärforschung der Österreichischen Akademie der Wissenschaften: Vienna, Austria, 1976; p. 113. [Google Scholar]
- Fink, J. Die Gliederung des Jungpleistozäns in Niederösterreich. Mitteilungen Der Geol. Ges. Wien. 1961, 54, 1–25. [Google Scholar]
- van Husen, V. Quartär bis oberstes Neogen. In Erläuterungen zur Geologischen Karte von Oberösterreich 1:200.000; Rupp, C., Linner, M., Mandl, G.W., Eds.; Geologische Bundesanstalt: Vienna, Austria, 2011; pp. 121–152. [Google Scholar]
- Kolmer, H. Über Lößsedimente des Murtales. Mitteilungen Des Naturwissenschaftlichen Ver. Steiermark. 1968, 98, 11–15. [Google Scholar]
- Harlfinger, O.; Knees, G. Klimahandbuch der österreichischen Bodenschätzung. Mitt. Der Österr. Bodenkundl. Ges. 1999, 58, 1–196. [Google Scholar]
- Hiebl, J.; Frei, C. Daily temperature grids for Austria since 1961–concept, creation and applicability. Theor. Appl. Climatol. 2016, 124, 161–178. [Google Scholar] [CrossRef]
- Hiebl, J.; Frei, C. Daily precipitation grids for Austria since 1961–development and evaluation of a spatial dataset for hydroclimatic monitoring and modelling. Theor. Appl. Climatol. 2018, 132, 327–345. [Google Scholar] [CrossRef]
- Zechmeister, H.G.; Kropik, M. Rarities from the xerothermic flora of eastern Austria: Rediscoveries of four bryophyte species supposedly extinct in Austria. Herzogia 2021, 34, 189–196. [Google Scholar]
- Zechmeister, H.G.; Kropik, M. The Bryophyte Flora of Vienna. Plants 2023, 12, 3002. [Google Scholar] [CrossRef]
- Zechmeister, H.G.; Kropik, M.; Sagmeister, P. Eine erste kommentierte Checkliste der Moose des Burgenlandes (Österreich) inklusive 39 Neufunde. Neilreichia 2025, 14, 75–104. [Google Scholar]
- Zechmeister, H.G.; Tribsch, A.; Moser, D.; Wrbka, T. Distribution of endangered bryophytes in Austrian cultural landscapes. Biol. Conserv. 2002, 103, 173–182. [Google Scholar] [CrossRef]
- QGIS. Geographic Information System; Version 3.30; Open Source Geospatial Foundation: Beaverton, OR, USA, 2023. [Google Scholar]
- Hennekens, S.M.; Schaminée, J.H.J. TURBOVEG, a Comprehensive Data Base Management System for Vegetation Data. J. Veg. Sci. 2001, 12, 589–591. [Google Scholar] [CrossRef]
- Tichý, L. JUICE, software for vegetation classification. J. Veg. Sci. 2002, 13, 451–453. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing; Version 4.4.3; R Foundation for Statistical Computing: Vienna, Austria, 2025.


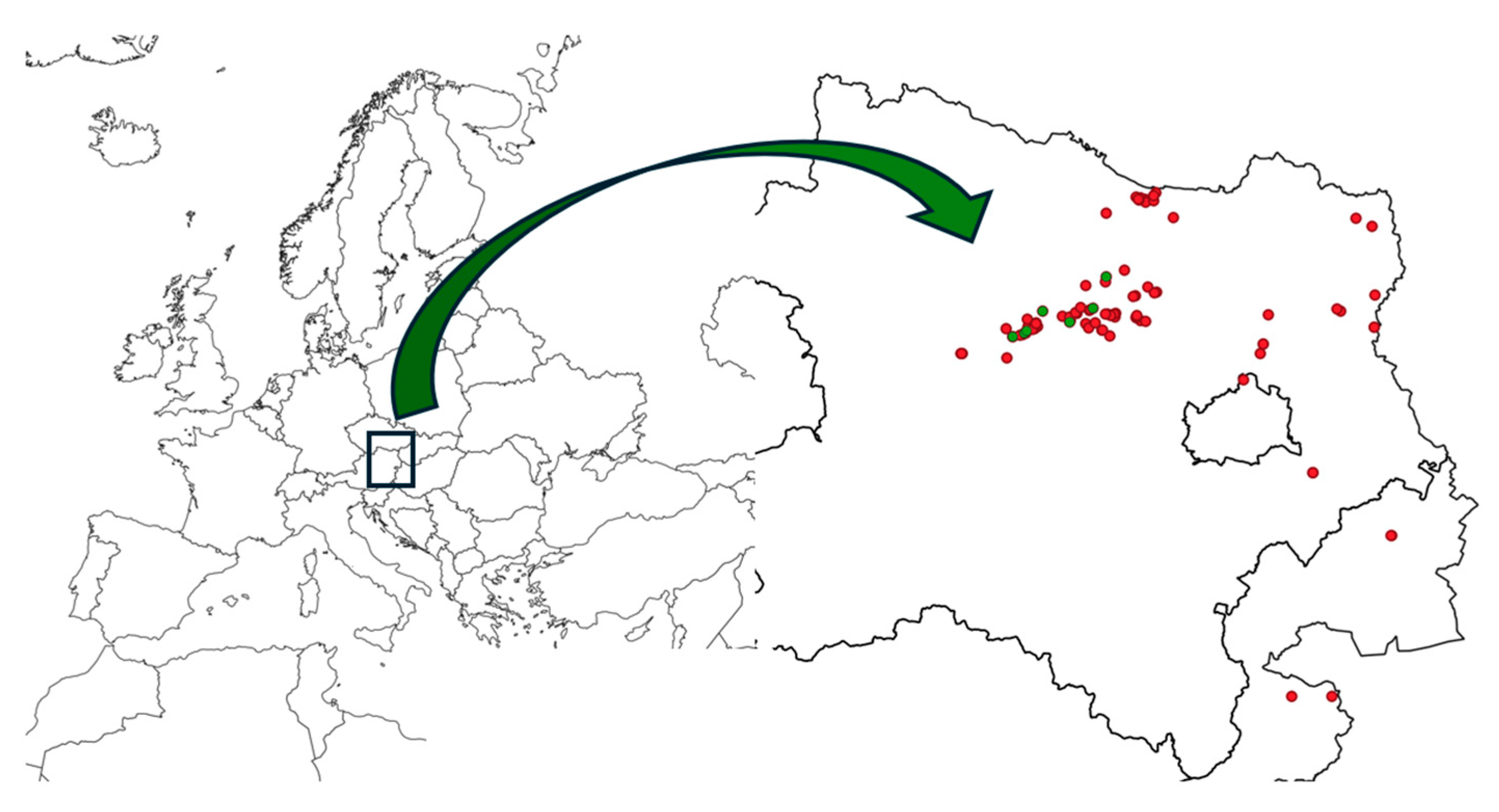
| Site | Nr. of Patches | Area Occupied | Sporophytes |
|---|---|---|---|
| Kranberg O Hollenstein * | 1 | 1 | n |
| Ottenthal N | 8 | 10 | y |
| Gebling SW | 15 | 40 | n |
| Unterrohrendorf-Schnabl | 20 | 30 | n |
| Gedersdorf W | 25 | 50 | n |
| Fels am Wagram N | 4 | 10 | y |
| Straß im Straßerthal, Geißberg | 1 | 5 | n |
| Species | Cluster | IndVal | p_BH |
|---|---|---|---|
| Aloina obliquifolia | 5 | 0.906 | 0.002 |
| Amblystegium serpens | 1 | 0.816 | 0.002 |
| Didymodon glaucus | 2 | 1 | 0.002 |
| Oxyrrhynchium schleicheri | 1 | 0.899 | 0.002 |
| Brachythecium rutabulum | 1 | 0.804 | 0.005 |
| Oxyrrhynchium hians var. hians | 1 | 0.77 | 0.005 |
| Hilpertia velenovskyi | 6 | 0.734 | 0.013 |
| Pseudocrossidium hornschuchianum | 3 | 0.671 | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zechmeister, H.G.; Kropik, M. Bryophytes of the Loess Cliffs in the Pannonian Area of Austria. Plants 2025, 14, 3128. https://doi.org/10.3390/plants14203128
Zechmeister HG, Kropik M. Bryophytes of the Loess Cliffs in the Pannonian Area of Austria. Plants. 2025; 14(20):3128. https://doi.org/10.3390/plants14203128
Chicago/Turabian StyleZechmeister, Harald G., and Michaela Kropik. 2025. "Bryophytes of the Loess Cliffs in the Pannonian Area of Austria" Plants 14, no. 20: 3128. https://doi.org/10.3390/plants14203128
APA StyleZechmeister, H. G., & Kropik, M. (2025). Bryophytes of the Loess Cliffs in the Pannonian Area of Austria. Plants, 14(20), 3128. https://doi.org/10.3390/plants14203128







