Differential Phosphorus Acquisition Strategies and Adaptive Mechanisms Evolved by Three Lespedeza Species to Tackle Phosphorus Deficiency
Abstract
1. Introduction
2. Results
2.1. Biomass Allocation and Root Morphological Characteristics
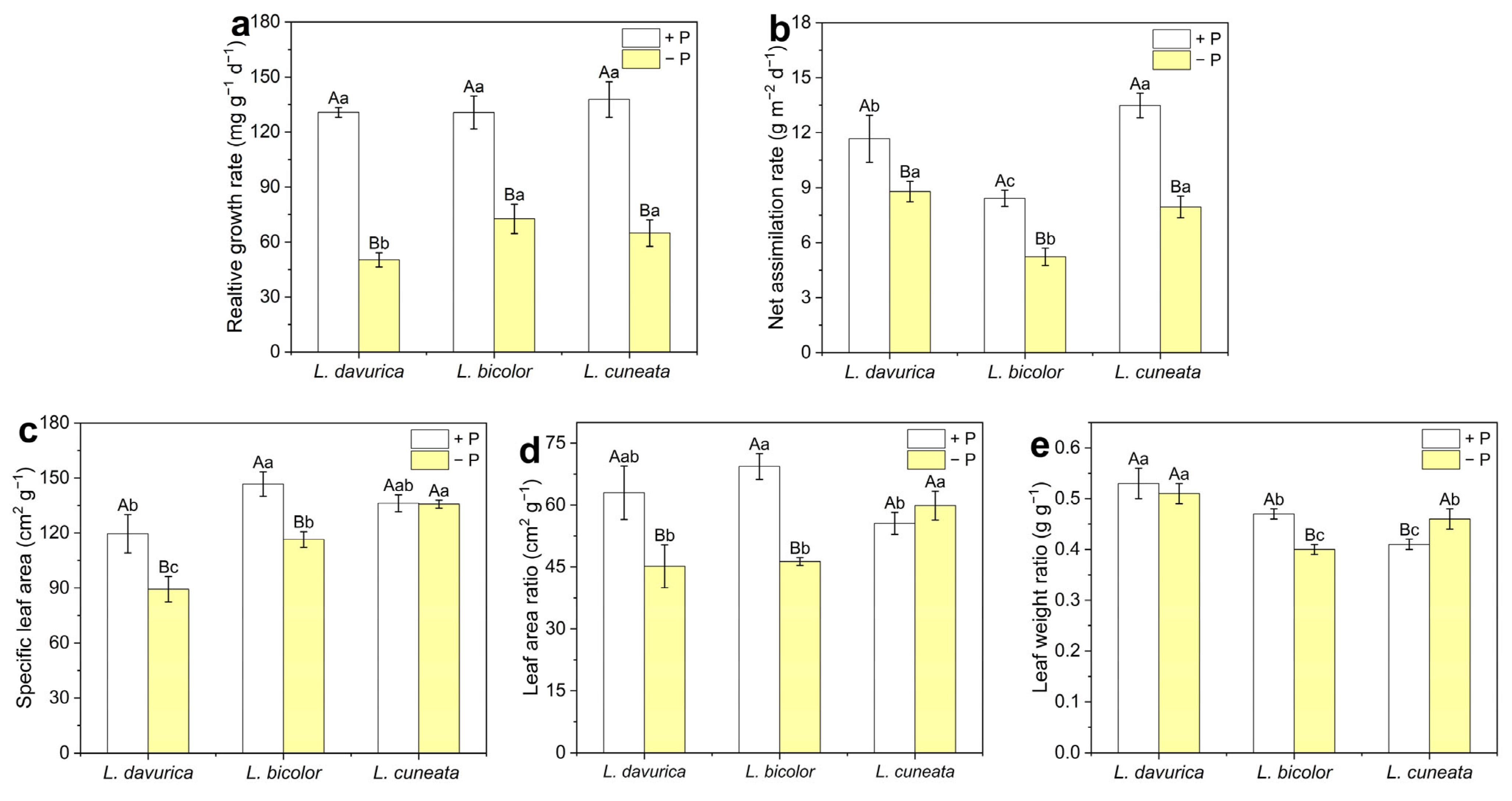
2.2. Growth Analysis
2.3. Phosphorus Allocation Patterns
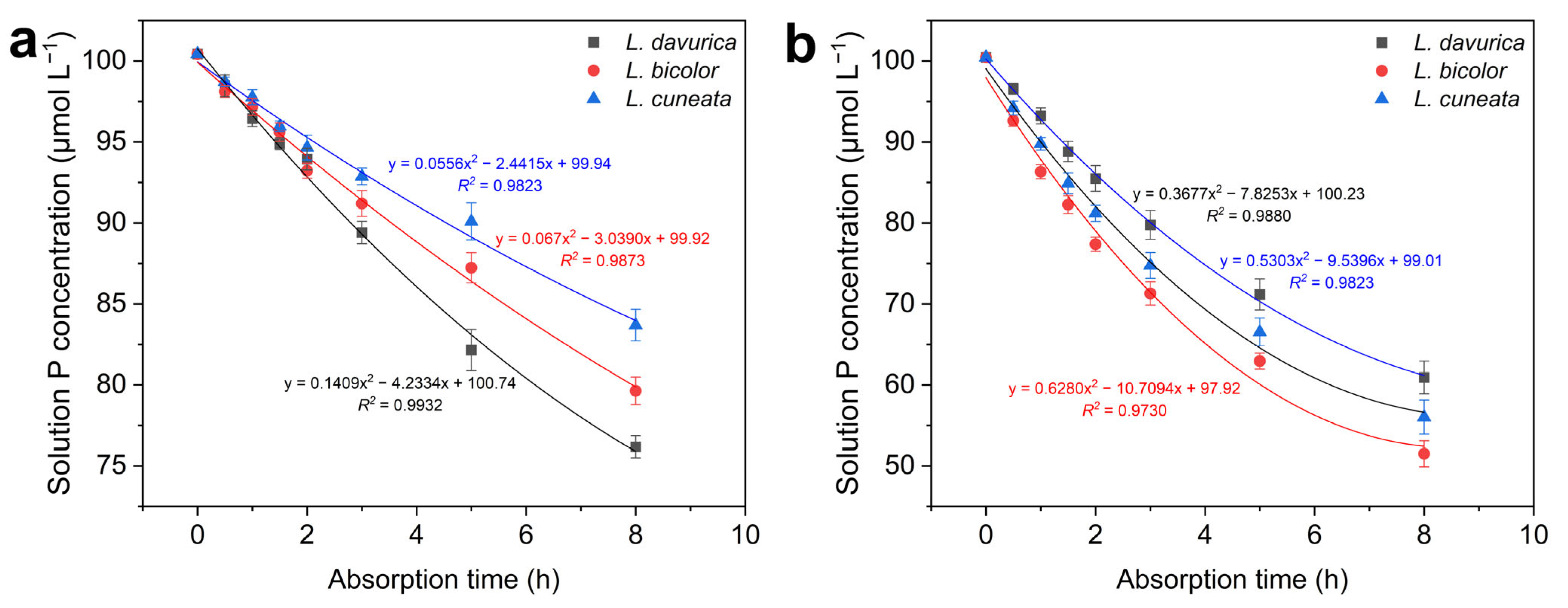
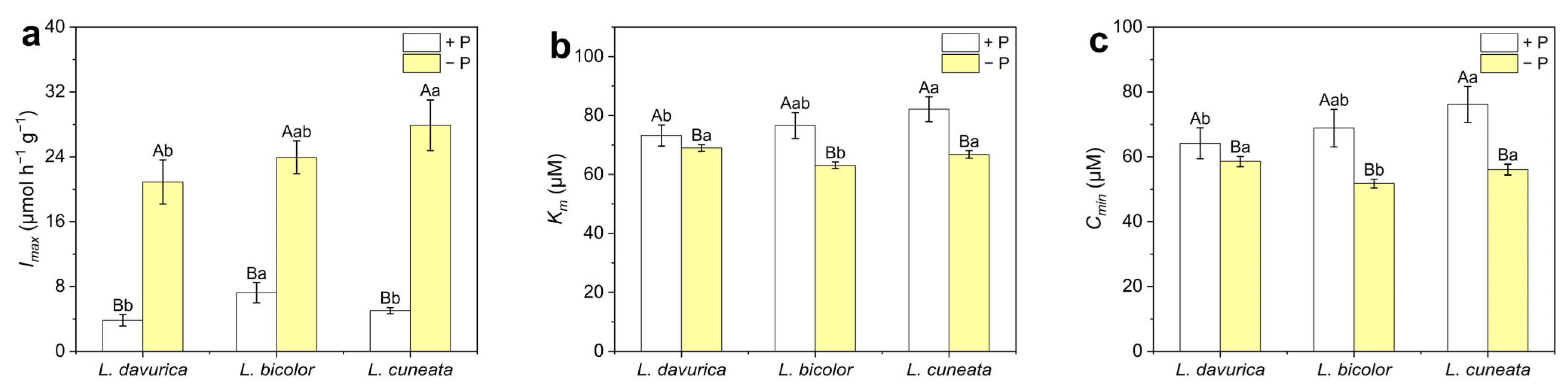
2.4. Phosphorus Absorption Kinetics
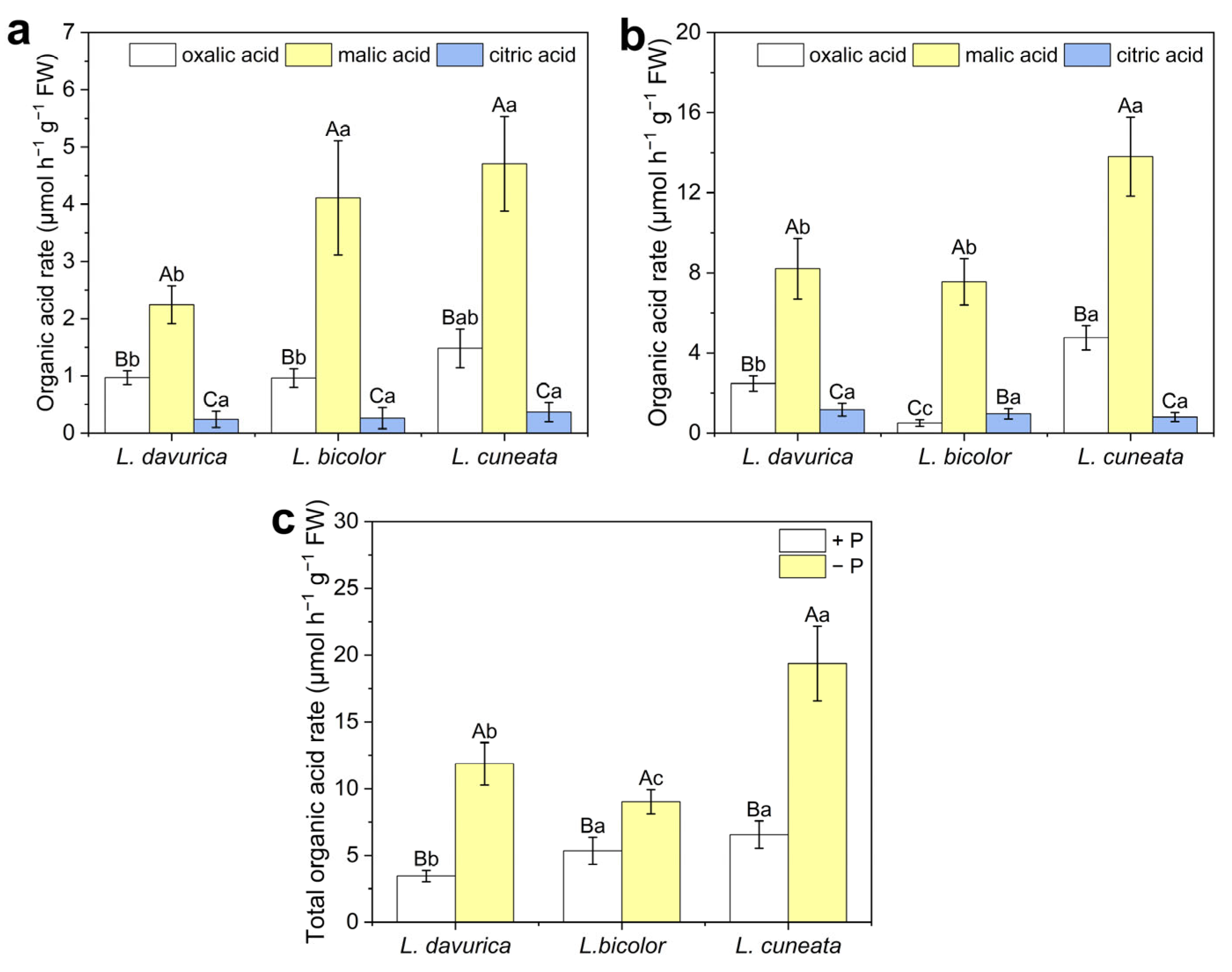
2.5. Acid Phosphatase Activity and Organic Acid Exudation
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Growth Conditions, and Phosphate Supplementation
4.2. Morphological Parameters and Growth Analyses
4.3. Root Exudate Collection
4.4. Measurement of Organic Acids and Acid Phosphatase Activity
4.5. Absorption Kinetics Test
4.6. Phosphorus Measurement
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bechtaoui, N.; Rabiu, M.K.; Raklami, A.; Oufdou, K.; Hafidi, M.; Jemo, M. Phosphate-Dependent Regulation of Growth and Stresses Management in Plants. Front. Plant Sci. 2021, 12, 679916. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, Y.; Jin, C.; Wang, J.; Wang, L.; Wu, J. Physiological and Morphological Responses of Hydroponically Grown Pear Rootstock Under Phosphorus Treatment. Front. Plant Sci. 2021, 12, 696045. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, M.S.; AbdElgawad, H.; Ven, A.; Verryckt, L.T.; Wieneke, S.; Janssens, I.A.; Vicca, S. Phosphorus Stress Strongly Reduced Plant Physiological Activity, but only Temporarily, in A Mesocosm Experiment with Zea mays Colonized by Arbuscular Mycorrhizal Fungi. Biogeosciences 2022, 19, 2353–2364. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of Soil Inorganic P in The Rhizosphere as Affected by Root-Induced Chemical Changes: A Review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Hou, E.; Luo, Y.; Kuang, Y.; Chen, C.; Lu, X.; Jiang, L.; Luo, X.; Wen, D. Global Meta-Analysis Shows Pervasive Phosphorus Limitation of Aboveground Plant Production in Natural Terrestrial Ecosystems. Nat. Commun. 2020, 11, 637. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.R.; Shane, M.W.; White, P.J.; et al. Opportunities for Improving Phosphorus-Use Efficiency in Crop Plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef]
- Lei, K.Y.; Creber, H.; Bol, R.; Tietema, A.; Sohi, S.P. Preferences of Pinus sylvestris Seedling Roots for Different Phosphorus Sources Under Phosphorus-Deficient Conditions. Plant Soil 2023, 482, 229–244. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Pendall, E.; Morgan, J.A.; Blumenthal, D.M.; Carrillo, Y.; LeCain, D.R.; Follett, R.F.; Williams, D.G. Climate Change Alters Stoichiometry of Phosphorus and Nitrogen in A Semiarid Grassland. New Phytol. 2012, 196, 807–815. [Google Scholar] [CrossRef]
- Hu, W.; Tan, J.; Shi, X.; Lock, T.R.; Kallenbach, R.L.; Yuan, Z. Nutrient Addition and Warming Alter the Soil Phosphorus Cycle in Grasslands: A Global Meta-Analysis. J. Soil. Sediment. 2022, 22, 2608–2619. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, X.; Yuan, P.; Han, B.; Wu, T.; Din, I.; Wang, C.; Hammond, J.P.; Wang, S.; Ding, G.; et al. Root Morphological Adaptation and Leaf Lipid Remobilization Drive Differences in Phosphorus Use Efficiency in Rapeseed Seedlings. Crop J. 2025, 13, 524–535. [Google Scholar] [CrossRef]
- Suriyagoda, L.D.B.; Lambers, H.; Renton, M.; Ryan, M.H. Growth, Carboxylate Exudates and Nutrient Dynamics in Three Herbaceous Perennial Plant Species Under Low, Moderate and High Phosphorus Supply. Plant Soil 2012, 358, 105–117. [Google Scholar] [CrossRef]
- Heuer, S.; Gaxiola, R.; Schilling, R.; Herrera-Estrella, L.; López-Arredondo, D.; Wissuwa, M.; Delhaize, E.; Rouached, H. Improving Phosphorus Use Efficiency: A Complex Trait with Emerging Opportunities. Plant J. 2017, 90, 868–885. [Google Scholar] [CrossRef]
- Tshewang, S.; Rengel, Z.; Siddique, K.H.M.; Solaiman, Z.M. Growth, Rhizosphere Carboxylate Exudation, and Arbuscular Mycorrhizal Colonisation in Temperate Perennial Pasture Grasses Varied with Phosphorus Application. Agronomy 2020, 10, 2017. [Google Scholar] [CrossRef]
- Liu, D. Root Developmental Responses to Phosphorus Nutrition. J. Integr. Plant Biol. 2021, 63, 1065–1090. [Google Scholar] [CrossRef]
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root Structure and Functioning for Efficient Acquisition of Phosphorus: Matching Morphological and Physiological Traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Tang, H.; Li, H.; Zhang, F.; Rengel, Z.; Whalley, W.R.; Shen, J. Major Crop Species Show Differential Balance Between Root Morphological and Physiological Responses to Variable Phosphorus Supply. Front. Plant Sci. 2016, 7, 1939. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.S.; Janos, D.P. Plant Growth, Phosphorus Nutrition, and Root Morphological Responses to Arbuscular Mycorrhizas, Phosphorus Fertilization, and Intraspecific Density. Mycorrhiza 2005, 15, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jianbo, S.; Caixian, T.; Rengel, Z. Root Morphology, Proton Release, and Carboxylate Exudation in Lupin in Response to Phosphorus Deficiency. J. Plant Nutr. 2008, 31, 557–570. [Google Scholar] [CrossRef]
- Kidd, D.R.; Ryan, M.H.; Haling, R.E.; Lambers, H.; Sandral, G.A.; Yang, Z.; Culvenor, R.A.; Cawthray, G.R.; Stefanski, A.; Simpson, R.J. Rhizosphere Carboxylates and Morphological Root Traits in Pasture Legumes and Grasses. Plant Soil 2016, 402, 77–89. [Google Scholar] [CrossRef]
- Marschener, H. Role of Root Growth, Arbuscular Mycorrhiza, and Root Exudates for the Efficiency in Nutrient Acquisition. Field Crop. Res. 1998, 56, 203–207. [Google Scholar] [CrossRef]
- Lambers, H. Phosphorus Acquisition and Utilization in Plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]
- Gerke, J. Improving Phosphate Acquisition from Soil via Higher Plants While Approaching Peak Phosphorus Worldwide: A Critical Review of Current Concepts and Misconceptions. Plants 2024, 13, 3478. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Oki, Y.; Adachi, T.; Murata, Y.; Khan, M.H.R. Relative Phosphorus Utilization Efficiency, Growth Response, and Phosphorus Uptake Kinetics of Brassica Cultivars Under a Phosphorus Stress Environment. Commun. Soil Sci. Plan. 2007, 38, 1061–1085. [Google Scholar] [CrossRef]
- Wang, R.; Funayama-Noguchi, S.; Xiong, Z.; Staudinger, C.; Wasaki, J. Phosphorus Absorption Kinetics and Exudation Strategies of Roots Developed by Three Lupin Species to Tackle P Deficiency. Planta 2023, 259, 29. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.S.; Sharif, M.O.; Lee, H.Y. Evaluating the Effect of Bacterial Inoculation and Fertilization on the Soil Nutrient Status of Coal Mine Soil by Growing Soybean (Glycine max) and Shrub Lespedeza (Lespedeza bicolor). Sustainability 2018, 10, 4793. [Google Scholar] [CrossRef]
- Chen, L.; Ma, P.; Li, J.; Liu, J.; Guo, F.; Wang, Y.; Zhang, J. Lespedeza potaninii Vass Seed Yield Response to Plant Density and Phosphate Fertilization in Northwest China. Eur. J. Agron. 2024, 156, 127173. [Google Scholar] [CrossRef]
- Li, X.L.; Lv, X.Y.; Ji, J.B.; Wang, W.D.; Wang, J.; Wang, C.; He, H.B.; Ben, A.L.; Liu, T.L. Complete Genome Sequence of Nguyenibacter sp. L1, A Phosphate Solubilizing Bacterium Isolated from Lespedeza bicolor Rhizosphere. Front. Microbiol. 2023, 14, 1257442. [Google Scholar] [CrossRef]
- Xu, B.; Xu, W.; Wang, Z.; Chen, Z.; Palta, J.A.; Chen, Y. Accumulation of N and P in the Legume Lespedeza davurica in Controlled Mixtures with the Grass Bothriochloa ischaemum under Varying Water and Fertilization Conditions. Front. Plant Sci. 2018, 9, 165. [Google Scholar] [CrossRef]
- Abdolzadeh, A.; Wang, X.; Veneklaas, E.J.; Lambers, H. Effects of Phosphorus Supply on Growth, Phosphate Concentration and Cluster-Root Formation in Three Lupinus Species. Ann. Bot. 2009, 105, 365–374. [Google Scholar] [CrossRef]
- Wu, A.; Fang, Y.; Liu, S.; Wang, H.; Xu, B.; Zhang, S.; Deng, X.; Palta, J.A.; Siddique, K.H.M.; Chen, Y. Root Morphology and Rhizosheath Acid Phosphatase Activity in Legume and Graminoid Species Respond Differently to Low Phosphorus Supply. Rhizosphere 2021, 19, 100391. [Google Scholar] [CrossRef]
- Aslam, M.M.; Karanja, J.K.; Yuan, W.; Zhang, Q.; Zhang, J.; Xu, W. Phosphorus Uptake is Associated with the Rhizosheath Formation of Mature Cluster Roots in White Lupin Under Soil Drying and Phosphorus Deficiency. Plant Physiol. Biochem. 2021, 166, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Veneklaas, E.J.; Pearse, S.J.; Lambers, H. Interactions among Cluster-Root Investment, Leaf Phosphorus Concentration, and Relative Growth Rate in Two Lupinus Species. Am. J. Bot. 2015, 102, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Roumet, C.; Urcelay, C.; Díaz, S. Suites of Root Traits Differ between Annual and Perennial Species Growing in the Field. New Phytol. 2006, 170, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Mommer, L.; De Vries, F.T. Going Underground: Root Traits As Drivers of Ecosystem Processes. Trends Ecol. Evol. 2014, 29, 692–699. [Google Scholar] [CrossRef]
- Irfan, M.; Aziz, T.; Maqsood, M.A.; Bilal, H.M.; Siddique, K.H.M.; Xu, M. Phosphorus (P) Use Efficiency in Rice is Linked to Tissue-Specific Biomass and P Allocation Patterns. Sci. Rep. 2020, 10, 4278. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Oki, Y.; Adachi, T. Intraspecific Variations of Phosphorus Absorption and Remobilization, P Forms, and Their Internal Buffering in Brassica Cultivars Exposed to a P-Stressed Environment. J. Integr. Plant Biol. 2008, 50, 703–716. [Google Scholar] [CrossRef]
- Hodge, A. The Plastic Plant: Root Responses to Heterogeneous Supplies of Nutrients. New Phytol. 2004, 162, 9–24. [Google Scholar] [CrossRef]
- Plaxton, W.C.; Tran, H.T. Metabolic Adaptations of Phosphate-Starved Plants. Plant Physiol. 2011, 156, 1006–1015. [Google Scholar] [CrossRef]
- Bhadouria, J.; Giri, J. Purple Acid Phosphatases: Roles in Phosphate Utilization and New Emerging Functions. Plant Cell Rep. 2022, 41, 33–51. [Google Scholar] [CrossRef]
- Rodgers, C.O.; Barneix, A.J. Cultivar Differences in the Rate of Nitrate Uptake by Intact Wheat Plants as Related to Growth Rate. Physiol. Plant. 1988, 72, 121–126. [Google Scholar] [CrossRef]
- Khandan-Mirkohi, A.; Schenk, M.K. Characteristics of Phosphorus Uptake Kinetics of Poinsettia and Marigold. Sci. Hortic. 2009, 122, 251–257. [Google Scholar] [CrossRef]
- Cacco, G.; Ferrari, G.; Saccomani, M. Pattern of Sulfate Uptake During Root Elongation in Maize: Its Correlation with Productivity. Physiol. Plant. 1980, 48, 375–378. [Google Scholar] [CrossRef]
- Barrow, N.J.; Roy, D.; Debnath, A. Evaluating the Benefits of Legacy Phosphate. Plant Soil 2022, 480, 561–570. [Google Scholar] [CrossRef]
- Almeida, D.S.; Delai, L.B.; Sawaya, A.C.H.F.; Rosolem, C.A. Exudation of Organic Acid Anions by Tropical Grasses in Response to Low Phosphorus Availability. Sci. Rep. 2020, 10, 16955. [Google Scholar] [CrossRef] [PubMed]
- Louw-Gaume, A.E.; Schweizer, N.; Rao, I.M.; Gaume, A.J.; Frossard, E. Temporal Differences in Plant Growth and Root Exudation of Two Brachiaria grasses in Response to Low Phosphorus Supply. Trop. Grassl.-Forrajes Trop. 2017, 5, 103–116. [Google Scholar] [CrossRef]
- Wang, Y.; Krogstad, T.; Clarke, J.L.; Hallama, M.; Øgaard, A.F.; Eich-Greatorex, S.; Kandeler, E.; Clarke, N. Rhizosphere Organic Anions Play a Minor Role in Improving Crop Species’ Ability to Take Up Residual Phosphorus (P) in Agricultural Soils Low in P Availability. Front. Plant Sci. 2016, 7, 1664. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Krogstad, T.; Clarke, N.; Falk Øgaard, A.; Liu Clarke, J. Impact of Phosphorus on Rhizosphere Organic Anions of Wheat at Different Growth Stages Under Field Conditions. AoB Plants 2017, 9, plx008. [Google Scholar] [CrossRef]
- Wang, Y.; Lambers, H. Root-Released Organic Anions in Response to Low Phosphorus Availability: Recent Progress, Challenges and Future Perspectives. Plant Soil 2020, 447, 135–156. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, X.; Liu, G.; Wang, T.; Zhou, H.; Tian, J.; Yao, Q.; He, J.; Tian, J.; Liang, C. Low-Phosphorus Stress Induces GmSTOP1-3-mediated Organic Acid Exudation to Recruit Phosphate-Solubilizing Bacteria for Organic Phosphorus Mineralization in Soybean Rhizosphere. J. Integr. Agr. 2025; in press. [Google Scholar] [CrossRef]
- Parra-Almuna, L.; Pontigo, S.; Ruiz, A.; González, F.; Ferrol, N.; Mora, M.D.; Cartes, P. Dissecting the Roles of Phosphorus Use Efficiency, Organic Acid Anions, and Aluminum-Responsive Genes Under Aluminum Toxicity and Phosphorus Deficiency in Ryegrass Plants. Plants 2024, 13, 929. [Google Scholar] [CrossRef]
- Yang, T.Y.; Qi, Y.P.; Huang, H.Y.; Wu, F.L.; Huang, W.T.; Deng, C.L.; Yang, L.T.; Chen, L.S. Interactive Effects of pH and Aluminum on the Secretion of Organic Acid Anions by Roots and Related Metabolic Factors in Citrus sinensis Roots and Leaves. Environ. Pollut. 2020, 262, 114303. [Google Scholar] [CrossRef]
- Neumann, G.; Massonneau, A.; Langlade, N.; Dinkelaker, B.; Hengeler, C.; Römheld, V.; Martinoia, E. Physiological Aspects of Cluster Root Function and Development in Phosphorus-deficient White Lupin (Lupinus albus L.). Ann. Bot. 2000, 85, 909–919. [Google Scholar] [CrossRef]
- Wasaki, J.; Yamamura, T.; Shinano, T.; Osaki, M. Secreted Acid Phosphatase is Expressed in Cluster Roots of Lupin in Response to Phosphorus Deficiency. Plant Soil 2003, 248, 129–136. [Google Scholar] [CrossRef]
- Lambers, H.; Martinoia, E.; Renton, M. Plant Adaptations to Severely Phosphorus-Impoverished Soils. Curr. Opin. Plant Biol. 2015, 25, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Honvault, N.; Houben, D.; Firmin, S.; Meglouli, H.; Laruelle, F.; Fontaine, J.; Lounès-Hadj Sahraoui, A.; Coutu, A.; Lambers, H.; Faucon, M.P. Interactions Between Below-Ground Traits and Rhizosheath Fungal and Bacterial Communities for Phosphorus Acquisition. Funct. Ecol. 2021, 35, 1603–1619. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Shen, W.; Kuzyakov, Y. Phosphatase Activity and Acidification in Lupine and Maize Rhizosphere Depend on Phosphorus Availability and Root Properties: Coupling Zymography with Planar Optodes. Appl. Soil Ecol. 2021, 167, 104029. [Google Scholar] [CrossRef]
- Aslam, M.M.; Pueyo, J.J.; Pang, J.; Yang, J.; Chen, W.; Chen, H.; Waseem, M.; Li, Y.; Zhang, J.; Xu, W. Root Acid Phosphatases and Rhizobacteria Synergistically Enhance White Lupin and Rice Phosphorus Acquisition. Plant Physiol. 2022, 190, 2449–2465. [Google Scholar] [CrossRef]
- Ma, X.; Mason-Jones, K.; Liu, Y.; Blagodatskaya, E.; Kuzyakov, Y.; Guber, A.; Dippold, M.A.; Razavi, B.S. Coupling Zymography with pH Mapping Reveals A Shift in Lupine Phosphorus Acquisition Strategy Driven by Cluster Roots. Soil Biol. Biochem. 2019, 135, 420–428. [Google Scholar] [CrossRef]
- Touhami, D.; McDowell, R.W.; Condron, L.M. Role of Organic Anions and Phosphatase Enzymes in Phosphorus Acquisition in the Rhizospheres of Legumes and Grasses Grown in a Low Phosphorus Pasture Soil. Plants 2020, 9, 1185. [Google Scholar] [CrossRef]
- Richardson, A.E.; George, T.S.; Hens, M.; Delhaize, E.; Ryan, P.R.; Simpson, R.J.; Hocking, P.J. Organic Anions Facilitate the Mobilization of Soil Organic Phosphorus and Its Subsequent Lability to Phosphatases. Plant Soil 2022, 476, 161–180. [Google Scholar] [CrossRef]
- Hunt, R. Plant Growth Analysis: The Rationale Behind the Use of the Fitted Mathematical Function. Ann. Bot. 1979, 43, 245–249. [Google Scholar] [CrossRef]
- Zhang, A.; Fang, Y.L.; Meng, J.F.; Wang, H.; Chen, S.X.; Zhang, Z.W. Analysis of Low Molecular Weight Organic Acids in Several Complex Liquid Biological Systems via HPLC with Switching Detection Wavelength. J. Food Compos. Anal. 2011, 24, 449–455. [Google Scholar] [CrossRef]
- Claassen, N.; Barber, S.A. A Method for Characterizing the Relation between Nutrient Concentration and Flux into Roots of Intact Plants. Plant Physiol. 1974, 54, 564–568. [Google Scholar] [CrossRef]
- Epstein, E. Dual Pattern of Ion Absorption by Plant Cells and by Plants. Nature 1966, 212, 1324–1327. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal Chim Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Shi, H.; Zuo, G.; Li, S.; Chen, Y.; Wang, S. Differential Phosphorus Acquisition Strategies and Adaptive Mechanisms Evolved by Three Lespedeza Species to Tackle Phosphorus Deficiency. Plants 2025, 14, 3124. https://doi.org/10.3390/plants14203124
Li J, Shi H, Zuo G, Li S, Chen Y, Wang S. Differential Phosphorus Acquisition Strategies and Adaptive Mechanisms Evolved by Three Lespedeza Species to Tackle Phosphorus Deficiency. Plants. 2025; 14(20):3124. https://doi.org/10.3390/plants14203124
Chicago/Turabian StyleLi, Jingchong, Hao Shi, Guanqiang Zuo, Shasha Li, Yafei Chen, and Shiwen Wang. 2025. "Differential Phosphorus Acquisition Strategies and Adaptive Mechanisms Evolved by Three Lespedeza Species to Tackle Phosphorus Deficiency" Plants 14, no. 20: 3124. https://doi.org/10.3390/plants14203124
APA StyleLi, J., Shi, H., Zuo, G., Li, S., Chen, Y., & Wang, S. (2025). Differential Phosphorus Acquisition Strategies and Adaptive Mechanisms Evolved by Three Lespedeza Species to Tackle Phosphorus Deficiency. Plants, 14(20), 3124. https://doi.org/10.3390/plants14203124





