RAS, a Pentatricopeptide Repeat Protein, Interacts with OsTRX z to Regulate Chloroplast Gene Transcription and RNA Processing
Abstract
1. Introduction
2. Results
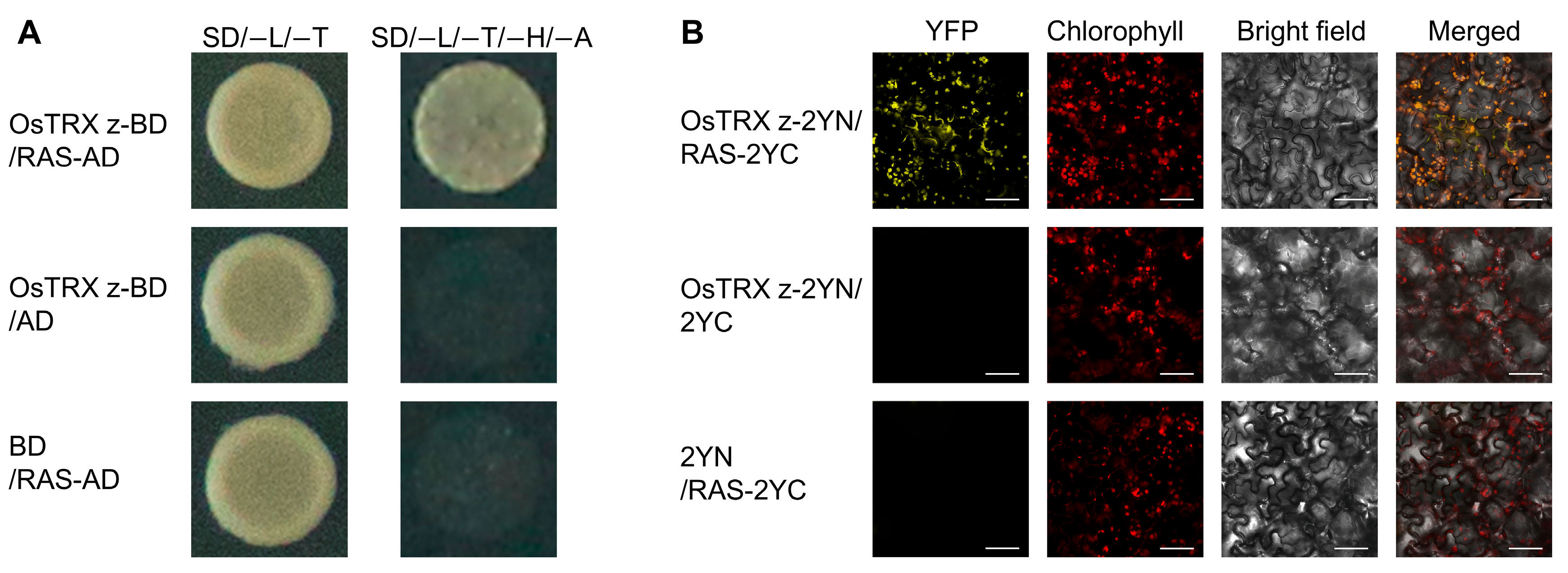
2.1. OsTRX z Interacts with RAS
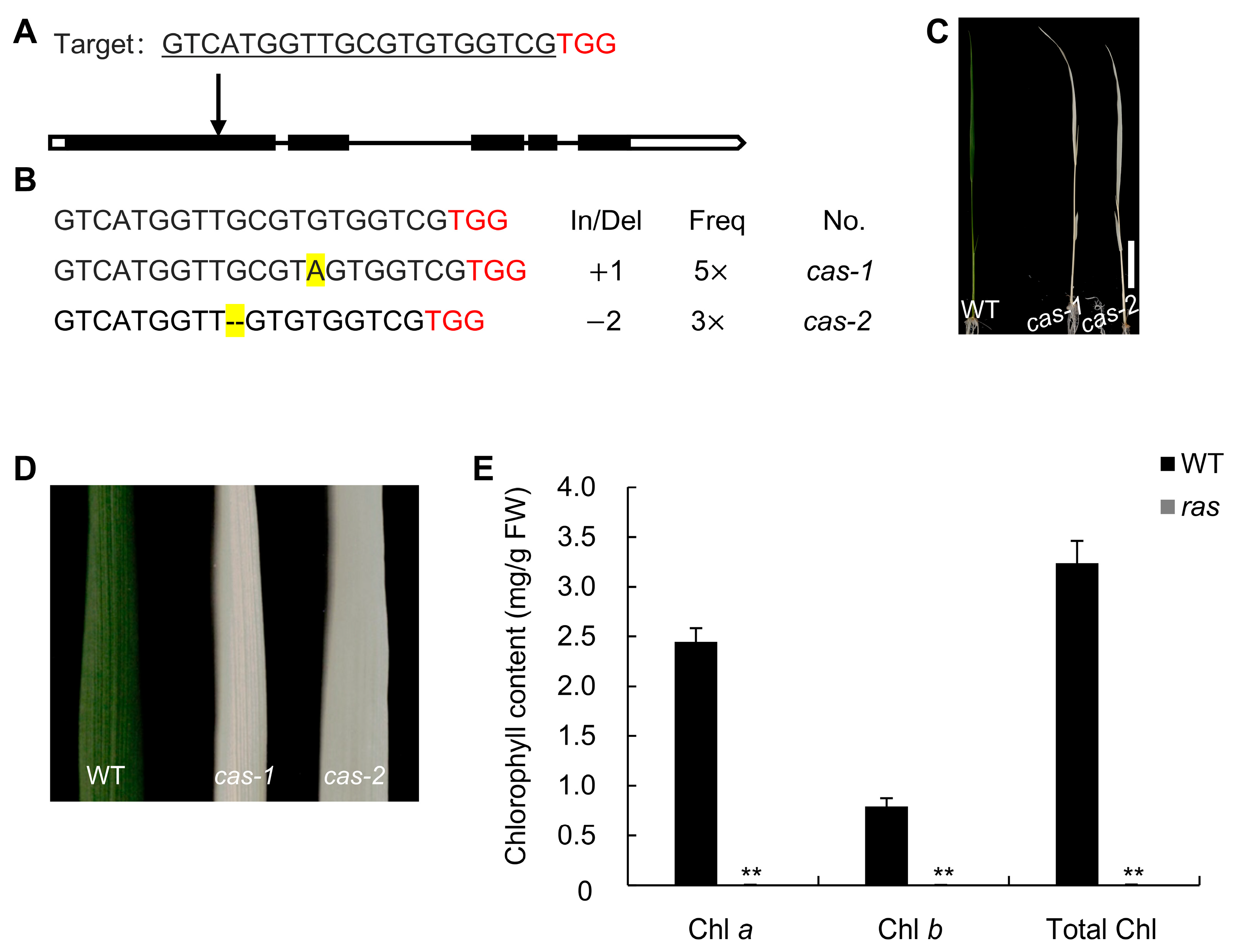
2.2. Characterization of the Ras Mutant
2.3. Subcellular Localization and Expression Pattern Analysis
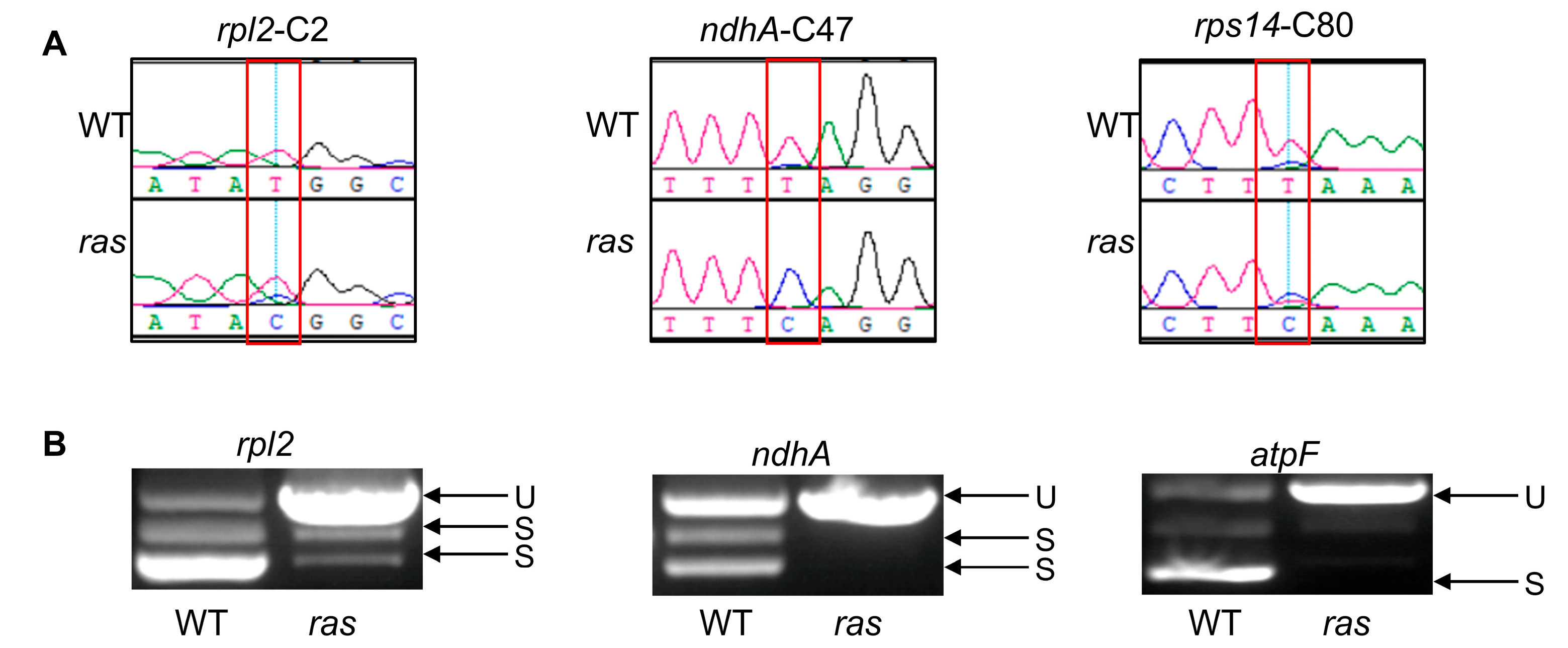
2.4. RAS Is Essential for the RNA Editing and RNA Splicing of Chloroplast Transcripts
2.5. The Ras Mutant Demonstrates Modified Expression Levels of Genes Associated with Chloroplast Development
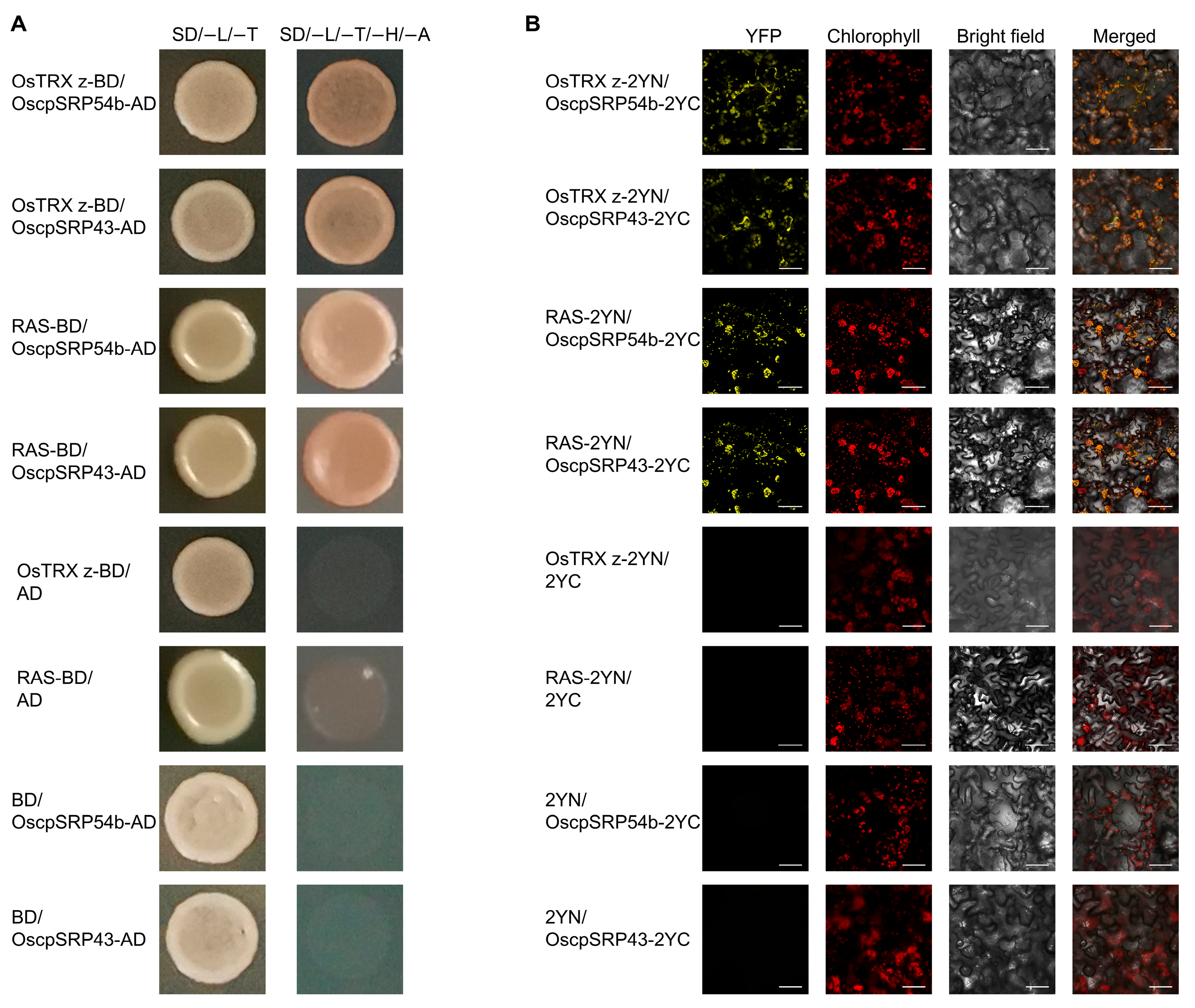
2.6. OsTRX z and RAS Are Translocated to Their Destinations via an SRP-Dependent Pathway
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Pigment Quantification
4.3. Transmission Electron Microscopy (TEM) Assays
4.4. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.5. Subcellular Localization
4.6. Chloroplast RNA Splicing and RNA Editing
4.7. Yeast Two-Hybrid (Y2H) and Bimolecular Fluorescence Complementation (BiFC)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, Q.B.; Huang, C.; Yang, Z.N. Nuclear-encoded factors associated with the chloroplast transcription machinery of higher plants. Front. Plant Sci. 2014, 5, 316. [Google Scholar] [CrossRef]
- Börner, T.; Aleynikova, A.Y.; Zubo, Y.O.; Kusnetsov, V.V. Chloroplast RNA polymerases: Role in chloroplast biogenesis. Biochim. Biophys. Acta 2015, 1847, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Arsova, B.; Hoja, U.; Wimmelbacher, M.; Greiner, E.; Ustun, S.; Melzer, M.; Petersen, K.; Lein, W.; Bornke, F. Plastidial thioredoxin z interacts with two fructokinase-like proteins in a thiol-dependent manner: Evidence for an essential role in chloroplast development in Arabidopsis and Nicotiana benthamiana. Plant Cell 2010, 22, 1498–1515. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, S.; Qiu, Z.; Zhao, J.; Nie, W.; Lin, H.; Zhu, Z.; Zeng, D.; Qian, Q.; Zhu, L. FRUCTOKINASE-LIKE PROTEIN 1 interacts with TRXz to regulate chloroplast development in rice. J. Integr. Plant Biol. 2018, 60, 94–111. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Shao, G.; Qiu, J.; Jiao, G.; Sheng, Z.; Xie, L.; Wu, Y.; Tang, S.; Wei, X.; Hu, P. White Leaf and Panicle 2, encoding a PEP-associated protein, is required for chloroplast biogenesis under heat stress in rice. J. Exp. Bot. 2017, 68, 5147–5160. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, T.; Yu, J.; Wu, T.; Wang, X.; Chen, G.; Tian, Y.; Zhang, H.; Wang, Y.; Terzaghi, W.; et al. TSV, a putative plastidic oxidoreductase, protects rice chloroplasts from cold stress during development by interacting with plastidic thioredoxin Z. New Phytol. 2017, 215, 240–255. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Ren, Y.; Duan, E.; Zhu, X.; Hao, Y.; Zhu, J.; Chen, R.; Lei, J.; Teng, X.; et al. White panicle2 encoding thioredoxin z, regulates plastid RNA editing by interacting with multiple organellar RNA editing factors in rice. New Phytol. 2021, 229, 2693–2706. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Zhang, M.; Ai, P. A chloroplast-localized pentatricopeptide repeat protein involved in RNA editing and splicing and its effects on chloroplast development in rice. BMC Plant Biol. 2022, 22, 437. [Google Scholar] [CrossRef]
- Qiu, Z.; Chen, D.; Teng, L.; Guan, P.; Yu, G.; Zhang, P.; Song, J.; Zeng, Q.; Zhu, L. OsWHY1 interacts with OsTRX z and is essential for early chloroplast development in rice. Rice 2022, 15, 50. [Google Scholar] [CrossRef]
- Wang, X.; An, Y.; Xu, P.; Xiao, J. Functioning of PPR proteins in organelle RNA metabolism and chloroplast biogenesis. Front. Plant Sci. 2021, 12, 627501. [Google Scholar] [CrossRef]
- Li, X.; Sun, M.; Liu, S.; Teng, Q.; Li, S.; Jiang, Y. Functions of PPR proteins in plant growth and development. Int. J. Mol. Sci. 2021, 22, 11274. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Prasad, A.M.; Srinivasan, R. Pentatricopeptide repeat proteins and their emerging roles in plants. Plant Physiol. Biochem. 2007, 45, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Barkan, A.; Small, I. Pentatricopeptide repeat proteins in plants. Annu. Rev. Plant Biol. 2014, 65, 415–442. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zou, Y.; Hu, J.; Ding, Y. Genome-wide analysis of the rice PPR gene family and their expression profiles under different stress treatments. BMC Genom. 2018, 19, 720. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, C. The Enigmatic Roles of PPR-SMR Proteins in Plants. Adv. Sci. 2019, 6, 1900361. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Tian, Y.; Teng, X.; Lv, Z.; Lei, J.; Duan, E.; Dong, H.; Yang, X.; Zhang, Y.; et al. Rice FLOURY ENDOSPERM22, encoding a pentatricopeptide repeat protein, is involved in both mitochondrial RNA splicing and editing and is crucial for endosperm development. J. Integr. Plant Biol. 2023, 65, 755–771. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Y.; Fang, Q.; Zhu, Y.; Zhang, Y.; Liu, X.; Lin, Y.; Barkan, A.; Zhou, F. The PPR-SMR protein ATP4 is required for editing the chloroplast rps8 mRNA in rice and maize. Plant Physiol. 2020, 184, 2011–2021. [Google Scholar] [CrossRef]
- Liu, S.; Melonek, J.; Boykin, L.M.; Small, I.; Howell, K.A. PPR-SMRs: Ancient proteins with enigmatic functions. RNA Biol. 2013, 10, 1501–1510. [Google Scholar] [CrossRef]
- Schmitz-Linneweber, C.; Small, I. Pentatricopeptide repeat proteins: A socket set for organelle gene expression. Trends Plant Sci. 2008, 13, 663–670. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, C.; Wang, Y.; He, M.; Li, Z.; Shen, L.; Li, Q.; Zhu, L.; Ren, D.; Hu, J.; et al. OsPPR11 encoding P-type PPR protein that affects group II intron splicing and chloroplast development. Plant Cell Rep. 2023, 42, 421–431. [Google Scholar] [CrossRef]
- Meng, L.; Du, M.; Zhu, T.; Li, G.; Ding, Y.; Zhang, Q. PPR proteins in plants: Roles, mechanisms, and prospects for rice research. Front. Plant Sci. 2024, 15, 1416742. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, Y.; He, M.; Li, Z.; Shen, L.; Li, Q.; Ren, D.; Hu, J.; Zhu, L.; Zhang, G.; et al. OsPPR9 encodes a DYW-type PPR protein that affects editing efficiency of multiple RNA editing sites and is essential for chloroplast development. J. Integr. Agric. 2023, 22, 972–980. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, Y.; Ai, P. OsTHA8 encodes a pentatricopeptide repeat protein required for RNA editing and splicing during rice chloroplast development. Crop J. 2023, 11, 1353–1367. [Google Scholar] [CrossRef]
- Lan, J.; Lin, Q.; Zhou, C.; Liu, X.; Miao, R.; Ma, T.; Chen, Y.; Mou, C.; Jing, R.; Feng, M.; et al. Young Leaf White Stripe encodes a P-Type PPR protein required for chloroplast development. J. Integr. Plant Biol. 2023, 65, 1687–1702. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Zhang, Y.; Zhao, W.; Yang, J.; Fan, Y. A novel PLS-DYW type PPR protein OsASL is essential for chloroplast development in rice. Plant Sci. 2024, 345, 112134. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, Y.; Zhang, Q.; Chen, C.; Qian, Q.; Guo, L. WAL3 encoding a PLS-type PPR protein regulates chloroplast development in rice. Plant Sci. 2022, 323, 111382. [Google Scholar] [CrossRef]
- Qin, T.; Zhao, P.; Sun, J.; Zhao, Y.; Zhang, Y.; Yang, Q.; Wang, W.; Chen, Z.; Mai, T.; Zou, Y.; et al. Research progress of PPR proteins in RNA editing, stress Rresponse, plant growth and development. Front. Genet. 2021, 12, 765580. [Google Scholar] [CrossRef]
- Qiu, T.; Zhao, X.; Feng, H.; Qi, L.; Yang, J.; Peng, Y.L.; Zhao, W. OsNBL3, a mitochondrion-localized pentatricopeptide repeat protein, is involved in splicing nad5 intron 4 and its disruption causes lesion mimic phenotype with enhanced resistance to biotic and abiotic stresses. Plant Biotechnol. J. 2021, 19, 2277–2290. [Google Scholar] [CrossRef]
- Zhou, L.; Mao, Y.C.; Yang, Y.M.; Wang, J.J.; Zhong, X.; Han, Y.; Zhang, Y.F.; Shi, Q.S.; Huang, X.h.; Meyers, B.C.; et al. Temperature and light reverse the fertility of rice P/TGMS line ostms19 via reactive oxygen species homeostasis. Plant Biotechnol. J. 2024, 22, 2020–2032. [Google Scholar] [CrossRef]
- Wimmelbacher, M.; Bornke, F. Redox activity of thioredoxin z and fructokinase-like protein 1 is dispensable for autotrophic growth of Arabidopsis thaliana. J. Exp. Bot. 2014, 65, 2405–2413. [Google Scholar] [CrossRef]
- Diaz, M.G.; Hernandez-Verdeja, T.; Kremnev, D.; Crawford, T.; Dubreuil, C.; Strand, A. Redox regulation of PEP activity during seedling establishment in Arabidopsis thaliana. Nat. Commun. 2018, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, C.; Ai, P.; Cui, X.; Zhang, Z. ALM1, encoding a Fe-superoxide dismutase, is critical for rice chloroplast biogenesis and drought stress response. Crop J. 2021, 9, 1018–1029. [Google Scholar] [CrossRef]
- Wang, D.; Liu, H.; Zhai, G.; Wang, L.; Shao, J.; Tao, Y. OspTAC2 encodes a pentatricopeptide repeat protein and regulates rice chloroplast development. J. Genet. Genom. 2016, 43, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, L.; Dai, L.; Gao, Y.; Zou, W.; Lu, X.; Wang, C.; Zhang, G.; Ren, D.; Hu, J.; et al. PALE-GREEN LEAF12 encodes a novel pentatricopeptide repeat protein required for chloroplast development and 16S rRNA processing in rice. Plant Cell Physiol. 2019, 60, 587–598. [Google Scholar] [CrossRef]
- Hutin, C.; Havaux, M.; Carde, J.P.; Kloppstech, K.; Meiherhoff, K.; Hoffman, N.; Nussaume, L. Double mutation cpSRP43–/cpSRP54– is necessary to abolish the cpSRP pathway required for thylakoid targeting of the light-harvesting chlorophyll proteins. Plant J. 2002, 29, 531–543. [Google Scholar] [CrossRef]
- Shi, Y.; He, Y.; Lv, X.; Wei, Y.; Zhang, X.; Xu, X.; Li, L.; Wu, J.L. Chloroplast SRP54s are essential for chloroplast development in rice. Rice 2020, 13, 54. [Google Scholar] [CrossRef]
- Lv, X.G.; Shi, Y.F.; Xu, X.; Wei, Y.L.; Wang, H.M.; Zhang, X.B.; Wu, J.L. Oryza sativa chloroplast signal recognition particle 43 (OscpSRP43) is required for chloroplast development and photosynthesis. PLoS ONE 2015, 10, e0143249. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, L.; Lu, C. Chloroplast gene expression: Recent advances and perspectives. Plant Commun. 2023, 4, 100611. [Google Scholar] [CrossRef]
- Pfalz, J.; Liere, K.; Kandlbinder, A.; Dietz, K.J.; Oelmuller, R. pTAC2, -6, and -12 are components of the transcriptionally active plastid chromosome that are required for plastid gene expression. Plant Cell 2006, 18, 176–197. [Google Scholar] [CrossRef]
- Shiina, T.; Tsunoyama, Y.; Nakahira, Y.; Khan, M.S. Plastid RNA polymerases, promoters, and transcription regulators in higher plants. Int. Rev. Cytol. 2005, 244, 1–68. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, Y.; Zhou, K.; Liu, L.; Wang, J.; Xu, Y.; Zhang, H.; Zhang, L.; Feng, Z.; Wang, L.; et al. WHITE STRIPE LEAF4 encodes a novel P-Type PPR protein required for chloroplast biogenesis during early leaf development. Front. Plant Sci. 2017, 8, 1116. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, W.; Wen, K.; Chen, G.; Sun, J.; Tian, Y.; Tang, W.; Yu, J.; An, H.; Wu, T.; et al. OsPPR6, a pentatricopeptide repeat protein involved in editing and splicing chloroplast RNA, is required for chloroplast biogenesis in rice. Plant Mol. Biol. 2017, 95, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Jiang, Q.; Xu, J.; Zhang, J.; Teng, S.; Lin, D.; Dong, Y. Disruption of the rice plastid ribosomal protein s20 leads to chloroplast developmental defects and seedling lethality. G3 2013, 3, 1769–1777. [Google Scholar] [CrossRef]
- Zhao, D.S.; Zhang, C.Q.; Li, Q.F.; Yang, Q.Q.; Gu, M.H.; Liu, Q.Q. A residue substitution in the plastid ribosomal protein L12/AL1 produces defective plastid ribosome and causes early seedling lethality in rice. Plant Mol. Biol. 2016, 91, 161–177. [Google Scholar] [CrossRef]
- Qiu, Z.; Chen, D.; He, L.; Zhang, S.; Yang, Z.; Zhang, Y.; Wang, Z.; Ren, D.; Qian, Q.; Guo, L.; et al. The rice white green leaf 2 gene causes defects in chloroplast development and affects the plastid ribosomal protein S9. Rice 2018, 11, 39. [Google Scholar] [CrossRef]
- Huang, W.; Zhu, Y.; Wu, W.; Li, X.; Zhang, D.; Yin, P.; Huang, J. The pentatricopeptide repeat protein SOT5/EMB2279 is required for plastid rpl2 and trnK intron splicing. Plant Physiol. 2018, 177, 684–697. [Google Scholar] [CrossRef]
- Tan, J.; Tan, Z.; Wu, F.; Sheng, P.; Heng, Y.; Wang, X.; Ren, Y.; Wang, J.; Guo, X.; Zhang, X.; et al. A novel chloroplast-localized pentatricopeptide repeat protein involved in splicing affects chloroplast development and abiotic stress response in rice. Mol. Plant 2014, 7, 1329–1349. [Google Scholar] [CrossRef]
- Ziehe, D.; Dunschede, B.; Schunemann, D. Molecular mechanism of SRP-dependent light-harvesting protein transport to the thylakoid membrane in plants. Photosynth. Res. 2018, 138, 303–313. [Google Scholar] [CrossRef]
- Sjuts, I.; Soll, J.; Bölter, B. Import of soluble proteins into chloroplasts and potential regulatory mechanisms. Front. Plant Sci. 2017, 8, 168. [Google Scholar] [CrossRef]
- Flores-Pérez, Ú.; Jarvis, P. Molecular chaperone involvement in chloroplast protein import. Biochim. Biophys. Acta 2013, 1833, 332–340. [Google Scholar] [CrossRef]
- Walter, B.; Pieta, T.; Schünemann, D. Arabidopsis thaliana mutants lacking cpFtsY or cpSRP54 exhibit different defects in photosystem II repair. Front. Plant Sci. 2015, 6, 250. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arnon, D. Copper enzymes in isolated chloroplasts. polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Qiu, Z.; He, L.; Hou, L.; Li, M.; Zhang, G.; Wang, X.; Chen, G.; Hu, J.; Gao, Z.; et al. The rice LRR-like1 protein YELLOW AND PREMATURE DWARF 1 is involved in leaf senescence induced by high light. J. Exp. Bot. 2021, 72, 1589–1605. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Z.; Wen, S.; Sun, P.; Chen, D.; Wang, C.; Song, X.; Xiao, L.; Zhang, P.; Zhao, D.; Wen, C.; et al. RAS, a Pentatricopeptide Repeat Protein, Interacts with OsTRX z to Regulate Chloroplast Gene Transcription and RNA Processing. Plants 2025, 14, 247. https://doi.org/10.3390/plants14020247
Qiu Z, Wen S, Sun P, Chen D, Wang C, Song X, Xiao L, Zhang P, Zhao D, Wen C, et al. RAS, a Pentatricopeptide Repeat Protein, Interacts with OsTRX z to Regulate Chloroplast Gene Transcription and RNA Processing. Plants. 2025; 14(2):247. https://doi.org/10.3390/plants14020247
Chicago/Turabian StyleQiu, Zhennan, Shiyong Wen, Peinan Sun, Dongdong Chen, Chunmiao Wang, Xiliang Song, Liying Xiao, Peiliang Zhang, Dongying Zhao, Cuiping Wen, and et al. 2025. "RAS, a Pentatricopeptide Repeat Protein, Interacts with OsTRX z to Regulate Chloroplast Gene Transcription and RNA Processing" Plants 14, no. 2: 247. https://doi.org/10.3390/plants14020247
APA StyleQiu, Z., Wen, S., Sun, P., Chen, D., Wang, C., Song, X., Xiao, L., Zhang, P., Zhao, D., Wen, C., Guan, P., Du, X., Sun, Y., Xu, C., & Song, J. (2025). RAS, a Pentatricopeptide Repeat Protein, Interacts with OsTRX z to Regulate Chloroplast Gene Transcription and RNA Processing. Plants, 14(2), 247. https://doi.org/10.3390/plants14020247





