The Function of Two Brassica napus β-Ketoacyl-CoA Synthases on the Fatty Acid Composition
Abstract
1. Introduction
2. Results
2.1. Bioinformatics Analysis of BnaKCSes
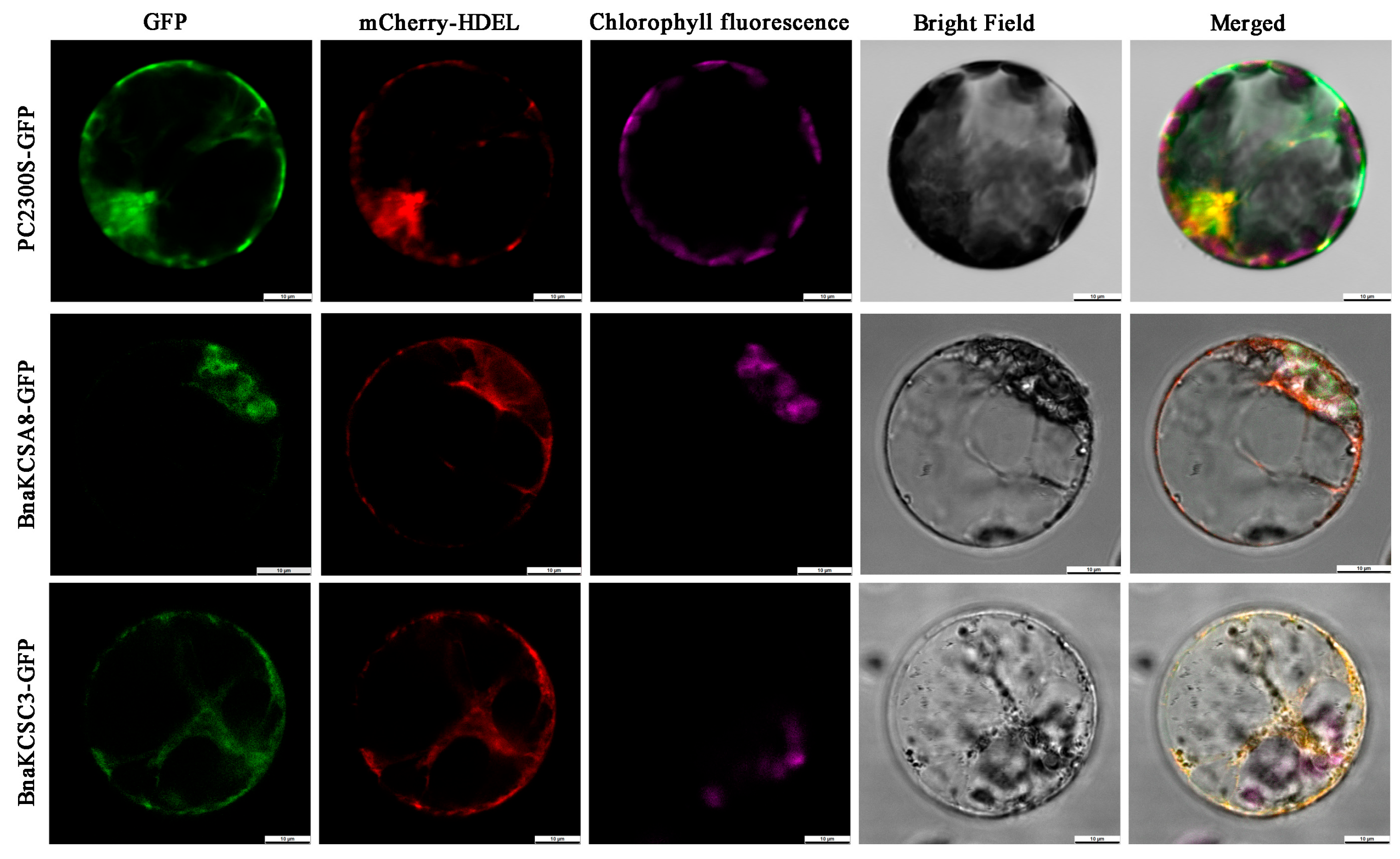
2.2. Gene Cloning and Subcellular Localization
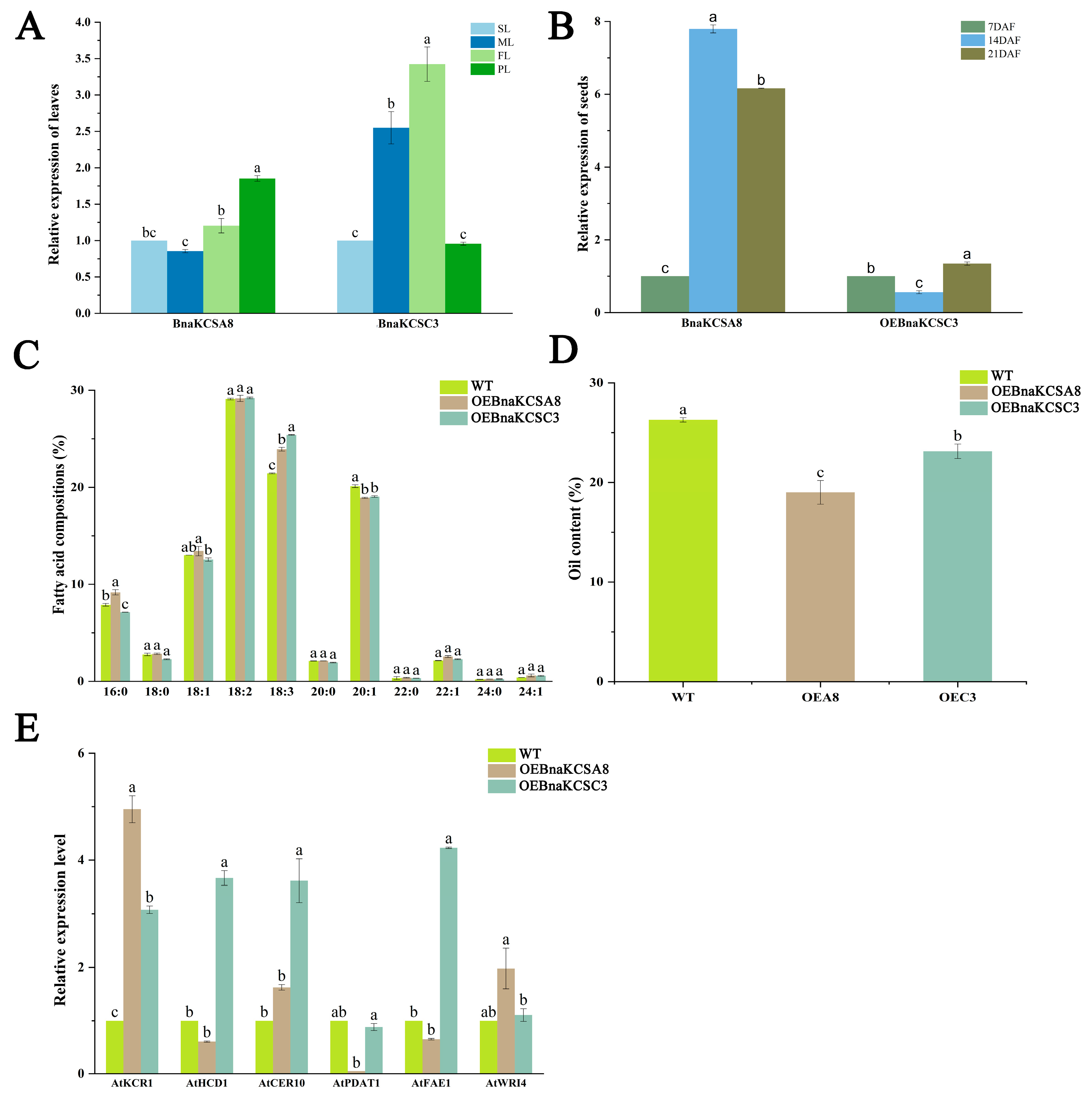
2.3. Expression Characteristics of BnaKCSes
2.4. Function of BnaKCSes in Yeast
2.5. Function of BnaKCSes in Arabidopsis
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Bioinformatics Analysis of BnaKCSes
4.3. BnaKCS Genes Cloning
4.4. Subcellular Localization
4.5. Yeast Cell Transformation
4.6. Overexpression of BnaKCSes in A. thaliana
4.7. Lipid Extraction
4.8. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
4.9. Data Presentation and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, Q.; Hua, W.; Yin, Y.; Zhang, X.; Liu, L.; Shi, J.; Zhao, Y.; Qin, L.; Chen, C.; Wang, H. Rapeseed research and production in China. Crop J. 2017, 5, 127–135. [Google Scholar] [CrossRef]
- Friedt, W.; Tu, J.; Fu, T. Academic and Economic Importance of Brassica napus Rapeseed. In The Brassica napus Genome; Liu, S., Snowdon, R., Chalhoub, B., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–20. [Google Scholar]
- Lin, Y.; Xuan, S.; Shuanshi, X.; Zhengang, G.; Du Chunfang. Variation and Correlation Analysis of Oleic Acid, Linoleic Acid, Linolenic Acid and ProteinContent in Brassica napus Seeds. J. Chin. Cereals Oils Assoc. 2021, 36, 6. [Google Scholar] [CrossRef]
- Haslam, T.M.; Kunst, L. Extending the story of very-long-chain fatty acid elongation. Plant Sci. 2013, 210, 93–107. [Google Scholar] [CrossRef] [PubMed]
- De Bigault, D.G.A.; Cacas, J.L. How Very-Long-Chain Fatty Acids Could Signal Stressful Conditions in Plants? Front. Plant Sci. 2016, 7, 1490. [Google Scholar] [CrossRef]
- Kogure, K.; Watanabe, A.; Ito, Y. Interaction of ONION2 ketoacyl CoA synthase with ketoacyl CoA reductase of rice. Mol. Biol. Rep. 2022, 49, 1643–1647. [Google Scholar] [CrossRef]
- Yang, L.; Fang, J.; Wang, J.; Hui, S.; Zhou, L.; Xu, B.; Chen, Y.; Zhang, Y.; Lai, C.; Jiao, G.; et al. Genome-wide identification and expression analysis of 3-ketoacyl-CoA synthase gene family in rice (Oryza sativa L.) under cadmium stress. Front. Plant Sci. 2023, 14, 1222288. [Google Scholar] [CrossRef]
- Joubès, J.; Raffaele, S.; Bourdenx, B.; Garcia, C.; Laroche-Traineau, J.; Moreau, P.; Domergue, F.; Lessire, R. The VLCFA elongase gene family in Arabidopsis thaliana: Phylogenetic analysis, 3D modelling and expression profiling. Plant Mol. Biol. 2008, 67, 547. [Google Scholar] [CrossRef]
- Guo, H.S.; Zhang, Y.M.; Sun, X.Q.; Li, M.M.; Hang, Y.Y.; Xue, J.Y. Evolution of the KCS gene family in plants: The history of gene duplication, sub/neofunctionalization and redundancy. Mol. Genet. Genom. 2016, 291, 739–752. [Google Scholar] [CrossRef]
- Campbell, A.A.; Stenback, K.E.; Flyckt, K.; Hoang, T.; Perera, M.; Nikolau, B.J. A single-cell platform for reconstituting and characterizing fatty acid elongase component enzymes. PLoS ONE 2019, 14, e213620. [Google Scholar] [CrossRef]
- Huai, D.; Xue, X.; Li, Y.; Wang, P.; Li, J.; Yan, L.; Chen, Y.; Wang, X.; Liu, N.; Kang, Y.; et al. Genome-Wide Identification of Peanut KCS Genes Reveals that AhKCS1 and AhKCS28 are Involved in Regulating VLCFA Contents in Seeds. Front. Plant Sci. 2020, 11, 406. [Google Scholar] [CrossRef]
- Rizwan, H.M.; Shaozhong, F.; Li, X.; Bilal, A.M.; Yousef, A.F.; Chenglong, Y.; Shi, M.; Jaber, M.; Anwar, M.; Hu, S.Y.; et al. Genome-Wide Identification and Expression Profiling of KCS Gene Family in Passion Fruit (Passiflora edulis) Under Fusarium kyushuense and Drought Stress Conditions. Front. Plant Sci. 2022, 13, 872263. [Google Scholar] [CrossRef] [PubMed]
- Denic, V.; Weissman, J.S. A molecular caliper mechanism for determining very long-chain fatty acid length. Cell 2007, 130, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Huai, D.; Zhang, Y.; Zhang, C.; Cahoon, E.B.; Zhou, Y. Combinatorial Effects of Fatty Acid Elongase Enzymes on Nervonic Acid Production in Camelina sativa. PLoS ONE 2015, 10, e131755. [Google Scholar] [CrossRef] [PubMed]
- Ozseyhan, M.E.; Kang, J.; Mu, X.; Lu, C. Mutagenesis of the FAE1 genes significantly changes fatty acid composition in seeds of Camelina sativa. Plant Physiol. Biochem. 2018, 123, 1–7. [Google Scholar] [CrossRef]
- Katavic, V.; Friesen, W.; Barton, D.L.; Gossen, K.K.; Giblin, E.M.; Luciw, T.; An, J.; Zou, J.; Mackenzie, S.L.; Keller, W.A. Improving Erucic Acid Content in Rapeseed through Biotechnology. Crop Sci. 2001, 41, 739–747. [Google Scholar] [CrossRef]
- James, D.J.; Lim, E.; Keller, J.; Plooy, I.; Ralston, E.; Dooner, H.K. Directed tagging of the Arabidopsis FATTY ACID ELONGATION1 (FAE1) gene with the maize transposon activator. Plant Cell 1995, 7, 309–319. [Google Scholar] [CrossRef]
- Lee, S.B.; Jung, S.J.; Go, Y.S.; Kim, H.U.; Kim, J.K.; Cho, H.J.; Park, O.K.; Suh, M.C. Two Arabidopsis 3-ketoacyl CoA synthase genes, KCS20 and KCS2/DAISY, are functionally redundant in cuticular wax and root suberin biosynthesis, but differentially controlled by osmotic stress. Plant J. 2009, 60, 462–475. [Google Scholar] [CrossRef]
- Pruitt, R.E.; Vielle-Calzada, J.P.; Ploense, S.E.; Grossniklaus, U.; Lolle, S.J. FIDDLEHEAD, a gene required to suppress epidermal cell interactions in Arabidopsis, encodes a putative lipid biosynthetic enzyme. Proc. Natl. Acad. Sci. USA 2000, 97, 1311–1316. [Google Scholar] [CrossRef]
- Yephremov, A.; Wisman, E.; Huijser, P.; Huijser, C.; Saedler, W.H. Characterization of the FIDDLEHEAD Gene of Arabidopsis Reveals a Link between Adhesion Response and Cell Differentiation in the Epidermis. Plant Cell 1999, 11, 2187–2201. [Google Scholar] [CrossRef]
- Kim, R.J.; Han, S.; Kim, H.J.; Hur, J.H.; Suh, M.C. Tetracosanoic acids produced by 3-ketoacyl-CoA synthase 17 are required for synthesizing seed coat suberin in Arabidopsis. J. Exp. Bot. 2024, 75, 1767–1780. [Google Scholar] [CrossRef]
- Wang, N.; Shi, L.; Tian, F.; Ning, H.; Wu, X. Assessment of FAE1 polymorphisms in three Brassica species using EcoTILLING and their association with differences in seed erucic acid contents. BMC Plant Biol. 2010, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Lang, C.; Wang, F.; Wu, X.; Liu, R.; Zheng, T.; Zhang, D.; Chen, J.; Wu, G. Depressed expression of FAE1 and FAD2 genes modifies fatty acid profiles and storage compounds accumulation in Brassica napus seeds. Plant Sci. 2017, 263, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, S.; Xu, Y.; Li, S.; Ni, Y. Overexpression of BnKCS1-1, BnKCS1-2, and BnCER1-2 promotes cuticular wax production and increases drought tolerance in Brassica napus. Crop J. 2019, 8, 26–37. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, D.; Li, X.; Zhou, B.; Chang, T.; Hong, B.; Guan, C.; Guan, M. Integrated Analysis of lncRNA-mRNA Regulatory Networks Related to Lipid Metabolism in High-Oleic-Acid Rapeseed. Int. J. Mol. Sci. 2023, 24, 6277. [Google Scholar] [CrossRef] [PubMed]
- Sagar, M.; Pandey, N.; Qamar, N.; Singh, B.; Shukla, A. Domain analysis of 3 Keto Acyl-CoA synthase for structural variations in Vitis vinifera and Oryza brachyantha using comparative modelling. Interdiscip. Sci. Comput. Life Sci. 2015, 7, 7–20. [Google Scholar] [CrossRef]
- Raboanatahiry, N.; Li, H.; Yu, L.; Li, M. Rapeseed (Brassica napus): Processing, Utilization, and Genetic Improvement. Agronomy 2021, 11, 1776. [Google Scholar] [CrossRef]
- Batista, C.; Barros, L.; Carvalho, A.M.; Ferreira, I.C. Nutritional and nutraceutical potential of rape (Brassica napus L. var. napus) and “tronchuda” cabbage (Brassica oleraceae L. var. costata) inflorescences. Food Chem. Toxicol. 2011, 49, 1208–1214. [Google Scholar] [CrossRef]
- Nicolosi, R.J.; Woolfrey, B.; Wilson, T.A.; Scollin, P.; Handelman, G.; Fisher, R. Decreased aortic early atherosclerosis and associated risk factors in hypercholesterolemic hamsters fed a high- or mid-oleic acid oil compared to a high-linoleic acid oil. J. Nutr. Biochem. 2004, 15, 540–547. [Google Scholar] [CrossRef]
- Rudkowska, I.; Roynette, C.E.; Nakhasi, D.K.; Jones, P.J.H. Phytosterols mixed with medium-chain triglycerides and high-oleic canola oil decrease plasma lipids in overweight men. Metab. Clin. Exp. 2006, 55, 391–395. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Passi, S.J.; Misra, A. Overview of trans fatty acids: Biochemistry and health effects. Diabetes Metab. Syndr. 2011, 5, 161–164. [Google Scholar] [CrossRef]
- Menaa, F.; Menaa, A.; Menaa, B.; Tréton, J. Trans-fatty acids, dangerous bonds for health? A background review paper of their use, consumption, health implications and regulation in France. Eur. J. Nutr. 2013, 52, 1289–1302. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Jefferis, B.J.; Lennon, L.; Papacosta, O.; Whincup, P.H.; Hingorani, A.D. Serum Conjugated Linoleic Acid and Risk of Incident Heart Failure in Older Men: The British Regional Heart Study. J. Am. Heart Assoc. 2018, 7, e6653. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, K.; Zhang, Z.; Yu, Y.; Wan, J.; He, H.; Fan, C. Targeted mutagenesis of flavonoid biosynthesis pathway genes reveals functional divergence in seed coat colour, oil content and fatty acid composition in Brassica napus L. Plant Biotechnol. J. 2024, 22, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Sakhno, L.O. Variability in the fatty acid composition of rapeseed oil: Classical breeding and biotechnology. Cytol. Genet. 2010, 44, 389–397. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Wu, W. Understanding fatty acid composition and lipid profile of rapeseed oil in response to nitrogen management strategies. Food Res. Int. 2023, 165, 112565. [Google Scholar] [CrossRef]
- Guo, Y.; Mietkiewska, E.; Francis, T.; Katavic, V.; Brost, J.M.; Giblin, M.; Barton, D.L.; Taylor, D.C. Increase in nervonic acid content in transformed yeast and transgenic plants by introduction of a Lunaria annua L. 3-ketoacyl-CoA synthase (KCS) gene. Plant Mol. Biol. 2009, 69, 565–575. [Google Scholar] [CrossRef]
- Taylor, D.C.; Francis, T.; Guo, Y.; Brost, J.M.; Katavic, V.; Mietkiewska, E.; Giblin, E.M.; Lozinsky, S.; Hoffman, T. Molecular cloning and characterization of a KCS gene from Cardamine graeca and its heterologous expression in Brassica oilseeds to engineer high nervonic acid oils for potential medical and industrial use. Plant Biotechnol. J. 2009, 7, 925–938. [Google Scholar] [CrossRef]
- Guo, W.; Wu, Q.; Yang, L.; Hu, W.; Liu, D.; Liu, Y. Ectopic Expression of CsKCS6 From Navel Orange Promotes the Production of Very-Long-Chain Fatty Acids (VLCFAs) and Increases the Abiotic Stress Tolerance of Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 564656. [Google Scholar] [CrossRef]
- Li, Q.; Shao, J.; Tang, S.; Shen, Q.; Wang, T.; Chen, W.; Hong, Y. Wrinkled1 Accelerates Flowering and Regulates Lipid Homeostasis between Oil Accumulation and Membrane Lipid Anabolism in Brassica napus. Front. Plant Sci. 2015, 6, 1015. [Google Scholar] [CrossRef]
- Fei, W.; Yang, S.; Hu, J.; Yang, F.; Qu, G.; Peng, D.; Zhou, B. Research advances of WRINKLED1 (WRI1) in plants. Funct. Plant Biol. 2020, 47, 185–194. [Google Scholar] [CrossRef]
- Nelson, B.K.; Cai, X.; Nebenführ, A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 2010, 51, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.H.; Shen, S.C.; Lee, L.Y.; Lee, S.H.; Chan, M.T.; Lin, C.S. Tape-Arabidopsis Sandwich—A Simpler Arabidopsis Protoplast Isolation Method. Plant Methods 2009, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, X.; Wang, R.; Chen, B.; Jiang, J.; Win, A.N.; Chai, Y. Cloning and expression of Perilla frutescens FAD2 gene and polymorphism analysis among cultivars. Acta Physiol. Plant. 2017, 39, 84. [Google Scholar] [CrossRef]
- Zhang, C.L.; Zhang, Y.L.; Hu, X.; Xiao, X.; Hao, Y.J. An apple long-chain acyl-CoA synthetase, MdLACS4, induces early flowering and enhances abiotic stress resistance in Arabidopsis. Plant Sci. 2020, 297, 110529. [Google Scholar] [CrossRef]
- Katavic, V.; Barton, D.L.; Giblin, E.M.; Reed, D.W.; Kumar, A.; Taylor, D.C. Gaining insight into the role of serine 282 in B. napus FAE1 condensing enzyme. Febs Lett. 2004, 562, 118–124. [Google Scholar] [CrossRef]
- Lian, J.; Lu, X.; Yin, N.; Ma, L.; Lu, J.; Liu, X.; Li, J.; Lu, J.; Lei, B.; Wang, R.; et al. Silencing of BnTT1 family genes affects seed flavonoid biosynthesis and alters seed fatty acid composition in Brassica napus. Plant Sci. 2017, 254, 32–47. [Google Scholar] [CrossRef]
- Li, F.; Wu, X.; Lam, P.; Bird, D.; Zheng, H.; Samuels, L.; Jetter, R.; Kunst, L. Identification of the wax ester synthase/acyl-coenzyme A: Diacylglycerol acyltransferase WSD1 required for stem wax ester biosynthesis in Arabidopsis. Plant Physiol. 2008, 148, 97–107. [Google Scholar] [CrossRef]
- Kenneth, S.T.L. Analysis of Relative Gene Expression Data Using RT-qPCR pdf. Methods 2001, 25, 402–408. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Zhou, B.; Hong, B.; Mao, J.; Chen, H.; Wu, J.; Liao, L.; Guan, C.; Guan, M. The Function of Two Brassica napus β-Ketoacyl-CoA Synthases on the Fatty Acid Composition. Plants 2025, 14, 202. https://doi.org/10.3390/plants14020202
Zhao D, Zhou B, Hong B, Mao J, Chen H, Wu J, Liao L, Guan C, Guan M. The Function of Two Brassica napus β-Ketoacyl-CoA Synthases on the Fatty Acid Composition. Plants. 2025; 14(2):202. https://doi.org/10.3390/plants14020202
Chicago/Turabian StyleZhao, Dongfang, Bingqian Zhou, Bo Hong, Jiajun Mao, Hu Chen, Junjie Wu, Li Liao, Chunyun Guan, and Mei Guan. 2025. "The Function of Two Brassica napus β-Ketoacyl-CoA Synthases on the Fatty Acid Composition" Plants 14, no. 2: 202. https://doi.org/10.3390/plants14020202
APA StyleZhao, D., Zhou, B., Hong, B., Mao, J., Chen, H., Wu, J., Liao, L., Guan, C., & Guan, M. (2025). The Function of Two Brassica napus β-Ketoacyl-CoA Synthases on the Fatty Acid Composition. Plants, 14(2), 202. https://doi.org/10.3390/plants14020202




