Prohexadione-Calcium Reduced Stem and Tiller Damage and Maintained Yield by Improving the Photosynthetic and Antioxidant Capacity of Rice (Oryza sativa L.) Under NaCl Stress
Abstract
1. Introduction
2. Results
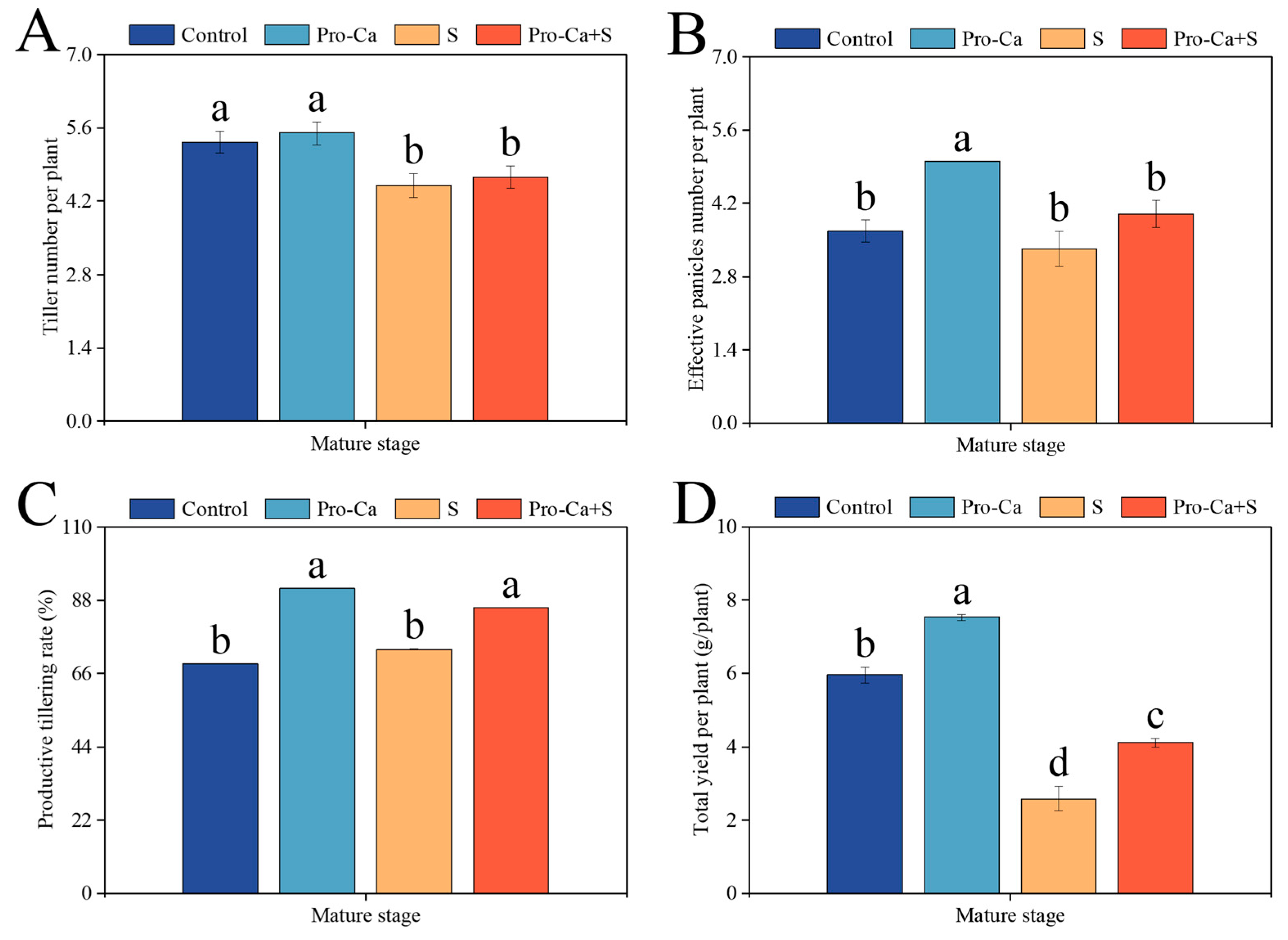
2.1. Effect of Pro-Ca on the Main Stem and Tiller Yield and Panicle Traits Under NaCl Stress
2.2. Effect of Pro-Ca on the Growth Relationship Between the Main Stem and Tiller Leaves at the Tillering Stage Under NaCl Stress
2.3. Effect of Pro-Ca on Morphological Characters of the Main Stem and the Tiller at the Fourth Leaf Axil Under NaCl Stress
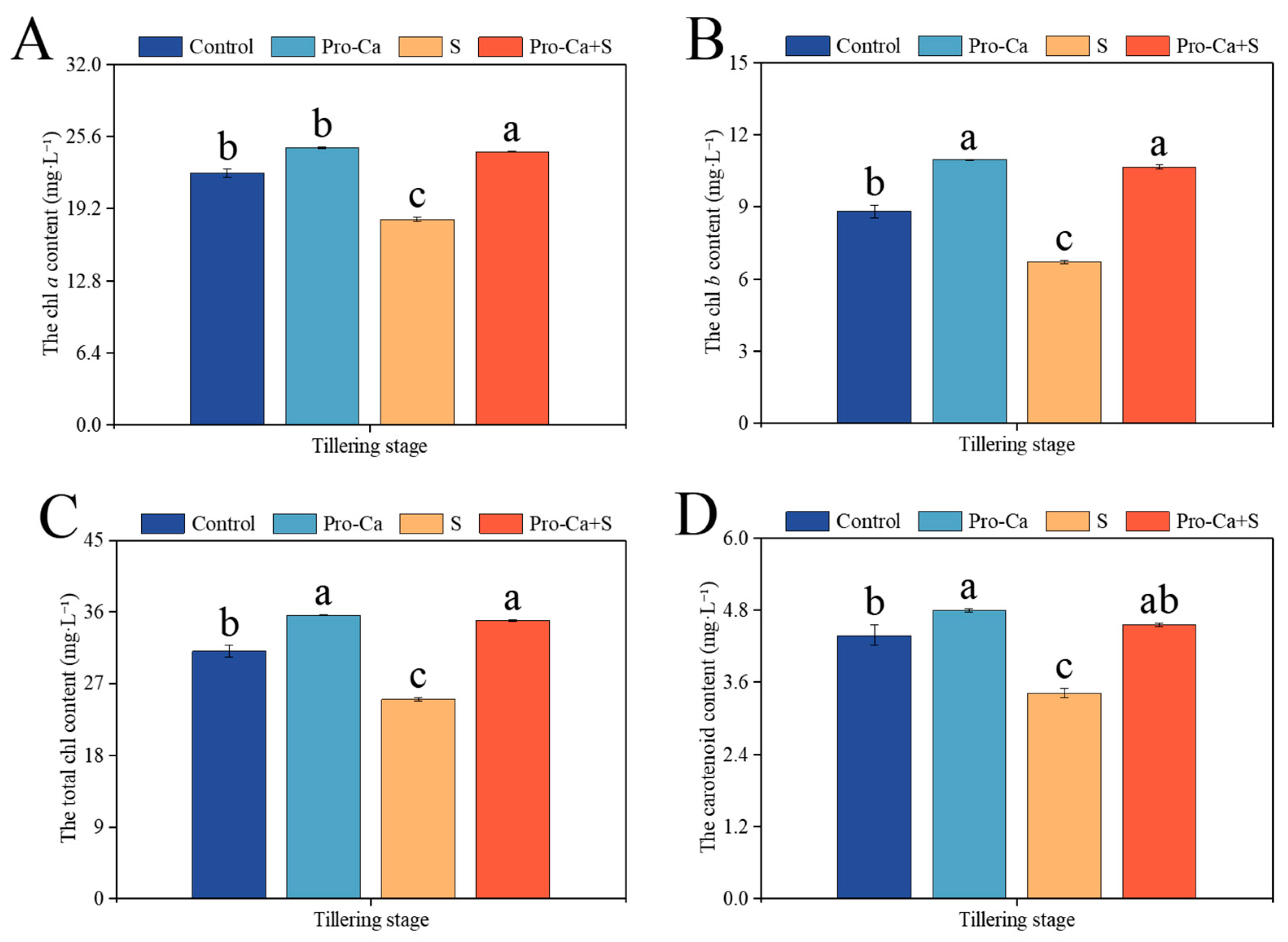
2.4. Effect of Pro-Ca on Photosynthetic Pigment Content Under NaCl Stress
2.5. Effect of Pro-Ca on Gas Exchange Parameters Under NaCl Stress
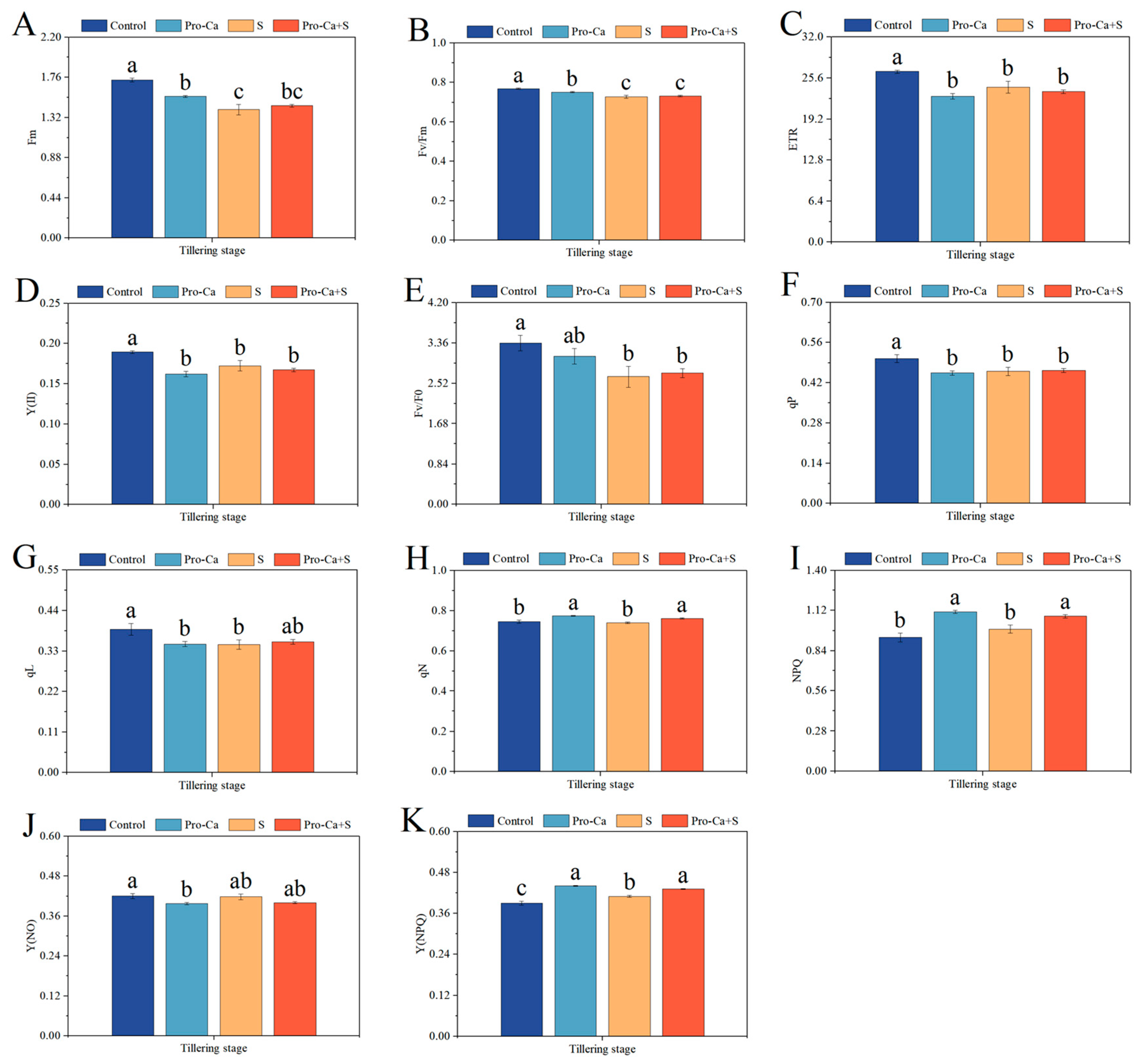
2.6. Effect of Pro-Ca on Chlorophyll Fluorescence Parameters Under NaCl Stress
2.7. Pro-Ca Increased the Activity of Antioxidant Enzymes in Rice at the Tillering Stage Under NaCl Stress
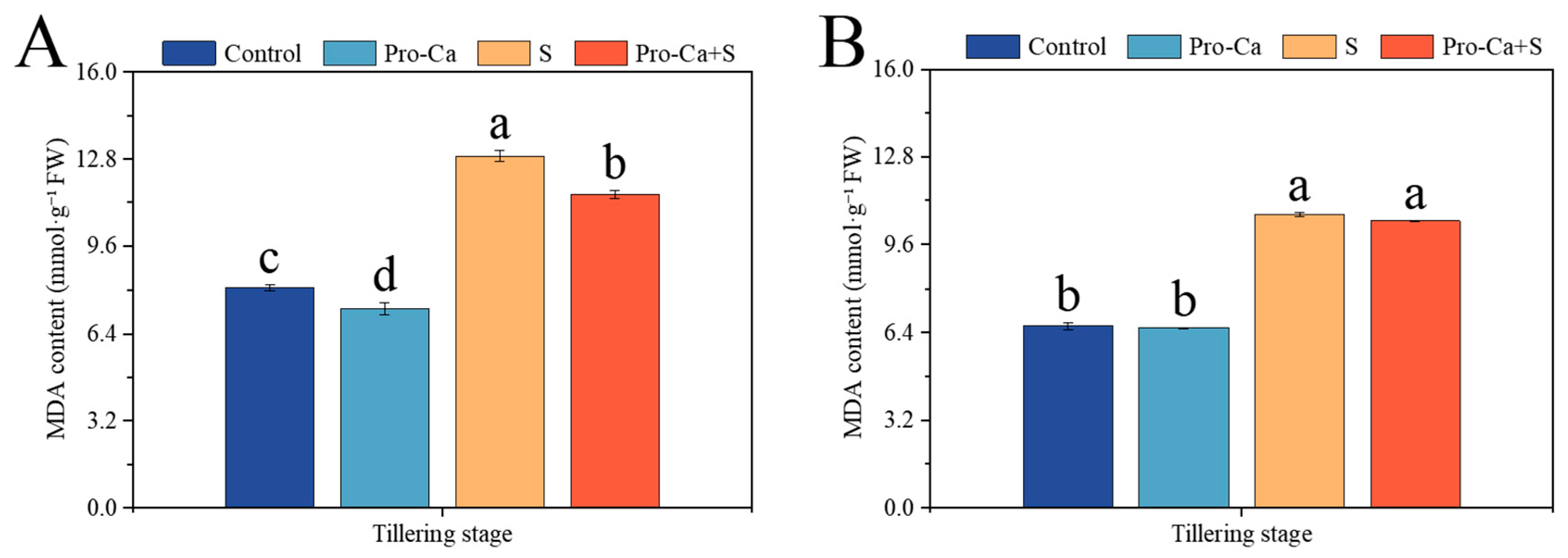
2.8. Effect of Pro-Ca on MDA Content Under NaCl Stress
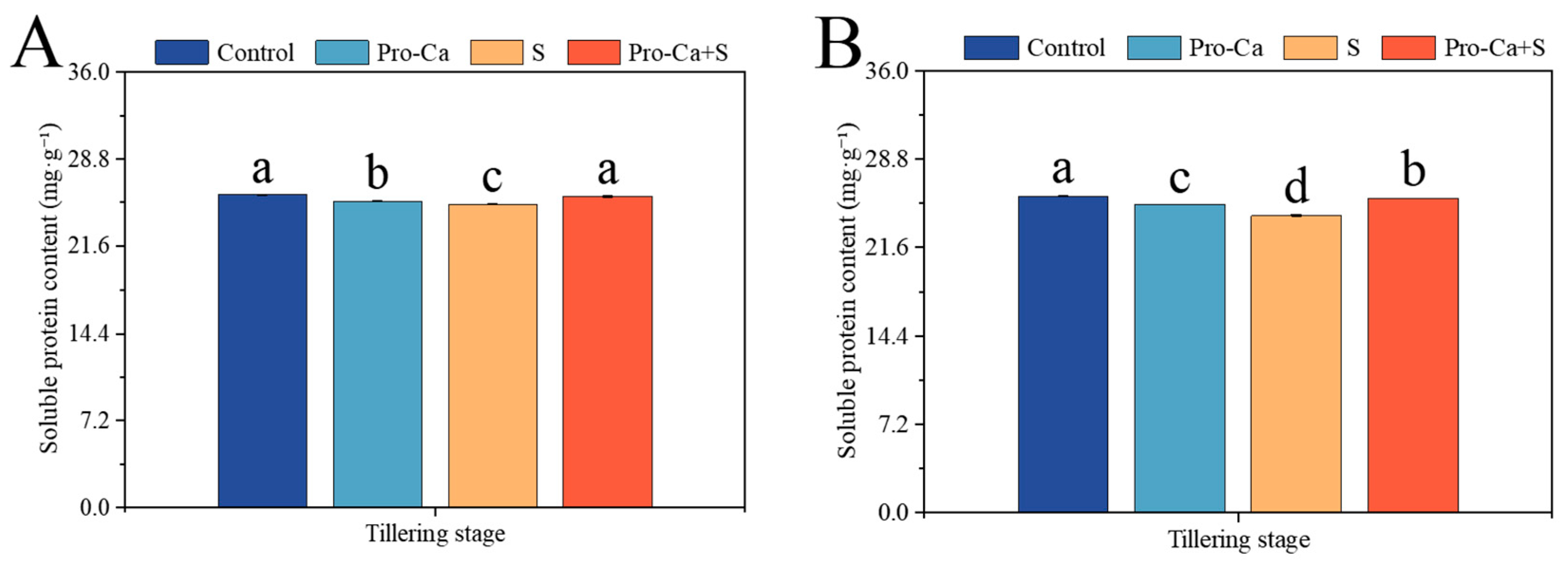
2.9. Effect of Pro-Ca on Soluble Protein Content Under NaCl Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Test Conditions
4.2. Yield and Panicle Traits
4.3. Morphological Parameters
4.4. Photosynthetic Pigment Content
4.5. Gas Exchange Parameters
4.6. Chlorophyll Fluorescence
4.7. Antioxidant Enzyme Activity
4.8. Malondialdehyde Content
4.9. Soluble Protein Content
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kromdijk, J.; Long, S.P. One Crop Breeding Cycle from Starvation? How Engineering Crop Photosynthesis for Rising CO2 and Temperature Could Be One Important Route to Alleviation. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152578. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). The Future of Food and Agriculture—Trends and Challenges; Food and Agriculture Organization of the United Nations, Agricultural Development Economics Division (ESA): Rome, Italy, 2017. [Google Scholar]
- Tester, M.; Davenport, R. Na+ Tolerance and Na+ Transport in Higher Plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Walthall, C.L.; Hatfield, J.; Backlund, P.; Lengnick, L.; Marshall, E.; Walsh, M.; Adkins, S.; Aillery, M.; Ainsworth, E.A.; Ammann, C.; et al. Climate Change and Agriculture in the United States: Effects and Adaptation; USDA: Washington, DC, USA, 2012.
- Gao, J.; Xu, G.; Xu, P. Gills Full-Length Transcriptomic Analysis of Osmoregulatory Adaptive Responses to Salinity Stress in Coilia Nasus. Ecotoxicol. Environ. Saf. 2021, 226, 112848. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Singh, B.; Mishra, S.; Singh, A.K.; Singh, N.K. Candidate Gene Based Association Analysis of Salt Tolerance in Traditional and Improved Varieties of Rice (Oryza sativa L.). J. Plant Biochem. Biotechnol. 2019, 28, 76–83. [Google Scholar] [CrossRef]
- Wu, K.; Wang, S.; Song, W.; Zhang, J.; Wang, Y.; Liu, Q.; Yu, J.; Ye, Y.; Li, S.; Chen, J.; et al. Enhanced Sustainable Green Revolution Yield via Nitrogen-Responsive Chromatin Modulation in Rice. Science 2020, 367, eaaz2046. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.; Zhang, R.; Feng, N.; Zheng, D. Photosynthetic Responses to Salt Stress in Two Rice (Oryza sativa L.) Varieties. Agronomy 2024, 14, 2134. [Google Scholar] [CrossRef]
- Sun, B.-R.; Fu, C.-Y.; Fan, Z.-L.; Chen, Y.; Chen, W.-F.; Zhang, J.; Jiang, L.-Q.; Lv, S.; Pan, D.-J.; Li, C. Genomic and Transcriptomic Analysis Reveal Molecular Basis of Salinity Tolerance in a Novel Strong Salt-Tolerant Rice Landrace Changmaogu. Rice 2019, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.; Juraimi, A.S.; Hanafi, M.; Ismail, M.R.; Selamat, A.; Rafii, M.; Latif, M. Biochemical and Anatomical Changes and Yield Reduction in Rice (Oryza sativa L.) under Varied Salinity Regimes. BioMed Res. Int. 2014, 2014, 208584. [Google Scholar] [CrossRef]
- Machado, R.; Serralheiro, R. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Razzaq, A.; Ali, A.; Safdar, L.B.; Zafar, M.M.; Rui, Y.; Shakeel, A.; Shaukat, A.; Ashraf, M.; Gong, W.; Yuan, Y. Salt Stress Induces Physiochemical Alterations in Rice Grain Composition and Quality. J. Food Sci. 2020, 85, 14–20. [Google Scholar] [CrossRef]
- Wegner, L.H.; Stefano, G.; Shabala, L.; Rossi, M.; Mancuso, S.; Shabala, S. Sequential Depolarization of Root Cortical and Stelar Cells Induced by an Acute Salt Shock—Implications for Na+ and K+ Transport into Xylem Vessels: Salt Shock Depolarization of Root Cells. Plant Cell Environ. 2011, 34, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Ahanger, M.A.; Zhou, J.; Ahmed, N.; Wei, C.; Yuan, S.; Ashraf, M.; Zhang, L. Beneficial Role of Acetylcholine in Chlorophyll Metabolism and Photosynthetic Gas Exchange in Nicotiana Benthamiana Seedlings under Salinity Stress. Plant Biol. 2020, 22, 357–365. [Google Scholar] [CrossRef]
- Farooq, M.; Gogoi, N.; Hussain, M.; Barthakur, S.; Paul, S.; Bharadwaj, N.; Migdadi, H.M.; Alghamdi, S.S.; Siddique, K.H.M. Effects, Tolerance Mechanisms and Management of Salt Stress in Grain Legumes. Plant Physiol. Biochem. 2017, 118, 199–217. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yu, C.; Zhou, L.; Chen, Y.; Liu, A.; Jin, J.; Hong, J.; Qi, Y.; Jiang, D. Rubisco Decrease Is Involved in Chloroplast Protrusion and Rubisco-Containing Body Formation in Soybean (Glycine max.) under Salt Stress. Plant Physiol. Biochem. 2014, 74, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Killi, D.; Haworth, M. Diffusive and Metabolic Constraints to Photosynthesis in Quinoa during Drought and Salt Stress. Plants 2017, 6, 49. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Yamamoto, H.; Allakhverdiev, S.I.; Inaba, M.; Yokota, A.; Murata, N. Oxidative Stress Inhibits the Repair of Photodamage to the Photosynthetic Machinery. EMBO J. 2001, 20, 5587–5594. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, G.; Iglesias-Moya, J.; García, A.; Martínez, J.; Romero, J.; Regalado, J.J.; Martínez, C.; Valenzuela, J.L.; Jamilena, M. Involvement of Ethylene Receptors in the Salt Tolerance Response of Cucurbita Pepo. Hortic. Res. 2021, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, Distribution and Roles of Osmoprotective Compounds Accumulated in Halophytes under Abiotic Stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity Tolerance of Crops—What Is the Cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Huang, Y.; Sun, S.; Sun, J.; Cao, H.; Shabala, S.; Bie, Z. Root Respiratory Burst Oxidase Homologue-Dependent H2O2 Production Confers Salt Tolerance on a Grafted Cucumber by Controlling Na+ Exclusion and Stomatal Closure. J. Exp. Bot. 2018, 69, 3465–3476. [Google Scholar] [CrossRef]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.; Cuypers, A. Plant Sugars Are Crucial Players in the Oxidative Challenge during Abiotic Stress: Extending the Traditional Concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Hanelt, D. 9—Photosynthesis Assessed by Chlorophyll Fluorescence. In Bioassays; Häder, D.-P., Erzinger, G.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 169–198. ISBN 978-0-12-811861-0. [Google Scholar]
- Johnson, M.P. Photosynthesis. Essays Biochem 2016, 60, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Bussotti, F.; Gerosa, G.; Digrado, A.; Pollastrini, M. Selection of Chlorophyll Fluorescence Parameters as Indicators of Photosynthetic Efficiency in Large Scale Plant Ecological Studies. Ecol. Indic. 2020, 108, 105686. [Google Scholar] [CrossRef]
- Chen, T.; Shabala, S.; Niu, Y.; Chen, Z.-H.; Shabala, L.; Meinke, H.; Venkataraman, G.; Pareek, A.; Xu, J.; Zhou, M. Molecular Mechanisms of Salinity Tolerance in Rice. Crop J. 2021, 9, 506–520. [Google Scholar] [CrossRef]
- Soleimani Aghdam, M. Mitigation of Postharvest Chilling Injury in Tomato Fruit by Prohexadione Calcium. J. Food Sci. Technol. 2013, 50, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Zahid, G.; Iftikhar, S.; Shimira, F.; Ahmad, H.M.; Aka Kaçar, Y. An Overview and Recent Progress of Plant Growth Regulators (PGRs) in the Mitigation of Abiotic Stresses in Fruits: A Review. Sci. Hortic. 2023, 309, 111621. [Google Scholar] [CrossRef]
- Du, X.; Du, Y.; Feng, N.; Zheng, D.; Zhou, H.; Huo, J. Exogenous Uniconazole Promotes Physiological Metabolism and Grain Yield of Rice under Salt Stress. Front. Plant Sci. 2024, 15, 1459121. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, J.; Dai, Z.; Chen, Y.; Shao, Z.; Wang, C.; Jin, X.; Wang, Y.; Feng, L. Prohexadione-Calcium Improves Grape Quality by Regulating Endogenous Hormones, Sugar and Acid Metabolism and Related Enzyme Activities in Grape Berries. BMC Plant Biol. 2024, 24, 122. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, D.; Feng, N.; Qiu, Q.-S.; Zhou, H.; Liu, M.; Li, Y.; Meng, F.; Huang, X.; Huang, A.; et al. Prohexadione Calcium Enhances Rice Growth and Tillering under NaCl Stress. Peerj 2023, 11, e14804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zheng, D.; Feng, N.; Qiu, Q.-S.; Zhou, H.; Meng, F.; Huang, X.; Huang, A.; Li, Y. Prohexadione-Calcium Alleviates the Leaf and Root Damage Caused by Salt Stress in Rice (Oryza sativa L.) at the Tillering Stage. PLoS ONE 2023, 18, e0279192. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, I.J.; Hamayun, M.; Kim, J.T.; Won, J.G.; Hwang, I.C.; Kim, K.U. Effect of Prohexadione Calcium on Growth Components and Endogenous Gibberellins Contents of Rice (Oryza sativa L.). J. Agron. Crop Sci. 2007, 193, 445–451. [Google Scholar] [CrossRef]
- Feng, N.; Yu, M.; Li, Y.; Jin, D.; Zheng, D. Prohexadione-Calcium Alleviates Saline-Alkali Stress in Soybean Seedlings by Improving the Photosynthesis and up-Regulating Antioxidant Defense. Ecotoxicol. Environ. Saf. 2021, 220, 112369. [Google Scholar] [CrossRef]
- Bekheta, M.A.; Abdelhamid, M.T.; El-Morsi, A.A. Physiological Response of Vicia Faba to Prohexadione-Calcium under Saline Conditions Resposta Fisiológica de Vicia Faba a Prohexadiona-Cálcio Sob Condições Salinas. Planta Daninha 2009, 27, 769–779. [Google Scholar] [CrossRef]
- Huang, X.; Zheng, D.; Feng, N.; Huang, A.; Zhang, R.; Meng, F.; Jie, Y.; Mu, B.; Mu, D.; Zhou, H. Effects of Prohexadione Calcium Spraying during the Booting Stage on Panicle Traits, Yield, and Related Physiological Characteristics of Rice under Salt Stress. PeerJ 2023, 11, e14673. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Ismail, A.M. Responses of Photosynthesis, Chlorophyll Fluorescence and ROS-Scavenging Systems to Salt Stress during Seedling and Reproductive Stages in Rice. Ann. Bot-Lond. 2007, 99, 1161–1173. [Google Scholar] [CrossRef]
- Yang, J.; Peng, S.; Visperas, R.M.; Sanico, A.L.; Zhu, Q.; Gu, S. Grain Filling Pattern and Cytokinin Content in the Grains and Roots of Rice Plants. Plant Growth Regul. 2000, 30, 261–270. [Google Scholar] [CrossRef]
- Abdullah, Z.; Khan, M.A.; Flowers, T.J. Causes of Sterility in Seed Set of Rice under Salinity Stress. J. Agron. Crop Sci. 2001, 187, 25–32. [Google Scholar] [CrossRef]
- Chakraborty, K.; Chattaopadhyay, K.; Nayak, L.; Ray, S.; Yeasmin, L.; Jena, P.; Gupta, S.; Mohanty, S.K.; Swain, P.; Sarkar, R.K. Ionic Selectivity and Coordinated Transport of Na+ and K+ in Flag Leaves Render Differential Salt Tolerance in Rice at the Reproductive Stage. Planta 2019, 250, 1637–1653. [Google Scholar] [CrossRef] [PubMed]
- Lokuruge, P.; Tar’an, B.; Harms, T.; Howard, R.; Bandara, M. Effect of Prohexadione Calcium on Vegetative Growth, Seed Maturity and Seed Yield of the Kabuli Chickpea Cultivar CDC Frontier. Can. J. Plant Sci. 2015, 95, 571–578. [Google Scholar] [CrossRef]
- Yeo, A.R.; Yeo, M.E.; Caporn, S.J.M.; Lachno, D.R.; Flowers, T.J. The Use of 14C-Ethane Diol as a Quantitative Tracer for the Transpirational Volume Flow of Water and an Investigation of the Effects of Salinity upon Transpiration, Net Sodium Accumulation and Endogenous ABA in Individual Leaves of Oryza sativa L. J. Exp. Bot. 1985, 36, 1099–1109. [Google Scholar] [CrossRef]
- Tao, Z.; Wang, D.; Ma, S.; Yang, Y.; Zhao, G.; Chang, X. Light Interception and Radiation Use Efficiency Response to Tridimensional Uniform Sowing in Winter Wheat. J. Integr. Agric. 2018, 17, 566–578. [Google Scholar] [CrossRef]
- Ashraf, M.Y.; Iqbal, N.; Ashraf, M.; Akhter, J. Modulation of physiological and biochemical metabolites in salt stressed rice by foliar application of zinc. J. Plant Nutr. 2014, 37, 447–457. [Google Scholar] [CrossRef]
- Wang, Y.; Nii, N. Changes in Chlorophyll, Ribulose Bisphosphate Carboxylase-Oxygenase, Glycine Betaine Content, Photosynthesis and Transpiration in Amaranthus Tricolor Leaves during Salt Stress. J. Hortic. Sci. Biotechnol. 2000, 75, 623–627. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Latif, H.H. Improvement of Drought Tolerance of Soybean Plants by Using Methyl Jasmonate. Physiol. Mol. Biol. Plants 2017, 23, 545–556. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.; Tran, L.-S.P. Impacts of Priming with Silicon on the Growth and Tolerance of Maize Plants to Alkaline Stress. Front. Plant Sci. 2016, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Mori, I.C. Stomatal Regulation of Plant Water Status. In Plant Abiotic Stress; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 47–67. ISBN 978-1-118-76437-4. [Google Scholar]
- de Santana, T.A.; de Oliveira, P.S.; da Silva, L.D.; Laviola, B.G.; Almeida, A.-A.F.; Gomes, F.P. Water Use Efficiency and Consumption in Different Brazilian Genotypes of Jatropha Curcas L. Subjected to Soil Water Deficit. Biomass Bioenerg. 2015, 75, 119–125. [Google Scholar] [CrossRef]
- Cramer, G.R.; Quarrie, S.A. Abscisic Acid Is Correlated with the Leaf Growth Inhibition of Four Genotypes of Maize Differing in Their Response to Salinity. Funct Plant Biol. 2002, 29, 111–115. [Google Scholar] [CrossRef]
- Han, J.-M.; Zhang, W.-F.; Xiong, D.-L.; Flexas, J.; Zhang, Y.-L. Mesophyll Conductance and Its Limiting Factors in Plant Leaves. Chin. J. Plant Ecol. 2017, 41, 914. [Google Scholar] [CrossRef]
- Ensminger, I.; Busch, F.; Huner, N.P.A. Photostasis and Cold Acclimation: Sensing Low Temperature through Photosynthesis. Physiol. Plant. 2006, 126, 28–44. [Google Scholar] [CrossRef]
- Fracheboud, Y.; Haldimann, P.; Leipner, J.; Stamp, P. Chlorophyll Fluorescence as a Selection Tool for Cold Tolerance of Photosynthesis in Maize (Zea mays L.). J. Exp. Bot. 1999, 50, 1533–1540. [Google Scholar] [CrossRef]
- Riva-Roveda, L.; Escale, B.; Giauffret, C.; Périlleux, C. Maize Plants Can Enter a Standby Mode to Cope with Chilling Stress. BMC Plant Biol. 2016, 16, 212. [Google Scholar] [CrossRef] [PubMed]
- Tietz, S.; Hall, C.C.; Cruz, J.A.; Kramer, D.M. NPQ(T): A Chlorophyll Fluorescence Parameter for Rapid Estimation and Imaging of Non-Photochemical Quenching of Excitons in Photosystem-II-Associated Antenna Complexes. Plant Cell Environ. 2017, 40, 1243–1255. [Google Scholar] [CrossRef]
- Pospíšil, P. Production of Reactive Oxygen Species by Photosystem II. Biochim. Biophys. Acta (BBA) Bioenerg. 2009, 1787, 1151–1160. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Mishra, P.; Bhoomika, K.; Dubey, R.S. Differential Responses of Antioxidative Defense System to Prolonged Salinity Stress in Salt-Tolerant and Salt-Sensitive Indica Rice (Oryza sativa L.) Seedlings. Protoplasma 2013, 250, 3–19. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, D.; Feng, N.; Zhou, H.; Mu, D.; Zhao, L.; Shen, X.; Rao, G.; Meng, F.; Huang, A. Physiological Mechanisms of ABA-Induced Salinity Tolerance in Leaves and Roots of Rice. Sci. Rep. 2022, 12, 8228. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, H.; Feng, N.; Zheng, D.; Ma, G.; Feng, S.; Liu, M.; Yu, M.; Huang, X.; Huang, A. Physiological and Transcriptome Analysis Reveals That Prohexadione-Calcium Promotes Rice Seedling’s Development under Salt Stress by Regulating Antioxidant Processes and Photosynthesis. PloS ONE 2023, 18, e0286505. [Google Scholar] [CrossRef]
- Mu, D.; Feng, N.; Zheng, D.; Zhou, H.; Liu, L.; Chen, G.; Mu, B. Physiological Mechanism of Exogenous Brassinolide Alleviating Salt Stress Injury in Rice Seedlings. Sci. Rep. 2022, 12, 20439. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring Control: The Evolution of ROS-Induced Oxidative Stress and Redox Signaling Pathways in Plant Stress Responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mao, B.; Yuan, D.; Chu, C.; Duan, M. Salt Tolerance in Rice: Physiological Responses and Molecular Mechanisms. Crop J. 2022, 10, 13–25. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Kuo, W.R.; Yeh, N.C.; Fujimoto, N.; Lin, H.C. Physicochemical Properties and Fertility Index of Culture Media Containing Distillery Residue Biochar and Their Applications to Plug Seedling. J. Fac. Agric. Kyushu Univ. 2019, 64, 127–135. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Plant Cell Membranes; Academic Press: Cambridge, MA, USA, 1987. [Google Scholar]
- Huo, J.; Yu, M.; Feng, N.; Zheng, D.; Zhang, R.; Xue, Y.; Khan, A.; Zhou, H.; Mei, W.; Du, X.; et al. Integrated Transcriptome and Metabolome Analysis of Salinity Tolerance in Response to Foliar Application of Choline Chloride in Rice (Oryza sativa L.). Front. Plant Sci. 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yang, C.; Xu, K.; Zhang, Z.; Dayong, L.; Zhihai, W.; Chen, Z. Development of Yield and Some Photosynthetic Characteristics during 82 Years of Genetic Improvement of Soybean Genotypes in Northeast China. Aust. J. Crop Sci. 2012, 6, 1416–1422. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Klapheck, S.; Zimmer, I.; Cosse, H. Scavenging of Hydrogen Peroxide in the Endosperm of Ricinus Communis by Ascorbate Peroxidase. Plant Cell Physiol. 1990, 31, 1005–1013. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- He, J.; Ren, Y.; Chen, X.; Chen, H. Protective Roles of Nitric Oxide on Seed Germination and Seedling Growth of Rice (Oryza sativa L.) under Cadmium Stress. Ecotoxicol. Environ. Saf. 2014, 108, 114–119. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]



| Parts | Treatment | Panicle Length (cm) | Number of Primary Branches | Number of Secondary Branches | The Number of Filled Grains | 1000-Grain Weight (g) | Seed Setting Percentage (%) | Yield (g·plant−1) |
|---|---|---|---|---|---|---|---|---|
| Main stem | Control | 24.53 ± 0.18 a | 11.00 ± 0.58 a | 8.00 ± 0.58 a | 43.67 ± 0.67 b | 26.57 ± 0.04 b | 54.25 ± 1.56 c | 1.16 ± 0.02 b |
| Pro-Ca | 22.33 ± 0.49 b | 10.00 ± 0.00 a | 5.67 ± 0.33 b | 52.67 ± 1.45 a | 27.67 ± 0.06 a | 81.05 ± 0.98 a | 1.46 ± 0.04 a | |
| S | 20.53 ± 0.44 c | 7.67 ± 0.88 b | 8.33 ± 0.88 a | 30.67 ± 1.20 c | 17.59 ± 0.24 d | 64.01 ± 4.09 b | 0.54 ± 0.02 d | |
| Pro-Ca + S | 16.83 ± 0.33 d | 9.67 ± 0.33 a | 5.67 ± 0.33 b | 40.67 ± 1.76 b | 24.54 ± 0.33 c | 65.24 ± 0.36 b | 1.00 ± 0.06 c | |
| Tiller | Control | 22.87 ± 2.42 b | 10.00 ± 0.00 a | 7.67 ± 0.33 a | 41.33 ± 1.20 b | 25.56 ± 0.50 ab | 54.85 ± 0.34 d | 4.79 ± 0.21 b |
| Pro-Ca | 24.73 ± 0.09 a | 8.33 ± 0.33 b | 6.00 ± 0.00 b | 50.33 ± 0.33 a | 26.71 ± 0.10 a | 69.93 ± 0.76 a | 6.07 ± 0.05 a | |
| S | 19.27 ± 0.64 c | 7.33 ± 0.33 c | 4.33 ± 0.33 c | 25.00 ± 2.00 d | 24.23 ± 0.52 c | 67.58 ± 0.08 b | 2.04 ± 0.32 d | |
| Pro-Ca + S | 19.00 ± 0.06 c | 7.00 ± 0.00 c | 3.00 ± 0.00 d | 31.67 ± 0.33 c | 24.53 ± 0.22 bc | 65.98 ± 0.39 c | 3.11 ± 0.06 c |
| Parts | Treatment | Plant Height (cm) | Root Length (cm) | Stem Diameter (mm) | Leaf Area (mm2) |
|---|---|---|---|---|---|
| Main stem | Control | 97.00 ± 0.23 a | 28.73 ± 0.49 b | 7.13 ± 0.09 a | 8972.97 ± 91.81 b |
| Pro-Ca | 76.40 ± 0.12 b | 31.70 ± 0.23 a | 6.93 ± 0.03 a | 10,462.33 ± 80.74 a | |
| S | 76.70 ± 0.06 b | 28.53 ± 0.18 b | 5.37 ± 0.15 b | 3738.67 ± 28.77 d | |
| Pro-Ca + S | 60.40 ± 0.06 c | 26.70 ± 0.12 c | 5.37 ± 0.09 b | 4400.87 ± 13.91 c | |
| Tiller | Control | 79.91 ± 0.28 a | 25.61 ± 0.06 a | 5.23 ± 0.01 b | 4132.58 ± 64.03 b |
| Pro-Ca | 65.52 ± 0.39 b | 22.59 ± 0.09 b | 5.73 ± 0.01 a | 4928.43 ± 54.58 a | |
| S | 49.44 ± 0.89 c | 18.23 ± 0.69 c | 3.83 ± 0.04 d | 1389.23 ± 36.74 d | |
| Pro-Ca + S | 46.21 ± 0.06 d | 22.58 ± 0.10 b | 4.50 ± 0.03 c | 2453.49 ± 82.88 c |
| Parts | Treatment | Shoot Dry Weight (mg) | Root Dry Weight (mg) | Root Shoot Ratio (%) | Seedling Index (10−2) |
|---|---|---|---|---|---|
| Main stem | Control | 874 ± 1 a | 263 ± 6 a | 40.8 ± 0.2 d | 42.6 ± 1.1 b |
| Pro-Ca | 770 ± 10 b | 273 ± 1 a | 53.4 ± 0.2 c | 46.4 ± 0.1 a | |
| S | 613 ± 11 c | 200 ± 2 c | 58.0 ± 0.3 b | 32.1 ± 0.5 d | |
| Pro-Ca + S | 630 ± 13 c | 210 ± 2 b | 61.3 ± 0.2 a | 35.4 ± 0.4 c | |
| Tiller | Control | 359 ± 5 a | 66 ± 2 a | 27.2 ± 1.0 ab | 10.6 ± 0.4 a |
| Pro-Ca | 276 ± 5 b | 51 ± 0 b | 24.8 ± 0.3 b | 8.9 ± 0.0 b | |
| S | 238 ± 2 d | 38 ± 3 c | 17.5 ± 0.2 c | 6.5 ± 0.5 d | |
| Pro-Ca + S | 253 ± 2 c | 42 ± 1 c | 29.3 ± 1.3 a | 7.8 ± 0.2 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, W.; Yang, S.; Xiong, J.; Khan, A.; Zhao, L.; Du, X.; Huo, J.; Zhou, H.; Sun, Z.; Yang, X.; et al. Prohexadione-Calcium Reduced Stem and Tiller Damage and Maintained Yield by Improving the Photosynthetic and Antioxidant Capacity of Rice (Oryza sativa L.) Under NaCl Stress. Plants 2025, 14, 188. https://doi.org/10.3390/plants14020188
Mei W, Yang S, Xiong J, Khan A, Zhao L, Du X, Huo J, Zhou H, Sun Z, Yang X, et al. Prohexadione-Calcium Reduced Stem and Tiller Damage and Maintained Yield by Improving the Photosynthetic and Antioxidant Capacity of Rice (Oryza sativa L.) Under NaCl Stress. Plants. 2025; 14(2):188. https://doi.org/10.3390/plants14020188
Chicago/Turabian StyleMei, Wanqi, Shaoxia Yang, Jian Xiong, Aaqil Khan, Liming Zhao, Xiaole Du, Jingxin Huo, Hang Zhou, Zhiyuan Sun, Xiaohui Yang, and et al. 2025. "Prohexadione-Calcium Reduced Stem and Tiller Damage and Maintained Yield by Improving the Photosynthetic and Antioxidant Capacity of Rice (Oryza sativa L.) Under NaCl Stress" Plants 14, no. 2: 188. https://doi.org/10.3390/plants14020188
APA StyleMei, W., Yang, S., Xiong, J., Khan, A., Zhao, L., Du, X., Huo, J., Zhou, H., Sun, Z., Yang, X., Yue, N., Feng, N., & Zheng, D. (2025). Prohexadione-Calcium Reduced Stem and Tiller Damage and Maintained Yield by Improving the Photosynthetic and Antioxidant Capacity of Rice (Oryza sativa L.) Under NaCl Stress. Plants, 14(2), 188. https://doi.org/10.3390/plants14020188






