Lomatium Species of the Intermountain Western United States: A Chemotaxonomic Investigation Based on Essential Oil Compositions
Abstract
1. Introduction
2. Results and Discussion
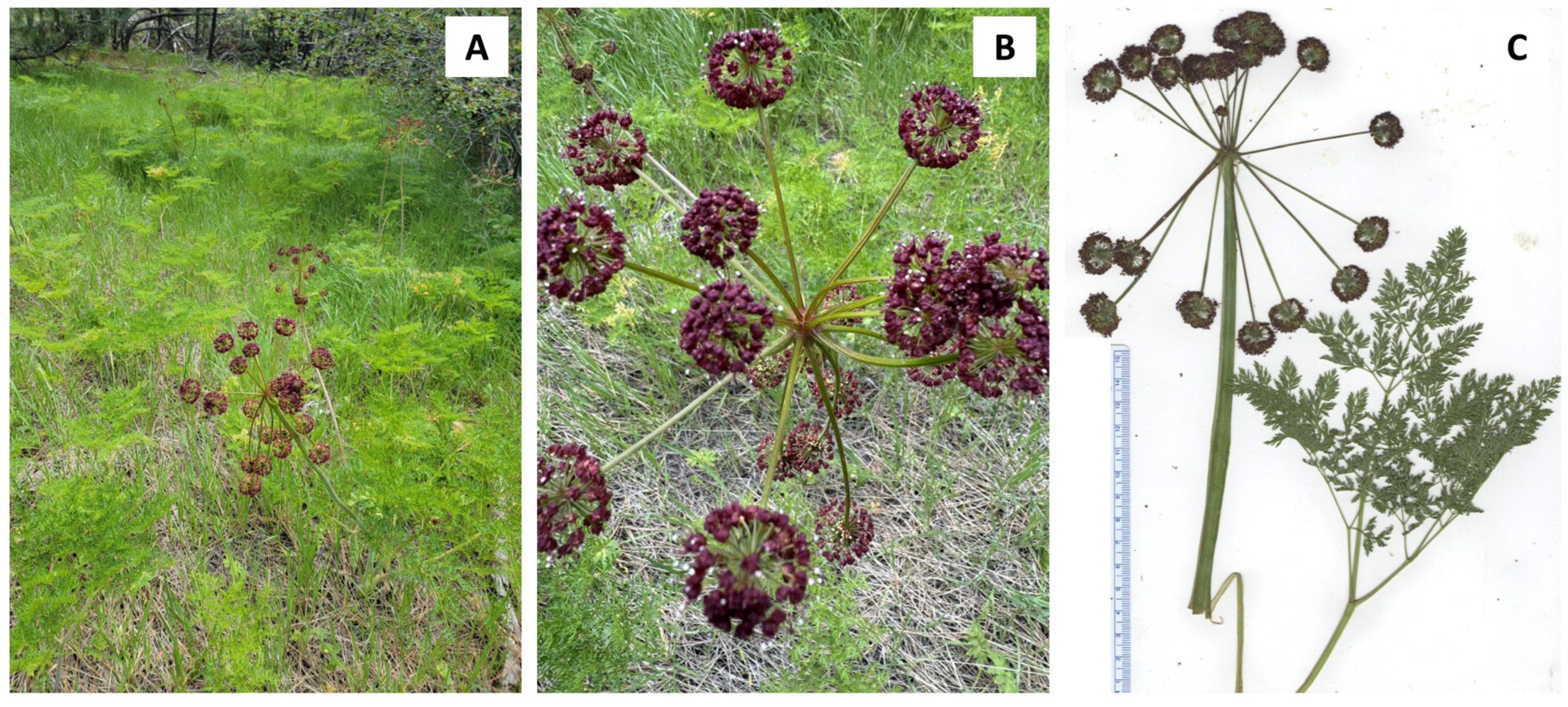
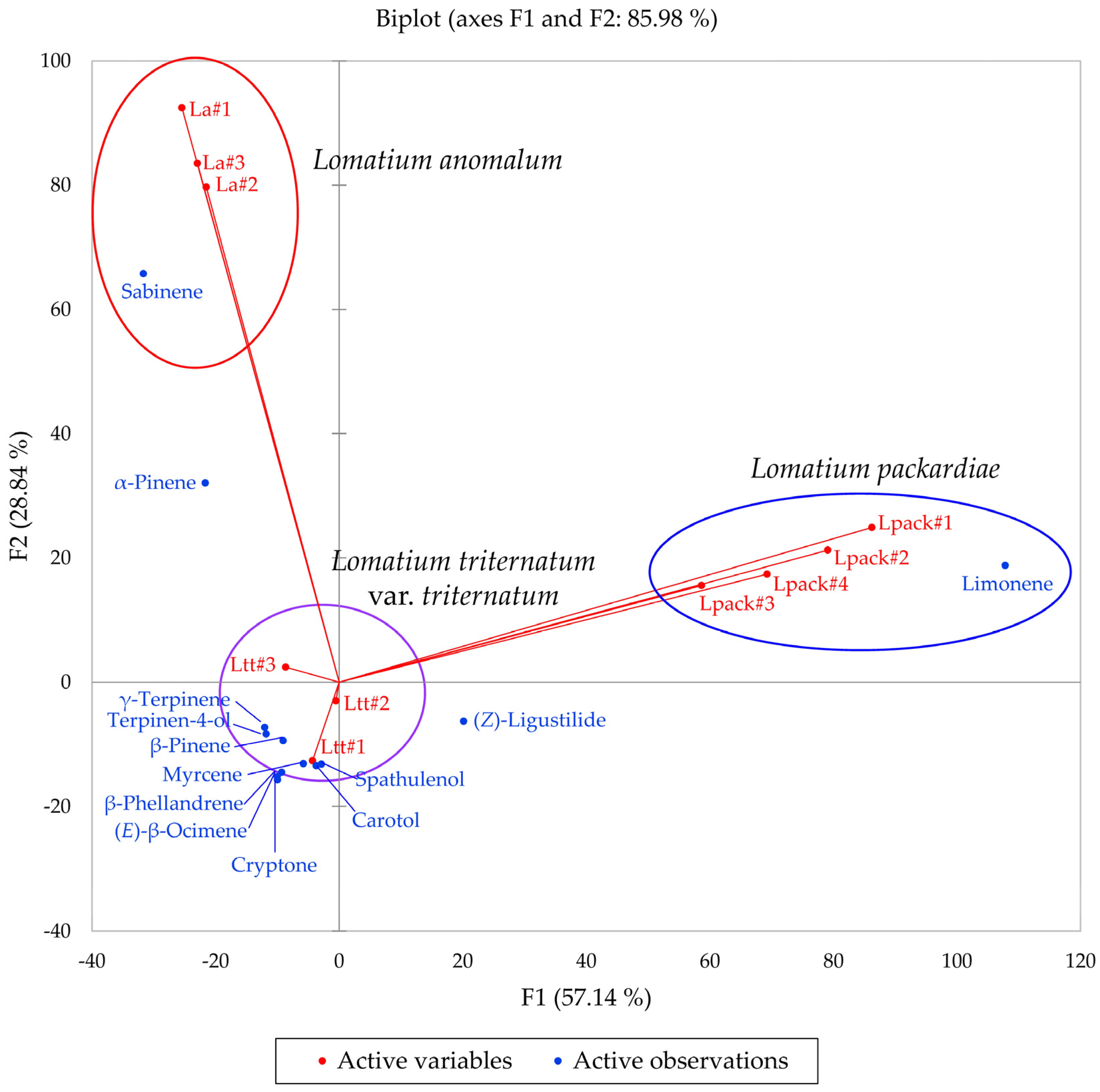
2.1. Lomatium anomalum (L. triternatum Complex)
2.2. Lomatium packardiae (L. triternatum Complex)
2.3. Lomatium triternatum var. triternatum (L. triternatum Complex)
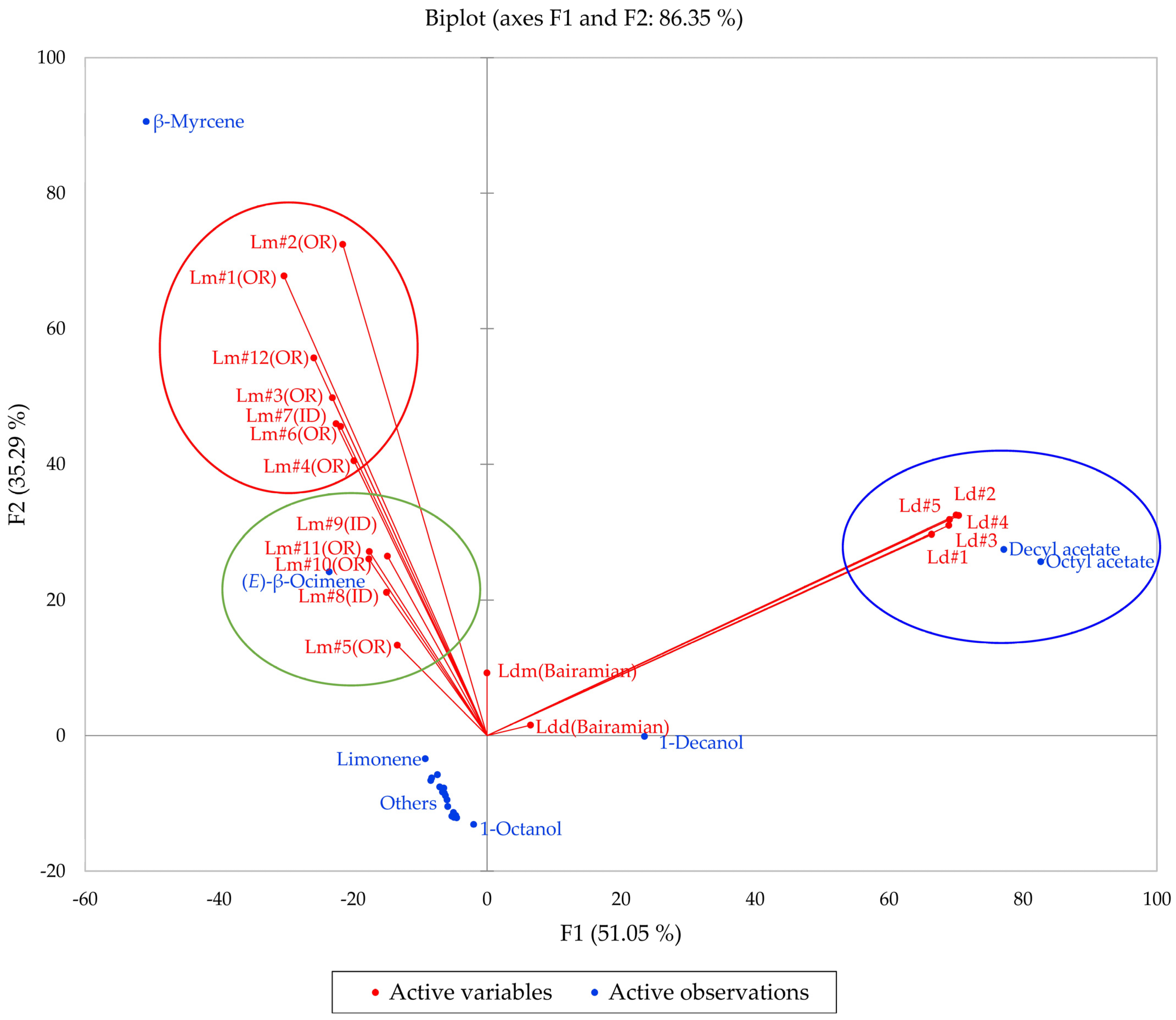
2.4. Lomatium dissectum (Lomatium dissectum Complex)
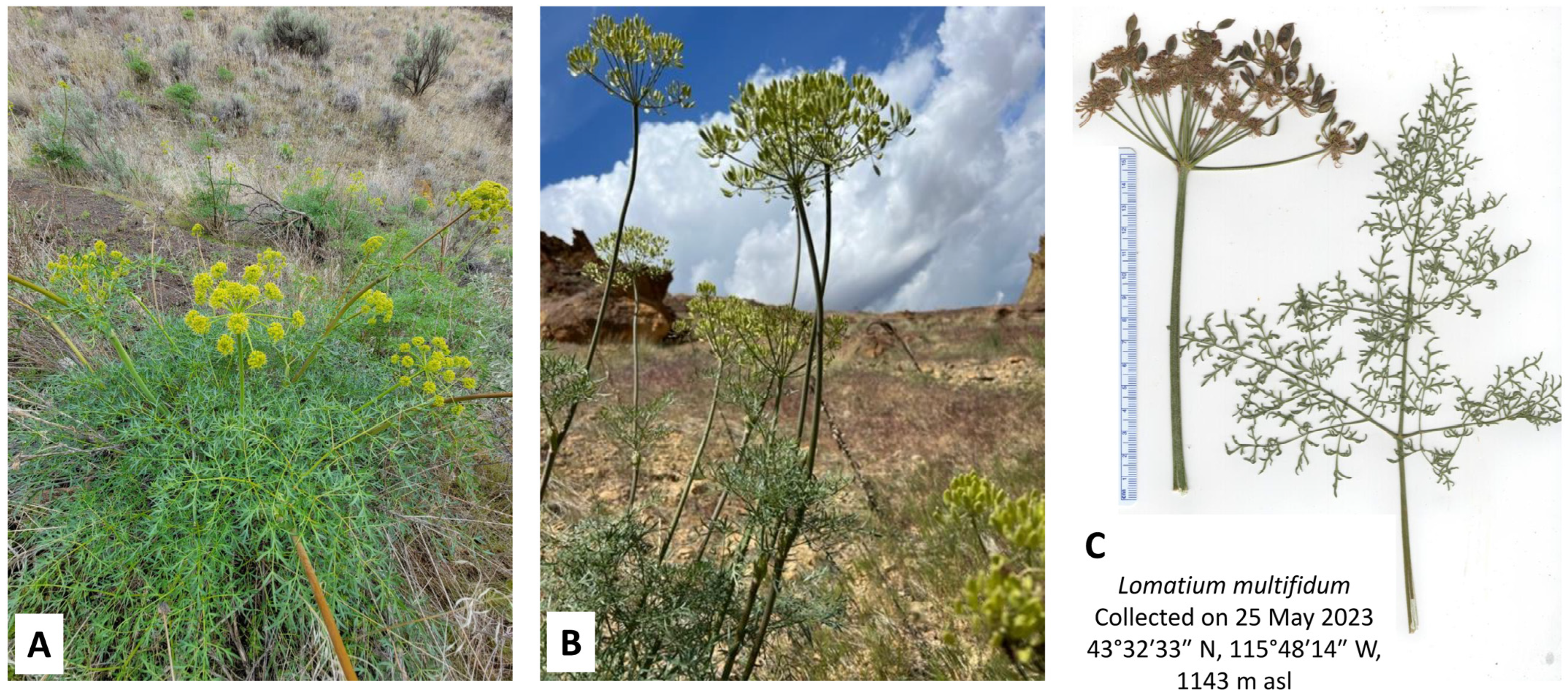
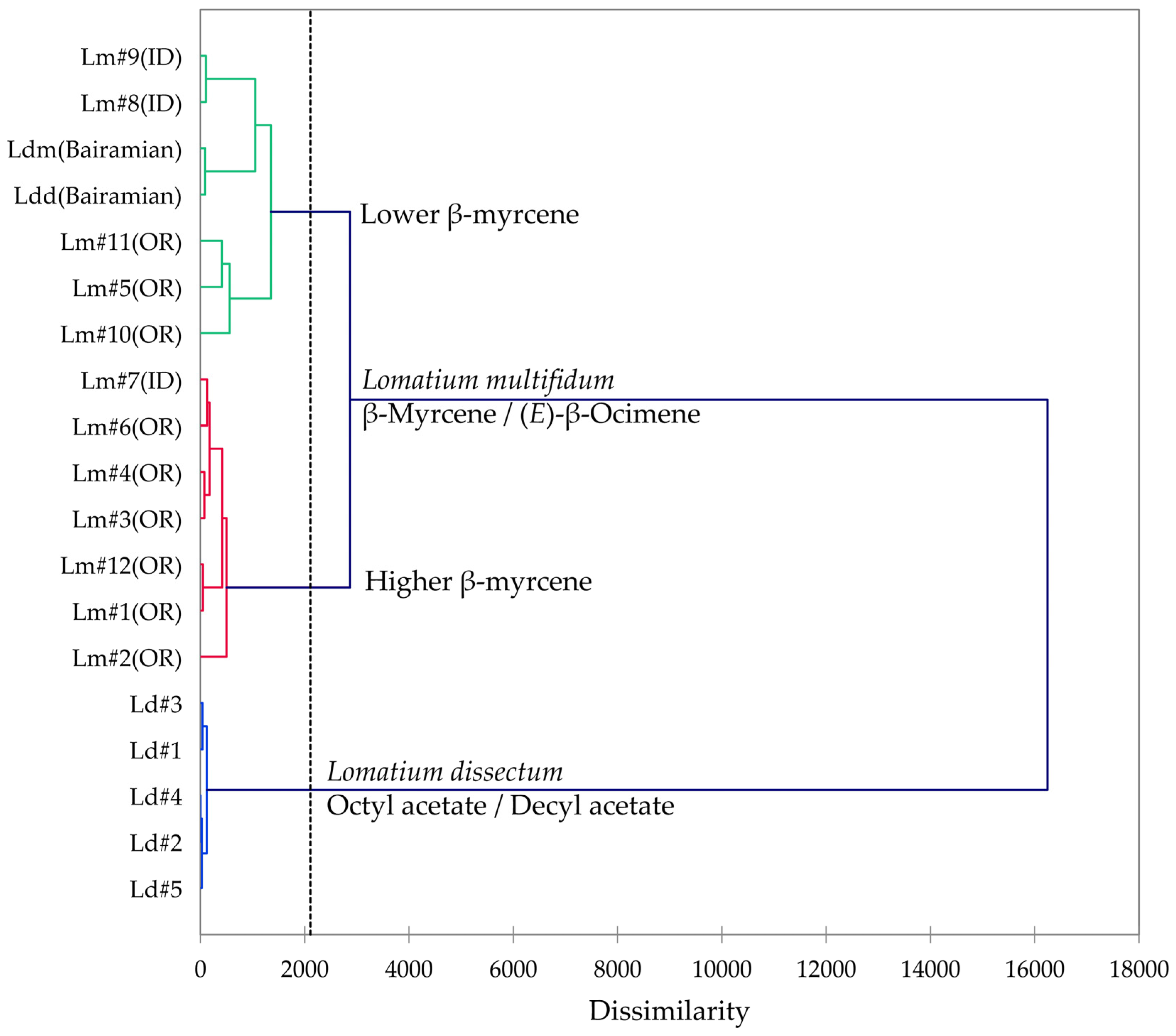
2.5. Lomatium multifidum (Lomatium dissectum Complex)
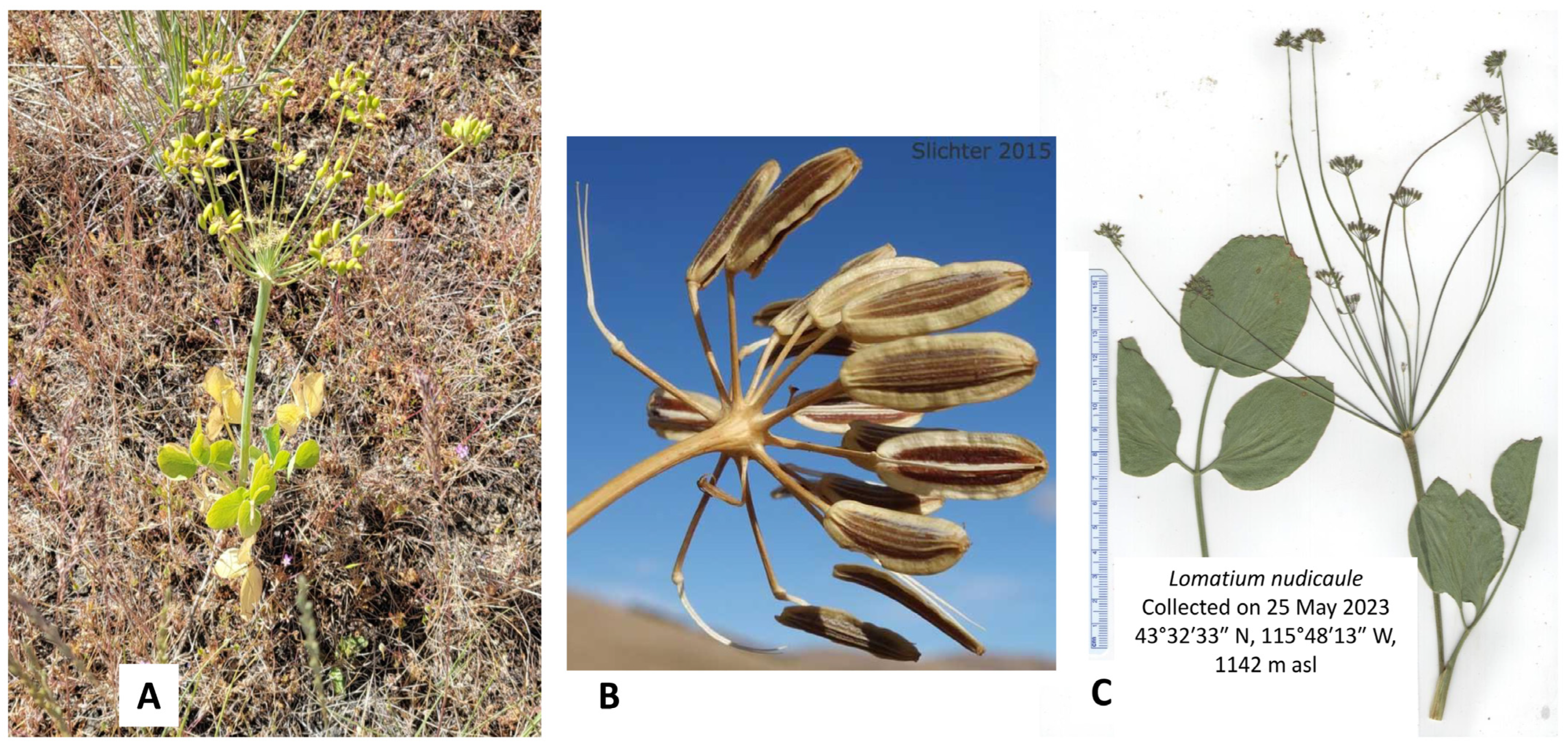
2.6. Lomatium nudicaule
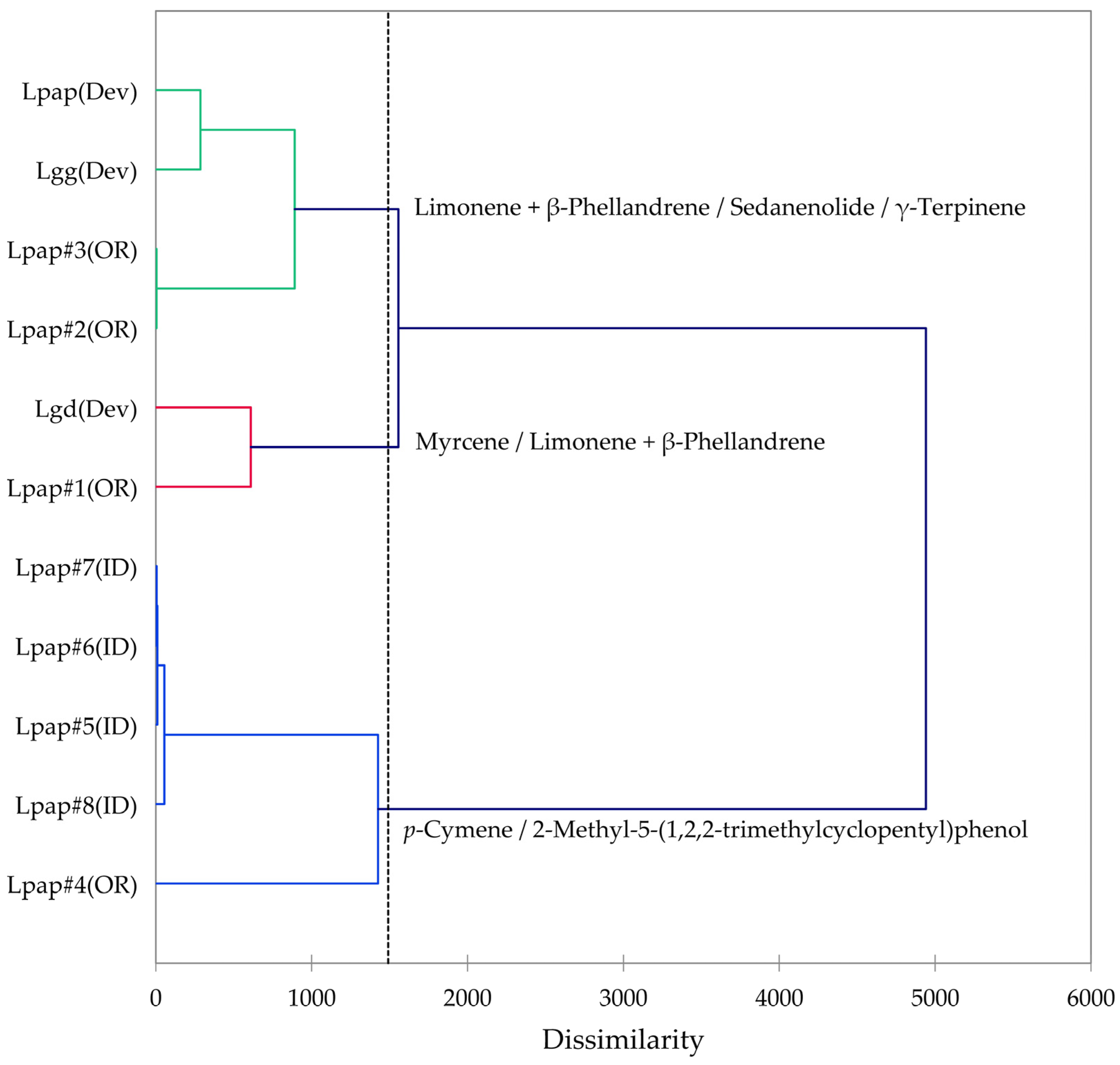
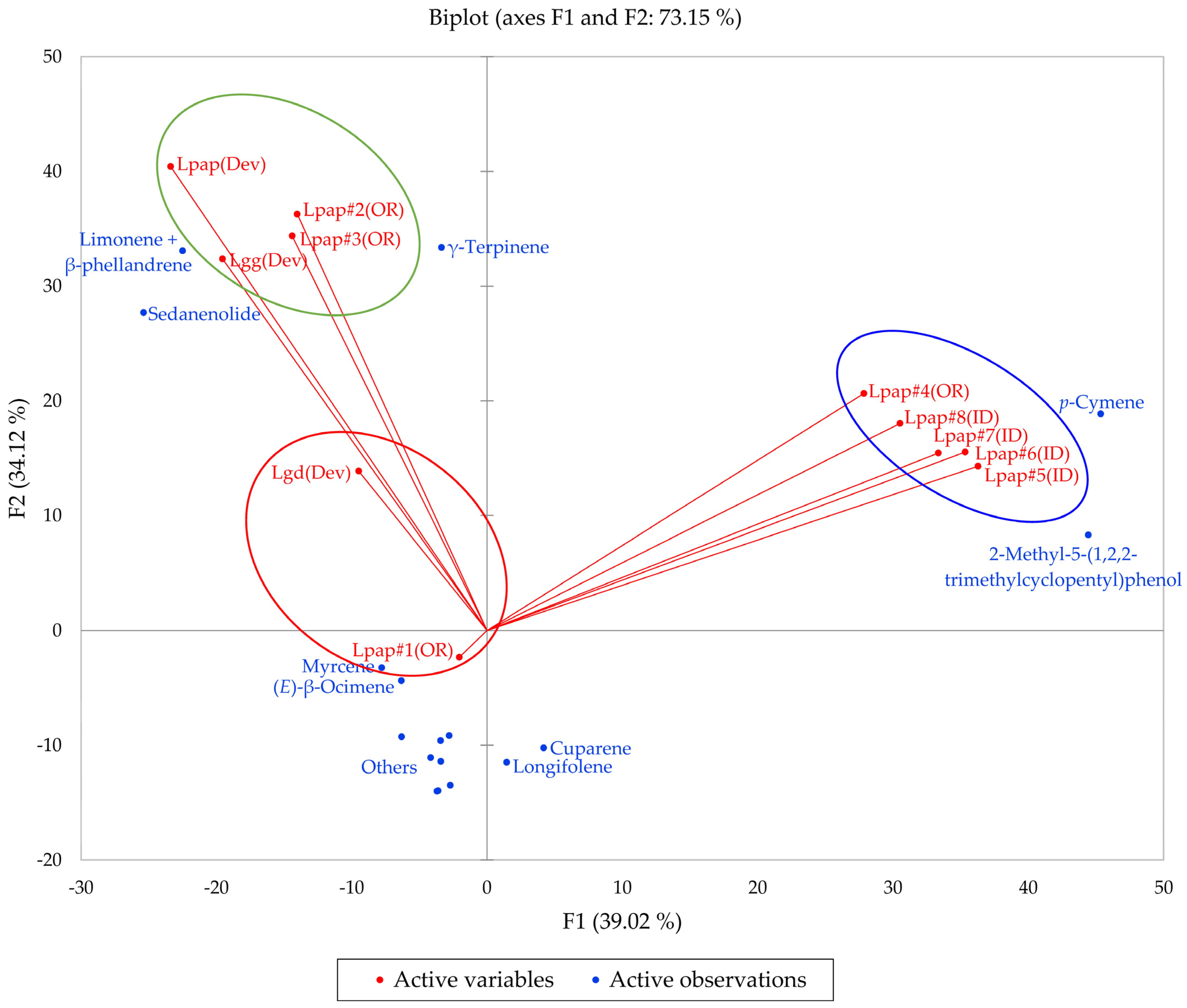
2.7. Lomatium papilioniferum (Lomatium grayi Complex)
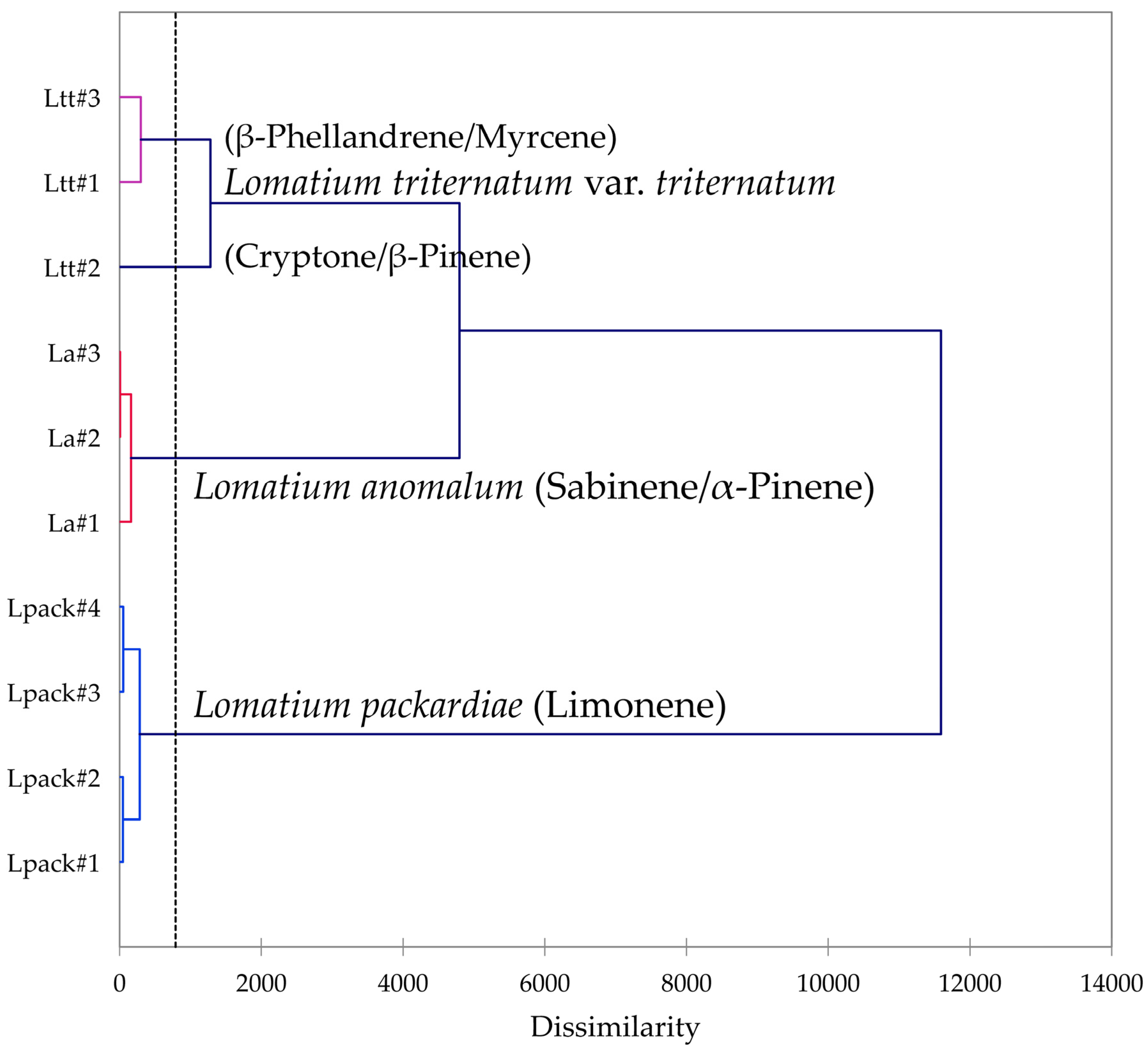
2.8. Analysis of Variance
2.9. Enantiomeric Distributions
3. Materials and Methods
3.1. Plant Collection and Identification
3.2. Hydrodistillation
3.3. Gas Chromatographic Analysis
3.4. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Flora Online. Lomatium Raf. Available online: https://www.worldfloraonline.org/taxon/wfo-4000022127 (accessed on 26 May 2024).
- Stevens, M.; Mansfield, D.H.; Smith, J.F.; Feist, M.A.E. Resolving the anomaly of Lomatium anomalum: Discovery of a new species in southwestern Idaho (U.S.A.), Lomatium andrusianum (Apiaceae). J. Bot. Res. Inst. Tex. 2018, 12, 1–15. [Google Scholar] [CrossRef]
- Ottenlips, M.V.; Mansfield, D.H.; Buerki, S.; Feist, M.A.E.; Downie, S.R.; Dodsworth, S.; Forest, F.; Plunkett, G.M.; Smith, J.F. Resolving species boundaries in a recent radiation with the Angiosperms353 probe set: The Lomatium packardiae/L. anomalum clade of the L. triternatum (Apiaceae) complex. Am. J. Bot. 2021, 108, 1217–1233. [Google Scholar] [CrossRef] [PubMed]
- Moerman, D.E. Native American Ethnobotany; Timber Press, Inc.: Portland, OR, USA, 1998; ISBN 978-0-88192-453-4. [Google Scholar]
- Turner, M.; Gustafson, P. Wildflowers of the Pacific Northwest; Timber Press, Inc.: Portland, OR, USA, 2006; ISBN 978-0-88192-745-0. [Google Scholar]
- Kartesz, J.T. The Biota of North America Program (BONAP). Available online: https://bonap.net/MapGallery/County/Lomatium%20triternatum.png (accessed on 9 June 2024).
- Tilley, D.; St. John, L.; Ogle, D.; Shaw, N. Plant Guide for Nineleaf Biscuitroot (Lomatium triternatum); United States Department of Agriculture, Natural Resources Conservation Service, Idaho Plant Materials Center: Aberdeen, ID, USA, 2010. [Google Scholar]
- Gucker, C.L.; Shaw, N.L. Nineleaf Biscuitroot: Lomatium triternatum (Pursh) J.M. Coult. & Rose. In Western Forbs: Biology, Ecology, and Use in Restoration; Gucker, C.L., Shaw, N.L., Eds.; Great Basin Fire Science Exchange: Reno, NV, USA, 2020; p. 21. [Google Scholar]
- Lesica, P.; Kittelson, P.M. Morphological and ecological segregation of two sympatric Lomatium triternatum (Apiaceae) varieties in Montana. Madroño 2023, 60, 211–216. [Google Scholar] [CrossRef]
- Smith, J.F.; Mansfield, D.H.; Stevens, M.; Sosa, E.; Feist, M.A.E.; Downie, S.R.; Plunkett, G.M.; Darrach, M. Try Tri again? Resolving species boundaries in the Lomatium triternatum (Apiaceae) complex. J. Syst. Evol. 2018, 56, 218–230. [Google Scholar] [CrossRef]
- Tilley, D.; St. John, L.; Ogle, D.; Shaw, N. Plant Guide for Gray’s Biscuitroot (Lomatium grayi); United States Department of Agriculture, Natural Resources Conservation Service, Idaho Plant Materials Center: Aberdeen, ID, USA, 2011. [Google Scholar]
- Kartesz, J.T. The Biota of North America Program (BONAP). Available online: https://bonap.net/MapGallery/County/Lomatium%20grayi.png (accessed on 2 June 2024).
- Alexander, J.A.; Whaley, W.; Blain, N. The Lomatium grayi complex (Apiaceae) of the western United States: A taxonomic revision based on morphometic, essential oil composition, and larva-host coevolution studies. J. Bot. Res. Inst. Tex. 2018, 12, 387–444. [Google Scholar] [CrossRef]
- Feist, M.A.E.; Smith, J.F.; Mansfield, D.H.; Darrach, M.; McNeill, R.P.; Downie, S.R.; Plunkett, G.M.; Wilson, B.L. New combinations in Lomatium (Apiaceae, subfamily Apioideae). Phytotaxa 2017, 316, 95–98. [Google Scholar] [CrossRef]
- Constance, L.; Wetherwax, M. Lomatium dissectum. Available online: https://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=31405 (accessed on 1 June 2024).
- Intermountain Region Herbarium Network. Lomatium multifidum. Available online: https://www.intermountainbiota.org/portal/collections/list.php (accessed on 29 May 2024).
- World Flora Online. Lomatium multifidum (Nutt.) R.P. McNeill & Darrach. Available online: http://www.worldfloraonline.org/taxon/wfo-0001423732 (accessed on 29 May 2024).
- Kartesz, J.T. The Biota of North America Program (BONAP). Available online: https://bonap.net/MapGallery/County/Lomatium%20nudicaule.png (accessed on 6 June 2024).
- Schlichter, P. Lomatium nudicaule. Available online: http://science.halleyhosting.com/nature/gorge/5petal/pars/lomatium/nudicaule/nudicaule10-18-2015.jpg (accessed on 6 June 2024).
- Beauchamp, P.S.; Chea, E.; Dimaano, J.G.; Dev, V.; Ly, B.; Miranda, A.E.; Whaley, W.H. Essential oil composition of six Lomatium species attractive to Indra swallowtail butterfly (Papilio indra): Principal component analysis against essential oil composition of Lomatium dissectum var. multifidum. J. Essent. Oil Res. 2009, 21, 535–542. [Google Scholar] [CrossRef]
- Asuming, W.A.; Beauchamp, P.S.; Descalzo, J.T.; Dev, B.C.; Dev, V.; Frost, S.; Ma, C.W. Essential oil composition of four Lomatium Raf. species and their chemotaxonomy. Biochem. Syst. Ecol. 2005, 33, 17–26. [Google Scholar] [CrossRef]
- Bairamian, S.; Beauchamp, P.S.; Descalzo, J.T.; Dev, B.C.; Dev, V.; Frost, S.C.; Nguyen, C.V. California Lomatiums part III. Composition of the hydrodistilled oils from two varieties of Lomatium dissectum. Isolation of a new hydrocarbon. J. Essent. Oil Res. 2004, 16, 461–468. [Google Scholar] [CrossRef]
- Beauchamp, P.S.; Dev, B.C.; Dev, V. California Lomatiums, part VI. Composition of the essential oils of Lomatium foeniculaceum ssp. fimbriatu. J. Essent. Oil Res. 2006, 18, 666–669. [Google Scholar] [CrossRef]
- Dev, V.; Ly, B.; Miranda, A.E.; Whaley, W. Lomatium grayi and Indra swallowtail butterfly. Composition of the essential oils of three varieties of Lomatium grayi (J. M. Coult et Rose) J. M. Coult et Rose. J. Essent. Oil Res. 2007, 19, 244–248. [Google Scholar] [CrossRef]
- Beauchamp, P.S.; Dev, V.; Tran, H.D.; Whaley, W.H. California Lomatiums, part VIII. Analysis of essential oils of Lomatium marginatum (Benth.) Coult. & Rose var. purpureum Jepson. isolation of (Z)-β-lomatene, a new sesquiterpene hydrocarbon. J. Essent. Oil Res. 2011, 23, 112–118. [Google Scholar] [CrossRef]
- Beauchamp, P.S.; Dev, V.; Kittisanthanon, K.; Ly, B. California Lomatiums, part X. Comparison of composition of the hydrodistilled oils from two subspecies of Lomatium mohavense. Nat. Prod. Res. 2011, 25, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, P.S.; Dev, B.C.; Dev, V.; Midland, S.L.; Sims, J.J. California Lomatiums, part VII. Analysis of the essential oils of Lomatium nevadense (Watson) J. Coulter et Rose var. parishii (J. Coulter et Rose) Jepson. Isolation of trans-dauc-8-en-11-ol, a new sesquiterpene alcohol and naturally occurring 2′,3′,3′-trimethyl-2′,3′-dihydroangelicin. J. Essent. Oil Res. 2007, 19, 117–124. [Google Scholar] [CrossRef]
- Beauchamp, P.S.; Descalzo, J.T.; Dev, B.C.; Dev, V.; Nguyen, C.V.; Midland, S.L.; Sims, J.J.; Tham, F.S. California Lomatiums, part IV: Composition of the essential oils of Lomatium rigidum (M.E. Jones) Jepson. Structures of two new funebrene epimers and a tridecatriene. J. Essent. Oil Res. 2004, 16, 571–578. [Google Scholar] [CrossRef]
- Dev, V.; Whaley, W.H.; Bailey, S.R.; Chea, E.; Dimaano, J.G.; Jogani, D.K.; Ly, B.; Eggett, D. Essential oil composition of nine Apiaceae species from western United States that attract the female Indra Swallowtail butterfly (Papilio indra). Biochem. Syst. Ecol. 2010, 38, 538–547. [Google Scholar] [CrossRef]
- Bedrossian, A.; Beauchamp, P.E.; Dev, V.; Kwan, S.; Munevar-Mendoza, E.; Okoreeh, E.K.; Moore, P.E. Composition of the essential oil of Lomatium torreyi. J. Essent. Oil Res. 1998, 10, 473–477. [Google Scholar] [CrossRef]
- New York Botanical Garden. Starr Virtual Herbarium. Available online: https://sweetgum.nybg.org/science/vh/specimen-list/?SummaryData=Lomatium+anomalum (accessed on 9 June 2024).
- New York Botanical Garden. Starr Virtual Herbarium. Available online: https://sweetgum.nybg.org/science/vh/specimen-list/?SummaryData=Lomatium+packardiae (accessed on 9 June 2024).
- New York Botanical Garden. Starr Virtual Herbarium. Available online: https://sweetgum.nybg.org/science/vh/specimen-list/?SummaryData=Lomatium+triternatum (accessed on 20 May 2024).
- Intermountain Region Herbarium Network. Lomatium triternatum. Available online: https://www.intermountainbiota.org/portal/collections/list.php (accessed on 20 May 2024).
- New York Botanical Garden. Starr Virtual Herbarium. Available online: https://sweetgum.nybg.org/science/vh/specimen-list/?SummaryData=Lomatium+papilioniferum (accessed on 2 June 2024).
- New York Botanical Garden. Starr Virtual Herbarium. Available online: https://sweetgum.nybg.org/science/vh/specimen-list/?SummaryData=Lomatium+dissectum (accessed on 1 June 2024).
- New York Botanical Garden. Starr Virtual Herbarium. Available online: https://sweetgum.nybg.org/science/vh/specimen-list/?SummaryData=Lomatium+multifidum (accessed on 1 June 2024).
- Mathias, M.E. A revision of the genus Lomatium. Ann. Mo. Bot. Gard. 1938, 25, 225–297. [Google Scholar] [CrossRef]
- Gucker, C.L.; Shaw, N.L. Barestem biscuitroot: Lomatium nudicaule (Pursh) J.M. Coult. & Rose. In Western Forbs: Biology, Ecology, and Use in Restoration; Gucker, C.L., Shaw, N.L., Eds.; Great Basin Fire Science Exchange: Reno, NV, USA, 2021; p. 17. [Google Scholar]
- Tilley, D.; St. John, L. Plant Guide for Barestem Biscuitroot (Lomatium nudicaule); United States Department of Agriculture, Natural Resources Conservation Service, Idaho Plant Materials Center: Aberdeen, ID, USA, 2012. [Google Scholar]
- New York Botanical Garden. Starr Virtual Herbarium. Available online: https://sweetgum.nybg.org/science/vh/specimen-list/?SummaryData=Lomatium+nudicaule (accessed on 8 June 2024).
- Likens, S.T.; Nickerson, G.B. Detection of certain hop oil constituents in brewing products. Proc. Annu. Meet. Am. Soc. Brew. Chem. 1964, 22, 5–13. [Google Scholar] [CrossRef]
- Au-Yeung, C.Y.; MacLeod, A.J. A comparison of the efficiency of the Likens and Nickerson extractor for aqueous, lipid/aqueous, and lipid samples. J. Agric. Food Chem. 1981, 29, 502–505. [Google Scholar] [CrossRef]
- Bouseta, A.; Collin, S. Optimized Likens-Nickerson methodology for quantifying honey flavors. J. Agric. Food Chem. 1995, 43, 1890–1897. [Google Scholar] [CrossRef]
- Poudel, A.; Dosoky, N.S.; Satyal, P.; Swor, K.; Setzer, W.N. Essential oil composition of Grindelia squarrosa from southern Idaho. Molecules 2023, 28, 3854. [Google Scholar] [CrossRef] [PubMed]
- van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- Mondello, L. FFNSC 3; Shimadzu Scientific Instruments: Columbia, MD, USA, 2016. [Google Scholar]
- NIST20; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2020.
- Satyal, P. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils. Ph.D. Dissertation, University of Alabama in Huntsville, Huntsville, AL, USA, 2015. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1974; ISBN 0-13-084542-6. [Google Scholar]




| Lomatium Species | Collection Site | Plant Tissue | Major Components (>5%) | Ref. |
|---|---|---|---|---|
| Lomatium brandegeei J.F. Macbr. | Slate Peak, Washington | Aerial parts | α-pinene (9.2%), β-phellandrene (60.9%) | [20] |
| Lomatium dasycarpum (Torr. & A. Gray) J.M. Coult. & Rose | Trinity National Forest, California | Leaves and stems | 3-methyl-2-buten-1-yl 3-methylbutyrate (22.3%), lavandulyl 2-methylbutyrate (16.9%), senkyunolide (9.8%) | [21] |
| Lomatium dissectum (Nutt.) Mathias & Constance var. dissectum | Six Rivers National Forest, California | Aerial parts | 1-octanol (9.0%), octyl acetate (5.3%), palmitic acid (15.3%) | [22] |
| Lomatium dissectum var. multifidum (Nutt.) Mathias & Constance (syn. Lomatium multifidum (Nutt.) R.P. McNeill & Darrach) | San Bernardino National Forest, California | Aerial parts | (3Z)-hexenol (18.5%), myrcene (6.0%), palmitic acid (8.6%) | [22] |
| Lomatium eastwoodiae (J.M. Coult. & Rose) J.F. Macbr. | Black Ridge, Colorado | Aerial parts | α-pinene (6.2%), myrcene (5.1%), limonene + β-phellandrene (12.9%), (E)-β-caryophyllene (12.2%), germacrene D 95.2%) | [20] |
| Lomatium foeniculaceum subsp. fimbriatum W.L. Theob. | Inyo National Forest, California | Leaves and stems | (3Z)-hexenol (6.5%), limonene + β-phellandrene (6.8%), terpinolene (6.7%), germacrene D (15.9%), (Z)-ligustilide (13.1%) | [23] |
| Lomatium graveolens (S. Watson) J.M. Coult. & Rose | Provo Peak, Utah | Aerial parts | β-pinene (21.6%), limonene + β-phellandrene (33.2%), osthole (5.2%) | [20] |
| Lomatium grayi “new variety” (based on the reported collection site, this is probably Lomatium papilioniferum J.A. Alexander & Whaley) [13] | Elko County, Nevada | Aerial parts | limonene + β-phellandrene (17.7%), γ-terpinene (16.1%), senkyunolide (44.0%) | [24] |
| Lomatium grayi (J.M. Coult & Rose) J.M. Coult. & Rose var. grayi | Utah County, Utah | Aerial parts | myrcene (8.4%), limonene + β-phellandrene (27.2%), γ-terpinene (10.4%), senkyunolide (24.4%) | [24] |
| Lomatium grayi var. depauperatum (M.E. Jones) Mathias | Juab County, Utah | Aerial parts | myrcene (8.1%), limonene + β-phellandrene (20.8%), (Z)-β-ocimene (18.9%), (Z)-ligustilide (6.7%) | [24] |
| Lomatium howelii (S. Watson) Jeps. | Eight Dollar Mountain, Oregon | Aerial parts | (E)-β-ocimene (5.8%), octyl acetate (24.8%), citronellyl acetate (7.1%), decyl acetate (6.7%), lauryl acetate (5.1%) | [20] |
| Lomatium howelii (S. Watson) Jeps. | Low Divide, California | Aerial parts | 1-octanol (11.4%), octyl acetate (23.5%), citronellyl acetate (6.0%), germacrene D (6.0%) | [20] |
| Lomatium junceum Barneby & N.H. Holmgren | Emery County, Utah | Aerial parts | α-pinene (24.3%), β-pinene (29.3%), limonene + β-phellandrene (11.3%) | [20] |
| Lomatium lucidum Jeps. | San Bernardino National Forest, California | Leaves and stems | limonene + β-phellandrene (11.5%), decanal (15.7%), bornyl/isobornyl acetate (6.1%), dodecanal (9.4%), α-humulene | [21] |
| Lomatium macrocarpum J.M. Coult. & Rose | Six Rivers National Forest, California | Leaves and stems | (3Z)-hexenol (9.2%), (E)-β-caryophyllene (12.6%), palmitic acid (9.0%), linoleic acid (5.2%) | [21] |
| Lomatium marginatum var. purpureum (Jeps.) Jeps. | Lake County, California | Leaves and stems | (3Z)-hexenol (10.3%), (Z)-β-lomatene (12.9%), (E)-β-caryophyllene (9.3%) | [25] |
| Lomatium mohavense (J.M. Coult. & Rose) J.M. Coult. & Rose subsp. mohavense | Grant, California | Leaves and stems | limonene + β-phellandrene (6.0%), trans-β-elemene (17.8%), (E)-β-caryophyllene (7.8%), germacrene D (10.8%), bicyclogermacrene (6.2%) | [26] |
| Lomatium mohavense subsp. longilobum W.L. Theob. | Acton, California | Leaves and stems | (3Z)-hexenol (7.5%), limonene + β-phellandrene (6.5%), β-sinensal (6.8%), iso-α-sinensal (19.3%), α-sinensal (5.4%), iso-α-sinensyl acetate (5.7%) | [26] |
| Lomatium nevadense (S. Watson) J.M. Coult. & Rose var. parishii (J.M. Coult. & Rose) Jeps. | Bishop, California | Leaves and stems | (E)-β-ocimene (5.1%), (E)-β-caryophyllene (10.3%), germacrene D (10.7%), bicyclogermacrene (7.0%), | [27] |
| Lomatium parryi (S. Watson) J.F. Macbr. | Pine Valley Mountains, Utah | Aerial parts | limonene + β-phellandrene (12.8%), bornyl acetate (18.6%) | [20] |
| Lomatium rigidum (M.E. Jones) Jeps. | Eastern Sierra Nevada Mountains, California | Leaves and stems | limonene + β-phellandrene (9.1%), δ-cadinene (12.4%), τ-cadinol + τ-muurolol (9.0%), α-cadinol (16.4%), (Z)-falcarinol (10.8%) | [28] |
| Lomatium rigidum (M.E. Jones) Jeps. | Bishop Canyon, California | Aerial parts | α-pinene (6.9%), limonene + β-phellandrene (28.6%), cryptone (5.6%), osthole (10.9%) | [29] |
| Lomatium scabrum (J.M. Coult. & Rose) Mathias var. tripinnatum Goodrich | St. George, Utah | Aerial parts | myrcene (8.2%), limonene + β-phellandrene (26.0%), (Z)-β-ocimene (11.8%) | [29] |
| Lomatium torreyi (J.M. Coult. & Rose) J.M. Coult. & Rose | Yosemite National Park, California | Aerial parts | β-phellandrene (12.9%), (Z)-β-ocimene (14.2%), (Z)-ligustilide (42.4%) | [30] |
| Lomatium utriculatum (Nutt. ex Torr. & A. Gray) J.M. Coult. & Rose | Six Rivers National Forest, California | Leaves and stems | (Z)-ligustilide (19.6%), palmitic acid (15.3%) | [21] |
| RIcalc | RIdb | Compound | La#1 | La#2 | La#3 |
|---|---|---|---|---|---|
| 800 | 801 | Hexanal | tr | tr | tr |
| 832 | 831 | Furfural | - | - | tr |
| 850 | 849 | (2E)-Hexenal | 0.1 | - | tr |
| 853 | 853 | (3Z)-Hexenol | tr | - | tr |
| 903 | 906 | Heptanal | - | tr | tr |
| 926 | 927 | α-Thujene | 0.3 | 0.8 | 0.7 |
| 934 | 933 | α-Pinene | 37.6 | 21.9 | 23.6 |
| 950 | 950 | Camphene | 0.1 | 0.1 | 0.1 |
| 975 | 972 | Sabinene | 48.2 | 48.0 | 49.9 |
| 980 | 978 | β-Pinene | 2.2 | 3.2 | 3.7 |
| 990 | 989 | Myrcene | 0.3 | 0.9 | 1.4 |
| 992 | 990 | Dehydro-1,8-cineole | tr | tr | tr |
| 1005 | 1004 | p-Mentha-1(7),8-diene | tr | tr | tr |
| 1007 | 1007 | α-Phellandrene | tr | tr | tr |
| 1010 | 1009 | δ-3-Carene | - | tr | tr |
| 1017 | 1018 | α-Terpinene | 0.5 | 1.5 | 1.3 |
| 1025 | 1025 | p-Cymene | 0.3 | 0.8 | 0.4 |
| 1030 | 1030 | Limonene | 0.7 | 1.7 | 1.2 |
| 1031 | 1031 | β-Phellandrene | 0.9 | 1.8 | 2.0 |
| 1032 | 1032 | 1,8-Cineole | - | - | tr |
| 1034 | 1034 | (Z)-β-Ocimene | tr | tr | tr |
| 1043 | 1045 | Phenylacetaldehyde | - | tr | tr |
| 1045 | 1045 | (E)-β-Ocimene | 0.1 | 0.6 | 0.7 |
| 1058 | 1058 | γ-Terpinene | 2.7 | 7.0 | 4.7 |
| 1070 | 1069 | cis-Sabinene hydrate | 0.4 | 0.7 | 0.7 |
| 1072 | 1071 | Dehydromyrcenol | - | - | tr |
| 1086 | 1086 | Terpinolene | 0.5 | 1.3 | 0.9 |
| 1091 | 1093 | p-Cymenene | - | tr | tr |
| 1097 | 1099 | 6-Camphenone | tr | tr | tr |
| 1100 | 1101 | Linalool | 0.1 | tr | 0.1 |
| 1101 | 1101 | trans-Sabinene hydrate | 0.4 | 0.6 | 0.6 |
| 1105 | 1107 | Nonanal | - | - | tr |
| 1107 | 1107 | 1-Octen-3-yl acetate | - | - | tr |
| 1113 | 1113 | p-Mentha-1,3,8-triene | - | 0.1 | tr |
| 1114 | 1112 | (E)-2,4-Dimethylhepta-2,4-dienal | - | - | tr |
| 1121 | 1122 | trans-p-Mentha-2,8-dien-1-ol | - | - | tr |
| 1125 | 1124 | cis-p-Menth-2-en-1-ol | 0.2 | 0.3 | 0.3 |
| 1135 | 1135 | 2-Vinylanisole | - | 0.1 | tr |
| 1143 | 1142 | trans-p-Menth-2-en-1-ol | 0.1 | 0.2 | 0.2 |
| 1146 | 1145 | trans-Verbenol | tr | tr | tr |
| 1150 | 1152 | 1,4-Dimethyl-4-acetylcyclohexene | tr | 0.1 | tr |
| 1179 | 1179 | 2-Isopropenyl-5-methyl-4-hexenal | tr | tr | tr |
| 1181 | 1180 | Terpinen-4-ol | 2.6 | 5.1 | 4.8 |
| 1187 | 1186 | p-Cymen-8-ol | tr | 0.1 | tr |
| 1195 | 1195 | α-Terpineol | 0.2 | 0.2 | 0.2 |
| 1197 | 1196 | cis-Piperitol | tr | tr | tr |
| 1210 | 1209 | trans-Piperitol | tr | tr | tr |
| 1224 | 1231 | trans-Chrysenthenyl acetate | 0.1 | 0.2 | 0.1 |
| 1240 | 1240 | Ascaridole | - | - | 0.1 |
| 1305 | 1306 | iso-Ascaridole | - | - | 0.1 |
| 1309 | 1309 | p-Vinylguaiacol | tr | tr | tr |
| 1351 | 1357 | Eugenol | 0.1 | - | - |
| 1376 | 1377 | α-Copaene | tr | tr | tr |
| 1384 | 1382 | β-Bourbonene | - | tr | tr |
| 1390 | 1390 | trans-β-Elemene | - | tr | tr |
| 1400 | 1403 | Methyl eugenol | tr | tr | - |
| 1411 | 1412 | Longifolene | tr | 0.3 | 0.1 |
| 1420 | 1417 | (E)-β-Caryophyllene | 0.2 | 0.1 | 0.1 |
| 1431 | 1432 | γ-Elemene | tr | tr | 0.1 |
| 1433 | 1432 | trans-α-Bergamotene | 0.1 | 0.1 | 0.1 |
| 1453 | 1452 | (E)-β-Farnesene | - | tr | tr |
| 1456 | 1454 | α-Humulene | tr | tr | tr |
| 1481 | 1480 | Germacrene D | 0.2 | 0.2 | 0.3 |
| 1496 | 1497 | Bicyclogermacrene | 0.1 | 0.3 | 0.2 |
| 1518 | 1518 | δ-Cadinene | tr | tr | tr |
| 1559 | 1557 | Germacrene B | tr | 0.1 | 0.1 |
| 1561 | 1560 | (E)-Nerolidol | 0.1 | 0.1 | tr |
| 1577 | 1576 | Spathulenol | - | tr | tr |
| 1603 | 1601 | Carotol | - | 0.1 | - |
| 1669 | 1669 | (2E,6Z)-Farnesol | 0.1 | tr | - |
| 1728 | 1730 | (Z)-Ligustilide | - | 0.8 | 0.5 |
| 1779 | 1776 | 2-Methyl-5-(1,2,2-trimethylcyclopentyl)phenol | - | - | 0.1 |
| 1788 | 1790 | (E)-Ligustilide | - | tr | tr |
| 1842 | 1841 | Phytone | - | tr | tr |
| 2149 | 2143 | Serratol | - | 0.1 | - |
| 2300 | 2300 | Tricosane | 0.1 | tr | tr |
| 2400 | 2400 | Tetracosane | - | - | 0.5 |
| 2500 | 2500 | Pentacosane | 0.1 | 0.1 | tr |
| 2575 | 2595 | Selinidin | 0.3 | - | - |
| 2700 | 2700 | Heptacosane | 0.2 | 0.2 | 0.1 |
| Monoterpene hydrocarbons | 94.3 | 89.9 | 90.6 | ||
| Oxygenated monoterpenoids | 3.9 | 7.4 | 7.2 | ||
| Sesquiterpene hydrocarbons | 0.6 | 1.0 | 0.9 | ||
| Oxygenated sesquiterpenoids | 0.1 | 0.2 | 0.1 | ||
| Diterpenoids | 0.0 | 0.1 | 0.0 | ||
| Benzenoid aromatics | 0.4 | 0.1 | 0.0 | ||
| Others | 0.5 | 1.0 | 1.1 | ||
| Total identified | 99.9 | 99.7 | 100.0 |
| RIcalc | RIdb | Compounds | Lpack#1 | Lpack#2 | Lpack#3 | Lpack#4 |
|---|---|---|---|---|---|---|
| 925 | 925 | α-Thujene | tr | tr | 0.1 | 0.1 |
| 933 | 932 | α-Pinene | 0.5 | 0.4 | 1.5 | 1.5 |
| 949 | 950 | Camphene | tr | tr | tr | tr |
| 972 | 971 | Sabinene | 0.1 | 0.1 | 2.3 | 0.4 |
| 977 | 978 | β-Pinene | 1.6 | 0.6 | 2.1 | 2.3 |
| 989 | 989 | Myrcene | 2.4 | 3.1 | 3.6 | 3.1 |
| 990 | 990 | Dehydro-1,8-cineole | - | - | tr | tr |
| 1005 | 1004 | p-Mentha-1(7),8-diene | 0.1 | 0.1 | tr | 0.1 |
| 1007 | 1006 | α-Phellandrene | 0.1 | 0.7 | 0.2 | 0.4 |
| 1009 | 1008 | δ-3-Carene | tr | tr | tr | tr |
| 1017 | 1018 | α-Terpinene | tr | tr | tr | tr |
| 1025 | 1025 | p-Cymene | 0.1 | 0.1 | 0.1 | tr |
| 1030 | 1030 | Limonene | 72.2 | 65.0 | 48.6 | 57.8 |
| 1031 | 1031 | β-Phellandrene | 4.4 | 6.2 | 5.1 | 5.4 |
| 1035 | 1034 | (Z)-β-Ocimene | - | - | 0.1 | 0.1 |
| 1044 | 1045 | Phenylacetaldehyde | tr | tr | tr | tr |
| 1045 | 1045 | (E)-β-Ocimene | 0.1 | 0.1 | 2.1 | 1.7 |
| 1057 | 1057 | γ-Terpinene | tr | tr | 0.3 | tr |
| 1070 | 1069 | cis-Sabinene hydrate | tr | tr | tr | tr |
| 1071 | 1071 | Dihydromyrcenol | tr | tr | - | - |
| 1085 | 1086 | Terpinolene | tr | tr | tr | tr |
| 1090 | 1090 | 6,7-Epoxymyrcene | tr | tr | - | - |
| 1098 | 1098 | Perillene | tr | tr | - | - |
| 1099 | 1101 | Linalool | tr | tr | tr | tr |
| 1101 | 1101 | trans-Sabinene hydrate | tr | tr | tr | tr |
| 1105 | 1104 | Nonanal | tr | tr | tr | tr |
| 1122 | 1122 | trans-p-Mentha-2,8-dien-1-ol | 0.1 | tr | tr | tr |
| 1125 | 1124 | cis-p-Menth-2-en-1-ol | tr | 0.1 | 0.1 | 0.1 |
| 1127 | 1127 | α-Campholenal | tr | - | - | - |
| 1131 | 1131 | Limona ketone | tr | tr | - | - |
| 1133 | 1134 | cis-Limonene oxide | 0.2 | tr | tr | tr |
| 1136 | 1137 | cis-p-Mentha-2,8-dien-1-ol | tr | tr | - | - |
| 1137 | 1137 | trans-Limonene oxide | 0.1 | tr | tr | tr |
| 1142 | 1142 | trans-p-Menth-2-en-1-ol | tr | tr | tr | tr |
| 1145 | 1146 | Oxophorone | - | - | 0.1 | tr |
| 1156 | 1156 | Pentylbenzene | tr | tr | - | tr |
| 1158 | 1161 | Pentylcyclohexa-1,3-diene | 0.1 | 0.2 | 0.1 | 0.2 |
| 1180 | 1180 | Terpinen-4-ol | tr | tr | 0.2 | tr |
| 1187 | 1187 | Cryptone | 0.3 | 0.1 | tr | tr |
| 1195 | 1195 | α-Terpineol | tr | tr | tr | tr |
| 1197 | 1198 | cis-Piperitol | - | - | tr | tr |
| 1203 | 1202 | cis-Sabinol | tr | tr | - | - |
| 1218 | 1218 | trans-Carveol | tr | tr | - | - |
| 1242 | 1242 | Cuminal | tr | - | - | - |
| 1243 | 1246 | Carvone | tr | tr | - | - |
| 1265 | 1265 | Dec-(2E)-enal | tr | tr | - | - |
| 1277 | 1277 | Phellandral | tr | tr | - | - |
| 1286 | 1286 | α-Terpinen-7-al | tr | tr | - | - |
| 1288 | 1286 | trans-Sabinyl acetate | - | - | 0.1 | - |
| 1338 | 1339 | 3-Oxo-p-menth-1-en-7-al | tr | tr | - | - |
| 1378 | 1380 | Daucene | tr | tr | 0.2 | 0.2 |
| 1388 | 1390 | trans-β-Elemene | - | tr | 0.1 | tr |
| 1410 | 1410 | Dodecanal | - | - | tr | tr |
| 1417 | 1417 | (E)-β-Caryophyllene | tr | tr | 0.1 | tr |
| 1417 | 1416 | β-Funebrene | - | tr | tr | tr |
| 1429 | 1430 | γ-Elemene | tr | tr | 0.1 | tr |
| 1433 | 1433 | trans-α-Bergamotene | - | - | tr | tr |
| 1452 | 1452 | (E)-β-Farnesene | 0.1 | 0.1 | 0.3 | 0.2 |
| 1454 | 1454 | α-Humulene | - | - | tr | tr |
| 1472 | 1473 | Dauca-5,8-diene | - | - | 0.2 | - |
| 1475 | 1475 | γ-Muurolene | - | - | - | 0.2 |
| 1480 | 1480 | Germacrene D | 0.1 | 0.2 | 1.4 | 0.7 |
| 1494 | 1494 | α-Zingiberene | tr | 0.1 | 0.1 | 0.1 |
| 1495 | 1497 | Bicyclogermacrene | - | - | 0.3 | tr |
| 1501 | 1504 | iso-Daucene | - | - | tr | tr |
| 1507 | 1508 | β-Bisabolene | - | - | tr | tr |
| 1511 | 1512 | α-Alaskene | - | tr | tr | tr |
| 1513 | 1512 | γ-Cadinene | - | - | tr | - |
| 1518 | 1518 | δ-Cadinene | - | - | tr | tr |
| 1523 | 1523 | β-Sesquiphellandrene | 0.5 | 1.0 | 1.1 | 0.9 |
| 1557 | 1557 | Germacrene B | tr | 0.1 | 0.1 | 0.1 |
| 1576 | 1574 | Germacra-1(10),5-dien-4β-ol | - | - | - | tr |
| 1577 | 1576 | Spathulenol | tr | - | 0.1 | - |
| 1581 | 1584 | 10-epi-Juneol | tr | tr | tr | tr |
| 1582 | 1587 | Caryophyllene oxide | - | - | - | - |
| 1601 | 1601 | Carotol | 2.2 | 0.2 | 7.0 | 7.2 |
| 1612 | 1615 | Zingiberenol | tr | tr | tr | tr |
| 1613 | 1613 | Tetradecanal | - | tr | tr | tr |
| 1649 | 1649 | 3-Butylphthalide | - | - | - | 0.1 |
| 1655 | 1655 | α-Cadinol | - | - | tr | tr |
| 1662 | 1664 | ar-Turmerone | tr | - | 0.1 | - |
| 1668 | 1669 | (3Z)-Butylidene phthalide | 0.6 | 0.3 | 0.1 | 0.2 |
| 1668 | 1668 | α-Turmerone | - | - | 0.5 | - |
| 1687 | 1687 | Himachal-4-en-1β-ol | - | 0.1 | 0.1 | 0.1 |
| 1700 | 1699 | Curlone B (=β-Turmerone) | tr | - | 0.2 | - |
| 1712 | 1712 | Senkyunolide (=Sedanenolide) | - | - | - | 0.1 |
| 1712 | 1719 | (3E)-Butylidene phthalide | 0.2 | 0.2 | 0.2 | 0.1 |
| 1729 | 1730 | (Z)-Ligustilide | 12.3 | 18.0 | 19.1 | 15.4 |
| 1781 | 1776 | 2-Methyl-5-(1,2,2-trimethylcyclopentyl)phenol | - | 0.1 | tr | 0.1 |
| 1788 | 1790 | (E)-Ligustilide | 1.0 | 2.6 | 1.2 | 1.1 |
| 2038 | 2037 | (Z)-Falcarinol | - | tr | 0.1 | 0.2 |
| 2300 | 2300 | Tricosane | tr | tr | tr | tr |
| 2400 | 2400 | Tetracosane | tr | tr | tr | tr |
| 2500 | 2500 | Pentacosane | 0.1 | 0.1 | 0.3 | 0.1 |
| 2600 | 2600 | Hexacosane | - | - | tr | tr |
| 2700 | 2700 | Heptacosane | tr | tr | 0.2 | tr |
| Monoterpene hydrocarbons | 81.5 | 76.4 | 66.1 | 72.9 | ||
| Oxygenated monoterpenoids | 0.7 | 0.2 | 0.5 | 0.1 | ||
| Sesquiterpene hydrocarbons | 0.7 | 1.4 | 4.0 | 2.3 | ||
| Oxygenated sesquiterpenoids | 2.2 | 0.3 | 7.8 | 7.3 | ||
| Benzenoid aromatics | 14.1 | 21.2 | 20.5 | 16.9 | ||
| Others | 0.1 | 0.3 | 0.7 | 0.5 | ||
| Total identified | 99.2 | 99.8 | 99.7 | 100.0 |
| RIcalc | RIdb | Compounds | Ltt#1 | Ltt#2 | Ltt#3 |
|---|---|---|---|---|---|
| 925 | 925 | α-Thujene | 0.1 | 0.2 | 0.6 |
| 933 | 932 | α-Pinene | 0.6 | 5.7 | 9.1 |
| 949 | 950 | Camphene | tr | 0.3 | 0.2 |
| 972 | 971 | Sabinene | 5.6 | 2.1 | 9.9 |
| 977 | 978 | β-Pinene | 0.5 | 10.6 | 11.9 |
| 989 | 989 | Myrcene | 12.7 | 2.9 | 14.1 |
| 990 | 990 | Dehydro-1,8-cineole | 0.1 | 0.4 | 0.2 |
| 1005 | 1004 | p-Mentha-1(7),8-diene | 0.4 | 0.5 | 0.2 |
| 1007 | 1006 | α-Phellandrene | 0.2 | - | 0.4 |
| 1009 | 1008 | δ-3-Carene | 0.1 | - | tr |
| 1017 | 1018 | α-Terpinene | - | - | 0.1 |
| 1019 | 1024 | 2-Cyclohexene-1,4-dione | - | 0.6 | - |
| 1025 | 1025 | p-Cymene | 2.1 | 4.3 | 0.7 |
| 1030 | 1030 | Limonene | 1.6 | 4.7 | 1.1 |
| 1031 | 1031 | β-Phellandrene | 48.5 | 1.7 | 29.4 |
| 1035 | 1034 | (Z)-β-Ocimene | 0.5 | - | 0.5 |
| 1045 | 1045 | (E)-β-Ocimene | 8.2 | - | 9.2 |
| 1057 | 1057 | γ-Terpinene | 0.3 | - | 0.3 |
| 1070 | 1069 | cis-Sabinene hydrate | 0.1 | 0.3 | 0.1 |
| 1071 | 1071 | Dihydromyrcenol | 0.2 | 0.6 | tr |
| 1085 | 1086 | Terpinolene | - | - | 0.1 |
| 1090 | 1090 | 6,7-Epoxymyrcene | 0.1 | 0.4 | 0.1 |
| 1091 | 1091 | Rosefuran | 0.1 | - | - |
| 1095 | 1097 | α-Pinene oxide | 0.1 | - | tr |
| 1098 | 1098 | Perillene | tr | 0.2 | - |
| 1099 | 1101 | Linalool | 0.3 | 1.3 | 0.1 |
| 1101 | 1101 | trans-Sabinene hydrate | 0.1 | 0.1 | 0.1 |
| 1105 | 1104 | Nonanal | tr | 0.2 | tr |
| 1107 | 1109 | 1-Octen-3-yl acetate | 0.1 | 0.2 | - |
| 1125 | 1124 | cis-p-Menth-2-en-1-ol | 0.2 | 0.8 | 0.1 |
| 1127 | 1127 | α-Campholenal | - | 0.3 | - |
| 1129 | 1129 | (Z)-Myroxide | tr | - | tr |
| 1131 | 1131 | Limona ketone | - | - | - |
| 1133 | 1134 | cis-Limonene oxide | - | 0.4 | - |
| 1138 | 1138 | Benzeneacetonitrile | 0.1 | - | tr |
| 1139 | 1139 | (E)-Myroxide | 0.2 | - | 0.1 |
| 1139 | 1139 | Nopinone | - | 0.5 | - |
| 1141 | 1141 | trans-Pinocarveol | - | 0.6 | - |
| 1142 | 1142 | trans-p-Menth-2-en-1-ol | 0.1 | 0.5 | 0.1 |
| 1145 | 1146 | Oxophorone | 0.1 | - | tr |
| 1146 | 1146 | trans-Verbenol | - | 0.2 | tr |
| 1162 | 1164 | Pinocarvone | - | 0.6 | - |
| 1169 | 1169 | Rosefuran epoxide | 0.1 | - | - |
| 1180 | 1180 | Terpinen-4-ol | 0.5 | 0.8 | 0.7 |
| 1187 | 1187 | Cryptone | 3.7 | 17.9 | 0.8 |
| 1192 | 1192 | Methyl salicylate | 0.1 | - | - |
| 1195 | 1195 | α-Terpineol | 0.2 | 0.6 | 0.2 |
| 1196 | 1196 | Myrtenal | - | 0.8 | - |
| 1197 | 1195 | p-Menth-3-en-7-al | 0.2 | 0.8 | 0.1 |
| 1197 | 1198 | cis-Piperitol | - | - | - |
| 1203 | 1202 | cis-Sabinol | 0.1 | - | 0.1 |
| 1223 | 1223 | m-Cumenol | - | 0.5 | - |
| 1242 | 1242 | Cuminal | 0.3 | 2.8 | 0.1 |
| 1243 | 1246 | Carvone | - | 0.3 | - |
| 1254 | 1254 | Piperitone | 0.1 | 0.4 | - |
| 1264 | 1258 | trans-Piperitone epoxide | 0.2 | 0.5 | - |
| 1265 | 1265 | Dec-(2E)-enal | - | 0.5 | - |
| 1286 | 1286 | α-Terpinen-7-al | 0.1 | 0.2 | tr |
| 1291 | 1291 | p-Cymen-7-ol | 0.3 | 2.4 | 0.1 |
| 1322 | 1318 | 4-Hydroxycryptone | - | 1.5 | - |
| 1331 | 1330 | Bicycloelemene | - | - | 0.1 |
| 1338 | 1339 | 3-Oxo-p-menth-1-en-7-al | 0.4 | 2.4 | 0.1 |
| 1388 | 1390 | trans-β-Elemene | - | - | 0.1 |
| 1417 | 1417 | (E)-β-Caryophyllene | 0.1 | - | 1.1 |
| 1442 | --- | Unidentified a | 0.5 | 3.5 | 0.1 |
| 1448 | --- | Unidentified b | - | 1.2 | - |
| 1454 | 1454 | α-Humulene | - | - | 0.1 |
| 1475 | 1475 | γ-Muurolene | 0.2 | - | 0.1 |
| 1480 | 1480 | Germacrene D | 3.0 | - | 2.5 |
| 1491 | --- | Unidentified c | 0.5 | 2.5 | 0.1 |
| 1495 | 1497 | Bicyclogermacrene | - | - | 0.8 |
| 1507 | 1508 | β-Bisabolene | 0.1 | - | - |
| 1513 | 1512 | γ-Cadinene | 0.1 | - | tr |
| 1518 | 1518 | δ-Cadinene | 0.2 | - | 0.1 |
| 1576 | 1574 | Germacra-1(10),5-dien-4β-ol | 0.3 | - | 0.2 |
| 1577 | 1576 | Spathulenol | - | 6.3 | 0.3 |
| 1582 | 1587 | Caryophyllene oxide | - | 1.2 | 0.1 |
| 1632 | 1630 | Caryophylla-4(12),8(13)-dien-5α-ol | - | - | 0.1 |
| 1639 | 1644 | allo-Aromadendrene epoxide | 0.3 | - | 0.1 |
| 1642 | 1642 | τ-Cadinol | - | - | tr |
| 1643 | 1644 | τ-Muurolol | - | - | 0.1 |
| 1649 | 1649 | 3-Butylphthalide | - | 0.4 | - |
| 1655 | 1655 | α-Cadinol | 0.3 | 0.4 | 0.2 |
| 1662 | 1664 | ar-Turmerone | 0.2 | 0.3 | 0.1 |
| 1693 | 1686 | Shyobunol | 0.1 | - | - |
| 1712 | 1712 | Senkyunolide (=Sedanenolide) | 0.4 | - | 0.2 |
| 1806 | 1807 | Tetradecyl acetate | - | - | 0.1 |
| 1872 | 1875 | Oplopanonyl acetate | 0.1 | 0.5 | 0.1 |
| 1936 | 1933 | Beyerene | 0.2 | 0.7 | 0.1 |
| 2038 | 2037 | (Z)-Falcarinol | 0.1 | - | 0.1 |
| 2300 | 2300 | Tricosane | - | - | 0.1 |
| 2400 | 2400 | Tetracosane | - | - | 0.9 |
| 2500 | 2500 | Pentacosane | 0.1 | 0.3 | 0.2 |
| 2612 | 2610 | Auraptene | 1.0 | 1.3 | 0.9 |
| 2700 | 2700 | Heptacosane | 0.3 | 0.7 | 0.4 |
| Monoterpene hydrocarbons | 81.6 | 33.0 | 87.9 | ||
| Oxygenated monoterpenoids | 8.0 | 39.1 | 2.8 | ||
| Sesquiterpene hydrocarbons | 3.6 | 0.0 | 4.9 | ||
| Oxygenated sesquiterpenoids | 1.3 | 8.8 | 1.1 | ||
| Diterpenoids | 0.2 | 0.7 | 0.1 | ||
| Benzenoid aromatics | 1.1 | 1.7 | 0.9 | ||
| Others | 1.0 | 2.5 | 2.0 | ||
| Total identified | 96.8 | 85.6 | 99.7 |
| RIcalc | RIdb | Compounds | Ld#1 | Ld#2 | Ld#3 | Ld#4 | Ld#5 |
|---|---|---|---|---|---|---|---|
| 783 | 782 | Prenol | 0.1 | tr | 0.1 | 0.1 | 0.1 |
| 933 | 933 | α-Pinene | tr | tr | tr | 0.1 | tr |
| 950 | 950 | Camphene | tr | tr | tr | tr | tr |
| 973 | 972 | Sabinene | tr | tr | tr | tr | tr |
| 979 | 978 | β-Pinene | tr | tr | tr | 0.3 | tr |
| 990 | 991 | Myrcene | tr | tr | tr | tr | tr |
| 992 | 990 | Dehydro-1,8-cineole | tr | tr | tr | tr | tr |
| 1004 | 1006 | Octanal | tr | tr | tr | tr | tr |
| 1005 | 1005 | (3Z)-Hexenyl acetate | tr | tr | tr | tr | tr |
| 1007 | 1006 | α-Phellandrene | tr | tr | - | tr | tr |
| 1012 | 1012 | Hexyl acetate | tr | tr | 0.1 | 0.1 | 0.1 |
| 1025 | 1025 | p-Cymene | tr | tr | tr | tr | tr |
| 1029 | 1030 | Limonene | tr | tr | tr | tr | tr |
| 1031 | 1031 | β-Phellandrene | tr | tr | tr | tr | tr |
| 1033 | 1032 | 1,8-Cineole | tr | tr | tr | tr | tr |
| 1035 | 1034 | (Z)-β-Ocimene | - | - | tr | - | tr |
| 1044 | 1045 | Phenylacetaldehyde | tr | tr | tr | tr | tr |
| 1046 | 1046 | (E)-β-Ocimene | tr | tr | tr | tr | tr |
| 1058 | 1057 | γ-Terpinene | tr | tr | - | tr | - |
| 1070 | 1069 | 1-Octanol | 0.6 | 0.3 | 1.1 | 0.8 | 0.6 |
| 1086 | 1087 | Terpinolene | - | - | - | - | tr |
| 1092 | 1093 | 2-Nonanone | - | - | - | tr | - |
| 1100 | 1101 | Linalool | tr | tr | tr | tr | tr |
| 1105 | 1107 | Nonanal | tr | tr | tr | tr | tr |
| 1108 | 1107 | 1-Octen-3-yl acetate | tr | tr | - | tr | tr |
| 1124 | 1123 | Methyl octanoate | tr | tr | tr | tr | tr |
| 1143 | 1142 | Epoxyterpinolene | tr | - | - | - | tr |
| 1151 | 1152 | 1,4-Dimethyl-4-acetylcyclohexene | tr | - | - | - | tr |
| 1152 | 1152 | Nerol oxide | tr | - | - | - | - |
| 1158 | 1160 | Pentylcyclohexa-1,3-diene | - | tr | - | - | - |
| 1179 | 1179 | 2-isopropenyl-5-methyl-4-hexenal | tr | - | - | - | tr |
| 1181 | 1180 | Terpinen-4-ol | tr | tr | - | - | - |
| 1189 | 1189 | p-Cymen-8-ol | - | tr | - | - | - |
| 1196 | 1195 | α-Terpineol | tr | tr | tr | tr | tr |
| 1200 | 1202 | (2Z)-Octenyl acetate | tr | - | tr | - | - |
| 1207 | 1208 | Decanal | 0.7 | 0.3 | 0.4 | 0.2 | 0.3 |
| 1211 | 1211 | Octyl acetate | 41.1 | 43.3 | 48.4 | 42.4 | 37.8 |
| 1216 | 1217 | Coumaran | - | tr | tr | tr | tr |
| 1225 | 1231 | trans-Chrysanthenyl acetate | tr | tr | - | - | tr |
| 1255 | 1257 | 2-Phenethyl acetate | - | - | - | - | tr |
| 1263 | 1263 | (2E)-Decenal | - | - | - | tr | tr |
| 1273 | 1271 | 1-Decanol | 18.4 | 12.2 | 14.5 | 9.8 | 13.3 |
| 1284 | 1284 | Lavandulyl acetate | tr | - | - | - | - |
| 1293 | 1293 | 2-Undecanone | - | - | - | tr | tr |
| 1310 | 1309 | 1-Nonyl acetate | tr | tr | tr | tr | 0.1 |
| 1312 | 1310 | trans-Ocimenyl acetate | - | - | - | - | tr |
| 1359 | 1361 | Neryl acetate | 0.1 | tr | tr | - | tr |
| 1365 | 1367 | Decanoic acid | tr | - | - | - | - |
| 1376 | 1375 | α-Copaene | - | - | tr | - | - |
| 1379 | 1379 | (E)-β-Damascenone | - | - | tr | - | - |
| 1387 | 1384 | (5E)-Decen-1-yl acetate | tr | 0.1 | tr | tr | tr |
| 1388 | 1388 | (3Z)-Decen-1-yl acetate | tr | 0.1 | tr | tr | 0.1 |
| 1409 | 1408 | Decyl acetate | 37.2 | 42.0 | 33.9 | 43.2 | 45.8 |
| 1414 | 1414 | Acora-3,7(14)-diene | - | - | - | tr | - |
| 1419 | 1417 | (E)-β-Caryophyllene | 0.1 | tr | 0.1 | 0.1 | 0.1 |
| 1427 | 1428 | β-Duprezianene | - | - | - | tr | - |
| 1452 | 1452 | (E)-β-Farnesene | - | - | - | tr | - |
| 1456 | 1454 | α-Humulene | tr | - | tr | tr | tr |
| 1474 | 1476 | 1-Dodecanol | 1.3 | 1.0 | 1.0 | 0.7 | 1.3 |
| 1488 | 1489 | β-Selinene | - | - | - | tr | - |
| 1490 | 1489 | (Z,E)-α-Farnesene | - | tr | tr | - | tr |
| 1494 | 1494 | 2-Tridecanone | - | - | - | tr | tr |
| 1495 | 1497 | α-Selinene | - | - | - | tr | - |
| 1504 | 1504 | (E,E)-α-Farnesene | - | tr | tr | tr | tr |
| 1508 | 1507 | 1-Pentadecene | tr | tr | - | - | tr |
| 1511 | 1512 | α-Alaskene | - | tr | - | tr | - |
| 1518 | 1518 | δ-Cadinene | - | - | tr | - | - |
| 1523 | 1523 | β-Sesquiphellandrene | - | tr | - | tr | - |
| 1529 | 1528 | Kessane | - | tr | - | - | - |
| 1560 | 1561 | (E)-Nerolidol | tr | tr | tr | tr | tr |
| 1582 | 1582 | Octyl hexanoate | tr | tr | tr | 0.1 | tr |
| 1602 | 1601 | Carotol | - | tr | tr | - | - |
| 1608 | 1607 | 1-Dodecyl acetate | 0.3 | 0.3 | 0.2 | 0.4 | 0.4 |
| 1655 | 1655 | α-Cadinol | - | - | tr | tr | - |
| 1685 | 1686 | epi-α-Bisabolol | - | - | - | tr | - |
| 1704 | 1699 | β-Cedr-8-en-15-ol | - | - | - | 1.5 | - |
| 1720 | 1722 | 3-Isobutylidene phthalide | - | tr | tr | tr | - |
| 1727 | 1730 | (Z)-Ligustilide | - | 0.1 | tr | - | tr |
| 1777 | 1779 | Octyl octanoate | tr | tr | tr | 0.1 | tr |
| 1779 | 1780 | (Z)-Nerolidyl isobutyrate | tr | tr | - | - | - |
| 1958 | 1958 | Palmitic acid | tr | - | tr | - | - |
| 1975 | 1978 | Decyl octanoate | - | tr | tr | tr | tr |
| 2046 | 2050 | Bergaptene | tr | tr | tr | tr | tr |
| 2148 | 2149 | Incensyl acetate | tr | tr | tr | tr | - |
| 2198 | 2192 | Geranylgeraniol | tr | - | - | - | - |
| 2301 | 2300 | Tricosane | tr | tr | tr | tr | tr |
| 2501 | 2500 | Pentacosane | tr | tr | tr | tr | tr |
| 2700 | 2700 | Heptacosane | tr | tr | tr | tr | tr |
| Isoprenoids | 0.2 | 0.0 | 0.2 | 2.1 | 0.2 | ||
| Benzenoid aromatics | trace | 0.1 | trace | trace | trace | ||
| Fatty acid derivatives | 99.6 | 99.7 | 99.7 | 97.8 | 99.6 | ||
| Others | 0.0 | trace | 0.0 | 0.0 | 0.0 | ||
| Total identified | 99.8 | 99.7 | 99.8 | 99.9 | 99.8 |
| RIcalc | RIdb | Compound | Lm#1 (OR) | Lm#2 (OR) | Lm#3 (OR) | Lm#4 (OR) | Lm#5 (OR) | Lm#6 (OR) | Lm#7 (ID) | Lm#8 (ID) | Lm#9 (ID) | Lm#10 (OR) | Lm#11 (OR) | Lm#12 (OR) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 781 | 782 | 3-Methylbut-2-en-1-ol | - | - | 1.7 | 1.4 | 1.7 | - | - | - | - | 2.0 | 2.6 | 2.2 |
| 790 | 790 | 3-Methyl-2-butenal | - | - | 0.2 | 0.1 | - | - | - | - | - | - | - | 0.2 |
| 801 | 802 | Hexanal | - | - | - | - | - | - | - | - | - | tr | 0.1 | 0.1 |
| 850 | 850 | (2E)-Hexenal | 0.3 | 0.3 | 0.2 | 0.2 | - | - | - | - | - | 0.1 | 0.8 | 0.4 |
| 852 | 853 | (3Z)-Hexenol | - | - | - | - | - | - | - | - | - | - | 0.1 | - |
| 903 | 905 | Heptanal | - | - | 0.1 | 0.2 | - | 0.1 | 0.3 | tr | 0.1 | - | 0.1 | 0.1 |
| 920 | 921 | Hashishene | tr | 0.1 | 0.3 | 0.2 | 0.1 | 0.1 | 0.1 | tr | tr | 0.1 | 0.1 | 0.2 |
| 922 | 923 | Tricyclene | tr | tr | 0.1 | tr | - | - | - | - | - | tr | tr | tr |
| 933 | 933 | α-Pinene | 0.6 | 0.3 | 0.3 | 0.2 | 0.4 | - | tr | 0.1 | 0.3 | 0.3 | 1.2 | 1.4 |
| 947 | 948 | α-Fenchene | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | 0.1 |
| 949 | 950 | Camphene | 4.2 | 1.7 | 4.8 | 2.0 | 0.9 | 0.4 | tr | 0.5 | 0.5 | 0.5 | 3.7 | 2.5 |
| 952 | 955 | Propylbenzene | - | 0.1 | 1.0 | 0.8 | 0.5 | - | 3.5 | tr | - | - | 0.3 | 0.4 |
| 965 | 963 | 2-Methyl-(3E)-octen-5-yne | 0.1 | 0.3 | 0.6 | 0.2 | 0.1 | 7.6 | 6.9 | 5.5 | 7.8 | tr | - | 0.1 |
| 972 | 972 | Sabinene | - | - | - | - | - | - | - | - | - | 0.1 | 0.4 | 0.1 |
| 975 | 981 | α-Myrcene | - | 0.1 | - | - | - | tr | - | - | - | - | - | - |
| 978 | 978 | β-Pinene | - | - | - | - | - | - | - | - | - | 0.1 | 0.1 | 0.4 |
| 989 | 991 | β-Myrcene | 46.7 | 54.1 | 38.9 | 31.2 | 12.5 | 37.5 | 33.2 | 18.2 | 23.8 | 12.9 | 21.1 | 38.0 |
| 991 | 992 | 1,5,5-Trimethyl-3-methylene-1-cyclohexene | - | 0.1 | - | - | - | - | - | - | - | - | - | - |
| 991 | 990 | Dehydro-1,8-cineole | - | - | - | - | - | - | - | - | - | - | 0.2 | - |
| 992 | 986 | cis-m-Mentha-2,8-diene | - | - | - | - | - | 0.1 | - | - | - | - | - | - |
| 1004 | 1004 | p-Mentha-1(7),8-diene | - | - | - | - | - | - | - | - | - | 0.1 | 0.2 | - |
| 1007 | 1007 | α-Phellandrene | - | - | - | tr | 0.3 | - | - | - | - | 1.0 | tr | - |
| 1024 | 1025 | p-Cymene | 0.7 | 1.0 | 1.1 | 0.3 | 14.8 | 0.2 | 0.1 | 0.2 | 0.8 | 1.6 | 2.8 | 0.5 |
| 1029 | 1030 | Limonene | 3.3 | 2.8 | 4.5 | 1.8 | 8.8 | 1.7 | 0.7 | 5.2 | 14.0 | 1.3 | 3.8 | 2.8 |
| 1030 | 1031 | β-Phellandrene | 0.3 | 0.2 | 0.1 | 2.5 | 4.1 | 0.1 | 0.1 | tr | 0.1 | 19.6 | 21.3 | 0.2 |
| 1032 | 1032 | 1,8-Cineole | - | - | - | - | - | - | - | - | - | 0.1 | tr | tr |
| 1034 | 1033 | Benzyl alcohol | - | - | - | - | - | 0.1 | - | - | - | - | - | - |
| 1034 | 1034 | (Z)-β-Ocimene | 2.2 | 0.1 | 2.2 | 2.5 | 4.0 | 0.3 | 1.5 | 0.8 | 0.4 | 5.7 | 2.1 | 3.5 |
| 1044 | 1045 | Phenylacetaldehyde | - | - | 0.1 | - | 0.1 | - | - | tr | - | 0.1 | 0.1 | tr |
| 1045 | 1045 | (E)-β-Ocimene | 24.8 | 0.3 | 7.0 | 10.5 | 14.1 | 3.5 | 17.3 | 8.1 | 4.6 | 37.4 | 9.4 | 23.7 |
| 1051 | 1051 | 2,3,6-Trimethylhepta-1,5-diene | - | - | 0.1 | - | - | - | - | - | - | - | tr | tr |
| 1057 | 1057 | γ-Terpinene | 0.4 | 0.1 | 0.1 | 0.1 | 13.1 | - | - | 0.1 | 0.2 | 3.8 | 0.2 | - |
| 1062 | 1073 | p-Mentha-3,8-diene | - | - | 0.2 | 0.1 | - | 0.2 | 0.1 | 0.2 | 0.4 | - | - | - |
| 1086 | 1086 | Terpinolene | 0.5 | 0.1 | 0.1 | 0.1 | 6.5 | 0.1 | 0.1 | 1.5 | 3.8 | 0.1 | 0.1 | 0.1 |
| 1090 | 1090 | 6,7-Epoxymyrcene | 0.1 | 0.7 | 0.5 | 0.1 | - | 0.3 | 0.1 | - | - | - | 0.2 | 0.2 |
| 1091 | 1091 | p-Cymenene | - | - | - | - | - | - | - | 0.1 | 0.2 | - | - | - |
| 1091 | 1091 | Rosefuran | 0.1 | - | 0.4 | 0.2 | - | 0.1 | 0.1 | 0.1 | 0.1 | - | 0.2 | 0.4 |
| 1096 | 1097 | α-Pinene oxide | 0.1 | - | 0.5 | 0.3 | - | 0.2 | 0.1 | 0.1 | tr | tr | 0.2 | 0.5 |
| 1099 | 1098 | Perillene | tr | 0.5 | 0.3 | 0.1 | - | 0.2 | tr | tr | - | - | - | - |
| 1100 | 1101 | Linalool | 0.1 | 0.1 | 0.4 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 | 0.3 | 0.2 |
| 1104 | 1102 | 6-Methylhepta-3,5-dien-2-one | - | - | 0.1 | - | - | - | - | - | - | - | - | 0.2 |
| 1121 | 1119 | Myrcenol | 0.2 | - | - | - | - | - | - | - | - | - | - | - |
| 1124 | 1124 | cis-p-Menth-2-en-1-ol | - | - | - | - | - | - | - | - | - | 0.1 | 0.1 | - |
| 1129 | 1128 | (4E,6Z)-allo-Ocimene | - | - | - | 0.2 | 0.2 | - | 0.3 | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 |
| 1137 | 1138 | Benzeneacetonitrile | - | - | - | - | - | - | - | - | - | 0.1 | - | - |
| 1139 | 1139 | (E)-Myroxide | 0.2 | - | 0.3 | 0.2 | 0.2 | 0.1 | 0.1 | tr | tr | - | 0.4 | 0.6 |
| 1142 | 1142 | trans-p-Menth-2-en-1-ol | - | - | - | - | - | - | - | - | - | - | 0.1 | - |
| 1144 | 1142 | Epoxyterpinolene | - | - | - | - | - | - | - | 0.1 | 0.4 | - | - | - |
| 1148 | 1149 | Camphor | 0.1 | - | - | - | - | - | - | - | - | - | - | - |
| 1156 | 1156 | Pentylbenzene | - | - | - | - | - | 0.1 | 0.1 | - | - | - | - | 0.2 |
| 1156 | 1156 | Camphene hydrate | 0.3 | 0.2 | 0.1 | 0.1 | - | - | - | - | - | - | - | - |
| 1169 | 1169 | Rosefuran epoxide | - | - | 0.2 | 0.1 | - | 0.1 | - | - | - | - | - | 0.2 |
| 1173 | 1173 | Borneol | 0.2 | tr | 0.1 | - | - | - | - | - | - | - | - | 0.2 |
| 1179 | 1179 | 2-Isopropenyl-5-methyl-4-hexenal | - | - | - | - | 0.1 | - | - | 0.1 | 0.2 | - | - | - |
| 1181 | 1180 | Terpinen-4-ol | 0.1 | tr | - | - | - | - | - | - | tr | - | - | - |
| 1186 | 1188 | p-Methylacetophenone | - | 0.1 | 0.1 | - | - | 0.1 | - | tr | 0.2 | - | - | - |
| 1187 | 1187 | Cryptone | - | - | - | 0.2 | - | - | - | - | - | 0.1 | 2.3 | - |
| 1188 | 1188 | p-Cymen-8-ol | 0.1 | 0.2 | 0.2 | - | 0.3 | 0.1 | - | 0.7 | 1.6 | - | - | 0.2 |
| 1195 | 1195 | α-Terpineol | 0.2 | 0.1 | 0.2 | tr | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 |
| 1207 | 1206 | Decanal | - | 0.1 | - | - | - | - | - | - | - | - | - | - |
| 1208 | 1207 | (3E)-Octenyl acetate | 0.3 | - | - | - | - | - | 0.2 | 0.1 | 0.1 | tr | 0.1 | 0.1 |
| 1210 | 1211 | Octyl acetate | - | 0.3 | - | - | - | - | - | - | - | - | - | - |
| 1226 | 1231 | (3Z)-Hexenyl 2-methylbutanoate | - | - | - | - | - | - | - | - | - | - | - | 0.2 |
| 1229 | 1229 | Thymyl methyl ether | - | 0.1 | - | - | 1.2 | - | - | - | 0.1 | - | - | - |
| 1244 | 1244 | Linalyl acetate | - | 0.2 | - | - | - | - | - | - | - | - | - | - |
| 1254 | 1254 | Piperitone | 0.1 | 0.1 | - | - | - | - | - | - | - | - | - | - |
| 1272 | 1271 | Decanol | - | 2.7 | - | - | - | - | - | - | - | - | - | - |
| 1284 | 1282 | Bornyl acetate | 0.9 | 0.1 | 5.8 | 2.6 | 3.4 | 0.1 | - | - | 0.1 | 1.1 | 2.9 | 3.9 |
| 1286 | --- | Unidentified a | - | - | 0.8 | tr | 0.8 | 0.2 | - | - | - | - | - | 2.4 |
| 1304 | 1302 | 4-Methylhexyl 2-methylbutanoate | - | - | - | - | - | - | - | - | - | - | - | 0.3 |
| 1307 | 1310 | cis-3-Butyl-4-vinyl cyclopentene | 0.1 | - | - | - | - | - | - | - | - | - | - | - |
| 1342 | 1343 | 2-(2,5-Dimethylphenyl)propanal | 0.1 | - | - | - | 0.3 | 0.1 | - | 0.2 | 1.4 | - | - | - |
| 1346 | 1346 | α-Terpinyl acetate | - | - | - | - | - | 0.1 | - | 0.2 | 0.3 | - | - | - |
| 1350 | 1348 | α-Longipinene | - | - | - | - | - | - | - | - | - | - | - | 0.1 |
| 1369 | 1367 | Cyclosativene | - | - | 0.2 | - | - | 0.1 | 0.1 | 0.2 | 0.1 | - | - | - |
| 1370 | 1370 | iso-Ledene | - | - | 0.2 | 0.7 | - | - | - | - | - | - | - | - |
| 1374 | 1372 | Longicyclene | - | - | 0.3 | 0.1 | - | 0.1 | 0.5 | 0.2 | 0.2 | - | - | 0.2 |
| 1376 | 1375 | α-Copaene | - | - | - | 0.1 | - | - | - | - | - | - | - | - |
| 1389 | 1390 | trans-β-Elemene | - | - | - | - | - | - | - | - | - | 0.1 | - | - |
| 1405 | 1411 | Thymohydroquinone dimethyl ether | - | - | - | - | - | - | - | - | - | - | - | 0.1 |
| 1406 | 1406 | α-Gurjunene | - | - | - | 0.1 | - | - | - | - | - | - | - | - |
| 1408 | 1408 | Decyl acetate | - | 7.7 | - | - | - | - | - | - | - | 0.2 | 1.7 | - |
| 1408 | 1405 | (Z)-β-Caryophyllene | - | - | - | - | - | - | - | - | - | - | - | 0.6 |
| 1409 | 1411 | Longifolene | - | - | 3.9 | 1.3 | 1.5 | 1.7 | 4.9 | 2.7 | 2.8 | - | 0.7 | 2.7 |
| 1410 | 1415 | β-Maaliene | - | - | - | 0.1 | - | - | - | - | - | - | - | - |
| 1415 | 1414 | α-Cedrene | - | - | 0.2 | 0.1 | - | - | 0.1 | 0.1 | 0.1 | - | - | - |
| 1419 | 1417 | (E)-β-Caryophyllene | 0.1 | 0.1 | 0.5 | 0.4 | 0.7 | 0.2 | 0.2 | 0.3 | 0.2 | 0.5 | 0.4 | 0.1 |
| 1423 | 1423 | β-Cedrene | - | - | 0.1 | 0.1 | - | - | 0.1 | 0.1 | 0.1 | tr | 0.2 | - |
| 1427 | 1430 | γ-Maaliene | - | - | - | 0.2 | - | - | - | - | - | - | - | - |
| 1429 | 1430 | γ-Elemene | - | - | - | - | - | - | - | - | - | 0.1 | - | - |
| 1433 | 1432 | trans-α-Bergamotene | 0.1 | - | 0.3 | 0.3 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 | 0.5 | 0.1 |
| 1434 | 1435 | α-Maaliene | - | - | - | 0.1 | - | - | - | - | - | - | - | - |
| 1434 | 1436 | α-Guaiene | - | - | - | - | - | - | - | 0.1 | 0.1 | 0.3 | - | - |
| 1436 | 1433 | β-Copaene | - | - | - | 0.1 | - | - | - | - | - | - | - | - |
| 1438 | 1438 | Aromadendrene | - | - | 0.5 | 1.7 | - | - | - | - | - | - | - | - |
| 1439 | 1438 | α-Guaiene | - | - | 0.4 | 1.2 | - | - | - | - | - | - | - | - |
| 1440 | 1440 | Guaia-6,9-diene | - | - | - | - | - | - | - | tr | tr | - | - | - |
| 1446 | 1446 | cis-Muurola-3,5-diene | - | - | - | 0.3 | - | - | - | - | - | - | - | - |
| 1447 | 1447 | Geranylacetone | - | - | - | - | - | - | - | - | - | 0.1 | 0.1 | 0.1 |
| 1449 | 1449 | α-Himachalene | - | - | 0.3 | 0.1 | - | 0.1 | 0.4 | 0.3 | 0.2 | - | 0.2 | 0.2 |
| 1452 | 1452 | (E)-β-Farnesene | - | - | 0.5 | 0.4 | 0.2 | 0.4 | 0.3 | 0.8 | 0.5 | 0.1 | 0.8 | 0.2 |
| 1453 | 1453 | Prezizaene | - | - | - | - | - | - | - | - | 0.2 | - | - | - |
| 1455 | 1454 | α-Humulene | 0.8 | 0.9 | tr | - | - | - | - | tr | 0.1 | 0.1 | 0.1 | - |
| 1457 | 1451 | Amorpha-4,11-diene | - | - | - | - | - | - | 0.1 | 0.1 | 0.1 | - | - | - |
| 1459 | 1458 | allo-Aromadendrene | - | - | 0.2 | 0.5 | - | - | - | - | - | - | - | - |
| 1465 | 1465 | Bornyl butyrate | - | - | - | - | - | - | - | - | 0.1 | - | - | - |
| 1473 | 1474 | Selina-4,11-diene | - | - | - | - | - | 0.2 | - | 0.1 | - | 0.1 | - | - |
| 1472 | 1475 | γ-Gurjunene | - | - | 0.2 | 0.5 | - | - | - | - | - | - | - | - |
| 1474 | 1475 | Dodecanol | - | 0.2 | - | - | - | - | - | - | - | - | - | - |
| 1478 | 1479 | α-Amorphene | - | 0.1 | - | - | - | - | - | 0.9 | 0.5 | - | - | - |
| 1479 | 1480 | γ-Himachalene | - | - | 0.2 | - | - | - | 0.2 | - | - | - | - | - |
| 1481 | 1482 | ar-Curcumene | - | - | 0.1 | - | - | 0.1 | 0.5 | 0.3 | 0.7 | - | - | - |
| 1486 | 1488 | δ-Selinene | - | - | - | 0.3 | - | - | - | - | - | - | - | - |
| 1488 | 1489 | β-Selinene | - | - | 0.2 | 0.6 | - | 0.3 | - | 0.1 | - | 0.2 | - | - |
| 1489 | 1489 | (Z,E)-α-Farnesene | 3.2 | 1.4 | - | - | - | 0.5 | 0.2 | - | - | - | - | - |
| 1490 | 1491 | Viridiflorene | - | - | 1.0 | 5.9 | 0.1 | - | - | - | - | - | - | - |
| 1495 | 1497 | α-Selinene | - | 0.1 | - | 0.2 | - | 0.2 | 0.1 | 0.1 | - | 0.1 | - | - |
| 1498 | 1497 | Capillene | - | - | - | - | 0.5 | - | - | - | - | - | - | - |
| 1498 | 1500 | α-Muurolene | - | 0.1 | - | - | - | - | 0.1 | tr | - | - | - | - |
| 1499 | 1503 | β-Himachalene | - | - | 0.2 | 0.1 | - | - | 0.2 | 0.3 | 0.2 | - | - | - |
| 1501 | 1505 | α-Bulnesene | - | - | 0.2 | 0.5 | - | - | - | - | - | 0.2 | - | - |
| 1502 | 1504 | Epizonarene | - | - | - | - | - | - | - | 0.1 | - | - | - | - |
| 1503 | 1504 | (E,E)-α-Farnesene | 0.3 | 0.3 | - | - | - | - | - | - | - | - | - | - |
| 1504 | 1501 | β-Dihydroagarofuran | - | - | - | - | - | 0.2 | - | 0.1 | - | - | - | - |
| 1506 | 1508 | β-Bisabolene | - | - | 0.4 | 0.2 | 0.1 | 0.1 | 0.3 | 1.2 | 1.2 | 0.1 | 0.6 | 0.2 |
| 1509 | 1511 | β-Curcumene | - | - | - | - | - | - | - | - | 0.2 | - | - | - |
| 1509 | 1511 | (Z)-γ-Bisabolene | - | 0.2 | - | 0.1 | - | 1.9 | 3.7 | 1.0 | 0.1 | - | - | - |
| 1512 | 1514 | Sesquicineole | - | - | - | - | - | - | - | 0.1 | 0.1 | - | - | - |
| 1516 | 1518 | Bornyl isovalerate | - | - | - | - | - | - | - | - | 0.1 | - | - | - |
| 1517 | 1519 | Nootkatene | - | - | - | 0.3 | - | - | - | - | - | - | - | - |
| 1518 | 1518 | δ-Cadinene | - | 0.1 | - | - | - | - | - | - | - | - | - | - |
| 1523 | 1523 | β-Sesquiphellandrene | - | - | - | - | - | - | - | - | - | - | 0.2 | - |
| 1529 | 1528 | (E)-γ-Bisabolene | - | 0.1 | - | - | - | - | 0.2 | 0.4 | 0.1 | - | - | - |
| 1537 | 1540 | Selina-4(15),7(11)-diene | - | - | - | - | - | - | - | 0.6 | 0.2 | 0.4 | - | - |
| 1540 | 1540 | (E)-α-Bisabolene | - | - | - | - | - | 0.1 | 0.2 | 0.6 | 0.6 | - | 0.2 | - |
| 1542 | 1542 | Selina-3,7(11)-diene | - | - | - | - | - | 0.1 | - | 0.7 | 0.2 | 0.3 | - | - |
| 1548 | 1549 | α-Elemol | - | - | - | - | - | - | - | - | - | - | 0.6 | - |
| 1558 | 1560 | Germacrene B | - | - | - | - | - | - | - | - | - | 0.2 | - | - |
| 1560 | 1560 | (E)-Nerolidol | 0.2 | 1.3 | 0.9 | 0.4 | 0.3 | 2.8 | 0.9 | 0.6 | 0.3 | 0.1 | 1.0 | 0.8 |
| 1562 | 1564 | epi-Globulol | - | - | 0.5 | 1.3 | - | - | - | - | - | - | - | - |
| 1570 | 1568 | Palustrol | - | - | - | 0.9 | - | - | - | - | - | - | - | - |
| 1570 | 1570 | Neryl 2-methylbutanoate | - | - | - | - | - | - | - | - | - | - | - | 0.1 |
| 1576 | 1575 | Caryolan-8-ol | - | - | 0.3 | - | - | - | - | 0.1 | - | - | - | - |
| 1576 | 1578 | Spathulenol | - | - | 1.0 | 0.5 | - | - | - | - | - | - | - | - |
| 1581 | 1582 | Caryophyllene oxide | - | - | 0.4 | - | - | 0.1 | - | - | - | - | - | - |
| 1582 | --- | Unidentified b | - | - | - | 1.2 | - | - | - | - | - | - | - | - |
| 1585 | 1592 | Globulol | - | - | 2.3 | 5.6 | 0.1 | 0.1 | - | - | - | - | - | - |
| 1594 | 1594 | Viridiflorol | - | - | - | 0.3 | - | - | - | - | - | - | - | - |
| 1595 | 1596 | (E)-β-Elemenone | 0.2 | - | - | - | - | - | - | - | - | - | - | - |
| 1595 | 1593 | Guaiol | - | - | - | - | - | 0.1 | - | 0.1 | - | 0.3 | - | - |
| 1596 | 1596 | Geranyl 2-methylbutanoate | - | - | - | - | - | - | - | - | - | - | - | 0.4 |
| 1597 | 1596 | Cubeban-11-ol | - | - | 0.3 | 0.9 | - | - | - | - | - | - | - | - |
| 1602 | 1601 | Longiborneol | - | - | - | 0.2 | - | - | - | - | - | - | - | - |
| 1604 | 1604 | Humulol | 0.9 | 2.0 | - | - | - | - | - | - | - | - | - | - |
| 1605 | 1607 | 5-epi-7-epi-α-Eudesmol | - | - | - | - | - | 0.1 | - | - | - | - | - | - |
| 1606 | 1609 | Rosifoliol | - | - | - | 0.4 | - | - | - | - | - | - | - | - |
| 1608 | 1610 | Cedrol | - | - | 0.2 | 0.2 | - | - | - | - | - | - | 0.4 | - |
| 1609 | 1613 | Humulene epoxide II | - | 0.4 | - | - | - | - | - | - | - | - | - | - |
| 1616 | 1613 | Ledol | - | - | - | - | - | 0.2 | - | - | - | - | - | - |
| 1624 | 1624 | epi-γ-Eudesmol | - | - | 1.0 | 2.6 | 0.1 | 2.6 | 1.6 | 1.4 | - | - | - | - |
| 1626 | 1624 | Selina-6-en-4β-ol | - | 0.1 | - | - | - | - | - | - | - | - | - | - |
| 1630 | 1632 | γ-Eudesmol | - | - | - | - | - | 4.1 | 2.6 | 1.9 | tr | 0.1 | 2.8 | - |
| 1637 | 1638 | Gossonorol | - | - | - | - | - | 0.1 | 0.1 | - | - | - | - | - |
| 1645 | 1645 | Agarospirol (=Hinesol) | - | - | - | - | - | 0.1 | 0.3 | 0.3 | - | - | - | - |
| 1648 | 1644 | Selina-3,11-dien-6α-ol | - | - | - | - | - | - | - | - | - | 0.1 | - | - |
| 1649 | 1649 | 3-Butylphthalide | - | 0.1 | - | - | - | - | - | - | - | - | - | - |
| 1653 | 1650 | Valerianol | - | - | - | - | - | 1.1 | 0.9 | 1.1 | - | - | - | - |
| 1653 | 1655 | α-Bisabolol oxide B | - | - | - | - | - | - | - | - | 0.6 | - | - | - |
| 1654 | 1655 | α-Cadinol | - | 0.1 | - | - | - | - | - | - | - | - | - | - |
| 1655 | 1655 | α-Eudesmol | - | - | - | - | - | 6.5 | 4.4 | 3.6 | - | 0.6 | 5.0 | 0.2 |
| 1658 | 1660 | neo-Intermedeol | - | - | - | - | - | 0.3 | - | 0.2 | - | - | - | - |
| 1662 | 1664 | ar-Turmerone | - | 0.1 | - | - | - | - | - | - | - | - | - | - |
| 1670 | 1671 | β-Bisabolol | - | - | - | - | - | 0.1 | 1.5 | - | 0.1 | - | - | - |
| 1671 | 1668 | Intermedeol | - | - | 0.6 | - | - | 0.1 | - | 0.2 | 0.1 | - | - | - |
| 1675 | 1673 | Bulnesol | - | - | - | 0.5 | - | - | - | - | - | - | - | - |
| 1685 | 1686 | epi-α-Bisabolol | - | - | 0.3 | - | - | 0.2 | 0.3 | - | - | - | 0.3 | - |
| 1686 | 1688 | α-Bisabolol | - | - | 0.3 | - | - | 0.1 | 0.2 | 22.0 | 26.3 | 0.1 | 0.5 | - |
| 1692 | 1692 | Civetone | - | 0.1 | - | - | - | 0.1 | - | - | - | - | - | - |
| 1692 | 1694 | Germacrone | - | - | - | - | - | - | - | - | - | - | 0.3 | - |
| 1697 | 1696 | Juniper camphor | - | - | - | - | - | - | - | 0.2 | - | 0.2 | - | - |
| 1707 | 1701 | β-Sinensal | - | - | - | - | - | - | 0.2 | - | - | - | - | - |
| 1712 | 1711 | 14-Hydroxy-α-humulene | 1.8 | 1.4 | - | - | - | - | - | - | - | - | - | - |
| 1712 | 1712 | Senkyunolide (=Sedanenolide) | - | - | 0.2 | - | 0.1 | - | - | - | - | - | - | - |
| 1720 | 1722 | 3-Isobutylidene phthalide | - | - | - | - | - | - | - | - | - | - | - | 0.9 |
| 1722 | 1720 | Longifolol | - | 0.3 | - | - | - | - | - | - | - | - | - | - |
| 1741 | 1742 | β-Bergamotol | 1.5 | 0.4 | - | - | - | - | - | - | - | - | - | - |
| 1742 | 1742 | (6S,7R)-Bisabolone | - | - | - | - | - | - | - | - | - | - | 0.2 | - |
| 1760 | 1760 | α-Sinensal | 0.1 | 0.2 | - | - | - | - | - | - | - | - | - | - |
| 1767 | 1769 | Benzyl benzoate | - | - | - | - | - | - | - | - | 0.2 | - | - | - |
| 1767 | 1765 | Eudesmyl acetate | - | - | - | - | - | 2.3 | 0.2 | 0.7 | - | - | - | - |
| 1779 | 1776 | δ-Cuparenol | - | - | - | - | - | - | - | - | - | 4.8 | - | - |
| 1783 | 1784 | Agarospyryl acetate | - | - | - | - | - | 5.3 | 0.5 | 1.6 | - | - | - | - |
| 1878 | 1879 | 4-Phytadiene | - | 0.1 | - | - | - | - | - | - | - | - | - | - |
| 1932 | 1933 | Beyerene | - | 0.1 | 0.1 | - | - | 0.1 | - | - | - | - | - | - |
| 1939 | 1938 | Hexadecalact-16-one | - | - | - | - | - | - | - | - | - | - | 1.8 | - |
| 1944 | 1946 | m-Camphorene | - | 0.3 | 0.2 | 0.1 | - | 0.3 | - | - | - | - | - | - |
| 1959 | --- | Unidentified c | 1.4 | 6.0 | 0.9 | 2.6 | 2.2 | 6.2 | 4.7 | 6.7 | 0.1 | 0.3 | - | - |
| 1959 | 1958 | Palmitic acid | - | - | - | - | - | - | - | - | - | 0.2 | 1.9 | - |
| 1961 | --- | 2-Methyl-4,5-nonadiene d | - | 0.8 | 0.2 | 0.5 | 0.3 | 0.8 | 1.1 | 1.2 | - | - | - | - |
| 1979 | 1984 | p-Camphorene | - | 0.1 | 0.1 | - | - | 0.1 | - | - | - | - | - | - |
| 1981 | 1985 | Vinyl palmitate | - | 0.3 | - | - | - | - | - | - | - | - | - | - |
| 2028 | --- | Unidentified e | - | 0.7 | 0.2 | 0.5 | 1.0 | 0.4 | 0.3 | 0.7 | - | - | - | - |
| 2128 | 2128 | Linoleic acid | - | - | - | - | - | 0.2 | - | - | - | - | 0.2 | - |
| 2148 | 2143 | Serratol | - | - | - | - | - | - | - | - | - | 0.4 | 0.1 | 0.2 |
| 2164 | 2164 | Ethyl linoleate | 0.3 | 1.0 | 0.2 | 0.7 | 0.4 | 0.6 | 0.2 | 0.6 | - | 0.2 | - | - |
| 2203 | --- | Suberosin d | - | - | 0.9 | 0.4 | 2.2 | - | - | - | - | - | - | - |
| 2205 | --- | Unidentified f | - | - | - | - | - | - | - | - | - | - | - | 1.0 |
| 2300 | 2300 | Tricosane | - | - | - | - | - | - | - | - | - | - | 0.2 | 0.1 |
| 2500 | 2500 | Pentacosane | 0.1 | 0.1 | 0.2 | - | 0.2 | tr | - | - | - | 0.1 | 0.4 | 0.1 |
| 2700 | 2700 | Heptacosane | - | - | 0.2 | - | 0.4 | - | - | - | - | 0.2 | 0.4 | 0.1 |
| Monoterpene hydrocarbons | 83.7 | 61.0 | 59.8 | 52.0 | 79.7 | 44.0 | 53.5 | 35.1 | 49.1 | 84.9 | 66.4 | 73.6 | ||
| Oxygenated monoterpenoids | 3.0 | 2.5 | 8.9 | 3.8 | 5.4 | 1.5 | 0.8 | 1.4 | 3.1 | 1.5 | 7.0 | 7.4 | ||
| Sesquiterpene hydrocarbons | 4.5 | 3.3 | 9.9 | 16.5 | 2.6 | 6.2 | 12.6 | 11.3 | 8.8 | 2.8 | 3.8 | 4.3 | ||
| Oxygenated sesquiterpenoids | 4.7 | 6.3 | 8.2 | 13.8 | 0.5 | 26.5 | 13.8 | 34.1 | 27.5 | 6.2 | 11.0 | 1.0 | ||
| Diterpenes | 0.0 | 0.6 | 0.4 | 0.1 | 0.0 | 0.4 | 0.0 | 0.0 | 0.0 | 0.4 | 0.1 | 0.2 | ||
| Benzenoid aromatics | 0.1 | 0.3 | 2.0 | 1.2 | 3.6 | 0.3 | 3.6 | 0.2 | 1.8 | 0.2 | 0.3 | 1.5 | ||
| Others | 1.2 | 13.8 | 3.9 | 3.3 | 3.1 | 9.7 | 8.6 | 7.5 | 8.0 | 3.0 | 10.6 | 4.3 | ||
| Total identified | 97.2 | 87.9 | 93.0 | 90.6 | 94.8 | 88.5 | 92.9 | 89.6 | 98.3 | 98.9 | 99.3 | 92.3 |
| RIcalc | RIdb | Compound | Ln#1 | Ln#2 | Ln#3 | Ln#4 | Ln#5 | Ln#6 | Ln#7 |
|---|---|---|---|---|---|---|---|---|---|
| 926 | 927 | α-Thujene | tr | tr | 0.1 | tr | tr | tr | tr |
| 933 | 933 | α-Pinene | 1.3 | 0.8 | 1.0 | 0.5 | 0.9 | 0.4 | 0.4 |
| 950 | 950 | Camphene | tr | tr | tr | tr | tr | tr | tr |
| 973 | 970 | 3,7,7-Trimethylcyclohepta-1,3,5-triene | - | 0.1 | 0.2 | - | - | - | - |
| 974 | 972 | Sabinene | 0.4 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 |
| 979 | 978 | β-Pinene | 2.1 | 1.3 | 0.5 | 0.9 | 2.0 | 1.0 | 1.2 |
| 990 | 991 | Myrcene | 5.4 | 6.1 | 2.3 | 2.8 | 4.4 | 1.1 | 0.7 |
| 999 | --- | 3,4-Dimethylenecyclopentanone a | tr | 0.1 | - | - | - | - | - |
| 1005 | 1004 | p-Mentha-1(7),8-diene | 0.4 | 0.3 | 0.3 | 0.2 | 0.3 | 0.2 | 0.1 |
| 1007 | 1007 | α-Phellandrene | 2.3 | 0.1 | 0.1 | 1.7 | 2.5 | 1.1 | 0.4 |
| 1010 | 1009 | δ-3-Carene | 0.8 | 5.2 | 12.6 | 1.3 | 1.9 | 0.5 | 0.2 |
| 1018 | 1018 | α-Terpinene | 0.1 | tr | - | 0.1 | 0.2 | tr | tr |
| 1020 | 1022 | m-Cymene | 0.2 | tr | tr | - | - | - | - |
| 1025 | 1025 | p-Cymene | 0.2 | 2.3 | 4.7 | 0.3 | 0.4 | 0.2 | 0.2 |
| 1030 | 1030 | Limonene | 0.1 | 1.0 | 2.5 | 0.5 | 0.4 | 0.2 | 0.3 |
| 1032 | 1031 | β-Phellandrene | 44.7 | 33.3 | 16.5 | 35.8 | 45.7 | 30.3 | 16.0 |
| 1035 | 1034 | (Z)-β-Ocimene | 0.2 | 0.8 | 1.7 | 0.2 | 0.1 | 0.3 | 1.8 |
| 1047 | 1046 | (E)-β-Ocimene | 9.6 | 3.6 | 6.0 | 3.3 | 3.7 | 5.9 | 9.9 |
| 1058 | 1057 | γ-Terpinene | 0.1 | 0.1 | tr | 0.1 | 0.1 | tr | tr |
| 1072 | 1072 | p-Cresol | - | 0.2 | 0.3 | - | - | - | - |
| 1074 | 1073 | α-Pinene oxide | - | - | 0.2 | - | - | - | 0.1 |
| 1082 | 1080 | m-Cymenene | - | - | 0.1 | - | - | - | - |
| 1086 | 1086 | Terpinolene | 1.4 | 1.2 | 1.7 | 1.8 | 2.9 | 0.7 | 0.3 |
| 1091 | 1091 | p-Cymenene | - | 0.8 | 1.4 | - | tr | - | tr |
| 1093 | 1091 | Rosefuran | - | - | - | - | - | tr | 0.1 |
| 1096 | 1097 | α-Pinene oxide | - | - | - | tr | tr | tr | 0.1 |
| 1100 | 1101 | Linalool | 0.6 | 0.2 | 0.2 | 0.2 | 0.2 | 0.5 | - |
| 1103 | 1101 | 6-Methylhepta-3,5-dien-2-one | - | - | 0.1 | - | - | - | - |
| 1104 | 1104 | 2-Methylbutyl 2-methylbutyrate | - | - | - | tr | tr | 0.1 | - |
| 1125 | 1124 | cis-p-Menth-2-en-1-ol | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | tr | tr |
| 1127 | 1131 | Cyclooctanone | - | 0.1 | 0.5 | - | - | - | - |
| 1128 | 1127 | allo-Ocimene | - | - | - | - | - | - | 0.1 |
| 1136 | 1130 | (Z)-Myroxide | - | 0.1 | 0.3 | - | - | - | tr |
| 1139 | 1339 | 3-Oxo-p-menth-1-en-7-al | tr | 0.5 | 0.3 | - | - | - | - |
| 1140 | 1141 | (E)-Myroxide | 0.1 | 0.6 | 1.3 | - | - | - | tr |
| 1143 | 1142 | trans-p-Menth-2-en-1-ol | 0.1 | - | - | 0.1 | 0.1 | tr | tr |
| 1158 | 1156 | Pentylbenzene | - | - | - | tr | tr | tr | tr |
| 1159 | 1161 | 5-Pentylcyclohexa-1,3-diene | 0.3 | - | - | 0.3 | 0.1 | 0.2 | 0.2 |
| 1175 | 1175 | (3E,5Z)-1,3,5-Undecatriene | 0.1 | - | - | 0.1 | 0.1 | 0.1 | tr |
| 1178 | 1180 | (E)-Isocitral | - | - | - | - | tr | tr | - |
| 1179 | 1180 | Terpinen-4-ol | tr | 0.1 | - | tr | tr | tr | - |
| 1183 | 1188 | p-Methylacetophenone | - | 0.2 | 0.6 | - | - | - | - |
| 1185 | 1187 | Cryptone | 0.8 | 6.4 | 7.7 | 0.4 | 0.5 | 0.4 | 0.3 |
| 1185 | 1185 | (3E,5E)-1,3,5-Undecatriene | - | - | - | tr | tr | tr | tr |
| 1194 | 1195 | α-Terpineol | 0.1 | - | - | tr | tr | tr | - |
| 1196 | 1195 | trans-4-Caranone | 0.1 | 0.7 | 0.3 | - | - | - | - |
| 1197 | 1195 | p-Menth-3-en-7-al | - | 0.5 | 0.3 | 0.1 | 0.1 | 0.1 | tr |
| 1201 | 1202 | cis-Sabinol | 0.1 | 0.4 | 0.6 | 0.1 | 0.1 | 0.1 | tr |
| 1208 | 1208 | trans-Piperitol | tr | - | - | - | tr | - | - |
| 1224 | 1227 | Citronellol | 0.6 | 0.5 | 1.0 | 0.3 | 0.4 | 0.1 | - |
| 1241 | 1247 | Cuminal | - | 0.3 | 0.2 | - | - | - | - |
| 1249 | 1254 | cis-Piperitone epoxide | - | 0.2 | - | - | - | - | - |
| 1263 | 1265 | (2E)-Decenal | - | 0.2 | - | - | - | - | - |
| 1272 | 1294 | p-Mentha-1,5-diene-7-ol | 0.1 | 0.5 | 0.4 | 0.1 | 0.1 | 0.1 | - |
| 1277 | 1277 | Phellandral | - | 0.3 | 0.2 | - | - | - | - |
| 1286 | 1286 | α-Terpinen-7-al | tr | 0.1 | 0.2 | - | - | - | - |
| 1291 | 1291 | p-Cymen-7-ol | 0.1 | 0.5 | 0.4 | tr | tr | tr | - |
| 1308 | 1313 | Phthalic anhydride | - | 1.2 | 1.7 | - | - | - | - |
| 1320 | 1318 | 3-Hydroxycineole | - | 0.2 | 0.4 | - | - | - | - |
| 1321 | 1320 | Methyl geranate | - | - | - | tr | tr | tr | - |
| 1322 | 1318 | 4-Hydroxycryptone | - | 0.1 | 0.2 | - | - | - | - |
| 1336 | 1336 | δ-Elemene | - | - | - | - | tr | tr | tr |
| 1339 | 1339 | 3-Oxo-p-menth-1-en-7-al | - | - | - | tr | tr | tr | tr |
| 1343 | --- | Unidentified b | 0.4 | 2.0 | 2.2 | 0.6 | 0.5 | 0.8 | 0.9 |
| 1375 | 1375 | α-Copaene | tr | - | - | - | - | - | - |
| 1382 | 1383 | cis-β-Elemene | - | - | - | - | tr | 0.1 | tr |
| 1389 | 1390 | trans-β-Elemene | 0.1 | - | - | 0.5 | 0.3 | 0.7 | 0.8 |
| 1420 | 1417 | (E)-β-Caryophyllene | 0.1 | 0.1 | 0.3 | 0.3 | 0.3 | 0.8 | 0.1 |
| 1429 | 1427 | γ-Elemene | 0.6 | 0.1 | 0.2 | 3.0 | 1.7 | 3.9 | 3.7 |
| 1452 | 1452 | (E)-β-Farnesene | 0.1 | 0.7 | 0.7 | 0.1 | 0.2 | 0.2 | 0.4 |
| 1456 | 1454 | α-Humulene | - | - | - | 0.1 | 0.1 | 0.1 | 0.1 |
| 1465 | 1463 | γ-Decalactone | - | - | - | 0.1 | tr | - | 0.1 |
| 1479 | 1480 | Germacrene D | 0.1 | - | - | 0.7 | 0.4 | 1.0 | 0.9 |
| 1485 | 1483 | Phenylethyl 2-methylbutyrate | 0.1 | 0.1 | - | 0.1 | 0.1 | 0.1 | 0.1 |
| 1489 | 1489 | β-Selinene | - | - | - | 0.1 | tr | 0.1 | 0.1 |
| 1490 | 1493 | Phenylethyl 3-methylbutanoate | - | - | - | - | - | - | 0.1 |
| 1537 | 1540 | Selina-4(15),7(11)-diene | - | - | - | - | - | 0.1 | - |
| 1542 | 1541 | (E)-α-Bisabolene | tr | 0.1 | tr | - | - | - | - |
| 1549 | 1549 | α-Elemol | - | - | - | - | - | - | 0.1 |
| 1551 | --- | 7-Hydroxypiperitone a | - | 0.3 | 0.3 | - | - | - | - |
| 1560 | 1557 | Germacrene B | 1.3 | 0.2 | 0.4 | 4.5 | 2.6 | 6.4 | 9.3 |
| 1574 | 1572 | Citronellyl 2-methylbutyrate | 0.1 | 0.4 | 0.3 | - | 0.1 | 0.1 | 0.1 |
| 1586 | 1587 | Caryophyllene oxide | tr | 0.1 | 0.4 | - | tr | 0.1 | - |
| 1596 | 1596 | Geranyl 2-methylbutyrate | tr | 0.1 | - | - | tr | - | - |
| 1633 | 2632 | Tetracosanal | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | - |
| 1654 | 1649 | 3-Butyl phthalide | - | 0.1 | 0.1 | - | - | - | - |
| 1668 | 1669 | (2E,6Z)-Farnesol | 2.1 | 1.1 | 2.5 | 0.2 | 0.5 | 0.3 | - |
| 1670 | 1669 | (3Z)-Butylidene phthalide | 0.5 | 2.1 | 2.1 | 0.4 | 0.3 | 0.5 | 1.1 |
| 1675 | 1674 | γ-Dodecalactone | - | - | - | 0.1 | tr | - | - |
| 1684 | 1684 | (2Z,6Z)-Farnesal | tr | 0.1 | 0.2 | - | - | - | - |
| 1693 | 1692 | (2Z,6Z)-Farnesol | 0.1 | - | - | - | - | - | - |
| 1707 | 1705 | 14-Hydroxy-4,5-dihydrocaryophyllene | tr | - | - | - | - | - | - |
| 1713 | 1716 | (2E,6E)-Farnesol | - | 0.1 | 0.1 | - | - | - | - |
| 1714 | 1712 | (Z)-Sedanenolide | 0.2 | - | - | - | 0.3 | 0.5 | - |
| 1719 | 1719 | (3E)-Butylidene phthalide | 0.2 | 0.6 | 1.4 | 0.3 | - | - | 0.4 |
| 1722 | 1722 | 3-Isobutylidene phthalide | - | - | 2.8 | 0.2 | - | - | - |
| 1728 | 1730 | (Z)-Ligustilide | 17.4 | 8.6 | 5.6 | 33.2 | 22.4 | 33.0 | 47.1 |
| 1772 | 1772 | α-Costol | - | - | 0.3 | 0.2 | 0.1 | 0.4 | - |
| 1788 | 1790 | (E)-Ligustilide | 0.9 | 0.2 | 0.2 | 2.9 | 2.0 | 4.8 | 1.8 |
| 1807 | --- | Unidentified oxygenated sesquiterpenoid c | 0.5 | 2.2 | 1.3 | 0.6 | 0.3 | 0.8 | 0.9 |
| 1933 | 1928 | Methyl linolenate | tr | 0.1 | - | - | - | - | - |
| 1959 | 1958 | Palmitic acid | 0.2 | 0.4 | 0.3 | - | - | - | - |
| 2004 | 2005 | Senkyunolide H | - | 2.6 | 1.8 | - | - | - | - |
| 2036 | 2037 | (Z)-Falcarinol | 0.1 | - | - | - | - | - | - |
| 2128 | 2128 | (Z,Z)-Linoleic acid | 0.1 | - | - | - | - | - | - |
| 2300 | 2300 | Tricosane | 0.1 | 0.2 | 0.1 | - | - | - | - |
| 2439 | 2442 | 2-Methyltetracosane | - | - | 1.2 | - | - | - | - |
| 2500 | 2500 | Pentacosane | 0.3 | 0.3 | 0.4 | 0.1 | 0.1 | 0.1 | tr |
| 2700 | 2700 | Heptacosane | 0.2 | 0.2 | 0.2 | - | - | - | - |
| 2800 | 2800 | Octacosane | tr | - | - | - | - | - | - |
| 2838 | 2833 | Hexacosanal | 0.7 | - | - | - | - | - | - |
| Monoterpene hydrocarbons | 69.4 | 57.2 | 51.7 | 49.7 | 65.6 | 42.0 | 31.6 | ||
| Oxygenated monoterpenoids | 2.7 | 13.1 | 14.8 | 1.2 | 1.6 | 1.4 | 0.6 | ||
| Sesquiterpene hydrocarbons | 2.3 | 1.1 | 1.6 | 9.3 | 5.5 | 13.4 | 15.2 | ||
| Oxygenated sesquiterpenoids | 2.2 | 1.3 | 3.5 | 0.4 | 0.6 | 0.8 | 0.1 | ||
| Benzenoid aromatics | 19.2 | 13.4 | 14.6 | 37.0 | 24.8 | 38.4 | 1.7 | ||
| Others | 2.5 | 4.3 | 4.7 | 0.7 | 0.6 | 1.1 | 49.2 | ||
| Total identified | 98.3 | 90.4 | 90.8 | 98.3 | 98.7 | 97.2 | 98.3 |
| RIcalc | RIdb | Compound | Lpap#1 (OR) | Lpap#2 (OR) | Lpap#3 (OR) | Lpap#4 (OR) | Lpap#5 (ID) | Lpap#6 (ID) | Lpap#7 (ID) | Lpap#8 (ID) |
|---|---|---|---|---|---|---|---|---|---|---|
| 808 | 806 | Hexanal | - | - | - | - | - | 0.1 | - | - |
| 849 | 849 | (2E)-Hexenal | 0.2 | tr | tr | tr | - | - | - | - |
| 908 | 906 | Heptanal | - | - | - | - | 0.1 | 0.2 | 0.1 | 0.1 |
| 920 | 921 | Hashishene | tr | - | - | - | - | - | - | - |
| 922 | 923 | Tricyclene | tr | tr | tr | tr | - | - | - | - |
| 925 | 925 | α-Thujene | tr | 0.1 | 0.1 | 0.1 | - | - | - | - |
| 933 | 933 | α-Pinene | 0.7 | 1.8 | 2.1 | 2.1 | tr | tr | 0.1 | 0.2 |
| 947 | 948 | α-Fenchene | tr | - | - | - | - | - | - | - |
| 949 | 950 | Camphene | 1.7 | 0.8 | 1.0 | 0.7 | 0.2 | tr | 0.8 | 0.8 |
| 951 | 955 | Propylbenzene | - | - | - | - | tr | 0.5 | 0.1 | 0.7 |
| 965 | 963 | 2-Methyl-(3E)-octen-5-yne | 0.2 | - | - | - | - | - | 0.2 | 0.2 |
| 970 | 972 | Tetrahydrofurfuryl acetate | 0.1 | - | - | tr | - | - | - | - |
| 972 | 971 | Sabinene | 0.2 | 0.7 | 0.9 | 0.6 | - | - | - | - |
| 978 | 978 | β-Pinene | 0.1 | 0.3 | 0.3 | 0.4 | - | tr | tr | tr |
| 984 | 984 | 6-Methylhept-5-en-2-one | - | - | - | - | tr | 0.1 | 0.1 | tr |
| 989 | 989 | Myrcene | 27.5 | 5.4 | 6.8 | 3.1 | 0.2 | 0.3 | 0.4 | 0.6 |
| 990 | 990 | Dehydro-1,8-cineole | - | tr | tr | tr | - | - | - | - |
| 1000 | 1000 | δ-2-Carene | tr | tr | tr | 0.1 | - | - | - | - |
| 1005 | 1004 | p-Mentha-1(7),8-diene | 0.1 | 0.1 | 0.1 | 0.1 | - | - | - | - |
| 1007 | 1007 | α-Phellandrene | - | 1.1 | 1.1 | - | tr | tr | tr | tr |
| 1009 | 1009 | δ-3-Carene | tr | 0.4 | 0.4 | 0.1 | tr | tr | tr | tr |
| 1017 | 1017 | α-Terpinene | - | 0.3 | 0.3 | - | 0.1 | 0.2 | 0.3 | 0.3 |
| 1019 | 1016 | Tetrahydro-2-furanmethanol acetate | - | - | - | 0.1 | - | - | - | - |
| 1025 | 1025 | p-Cymene | 6.0 | 3.1 | 2.6 | 47.8 | 22.9 | 20.9 | 20.4 | 21.1 |
| 1029 | 1030 | Limonene | 2.4 | 1.6 | 2.0 | 3.0 | 0.2 | 0.2 | 0.6 | 0.6 |
| 1032 | 1031 | β-Phellandrene | 0.8 | 23.8 | 23.2 | 5.7 | tr | tr | tr | tr |
| 1032 | 1032 | 1,8-Cineole | - | - | - | - | tr | tr | tr | tr |
| 1033 | 1033 | Benzyl alcohol | - | - | - | - | - | tr | tr | tr |
| 1035 | 1034 | (Z)-β-Ocimene | 0.2 | 0.2 | 0.2 | 0.2 | tr | - | tr | tr |
| 1043 | 1043 | Phenylacetaldehyde | - | - | - | - | tr | tr | tr | tr |
| 1046 | 1046 | (E)-β-Ocimene | 0.7 | 7.2 | 7.2 | 2.9 | 0.3 | tr | 0.1 | tr |
| 1051 | 1051 | 2,3,6-Trimethylhepta-1,5-diene | 0.3 | - | - | - | - | - | - | - |
| 1058 | 1058 | γ-Terpinene | 0.1 | 30.9 | 28.6 | 3.1 | 7.3 | 9.1 | 10.3 | 15.1 |
| 1070 | 1069 | cis-Linalool oxide (furanoid) | - | - | - | 0.2 | - | - | - | - |
| 1071 | 1072 | Dihydromyrcenol | 0.2 | - | - | - | - | - | - | - |
| 1085 | 1086 | Terpinolene | - | 2.5 | 2.4 | - | 0.2 | 0.2 | 0.3 | 0.4 |
| 1086 | 1086 | trans-Linalool oxide (furanoid) | - | - | - | 0.1 | - | - | - | - |
| 1090 | 1090 | 6,7-Epoxymyrcene | 0.7 | - | - | 0.4 | - | - | - | - |
| 1090 | 1091 | p-Cymenene | - | - | - | - | tr | 0.1 | tr | tr |
| 1091 | 1091 | Rosefuran | 0.4 | - | - | 0.1 | - | - | - | - |
| 1095 | 1097 | α-Pinene oxide | 0.1 | - | - | 0.1 | - | - | - | - |
| 1098 | 1098 | Perillene | 0.6 | - | - | - | - | - | - | - |
| 1100 | 1101 | Linalool | 0.5 | 1.4 | 1.8 | 0.4 | tr | tr | 0.1 | tr |
| 1103 | 1102 | 6-Methylhepta-3,5-dien-2-one | 0.4 | - | - | 0.1 | tr | tr | tr | tr |
| 1105 | 1107 | Nonanal | 0.1 | - | - | - | tr | tr | tr | tr |
| 1120 | 1119 | Myrcenol | 0.2 | - | - | - | - | - | - | - |
| 1121 | 1121 | Isopentylbenzene | - | - | - | - | - | tr | tr | tr |
| 1122 | 1121 | (3E,5E)-1,3,5-Undecatriene | - | - | - | - | - | tr | tr | tr |
| 1124 | 1124 | cis-p-Menth-2-en-1-ol | - | 0.1 | 0.1 | 0.1 | - | - | - | - |
| 1127 | 1126 | α-Campholenal | - | - | - | 0.1 | - | - | - | - |
| 1129 | 1130 | (Z)-Myroxide | 0.1 | - | - | tr | - | - | - | - |
| 1133 | 1132 | cis-Limonene oxide | - | - | - | tr | - | - | - | - |
| 1137 | 1138 | trans-Limonene oxide | - | - | - | 0.1 | - | - | - | - |
| 1139 | 1139 | (E)-Myroxide | 0.5 | - | - | 0.4 | - | - | - | - |
| 1142 | 1142 | Epoxyterpinolene | - | 0.1 | 0.1 | - | - | - | - | - |
| 1143 | 1142 | trans-p-Menth-2-en-1-ol | - | - | - | 0.2 | - | - | - | - |
| 1147 | 1149 | Camphor | 0.2 | - | - | 0.1 | - | - | - | - |
| 1156 | 1156 | Pentylbenzene | 0.1 | tr | tr | 0.5 | - | - | - | - |
| 1156 | 1156 | Camphene hydrate | 0.2 | - | - | - | - | - | tr | tr |
| 1156 | 1156 | Pentylbenzene | - | - | - | - | tr | 0.1 | - | tr |
| 1157 | 1161 | 5-Pentylcyclohexa-1,3-diene | - | 0.9 | 1.0 | - | 0.1 | 0.2 | 0.1 | 0.1 |
| 1159 | 1163 | (2E)-Nonenal | - | - | - | - | - | tr | - | tr |
| 1162 | --- | 2-Propylphenyl methyl ether a | - | - | - | - | - | 0.3 | - | 0.1 |
| 1162 | 1162 | (E,E)-2,6-Dimethyl-3,5,7-octatriene-2-ol | - | tr | tr | 0.6 | 0.1 | - | tr | - |
| 1169 | 1169 | Rosefuran epoxide | 0.1 | - | - | 0.1 | - | - | - | - |
| 1172 | 1173 | Borneol | 0.1 | - | - | - | - | - | - | - |
| 1174 | 1175 | (3E,5Z)-1,3,5-Undecatriene | - | tr | tr | - | - | - | - | - |
| 1180 | 1180 | Terpinen-4-ol | 0.1 | 0.1 | 0.1 | 0.1 | tr | - | 0.1 | 0.1 |
| 1185 | 1188 | p-Methylacetophenone | tr | - | - | - | - | - | - | - |
| 1186 | 1189 | p-Cymen-8-ol | - | - | - | - | 0.1 | 0.6 | 0.1 | tr |
| 1187 | 1187 | Cryptone | 0.7 | 0.2 | 0.2 | 2.7 | - | - | - | - |
| 1189 | 1195 | trans-4-Caranone | - | - | - | 0.1 | - | - | - | - |
| 1195 | 1195 | α-Terpineol | 0.2 | tr | tr | - | tr | - | tr | tr |
| 1197 | 1205 | cis-4-Caranone | - | tr | tr | 0.3 | - | - | - | - |
| 1203 | 1202 | cis-Sabinol | - | tr | tr | - | - | - | - | - |
| 1207 | 1208 | Verbenone | - | - | - | 0.1 | - | - | - | - |
| 1207 | 1208 | Decanal | 0.1 | - | - | - | - | - | - | - |
| 1209 | 1209 | trans-Piperitol | - | - | - | tr | - | - | - | - |
| 1209 | 1207 | (3E)-Octenyl acetate | 0.2 | - | - | - | - | - | - | - |
| 1210 | 1211 | Octyl acetate | 0.1 | - | - | - | - | - | - | - |
| 1223 | 1231 | trans-Chrysanthenyl acetate | - | tr | tr | - | - | - | - | - |
| 1223 | 1224 | Thymyl methyl ether | - | - | - | - | 0.3 | 0.2 | 0.2 | 0.3 |
| 1238 | 1238 | Carvacryl methyl ether | - | - | - | - | 0.4 | 0.3 | 0.4 | 0.5 |
| 1242 | 1242 | Cuminaldehyde | - | - | - | 0.1 | - | - | - | - |
| 1254 | 1254 | Piperitone | 0.7 | 3.3 | 4.1 | 5.9 | - | - | - | - |
| 1265 | 1265 | (2E)-Decenal | - | - | - | 0.2 | - | - | - | - |
| 1273 | 1271 | 1-Decanol | 2.2 | - | - | - | - | - | - | - |
| 1283 | 1282 | Bornyl acetate | 0.1 | - | - | - | 0.7 | 0.1 | 3.9 | 2.9 |
| 1285 | 1287 | Limonene dioxide | - | - | - | 0.1 | - | - | - | - |
| 1286 | 1287 | iso-Bornyl acetate | - | - | - | - | - | - | tr | tr |
| 1289 | 1289 | Thymol | - | 0.1 | 0.1 | 0.1 | tr | tr | tr | tr |
| 1290 | 1289 | (9Z)-Tetradecenal | 0.1 | - | - | - | - | - | - | - |
| 1292 | 1291 | p-Cymen-7-ol | - | - | - | 0.2 | - | - | - | - |
| 1298 | 1300 | Carvacrol | - | tr | tr | 0.1 | tr | tr | tr | 0.1 |
| 1314 | 1321 | 2-Methyl-5-(propan-2-ylidene)cyclohexane-1,4-diol | - | - | - | 0.2 | - | - | - | - |
| 1323 | 1318 | 4-Hydroxycryptone | - | - | - | 0.1 | - | - | - | - |
| 1339 | 1339 | 3-Oxo-p-Menth-1-en-7-al | - | - | - | 0.1 | - | - | - | - |
| 1342 | 1343 | 2-(2,5-Dimethylphenyl)propanal | - | - | - | 0.2 | - | - | - | - |
| 1350 | 1352 | α-Longipinene | - | - | - | - | 0.1 | 0.1 | 0.1 | 0.2 |
| 1369 | 1367 | Cyclosativene | - | - | - | - | 0.1 | 0.1 | 0.1 | 0.2 |
| 1372 | 1370 | iso-Ledene | - | - | - | - | tr | 0.1 | tr | tr |
| 1374 | 1372 | Longicyclene | - | - | - | - | 0.3 | 0.1 | 0.3 | 0.3 |
| 1375 | 1375 | α-Copaene | - | - | - | - | - | 0.1 | 0.1 | 0.1 |
| 1382 | 1383 | 2-epi-α-Funebrene | - | - | - | - | 0.4 | 0.4 | 0.4 | 0.5 |
| 1384 | 1385 | α-Duprezianene | - | - | - | - | 0.9 | 0.7 | 0.8 | 0.8 |
| 1390 | 1390 | β-Elemene | - | - | - | 0.1 | - | - | - | - |
| 1392 | 1392 | (Z)-Jasmone | - | tr | - | 0.1 | - | - | - | - |
| 1399 | 1403 | Methyl eugenol | - | - | - | - | 0.7 | 4.0 | 0.1 | 0.1 |
| 1402 | 1403 | α-Funebrene | - | - | - | - | 0.3 | 0.3 | 0.3 | 0.3 |
| 1405 | 1403 | di-epi-α-Cedrene | - | - | - | - | 0.2 | 0.1 | 0.1 | 0.1 |
| 1408 | 1408 | Isopropyl 4-ethylbenzoate | - | - | - | 0.1 | - | - | - | - |
| 1408 | 1409 | Decyl acetate | 6.0 | - | - | - | - | - | - | - |
| 1409 | 1411 | Longifolene | - | - | - | - | 3.6 | 1.6 | 3.3 | 3.5 |
| 1416 | 1414 | α-Cedrene | - | - | - | - | 0.2 | 0.1 | 0.1 | 0.1 |
| 1417 | 1416 | 2-epi-β-Funebrene | - | - | - | - | 0.3 | 0.3 | 0.4 | 0.4 |
| 1418 | 1417 | (E)-β-Caryophyllene | 0.1 | tr | 0.1 | - | 0.2 | 0.1 | 0.1 | 0.1 |
| 1424 | 1423 | β-Cedrene | - | - | - | - | 0.1 | 0.1 | 0.1 | 0.1 |
| 1426 | 1428 | β-Duprezianene | - | - | - | - | 0.1 | 0.1 | 0.1 | 0.1 |
| 1428 | 1427 | γ-Elemene | - | tr | tr | - | - | - | - | - |
| 1432 | 1432 | trans-α-Bergamotene | 0.1 | 0.2 | 0.2 | - | - | - | - | - |
| 1435 | 1433 | cis-Thujopsene | - | - | - | - | 0.1 | 0.1 | 0.1 | 0.1 |
| 1437 | 1437 | iso-Bazzanene | - | - | - | - | 0.2 | 0.1 | 0.1 | 0.1 |
| 1441 | 1442 | Guaia-6,9-diene | - | - | - | - | 0.1 | - | - | - |
| 1447 | 1447 | Geranylacetone | - | - | - | - | 0.3 | 0.7 | 0.4 | 0.4 |
| 1450 | 1449 | α-Himachalene | 1.2 | - | - | - | 0.6 | 0.2 | 0.5 | 0.4 |
| 1451 | 1451 | (E)-β-Farnesene | 0.1 | tr | tr | - | 1.5 | 1.1 | 1.7 | 2.1 |
| 1453 | 1450 | 1,2,2α,3,3,4,6,7,8,8α-Decahydro-2α,7,8-trimethylacenaphthylene | - | - | - | - | - | 0.6 | - | 0.7 |
| 1455 | 1454 | α-Humulene | 1.4 | tr | tr | - | - | - | - | - |
| 1457 | 1457 | allo-Aromadendrene | 0.5 | - | - | - | - | - | - | - |
| 1457 | 1461 | Amorpha-4,11-diene | - | - | - | - | 1.1 | 0.6 | 0.7 | 0.6 |
| 1459 | 1456 | 7-Isopropenyl-1-methyl-4-methylenedecahydroazulene | - | - | - | - | 0.1 | 0.1 | 0.1 | 0.1 |
| 1464 | 1463 | γ-Decalactone | - | 0.1 | 0.1 | 0.1 | - | - | - | - |
| 1466 | 1467 | β-Acoradiene | - | - | - | - | 0.1 | tr | - | - |
| 1473 | 1473 | γ-Selinene | - | - | - | - | - | tr | - | 0.1 |
| 1473 | 1474 | 10-epi-β-Acoradiene | - | - | - | - | 0.1 | - | - | - |
| 1474 | 1476 | 1-Dodecanol | 0.3 | - | - | - | - | - | - | - |
| 1474 | 1474 | 4-epi-α-Acoradiene | - | - | - | - | 0.2 | 0.1 | 0.1 | 0.1 |
| 1476 | 1477 | trans-Cadina-1(6),4-diene | - | - | - | - | 0.2 | 0.1 | - | 0.1 |
| 1478 | 1479 | α-Amorphene | 0.2 | - | - | - | 0.6 | tr | - | 0.3 |
| 1478 | 1480 | γ-Himachalene | - | - | - | - | - | - | 0.2 | - |
| 1480 | 1480 | Germacrene D | - | 0.6 | 0.6 | - | - | - | - | - |
| 1480 | 1480 | ar-Curcumene | - | - | - | - | 0.8 | 0.6 | 0.7 | 0.6 |
| 1485 | 1488 | 4-epi-(Z)-Dihydroagarofuran | - | - | - | - | 0.4 | - | - | - |
| 1488 | 1487 | β-Selinene | - | - | - | - | - | 0.1 | 0.1 | 0.1 |
| 1490 | 1491 | δ-Decalactone | - | 0.1 | 0.1 | 0.1 | - | - | - | - |
| 1490 | 1489 | (Z,E)-α-Farnesene | - | - | - | - | 0.2 | 0.2 | 0.1 | 0.4 |
| 1498 | 1499 | Benzyl tiglate | - | tr | - | 0.1 | - | - | - | - |
| 1498 | 1497 | α-Muurolene | 0.2 | - | - | - | tr | 0.1 | 0.1 | 0.1 |
| 1500 | 1503 | β-Himachalene | - | - | - | - | 0.3 | 0.1 | 0.3 | 0.3 |
| 1503 | 1504 | α-Cuprenene | - | - | - | - | 0.3 | 0.1 | 0.2 | 0.3 |
| 1506 | 1507 | Geranyl isobutyrate | - | 0.1 | 0.1 | - | - | - | - | - |
| 1506 | 1508 | β-Bisabolene | 0.1 | - | - | - | - | - | - | - |
| 1506 | 1506 | α-Chamigrene | - | - | - | - | 1.8 | 2.1 | 1.0 | 1.9 |
| 1508 | 1505 | Cuparene | - | - | - | - | 6.0 | 4.1 | 4.9 | 3.5 |
| 1512 | 1512 | γ-Cadinene | 0.3 | tr | tr | - | - | - | - | - |
| 1518 | 1523 | β-Guaiene | 0.7 | - | - | - | - | - | - | - |
| 1518 | 1518 | δ-Cadinene | 0.1 | tr | tr | - | 0.2 | 0.3 | 0.5 | 0.3 |
| 1519 | 1519 | trans-Calamenene | - | - | - | - | 0.1 | 0.1 | tr | - |
| 1522 | 1521 | Zonarene | - | - | - | - | - | 0.1 | 0.1 | - |
| 1526 | 1528 | (E)-γ-Bisabolene | - | - | - | - | 0.2 | 0.1 | 0.2 | 0.2 |
| 1528 | 1528 | Kessane | - | tr | tr | 0.1 | - | 0.1 | tr | - |
| 1534 | 1535 | γ-Cuprenene | - | - | - | - | 1.1 | 0.6 | 0.8 | 0.7 |
| 1541 | 1541 | α-Calacorene | - | - | - | - | 0.5 | 0.1 | 0.2 | 0.2 |
| 1546 | 1548 | Elemicin | - | - | - | - | - | 0.1 | - | - |
| 1547 | 1549 | α-Agarofuran | - | - | - | - | - | 0.1 | 0.1 | 0.1 |
| 1555 | 1555 | (Z)-Dihydronerolidol | 0.4 | - | - | - | - | - | - | - |
| 1558 | 1557 | Germacrene B | - | tr | tr | - | - | - | - | - |
| 1559 | 1560 | (E)-Nerolidol | 4.6 | tr | tr | - | 0.5 | 0.5 | 0.6 | 0.6 |
| 1569 | 1570 | (E)-Dihydronerolidol | 0.2 | - | - | - | - | - | - | - |
| 1575 | 1575 | Caryolan-8-ol | 0.2 | - | - | - | 0.1 | tr | - | - |
| 1576 | 1576 | Spathulenol | - | tr | tr | - | - | - | - | - |
| 1581 | 1587 | Caryophyllene oxide | 0.2 | - | - | 0.6 | - | - | - | - |
| 1582 | 1584 | 10-epi-Juneol | - | - | - | - | - | 0.4 | 0.1 | 0.3 |
| 1594 | 1595 | Geranyl 2-methylbutyrate | 0.2 | 0.1 | 0.2 | - | - | - | - | - |
| 1596 | 1593 | Guaiol | - | - | - | - | 1.2 | 0.3 | 0.2 | 0.1 |
| 1598 | 1596 | Humulene epoxide I | 0.4 | - | - | - | - | - | - | - |
| 1602 | 1602 | Geranyl isovalerate | - | 0.1 | 0.1 | - | - | - | - | - |
| 1602 | 1601 | Longiborneol | - | - | - | - | 0.2 | 0.1 | 0.1 | 0.2 |
| 1603 | 1604 | Humulol | 8.4 | tr | tr | - | - | - | - | - |
| 1606 | 1606 | Cedrol | - | - | - | - | 0.6 | 0.7 | 0.5 | 0.5 |
| 1609 | 1611 | Humulene epoxide II | 3.8 | - | - | 0.3 | - | - | - | - |
| 1613 | 1612 | 5-epi-7-epi-β-Eudesmol | - | - | - | - | 0.1 | - | - | - |
| 1621 | 1624 | epi-γ-Eudesmol | 0.2 | - | - | - | - | - | - | - |
| 1622 | 1624 | 10-epi-γ-Eudesmol | - | - | - | - | 1.1 | 3.6 | 2.1 | 1.7 |
| 1625 | 1624 | Selin-6-en-4β-ol | 0.1 | - | - | - | - | - | - | - |
| 1625 | 1631 | Eremoligenol | - | - | - | - | 0.2 | 0.4 | 0.3 | 0.2 |
| 1627 | 1628 | 1-epi-Cubenol | - | - | - | - | - | 0.1 | 0.1 | - |
| 1632 | 1633 | γ-Eudesmol | - | - | - | - | 0.2 | 0.4 | 0.2 | 0.2 |
| 1638 | 1644 | allo-Aromadendrene epoxide | - | 0.1 | 0.1 | - | - | - | - | - |
| 1641 | 1641 | τ-Muurolol | 0.1 | - | - | - | - | - | - | - |
| 1648 | 1649 | 3-Butylphthalide | 2.2 | 0.8 | 0.8 | 5.1 | - | - | - | - |
| 1652 | 1646 | Agarospirol (=Hinesol) | 0.1 | - | - | - | - | - | - | - |
| 1653 | 1652 | α-Eudesmol | 0.2 | - | - | - | - | - | - | - |
| 1653 | 1657 | Valerianol | - | - | - | - | 0.7 | 2.5 | 1.2 | 1.1 |
| 1654 | 1655 | α-Cadinol | 0.3 | - | - | - | - | - | - | - |
| 1655 | 1656 | β-Eudesmol | - | - | - | - | 1.2 | 2.1 | 1.1 | 0.8 |
| 1661 | 1664 | 7-epi-α-Eudesmol | - | - | - | - | - | 0.3 | 0.2 | 0.1 |
| 1665 | 1664 | Bulnesol | - | - | - | - | 0.8 | 0.1 | - | - |
| 1669 | 1671 | β-Bisabolol | - | - | - | - | 0.1 | 0.1 | 0.1 | 0.1 |
| 1672 | 1673 | Cadalene | - | - | - | - | 0.1 | - | - | - |
| 1676 | 1674 | γ-Dodecalactone | - | - | - | 0.1 | - | - | - | - |
| 1678 | 1676 | 1-Tetradecanol | - | - | - | - | - | 0.2 | - | 0.1 |
| 1684 | 1686 | epi-α-Bisabolol | 0.2 | - | - | - | - | - | - | - |
| 1686 | 1686 | α-Bisabolol | 0.1 | - | - | - | 1.0 | 0.2 | 1.8 | 0.1 |
| 1691 | 1686 | Octadec-(13Z)-enal | 0.3 | - | - | - | - | - | - | - |
| 1712 | 1712 | Sedanenolide (=Senkyunolide A) | 1.5 | 10.8 | 10.7 | 1.8 | - | - | - | - |
| 1728 | 1730 | (Z)-Ligustilide | - | tr | tr | - | - | - | - | - |
| 1756 | 1756 | Hexadec-(11E)-en-1-ol | 0.4 | - | - | - | - | - | - | - |
| 1781 | 1776 | 2-Methyl-5-(1,2,2-trimethylcyclopentyl)phenol | - | - | - | - | 30.5 | 31.5 | 29.3 | 24.9 |
| 1794 | 1796 | Hexadec-(9Z)-enal | 0.3 | - | - | - | - | - | - | - |
| 1802 | --- | Unidentified b | - | - | - | - | 0.8 | 0.7 | 1.8 | 1.3 |
| 1876 | 1878 | Hexadec-(2E)-enal | 0.5 | - | - | - | - | - | - | - |
| 1932 | 1933 | Beyerene | 0.3 | tr | tr | - | - | - | - | - |
| 1940 | 1938 | Hexadecanolact-16-one | - | - | - | - | 1.0 | 0.3 | 0.3 | 0.2 |
| 2035 | 2037 | (Z)-Falcarinol | - | tr | - | - | - | - | - | - |
| 2105 | 2106 | Phytol | 0.1 | tr | tr | - | - | - | - | - |
| 2300 | 2300 | Tricosane | 0.5 | tr | tr | - | - | - | - | - |
| 2500 | 2500 | Pentacosane | 0.5 | 0.1 | tr | tr | - | - | - | - |
| Monoterpene hydrocarbons | 40.6 | 80.5 | 79.3 | 69.9 | 31.4 | 30.9 | 33.2 | 39.2 | ||
| Oxygenated monoterpenoids | 5.4 | 5.4 | 6.7 | 10.3 | 1.5 | 1.2 | 4.7 | 3.8 | ||
| Sesquiterpene hydrocarbons | 4.8 | 0.7 | 0.9 | 0.1 | 23.2 | 15.6 | 18.9 | 20.3 | ||
| Oxygenated sesquiterpenoids | 19.7 | 0.1 | 0.1 | 0.9 | 39.1 | 43.4 | 37.6 | 30.9 | ||
| Diterpenoids | 0.4 | traces | traces | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| Benzenoid aromatics | 0.1 | traces | traces | 0.9 | 0.7 | 5.0 | 0.2 | 0.9 | ||
| Others | 16.8 | 12.9 | 12.8 | 10.5 | 1.6 | 1.6 | 1.1 | 1.0 | ||
| Total identified | 87.7 | 99.6 | 99.7 | 92.4 | 97.3 | 97.6 | 95.7 | 96.1 |
| Lomatium Species | Component Percentage (Means ± Standard Deviations) | |||||||
|---|---|---|---|---|---|---|---|---|
| Limonene | Sabinene | α-Pinene | β-Phellandrene | Myrcene | β-Pinene | Cryptone | (E)-β-Ocimene | |
| Lomatium anomalum | 1.2 ± 0.5 b | 48.7 ± 1.0 a | 27.7 ± 8.6 a | 1.6 ± 0.6 c | 0.9 ± 0.6 b | 3.0 ± 0.8 b | 0.0 b | 0.5 ± 0.3 ab |
| Lomatium dissectum var. dissectum | traces b | traces c | traces b | traces c | traces b | 0.1 ± 0.1 b | 0.0 b | traces b |
| Lomatium multifidum | 4.2 ± 3.8 b | 0.1 ± 0.1 c | 0.4 ± 0.4 b | 4.0 ± 7.8 c | 30.7 ± 13.2 a | 0.1 ± 0.1 b | 0.2 ± 0.6 | 13.4 ± 10.8 a |
| Lomatium nudicaule | 0.7 ± 0.9 b | 0.2 ± 0.1 c | 0.8 ± 0.3 b | 31.8 ± 12.0 a | 3.3 ± 2.1 b | 1.3 ± 0.6 b | 2.4 ± 3.3 ab | 6.0 ± 2.8 ab |
| Lomatium packardiae | 60.9 ± 10.1 a | 0.7 ±1.0 c | 1.0 ± 0.6 b | 5.3 ± 0.8 bc | 3.1 ± 0.5 b | 1.6 ± 0.8 b | 0.1 ± 0.1 b | 1.0 ± 1.1 b |
| Lomatium papilioniferum (Idaho) | 0.4 ± 0.2 b | 0.0 c | 0.1 ± 0.1 b | traces c | 0.4 ± 0.2 b | traces b | 0.0 b | 0.1 ± 0.1 b |
| Lomatium papilioniferum (Oregon) | 2.2 ± 0.6 b | 0.6 ± 0.3 c | 1.7 ± 0.7 b | 13.4 ± 11.9 abc | 10.7 ± 11.3 b | 0.3 ± 0.2 b | 0.9 ± 1.2 b | 4.5 ± 3.3 ab |
| Lomatium triternatum var. triternatum | 2.5 ± 2.0 b | 5.9 ± 3.9 b | 5.1 ± 4.3 b | 26.5 ± 23.5 ab | 9.9 ± 6.1 b | 7.7 ± 6.3 a | 7.5 ± 9.2 a | 5.8 ± 5.1 ab |
| octyl acetate | decyl acetate | p-cymene | γ-terpinene | sedanenolide | MTMCP | (Z)-ligustilide | δ-3-carene | |
| Lomatium anomalum | 0.0 b | 0.0 b | 0.5 ± 0.3 b | 4.8 ± 2.2 ab | 0.0 b | traces b | 0.4 ± 0.4 b | traces b |
| Lomatium dissectum var. dissectum | 42.6 ± 3.9 a | 40.4 ± 4.8 a | traces b | traces b | 0.0 b | 0.0 b | traces b | 0.0 b |
| Lomatium multifidum | traces b | 0.8 ± 2.2 b | 2.0 ± 4.1 b | 1.5 ± 3.8 b | traces b | 0.0 b | 0.0 b | 0.0 b |
| Lomatium nudicaule | 0.0 b | 0.0 b | 1.2 ± 1.7 b | 0.1 ± 0.1 b | 0.1 ± 0.1 b | 0.0 b | 23.9 ± 14.8 a | 3.2 ± 4.5 a |
| Lomatium packardiae | 0.0 b | 0.0 b | 0.1 ± 0.0 b | 0.1 ± 0.1 b | traces b | 0.1 ± 0.1 b | 16.2 ± 3.0 a | traces b |
| Lomatium papilioniferum (Idaho) | 0.0 b | 0.0 b | 21.4 ± 1.1 a | 10.5 ± 3.4 ab | 0.0 b | 29.0 ± 2.9 a | 0.0 b | traces b |
| Lomatium papilioniferum (Oregon) | traces b | 1.5 ± 3.0 b | 14.9 ± 22.0 ab | 15.7 ± 16.4 a | 6.2 ± 5.3 a | 0.0 b | traces b | 0.2 ± 0.2 b |
| Lomatium triternatum var. triternatum | 0.0 b | 0.0 b | 2.4 ± 1.8 b | 0.2 ± 0.2 b | 0.2 ± 0.2 b | 0.0 b | 0.0 b | traces b |
| Lomatium Species | Enantiomeric Distribution, (+): (−) | |||||||||
| α-Thujene | α-Pinene | Camphene | Sabinene | β-Pinene | α-Phellandrene | Limonene | β-Phellandrene | cis-Sabinene Hydrate | ||
| Lomatium triternatum complex | ||||||||||
| La#1 | 0.0: 100.0 | 98.8: 1.2 | - | 95.8: 4.2 | 68.0: 32.0 | - | 60.2: 39.8 | 94.9: 5.1 | 92.7: 7.3 | |
| La#2 | 0.0: 100.0 | 98.2: 1.8 | - | 95.1: 4.9 | 0.0: 100.0 | - | 56.3: 43.7 | 93.8: 6.2 | 92.9: 7.1 | |
| La#3 | 0.0: 100.0 | 98.5: 1.5 | - | 95.8: 4.2 | 0.0: 100.0 | - | 52.5: 47.5 | 95.0: 5.0 | 92.1: 7.9 | |
| Lpack#1 | - | 47.2: 52.8 | - | - | 66.7: 33.3 | 100.0: 0.0 | 99.3: 0.7 | 84.9: 15.1 | - | |
| Lpack#2 | - | 29.5: 70.5 | - | - | 28.8: 71.2 | 100.0: 0.0 | 99.1: 0.9 | 99.8: 0.2 | - | |
| Lpack#3 | - | 6.3: 93.7 | - | 5.1: 94.9 | 2.8: 97.2 | 94.3: 5.7 | 98.9: 1.1 | 96.0: 4.0 | - | |
| Lpack#4 | - | 6.6: 93.4 | - | 14.1: 85.9 | 2.1: 97.9 | 98.1: 1.9 | 99.1: 0.9 | 99.2: 0.8 | - | |
| Ltt#1 | - | 51.2: 48.8 | - | 5.7: 94.3 | 27.5: 72.5 | 100.0: 0.0 | 29.8: 70.2 | 96.4: 3.6 | - | |
| Ltt#2 | - | 5.4: 94.6 | - | 8.2: 91.8 | 2.7: 97.3 | - | 20.2: 79.8 | 86.1: 13.9 | - | |
| Ltt#3 | 0.0: 100.0 | 3.3: 96.7 | 8.8: 91.2 | 3.8: 96.2 | 2.2: 97.8 | 100.0: 0.0 | 24.7: 75.3 | 97.0: 3.0 | - | |
| Lomatium grayi complex | ||||||||||
| Lpap#1 (OR) | - | 30.3: 69.7 | 20.0: 80.0 | - | - | - | 26.8: 73.2 | 77.0: 23.0 | - | |
| Lpap#2 (OR) | 64.8: 35.2 | 24.0: 76.0 | 15.9: 84.1 | 53.1: 46.9 | 14.5: 85.5 | 87.1: 12.9 | 13.9: 86.1 | 85.6: 14.4 | - | |
| Lpap#3 (OR) | 62.2: 37.8 | 24.3: 75.7 | 15.5: 84.5 | 53.8: 46.2 | 16.0: 84.0 | 81.6: 18.4 | 12.0: 88.0 | 80.9: 19.1 | - | |
| Lpap#4 (OR) | 61.1: 38.9 | 13.7: 86.3 | 13.8: 86.2 | 70.6: 29.4 | 8.8: 91.2 | - | 11.3: 88.7 | 78.8: 21.2 | - | |
| Lpap#5 (ID) | - | - | 25.9: 74.1 | - | - | - | 34.5: 65.5 | - | - | |
| Lpap#6 (ID) | - | 52.5: 47.5 | - | - | - | - | 54.4: 45.7 | - | - | |
| Lpap#7 (ID) | - | 45.2: 54.8 | 27.4: 72.6 | - | - | - | 37.2: 62.8 | - | - | |
| Lpap#8 (ID) | - | 43.1: 56.9 | 23.4: 76.6 | - | - | - | 31.5: 68.5 | - | - | |
| Lomatium dissectum complex | ||||||||||
| Ld#1 | - | 60.2: 39.8 | - | - | 39.8: 60.2 | - | 79.3: 20.7 | 100.0: 0.0 | - | |
| Ld#2 | - | 33.1: 66.9 | - | - | 63.5: 36.5 | - | 62.0: 38.0 | 100.0: 0.0 | - | |
| Ld#3 | - | 54.9: 45.1 | - | - | - | - | 100.0: 0.0 | 100.0: 0.0 | - | |
| Ld#4 | - | 87.8: 12.2 | - | - | 95.7: 4.3 | - | 59.1: 40.9 | 100.0: 0.0 | - | |
| Ld#5 | - | 36.5: 63.5 | - | - | 79.8: 20.2 | - | 56.9: 43.1 | 100.0: 0.0 | - | |
| Lm#1 (OR) | - | 33.4: 66.6 | 19.8: 80.2 | - | - | - | 24.7: 75.3 | - | - | |
| Lm#2 (OR) | - | 35.9: 64.1 | 21.7: 78.3 | - | - | - | 35.6: 64.4 | - | - | |
| Lm#3 (OR) | - | 36.6: 63.4 | 26.9: 73.1 | - | - | - | 34.9: 65.1 | - | - | |
| Lm#4 (OR) | - | 42.4: 57.6 | 28.1: 71.9 | - | - | - | 35.6: 64.4 | 100.0: 0.0 | - | |
| Lm#5 (OR) | - | 46.2: 53.8 | 31.3: 68.7 | - | - | - | 46.5: 53.5 | 100.0: 0.0 | - | |
| Lm#6 (OR) | - | 45.1: 54.9 | 76.4: 23.6 | - | - | - | 55.5: 44.5 | - | - | |
| Lm#7 (ID) | - | - | - | - | - | - | 38.7: 61.3 | - | - | |
| Lm#8 (ID) | - | 41.9: 48.1 | 80.9: 19.1 | - | - | - | 51.7: 48.3 | - | - | |
| Lm#9 (ID) | - | 40.5: 59.5 | 54.4: 45.6 | - | - | - | 47.6: 52.4 | - | - | |
| Lm#10 (OR) | - | 65.5: 34.5 | 30.5: 69.5 | - | - | 100.0: 0.0 | 43.6: 56.4 | 100.0: 0.0 | - | |
| Lm#11 (OR) | - | 52.2: 47.8 | 24.5: 75.5 | - | - | - | 34.9: 65.1 | 100.0: 0.0 | - | |
| Lm#12 (OR) | - | 21.1: 78.9 | 24.7: 75.3 | - | - | - | 42.8: 57.2 | - | - | |
| Lomatium nudicaule | ||||||||||
| Ln#1 | - | 92.9: 7.1 | - | 100.0: 0.0 | 100.0: 0.0 | 100.0: 0.0 | 41.1: 58.9 | 100.0: 0.0 | - | |
| Ln#2 | - | 89.9: 10.1 | - | - | 94.1: 5.9 | - | 43.6: 56.4 | 99.9: 0.1 | - | |
| Ln#3 | - | 93.7: 6.3 | - | - | 90.3: 9.7 | - | 48.8: 51.2 | 100.0: 0.0 | - | |
| Ln#4 | - | 87.8: 12.2 | - | - | 92.3: 7.7 | 100.0: 0.0 | 43.7: 56.3 | 99.9: 0.1 | - | |
| Ln#5 | - | 91.5: 8.5 | - | - | 96.7: 3.3 | 100.0: 0.0 | 44.8: 55.2 | 99.9: 0.1 | - | |
| Ln#6 | - | 88.3: 11.7 | - | - | 94.6: 5.4 | 100.0: 0.0 | 43.2: 56.8 | 99.9: 0.1 | - | |
| Ln#7 | - | 90.9: 9.1 | - | - | 92.0: 8.0 | 100.0: 0.0 | 49.0: 51.0 | 100.0: 0.0 | - | |
| Lomatium Species | Enantiomeric Distribution, (+): (−) | |||||||||
| Linalool | trans-Sabinene Hydrate | Terpinen-4-ol | α-Terpineol | Piperitone | (E)-β-Caryophyllene | Germacrene D | β-Bisabolene | δ-Cadinene | (E)-Nerolidol | |
| Lomatium triternatum complex | ||||||||||
| La#1 | - | 93.1: 6.9 | 72.0: 28.0 | 59.3: 40.7 | - | 0.0: 100.0 | 0.0: 100.0 | - | - | - |
| La#2 | - | 95.4: 4.6 | 71.8: 28.2 | 55.8: 44.2 | - | 0.0: 100.0 | 0.0: 100.0 | - | - | - |
| La#3 | - | 95.7: 4.3 | 71.8: 28.2 | 55.5: 44.5 | - | 0.0: 100.0 | 0.0: 100.0 | - | - | - |
| Lpack#1 | - | - | - | - | - | - | - | - | - | - |
| Lpack#2 | - | - | - | - | - | - | 80.6: 19.4 | - | - | - |
| Lpack#3 | - | - | 29.4: 70.6 | - | - | 0.0: 100.0 | 24.6: 75.4 | - | - | - |
| Lpack#4 | - | - | - | - | - | - | 30.9: 69.1 | - | - | - |
| Ltt#1 | 56.8: 43.2 | - | 35.7: 64.3 | - | - | - | 0.0: 100.0 | - | - | - |
| Ltt#2 | 67.5: 32.5 | - | - | - | - | - | - | - | - | - |
| Ltt#3 | - | - | 30.4: 69.6 | - | - | 0.0: 100.0 | 0.0: 100.0 | - | - | - |
| Lomatium grayi complex | ||||||||||
| Lpap#1 (OR) | 67.7: 32.3 | - | - | 29.3: 70.7 | - | - | - | - | - | 7.4: 92.6 |
| Lpap#2 (OR) | 88.6: 11.4 | - | 67.6: 32.4 | 26.2: 73.8 | 0.3: 99.7 | - | 0.0: 100.0 | - | - | - |
| Lpap#3 (OR) | 90.1: 9.9 | - | 69.5: 30.5 | 27.2: 72.8 | 0.3: 99.7 | - | 0.0: 100.0 | - | - | - |
| Lpap#4 (OR) | 78.7: 21.3 | - | 67.5: 32.5 | - | 0.4: 99.6 | - | - | - | - | - |
| Lpap#5 (ID) | - | - | - | - | - | - | - | - | - | 30.8: 69.2 |
| Lpap#6 (ID) | - | - | - | - | - | 0.0: 100.0 | - | - | 100.0: 0.0 | 36.1: 63.9 |
| Lpap#7 (ID) | - | - | - | - | - | 0.0: 100.0 | - | - | 100.0: 0.0 | 28.8: 71.2 |
| Lpap#8 (ID) | - | - | - | - | - | 0.0: 100.0 | - | - | 100.0: 0.0 | 26.9: 73.1 |
| Lomatium dissectum complex | ||||||||||
| Ld#1 | - | - | - | - | - | 0.0: 100.0 | - | - | - | - |
| Ld#2 | - | - | - | - | - | 0.0: 100.0 | - | - | - | - |
| Ld#3 | - | - | - | - | - | 0.0: 100.0 | - | - | - | - |
| Ld#4 | - | - | - | - | - | 0.0: 100.0 | - | - | - | - |
| Ld#5 | - | - | - | - | - | 0.0: 100.0 | - | - | - | - |
| Lm#1 (OR) | - | - | - | 29.6: 70.4 | - | 0.0: 100.0 | - | - | - | - |
| Lm#2 (OR) | - | - | - | 30.3: 69.7 | - | 0.0: 100.0 | - | - | - | 7.7: 92.3 |
| Lm#3 (OR) | - | - | - | 26.4: 73.6 | - | 0.0: 100.0 | - | - | - | 5.0: 95.0 |
| Lm#4 (OR) | - | - | - | - | - | 0.0: 100.0 | - | - | - | - |
| Lm#5 (OR) | - | - | - | - | - | 0.0: 100.0 | - | - | - | 6.5: 93.5 |
| Lm#6 (OR) | - | - | - | - | - | 0.0: 100.0 | - | - | - | 15.2: 84.8 |
| Lm#7 (ID) | - | - | - | - | - | 0.0: 100.0 | - | 100.0: 0.0 | - | 18.7: 81.3 |
| Lm#8 (ID) | - | - | - | 28.2: 71.8 | - | 0.0: 100.0 | - | 25.6: 74.4 | - | 14.7: 85.3 |
| Lm#9 (ID) | - | - | - | 30.3: 69.7 | - | 0.0: 100.0 | - | 17.1: 82.9 | - | 11.0: 89.0 |
| Lm#10 (OR) | - | - | - | - | - | 0.0: 100.0 | - | - | - | - |
| Lm#11 (OR) | - | - | - | - | - | 0.0: 100.0 | - | 100.0: 0.0 | - | 8.3: 91.7 |
| Lm#12 (OR) | - | - | - | - | - | 0.0: 100.0 | - | 100.0: 0.0 | - | 8.5: 91.5 |
| Lomatium nudicaule | ||||||||||
| Ln#1 | 27.1: 72.9 | - | - | - | - | 0.0: 100.0 | 100.0: 0.0 | - | - | - |
| Ln#2 | 37.1: 62.9 | - | - | - | - | - | - | - | - | - |
| Ln#3 | 32.0: 68.0 | - | - | - | - | - | - | - | - | - |
| Ln#4 | 19.6: 80.4 | - | - | - | - | 0.0: 100.0 | 93.4: 6.6 | - | - | - |
| Ln#5 | 22.6: 77.4 | - | - | - | - | 0.0: 100.0 | 94.3: 5.7 | - | - | - |
| Ln#6 | 10.7: 89.3 | - | - | - | - | 0.0: 100.0 | 94.9: 5.1 | - | - | - |
| Ln#7 | - | - | - | - | - | 0.0: 100.0 | - | - | - | - |
| Enantiomer Percentage (Means ± Standard Deviations) | ||||||
|---|---|---|---|---|---|---|
| Lomatium Species | (+)-α-Pinene | (−)-Camphene | (+)-Sabinene | (+)-β-Pinene | (+)-Limonene | (+)-Linalool |
| Lomatium anomalum | 98.5 ± 0.3 a | - | 95.6 ± 0.4 a | 22.7 ± 39.3 bc | 56.3 ± 3.9 bc | - |
| Lomatium packardiae | 22.4 ± 19.8 c | - | 9.6 ± 6.4 c | 25.1 ± 30.4 bc | 99.1 ± 0.2 a | - |
| Lomatium triternatum var. triternatum | 20.0 ± 27.1 c | - | 5.9 ± 2.2 c | 10.8 ± 14.5 c | 24.9 ± 4.8 de | 62.2 ± 7.6 a |
| Lomatium papilioniferum (Oregon) | 23.1 ± 6.9 c | 83.7 ± 2.6 a | 59.2 ± 9.9 b | 13.1 ± 3.8 c | 16.0 ± 7.3 e | 81.3 ± 10.4 a |
| Lomatium papilioniferum (Idaho) | 46.9 ± 4.9 bc | 74.4 ± 2.0 a | - | - | 39.4 ± 10.3 cd | - |
| Lomatium dissectum | 54.5 ± 21.9 b | - | - | 69.7 ± 23.9 ab | 71.5 ± 18.2 b | - |
| Lomatium multifidum | 41.9 ± 11.2 bc | 61.9 ± 22.1 a | - | - | 41.0 ± 8.6 cd | - |
| Lomatium nudicaule | 90.7 ± 2.2 a | - | - | 94.3 ± 3.3 a | 44.9 ± 3.0 cd | 24.9 ± 9.4 b |
| Lomatium Species (Voucher Number) | Sample # | Collection Site | Collection Date | Mass Aerial Parts (g) | Mass Essential Oil (g) | Essential Oil Color | % |
|---|---|---|---|---|---|---|---|
| Lomatium anomalum Jones ex J.M. Coult. & Rose (voucher WNS-La-5379) | #1 | Near Grangeville, Idaho (45°55′29″ N, 116°8′19″ W, 1042 m asl) | 2 June 2022 | 72.25 | 1.214 | pale yellow | 1.68 |
| Lomatium anomalum Jones ex J.M. Coult. & Rose | #2 | Near Grangeville, Idaho (45°52′34″ N, 116°13′40″ W, 1079 m asl) | 30 May 2024 | 108.12 | 1.692 | colorless | 1.57 |
| Lomatium anomalum Jones ex J.M. Coult. & Rose | #3 | Near Grangeville, Idaho (45°52′34″ N, 116°13′40″ W, 1079 m asl) | 30 May 2024 | 97.72 | 1.594 | colorless | 1.63 |
| Lomatium dissectum (Nutt.) Mathias & Constance (voucher WNS-Ld-0181) | #1 | Near Grangeville, Idaho (45°52′34″ N, 116°13′40″ W, 1079 m asl) | 30 May 2024 | 94.80 | 2.401 | colorless | 2.53 |
| Lomatium dissectum (Nutt.) Mathias & Constance | #2 | Near Grangeville, Idaho (45°50′24″ N, 116°14′6″ W, 1275 m asl) | 30 May 2024 | 113.31 | 3.107 | colorless | 2.74 |
| Lomatium dissectum (Nutt.) Mathias & Constance | #3 | Near Grangeville, Idaho (45°50′24″ N, 116°14′6″ W, 1275 m asl) | 30 May 2024 | 238.04 | 4.623 | colorless | 1.94 |
| Lomatium dissectum (Nutt.) Mathias & Constance | #4 | Near Grangeville, Idaho (45°50′24″ N, 116°14′6″ W, 1275 m asl) | 30 May 2024 | 129.21 | 3.370 | colorless | 2.61 |
| Lomatium dissectum (Nutt.) Mathias & Constance | #5 | Near Grangeville, Idaho (45°50′24″ N, 116°14′6″ W, 1275 m asl) | 30 May 2024 | 198.53 | 4.070 | colorless | 2.05 |
| Lomatium multifidum (Nutt.) R.P. McNeill & Darrach (voucher WNS-Lm-7137) | #1 | Between Boggs Junction and Arlington, Oregon (45°41′23″ N, 120°30′0″ W, 97 m asl) | 17 April 2023 | 176.41 | 4.712 | yellow | 2.67 |
| Lomatium multifidum (Nutt.) R.P. McNeill & Darrach | #2 | Between Boggs Junction and Arlington, Oregon (45°41′23″ N, 120°30′0″ W, 97 m asl) | 17 April 2023 | 89.14 | 1.699 | yellow | 1.91 |
| Lomatium multifidum (Nutt.) R.P. McNeill & Darrach | #3 | Leslie Gulch, Oregon (43°18′22″ N, 117°17′31″ W, 955 m asl) | 27 May 2023 | 64.18 | 3.429 | yellow | 5.34 |
| Lomatium multifidum (Nutt.) R.P. McNeill & Darrach | #4 | Leslie Gulch, Oregon (43°18′22″ N, 117°17′31″ W, 955 m asl) | 27 May 2023 | 83.37 | 4.492 | yellow | 5.39 |
| Lomatium multifidum (Nutt.) R.P. McNeill & Darrach | #5 | Leslie Gulch, Oregon (43°18′22″ N, 117°17′31″ W, 955 m asl) | 27 May 2023 | 62.89 | 1.288 | yellow | 2.05 |
| Lomatium multifidum (Nutt.) R.P. McNeill & Darrach | #6 | Near Prairie, Idaho (43°32′33″ N, 115°48′14″ W, 1143 m asl) | 25 May 2023 | 38.87 | 2.141 | yellow | 5.51 |
| Lomatium multifidum (Nutt.) R.P. McNeill & Darrach | #7 | Near Prairie, Idaho (43°32′33″ N, 115°48′14″ W, 1143 m asl) | 25 May 2023 | 79.81 | 4.907 | yellow | 6.148 |
| Lomatium multifidum (Nutt.) R.P. McNeill & Darrach | #8 | Near Prairie, Idaho (43°32′33″ N, 115°48′14″ W, 1143 m asl) | 25 May 2023 | 43.88 | 1.583 | yellow | 3.61 |
| Lomatium multifidum (Nutt.) R.P. McNeill & Darrach | #9 | Near Prairie, Idaho (43°32′33″ N, 115°48′14″ W, 1143 m asl) | 25 May 2023 | 58.09 | 2.537 | yellow | 4.37 |
| Lomatium multifidum (Nutt.) R.P. McNeill & Darrach | #10 | Lake Owyhee, Oregon (43°36′33″ N, 117°15′15″ W, 841 m asl) | 8 May 2024 | 97.41 | 2.578 | colorless | 2.65 |
| Lomatium multifidum (Nutt.) R.P. McNeill & Darrach | #11 | Lake Owyhee, Oregon (43°36′33″ N, 117°15′15″ W, 841 m asl) | 8 May 2024 | 69.77 | 1.116 | pale yellow | 1.60 |
| Lomatium multifidum (Nutt.) R.P. McNeill & Darrach | #12 | Leslie Gulch, Oregon (43°18′26″ N, 117°17′32″ W, 952 m asl) | 11 May 2024 | 124.04 | 3.643 | colorless | 2.94 |
| Lomatium nudicaule (Nutt.) J.M. Coult. & Rose (voucher WNS-Ln-5374) | #1 | Boise Foothills, Idaho (43°32′45″ N, 115°48′15″ W, 1146 m asl) | 12 June 2022 | 191.37 | 0.564 | pale yellow | 0.30 |
| Lomatium nudicaule (Nutt.) J.M. Coult. & Rose | #2 | Boise Foothills, Idaho (43°32′45″ N, 115°48′15″ W, 1146 m asl) | 12 June 2022 | 46.03 | 0.100 | pale yellow | 0.22 |
| Lomatium nudicaule (Nutt.) J.M. Coult. & Rose | #3 | Boise Foothills, Idaho (43°32′45″ N, 115°48′15″ W, 1146 m asl) | 12 June 2022 | 42.06 | 0.063 | pale yellow | 0.15 |
| Lomatium nudicaule (Nutt.) J.M. Coult. & Rose | #4 | Near Prairie, Idaho (43°32′33″ N, 115°48′13″ W, 1142 m asl) | 25 May 2023 | 68.16 | 1.822 | colorless | 2.67 |
| Lomatium nudicaule (Nutt.) J.M. Coult. & Rose | #5 | Near Prairie, Idaho (43°32′33″ N, 115°48′13″ W, 1142 m asl) | 25 May 2023 | 67.14 | 1.845 | colorless | 2.75 |
| Lomatium nudicaule (Nutt.) J.M. Coult. & Rose | #6 | Near Prairie, Idaho (43°32′33″ N, 115°48′13″ W, 1142 m asl) | 25 May 2023 | 49.56 | 1.253 | pale yellow | 2.53 |
| Lomatium nudicaule (Nutt.) J.M. Coult. & Rose | #7 | Near Midvale, Idaho (44°26′45″ N, 116°48′3″ W, 963 m asl) | 21 May 2024 | 120.37 | 3.620 | colorless | 3.01 |
| Lomatium packardiae Cronquist (voucher WNS-Lpack-0173) | #1 | Near Midvale, Idaho (44°25′29″ N, 116°49′19″ W, 988 m asl) | 21 May 2024 | 70.22 | 1.287 | colorless | 1.83 |
| Lomatium packardiae Cronquist | #2 | Near Midvale, Idaho (44°26′42″ N, 116°48′1″ W, 963 m asl) | 21 May 2024 | 123.10 | 1.920 | colorless | 1.56 |
| Lomatium papilioniferum J.A. Alexander & Whaley (voucher WNS-Lpap-6926) | #1 | Between Boggs Junction and Arlington, Oregon (45°41′23″ N, 120°30′0″ W, 97 m asl) | 17 April 2023 | 90.10 | 0.179 | yellow | 0.20 |
| Lomatium papilioniferum J.A. Alexander & Whaley | #2 | Between Boggs Junction and Arlington, Oregon (45°41′23″ N, 120°30′0″ W, 97 m asl) | 17 April 2023 | 134.46 | 1.532 | yellow | 1.14 |
| Lomatium papilioniferum J.A. Alexander & Whaley | #3 | Between Boggs Junction and Arlington, Oregon (45°41′23″ N, 120°30′0″ W, 97 m asl) | 17 April 2023 | 112.70 | 2.208 | colorless | 1.96 |
| Lomatium papilioniferum J.A. Alexander & Whaley | #4 | Between Boggs Junction and Arlington, Oregon (45°41′23″ N, 120°30′0″ W, 97 m asl) | 17 April 2023 | 153.02 | 2.349 | pale yellow | 1.54 |
| Lomatium papilioniferum J.A. Alexander & Whaley | #5 | Near Mann Creek Reservoir, Idaho (44°23′43″ N, 116°53′45″ W, 900 m asl) | 21 May 2024 | 71.85 | 2.066 | yellow | 2.88 |
| Lomatium papilioniferum J.A. Alexander & Whaley | #6 | Near Mann Creek Reservoir, Idaho (44°23′43″ N, 116°53′45″ W, 900 m asl) | 21 May 2024 | 68.18 | 2.199 | yellow | 3.23 |
| Lomatium papilioniferum J.A. Alexander & Whaley | #7 | Near Mann Creek Reservoir, Idaho (44°24′5″ N, 116°53′53″ W, 883 m asl) | 21 May 2024 | 90.42 | 2.754 | yellow | 3.05 |
| Lomatium papilioniferum J.A. Alexander & Whaley | #8 | Near Mann Creek Reservoir, Idaho (44°24′4″ N, 116°53′53″ W, 884 m asl) | 21 May 2024 | 62.27 | 2.071 | yellow | 3.33 |
| Lomatium triternatum (Pursh) J.M. Coult. & Rose var. triternatum (voucher WNS-Ltt-7101) | #1 | Near Prairie, Idaho (43°30′25″ N, 115°55′35″ W, 1460 m asl) | 25 May 2023 | 30.30 | 0.538 | colorless | 1.77 |
| Lomatium triternatum (Pursh) J.M. Coult. & Rose | #2 | Near Prairie, Idaho (43°30′25″ N, 115°55′35″ W, 1460 m asl) | 25 May 2023 | 39.22 | 0.638 | colorless | 1.63 |
| Lomatium triternatum (Pursh) J.M. Coult. & Rose | #3 | Near Prairie, Idaho (43°30′25″ N, 115°55′35″ W, 1460 m asl) | 25 May 2023 | 27.06 | 0.367 | colorless | 1.36 |
| Lomatium triternatum (Pursh) J.M. Coult. & Rose | #4 | Near Arrowrock Reservoir, Idaho (43°36′41″ N, 115°49′59″ W, 984 m asl) | 9 May 2024 | 87.12 | 1.569 | pale yellow | 1.80 |
| Lomatium triternatum (Pursh) J.M. Coult. & Rose | #5 | Near Arrowrock Reservoir, Idaho (43°36′42″ N, 115°49′56″ W, 985 m asl) | 9 May 2024 | 48.25 | 1.038 | colorless | 2.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setzer, W.N.; Poudel, A.; Satyal, P.; Swor, K.; Shock, C.C. Lomatium Species of the Intermountain Western United States: A Chemotaxonomic Investigation Based on Essential Oil Compositions. Plants 2025, 14, 186. https://doi.org/10.3390/plants14020186
Setzer WN, Poudel A, Satyal P, Swor K, Shock CC. Lomatium Species of the Intermountain Western United States: A Chemotaxonomic Investigation Based on Essential Oil Compositions. Plants. 2025; 14(2):186. https://doi.org/10.3390/plants14020186
Chicago/Turabian StyleSetzer, William N., Ambika Poudel, Prabodh Satyal, Kathy Swor, and Clinton C. Shock. 2025. "Lomatium Species of the Intermountain Western United States: A Chemotaxonomic Investigation Based on Essential Oil Compositions" Plants 14, no. 2: 186. https://doi.org/10.3390/plants14020186
APA StyleSetzer, W. N., Poudel, A., Satyal, P., Swor, K., & Shock, C. C. (2025). Lomatium Species of the Intermountain Western United States: A Chemotaxonomic Investigation Based on Essential Oil Compositions. Plants, 14(2), 186. https://doi.org/10.3390/plants14020186








