Direct Hot Solid–Liquid Extraction (DH-SLE): A High-Yield Greener Technique for Lipid Recovery from Coffee Beans
Abstract
1. Introduction
2. Results
2.1. Lipid Extraction from Green Coffee Beans by Pressing
2.2. Lipid Extraction from Green Coffee Beans by Soxhlet
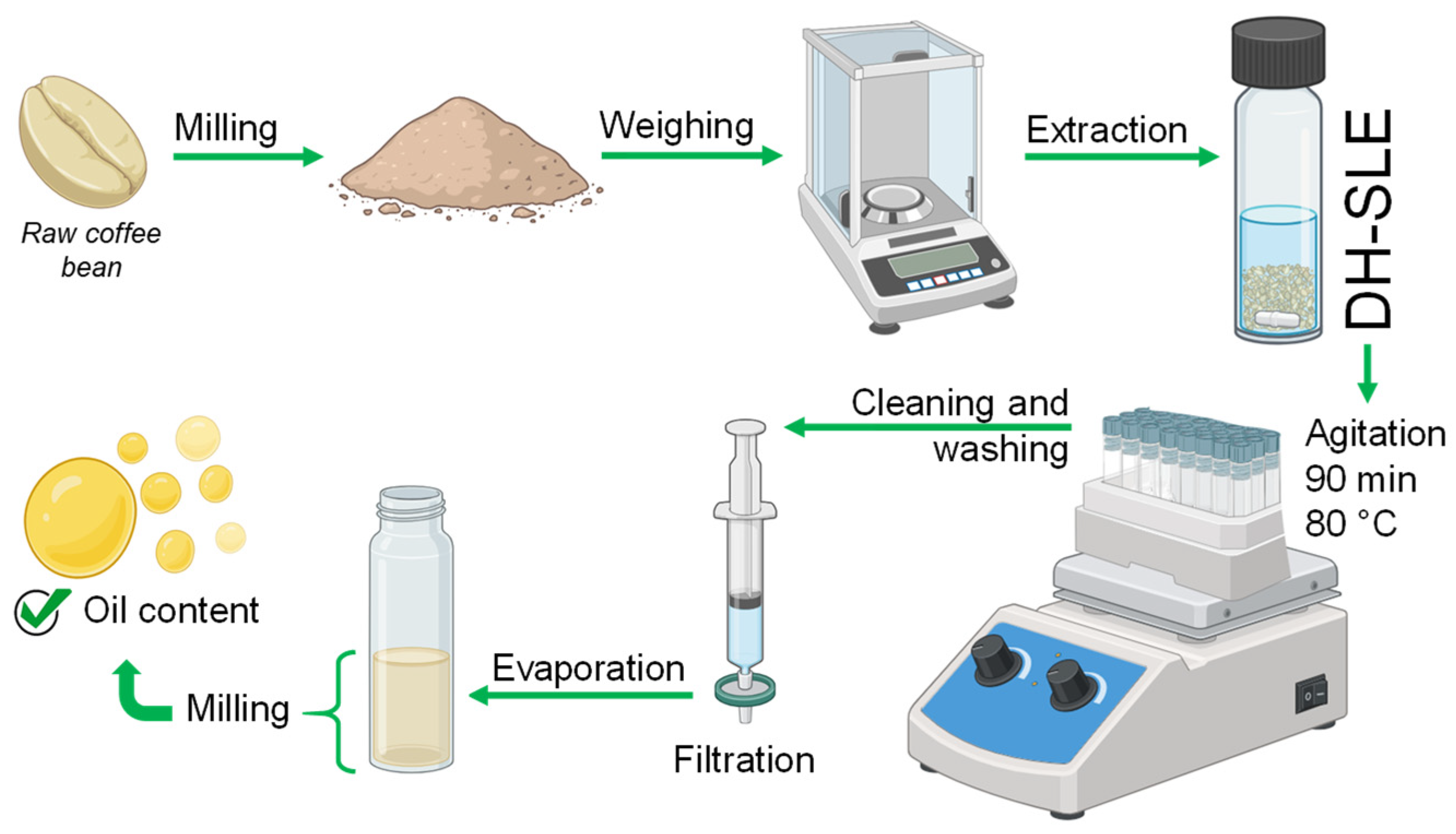
2.3. Lipid Extraction from Green Coffee Beans by Direct Hot Solid–Liquid Extraction (DH-SLE)
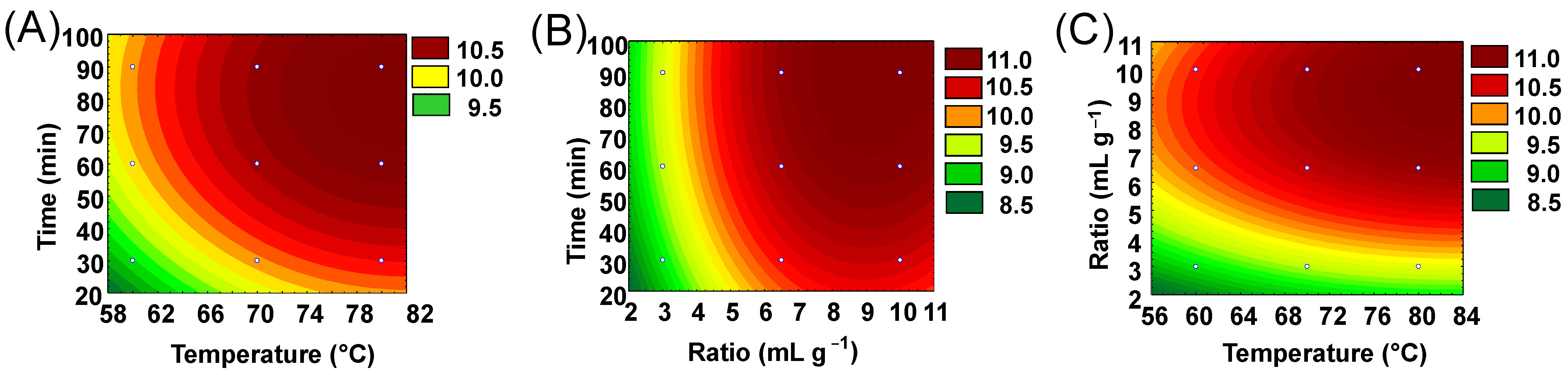
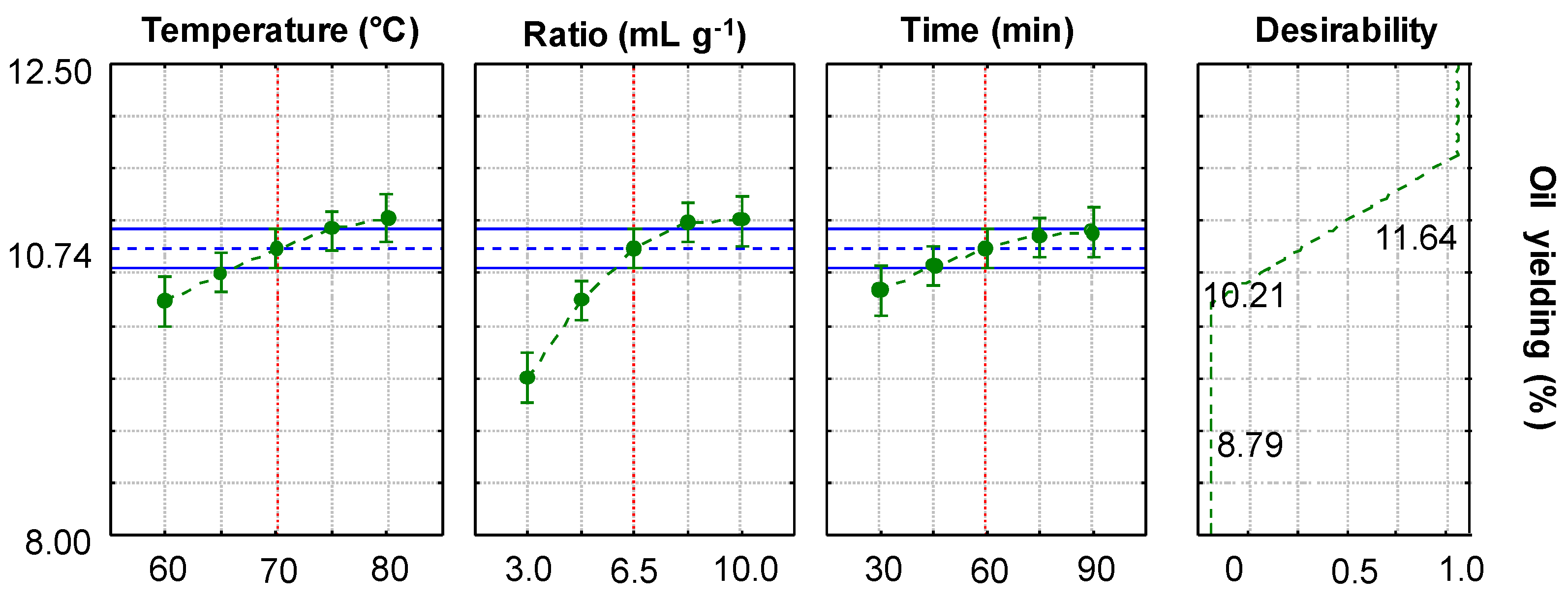
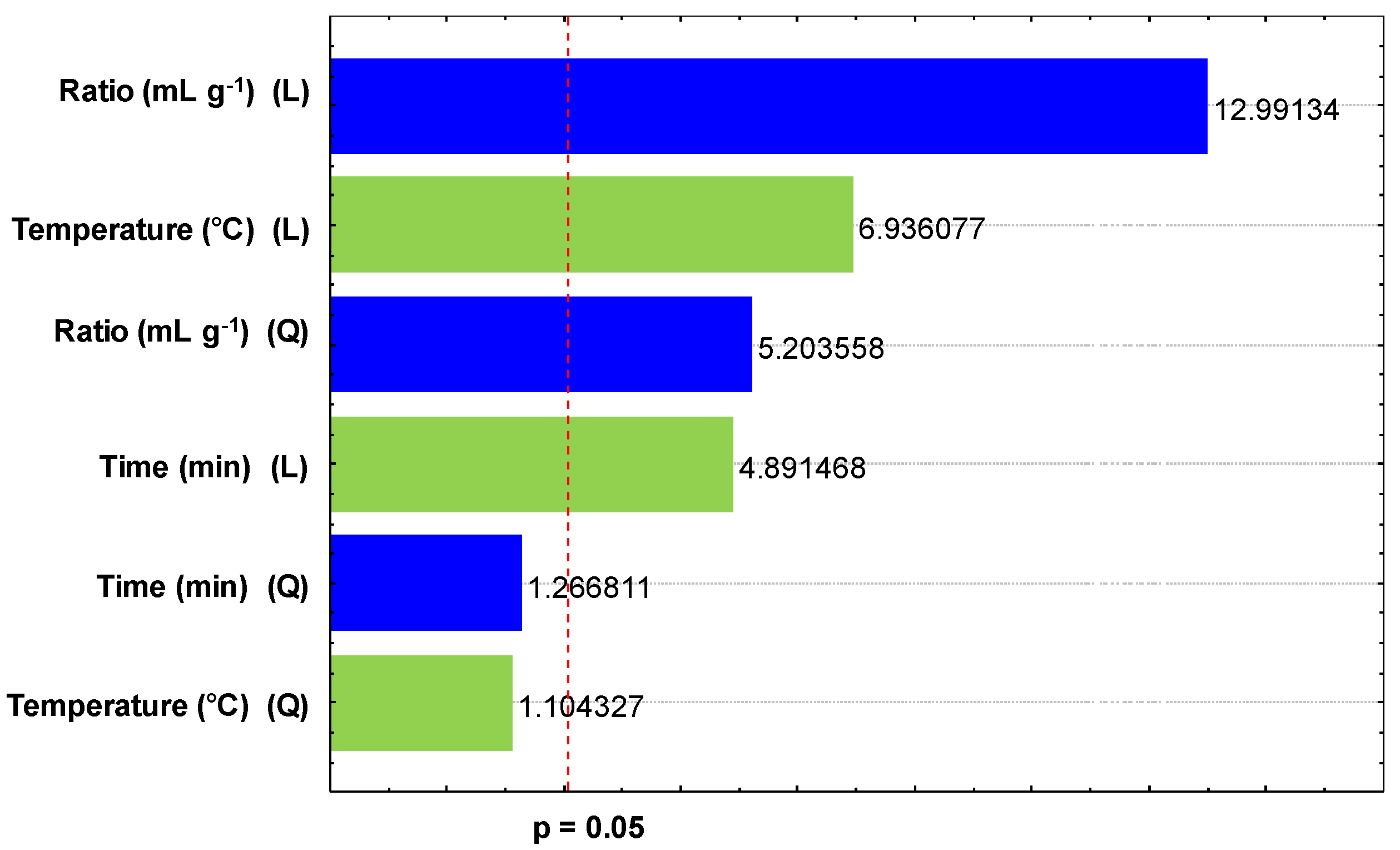
2.3.1. Experimental Design
2.3.2. Optimization of DH-SLE and Validation Against Soxhlet Extraction
2.3.3. Fatty Acid Composition
2.3.4. Compliance with the United Nations Sustainable Development Goals
2.3.5. Return on Investment (ROI) Analysis
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Solid–Liquid Extraction by Soxhlet
3.3. Direct Hot Solid–Liquid Extraction (DH-SLE)
3.4. Transesterification of Oils
3.5. FAME Analysis
3.6. Statistical Analysis
3.7. Return on Investment Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pereira, L.L.; Moreira, T.R. Food Engineering Series. In Quality Determinants in Coffee Production, 1st ed.; Pereira, L.L., Moreira, T.R., Eds.; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Freitas, V.V.; Borges, L.L.R.; Vidigal, M.C.T.R.; dos Santos, M.H.; Stringheta, P.C. Coffee: A comprehensive overview of origin, market, and the quality process. Trends Food Sci. Technol. 2024, 146, 104411. [Google Scholar] [CrossRef]
- Volsi, B.; Telles, T.S.; Caldarelli, C.E.; Camara, M.R.G. The Dynamics of Coffee Production in Brazil. PLoS ONE 2019, 14, e0219742. [Google Scholar] [CrossRef] [PubMed]
- Tieghi, H.; Pereira, L.A.; Viana, G.S.; Katchborian-Neto, A.; Santana, D.B.; Mincato, R.L.; Dias, D.F.; Chagas-Paula, D.A.; Soares, M.G.; de Araújo, W.G.; et al. Effects of Geographical Origin and Post-Harvesting Processing on the Bioactive Compounds and Sensory Quality of Brazilian Specialty Coffee Beans. Food Res. Int. 2024, 186, 114346. [Google Scholar] [CrossRef]
- Novaes, F.J.M.; Itabaiana Junior, I.; Sutili, F.K.; Marriott, P.J.; Bizzo, H.R.; de Aquino Neto, F.R.; de Souza, R.O.M.A.; Rezende, C.M. Lipase-Catalysed Esters Synthesis of Cafestol and Kahweol. Food Chem. 2018, 259, 226–233. [Google Scholar] [CrossRef]
- Speer, K.; Kölling-Speer, I. The Lipid Fraction of the Coffee Bean. Braz. J. Plant Physiol. 2006, 18, 201–216. [Google Scholar] [CrossRef]
- Ribeiro, R.C.; Teixeira, R.S.S.; de Rezende, C.M. Extrusion Pretreatment of Green Arabica Coffee Beans for Lipid Enhance Extraction. Ind. Crop. Prod. 2024, 221, 119318. [Google Scholar] [CrossRef]
- Burdan, F. Caffeine in Coffee. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: London, UK, 2015; pp. 201–207. [Google Scholar] [CrossRef]
- Gaspar, S.; Ramos, F. Caffeine: Consumption and Health Effects. In Encyclopedia of Food and Health, 1st ed.; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: London, UK, 2016; pp. 573–578. [Google Scholar]
- Hall, R.D.; Trevisan, F.; de Vos, R.C.H. Coffee Berry and Green Bean Chemistry Opportunities for Improving Cup Quality and Crop Circularity. Food Res. Int. 2022, 151, 110825. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, A.; Bondonno, C.P.; Liu, A.H.; Considine, M.J.; Rich, L.; Mas, E.; Croft, K.D.; Hodgson, J.M. Acute Effects of Chlorogenic Acid on Nitric Oxide Status, Endothelial Function, and Blood Pressure in Healthy Volunteers: A Randomized Trial. J. Agric. Food Chem. 2012, 60, 9130–9136. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In Vitro and in Vivo Antioxidant Properties of Chlorogenic Acid and Caffeic Acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Perrone, D.; Donangelo, C.M.; Farah, A. Fast simultaneous analysis of caffeine, trigonelline, nicotinic acid and sucrose in coffee by liquid chromatography–mass spectrometry. Food Chem. 2008, 110, 1030–1035. [Google Scholar] [CrossRef]
- Alcantara, G.M.R.N.; Dresch, D.; Melchert, W.R. Use of Non-Volatile Compounds for the Classification of Specialty and Traditional Brazilian Coffees Using Principal Component Analysis. Food Chem. 2021, 360, 130088. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Gonzalez Viejo, C.; Fuentes, S.; Dunshea, F.R.; Suleria, H.A.R. Evaluation of Spontaneous Fermentation Impact on the Physicochemical Properties and Sensory Profile of Green and Roasted Arabica Coffee by Digital Technologies. Food Res. Int. 2024, 176, 113800. [Google Scholar] [CrossRef] [PubMed]
- Toci, A.T.; Farah, A. Volatile Fingerprint of Brazilian Defective Coffee Seeds: Corroboration of Potential Marker Compounds and Identification of New Low Quality Indicators. Food Chem. 2014, 153, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Toledo, P.R.A.B.; Pezza, L.; Pezza, H.R.; Toci, A.T. Relationship Between the Different Aspects Related to Coffee Quality and Their Volatile Compounds. Com. Ver. Food Sci. Food Saf. 2016, 15, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Bagnulo, E.; Strocchi, G.; Bicchi, C.; Liberto, E. Industrial Food Quality and Consumer Choice: Artificial Intelligence-Based Tools in the Chemistry of Sensory Notes in Comfort Foods (Coffee, Cocoa and Tea). Trends Food Sci. Technol. 2024, 147, 104415. [Google Scholar] [CrossRef]
- Farah, A. Coffee Production, Quality and Chemistry, 1st ed.; R. S. of Chemistry: London, UK, 2019. [Google Scholar] [CrossRef]
- Novaes, F.J.M.; da Silva, M.A.E.; Silva, D.C.; de Aquino Neto, F.R.; Rezende, C.M. Extraction of Diterpene-Phytochemicals in Raw and Roasted Coffee Beans and Beverage Preparations and Their Relationship. Plants 2023, 12, 1580. [Google Scholar] [CrossRef]
- Eldesouki, S.; Qadri, R.; Abu Helwa, R.; Barqawi, H.; Bustanji, Y.; Abu-Gharbieh, E.; El-Huneidi, W. Recent Updates on the Functional Impact of Kahweol and Cafestol on Cancer. Molecules 2022, 27, 7332. [Google Scholar] [CrossRef] [PubMed]
- Kusumah, J.; Gonzalez de Mejia, E. Coffee constituents with antiadipogenic and antidiabetic potentials: A narrative review. Food Chem. Toxicol. 2022, 161, 112821. [Google Scholar] [CrossRef]
- Silva, M.A.E.; Brand, A.L.M.; Novaes, F.J.M.; Rezende, C.M. Cafestol, Kahweol and Their Acylated Derivatives: Antitumor Potential, Pharmacokinetics, and Chemopreventive Profile. Food Rev. Int. 2023, 39, 7048–7080. [Google Scholar] [CrossRef]
- Brand, A.L.M.; Lima, F.A.; Tinoco, N.A.B.; Mota, J.C.; Moreira, I.G.S.; Novaes, F.J.M.; Garrett, R.; Giorno, T.B.S.; Fernandes, P.D.; Rezende, C.M. βN-Alkanoyl-5-Hydroxytryptamines (Cn-5HTs) in Coffee: A Review. Food Rev. Int. 2022, 39, 4761–4780. [Google Scholar] [CrossRef]
- Giorno, T.B.S.; Lima, F.A.; Brand, A.L.M.; de Oliveira, C.M.; Rezende, C.M.; Fernandes, P.D. Characterization of βN-octadecanoyl-5-hydroxytryptamide anti-inflammatory effect. Molecules 2021, 26, 3709. [Google Scholar] [CrossRef] [PubMed]
- Novaes, F.J.M.; Oigman, S.S.; De Souza, R.O.M.A.; Rezende, C.M.; De Aquino Neto, F.R. New Approaches on the Analyses of Thermolabile Coffee Diterpenes by Gas Chromatography and its Relationship with Cup Quality. Talanta 2015, 139, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Oestreich-Janzen, S. Caracterización Físico-Química y Sensorial de dos Variedades de Café (Coffea arabica) del Occidente de Honduras. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Ripper, B.; Barreto, M.S.; Novaes, F.J.M.; de Godoy, M.G.; Freire, D.M.G.; de Rezende, C.M.; Nunes, J.C.; Perrone, D. Comprehensive Composition of Flavor Precursors in Kopi Luwak and Jacu Exotic Green Bioprocessed Coffees. Front. Sustain. Food Syst. 2022, 6, 233. [Google Scholar] [CrossRef]
- Clarke, R.J.; Macrae, R. Coffee: Volume 1: Chemistry; Elsevier Applied Science: Amsterdam, The Netherlands, 1985. [Google Scholar]
- Bosso, H.; Barbalho, S.M.; de Alvares Goulart, R.; Otoboni, A.M.M.B. Green coffee: Economic relevance and a systematic review of the effects on human health. Crit. Rev. Food Sci. Nutr. 2023, 63, 394–410. [Google Scholar] [CrossRef] [PubMed]
- Wagemaker, T.A.L.; Rijo, P.; Rodrigues, L.M.; Maia Campos, P.M.B.G.; Fernandes, A.S.; Rosado, C. Integrated approach in the assessment of skin compatibility of cosmetic formulations with green coffee oil. Int. J. Cosmet. Sci. 2015, 37, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Wagemaker, T.A.L.; Campos, P.M.B.G.M.; Fernandes, A.S.; Rijo, P.; Nicolai, M.; Roberto, A.; Rosado, C.; Reis, C.; Rodrigues, L.M.; Carvalho, C.R.L.; et al. Unsaponifiable matter from oil of green coffee beans: Cosmetic properties and safety evaluation. Drug Dev. Ind. Pharm. 2016, 42, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, R.G.; Anhaia, F.M.P.; Paula, J.T.; Meirelles, A.J.A.; Cabral, F.A. Coffee Industrial Residue: Sequential High Pressure Extraction and Conventional Methods. Braz. J. Chem. Eng. 2024, 41, 1–10. [Google Scholar] [CrossRef]
- Shelake, R.M.; Wagh, S.G.; Patil, A.M.; Červený, J.; Waghunde, R.R.; Kim, J.-Y. Heat Stress and Plant–Biotic Interactions: Advances and Perspectives. Plants 2024, 13, 2022. [Google Scholar] [CrossRef]
- Araújo, J.M.A.; Sandi, D. Extraction of Coffee Diterpenes and Coffee Oil Using Supercritical Carbon Dioxide. Food Chem. 2007, 101, 1087–1094. [Google Scholar] [CrossRef]
- AOAC. Method 14.029. Official Methods of Analysis of the Association of Official Agricultural Chemists, 10th ed.; AOAC: Association of Official Analytical Chemists, Inc.: Washington, DC, USA, 1965. [Google Scholar]
- Tsukui, A.; Santos Júnior, H.M.; Oigman, S.S.; De Souza, R.O.M.A.; Bizzo, H.R.; Rezende, C.M. Microwave-Assisted Extraction of Green Coffee Oil and Quantification of Diterpenes by HPLC. Food Chem. 2014, 164, 266–271. [Google Scholar] [CrossRef] [PubMed]
- German Society for Lipid Sciences (GSLS). Einheitsmethoden 1950–1975, No. B-1b; Wissenschaftliche Verlagsgesellschaft MbH: Stuttgart, Germany, 1952. [Google Scholar]
- Carrera, F.; Leon-Camacho, M.; Pablos, F.; Ganzáles, A.G. Authentication of Green Coffee Varieties According to Their Sterolic Profile. Anal. Chim. Acta 1998, 370, 131–139. [Google Scholar] [CrossRef]
- Topala, C.M.; Tataru, L.D. Infrared Spectra of Green Arabica Coffee Extraction using Supercritical Carbon Dioxide and Soxhlet Technique. Rev. Chim. 2015, 66, 1128–1131. [Google Scholar]
- Oliveira, É.R.; Silva, R.F.; Santos, P.R.; Queiroz, F. Potential of Alternative Solvents to Extract Biologically Active Compounds from Green Coffee Beans and its Residue from the Oil Industry. Food Bioprod. Process. 2019, 115, 47–58. [Google Scholar] [CrossRef]
- Coelho, E.G.; Bertarini, P.L.L.; Gomes, M.S.; Amaral, L.R.; Zotarelli, M.F.; Santos, L.D.; Santana, R.C. Physicochemical and Sensory Properties of Arabica Coffee Beans of Arara cv. Dried Using Different Methods. Foods 2024, 13, 642. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.S.; Franca, A.S.; Camargos, R.R.S.; Ferraz, V.P. Coffee Oil as a Potential Feedstock for Biodiesel Production. Bioresour. Technol. 2008, 99, 3244–3250. [Google Scholar] [CrossRef]
- Dong, W.; Chen, Q.; Wei, C.; Hu, R.; Long, Y.; Zong, Y.; Chu, Z. Comparison of the Effect of Extraction Methods on the Quality of Green Coffee Oil from Arabica Coffee Beans: Lipid Yield, Fatty Acid Composition, Bioactive Components, and Antioxidant Activity. Ultrason. Sonochem. 2021, 74, 105578. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, N.A.; Cornelio-Santiago, H.P.; Fukumasu, H.; de Oliveira, A.L. Green Coffee Extracts Rich in Diterpenes–Process Optimization of Pressurized Liquid Extraction Using Ethanol as Solvent. J. Food Eng. 2018, 224, 148–155. [Google Scholar] [CrossRef]
- De Oliveira, P.M.A.; De Almeida, R.H.; De Oliveira, N.A.; Bostyn, S.; Gonçalves, C.B.; De Oliveira, A.L. Enrichment of Diterpenes in Green Coffee Oil Using Supercritical Fluid Extraction-Characterization and Comparison with Green Coffee Oil from Pressing. J. Supercrit. Fluids 2014, 95, 137–145. [Google Scholar] [CrossRef]
- Ahangari, B.; Sargolzaei, J. Extraction of Lipids from Spent Coffee Grounds Using Organic Solvent and Supercritical Carbon Dioxide. J. Food Process. Preserv. 2013, 37, 1014–1021. [Google Scholar] [CrossRef]
- AOAC. Crude Fat in Feeds, Cereal Grains and Forages, 18th ed.; AOAC 2003.06-2006; Association of Official Analytical Chemists: Gaithersburgs, MD, USA, 2006. [Google Scholar]
- Soxhlet, F. Die Gewichtsanalytische Bestimmung des Milchfettes. Dinglers Polytech. J. 1879, 232, 461–465. [Google Scholar]
- Zygler, A.; Słomińska, M.; Namieśnik, J. Soxhlet Extraction and New Developments Such as Soxtec. In Comprehensive Sampling and Sample Preparation: Analytical Techniques for Scientists, 1st ed.; Pawliszyn, J., Ed.; Academic Press: London, UK, 2012; pp. 65–82. [Google Scholar] [CrossRef]
- Novaes, F.J.M.; de Faria, D.C.; Ferraz, F.Z.; Aquino Neto, F.R. Hansen Solubility Parameters Applied to the Extraction of Phytochemicals. Plants 2023, 12, 3008. [Google Scholar] [CrossRef]
- Yara-Varón, E.; Fabiano-Tixier, A.S.; Balcells, M.; Canela-Garayoa, R.; Bily, A.; Chemat, F. Is it Possible to Substitute Hexane with Green Solvents for Extraction of Carotenoids? A Theoretical Versus Experimental Solubility Study. RSC Adv. 2016, 6, 27750–27759. [Google Scholar] [CrossRef]
- Cascant, M.M.; Breil, C.; Garrigues, S.; de la Guardia, M.; Fabiano-Tixier, A.S.; Chemat, F.A. Green Analytical Chemistry Approach for Lipid Extraction: Computation Methods in the Selection of Green Solvents as Alternative to Hexane. Anal. Bioanal. Chem. 2017, 409, 3527–3539. [Google Scholar] [CrossRef]
- Li, Y.; Fine, F.; Fabiano-Tixier, A.S.; Abert-Vian, M.; Carre, P.; Pages, X.; Chemat, F. Evaluation of Alternative Solvents for Improvement of Oil Extraction from Rapeseeds. Comptes Rendus Chim. 2014, 17, 242–251. [Google Scholar] [CrossRef]
- López-Bascón-Bascon, M.A.; Luque de Castro, M.D. Soxhlet Extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 327–354. [Google Scholar] [CrossRef]
- Novaes, F.J.M.; Lima, F.A.; Calado, V.; Marriott, P.J.; de Aquino Neto, F.R.; Rezende, C.M. Isolating Valuable Coffee Diterpenes by Using an Inexpensive Procedure. Ind. Crop. Prod. 2020, 152, 112494. [Google Scholar] [CrossRef]
- Kerton, F.; Marriott, R. Alternative Solvents for Green Chemistry, 2nd ed.; RSC Green Chemistry Series; The Royal Society of Chemistry: Cambridge, UK, 2013; Volume 20. [Google Scholar] [CrossRef]
- Kail, B.W.; Link, D.D.; Morreale, B.D. Determination of Free Fatty Acids and Triglycerides by Gas Chromatography Using Selective Esterification Reactions. J. Chromatogr. Sci. 2012, 50, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Tazikeh, S.; Zendehboudi, S.; Ghafoori, S.; Lohi, A.; Mahinpey, N. Algal bioenergy production and utilization: Technologies, challenges, and prospects. J. Environ. Chem. Eng. 2022, 1010, 107863. [Google Scholar] [CrossRef]
- Zeng, X.; Danquah, M.K.; Chen, X.D.; Lu, Y. Microalgae bioengineering: From CO2 fixation to biofuel production. Renovar. Sustentar. Energia Rev. 2011, 15, 3252–3260. [Google Scholar] [CrossRef]
- Folmer, B.; Blank, I.; Farah, A.; Giuliano, P.; Sanders, D.; Wille, C. The Craft of Sciece of Coffee, 1st ed.; Academic Press: Cambridge, UK, 2017. [Google Scholar]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; A/RES/70/1; United Nations: New York, NY, USA, 2015. [Google Scholar]
- International Olive Council. Determination of Fatty Acid Methyl Esters by Gas Chromatography (COI-T.20-Doc.-No-33-Rev.-1-2017). 2017. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-T.20-Doc.-No-33-Rev.-1-2017.pdf (accessed on 29 May 2024).
- Phillips, P.P. Return on Investment (ROI) Basics, 1st ed.; ASTD Press: Alexandria, VA, USA, 2023. [Google Scholar]
- Phillips, J.J. ROI: The search for best practices. Train. Dev. 1996, 50, 42–48. [Google Scholar]
| Extraction Technique | Procedure Details | Yield (%) | Reference |
|---|---|---|---|
| Soxhlet | 20 g of GCB 200 mL hexane 6 h of extraction under reflux | 11.4 | [36] |
| 2–5 g of GCB 250–300 mL petroleum ether 8–16 h of extraction under reflux | Not informed | [37] | |
| 7.5 g of GCB 30 mL petroleum ether 4 h of extraction under reflux | 7.5–9.5 | [38] | |
| 30 g of GCB 300 mL hexane 4–16 h of extraction under reflux | 10.4–10.5 | [26] | |
| 7.5 g of GCB 30 mL petroleum ether 4 h of extraction under reflux | Not informed | [39] | |
| 50 g of GCB 250 mL hexane 8 h of extraction under reflux | 15 | [40] | |
| 50 g of GCB 100 mL hexane 7 h of extraction under reflux | 8.5 | [41] | |
| 2 g of GCB 10 mL solvent 3 h of extraction under reflux | 8.3 (acetone) 11.7 (ethanol) 6.4 (ethyl acetate) 8.8 (hexane) 10.2 (isopropanol) 7.7 (petroleum ether) | [42] | |
| 25 g of GCB 400 mL of petroleum ether 4 h of extraction under reflux | 9.5 | [23] | |
| 2 g of GCB petroleum ether 6 h of extraction under reflux | 14.0 | [43] | |
| Industrial Soxhlet 25 kg GCB Hexane 16 h of extraction under reflux | 10–12 | [44] | |
| 20 g GCB 150 mL hexane 4 h of extraction under reflux | 9.1–16.4 | [7] | |
| Microwave assisted extraction | 2 g of GCB 8 mL petroleum ether 45 °C; 10 min; 600 rpm | 5.9–7.6 | [38] |
| 10 g of GCB 100 mL ethanol; 60 °C; 30 min; 200 W | 9.3 | [45] | |
| Ultrasonic extraction | 10 g of GCB 300 mL ethanol 35 °C; 40 kHz; 50 min; 50 W | 9.1 | [45] |
| Microwave assisted ultrasonic extraction | 3.6 g of GCB 100 mL ethanol 60 °C; 350 W; 10 min | 10.6 | [45] |
| Accelerated solvent extraction | 22 g of GCB 30 mL ethanol 3 cycles; 10.3 Mpa | 6.6–9.8 | [46] |
| 20 g of GCB 40 mL ethanol 100 °C; 100 bar; 30 min | 6.3 | [45] | |
| CO2 Supercritical Fluid Extraction | 200 mg of GCB or RCB 60–90 °C; 235–380 bar; 25 min; 1.5 mL min−1 of CO2 | 10.3–14.0 | [36] |
| 90 g of GCB; 90 °C; 300 bar; 695.42 kg/m3; 6 h; CO2 at 5 g/min | 6.5 | [47] | |
| 90 g of GCB 70 °C; 300 bar 6 h; CO2 at 5 g/min | 8.8 | [46] | |
| Expeller screw press | 350 g of GCB Continuous screw press 5 mm outlet, 18 rpm | 5.3–7.0 | [7] |
| 1 kg of GCB 0.67 to 1.2 kg/h 18 rpm | 2.6–6.3 | [23] |
| Experiment | Yield (%) |
|---|---|
| Replicate 1 | 11.54 |
| Replicate 2 | 11.50 |
| Replicate 3 | 11.65 |
| Mean | 11.56 |
| Standard deviation | 0.08 |
| Coefficient of variation (%) | 0.67 |
| Factor | Levels | ||
|---|---|---|---|
| Low (−1) | Center (0) | High (+1) | |
| T, temperature (°C) | 60 | 70 | 80 |
| R, the ratio between solvent volume and the mass of ground raw coffee beans | 3:1 (2 mL:0.667 g−1) | 6.5:1 (2 mL:0.308 g−1) | 10:1 (2 mL:0.200 g−1) |
| t, time (min) | 30 | 60 | 90 |
| Experiment | T (°C) | R (mL g−1) | t (min) | Coffee Oil Yield (%) |
|---|---|---|---|---|
| 1 | 60 | 3.0 | 30 | 8.79 |
| 2 | 60 | 3.0 | 90 | 9.21 |
| 3 | 60 | 3.0 | 60 | 8.97 |
| 4 | 60 | 6.5 | 90 | 10.27 |
| 5 | 60 | 6.5 | 60 | 10.41 |
| 6 | 60 | 6.5 | 30 | 10.00 |
| 7 | 60 | 10.0 | 60 | 10.24 |
| 8 | 60 | 10.0 | 30 | 9.76 |
| 9 | 60 | 10.0 | 90 | 10.81 |
| 10 | 70 | 3.0 | 90 | 9.64 |
| 11 | 70 | 3.0 | 60 | 9.80 |
| 12 | 70 | 3.0 | 30 | 8.82 |
| 13 | 70 | 6.5 | 60 | 10.79 |
| 14 | 70 | 6.5 | 30 | 10.41 |
| 15 | 70 | 6.5 | 90 | 11.09 |
| 16 | 70 | 10.0 | 30 | 10.93 |
| 17 | 70 | 10.0 | 90 | 11.18 |
| 18 | 70 | 10.0 | 60 | 10.99 |
| 19 | 80 | 3.0 | 60 | 10.19 |
| 20 | 80 | 3.0 | 30 | 9.29 |
| 21 | 80 | 3.0 | 90 | 9.53 |
| 22 | 80 | 6.5 | 30 | 10.58 |
| 23 | 80 | 6.5 | 90 | 11.19 |
| 24 | 80 | 6.5 | 60 | 11.17 |
| 25 | 80 | 10.0 | 90 | 11.64 |
| 26 | 80 | 10.0 | 60 | 11.15 |
| 27 | 80 | 10.0 | 30 | 10.64 |
| 28 | 70 | 6.5 | 60 | 10.73 |
| 29 | 70 | 6.5 | 60 | 10.64 |
| 30 | 70 | 6.5 | 60 | 10.23 |
| Average value (Exp. 28–30) | 10.53 | |||
| Standard deviation (Exp. 28–30) | 0.27 | |||
| Coefficient of variation (%; Exp. 28–30) | 2.54 | |||
| Coffee Oil Yield (%) | Extraction Time (min) | ||
|---|---|---|---|
| 90 | 120 | 150 | |
| Replicate 1 | 11.75 | 11.73 | 11.38 |
| Replicate 2 | 11.74 | 77.83 | 11.49 |
| Replicate 3 | 11.29 | 11.34 | 11.50 |
| Average value | 11.59 | 11.63 | 11.46 |
| Standard deviation | 0.26 | 0.26 | 0.07 |
| Coefficient of variation (%) | 2.27 | 2.23 | 0.58 |
| Time (min) | Compound Name | Pressing (%) | Soxhlet (%) | DH-SLE (%) |
|---|---|---|---|---|
| 16.208 | Myristic acid (C14:0) | 0.10 ± 0.04 | 0.12 ± 0.06 | 0.07 ± 0.03 |
| 20.797 | Palmitic acid (C16:0) | 32.78 ± 2.14 | 32.93 ± 2.23 | 34.14 ± 3.54 |
| 25.637 | Stearic acid (C18:0) | 10.30 ± 0.72 | 9.95 ± 0.97 | 9.83 ± 1.26 |
| 26.101 | Oleic acid (C18:1) | 11.90 ± 0.45 | 10.92 ± 1.13 | 11.57 ± 0.94 |
| 27.383 | Linoleic acid (C18:2) | 36.97 ± 2.89 | 37.41 ± 2.73 | 37.07 ± 1.93 |
| 28.790 | Linolenic acid (C18:3) | 1.85 ± 0.20 | 1.35 ± 0.13 | 1.50 ± 0.26 |
| 30.593 | Arachidic acid (C20:0) | 3.97 ± 0.97 | 4.43 ± 0.27 | 3.70 ± 0.77 |
| 31.022 | Gadoleic acid (C20:1) | 0.46 ± 0.15 | 0.45 ± 0.18 | 0.40 ± 0.13 |
| 35.439 | Behenic acid (C22:0) | 0.92 ± 0.28 | 1.32 ± 0.42 | 0.86 ± 0.40 |
| 36.452 | Erucic acid C22:1) | 0.36 ± 0.10 | 0.67 ± 0.25 | 0.54 ± 0.13 |
| 40.097 | Lignoceric acid (C24:0) | 0.39 ± 0.13 | 0.46 ± 0.09 | 0.33 ± 0.11 |
| Sample Estimation | Soxhlet | DH-SLE |
|---|---|---|
| Sample mass (g) | 30 | 0.2 |
| Volume of n-Hexane (mL) | 300 | 2 |
| Extraction time (h) | 4–16 | 1.5 |
| Water consumption per hour (L) | 90 | 0 |
| Energy consumption (kWh) | 3.0 | 1.5 |
| Coffee oil yield (% w/w) | 11.5 | 11.6 |
| Item | DH-SLE (USD) | Soxhlet (USD) | Economy (USD) |
|---|---|---|---|
| Hexane cost | 0.21 | 31.30 | 31.09 |
| Energy cost | 0.18 | 0.36 | 0.18 |
| Water cost | 0.00 | 0.54 | 0.54 |
| Total by extraction | 0.39 | 32.20 | 31.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Faria, D.C.; de Queiroz, M.E.L.R.; Novaes, F.J.M. Direct Hot Solid–Liquid Extraction (DH-SLE): A High-Yield Greener Technique for Lipid Recovery from Coffee Beans. Plants 2025, 14, 185. https://doi.org/10.3390/plants14020185
de Faria DC, de Queiroz MELR, Novaes FJM. Direct Hot Solid–Liquid Extraction (DH-SLE): A High-Yield Greener Technique for Lipid Recovery from Coffee Beans. Plants. 2025; 14(2):185. https://doi.org/10.3390/plants14020185
Chicago/Turabian Stylede Faria, Daliane Cláudia, Maria Eliana Lopes Ribeiro de Queiroz, and Fábio Junior Moreira Novaes. 2025. "Direct Hot Solid–Liquid Extraction (DH-SLE): A High-Yield Greener Technique for Lipid Recovery from Coffee Beans" Plants 14, no. 2: 185. https://doi.org/10.3390/plants14020185
APA Stylede Faria, D. C., de Queiroz, M. E. L. R., & Novaes, F. J. M. (2025). Direct Hot Solid–Liquid Extraction (DH-SLE): A High-Yield Greener Technique for Lipid Recovery from Coffee Beans. Plants, 14(2), 185. https://doi.org/10.3390/plants14020185







