Bryophyte Flora of AlUla County (Saudi Arabia)—Distribution, Ecology, and Conservation
Abstract
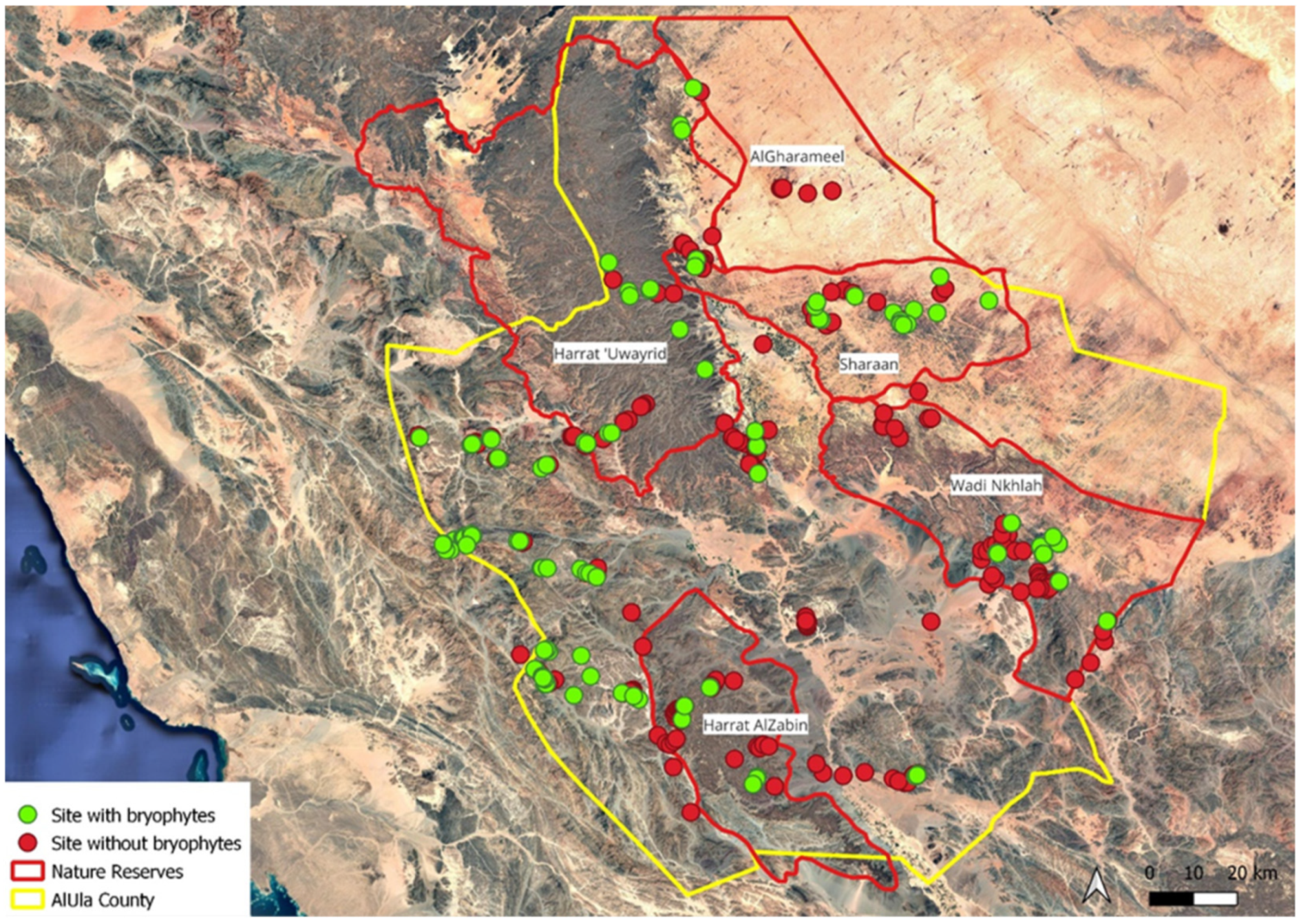
1. Introduction
2. Results
2.1. Acaulon triquetrum (Spruce) Müll. Hal. (Pottiaceae)
2.2. Aloina rigida (Hedw.) Limpr. (Pottiaceae)
2.3. Bryum dichotomum Hedw. (Bryaceae)
2.4. Clevea spathysii (Lindenb.) Müll. Frib. (Cleveaceae)
2.5. Genus Crossidium (Pottiaceae) (Crossidium aberrans Holz. & E. B. Bartram, C. crassinervium (De Not.) Jur., C. deserti W. Frey & Kürschner, C. squamiferum (Viv.) Jur.)
2.6. Didymodon desertorum (J. Froehl.) J. A. Jiménez & M. J. Cano (Pottiaceae)
2.7. Encalypta vulgaris Hedw. (Encalyptaceae)
2.8. Genus Entosthodon (Funariaceae) (E. cf. commutatus Durieu & Mont., E. duriaei Mont., and E. muhlenbergii (Turner) Fife)
2.9. Eucladium verticillatum (With.) Bruch & Schimp. (Pottiaceae)
2.10. Fissidens cf. arnoldii R. Ruthe (Fissidentaceae)
2.11. Funaria hygrometrica Hedw. (Funariaceae)
2.12. Genus Geheebia (Pottiaceae)
2.13. Genus Grimmia (Grimmiaceae)
2.14. Gymnostomiella vernicosa (Hook. ex Harv.) M. Fleisch. (Pottiaceae)
2.15. Genus Gymnostomum (Pottiaceae)
2.16. Gyroweisia tenuis (Hedw.) Schimp. (Pottiaceae)
2.17. Hymenostylium hildebrandtii (Müll. Hal.) R. H. Zander (Pottiaceae)
2.18. Genus Microbryum (Pottiaceae)
2.19. Molendoa handelii (Schiffn.) Brinda & R. H. Zander (Pottiaceae)
2.20. Plagiochasma rupestre (J. R. Forst. & G. Forst.) Steph. (Aytoniaceae)
2.21. Pterygoneurum ovatum (Hedw.) Dixon (Pottiaceae)
2.22. Genus Ptychostomum (Bryaceae)
2.23. Riccia cavernosa Hoffm. (Ricciaceae)
2.24. Riella affinis M. Howe & Underw. (Riellaceae)
2.25. Genus Syntrichia (Pottiaceae)
2.26. Genus Targionia (Targioniaceae)
2.27. Timmiella barbuloides (Brid.) Mönk. (Pottiaceae)
2.28. Genus Tortula (Pottiaceae)
2.29. Trichostomopsis australasiae (Hook. & Grev.) H. Rob. (Pottiaceae)
2.30. Vinealobryum vineale (Brid.) R. H. Zander (Pottiaceae)
2.31. Weissia condensa (Voit) Lindb. (Pottiaceae)
3. Discussion
4. Conservation
5. Materials and Methods
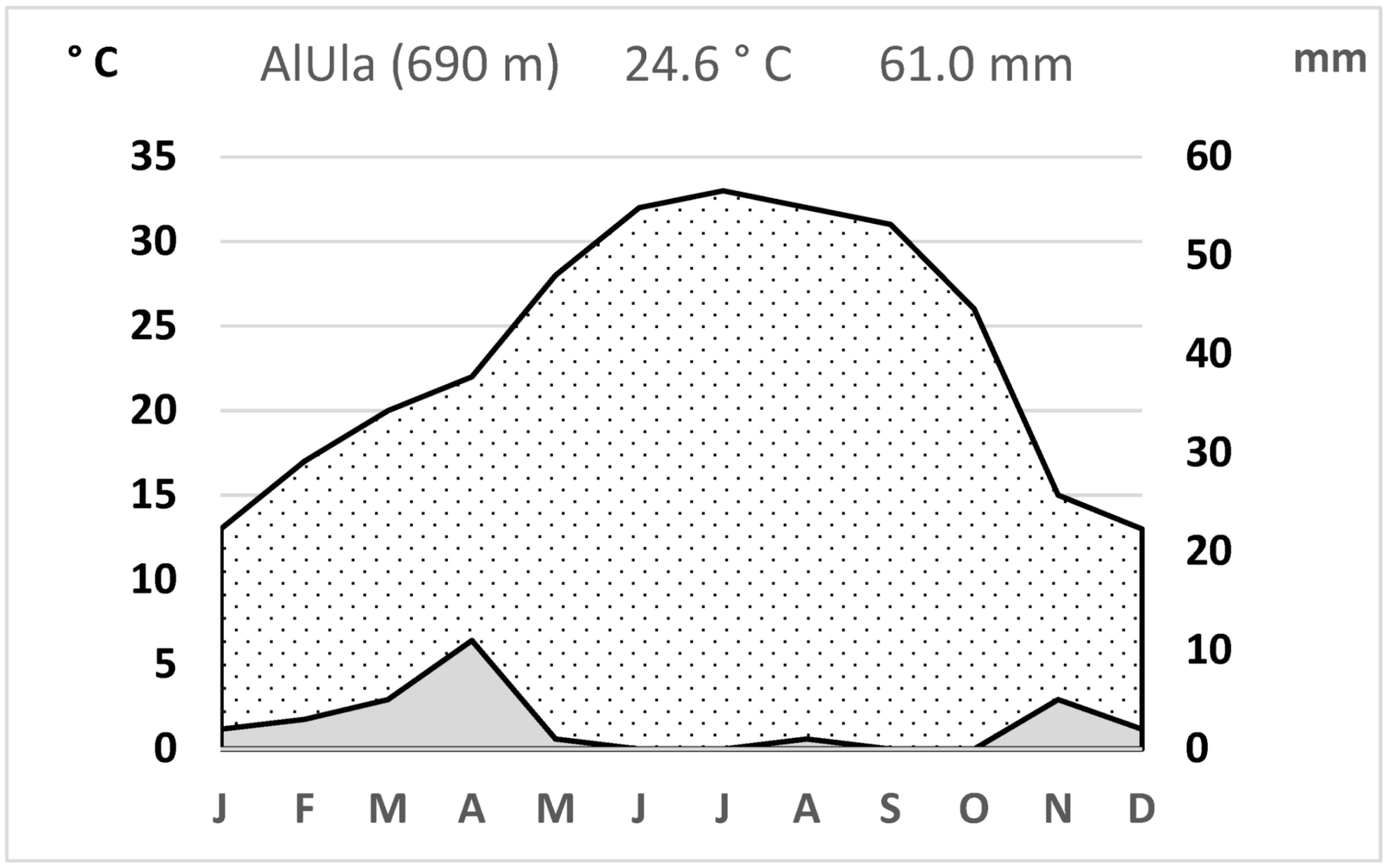
5.1. Study Site
- Lycium shawi–Ephedra foliata shrubland of the runnels and wadis of the Hijaz Mountains;
- Vegetation of the sandstone canyons and sandy gullies (Ficus salicifolia, F. palmata, Abutilon bidentatum, etc.);
- A sparse mixed herb and shrub community of the scree slopes (Ochradenus baccatus, Fagonia bruguieri, Forsskaolea tenacissima, and Tetraena simplex) housing crack micro-communities dominated by Parietaria alsinifolia, Isatis lusitanica, and Hemionitis pteridioides);
- The Retama raetam–Artemisia sieberi open shrubland of the high Hijaz Mountains, well developed in the Jabal AlWard from 1500 to 2000 m;
- Palm groves housing rich assemblages of ruderal communities (Aizoon canariense, Lysimachia arvensis, Calendula tripterocarpa, Malva parviflora, Bromus tectorum, etc.);
- Seasonal pools with Juncus rigidus, Typha sp., and Mentha longifolia and mainly Characeae species and green Algae;
- Seepage with Adiantum capillus veneris.
5.2. Surveys and Nomenclature
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Accepted Nomenclature | AlUla | Reference | Family | Taxonomy 1 | Taxonomy 2 |
|---|---|---|---|---|---|
| Acaulon triquetrum (Spruce) Müll. Hal. | x | [11] | Pottiaceae Hampe | Bryophyta | Moss |
| Alleniella besseri (Lobarz.) S. Olsson, Enroth & D. Quandt | [6] | Neckeraceae Schimp. | Bryophyta | Moss | |
| Aloina aloides (Koch ex Schultz) Kindb. | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Aloina rigida (Hedw.) Limpr. | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Anomobryum julaceum (Schrad. ex G. Gaertn., B. Mey. & Scherb.) Schimp. | [6] | Bryaceae Rchb. | Bryophyta | Moss | |
| Asterella persica (Steph.) M. Howe | [5] | Aytoniaceae Cavers | Marchantiophyta | Liverwort | |
| Bartramia aprica Müll. Hal. | [6] | Bartramiaceae Schwägr. | Bryophyta | Moss | |
| Brachymenium exile (Dozy & Molk.) Bosch. & Sande Lac. | [6] | Bryaceae Rchb. | Bryophyta | Moss | |
| Braunia secunda (Hook.) Bruch & Schimp. | [6] | Hedwigiaceae Schimp. | Bryophyta | Moss | |
| Bryum arachnoideum Müll. Hal. | [6] | Bryaceae Rchb. | Bryophyta | Moss | |
| Bryum argenteum Hedw. | [6] | Bryaceae Rchb. | Bryophyta | Moss | |
| Bryum dichotomum Hedw. | x | [6] | Bryaceae Rchb. | Bryophyta | Moss |
| Bryum radiculosum Brid. | [8] | Bryaceae Rchb. | Bryophyta | Moss | |
| Bryum violaceum Crundw. & Nyholm | [6] | Bryaceae Rchb. | Bryophyta | Moss | |
| Campylopus pilifer Brid. | [6] | Leucobryaceae Schimp. | Bryophyta | Moss | |
| Clevea spathysii (Lindenb.) Müll. Frib. | x | [11] | Cleveaceae Cavers | Marchantiophyta | Liverwort |
| Cratoneuron filicinum (Hedw.) Spruce | [6] | Amblystegiaceae Kindb. | Bryophyta | Moss | |
| Crossidium aberrans Holz. & E. B. Bartram | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Crossidium crassinervium (De Not.) Jur. | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Crossidium davidai Catches. | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Crossidium deserti W. Frey & Kürschner | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Crossidium laevipilum Thér. & Trab. | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Crossidium laxefilamentosum W. Frey & Kürschner | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Crossidium squamiferum (Viv.) Jur. | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Didymodon acutus (Brid.) K. Saito | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Didymodon cordatus Jur. | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Didymodon desertorum (J. Froehl.) J. A. Jiménez & M. J. Cano | x | [9] | Pottiaceae Hampe | Bryophyta | Moss |
| Didymodon rigidulus Hedw. | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Encalypta intermedia Jur. | [6] | Encalyptaceae Schimp. | Bryophyta | Moss | |
| Encalypta vulgaris Hedw. | x | [6] | Encalyptaceae Schimp. | Bryophyta | Moss |
| Entosthodon attenuatus (Dicks.) Bryhn | [6] | Funariaceae Schwägr. | Bryophyta | Moss | |
| Entosthodon cf. commutatus Durieu & Mont. | x | This paper | Funariaceae Schwägr. | Bryophyta | Moss |
| Entosthodon duriaei Mont. | x | [6] | Funariaceae Schwägr. | Bryophyta | Moss |
| Entosthodon muhlenbergii (Turner) Fife | x | [6] | Funariaceae Schwägr. | Bryophyta | Moss |
| Entosthodon pulchellus (H. Philib.) Brugués | [6] | Funariaceae Schwägr. | Bryophyta | Moss | |
| Eucladium verticillatum (With.) Bruch & Schimp. | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Exormotheca pustulosa Mitt. | [5] | Corsiniaceae Engl. | Marchantiophyta | Liverwort | |
| Fabronia abyssinica Müll. Hal. | [6] | Fabroniaceae Schimp. | Bryophyta | Moss | |
| Fabronia ciliaris (Brid.) Brid. | [6] | Fabroniaceae Schimp. | Bryophyta | Moss | |
| Fabronia pusilla Raddi | [6] | Fabroniaceae Schimp. | Bryophyta | Moss | |
| Fabronia socotrana Mitt. | [6] | Fabroniaceae Schimp. | Bryophyta | Moss | |
| Fissidens cf. arnoldii R. Ruthe | x | [6] | Fissidentaceae Schimp. | Bryophyta | Moss |
| Fissidens bryoides Hedw. subsp. bryoides | [6] | Fissidentaceae Schimp. | Bryophyta | Moss | |
| Fissidens bryoides Hedw. subsp. schmidii (Müll. Hal.) Nork | [6] | Fissidentaceae Schimp. | Bryophyta | Moss | |
| Fissidens crassipes Wilson ex Bruch & Schimp. subsp. crassipes | [6] | Fissidentaceae Schimp. | Bryophyta | Moss | |
| Fissidens crispulus Brid. | [6] | Fissidentaceae Schimp. | Bryophyta | Moss | |
| Fissidens crispus Mont. | [6] | Fissidentaceae Schimp. | Bryophyta | Moss | |
| Fissidens sciophyllus Mitt. | [6] | Fissidentaceae Schimp. | Bryophyta | Moss | |
| Fissidens viridulus (Sw.) Wahlenb. | [6] | Fissidentaceae Schimp. | Bryophyta | Moss | |
| Fontinalis hypnoides Hartm. | [6] | Fontinalaceae Schimp. | Bryophyta | Moss | |
| Fossombronia caespitiformis (Raddi) De Not. ex Rabenh. | [5] | Fossombroniaceae Hazsl. | Marchantiophyta | Liverwort | |
| Frullania caffraria Steph. | [5] | Frullaniaceae Lorch | Marchantiophyta | Liverwort | |
| Frullania obscurifolia Mitt. | [5] | Frullaniaceae Lorch | Marchantiophyta | Liverwort | |
| Frullania trinervis (Lehm.) Drège | [5] | Frullaniaceae Lorch | Marchantiophyta | Liverwort | |
| Funaria hygrometrica Hedw. | x | [11] | Funariaceae Schwägr. | Bryophyta | Moss |
| Geheebia lurida (Hornsch. ex Spreng.) J. A. Jiménez & M. J. Cano | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Geheebia siccula (M.J. Cano, Ros, García-Zam. & J. Guerra) J.A. Jiménez & M. J. Cano | x | This paper | Pottiaceae Hampe | Bryophyta | Moss |
| Geheebia tophacea (Brid.) R. H. Zander | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Grimmia anodon Bruch & Schimp. | x | [6] | Grimmiaceae Arn. | Bryophyta | Moss |
| Grimmia laevigata (Brid.) Brid. | [6] | Grimmiaceae Arn. | Bryophyta | Moss | |
| Grimmia lisae De Not. | [6] | Grimmiaceae Arn. | Bryophyta | Moss | |
| Grimmia orbicularis Bruch ex Wilson | x | [6] | Grimmiaceae Arn. | Bryophyta | Moss |
| Grimmia pulvinata (Hedw.) Sm. | [6] | Grimmiaceae Arn. | Bryophyta | Moss | |
| Grimmia tergestina Tomm. ex Bruch & Schimp. | [6] | Grimmiaceae Arn. | Bryophyta | Moss | |
| Grimmia trichophylla Grev. | [6] | Grimmiaceae Arn. | Bryophyta | Moss | |
| Gymnostomiella vernicosa (Hook. ex Harv.) M. Fleisch. | x | [11] | Pottiaceae Hampe | Bryophyta | Moss |
| Gymnostomum calcareum Nees & Hornsch. var. calcareum | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Gymnostomum mosis (Lorentz) Jur. & Milde | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Gymnostomum viridulum Brid. | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Gyroweisia reflexa (Brid.) Schimp. | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Gyroweisia tenuis (Hedw.) Schimp. | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Hydrogonium bolleanum (Müll. Hal.) A. Jaeger | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Hydrogonium fontanum (Müll. Hal.) A. Jaeger | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Hydrogonium orientale (F. Weber) Jan Kučera | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Hymenostylium hildebrandtii (Müll. Hal.) R. H. Zander | x | [7] | Pottiaceae Hampe | Bryophyta | Moss |
| Hymenostylium recurvirostrum (Hedw.) Dixon | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Hyophila involuta (Hook.) A. Jaeger | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Hypnum cupressiforme Hedw. var. cupressiforme | [6] | Hypnaceae Schimp. | Bryophyta | Moss | |
| Hypnum vaucheri Lesq. | [6] | Hypnaceae Schimp. | Bryophyta | Moss | |
| Imbribryum alpinum (Huds. ex With.) N. Pedersen | [6] | Bryaceae Rchb. | Bryophyta | Moss | |
| Lejeunea aethiopica E. W. Jones | [5] | Lejeuneaceae Rostovzev | Marchantiophyta | Liverwort | |
| Leptodon smithii (Hedw.) F. Weber & D. Mohr | [6] | Neckeraceae Schimp. | Bryophyta | Moss | |
| Leptophascum leptophyllum (Müll. Hal.) J. Guerra & M. J. Cano. | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Leucodon dracaenae Solms ex Venturi | [6] | Leucodontaceae Schimp. | Bryophyta | Moss | |
| Lunularia cruciata (L.) Dumort. ex Lindb. | [5] | Lunulariaceae H. Klinggr. | Marchantiophyta | Liverwort | |
| Mannia androgyna (L.) A. Evans | [5] | Aytoniaceae Cavers | Marchantiophyta | Liverwort | |
| Mannia fragrans (Balb.) Frye & L. Clark | [5] | Aytoniaceae Cavers | Marchantiophyta | Liverwort | |
| Metzgeria furcata (L.) Corda var. furcata | [5] | Metzgeriaceae H. Klinggr. | Marchantiophyta | Liverwort | |
| Microbryum davallianum (Sm.) R. H. Zander | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Microbryum rectum (With.) R. H. Zander | x | [11] | Pottiaceae Hampe | Bryophyta | Moss |
| Microbryum starckeanum (Hedw.) R. H. Zander | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Molendoa handelii (Schiffn.) Brinda & R. H. Zander | x | [11] | Pottiaceae Hampe | Bryophyta | Moss |
| Orthotrichum anomalum Hedw. | [6] | Orthotrichaceae Arn. | Bryophyta | Moss | |
| Orthotrichum diaphanum Schrad. ex Brid. | [6] | Orthotrichaceae Arn. | Bryophyta | Moss | |
| Oxymitra incrassata (Brot.) Sérgio & Sim-Sim | [10] | Oxymitraceae Müll. Frib. ex Grolle | Marchantiophyta | Liverwort | |
| Oxyrrhynchium speciosum (Brid.) Warnst. | [6] | Brachytheciaceae Schimp. | Bryophyta | Moss | |
| Philonotis caespitosa Jur. | [6] | Bartramiaceae Schwägr. | Bryophyta | Moss | |
| Physcomitrium niloticum (Delile) Müll. Hal. | [6] | Funariaceae Schwägr. | Bryophyta | Moss | |
| Plagiochasma appendiculatum Lehm. & Lindenb. | [5] | Aytoniaceae Cavers | Marchantiophyta | Liverwort | |
| Plagiochasma eximium (Schiffn.) Steph. | [5] | Aytoniaceae Cavers | Marchantiophyta | Liverwort | |
| Plagiochasma rupestre (J. R. Forst. & G. Forst.) Steph. | x | [5] | Aytoniaceae Cavers | Marchantiophyta | Liverwort |
| Plagiochila squamulosa Mitt. | [5] | Plagiochilaceae Müll. Frib. | Marchantiophyta | Liverwort | |
| Plaubelia involuta (Magill) R. H. Zander | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Pohlia melanodon (Brid.) A. J. Shaw | [6] | Mniaceae Schwägr. | Bryophyta | Moss | |
| Pseudocrossidium porphyreoneurum (Müll. Hal.) R. H. Zander | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Pseudocrossidium replicatum (Taylor) R. H. Zander | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Pseudoleskea leikipiae (Müll. Hal.) Paris | [6] | Pseudoleskeaceae Schimp. | Bryophyta | Moss | |
| Pseudoleskea leskeoides (Paris) Müll. Hal. | [6] | Pseudoleskeaceae Schimp. | Bryophyta | Moss | |
| Pseudoleskea plagiostoma Müll. Hal. var. attenuata Müll. Hal. | [6] | Pseudoleskeaceae Schimp. | Bryophyta | Moss | |
| Pseudoleskea plagiostoma Müll. Hal. var. plagiostoma | [6] | Pseudoleskeaceae Schimp. | Bryophyta | Moss | |
| Pterygoneurum ovatum (Hedw.) Dixon | x | [11] | Pottiaceae Hampe | Bryophyta | Moss |
| Ptychostomum capillare (Hedw.) Holyoak & N. Pedersen | x | [6] | Bryaceae Rchb. | Bryophyta | Moss |
| Ptychostomum cellulare (Hook.) D. Bell & Holyoak | x | [11] | Bryaceae Rchb. | Bryophyta | Moss |
| Ptychostomum cernuum (Hedw.) Hornsch. | [7] | Bryaceae Rchb. | Bryophyta | Moss | |
| Ptychostomum funkii (Schwägr.) J. R. Spence | [6] | Bryaceae Rchb. | Bryophyta | Moss | |
| Ptychostomum imbricatulum (Müll. Hal.) Holyoak & N. Pedersen | [6] | Bryaceae Rchb. | Bryophyta | Moss | |
| Ptychostomum moravicum (Podp.) Ros & Mazimpaka | [6] | Bryaceae Rchb. | Bryophyta | Moss | |
| Ptychostomum pseudotriquetrum (Hedw.) J. R. Spence & H. P. Ramsay | x | [6] | Bryaceae Rchb. | Bryophyta | Moss |
| Ptychostomum turbinatum (Hedw.) J. R. Spence | [6] | Bryaceae Rchb. | Bryophyta | Moss | |
| Racopilum capense Müll. Hal. ex Broth. | [6] | Racopilaceae Kindb. | Bryophyta | Moss | |
| Radula lindenbergiana Gottsche ex C. Hartm. | [5] | Radulaceae Müll. Frib. | Marchantiophyta | Liverwort | |
| Reboulia hemisphaerica (L.) Raddi | [5] | Aytoniaceae Cavers | Marchantiophyta | Liverwort | |
| Rhynchostegium riparioides (Hedw.) Cardot | [6] | Brachytheciaceae Schimp. | Bryophyta | Moss | |
| Riccia atromarginata Levier subsp. atromarginata | [5] | Ricciaceae Rchb. | Marchantiophyta | Liverwort | |
| Riccia cavernosa Hoffm. | x | [5] | Ricciaceae Rchb. | Marchantiophyta | Liverwort |
| Riccia congoana Steph. | [5] | Ricciaceae Rchb. | Marchantiophyta | Liverwort | |
| Riccia crenatodentata O. H. Volk | [5] | Ricciaceae Rchb. | Marchantiophyta | Liverwort | |
| Riccia crinita Taylor | [5] | Ricciaceae Rchb. | Marchantiophyta | Liverwort | |
| Riccia lamellosa Raddi | [5] | Ricciaceae Rchb. | Marchantiophyta | Liverwort | |
| Riccia sorocarpa Bisch. | [5] | Ricciaceae Rchb. | Marchantiophyta | Liverwort | |
| Riccia trabutiana Steph. | [5] | Ricciaceae Rchb. | Marchantiophyta | Liverwort | |
| Riella affinis M. Howe & Underw. | x | [11] | Riellaceae Engl. | Marchantiophyta | Liverwort |
| Schistidium crassipilum H. H. Blom | [6] | Grimmiaceae Arn. | Bryophyta | Moss | |
| Sciurohypnum flotowianum (Sendtn.) Ignatov & Huttunen | [6] | Brachytheciaceae Schimp. | Bryophyta | Moss | |
| Scorpiurium circinatum (Bruch) M. Fleisch. & Loeske | [6] | Brachytheciaceae Schimp. | Bryophyta | Moss | |
| Syntrichia caninervis Mitt. var. caninervis | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Syntrichia fragilis (Taylor) Ochyra | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Syntrichia laevipila Brid. | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Syntrichia princeps (De Not.) Mitt. | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Syntrichia rigescens (Broth. & Geh.) Ochyra | x | [11] | Pottiaceae Hampe | Bryophyta | Moss |
| Syntrichia ruralis (Hedw.) F. Weber & D. Mohr var. ruralis | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Targionia hypophylla L. subsp. hypophylla | x | [5] | Targioniaceae Dumort. | Marchantiophyta | Liverwort |
| Targionia hypophylla L. subsp. linealis W. Frey & Kürschner | [5] | Targioniaceae Dumort. | Marchantiophyta | Liverwort | |
| Targionia lorbeeriana Müll. Frib. | x | [5] | Targioniaceae Dumort. | Marchantiophyta | Liverwort |
| Timmiella barbuloides (Brid.) Mönk. | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Tortella humilis (Hedw.) Jenn. | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Tortella inclinata (R. Hedw.) Limpr. var. inclinata | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Tortella inflexa (Bruch) Broth. | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Tortella malacophylla (Müll. Hal.) Paris | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Tortella squarrosa (Brid.) Limpr. | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Tortula atrovirens (Sm.) Lindb. | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Tortula brevissima Schiffn. | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Tortula cuneifolia (Dicks.) Turner | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Tortula inermis (Brid.) Mont. | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Tortula mucronifera W. Frey, Kürschner & Ros | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Tortula muralis Hedw. var. muralis | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Tortula pallida (Lindb.) R. H. Zander | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Tortula revolvens (Schimp.) G. Roth | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Tortula viridifolia (Mitt.) Blockeel & A. J. E. Sm | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Trichostomopsis australasiae (Hook. & Grev.) H. Rob. | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Trichostomopsis umbrosa (Müll. Hal.) H. Rob. | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Trichostomum brachydontium Bruch | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Trichostomum crispulum Bruch | [6] | Pottiaceae Hampe | Bryophyta | Moss | |
| Vinealobryum vineale (Brid.) R. H. Zander | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Weissia condensa (Voit) Lindb. | x | [6] | Pottiaceae Hampe | Bryophyta | Moss |
| Weissia controversa Hedw. var. controversa | [6] | Pottiaceae Hampe | Bryophyta | Moss |
References
- Chaudhary, S.A. Flora of the Kingdom of Saudi Arabia; Ministry of Agriculture and Water: Riyadh, Saudi Arabia, 2001; Volume III. [Google Scholar]
- Kürschner, H.; Ghazanfar, S.A. Bryophytes and Lichens. In Vegetation of the Arabian Peninsula; Ghazanfar, S.A., Fisher, M., Eds.; Springer-Science+Business Media: Dordrecht, The Netherlands, 1998; pp. 99–124. [Google Scholar]
- Kürschner, H. Bryophyte Flora of the Arabian Peninsula and Socotra. Bryophyt. Bibl. 2000, 55, 1–131. [Google Scholar]
- Frey, W.; Kürschner, H. The First Records of Bryophytes from Saudi Arabia: Studies in Arabian Bryophytes. Lindbergia 1982, 8, 157–160. [Google Scholar]
- Kürschner, H.; Frey, W. Liverworts, Mosses and Hornworts of Southwest Asia: Marchantiophyta, Bryophyta, Anthocerotophyta, 2nd enlarged and revised revision. Nova Hedwig. Beih. 2020, 149, 1–267. [Google Scholar]
- Taha, M.A. An Annotated Checklist of Saudi Arabian Mosses. Egypt. Acad. J. Biolog. Sci. 2019, 10, 17–26. [Google Scholar] [CrossRef]
- Kürschner, H.; Frey, W. Bryophyte Novelties from the Arabian Peninsula and Socotra Island. Nova Hedwig. 2020, 110, 281–286. [Google Scholar] [CrossRef]
- Taha, M.A.; Abou-Salama, U.Y. Bryum radiculosum Brid. New to Arabian Peninsula with New Bryaceae Additions to South Hejaz Region in Saudi Arabia. Egypt. Acad. J. Biolog. Sci. 2020, 11, 91–101. [Google Scholar] [CrossRef]
- Jiménez, J.A.; Cano, M.J.; Kürschner, H.; Porley, R.D.; Guerra, J. Reappraisal of Barbula trifaria var. desertorum (J. Froehl.) S. Agnew (Pottiaceae, Bryophyta), Based on Morphological and Molecular Evidence. Nova Hedwig. 2022, 114, 9–21. [Google Scholar] [CrossRef]
- Al-Khulaidi, A.W.; Alzahrani, A.M.; Al-Namazi, A.A.; Al-Faify, E.A.; Alfaifi, M.M.; Al Sagheer, N.A.; Al-Gifri, A.N. New Plant Records for the Flora of Saudi Arabia. J. Threat. Taxa 2024, 16, 24899–24909. [Google Scholar] [CrossRef]
- Hugonnot, V.; Pépin, F.; Freedman, J. New Bryophyte Species for Saudi Arabia and the Arabian Peninsula from AlUla County. Nova Hedwig. 2025; submitted. [Google Scholar]
- Holyoak, D.T. European Bryaceae: A Guide to the Species of the Moss Family Bryaceae in Western and Central Europe and Macaronesia; Pisces Publications: Newbury, UK, 2021; 344p. [Google Scholar]
- Frey, W.; Kürschner, H. Bryophytes of the Arabian Peninsula and Socotra: Floristics, Phytogeography and Definition of the Xerothermic Pangaean Element. Nova Hedwig. 1988, 46, 37–120. [Google Scholar]
- Frey, W.; Kürschner, H. Studies in Arabian Bryophytes 3: Crossidium asirense (Pottiaceae), a New Species from Asir Mountains (Saudi Arabia). J. Bryol. 1984, 13, 25–31. [Google Scholar] [CrossRef]
- Frey, W.; Kürschner, H. Conspectus Bryophytorum Orientalum et Arabicorum: An Annotated Catalogue of the Bryophytes in Southwest Asia. Bryophyt. Bibl. 1991, 39, 1–181. [Google Scholar]
- Bruggeman-Nannenga, M.A. An Annotated List of Fissidens Species from the Yemen Arab Republic and Sultanate of Oman, with F. laxetexturatus nov. spec. Studies in Arabian Bryophytes 7. Nova Hedwig. 1987, 45, 113–117. [Google Scholar]
- Jiménez, J.A.; Cano, M.J.; Guerra, J. A Multilocus Phylogeny of the Moss Genus Didymodon and Allied Genera (Pottiaceae): Generic Delimitations and Their Implications for Systematics. J. Syst. Evol. 2022, 60, 281–304. [Google Scholar] [CrossRef]
- Kučera, J.; Blockeel, T.; Erzberger, P.; Papp, B.; Soldán, Z.; Vellak, K.; Werner, O.; Ros, R.M. The Didymodon tophaceus Complex (Pottiaceae, Bryophyta) Revisited: New Data Support the Subspecific Rank of Currently Recognized Species. Cryptogam. Bryol. 2018, 39, 241–257. [Google Scholar] [CrossRef]
- González-Mancebo, J.M.; Patiño, J.; Rodríguez-Romero, A.; Werner, O.; Ros, R.M. Gymnostomiella (Pottiaceae, Bryophyta) Revisited: New Insights Revealed by a Morphometric Analysis. Nova Hedwig. Beih. 2010, 138, 69–83. [Google Scholar]
- Sérgio, C.; Stow, S. Identity of the Endemic African Species Gymnostomiella monodii P. de la Varde (Pottiaceae, Musci) and New Records for Macaronesia (Cape Verde). J. Bryol. 2017, 39, 1–5. [Google Scholar] [CrossRef]
- Kürschner, H. Biogeography and Introduction to Vegetation. In Vegetation of the Arabian Peninsula; Ghazanfar, S.A., Fisher, M., Eds.; Springer: Dordrecht, The Netherlands, 1998; pp. 63–98. [Google Scholar]
- Frey, W.; Kürschner, H. A Desert Bryophyte Synusia from the Jabal Tuwayq Mountain Systems (Central Saudi Arabia) with the Description of Two New Crossidium Species (Pottiaceae). Nova Hedwig. 1987, 45, 119–136. [Google Scholar]
- Kürschner, H.; Ochyra, R. Two Remarkable Hygrophytic Mosses New to the Bryophyte Flora of Yemen (Studies in Arabian Bryophytes 25). Fragm. Flor. Geobot. 1999, 44, 269–275. [Google Scholar]
- El-Oqlah, A.; Frey, W.; Kürschner, H. Tortula rigescens Broth. et Geh. (Pottiaceae): A Remarkable Species New to the Moss Flora of Jordan. Lindbergia 1988, 14, 27–29. [Google Scholar]
- Gallego, M.T.; Cano, M.J.; Ros, R.M.; Guerra, J.; Rettig, J.H. New Taxonomic Data on a Circum-Tethyan Group of Syntrichia (Pottiaceae, Bryophyta): The S. caninervis Complex. Syst. Bot. 2002, 27, 643–653. [Google Scholar]
- Gallego, M.T. A Taxonomic Study of the Genus Syntrichia Brid. (Pottiaceae, Musci) in the Mediterranean Region and Macaronesia. J. Hattori Bot. Lab. 2005, 98, 47–122. [Google Scholar]
- Frey, W.; Kürschner, H. Targionia hypophylla L. ssp. linealis (Marchantiidae, Hepaticae), a New Subspecies from Saudi Arabia, and Further Discoveries. Studies in Arabian Bryophytes 17. Nova Hedwig. 1993, 57, 127–133. [Google Scholar]
- Frey, W.; Kürschner, H. Wüstenmoose: Anpassungen und Überlebensstrategien im Täglichen Kampf mit der Sonne. BIUZ 1998, 28, 231–240. [Google Scholar] [CrossRef]
- Ochi, H.; Kürschner, H. Bryum nanoapiculatum (Bryaceae, Musci): A Species New to the Moss Flora of the Arabian Peninsula. Nova Hedwig. 1988, 47, 359–361. [Google Scholar]
- Delgadillo Moya, C. Pseudaloina (Pottiaceae, Musci): A New Genus from Yemen. Bryologist 1982, 85, 401–404. [Google Scholar]
- Pursell, R.A.; Kürschner, H. Fissidens arabicus (Fissidentaceae): A New Species from the Asir Mountains (Saudi Arabia). Studies in Arabian Bryophytes 6. Nova Hedwig. 1987, 44, 101–103. [Google Scholar]
- Volk, O.H. Riccia crenatodentata (Marchantiales) sp. nov. aus Arabien. Studien über Bryophyten aus Arabien II. Nova Hedwig. 1988, 46, 27–35. [Google Scholar]
- Mitten, W. Muscineae. In I.B. Balfour, Botany of Socotra. Trans. R. Soc. Edinb. 1888, 31, 330–336, 445. [Google Scholar]
- Al-Gifri, A.N.; Kürschner, H.; Mies, B. New Records, Additions and a New Species, Sematophyllum socotrense Buck (Sematophyllaceae, Musci) to the Bryophyte Flora of Socotra (Yemen). Studies in Arabian Bryophytes 19. Nova Hedwig. 1995, 61, 467–480. [Google Scholar]
- Dixon, H.N. Bryophyta Nova (18 & 19). Ann. Bryol. 1934, 7, 157–158. [Google Scholar]
- Müller, C. Genera Muscorum Frondosorum; Eduard Kummer: Leipzig, Germany, 1901; 474p. [Google Scholar]
- Townsend, C.C. Bryophytes from Socotra. Trans. Br. Bryol. Soc. 1969, 5, 790–796. [Google Scholar] [CrossRef]
- Frey, W.; Kürschner, H.; Ros, R.M.; Guerra, J.; Cano, M.J. Tortula mucronifera (Pottiaceae, Musci), a New Xerophytic Species of the Arabian Peninsula and Jordan. Nova Hedwig. 1994, 59, 345–351. [Google Scholar]
- Zander, R.H. Genera of the Pottiaceae: Mosses of Harsh Environments. Bull. Buffalo Soc. Nat. Sci. 1993, 32, 378p. [Google Scholar]
- Hofmeister, W.; Smith, G. Bryophyte Development and Simple Structural Features. J. Exp. Bot. 2021, 73, 4306–4315. [Google Scholar]
- Rensing, S.A.; Lang, D. The Biology of Bryophytes: Evolution, Simplicity, and Adaptation. Plant Cell 2022, 34, 228–243. [Google Scholar]
- Kürschner, H.; Ochyra, R. Erpodium glaziovii (Erpodiaceae, Bryopsida) and Further Novelties from the Arabian Peninsula. Additions to the Bryophyte Flora of the Arabian Peninsula and Socotra 4. Willdenowia 2003, 33, 205–210. [Google Scholar] [CrossRef]
- Kürschner, H.; Buck, W.R.; Sollman, P. Two Tropical Species New to the Bryophyte Flora of the Arabian Peninsula. Additions to the Bryophyte Flora of the Arabian Peninsula and Socotra 2. Nova Hedwig. 2001, 73, 253–259. [Google Scholar] [CrossRef]
- Proctor, M.C.F. Physiological Ecology of Bryophytes. In Bryophyte Biology; Shaw, A.J., Goffinet, B., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 225–247. [Google Scholar]
- Stark, L.R. Mosses in Harsh Environments. In Bryophyte Biology, 2nd ed.; Goffinet, B., Shaw, A.J., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 330–357. [Google Scholar]
- Belnap, J. The World at Your Feet: Desert Biological Soil Crusts. Front. Ecol. Environ. 2003, 1, 181–189. [Google Scholar] [CrossRef]
- Zundel, C.; Frey, B. Biological Soil Crusts: Underappreciated Contributors to Dryland Ecosystem Resilience. Biol. Environ. Proc. R. Ir. Acad. 2013, 113, 1–23. [Google Scholar]
- Stark, L.R. Phenology and Reproductive Biology of Syntrichia caninervis (Pottiaceae) in the Mojave Desert. Bryologist 1997, 100, 13–20. [Google Scholar] [CrossRef]
- Alpert, P. Constraints of Tolerance: Why Are Desiccation-Tolerant Organisms So Small or Rare? J. Exp. Biol. 2006, 209, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Vanderpoorten, A.; Goffinet, B. Introduction to Bryophytes; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Drysdale, R.N.; Zanchetta, G.; Hellstrom, J. Chemical Evolution of a Travertine-Depositing River System: The Rapolano Terme Travertine System, Central Italy. Aquat. Geochem. 2003, 9, 275–298. [Google Scholar] [CrossRef]
- Pentecost, A.; Zhang, Z. Tufa Deposits from China. Quat. Int. 2001, 86, 119–131. [Google Scholar]
- Ford, T.D.; Pedley, H.M. A Review of Tufa and Travertine Deposits of the World. Earth-Sci. Rev. 1996, 41, 117–175. [Google Scholar] [CrossRef]
- Goudie, A.S. Arid and Semi-Arid Geomorphology; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Eldridge, D.J.; Greene, R.S.B. Microbiotic Soil Crusts: A Review of Their Roles in Soil and Ecological Processes in the Rangelands of Australia. Aust. J. Soil Res. 1994, 32, 389–415. [Google Scholar] [CrossRef]
- Jeffries, D.L.; Klopatek, J.M. Effects of Grazing on the Vegetation of the Blackbrush Association. J. Range Manag. 1987, 40, 390–392. [Google Scholar] [CrossRef]
- Proctor, M.C.F.; Oliver, M.J.; Wood, A.J.; Alpert, P.; Stark, L.R.; Cleavitt, N.L.; Mishler, B.D. Desiccation-Tolerance in Bryophytes: A Review. Bryologist 2007, 110, 595–621. [Google Scholar] [CrossRef]
- Zohary, M. Geobotanical Foundations of the Middle East; Fischer Verlag: Stuttgart, Germany, 1973. [Google Scholar]
- Hadley, D.G. Explanatory Notes to the Geological Map of the Sahl Al Matran Quadrangle; Sheet 26C; Saudi Arabian Deputy Ministry for Mineral Resources: Jiddah, Saudi Arabia, 1987. [Google Scholar]
- Rasul, N.M.A.; Stewart, I.C.F.; Nawab, Z.A. Introduction to the Red Sea: Its Origin, Structure, and Environment. In The Red Sea; Rasul, N., Stewart, I., Eds.; Springer Earth System Sciences; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Altherr, R.; Mertz-Kraus, R.; Volker, F.; Kreuzer, H.; Henjes-Kunst, F.; Lange, U. Geodynamic Setting of Upper Miocene to Quaternary Alkaline Basalts from Harrat al ‘Uwayrid (NW Saudi Arabia): Constraints from K-Ar Dating, Chemical and Sr-Nd-Pb Isotope Compositions, and Petrological Modeling. Lithos 2019, 330–331, 120–138. [Google Scholar] [CrossRef]
- Al-Bassam, A.M.; Kahlil, A.R.; Kassem, O.M. Using Updated DurovPwin Program for Hydro-Chemical Data Processing: Case Study of Al-Ula Area, Saudi Arabia. Recent Adv. Environ. Sci. Geosci. 2014, 75–81. [Google Scholar] [CrossRef]
- Cromer, P.J.; Pressey, R.L.; Hunter, M.L.; Schloss, C.A.; Buttrick, S.C.; Heller, N.E.; Tirpark, J.M.; Faith, D.P.; Cross, M.S.; Shaffer, M. Incorporating Geodiversity into Conservation Decisions. Conserv. Biol. 2015, 29, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J.J.; Ackerly, D.D.; Albano, C.M.; Anderson, M.G.; Dobrowski, S.Z.; Gill, J.L.; Heller, N.E.; Pressey, R.L.; Sanderson, E.W.; Weiss, S.B. The Theory Behind the Challenges of Conserving Nature’s Stage in a Time of Rapid Change. Conserv. Biol. 2025, 29, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Alahuhta, J.; Alahulkko, T.; Turkianinen, H.; Purola, L.; Akujärvi, A.; Lampinen, R.; Hjort, J. The Role of Geodiversity in Providing Ecosystem Services at Broad Scales. Ecol. Indic. 2018, 91, 47–56. [Google Scholar] [CrossRef]
- CMEP. Inventory and Vegetation of AlUla Flora Project; Centre for Middle Eastern Plants, Part of the Royal Botanic Garden Edinburgh, Royal Commission for AlUla: AlUla, Saudi Arabia, 2023; 115p. [Google Scholar]
- Kürschner, H.; Neef, R. Tayma Oasis (Saudi Arabia) and Its Surroundings—A First Synthesis of the Flora, Vegetation, Natural Resources, and Floral History. In Tayma I. Archaeological Exploration, Palaeoenvironment, Cultural Contact; Hausleiter, A., Eichmann, R., Al-Najem, M., Eds.; Archaeopress: Oxford, UK, 2018; pp. 88–125. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie, Grundzüge der Vegetationskunde, 3rd ed.; Springer: Wien, Austria, 1964. [Google Scholar]
- Frey, W.; Kürschner, H. Soziologie und Lebensstrategien Epiphytischer Bryophyten in Israel und Jordanien. Nova Hedwig. 1995, 61, 211–232. [Google Scholar]
- Hugonnot, V.; Pépin, F.; Freedman, J. Bryophyte Communities from AlUla County (Saudi Arabia). Nova Hedwig. 2025; submitted. [Google Scholar]
- Brinda, J.C.; Zander, R.H. A New Combination in the Genus Molendoa (Pottiaceae, Bryophyta). Phytotaxa 2020, 454, 300. [Google Scholar] [CrossRef]
- Rubasinghe, S.C.K. Phylogeny and Taxonomy of the Complex Thalloid Liverwort Family Cleveaceae Cavers. Ph.D. Thesis, University of Edinburgh, Edinburgh, UK, 2011; 264p. [Google Scholar]
- Segarra-Moragues, J.G.; Puche, F.; Gil-López, M.J. A Complete Description and Conservation Assessment of Riella affinis Howe & Underwood (Riellaceae, Sphaerocarpales) New to Continental Europe. Cryptogam. Bryol. 2019, 40, 297–307. [Google Scholar]
- Brugués, M.; Ros, R.M.; Sáez, L.; Cano, M.J. Entosthodon commutatus Durieu et Mont. (Funariaceae, Bryopsida), New to Europe and Morocco. Cryptogam. Bryol. 2010, 31, 271–276. [Google Scholar]



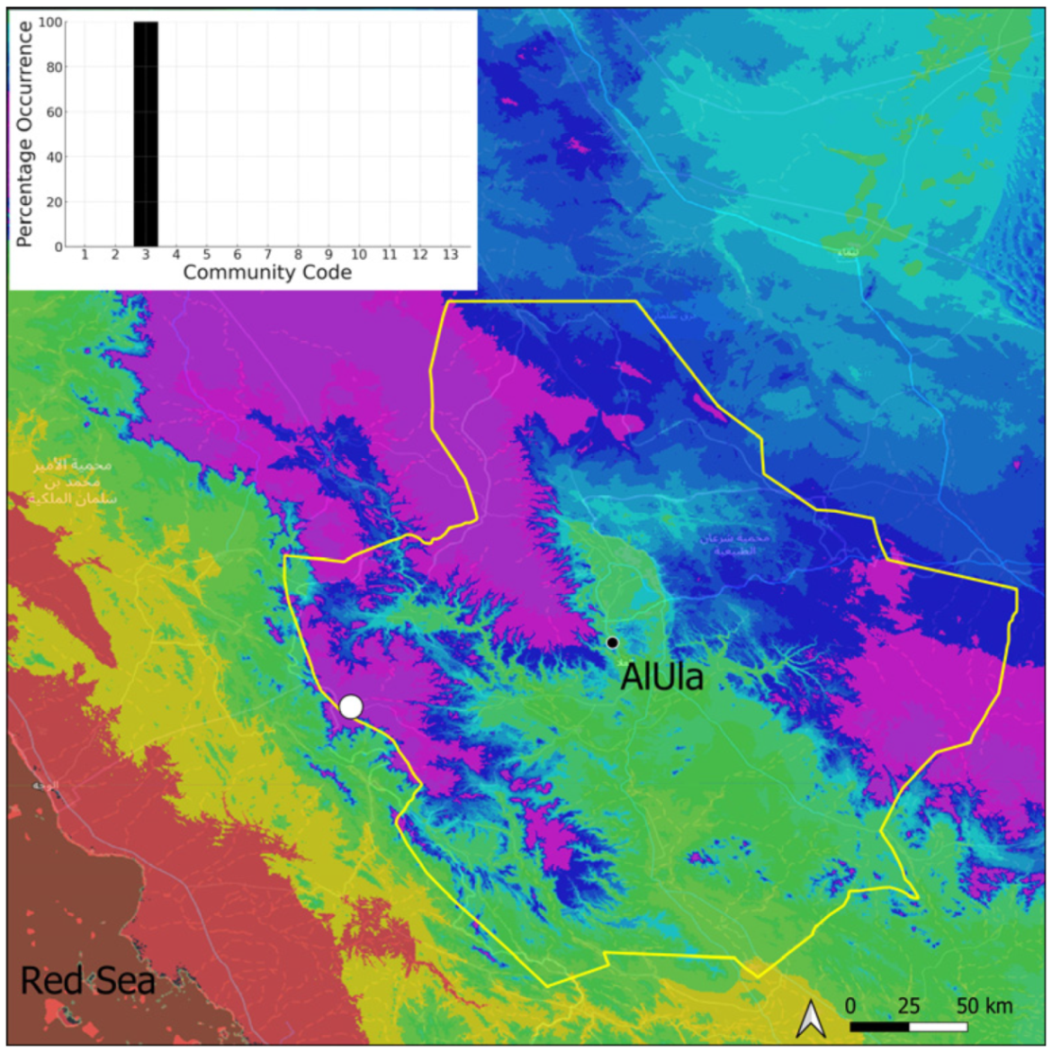
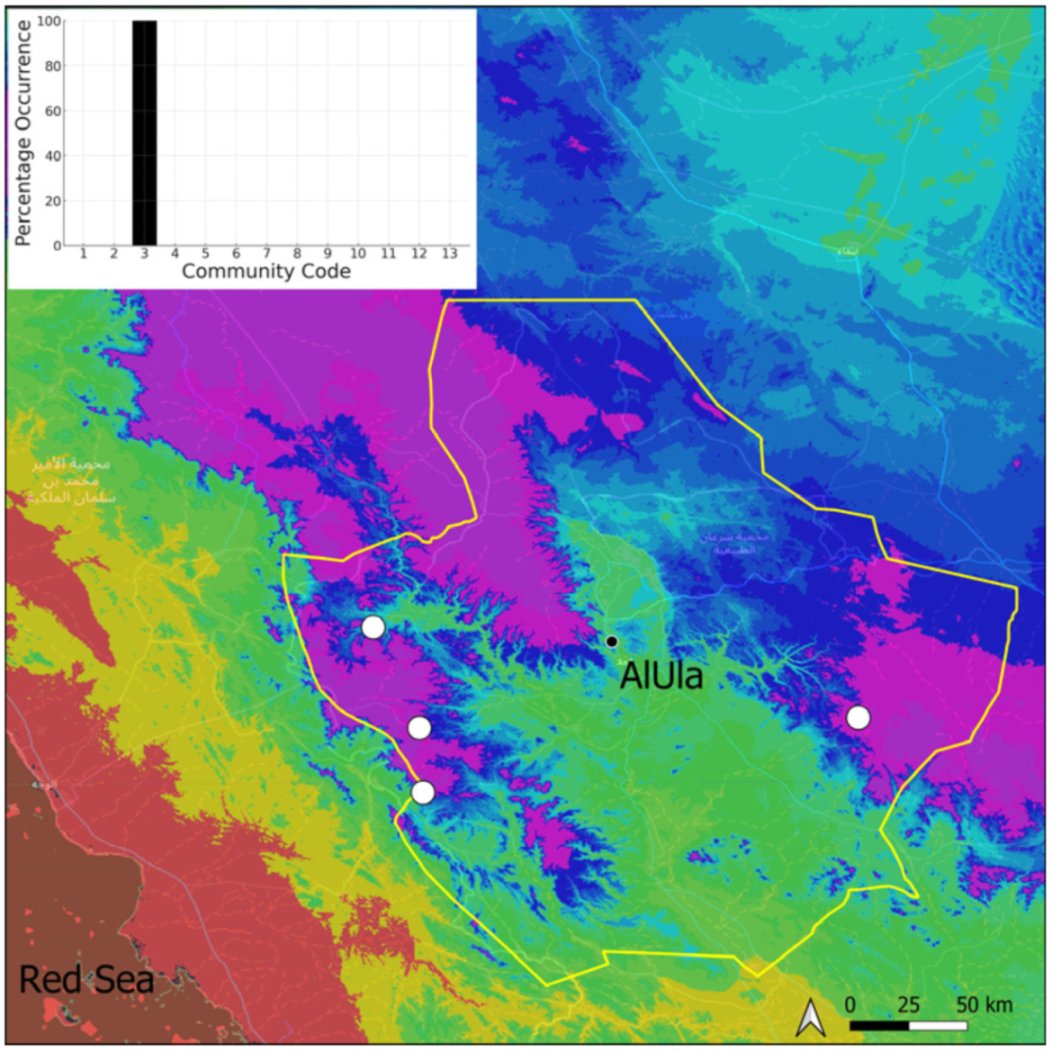
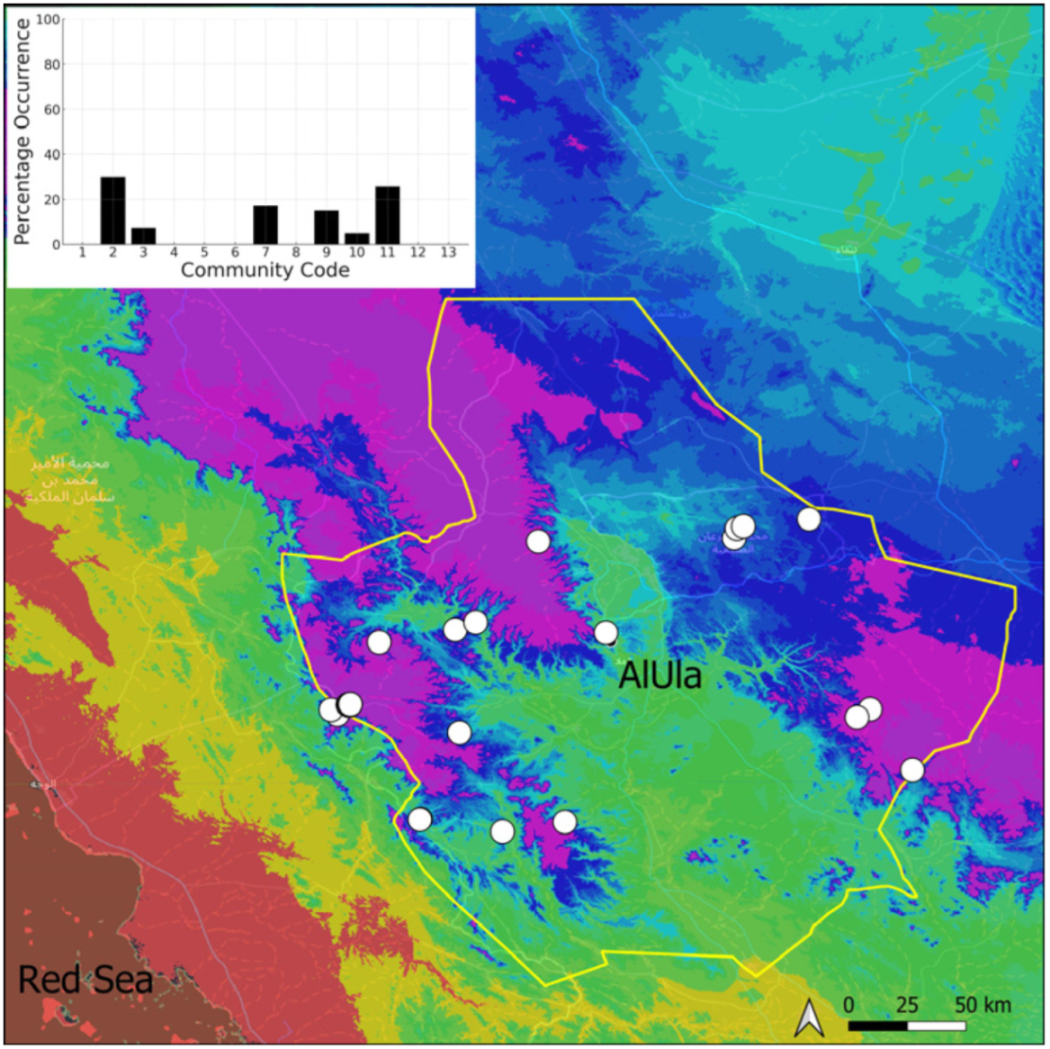
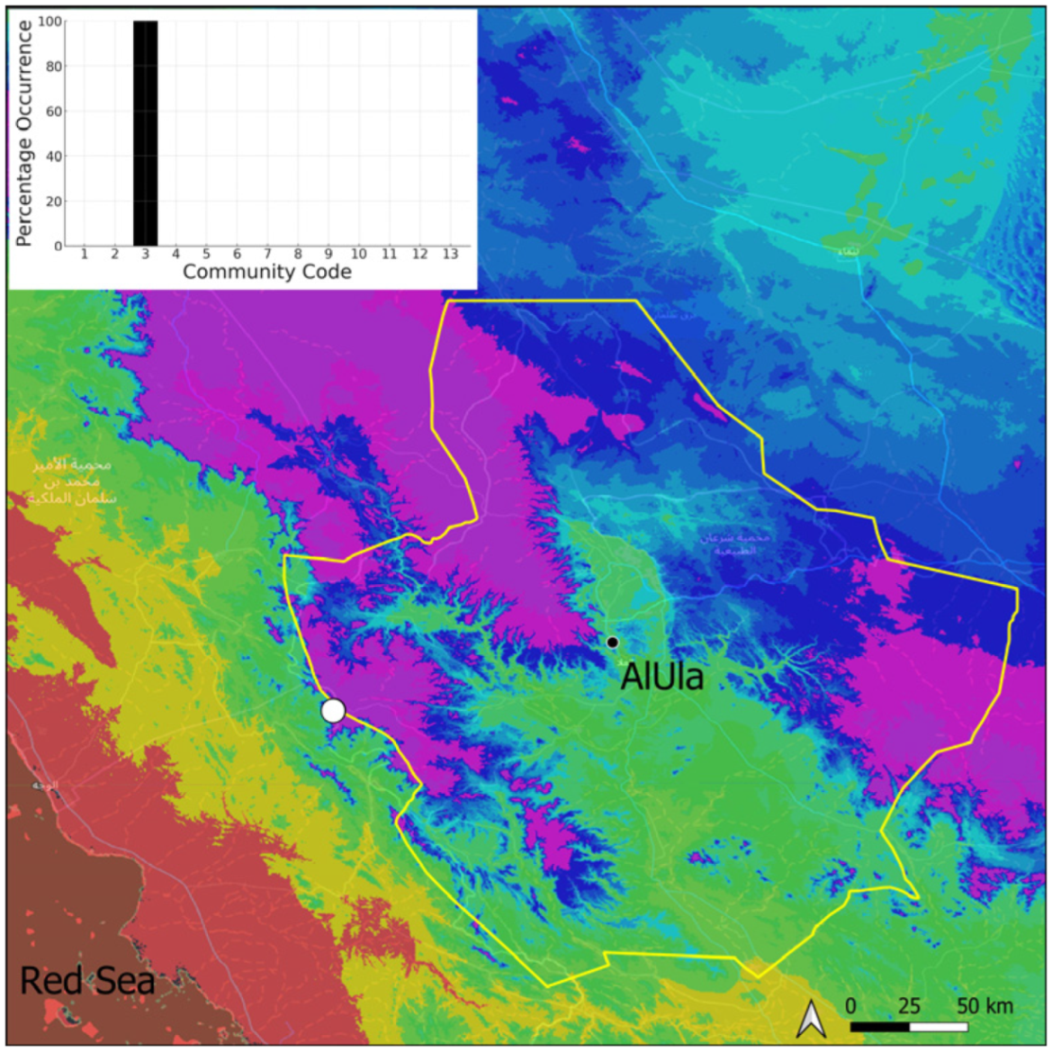
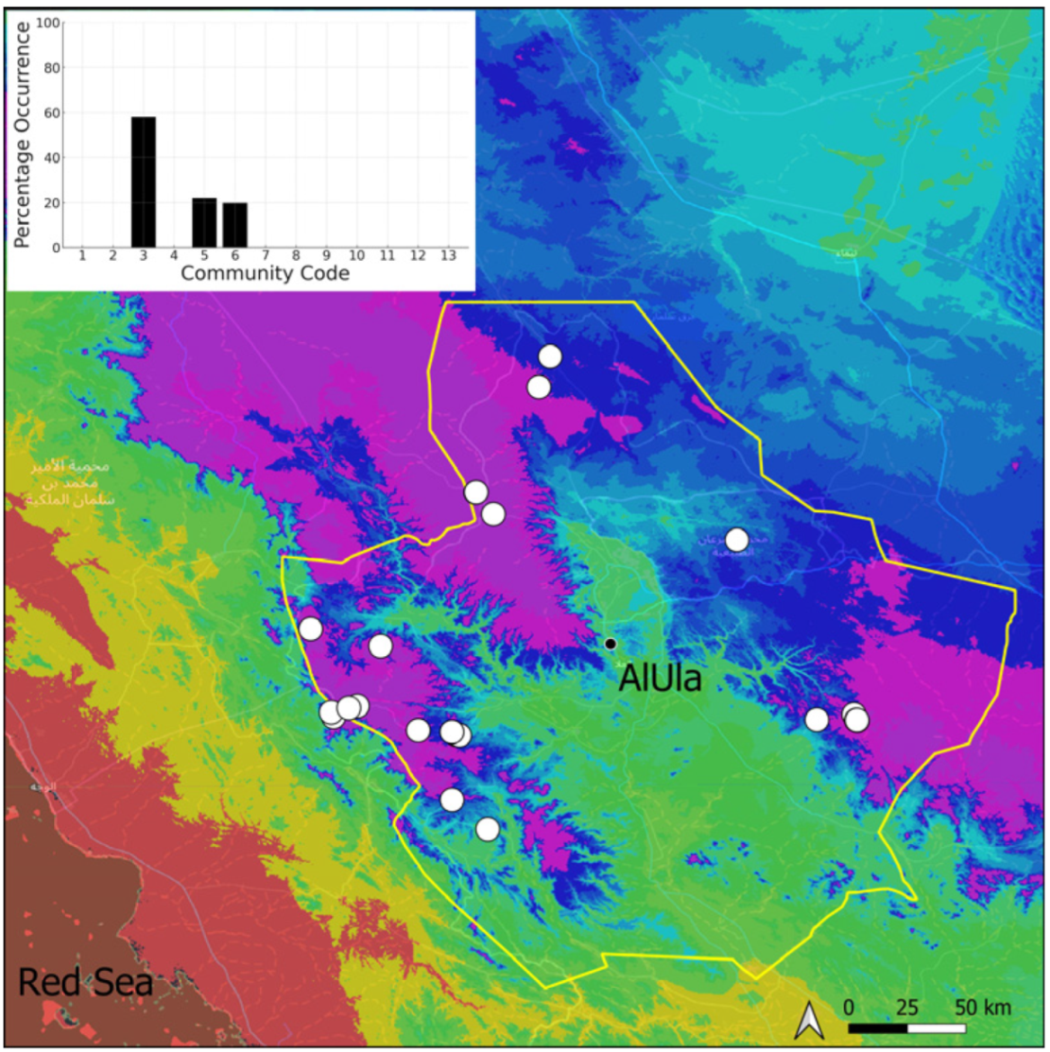
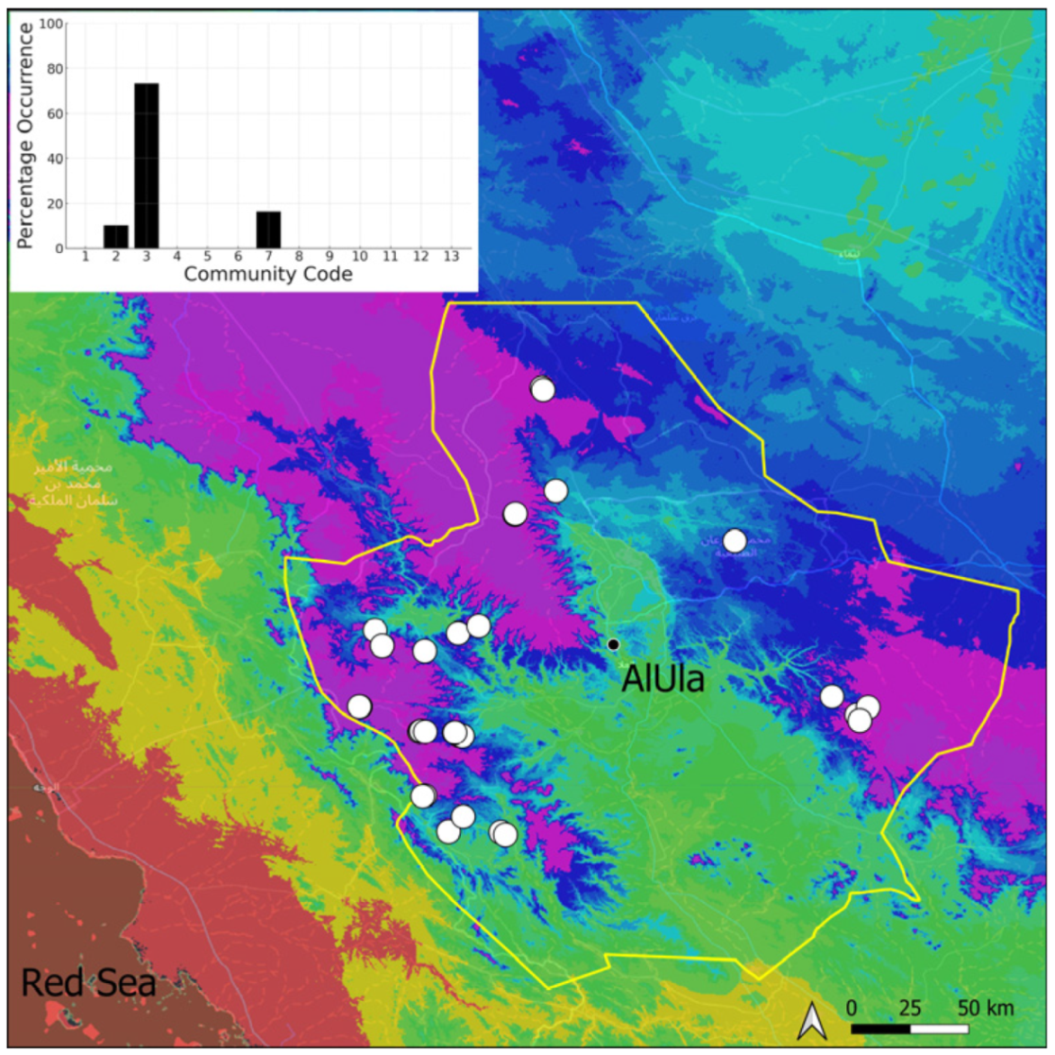
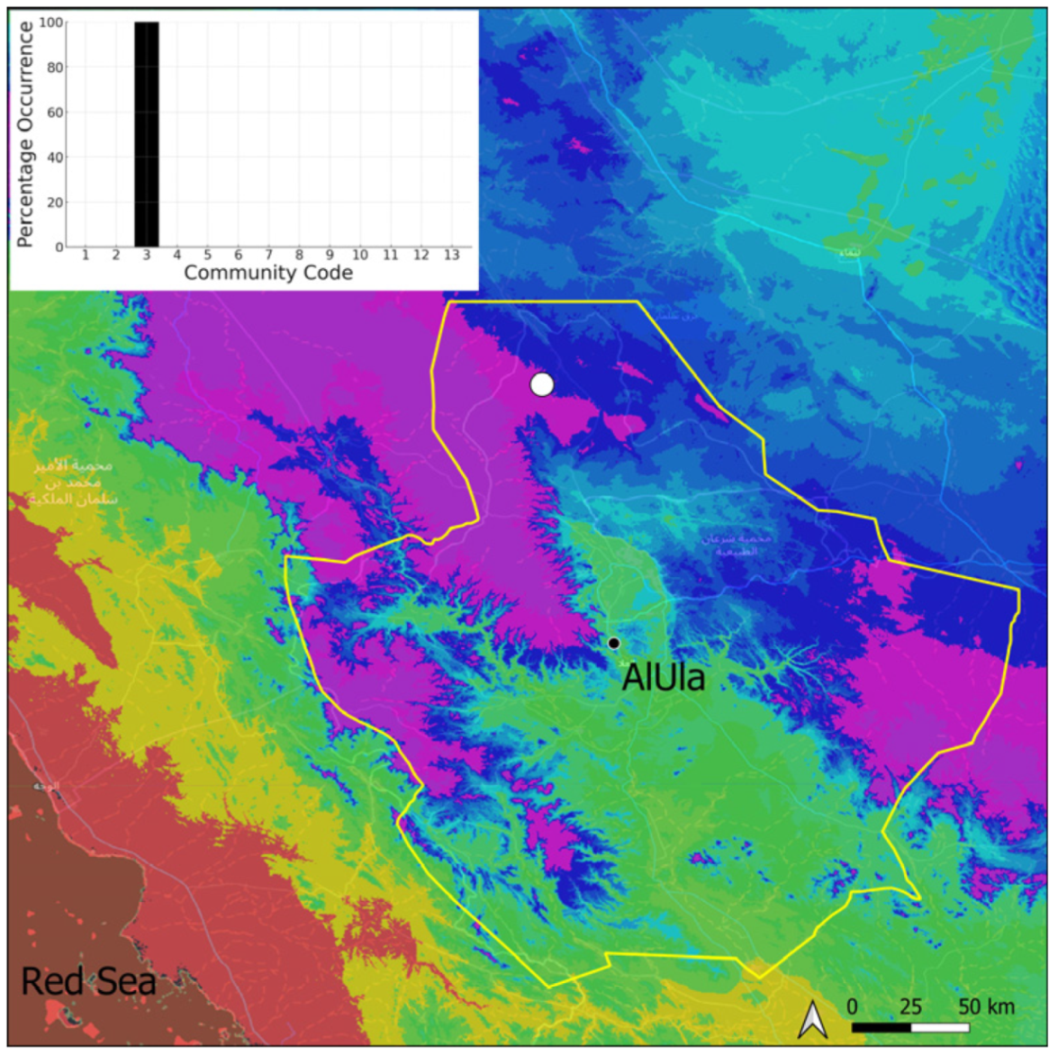


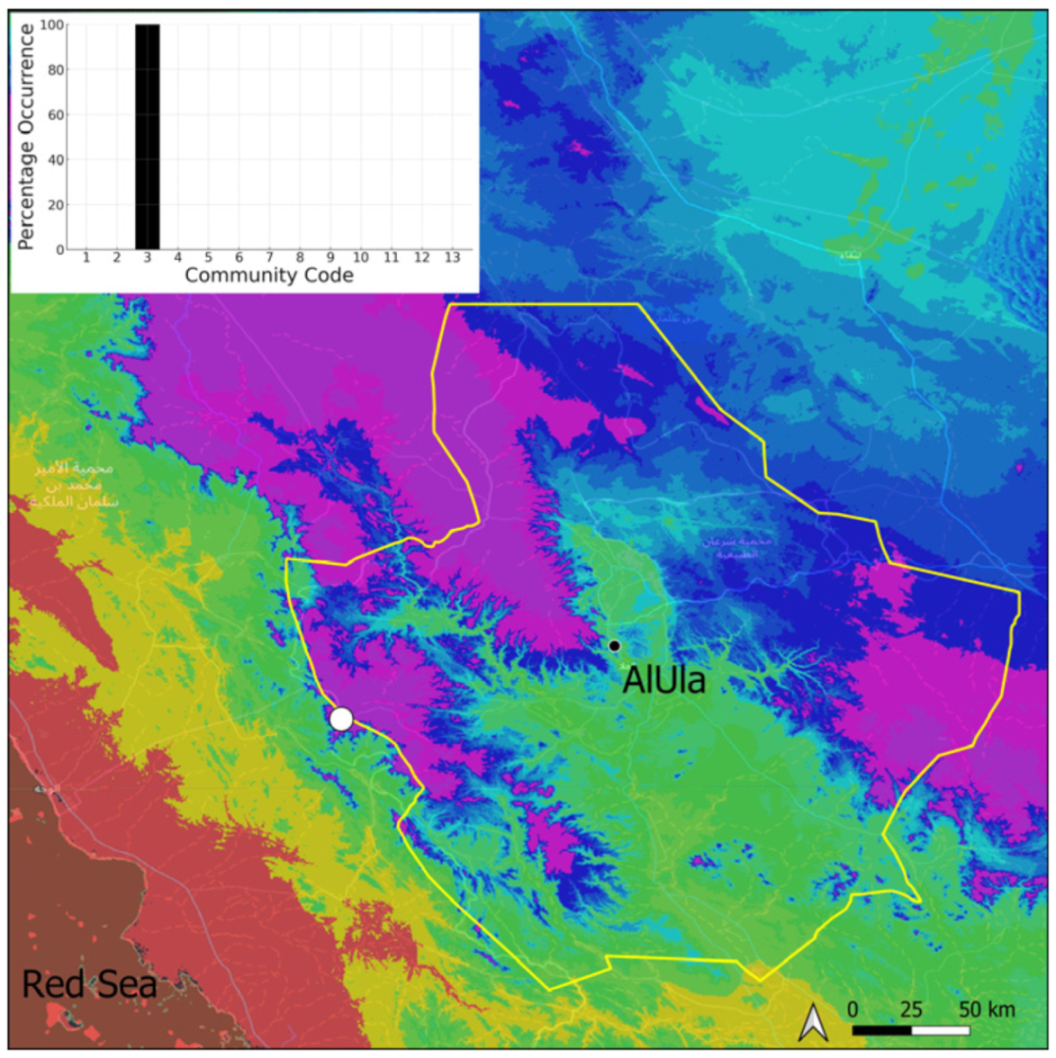
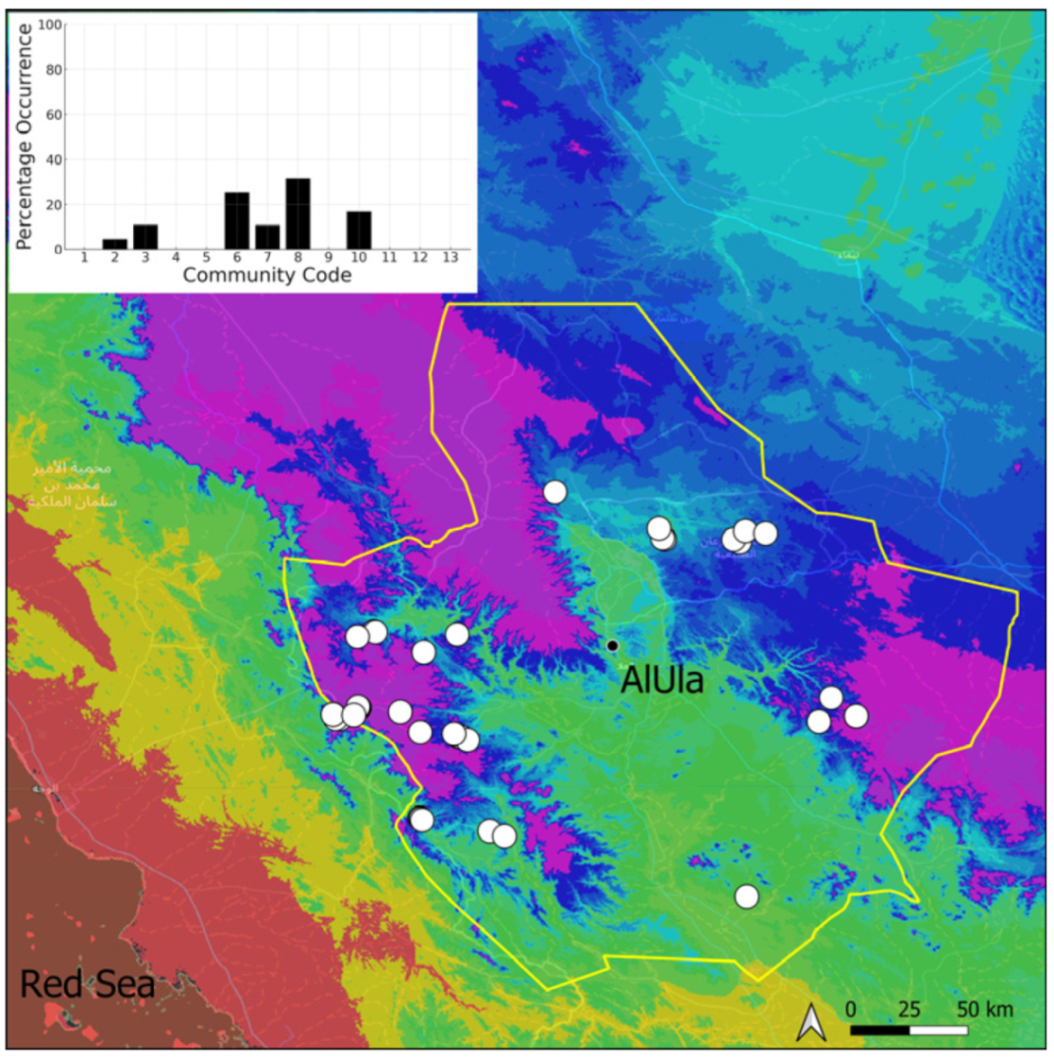
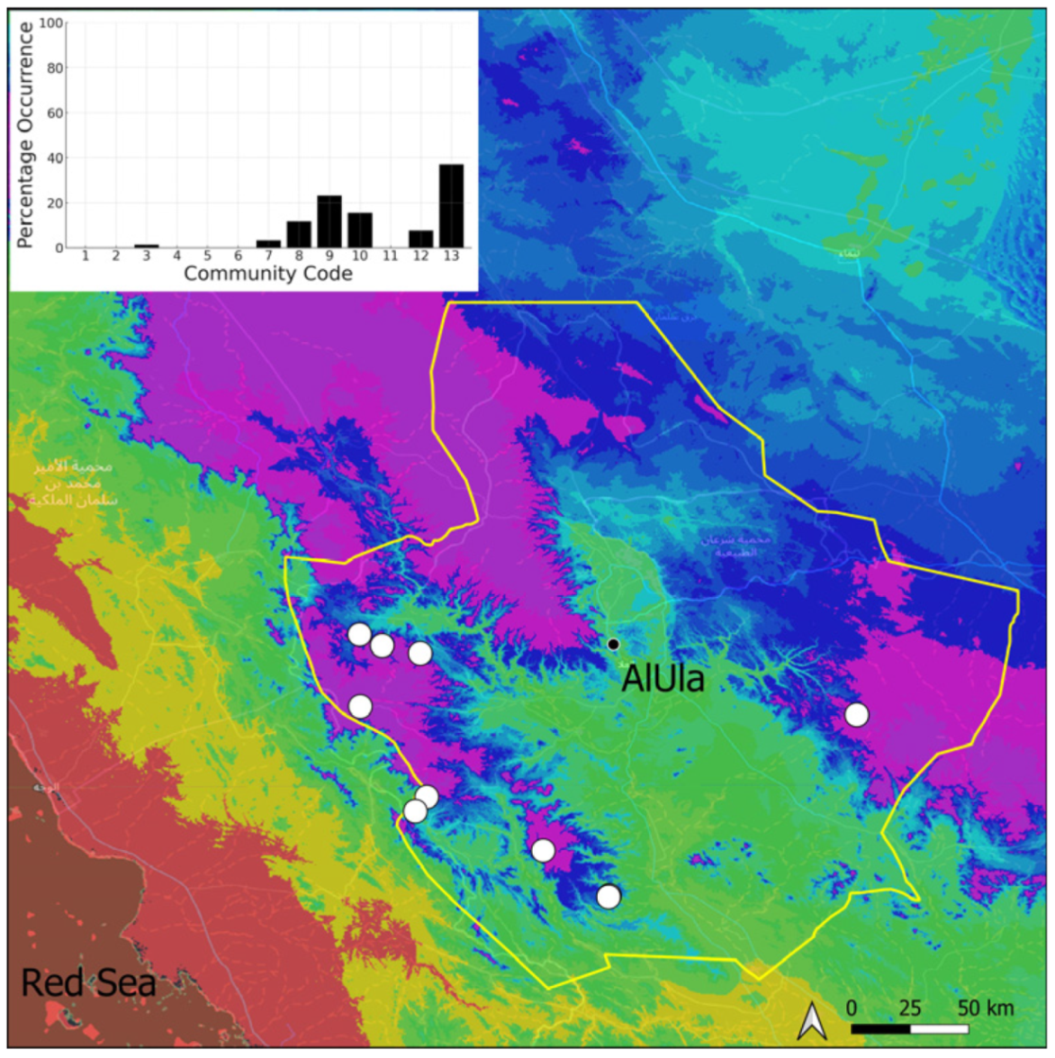
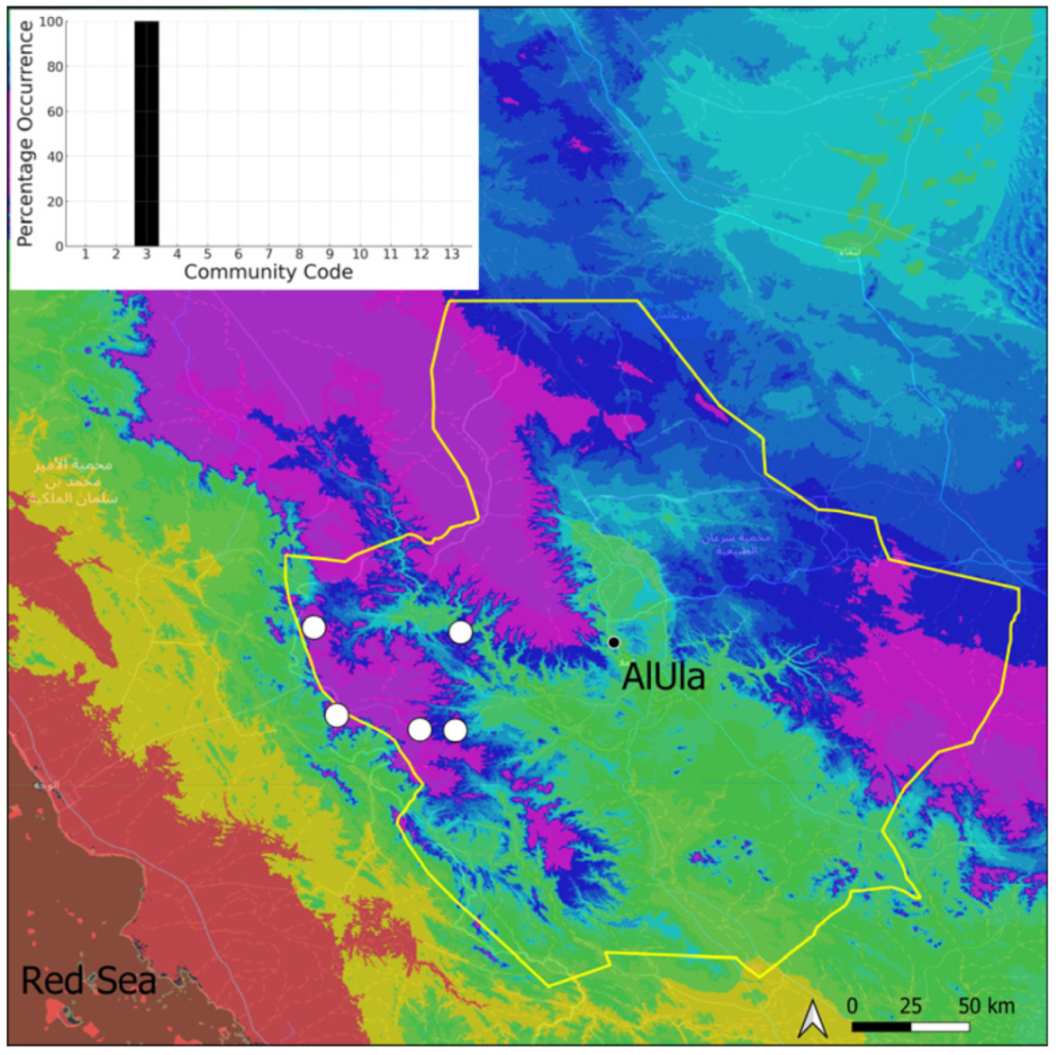

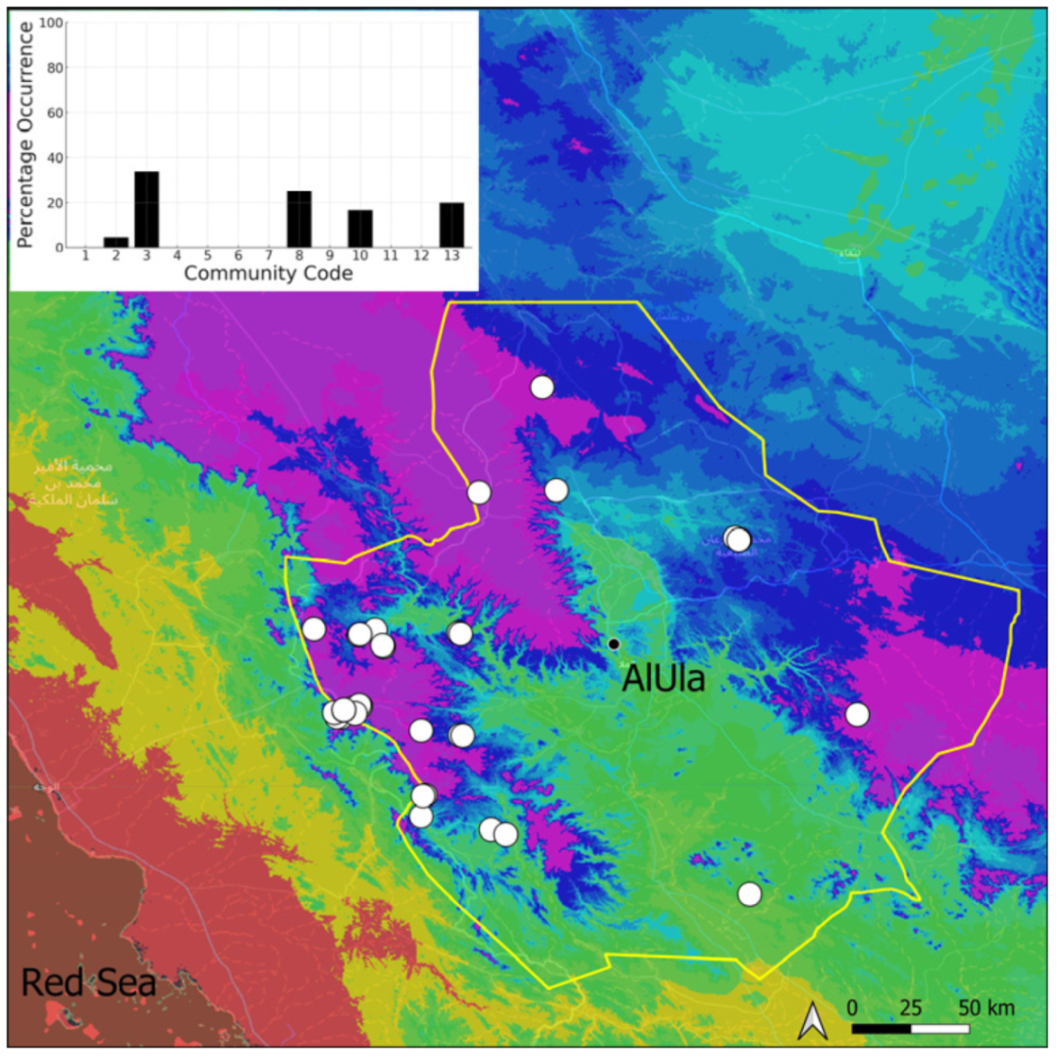
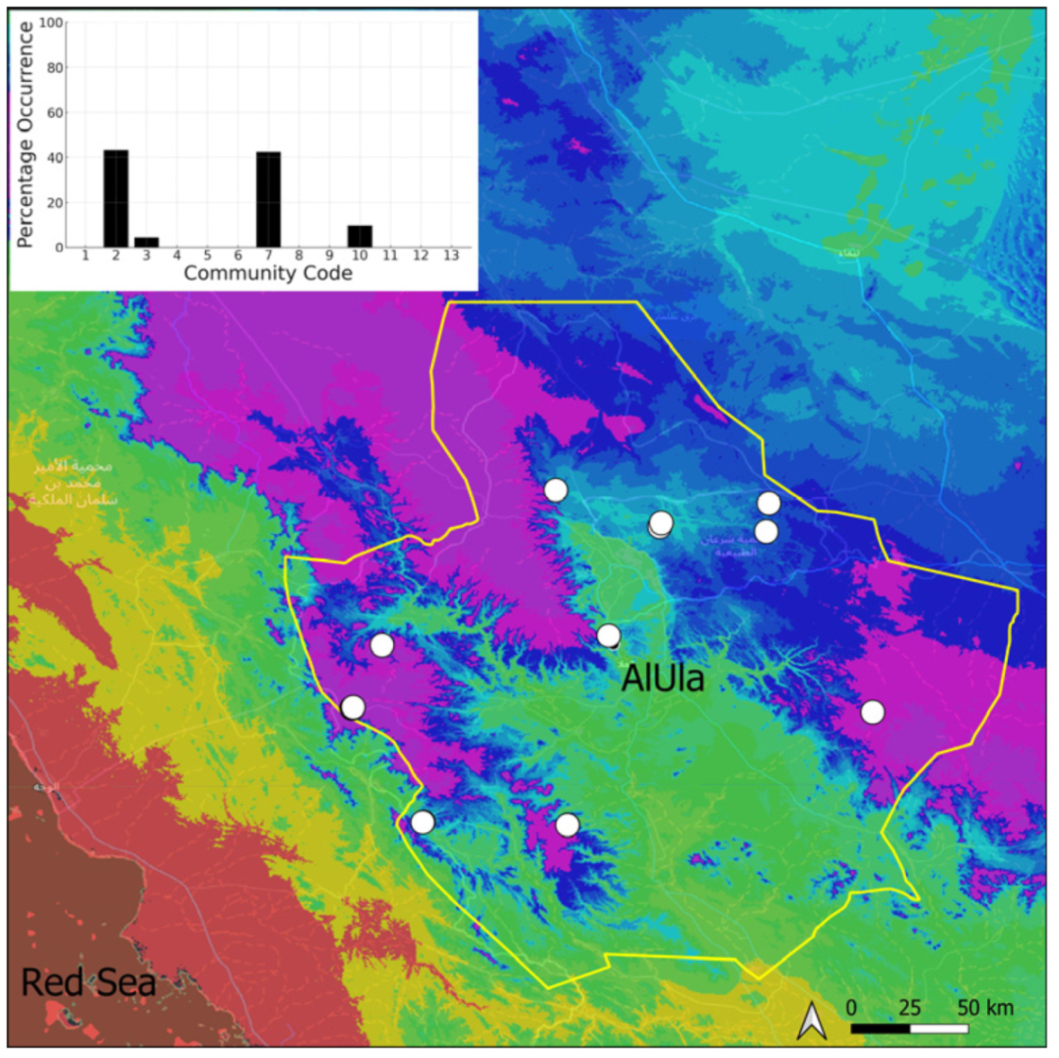
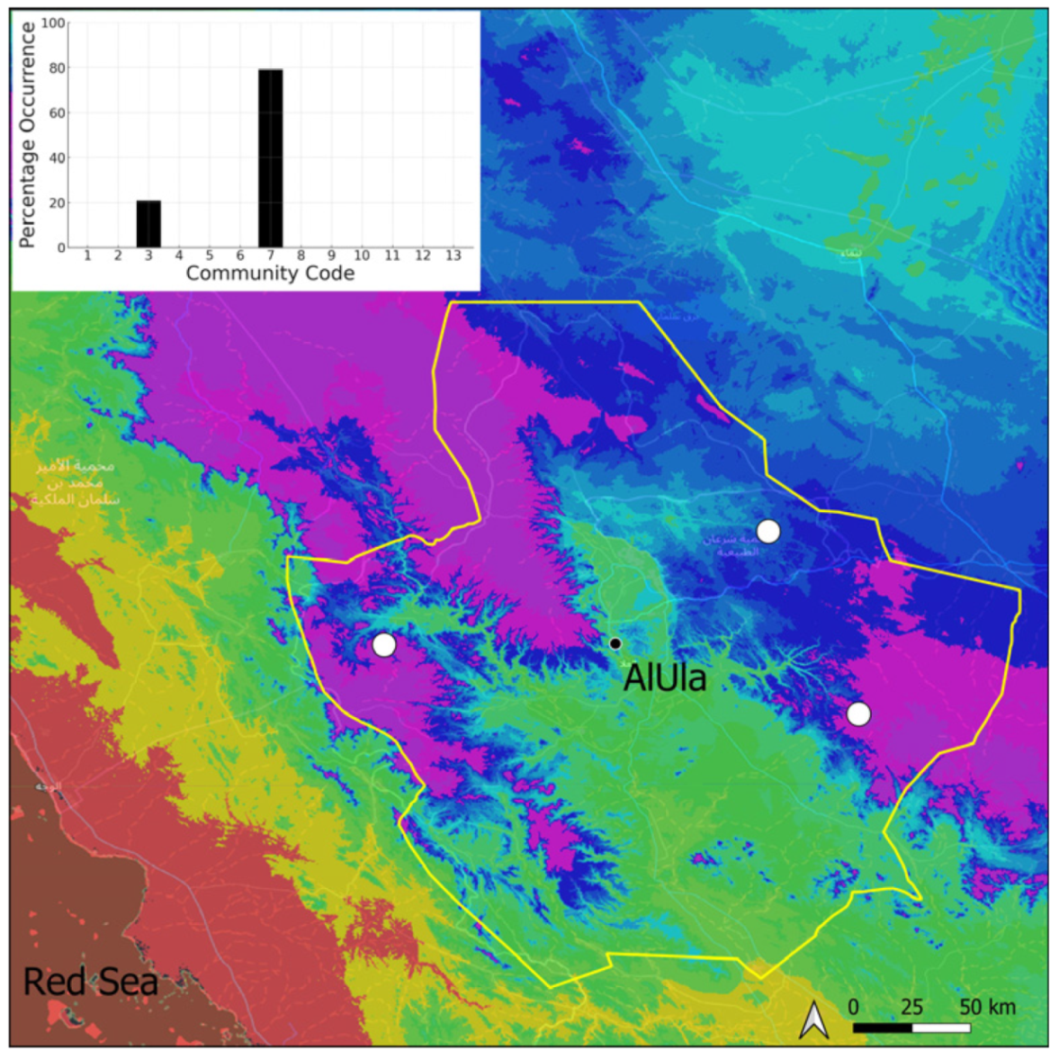
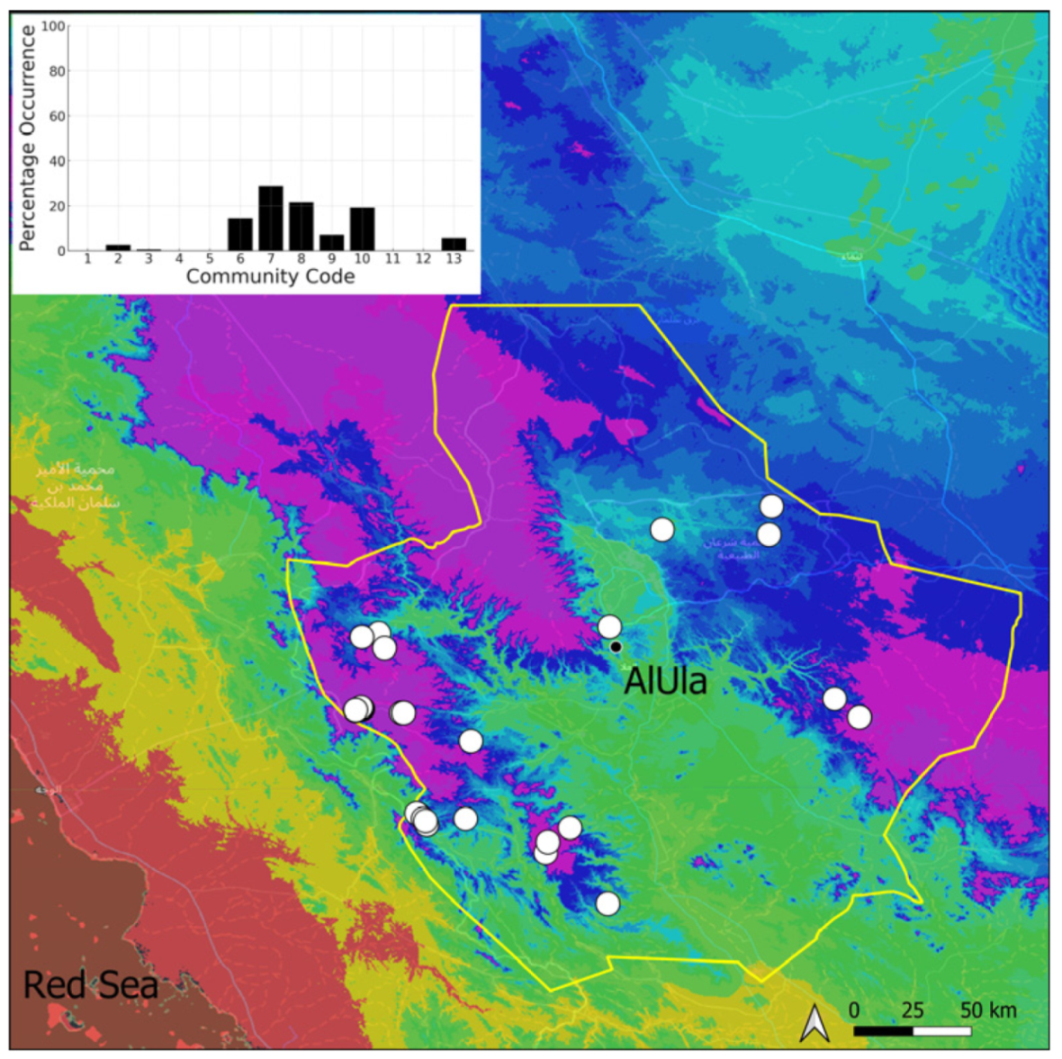
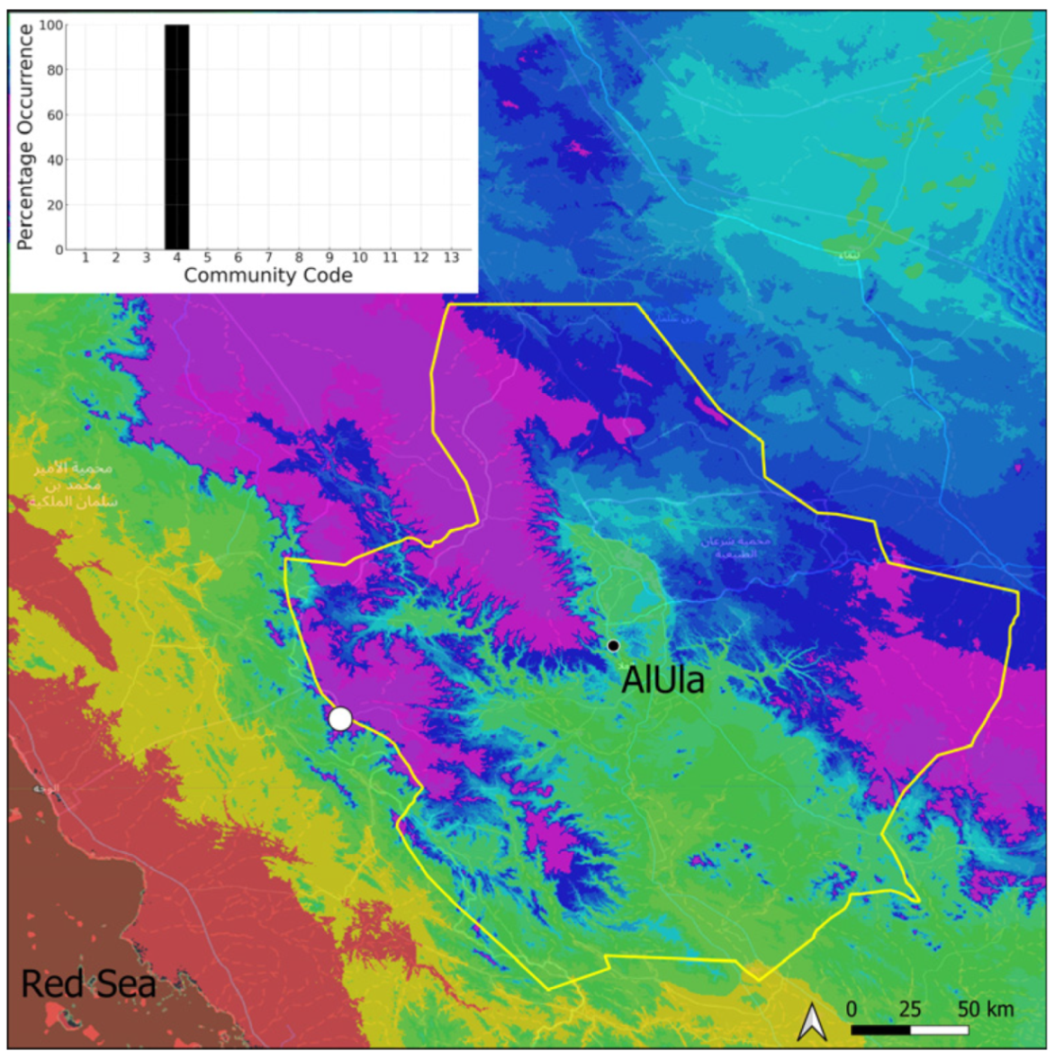
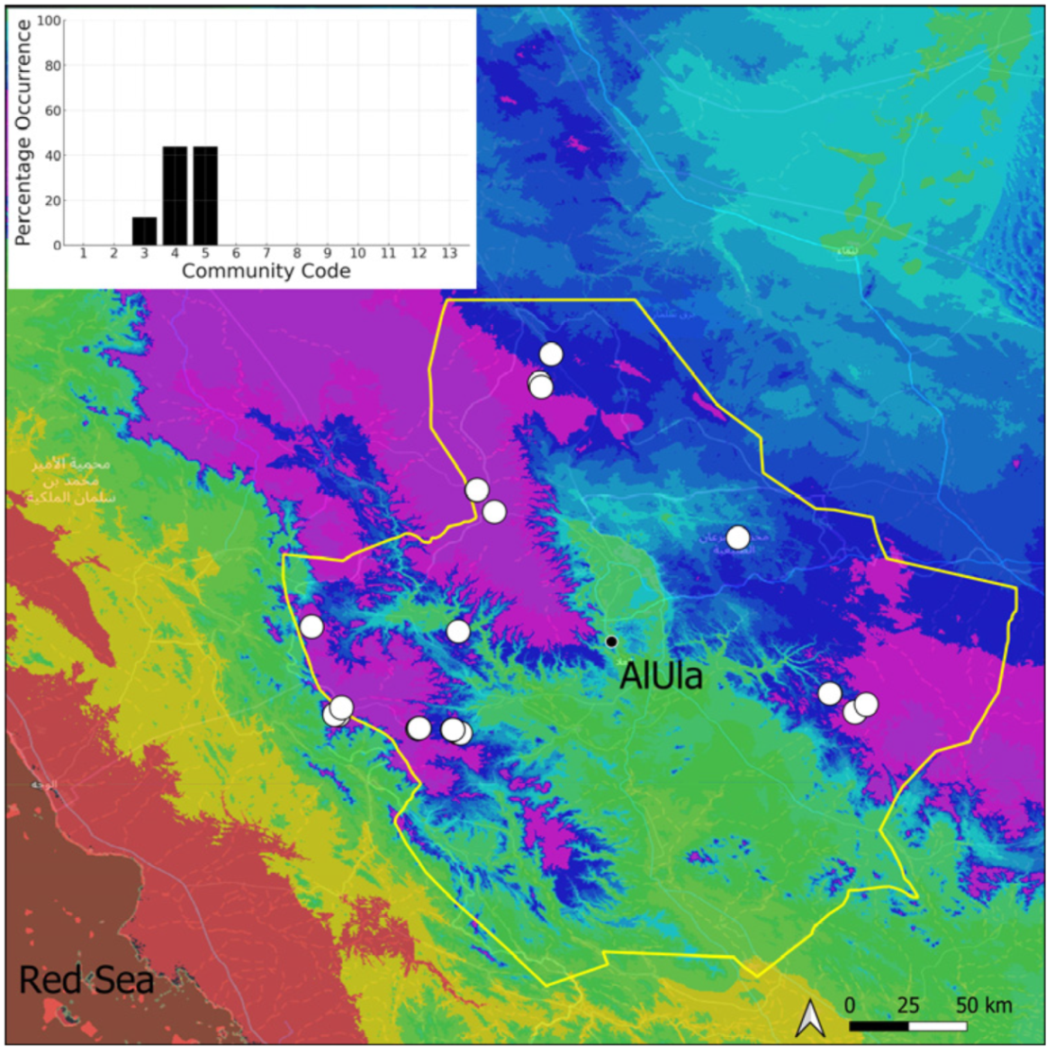
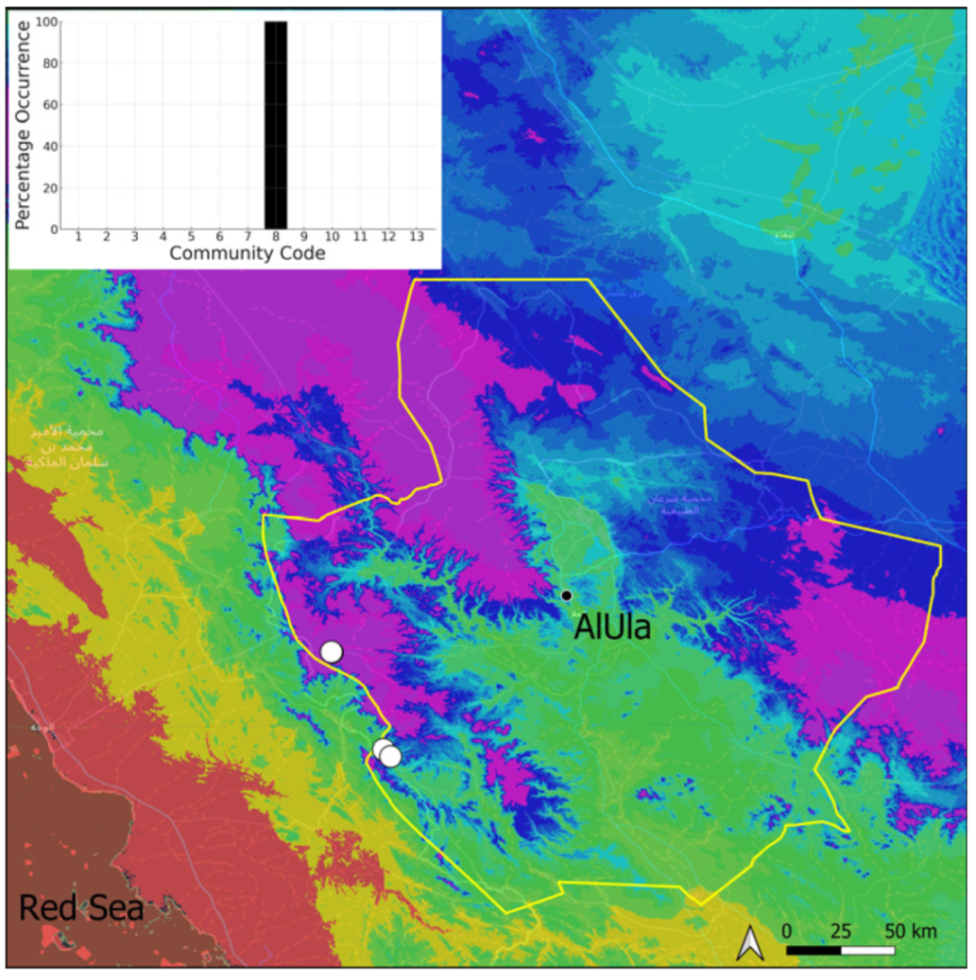

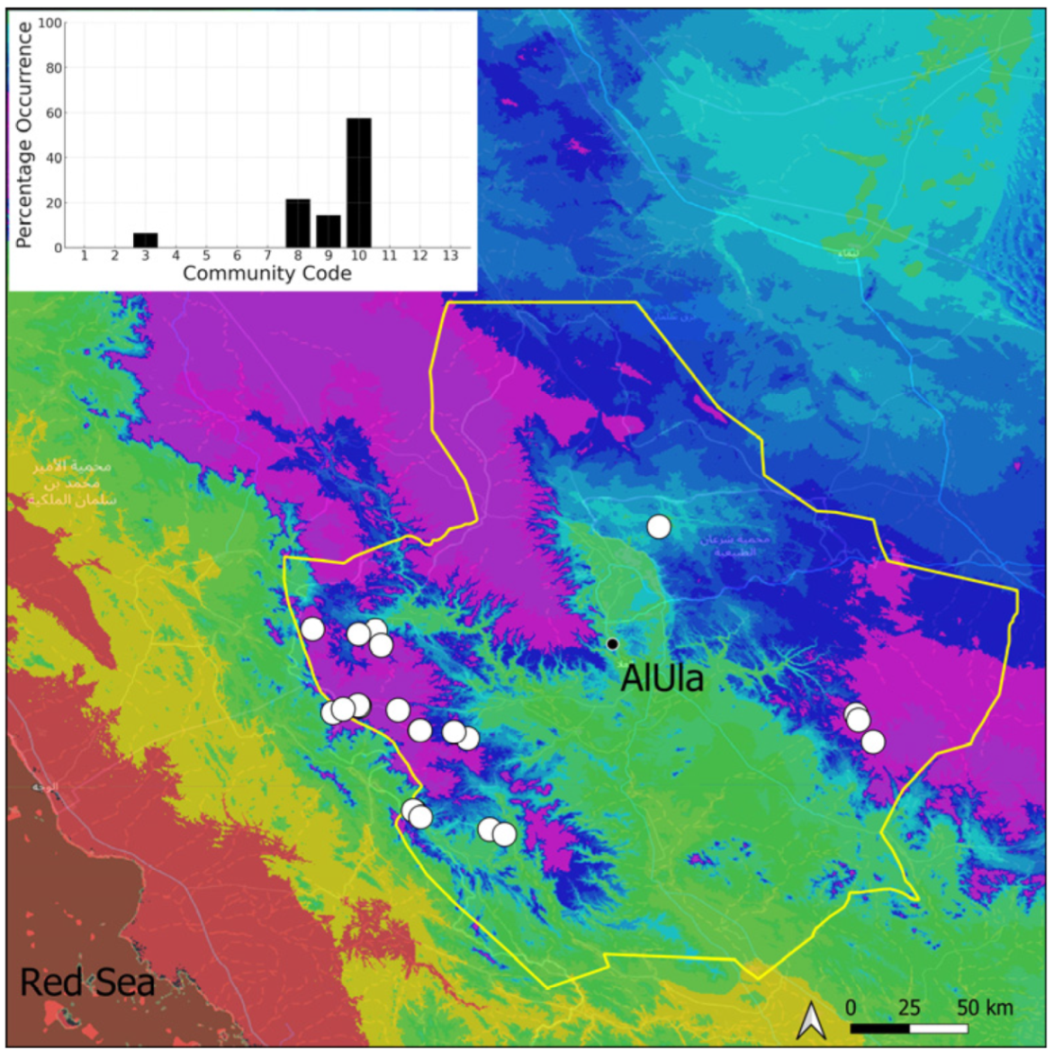
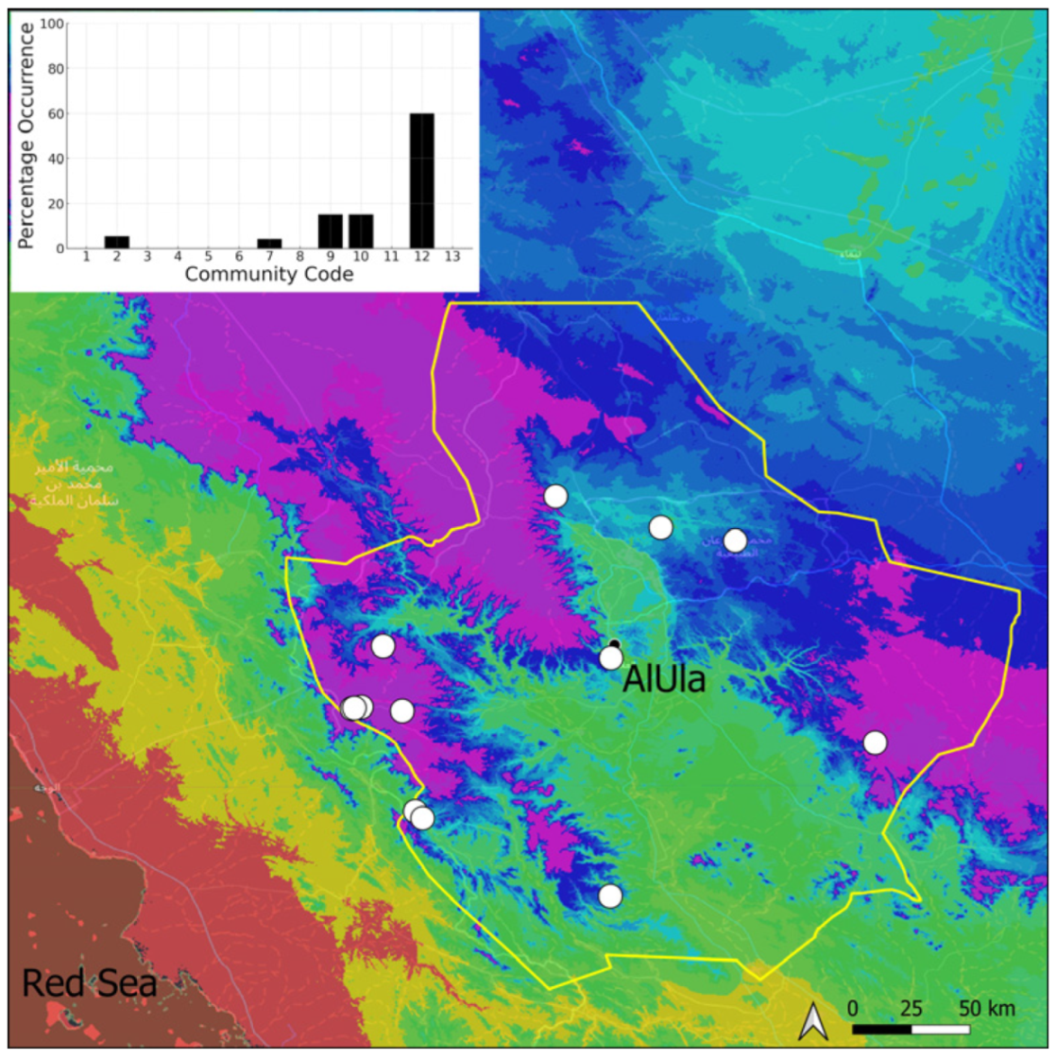
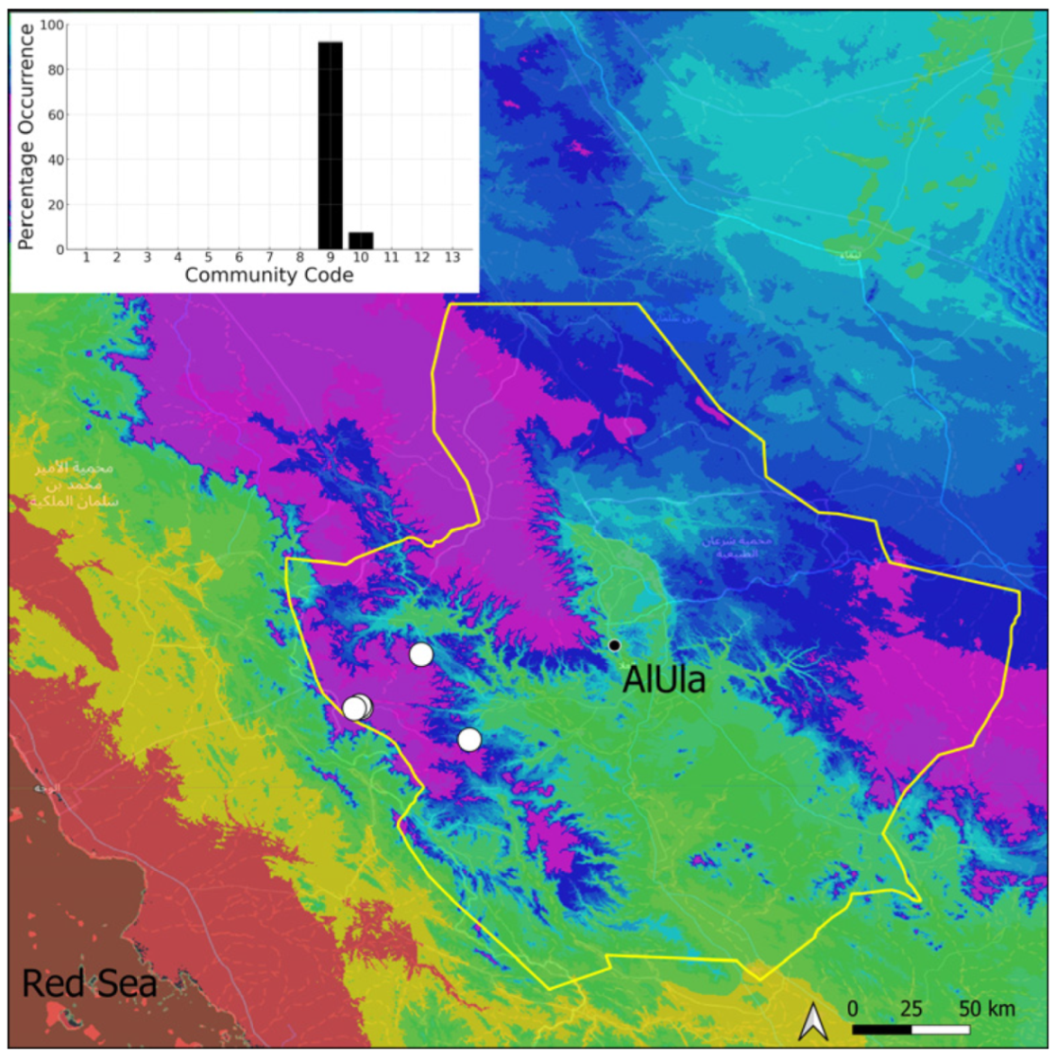


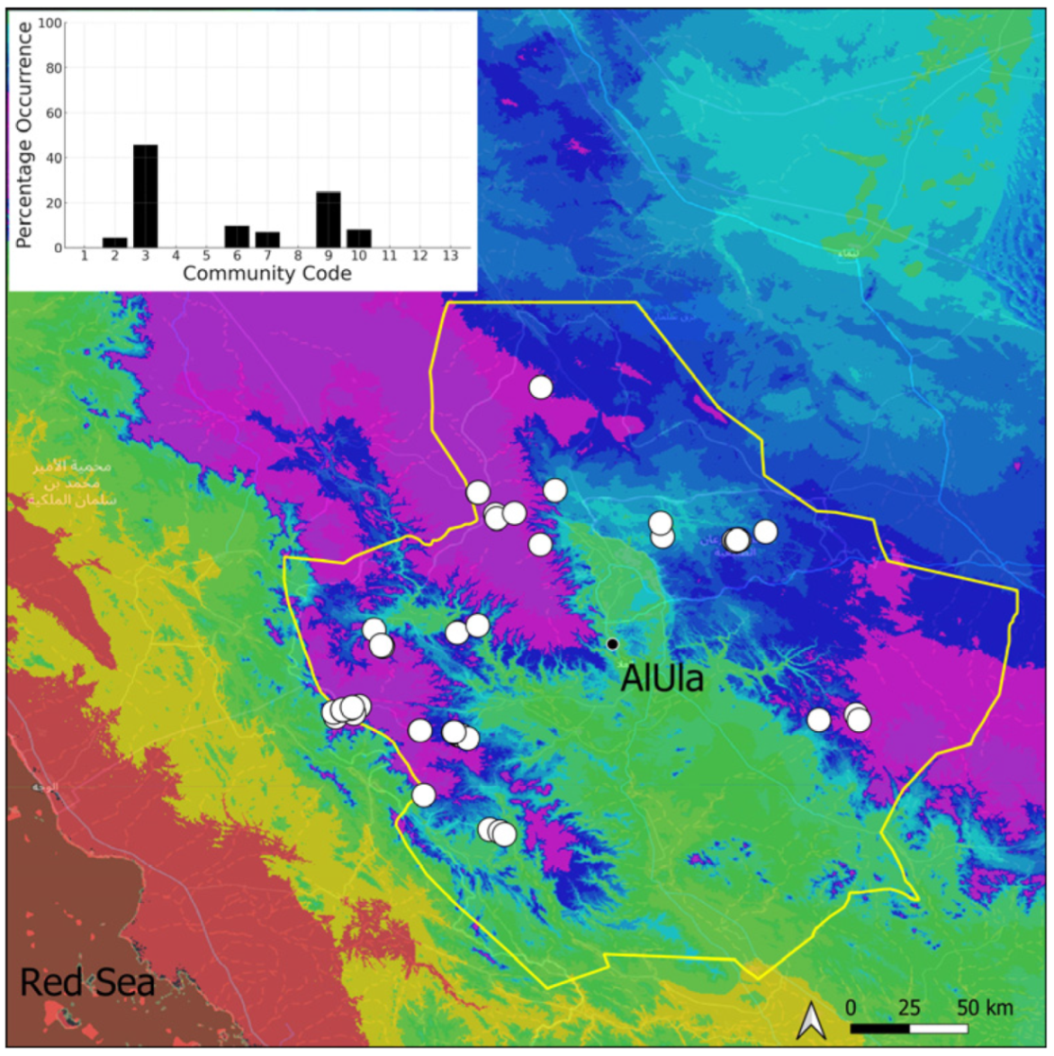
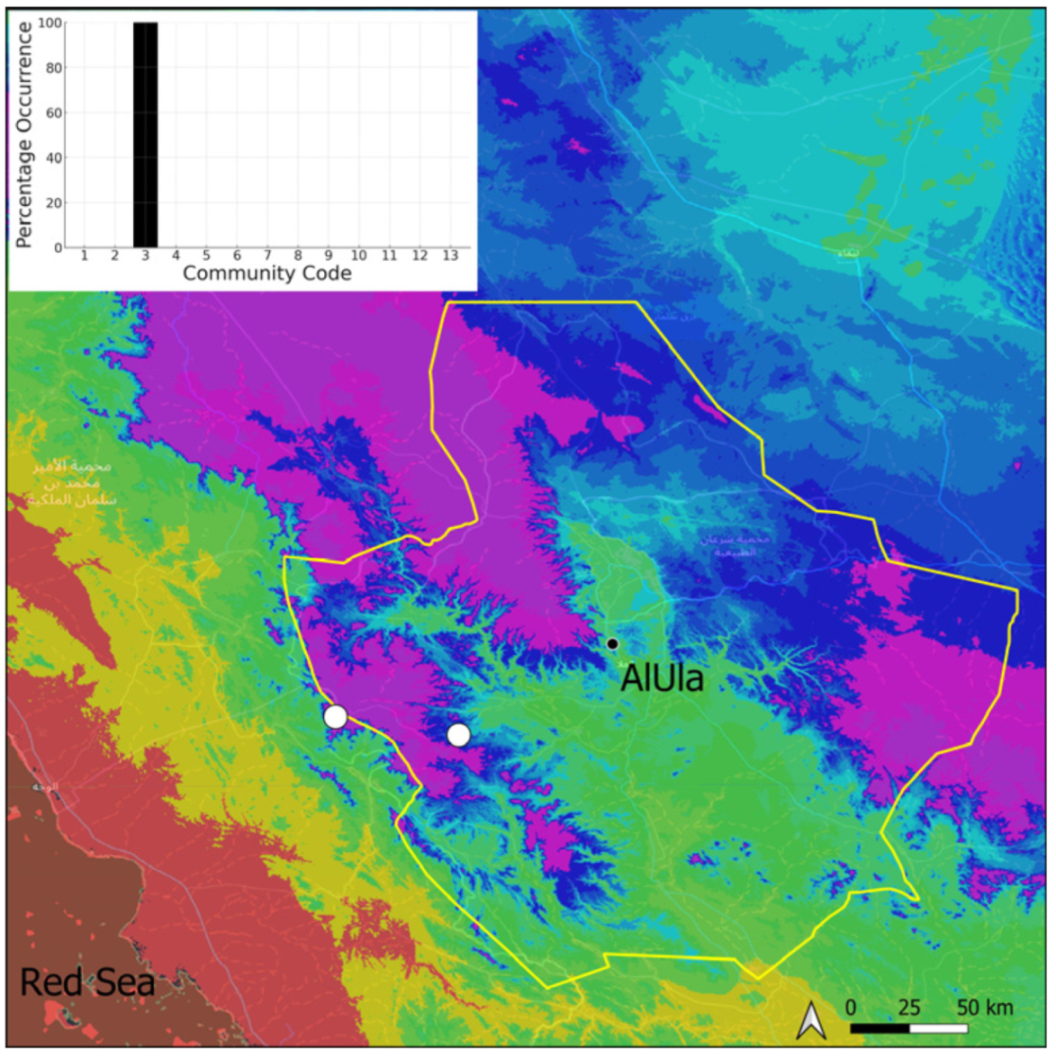
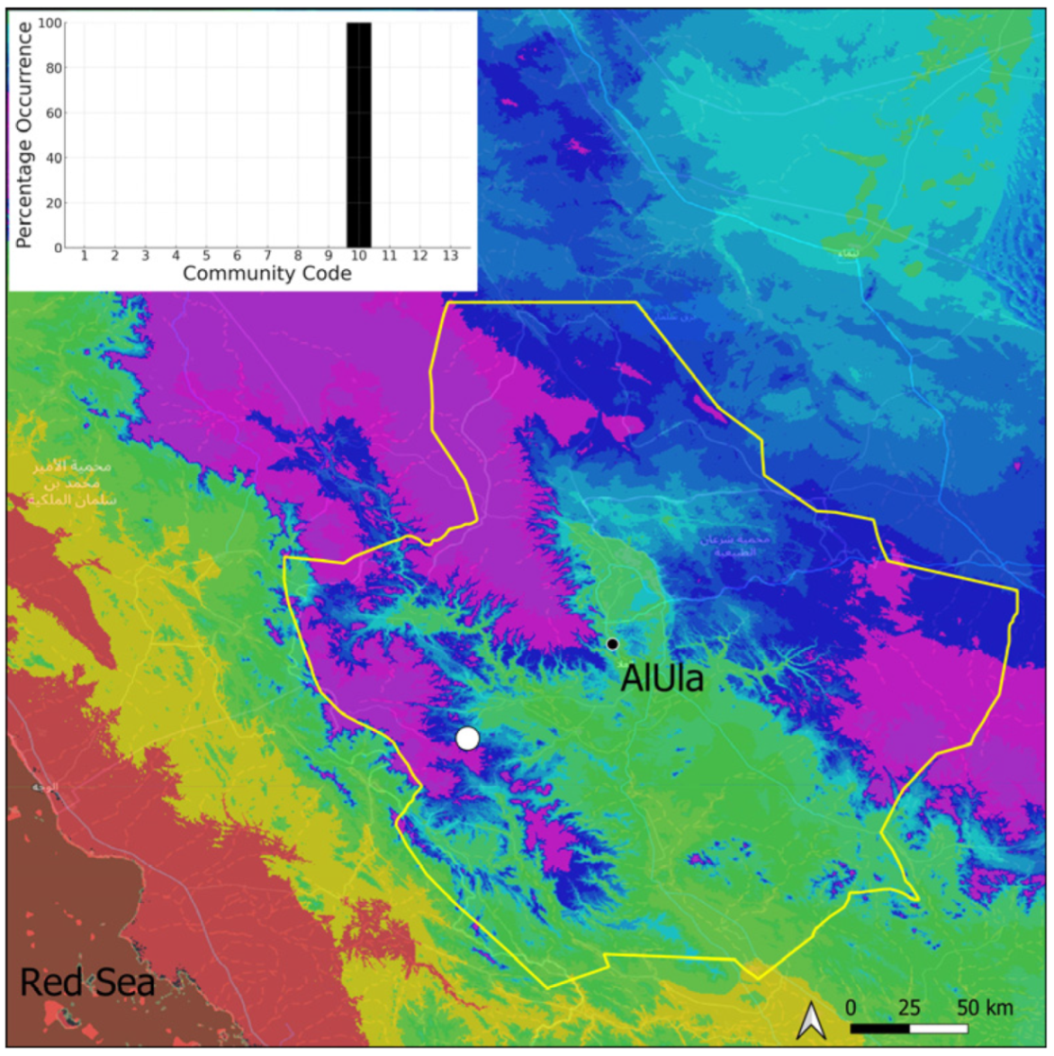
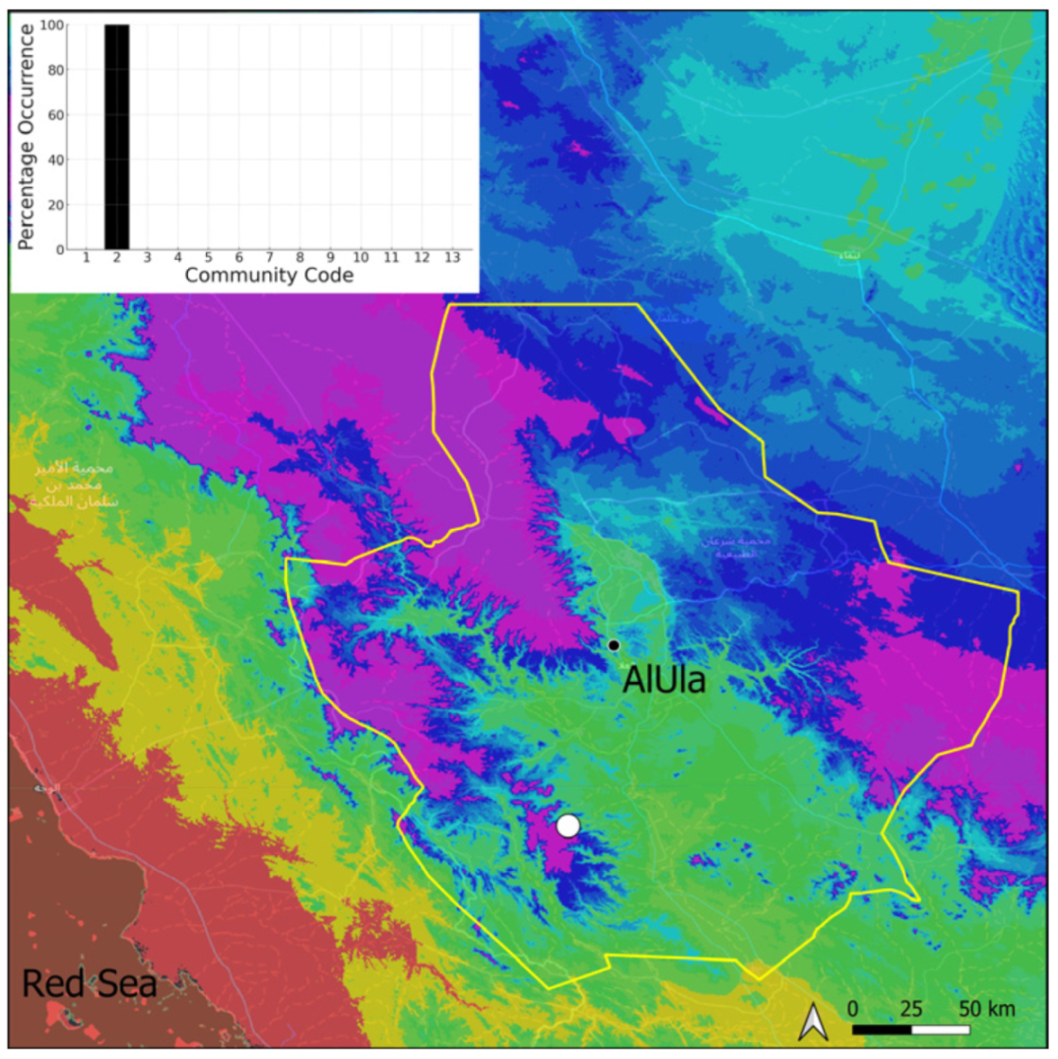
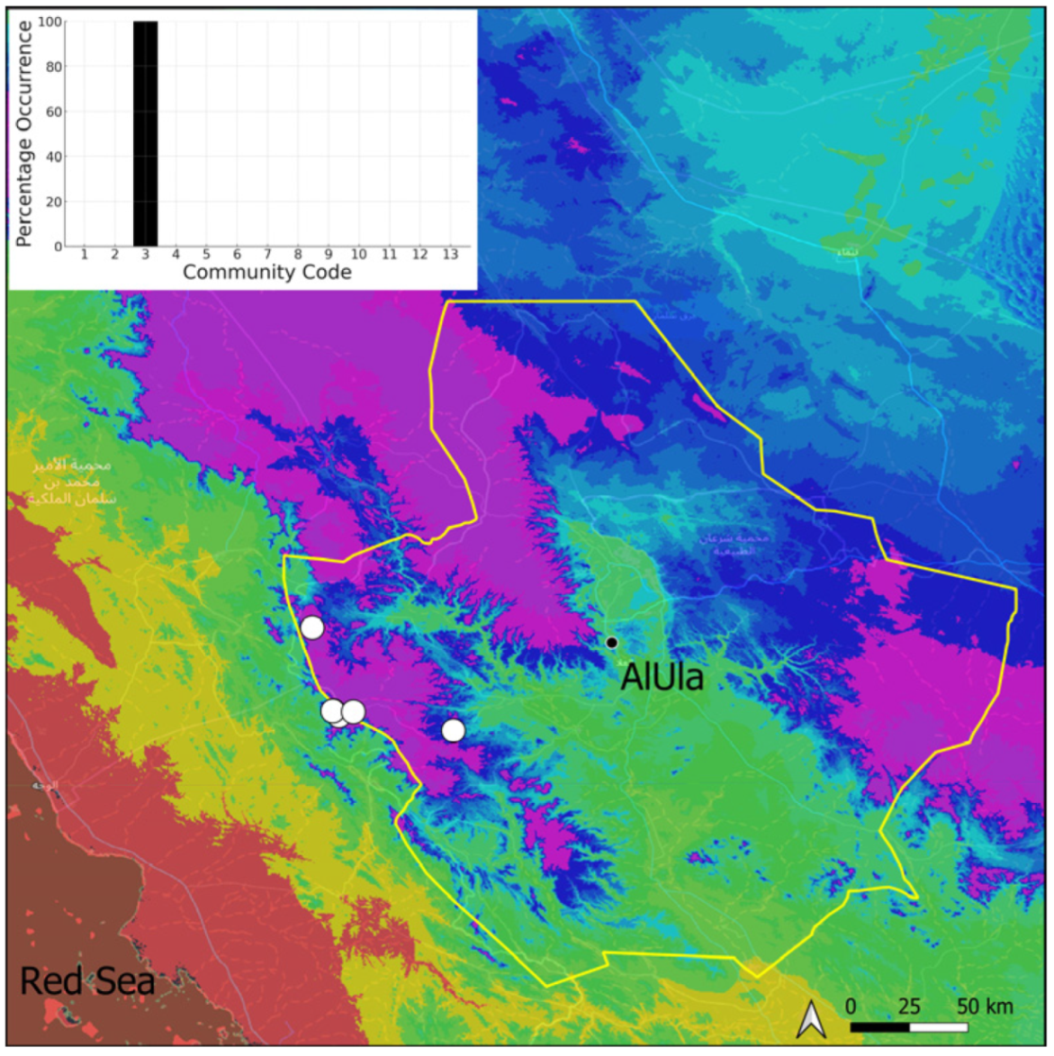
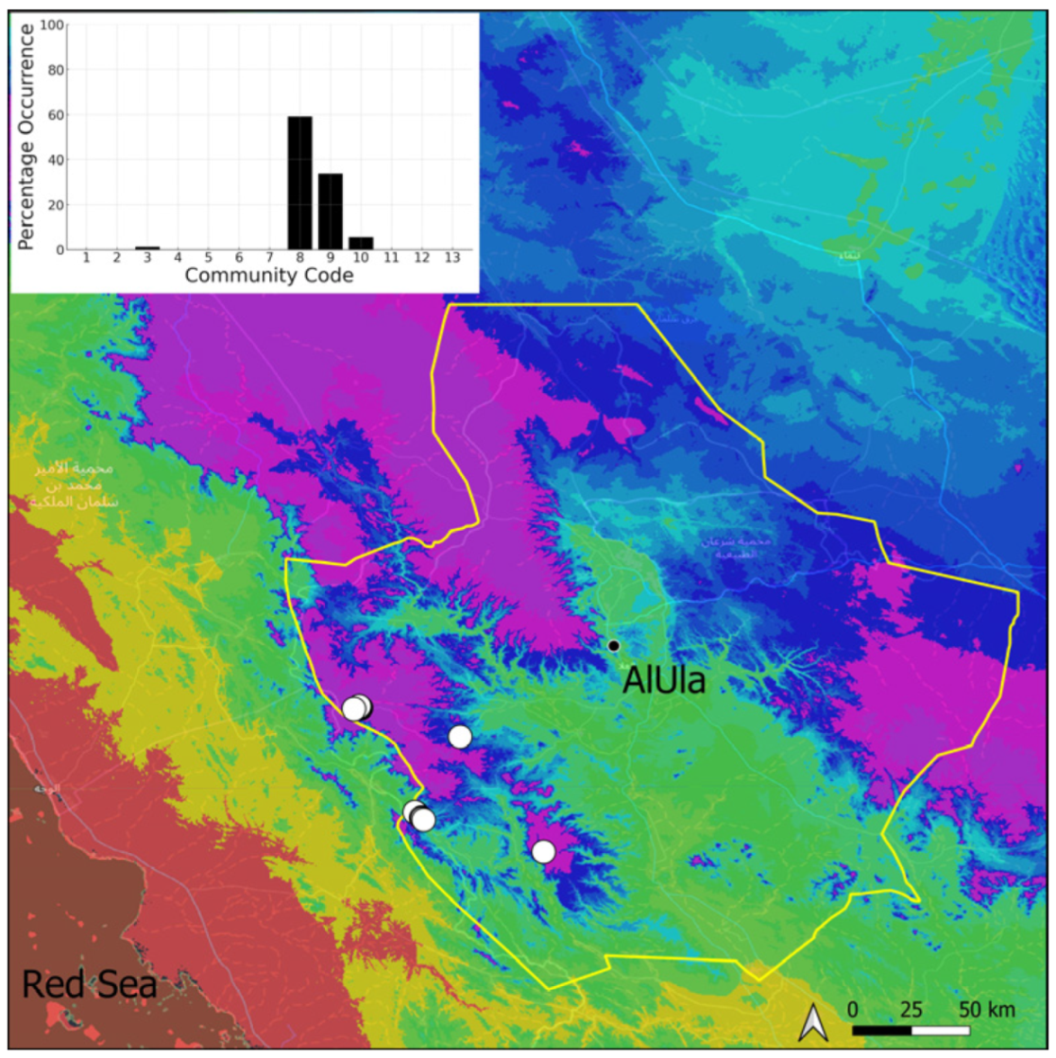
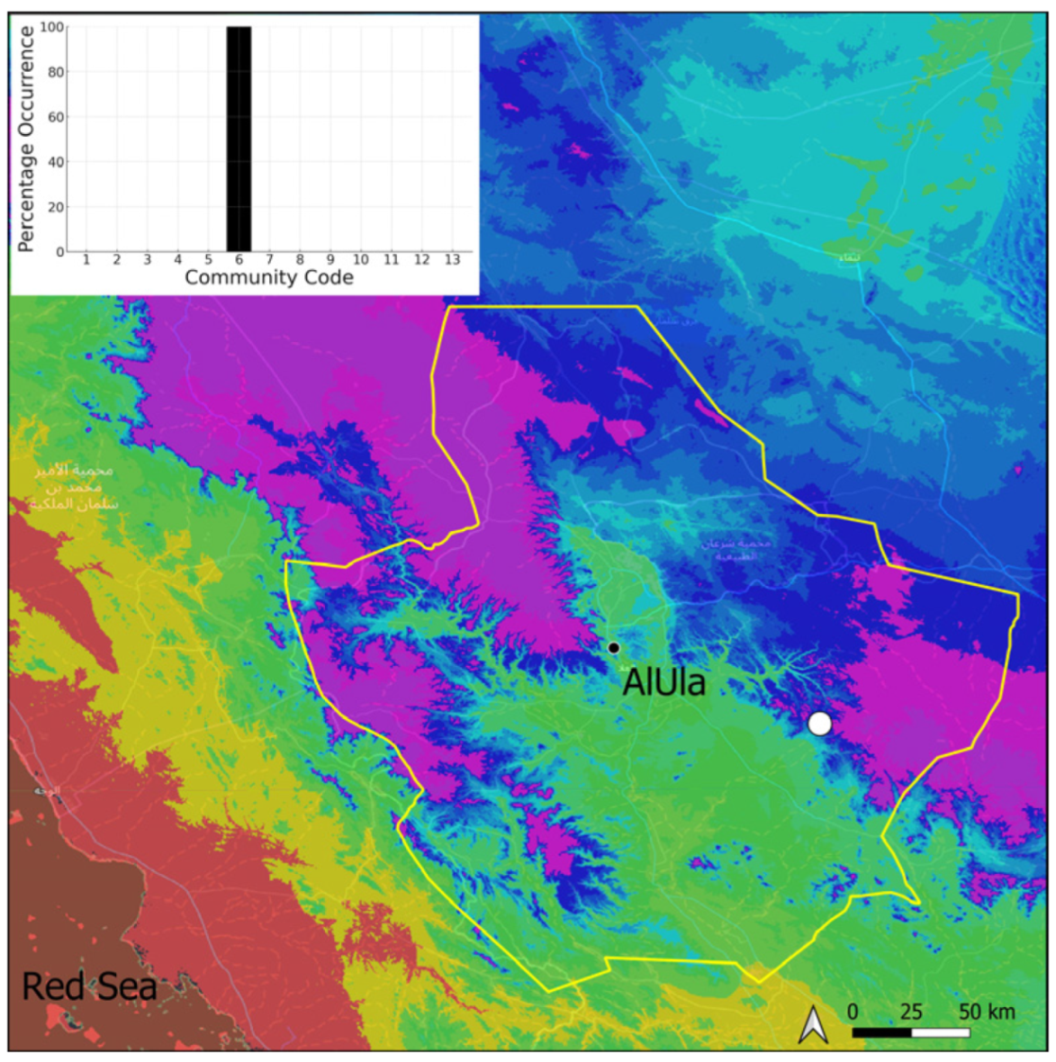

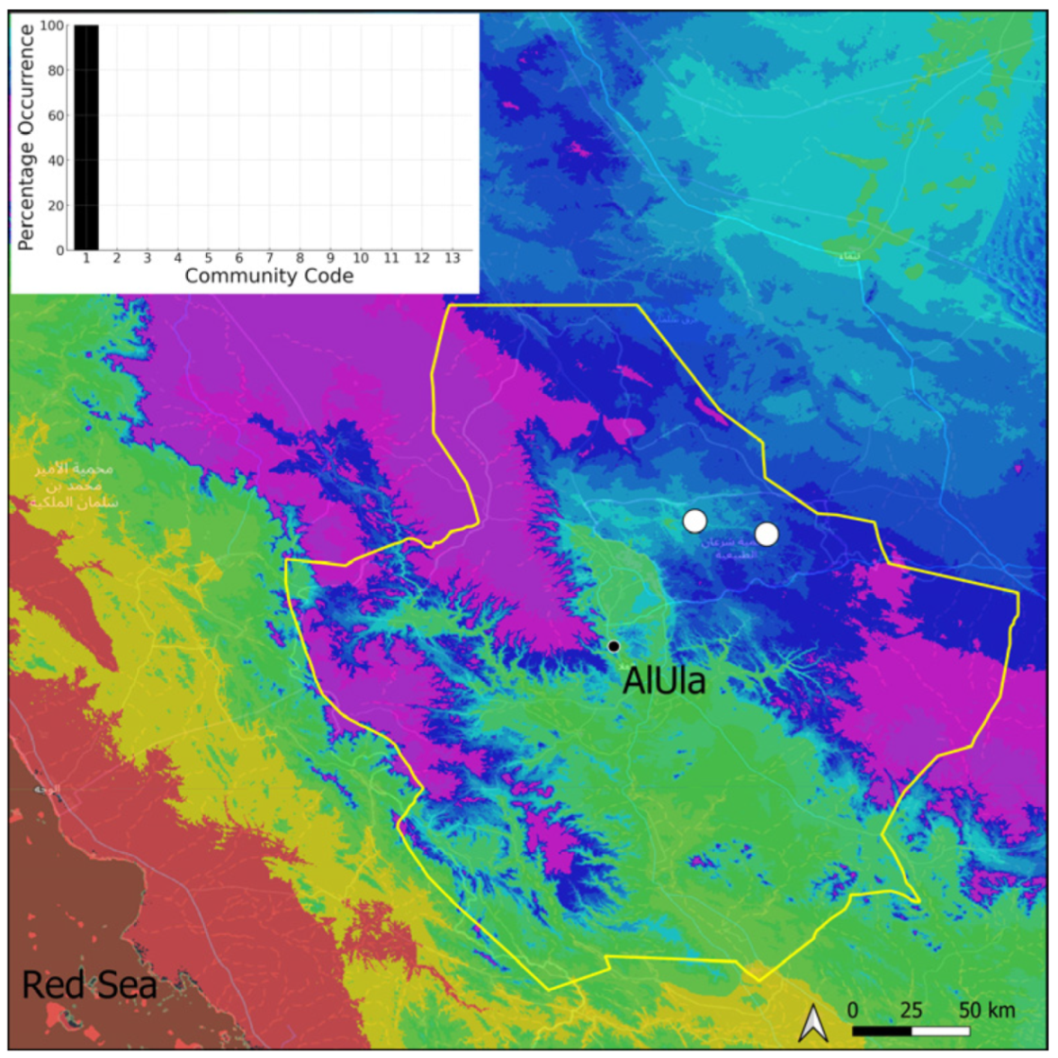

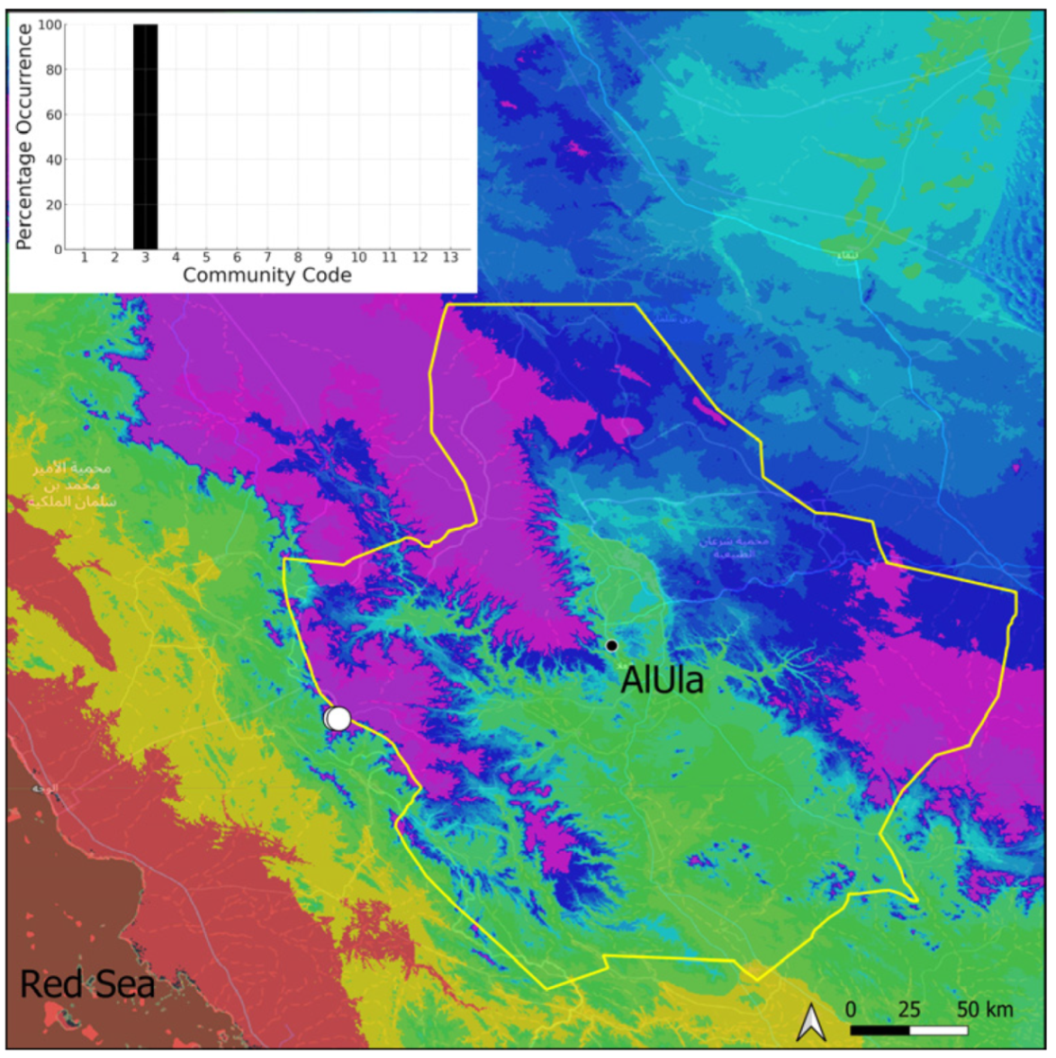
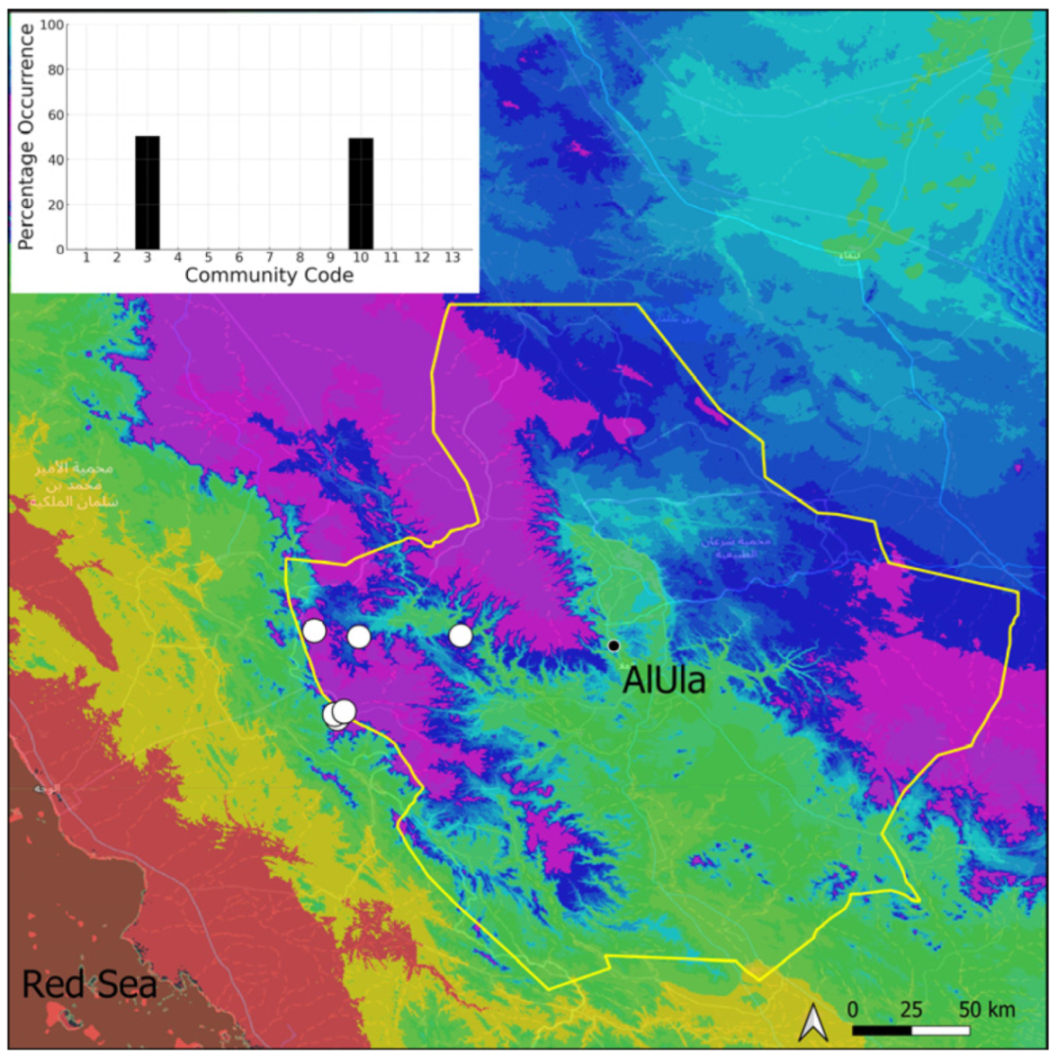
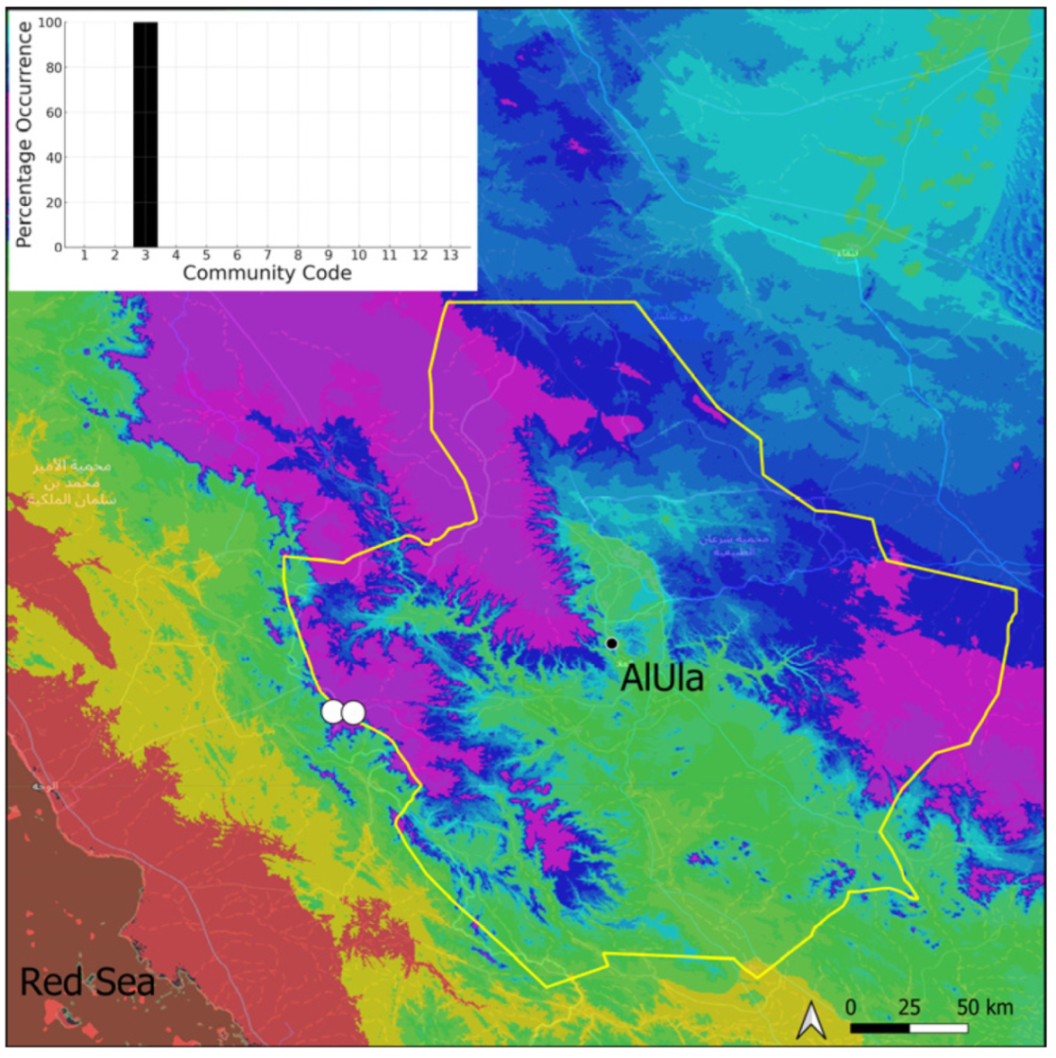
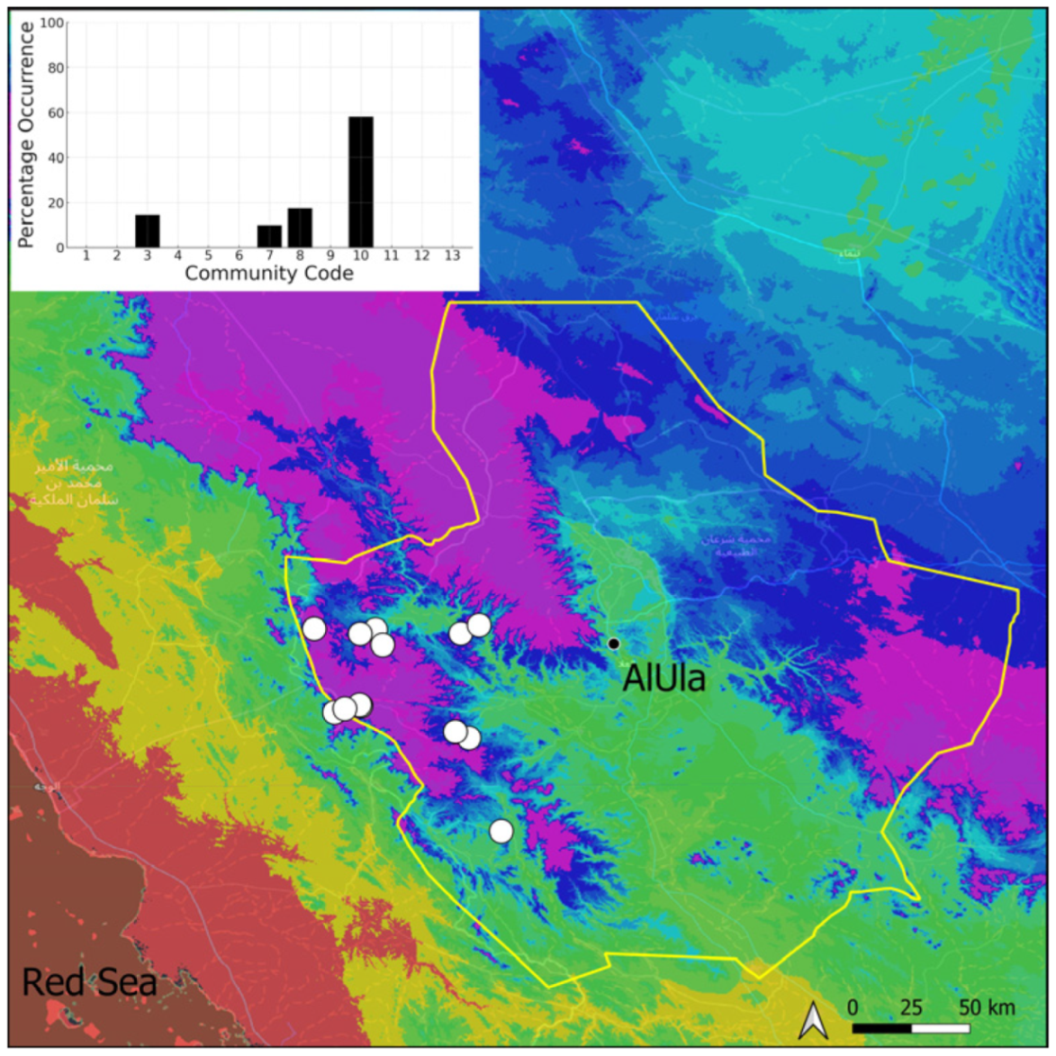
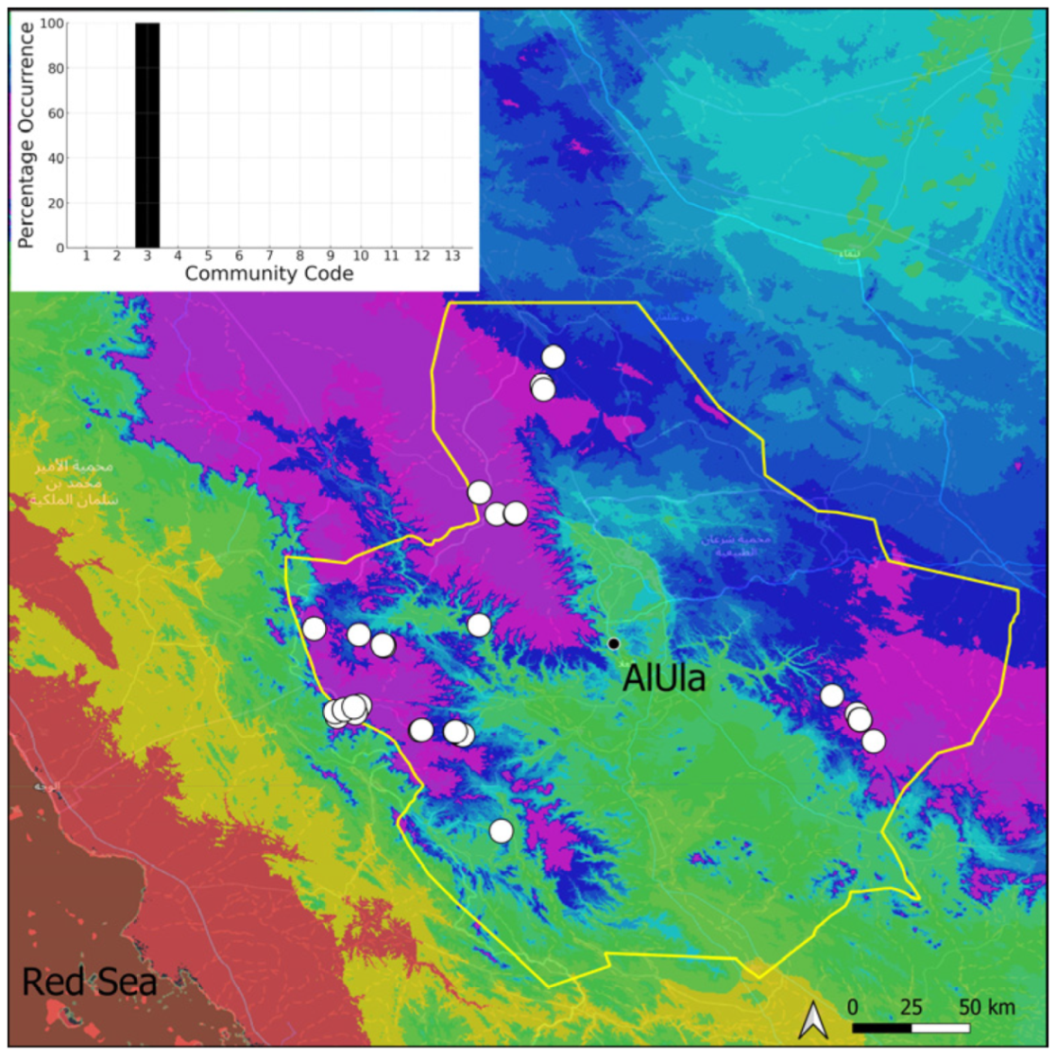
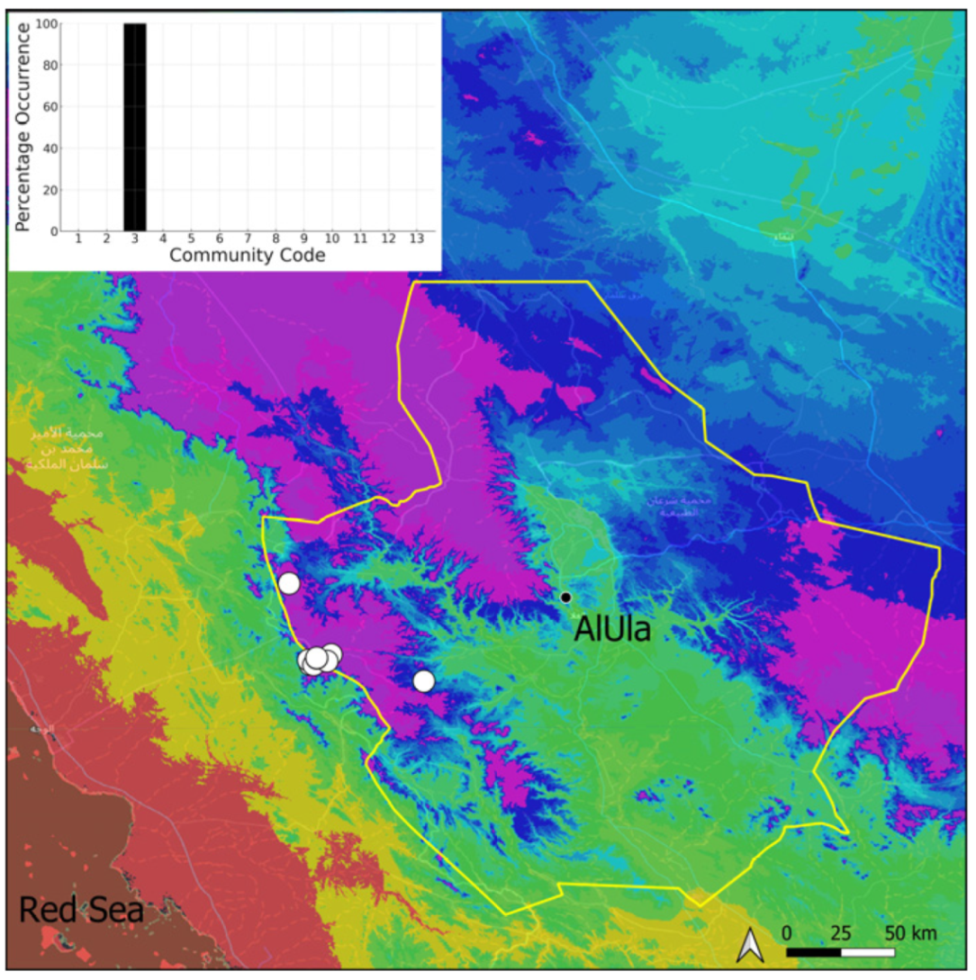
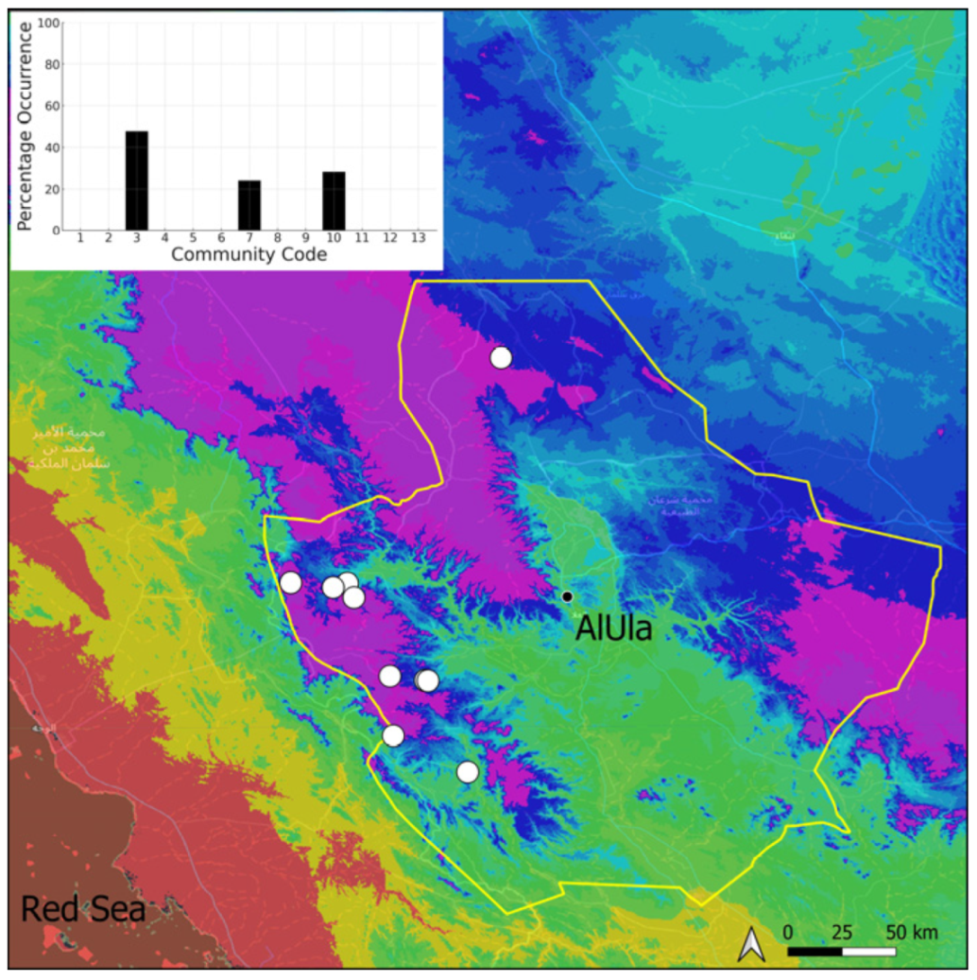



| Taxon | Number of Occurrences | Frequency in AlUla | Frequency on Arabian Peninsula | Heritage Value | Phylogeny | Family |
|---|---|---|---|---|---|---|
| Acaulon triquetrum | 1 | 0.12 | R | xx | M | Pottiaceae |
| Aloina rigida | 4 | 0.48 | C | M | Pottiaceae | |
| Bryum dichotomum | 33 | 3.92 | C | M | Bryaceae | |
| Bryum sp. | 51 | 6.06 | M | Bryaceae | ||
| Clevea spathysii | 2 | 0.24 | R | xx | L | Cleveaceae |
| Crossidium aberrans (Figure 1) | 33 | 3.92 | C | M | Pottiaceae | |
| Crossidium crassinervium | 36 | 4.28 | C | M | Pottiaceae | |
| Crossidium deserti | 1 | 0.12 | C | M | Pottiaceae | |
| Crossidium squamiferum | 30 | 3.56 | C | M | Pottiaceae | |
| Didymodon desertorum | 22 | 2.61 | C | M | Pottiaceae | |
| Encalypta vulgaris | 1 | 0.12 | C | M | Encalyptaceae | |
| Entosthodon cf. commutatus | 42 | 4.99 | M | Funariaceae | ||
| Entosthodon duriaei | 17 | 2.02 | C | M | Funariaceae | |
| Entosthodon muhlenbergii | 7 | 0.83 | C | M | Funariaceae | |
| Entosthodon sp. | 14 | 1.65 | M | Funariaceae | ||
| Eucladium verticillatum | 11 | 1.31 | C | x | M | Pottiaceae |
| Fissidens cf. arnoldii | 42 | 4.99 | C | M | Fissidentaceae | |
| Funaria hygrometrica | 18 | 2.14 | C | M | Funariaceae | |
| Geheebia siccula | 3 | 0.36 | R | x | M | Pottiaceae |
| Geheebia tophacea | 39 | 4.63 | C | M | Pottiaceae | |
| Grimmia anodon | 2 | 0.24 | R | xx | M | Grimmiaceae |
| Grimmia orbicularis | 41 | 4.87 | C | M | Grimmiaceae | |
| Gymnostomiella vernicosa | 5 | 0.59 | R | xxx | M | Pottiaceae |
| Gymnostomum calcareum | 6 | 0.71 | C | M | Pottiaceae | |
| Gymnostomum mosis (Figure 2) | 28 | 3.33 | C | M | Pottiaceae | |
| Gyroweisia tenuis | 13 | 1.54 | C | M | Pottiaceae | |
| Hymenostylium hildebrandtii | 5 | 0.59 | R | xxx | M | Pottiaceae |
| Microbryum davallianum | 4 | 0.48 | C | M | Pottiaceae | |
| Microbryum rectum | 1 | 0.12 | R | xx | M | Pottiaceae |
| Microbryum starckeanum | 54 | 6.41 | C | M | Pottiaceae | |
| Molendoa handelii | 2 | 0.24 | R | xx | M | Pottiaceae |
| Plagiochasma rupestre | 1 | 0.12 | C | L | Aytoniaceae | |
| Pterygoneurum ovatum | 1 | 0.12 | C | M | Pottiaceae | |
| Ptychostomum capillare | 7 | 0.83 | C | M | Bryaceae | |
| Ptychostomum cellulare | 12 | 1.43 | TR | xxx | M | Bryaceae |
| Ptychostomum pseudotriquetrum | 2 | 0.24 | TR | xx | M | Bryaceae |
| Riccia cavernosa | 32 | 3.80 | C | L | Ricciaceae | |
| Riella affinis | 2 | 0.24 | TR | xxx | L | Riellaceae |
| Syntrichia caninervis var. caninervis | 2 | 0.24 | C | M | Pottiaceae | |
| Syntrichia rigescens | 3 | 0.36 | R | x | M | Pottiaceae |
| Targionia hypophylla | 10 | 1.19 | C | L | Targioniaceae | |
| Targionia lorbeeriana | 2 | 0.24 | C | L | Targioniaceae | |
| Timmiella barbuloides | 18 | 2.14 | C | M | Pottiaceae | |
| Tortula atrovirens | 48 | 5.70 | C | M | Pottiaceae | |
| Tortula inermis | 17 | 2.02 | C | M | Pottiaceae | |
| Tortula mucronifera | 17 | 2.02 | C | M | Pottiaceae | |
| Tortula muralis | 20 | 2.38 | C | M | Pottiaceae | |
| Trichostomopsis australasiae | 52 | 6.18 | C | M | Pottiaceae | |
| Vinealobryum vineale | 8 | 0.95 | C | M | Pottiaceae | |
| Weissia condensa | 20 | 2.38 | C | M | Pottiaceae |
| Crossidium aberrans | Crossidium crassinervium | Crossidium deserti | Crossidium squamiferum | |
|---|---|---|---|---|
| Crossidium aberrans | 4 | 3 | ||
| Crossidium crassinervium | 4 | 8 | ||
| Crossidium deserti | 1 | |||
| Crossidium squamiferum | 3 | 8 | 1 |
| Accepted Nomenclature | Description Name | Family | Global Range | Diagnosis Reference |
|---|---|---|---|---|
| Bryum nanoapiculatum Ochi & Kürschner | Bryum nanoapiculatum Ochi & Kürschner | Bryaceae | Oman; Yemen | [29] |
| Crossidium davidai Catches. | Crossidium asirense W. Frey & Kürschner | Pottiaceae | Australia; Arabian Peninsula | [14] |
| Crossidium deserti W. Frey & Kürschner | Crossidium deserti W. Frey & Kürschner | Pottiaceae | Saudi Arabia | [13] |
| Crossidium laxefilamentosum W. Frey & Kürschner | Crossidium laxefilamentosum W. Frey & Kürschner | Pottiaceae | Arabian Peninsula; North Africa | [22] |
| Crossidium woodii (Delgad.) R. H. Zander | Pseudaloina woodii Delgad. | Pottiaceae | Yemen | [30] |
| Fissidens laxetexturatus Brugg.-Nann. | Fissidens laxetexturatus Brugg.-Nann. | Fissidentaceae | Oman, Yemen | [16] |
| Fissidens sciophyllus Mitt. | Fissidens arabicus Pursell & Kürschner | Fissidentaceae | Tropical Africa, and Arabian Peninsula | [31] |
| Riccia crenatodentata O. H. Volk | Riccia crenatodentata O. H. VOLK | Ricciaceae | Arabian Peninsula; | [32] |
| Schlotheimia balfourii Mitt. | Schlotheimia balfourii Mitt. | Orthotrichaceae | Yemen (Socotra) | [33] |
| Sematophyllum socotrense W. R. Buck | Sematophyllum socotrense W. R. Buck | Sematophyllaceae | Yemen (Socotra) | [34] |
| Splachnobryum aquaticum Müll. Hal. | Splachnobryum arabicum Dixon | Splachnobryaceae | Asia, Africa, and Arabian Peninsula | [35] |
| Targionia hypophylla subsp. linealis W. Frey & Kürschner | Targionia hypophylla subsp. linealis W. Frey & Kürschner | Targioniaceae | Arabian Peninsula | [27] |
| Tortella humilis (Hedw.) Jenn. | Barbula schweinfurthiana Müll. Hal. | Pottiaceae | Sub-cosmopolitan | [36] |
| Tortella smithii C. C. Towns. | Tortella smithii C. C. Towns. | Pottiaceae | Yemen (including Socotra) | [37] |
| Tortula mucronifera W. Frey, Kürschner & Ros | Tortula mucronifera W. Frey, Kürschner & Ros | Pottiaceae | Arabian Peninsula and Jordan | [38] |
| Weissia artocosana R. H. Zander | Weissia artocosana R. H. Zander | Pottiaceae | Yemen (Socotra) | [39] |
| Weissia socotrana Mitt. | Weissia socotrana Mitt. | Pottiaceae | Yemen (Socotra) | [33] |
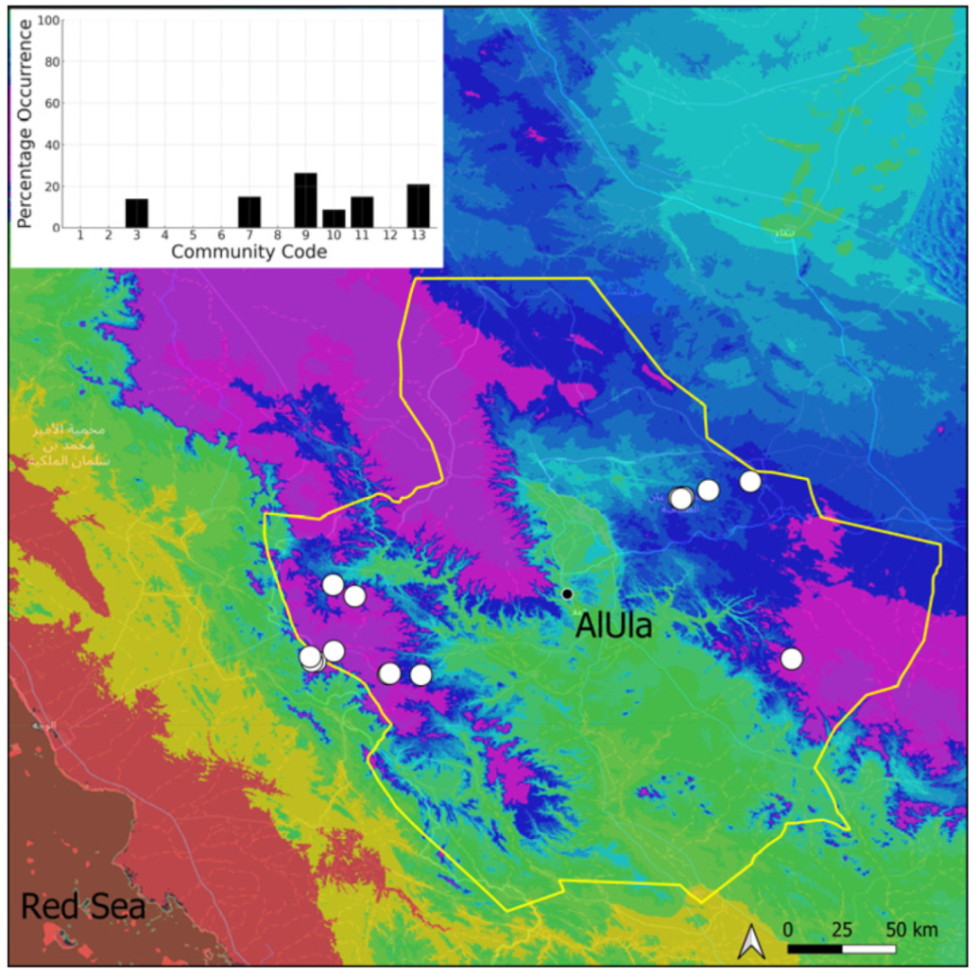
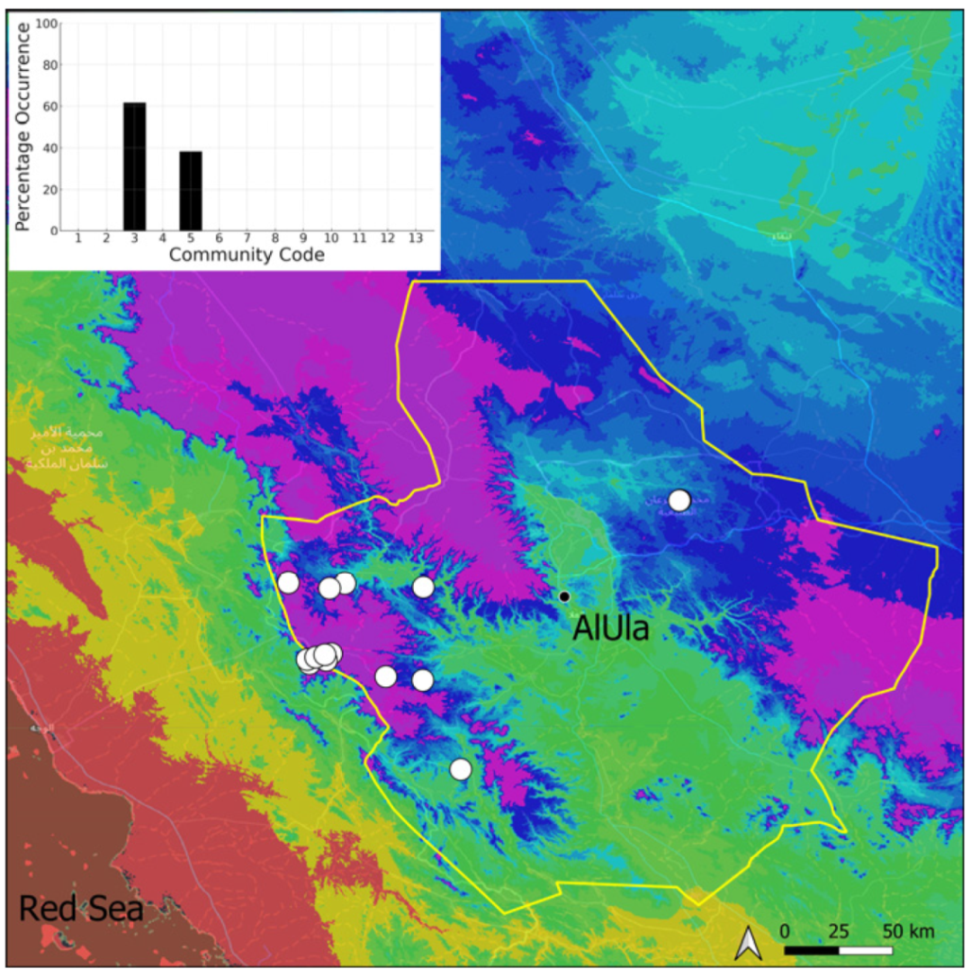
| Community Code | Ecology | Community Name | Description |
|---|---|---|---|
| 1 | Aquatic community | Riella affinis community | Monospecific, ephemeral community of shallow water bodies |
| 6 | Tufa communities | Eucladio verticillatae–Adiantetum capillusi-veneris Br.-BI. ex Horvatić 1934 | Community of active flowing tufa deposits |
| 7 | Geheebia tophacea community | Community of periodically drying tufa deposits | |
| 8 | Gymnostomiella vernicosa community | Community of tufa deposits with tropical affinities | |
| 9 | Hymenostylium hildebrandtii community | Community of tufa deposits in the minor beds of small temporary wadi | |
| 10 | Gymnostomum mosis community | Community of periodically drying tufa deposits | |
| 12 | Gyroweisia tenuis community | Community of periodically drying tufa deposits | |
| 13 | Entosthodon duriaei community | Community of periodically drying tufa deposits | |
| 2 | Terricolous communities | Riccia cavernosa community | Community of sandy shores of temporary wadis |
| 3 | Pottiaceae community | Pottiaceae-rich community of scree and the base of rock walls | |
| 11 | Bryum sp. community | Community of compacted, temporarily moist soils | |
| 4 | Saxicolous communities | Grimmia anodon community | Community of high-altitude granite boulders |
| 5 | Grimmia orbicularis community | Community of granite or sandstone boulders and rock walls |
| Current Nomenclature | Kürschner & Frey, 2020 | Followed Reference |
|---|---|---|
| Molendoa handelii (Schiffn.) Brinda & R. H. Zander | Anoectangium handelii Schiffn. | [71] |
| Clevea spathysii (Lindenb.) Müll. Frib. | Athalamia spathysii (Lindenb.) S. Hatt. | [72] |
| Ptychostomum capillare (Hedw.) Holyoak & N. Pedersen | Bryum capillare Hedw. | [12] |
| Ptychostomum pseudotriquetrum (Hedw.) J. R. Spence & H. P. Ramsay | Bryum pseudotriquetrum (Hedw.) G. Gaertn. | [12] |
| Ptychostomum torquescens (Bruch & Schimp.) Ros & Mazimpaka | Bryum torquescens Bruch & Schimp. | [12] |
| Trichostomopsis australasiae (Hook. & Grev.) H. Rob. | Didymodon australasiae (Hook. & Grev.) R. H. Zander | [17] |
| Geheebia siccula (M.J. Cano, Ros, García-Zam. & J. Guerra) J. A. Jiménez & M. J. Cano | Didymodon sicculus M. J. Cano, Ros, García-Zam. & J. Guerra | [17] |
| Geheebia tophacea (Brid.) R. H. Zander | Didymodon tophaceus (Brid.) Lisa | [17] |
| Vinealobryum vineale (Brid.) R. H. Zander | Didymodon vinealis (Brid.) R. H. Zander | [17] |
| Riella affinis M. Howe & Underw. | not mentioned | [73] |
| Entosthodon commutatus Durieu & Mont. | not mentioned | [74] |
| Didymodon desertorum (J. Froehl.) J. A. Jiménez & M. J. Cano | not recognized | [9] |
| Ptychostomum cellulare (Hook.) D. Bell & Holyoak | Plagiobryoides cellularis (Hook.) J. R. Spence | [12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hugonnot, V.; Pépin, F.; Freedman, J. Bryophyte Flora of AlUla County (Saudi Arabia)—Distribution, Ecology, and Conservation. Plants 2025, 14, 170. https://doi.org/10.3390/plants14020170
Hugonnot V, Pépin F, Freedman J. Bryophyte Flora of AlUla County (Saudi Arabia)—Distribution, Ecology, and Conservation. Plants. 2025; 14(2):170. https://doi.org/10.3390/plants14020170
Chicago/Turabian StyleHugonnot, Vincent, Florine Pépin, and Jan Freedman. 2025. "Bryophyte Flora of AlUla County (Saudi Arabia)—Distribution, Ecology, and Conservation" Plants 14, no. 2: 170. https://doi.org/10.3390/plants14020170
APA StyleHugonnot, V., Pépin, F., & Freedman, J. (2025). Bryophyte Flora of AlUla County (Saudi Arabia)—Distribution, Ecology, and Conservation. Plants, 14(2), 170. https://doi.org/10.3390/plants14020170






