Spatial and Temporal Variability Management for All Farmers: A Cell-Size Approach to Enhance Coffee Yields and Optimize Inputs
Abstract
1. Introduction
2. Results and Discussion
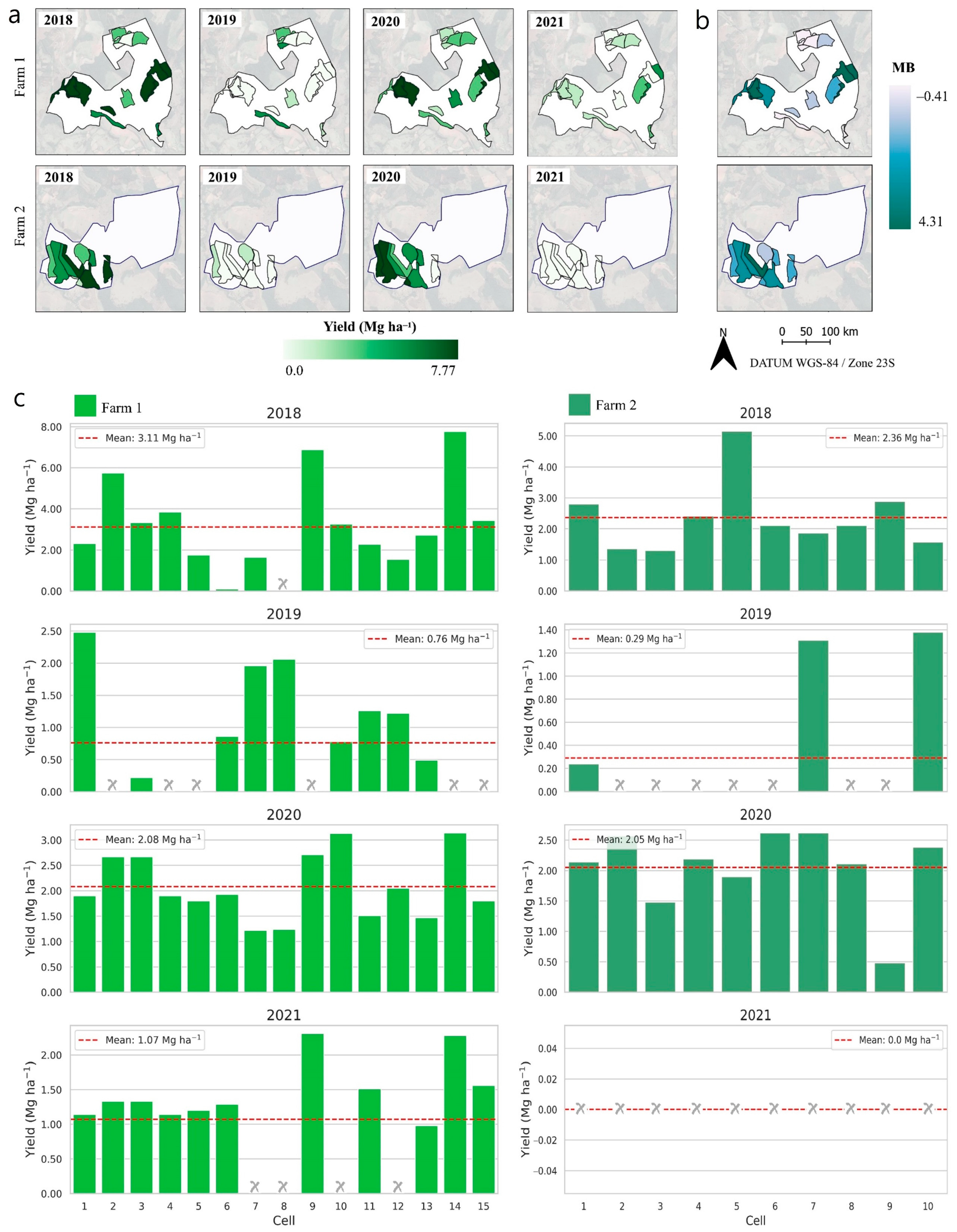
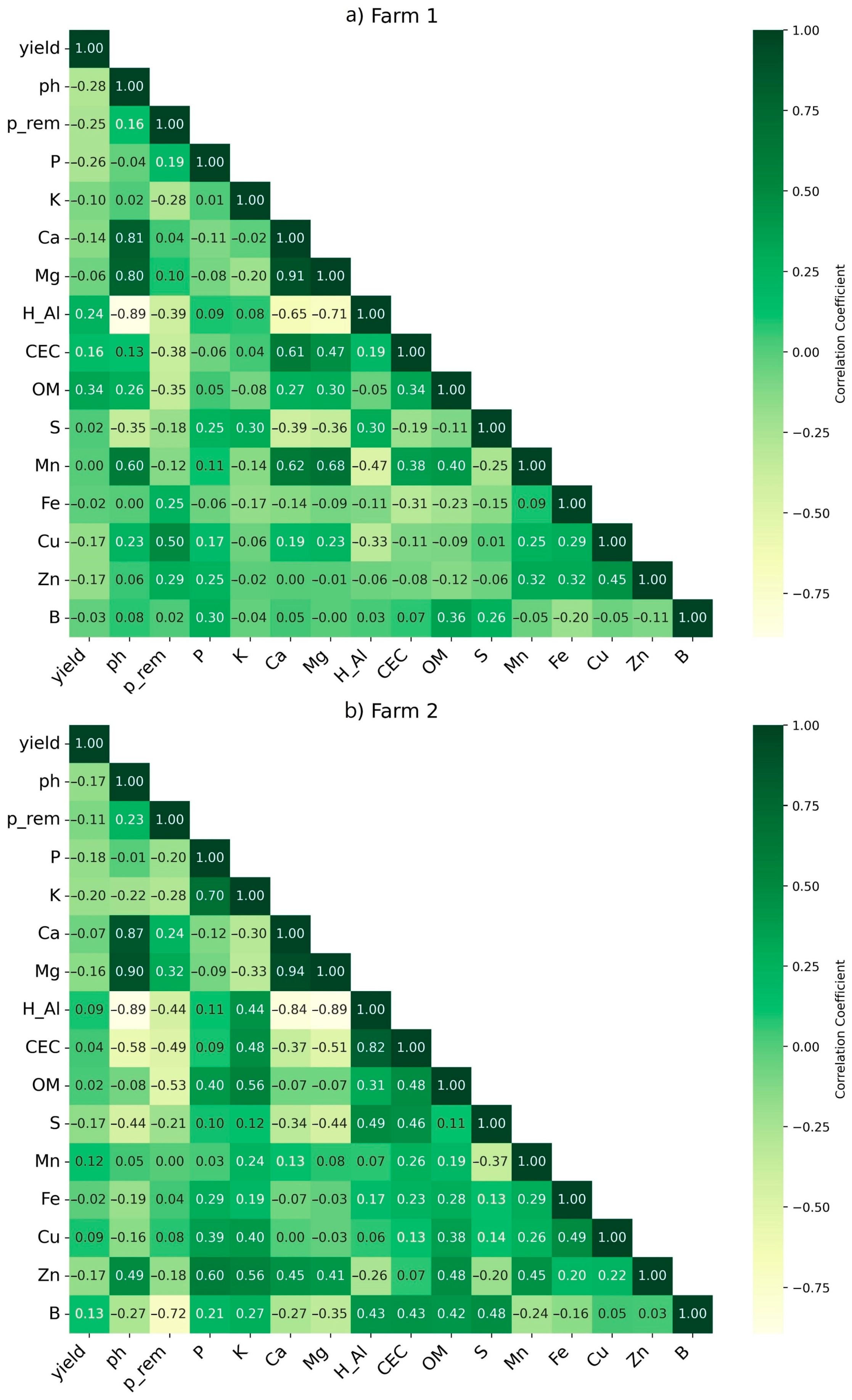
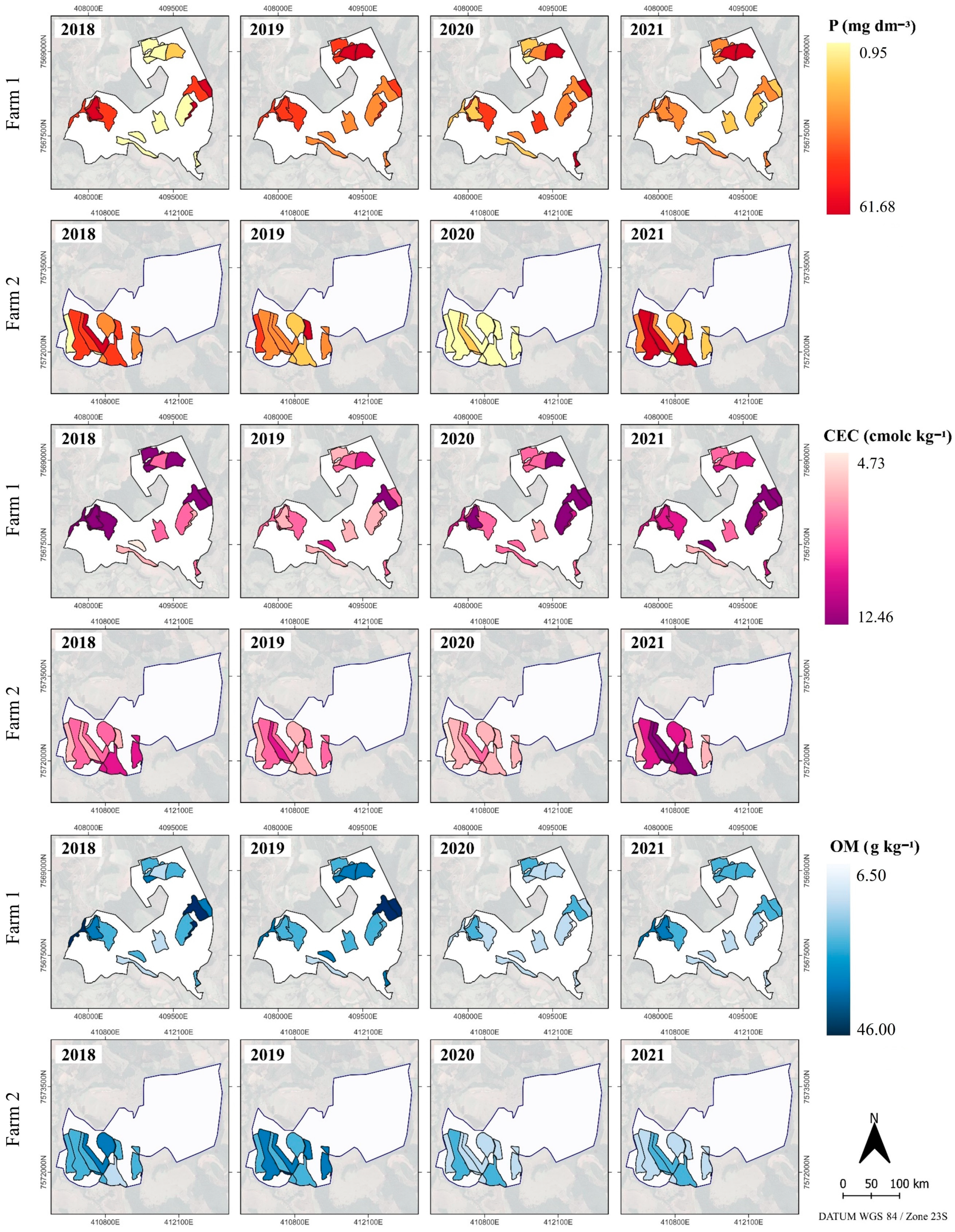
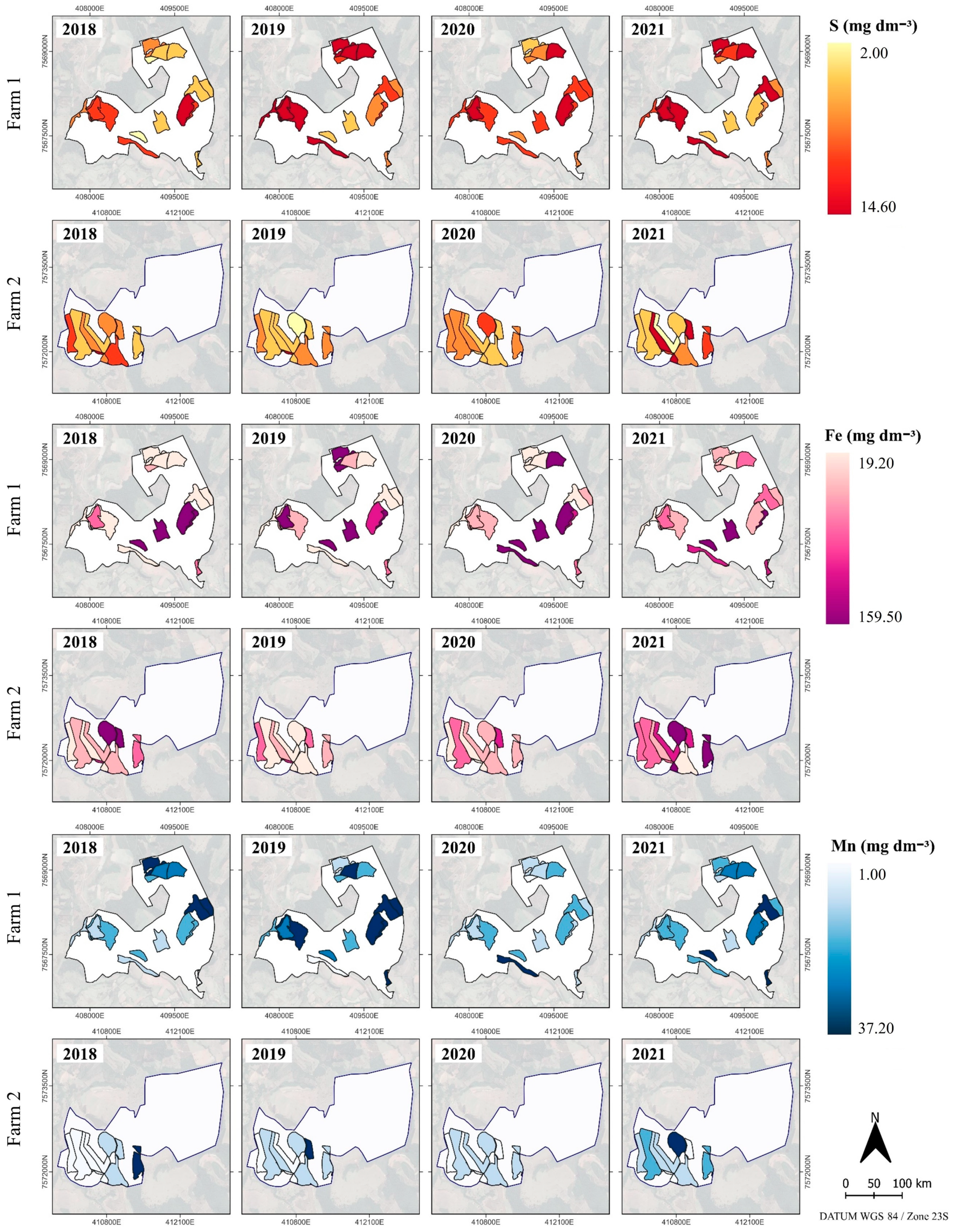
2.1. Soil Attributes and Yield Spatio-Temporal Variability
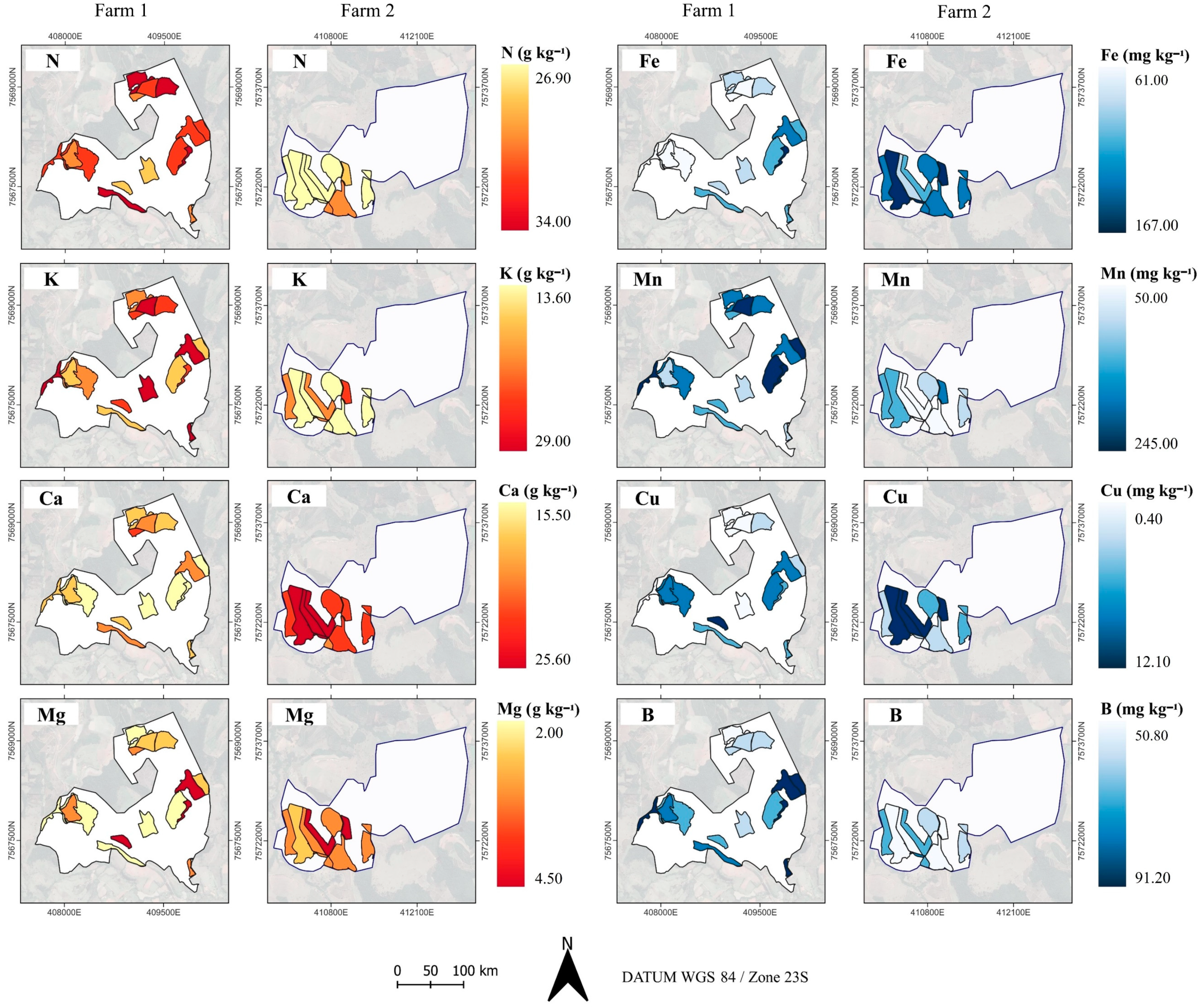
2.2. Spatio Characterization of Coffee Leaf Nutrient Content
2.3. Optimizing Fertilization in Cell-Size Resolution
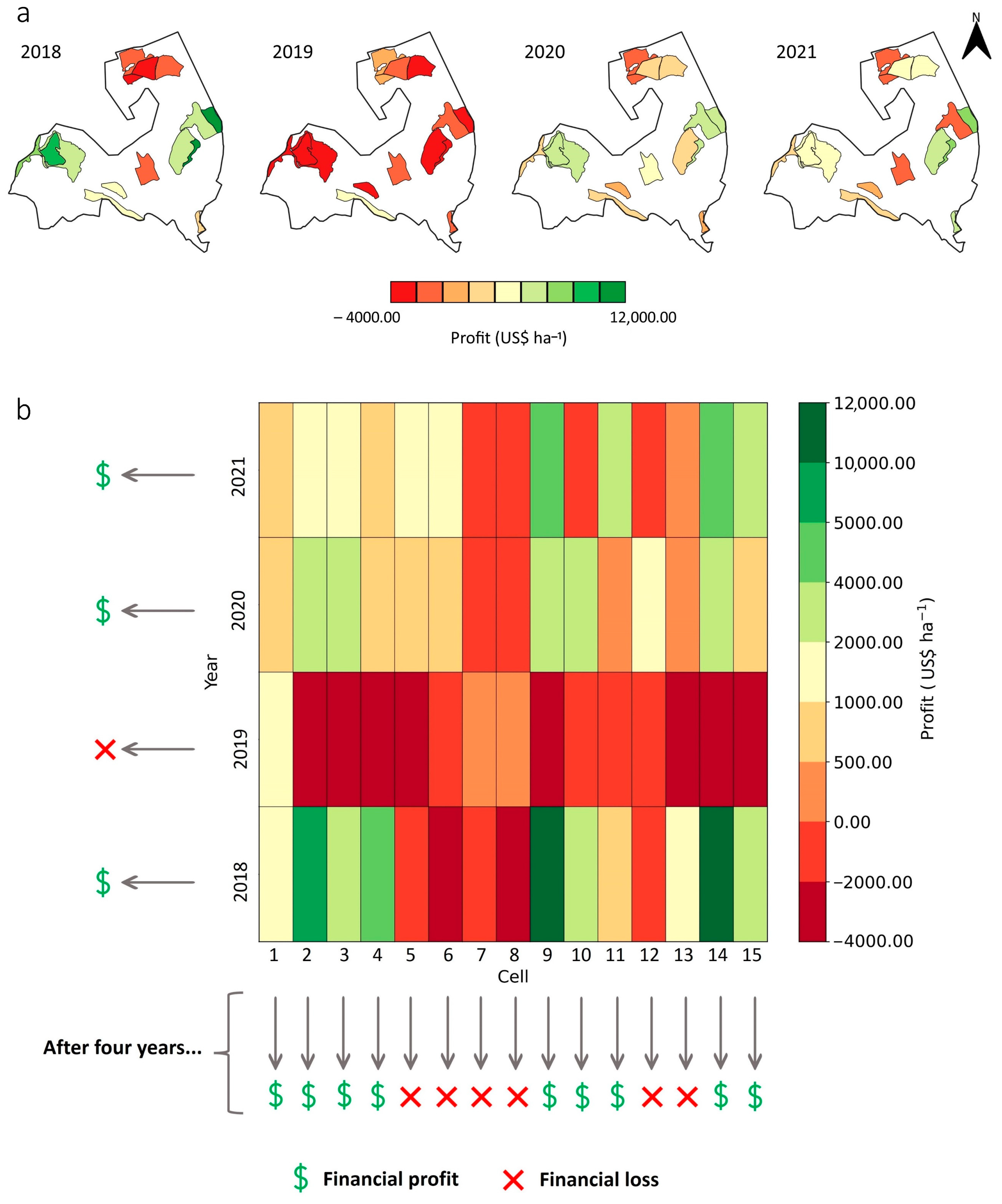
2.4. Profitability Maps at the Cell Level
2.5. Advantages, Limitations and Futures Studies
3. Materials and Methods
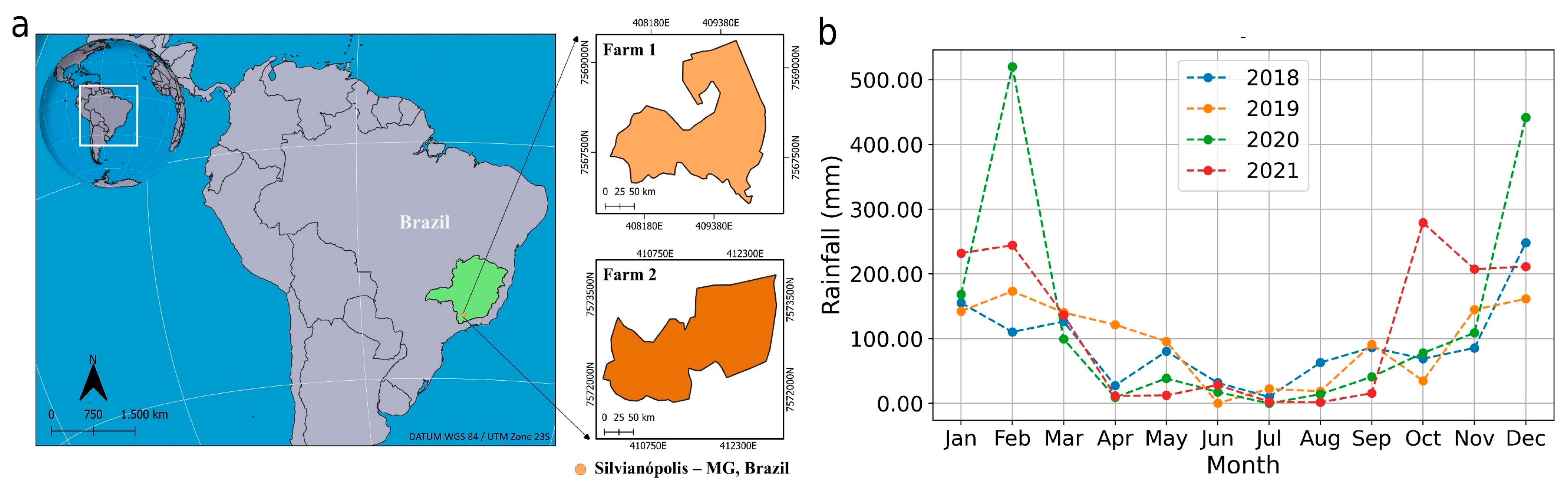
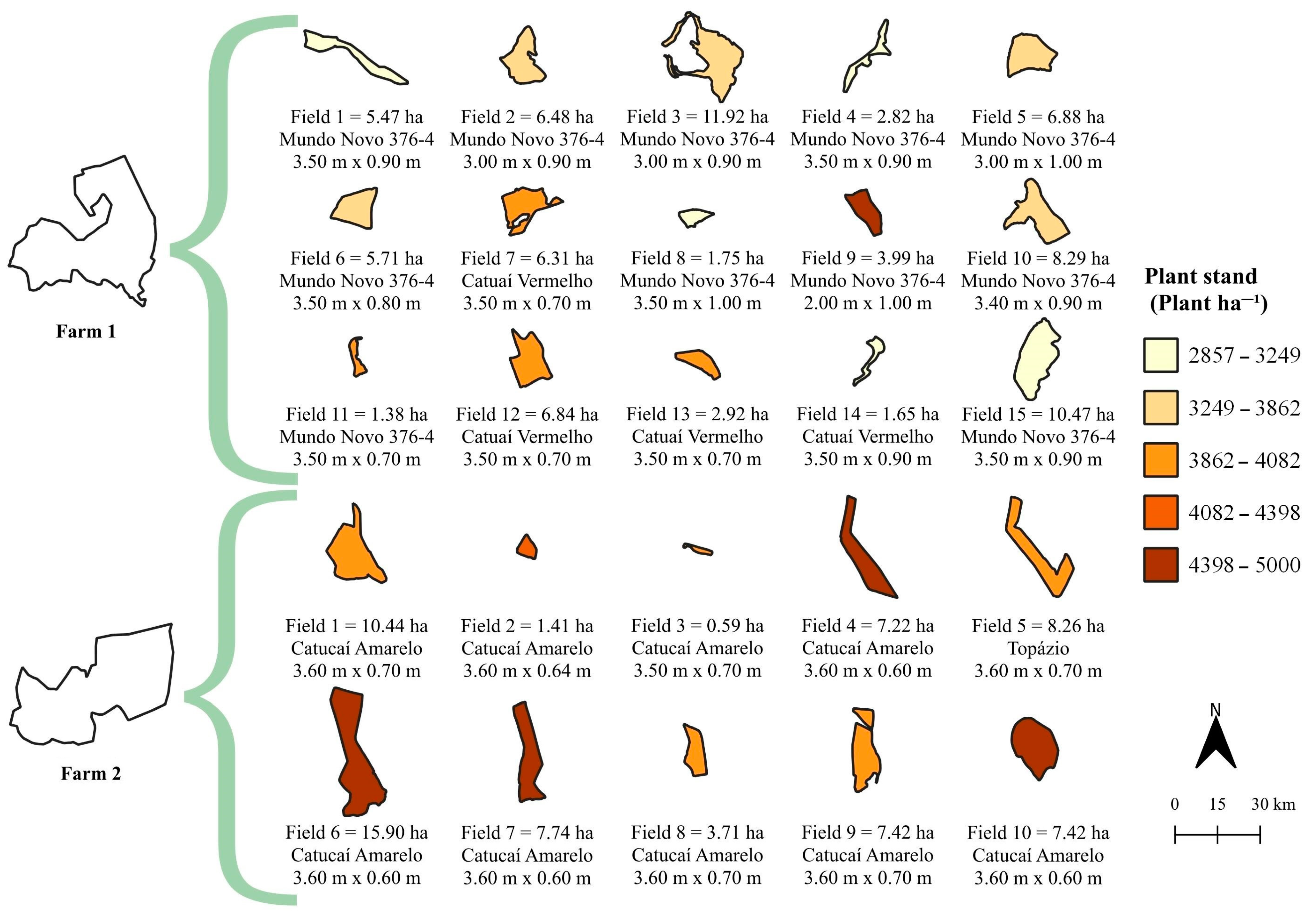
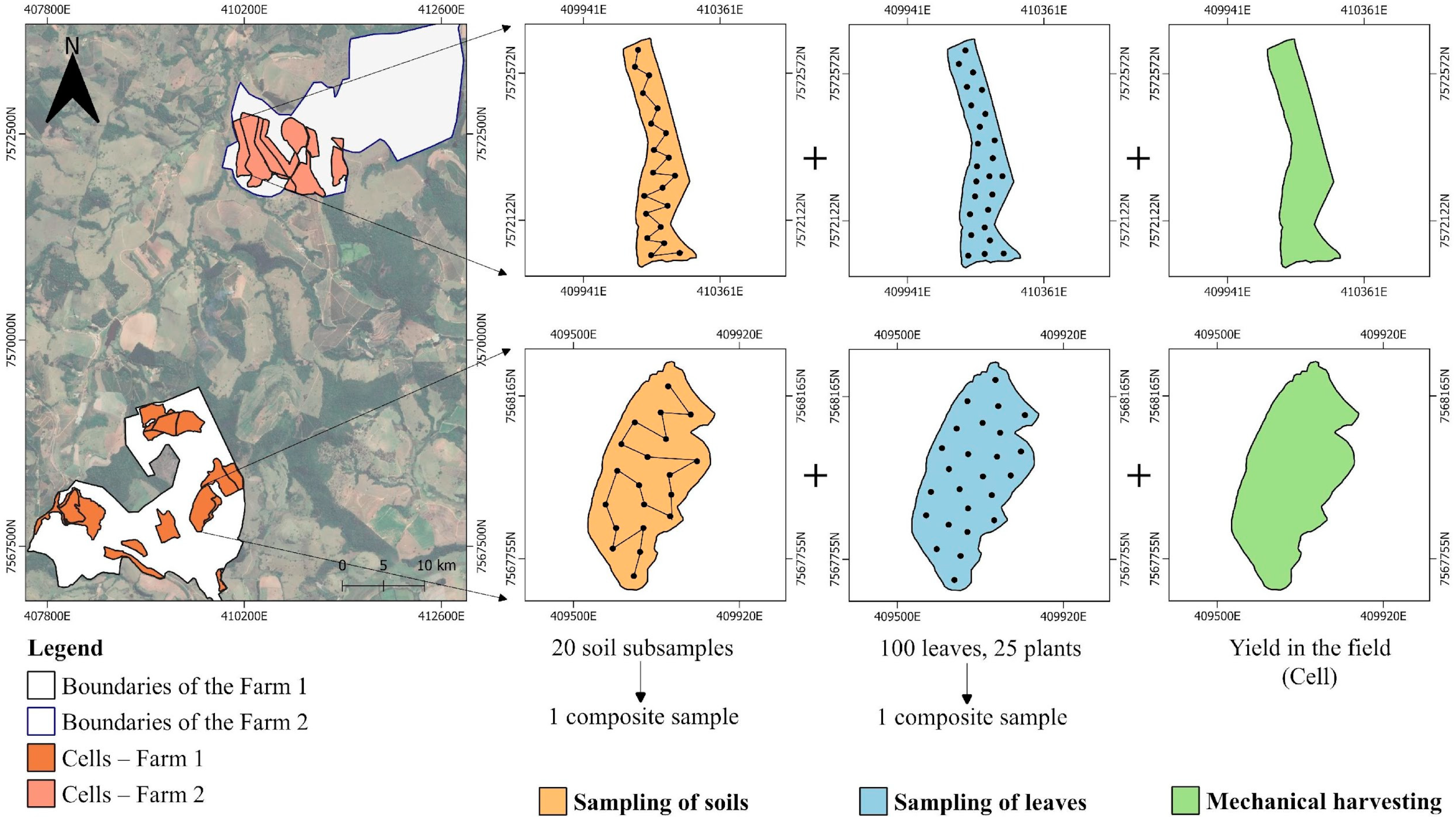
3.1. Data Collection
3.2. Optimizing Soil Fertilization
3.3. Economic Analysis
3.4. Statistical Analysis and Surface Mapping
4. Conclusions
- The cell-size sampling strategy identified high- and low-yielding regions over time, associating them with their biennial effect. Consequently, the identification of profitable areas can guide the management of resources and inputs in the subsequent harvest.
- The cell-size approach optimized the recommendation of potassium and phosphate fertilizers on farms, demonstrating that localized management can be carried out regardless of the spatial resolution data.
- Although this method was applied to coffee cultivation, cell sampling indicates that it is a strategy applicable to other crops and regions in various contexts.
- This methodology can be applied in scenarios with limited resources for high-density sampling, especially small- and medium-sized farmers, integrating them into the adoption of precision agriculture practices.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Department of Agriculture (USDA). Coffe. 2024. Available online: https://fas.usda.gov/data/commodities/coffee (accessed on 10 June 2024).
- Martinez, H.E.P.; de Andrade, S.A.L.; Santos, R.H.S.; Baptistella, J.L.C.; Mazzafera, P. Agronomic practices toward coffee sustainability. A review. Sci. Agric. 2024, 81, e20220277. [Google Scholar] [CrossRef]
- United Nations (UN). Transforming our World: The 2030 Agenda for Sustainable Development. 2015. Available online: https://sustainabledevelopment.un.org/content/documents/21252030%20Agenda%20for%20Sustainable%20Development%20web.pdf (accessed on 11 January 2024).
- Martello, M.; Molin, J.P.; Angnes, G.; Acorsi, M.G. Assessing the Temporal and Spatial Variability of Coffee Plantation Using RPA-Based RGB Imaging. Drones 2022, 6, 267. [Google Scholar] [CrossRef]
- Kazama, E.H.; Silva, R.P.; Tavares, T.O.; Correa, L.N.; Estevam, F.N.L.; Nicolau, F.E.A.; Maldonado Júnior, W. Methodology for selective coffee harvesting in management zones of yield and maturation. Precis. Agric. 2021, 22, 711–733. [Google Scholar] [CrossRef]
- Rosas, J.T.F.; Pinto, F.d.A.d.C.; de Queiroz, D.M.; Villar, F.M.d.M.; Valente, D.S.M.; Martins, R.N. Coffee ripeness monitoring using a UAV-mounted low-cost multispectral camera. Precis. Agric. 2022, 23, 300–318. [Google Scholar] [CrossRef]
- Bazame, H.C.; Molin, J.P.; Althoff, D.; Martello, M. Detection of coffee fruits on tree branches using computer vision. Sci. Agric. 2023, 80, e20220064. [Google Scholar] [CrossRef]
- Eron, F.; Noman, M.; Oliveira, R.R.; Chalfun-Junior, A. Computer Vision-Aided Intelligent Monitoring of Coffee: Towards Sustainable Coffee Production. Sci. Hortic. 2024, 327, 112847. [Google Scholar] [CrossRef]
- Pantoja-Gomez, L.M.; Corrêa, A.S.; Oliveira, L.O.; Guedes, R.N.C. Common Origin of Brazilian and Colombian Populations of the Neotropical Coffee Leaf Miner, Leucoptera coffeella (Lepidoptera: Lyonetiidae). J. Econ. Entomol. 2019, 112, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Nazir, T.; Javed, A.; Amin, S.T.; Jeribi, F.; Tahir, A. CoffeeNet: A deep learning approach for coffee plant leaves diseases recognition. Expert Syst. Appl. 2024, 237 Pt A, 121481. [Google Scholar] [CrossRef]
- Orlando, V.S.W.; Vieira, B.S.; Martins, G.D.; Lopes, E.A.; Assis, G.A.; Pereira, F.V.; Galo, M.L.B.; Rodrigues, L.S. Orbital multispectral imaging: A tool for discriminating management strategies for nematodes in coffee. Precis. Agric. 2024, 25, 2573–2588. [Google Scholar] [CrossRef]
- Silva, C.O.F.; Grego, C.R.; Manzione, R.L.; Oliveira, S.R.M.; Rodrigues, G.C.; Rodrigues, C.A.G.; Speranza, E.A.; Luchiari, A.; Koenigkan, L.V. Summarizing soil chemical variables into homogeneous management zones—Case study in a specialty coffee crop. Smart Agric. Technol. 2024, 7, 100418. [Google Scholar] [CrossRef]
- Vilela, E.F.; Castro, G.D.M.; Marin, D.B.; Santana, C.C.; Leite, D.H.; Matos, C.S.M.; Silva, C.A.; Lopes, I.P.C.; Queiroz, D.M.; Silva, R.A.; et al. Remote Monitoring of Coffee Leaf Miner Infestation Using Machine Learning. Agriengineering 2024, 6, 1697–1711. [Google Scholar] [CrossRef]
- Ramirez, G.M.; Zullo Júnior, J. Estimativa de parâmetros biofísicos de plantios de café a partir de imagens orbitais de alta resolução espacial. Eng. Agrícola 2010, 30, 468–479. [Google Scholar] [CrossRef]
- Santos, L.M.; Ferraz, G.A.S.; Carvalho, M.A.F.; Teodoro, S.A.; Campos, A.A.V.; Menicucci Neto, P. Use of RPA Images in the Mapping of the Chlorophyll Index of Coffee Plants. Sustainability 2022, 14, 13118. [Google Scholar] [CrossRef]
- Getachew, M.; Boeckx, P.; Verheyen, K.; Tolassa, T.; Tack, A.J.M.; Hylander, K.; Luca, S.; Zewdie, B.; De Frenne, P. Within and among farm variability of coffee quality of smallholders in southwest Ethiopia. Agrofor. Syst. 2023, 97, 883–905. [Google Scholar] [CrossRef]
- Molin, J.P.; Motomiya, A.V.A.; Frasson, F.R.; Faulin, G.D.C.; Tosta, W. Test procedure for variable rate fertilizer on coffee. Acta Scientiarum. Agronomy 2010, 32, 569–575. [Google Scholar] [CrossRef]
- Alves, M.C.; Sanches, L.; Pozza, E.A.; Pozza, A.A.A.; Silva, F.M. The role of machine learning on Arabica coffee crop yield based on remote sensing and mineral nutrition monitoring. Biosyst. Eng. 2022, 221, 81–104. [Google Scholar] [CrossRef]
- Aragão, O.O.; Jesus, E.C.; Oliveira-Longatti, S.M.; Souza, A.A.; Moreira, F.M.S. Physical, Chemical, and Microbiological Attributes as Discriminators of Coffee-Growing and Forest Sites in Different Soils in the Brazilian Atlantic Forest Biome. J. Soil Sci. Plant Nutr. 2023, 23, 6767–6776. [Google Scholar] [CrossRef]
- Mwendwa, S.M.; Mbuvi, J.P.; Kironchi, G.; Gachene, C.K.K. Assessing spatial variability of selected soil properties in Upper Kabete Campus coffee farm, University of Nairobi, Kenya. Heliyon 2022, 8, e10190. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, X.; Sun, W.; Cui, N.; Guo, J.; Chen, H.; Huang, W. Fertilizer Optimization Combined with Coffee Husk Returning to Improve Soil Environmental Quality and Young Coffee Tree Growth. J. Soil Sci. Plant Nutr. 2023, 24, 650–665. [Google Scholar] [CrossRef]
- Martello, M.; Molin, J.P.; Bazame, H.C.; Tavares, T.R.; Maldaner, L.F. Use of Active Sensors in Coffee Cultivation for Monitoring Crop Yield. Agronomy 2022, 12, 2118. [Google Scholar] [CrossRef]
- Zanella, M.A.; Martins, R.N.; Silva, F.M.; Carvalho, L.C.C.; Alves, M.C.; Rosas, J.T.F. Coffee yield prediction using high-resolution satellite imagery and crop nutritional status in Southeast Brazil. Remote Sens. Appl. Soc. Environ. 2024, 33, 101092. [Google Scholar] [CrossRef]
- Pereira, S.P.; Bartholo, G.F.; Baliza, D.P.; Sobreira, F.M.; Guimarães, R.J. Crescimento, produtividade e bienalidade do cafeeiro em função do espaçamento de cultivo. Pesqui. Agropecu. Bras. 2011, 46, 152–160. [Google Scholar] [CrossRef]
- Bernardes, T.; Moreira, M.A.; Adami, M.; Giarolla, A.; Rudorff, B.F.T. Monitoring Biennial Bearing Effect on Coffee Yield Using MODIS Remote Sensing Imagery. Remote Sens. 2012, 4, 2492–2509. [Google Scholar] [CrossRef]
- Kouadio, L.; Tixier, P.; Byrareddy, V.; Marcussen, T.; Mushtaq, S.; Rapidel, B.; Stone, R. Performance of a process-based model for predicting robusta coffee yield at the regional scale in Vietnam. Ecol. Model. 2021, 443, 109469. [Google Scholar] [CrossRef]
- Ferreira, G.F.P.; Lemos, O.L.; Soratto, R.P.; Perdoná, M.J. Spatial variability of leaf macronutrient concentration and fruit production of an Arabica coffee plantation using two sampling densities. Precis. Agric. 2022, 23, 1473–1488. [Google Scholar] [CrossRef]
- Kerry, R.; Ingram, B.; Oliver, M.; Frogbrook, Z. Soil sampling and sensed ancillary data requirements for soil mapping in precision agriculture II: Contour mapping of soil properties with sensed z-score data for comparison with management zone averages. Precis. Agric. 2024, 25, 1212–1234. [Google Scholar] [CrossRef]
- Gonçalves, M.G.M.; Avalos, F.A.P.; dos Reis, J.V.; Costa, M.V.; Silva, S.H.G.; Poggere, G.C.; Curi, N.; de Menezes, M.D. Pedology-based management class establishment: A study case in Brazilian coffee crops. Precis. Agric. 2022, 23, 1027–1050. [Google Scholar] [CrossRef]
- Morgan, M.T.; Ess, D.R. The Precision-Farming Guide for Agriculturists; John Deere Publishing: Moline, IL, USA, 1997. [Google Scholar]
- Molin, J.P.; Amaral, L.R.; Colaço, A.F. Agricultura de Precisão; Oficina de Textos: São Paulo, Brazil, 2015. [Google Scholar]
- Angnes, G.; Martello, M.; Faulin, G.D.C.; Molin, J.P.; Romanelli, T.L. Energy Efficiency of Variable Rate Fertilizer Application in Coffee Production in Brazil. Agriengineering 2021, 3, 815–826. [Google Scholar] [CrossRef]
- Latini, A.O.; Silva, D.P.; Souza, F.M.L.; Ferreira, M.C.; Moura, M.S.; Suarez, N.F. Reconciling coffee productivity and natural vegetation conservation in an agroecosystem landscape in Brazil. J. Nat. Conserv. 2020, 57, 125902. [Google Scholar] [CrossRef]
- d’Albertas, F.; Sparovek, G.; Pinto, L.F.G.; Hohlenwerger, C.; Metzger, J.P. Yield increases mediated by pollination and carbon payments can offset restoration costs in coffee landscapes. One Earth 2024, 7, 110–122. [Google Scholar] [CrossRef]
- Koutouleas, A.; Bosselmann, A.S.; Rahn, E. Is agroforestry a sustainable management system for future coffee production? In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2024. [Google Scholar] [CrossRef]
- Liu, K.; Harrison, M.T.; Yan, H.; Liu, D.L.; Meinke, H.; Hoogenboom, G.; Wang, B.; Peng, B.; Guan, K.; Jaegermeyr, J.; et al. Silver lining to a climate crisis in multiple prospects for alleviating crop waterlogging under future climates. Nat. Commun. 2023, 14, 765. [Google Scholar] [CrossRef] [PubMed]
- Companhia Nacional de Abastecimento (Conab). Acompanhamento da Safra Brasileira: Café. 2018. Available online: https://www.conab.gov.br/info-agro/safras/cafe (accessed on 5 May 2023).
- Companhia Nacional de Abastecimento (Conab). Acompanhamento da Safra Brasileira: Café. 2021. Available online: https://www.conab.gov.br/info-agro/safras/cafe/boletim-da-safra-de-cafe (accessed on 5 May 2023).
- Franco Junior, K.S.; Florentino, L.A.; Calegari, A.; Mantovani, J.R.; Caixeta, I.F.; Terra, A.B.C. Coverage plants in the management of skeletal coffee. Rev. Ceres 2022, 69, 247–255. [Google Scholar] [CrossRef]
- Filla, V.A.; Coelho, A.P.; Meirelles, F.C.; Cavalcante, A.G.; Oliveira, N.H.C.; Lemos, L.B. Responsiveness of Arabica coffee cultivars to skeleton pruning in a low-altitude region. Crop Sci. 2024, 64, 942–955. [Google Scholar] [CrossRef]
- Campbell, T.; Kalcsits, L. Strategies to overcome biennial bearing in apple—A review. Eur. J. Agron. 2024, 158, 127213. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Godoy, A.G.; Menezes-Silva, P.E.; Martins, S.C.V.; Sanglard, L.M.V.P.; Morais, L.E.; Torre-Neto, A.; Ghini, R. Sustained enhancement of photosynthesis in coffee trees grown under free-air CO2 enrichment conditions: Disentangling the contributions of stomatal, mesophyll, and biochemical limitations. J. Exp. Bot. 2016, 67, 341–352. [Google Scholar] [CrossRef]
- Pereira, A.A.; Morais, A.R.; Scalco, M.S.; Fernandes, T.J. Modelagem do diâmetro de copa do cafeeiro podado cultivado em diferentes densidades e regimes hídricos. Coffee Sci. 2016, 11, 495–501. [Google Scholar]
- Mao, C.; He, J.; Wen, X.; Xiang, Y.; Feng, J.; Shu, Y. Correlation and Pathway Analysis of the Carbon, Nitrogen, and Phosphorus in Soil-Microorganism-Plant with Main Quality Components of Tea (Camellia sinensis). Phyton-Int. J. Exp. Bot. 2024, 93, 487–502. [Google Scholar] [CrossRef]
- Basiru, S.; Mwanza, H.P.; Hijri, M. Analysis of Arbuscular Mycorrhizal Fungal Inoculant Benchmarks. Microorganisms 2021, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Fung, E.; Wang, J.; Zhao, X.; Farzamian, M.; Allred, B.; Clevenger, W.B.; Levenson, P.; Triantafilis, J. Mapping cation exchange capacity and exchangeable potassium using proximal soil sensing data at the multiple-field scale. Soil Tillage Res. 2023, 232, 105735. [Google Scholar] [CrossRef]
- Nascimento, M.S.; Barreto-Garcia, P.A.B.; Monroe, P.H.M.; Pereira, M.G.; Barros, W.T.; Nunes, M.R. Carbon in soil macroaggregates under coffee agroforestry systems: Modeling the effect of edaphic fauna and residue input. Appl. Soil Ecol. 2024, 202, 105604. [Google Scholar] [CrossRef]
- Shi, P.; Zhang, Y.; Li, P.; Li, Z.; Yu, K.; Ren, Z.; Xu, G.; Cheng, S.; Wang, F.; Ma, Y. Distribution of soil organic carbon impacted by land-use changes in a hilly watershed of the Loess Plateau, China. Sci. Total Environ. 2019, 652, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Wen, X.; Li, H.; Huang, C.; Jiang, Y.; Liu, D.; Sun, B. Influence of manure fertilization on soil phosphorous retention and clay mineral transformation: Evidence from a 16-year long-term fertilization experiment. Appl. Clay Sci. 2021, 204, 106021. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiong, W.; Xing, Y.; Sun, Y.; Lin, X.; Dong, Y. Long-Term Coffee Monoculture Alters Soil Chemical Properties and Microbial Communities. Sci. Rep. 2018, 8, 6116. [Google Scholar] [CrossRef]
- Wallace, A. The Law of the Maximum. Better Crops Plant Food 1993, 77, 20–22. [Google Scholar]
- Parecido, R.J.; Soratto, R.P.; Perdoná, M.J.; Gitari, H.I. Foliar-applied silicon may enhance fruit ripening and increase yield and nitrogen use efficiency of Arabica coffee. Eur. J. Agron. 2022, 140, 126602. [Google Scholar] [CrossRef]
- Khan, H.; Esau, T.J.; Farooque, A.A.; Zaman, Q.U.; Abbas, F.; Schumann, A.W. Soil spatial variability and its management with precision agriculture. In Precision Agriculture; Zaman, Q., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 19–36. [Google Scholar] [CrossRef]
- Nascimento, M.O.; Celestino, S.M.C.; Veiga, A.D.; Jesus, B.D.A.; Oliveira, L.L. Quality of Arabica coffee grown in Brazilian Savannah and impact of potassium sources. Food Res. Int. 2024, 188, 114500. [Google Scholar] [CrossRef]
- Lalani, B.; Lanza, G.; Leiva, B.; Mercado, L.; Haggar, J. Shade versus intensification: Trade-off or synergy for profitability in coffee agroforestry systems? Agric. Syst. 2024, 214, 103814. [Google Scholar] [CrossRef]
- Instituto Capixaba de Pesquisa, Assistência Técnica e Extensão Rural (Incaper). Poda Programada de Ciclo em Café Arábica—PPCA: Nova Tecnologia de Poda Para o Café Arábica; Documento nº 242; Incaper: Vitória, Brazil, 2016. [Google Scholar]
- Empresa Brasileira de Pesquisa Agropecuária (Embrapa). Boas Práticas Agrícolas Aplicadas à Lavoura Cafeeira Para o Estado de Minas Gerais; Embrapa Café: Brasília, Brazil, 2022. [Google Scholar]
- Khalfaoui, R.; Goodell, J.W.; Mefteh-Wali, S.; Chishti, M.Z.; Gozgor, G. Impact of climate risk shocks on global food and agricultural markets: A multiscale and tail connectedness analysis. Int. Rev. Financ. Anal. 2024, 93, 103206. [Google Scholar] [CrossRef]
- Xu, M.; Chen, C. Memon Aftab Ahmed. Market-oriented farmland transfer and outsourced machinery services: Evidence from China. Econ. Anal. Policy 2024, 81, 1214–1226. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Karp, F.H.S.; Adamchuk, V.; Dutilleul, P.; Melnitchouck, A. Comparative study of interpolation methods for low-density sampling. Precis. Agric. 2024, 25, 2776–2800. [Google Scholar] [CrossRef]
- Kerry, R.; Ingram, B.; Oliver, M.; Frogbrook, Z. Soil sampling and sensed ancillary data requirements for soil mapping in precision agriculture I. delineation of management zones to determine zone averages of soil properties. Precis. Agric. 2024, 25, 1181–1211. [Google Scholar] [CrossRef]
- Valente, D.S.M.; Pereira, G.W.; Queiroz, D.M.; Zandonadi, R.S.; Amaral, L.R.; Bottega, E.L.; Costa, M.M.; Coelho, A.L.F.; Grift, T. Accuracy of Various Sampling Techniques for Precision Agriculture: A Case Study in Brazil. Agriculture 2024, 14, 2198. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Simões, J.C.; Pelegrini, D.F. Diagnóstico da Cafeicultura Mineira—Regiões Tradicionais: Sul/Sudoeste de Minas, Zona da Mata, Triângulo Mineiro/Alto Paranaíba; Empresa de Pesquisa Agropecuária de Minas Gerais (EPAMIG): Belo Horizonte, Brazil, 2010. [Google Scholar]
- Misgana, Z.; Garedew, W.; Alemayehu, Y.; Bekeko, Z.; Nebiyu, A. The influence of shade tree species and coffee varieties on selected soil physicochemical properties in coffee-based farming system of southwestern Ethiopia. Trees For. People 2024, 17, 100650. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo; Embrapa: Brasília, Brazil, 2017. [Google Scholar]
- Abreu, C.A.; Abreu, M.F.; Van Raij, B.; Bataglia, O.C.; Andrade, J.C. Extraction of boron from soil by microwave heating for ICP OES determination. Commun. Soil Sci. Plant Anal. 1994, 25, 3321–3333. [Google Scholar] [CrossRef]
- Malavolta, E.; Yamada, T.; Guidolin, J.A. Nutrição e Adubação do Cafeeiro; Instituto Internacional da Potassa: Piracicaba, Brazil, 1982. [Google Scholar]
- Stevens, W.L. Análises estatísticas do ensaio de variedades de café. Bragantia 1949, 9, 103–123. [Google Scholar] [CrossRef]
- Matiello, J.B.; Santinato, R.; Almeida, S.R.; Garcia, A.W.R. Cultura de Café no Brasil, Manual de Recomendações; Fundação Procafé: Varginha, Brazil, 2024. [Google Scholar]
- Silva, V.A.; Rezende, J.C.; Carvalho, A.M.; Carvalho, G.R.; Rezende, T.T.; Ferreira, A.D. Recuperação de cultivares de café submetidas ao esqueletamento aos quatro anos e meio de idade. Coffee Sci. 2016, 11, 55–64. [Google Scholar]
- Ribeiro, A.C.; Guimarães, P.T.G.; Alvarez, V.V.H. Recomendações Para o Uso de Corretivos e Fertilizantes em Minas Gerais; Comissão de Fertilidade do Solo do Estado de Minas Gerais: Viçosa, Brazil, 1999. [Google Scholar]
- Centro de Estudos Avançados em Economia Aplicada (CEPEA-Esalq/USP). 2024. Available online: https://www.cepea.esalq.usp.br/br/indicador/cafe.aspx (accessed on 1 March 2024).
- Instituto de Pesquisa Econômica Aplicada (IPEA). 2024. Available online: https://www.ipea.gov.br/ (accessed on 1 March 2024).
- Kluyver, T.; Ragan-Kelley, B.; Pérez, F.; Granger, B.; Bussonnier, M.; Frederic, J.; Kyle, K.; Jessica, H.; Jason, G.; Sylvain, C.; et al. Jupyter notebooks–a publishing format for reproducible computational workflows. In Positioning and Power in Academic Publishing: Players, Agents and Agendas, Proceedings of the 20th International Conference on Electronic Publishing, Göttingen, Germany, 7–9 June 2016; ELPUB: Göttingen, Germany, 2016. [Google Scholar] [CrossRef]
- Python. The Python Standard Library. 2024. Available online: https://docs.python.org/3/library/index.html (accessed on 15 January 2024).
- QGIS.org. QGIS Geographic Information System. QGIS Association. 2024. Available online: https://www.qgis.org (accessed on 11 February 2024).
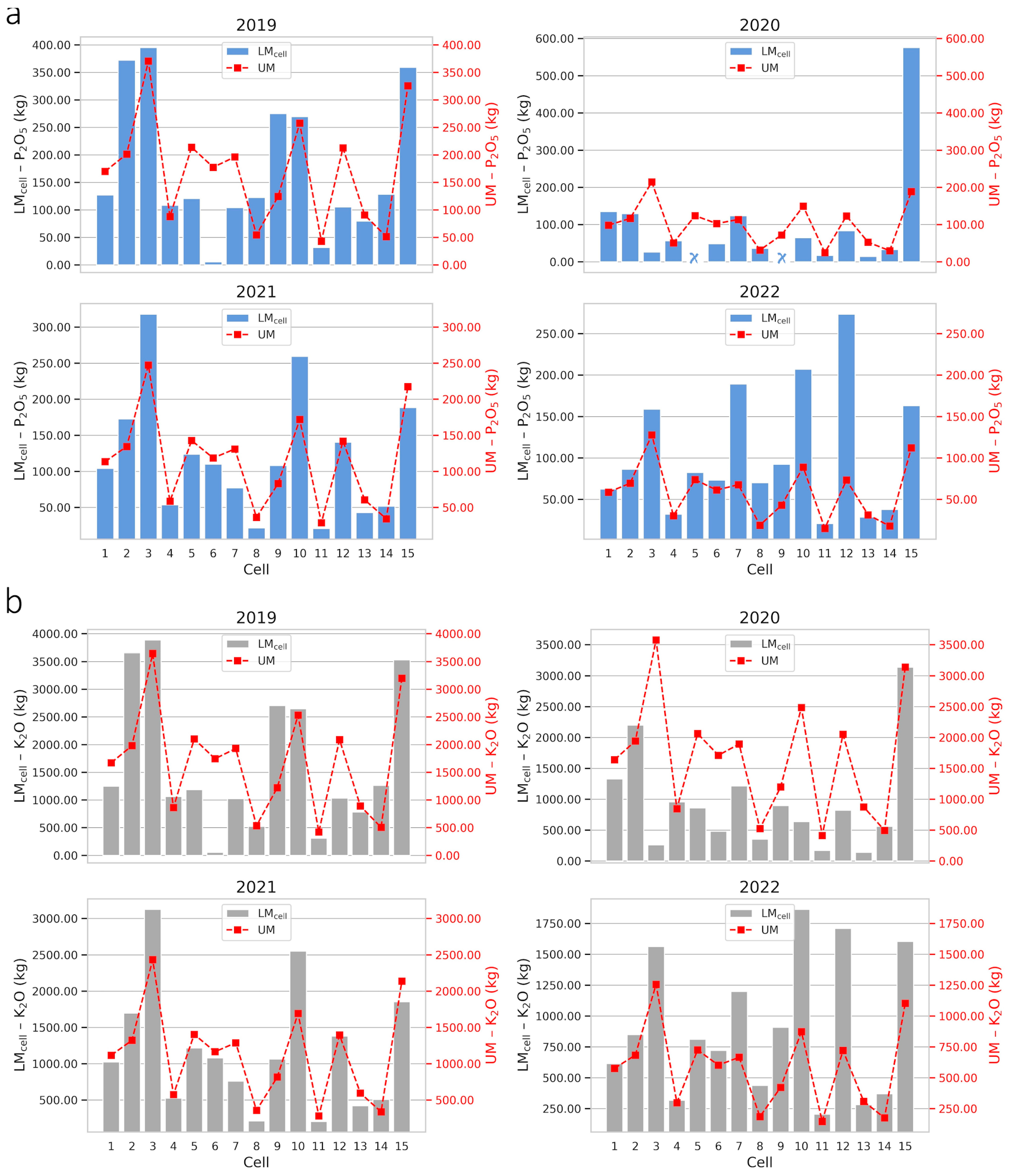
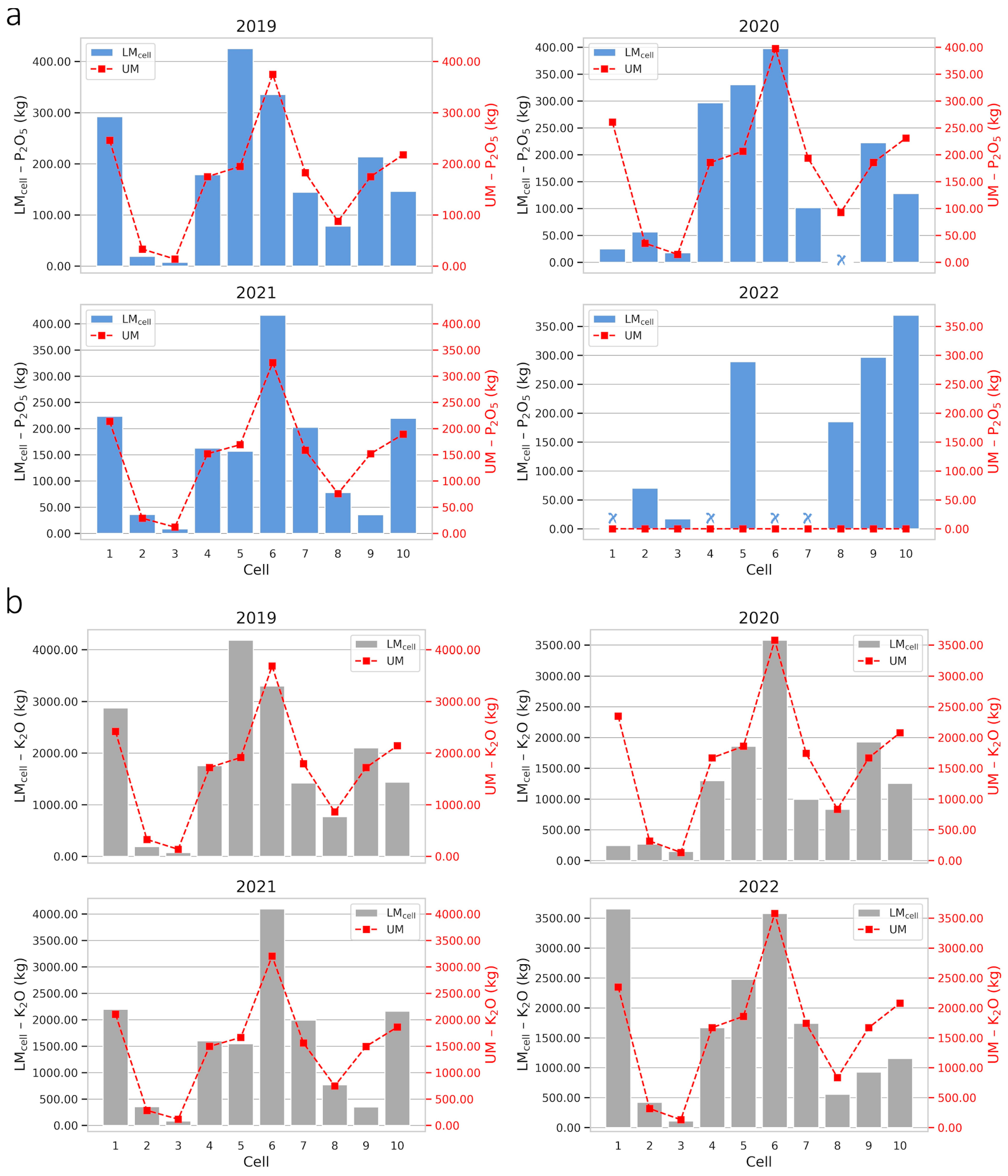
| Farm | Fertilizer | Management | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|---|
| 1 | P2O5 | UM (kg) | 2575.85 | 1491.79 | 1720.06 | 888.08 |
| LMcell (kg) | 2604.56 | 1345.39 | 1793.09 | 1578.56 | ||
| Difference (kg) | 28.71 | −146.40 | 73.03 | 690.49 | ||
| % | 1.11 | −9.81 | 4.25 | 77.75 | ||
| K2O | UM (kg) | 25,329.19 | 24,863.10 | 16,913.95 | 8732.76 | |
| LMcell (kg) | 24,931.94 | 14,037.45 | 17,632.04 | 13,455.99 | ||
| Difference (kg) | −397.25 | −10,825.65 | 718.09 | 4723.24 | ||
| % | −1.57 | −43.54 | 4.25 | 54.09 | ||
| 2 | P2O5 | UM (kg) | 1700.50 | 1803.00 | 1477.91 | 0.00 |
| LMcell (kg) | 1841.42 | 1575.37 | 1541.26 | 1228.76 | ||
| Difference (kg) | 140.92 | −227.63 | 63.35 | 1228.76 | ||
| % | 8.29 | −12.63 | 4.29 | 100.00 | ||
| K2O | UM (kg) | 16,721.61 | 16,227.00 | 14,532.80 | 16,227.00 | |
| LMcell (kg) | 18,107.33 | 12,410.81 | 15,155.73 | 16,292.85 | ||
| Difference (kg) | 1385.72 | −3816.19 | 622.93 | 65.85 | ||
| % | 8.29 | −23.52 | 4.29 | 0.41 |
| Variables | Unit | Farm 1 | Farm 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2020 | 2021 | 2018 | 2019 | 2020 | 2021 | ||
| pH H2O | - | 5.47 | 5.67 | 5.03 | 5.62 | 5.07 | 5.67 | 5.41 | 5.43 |
| P rem | mg dm−3 | 20.02 | 21.12 | 23.89 | 20.81 | 14.44 | 16.29 | 22.10 | 15.39 |
| P | mg dm−3 | 3.01 | 17.29 | 9.71 | 8.16 | 3.43 | 7.19 | 1.50 | 13.09 |
| K | mg dm−3 | 63.47 | 89.53 | 73.00 | 78.60 | 77.30 | 98.65 | 63.00 | 101.90 |
| Ca | cmolc dm−3 | 3.02 | 2.87 | 2.09 | 3.83 | 1.39 | 2.10 | 1.97 | 2.92 |
| Mg | cmolc dm−3 | 0.84 | 0.74 | 0.52 | 0.83 | 0.37 | 0.59 | 0.54 | 0.76 |
| H + Al | cmolc dm−3 | 3.83 | 3.32 | 4.89 | 3.53 | 5.00 | 3.45 | 3.21 | 4.12 |
| CEC | cmolc dm−3 | 7.94 | 7.08 | 7.68 | 8.36 | 6.96 | 6.43 | 5.88 | 8.05 |
| OM | g kg−1 | 30.60 | 31.20 | 16.70 | 22.10 | 28.10 | 31.90 | 17.70 | 21.30 |
| S | mg dm−3 | 4.22 | 6.60 | 4.98 | 5.07 | 4.13 | 3.57 | 3.58 | 4.62 |
| Mn | mg dm−3 | 17.18 | 19.79 | 11.73 | 17.37 | 8.33 | 8.54 | 5.51 | 10.25 |
| Fe | mg dm−3 | 53.03 | 58.47 | 62.43 | 64.79 | 55.97 | 41.52 | 42.49 | 66.10 |
| Cu | mg dm−3 | 0.77 | 1.18 | 1.08 | 0.93 | 1.19 | 0.94 | 1.00 | 1.64 |
| Zn | mg dm−3 | 1.36 | 1.79 | 2.56 | 1.98 | 1.36 | 2.90 | 0.94 | 3.04 |
| B | mg dm−3 | 0.47 | 0.69 | 0.34 | 0.45 | 0.76 | 0.57 | 0.39 | 0.36 |
| Variables | Year | |||
|---|---|---|---|---|
| 2018 | 2019 | 2020 | 2021 | |
| Effective operating cost (US$ ha−1) | 2583 | 2393 | 1831 | 1750 |
| Total operating cost (US$ ha−1) | 3266 | 3025 | 2315 | 2212 |
| Total cost (US$ ha−1) | 3637 | 3369 | 2577 | 2463 |
| Average coffee price (US$ bag−1) * | 120 | 107 | 105 | 178 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, E.R.O.d.; Silva, T.L.d.; Wei, M.C.F.; Souza, R.A.d.; Molin, J.P. Spatial and Temporal Variability Management for All Farmers: A Cell-Size Approach to Enhance Coffee Yields and Optimize Inputs. Plants 2025, 14, 169. https://doi.org/10.3390/plants14020169
Silva EROd, Silva TLd, Wei MCF, Souza RAd, Molin JP. Spatial and Temporal Variability Management for All Farmers: A Cell-Size Approach to Enhance Coffee Yields and Optimize Inputs. Plants. 2025; 14(2):169. https://doi.org/10.3390/plants14020169
Chicago/Turabian StyleSilva, Eudocio Rafael Otavio da, Thiago Lima da Silva, Marcelo Chan Fu Wei, Ricardo Augusto de Souza, and José Paulo Molin. 2025. "Spatial and Temporal Variability Management for All Farmers: A Cell-Size Approach to Enhance Coffee Yields and Optimize Inputs" Plants 14, no. 2: 169. https://doi.org/10.3390/plants14020169
APA StyleSilva, E. R. O. d., Silva, T. L. d., Wei, M. C. F., Souza, R. A. d., & Molin, J. P. (2025). Spatial and Temporal Variability Management for All Farmers: A Cell-Size Approach to Enhance Coffee Yields and Optimize Inputs. Plants, 14(2), 169. https://doi.org/10.3390/plants14020169







