Green Extraction of Bioactives from Curcuma longa Using Natural Deep Eutectic Solvents: Unlocking Antioxidative, Antimicrobial, Antidiabetic, and Skin Depigmentation Potentials
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterizations of NADES
2.2. Antioxidant Potential of Turmeric NADES Extracts
2.2.1. Total Phenolic Content (TPC) of Extracts
2.2.2. Radical Scavenging Activity
2.3. Antibacterial Activity of NADES Extracts
2.4. Inhibitory Effects of NADES Extracts on Tyrosinase Activity and Keratinocyte Survival
2.5. Inhibitory Effects of NADES Extracts on α-Amylase
2.6. Curcumin Content in NADES Extracts
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of NADESs
3.3. Extractions
3.4. FTIR (Fourier-Transform Infrared) Spectroscopy
3.5. Spectrophotometric Assays
3.5.1. Total Phenolic Content (TPC)
3.5.2. DPPH Radical Scavenging Assay
3.5.3. ABTS Radical Scavenging Assay
3.6. Agar Well Diffusion Test
3.7. Assessing of Skin-Related Effects
3.7.1. Tyrosinase Inhibition Assay
3.7.2. Keratinocyte Viability Assessment
3.8. α-Amylase Inhibition Assay
3.9. LC–MS Analysis of Curcumin
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Park, J.; Do, S.; Lee, M.; Ha, S.; Lee, K.-G. Preparation of Turmeric Powder with Various Extraction and Drying Methods. Chem. Biol. Technol. Agric. 2022, 9, 39. [Google Scholar] [CrossRef]
- Prasad, R.; Shivay, Y. Scientific and Medical Research Support Can Increase Export Earnings from Turmeric (Curcuma longa). Natl. Acad. Sci. Lett. 2021, 44, 481–483. [Google Scholar] [CrossRef]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An Anti-Inflammatory Molecule from a Curry Spice on the Path to Cancer Treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [PubMed]
- Shirsath, S.R.; Sable, S.S.; Gaikwad, S.G.; Sonawane, S.H.; Saini, D.R.; Gogate, P.R. Intensification of Extraction of Curcumin from Curcuma Amada Using Ultrasound Assisted Approach: Effect of Different Operating Parameters. Ultrason. Sonochem. 2017, 38, 437–445. [Google Scholar] [CrossRef]
- Ciucă, M.; Racovita, R. Curcumin: Overview of Extraction Methods, Health Benefits, and Encapsulation and Delivery Using Microemulsions and Nanoemulsions. Int. J. Mol. Sci. 2023, 24, 8874. [Google Scholar] [CrossRef]
- Manasa, P.; Kamble, A.; Chilakamarthi, U. Various Extraction Techniques of Curcumin—A Comprehensive Review. ACS Omega 2023, 8, 34868–34878. [Google Scholar] [CrossRef]
- Wakte, P.S.; Sachin, B.S.; Patil, A.A.; Mohato, D.M.; Band, T.H.; Shinde, D.B. Optimization of Microwave, Ultra-Sonic and Supercritical Carbon Dioxide Assisted Extraction Techniques for Curcumin from Curcuma longa. Sep. Purif. Technol. 2011, 79, 50–55. [Google Scholar] [CrossRef]
- Jiang, T.; Ghosh, R.; Charcosset, C. Extraction, Purification and Applications of Curcumin from Plant Materials—A Comprehensive Review. Trends Food Sci. Technol. 2021, 112, 419–430. [Google Scholar] [CrossRef]
- Schuh, L.; Reginato, M.; Florêncio, I.; Falcao, L.; Boron, L.; Gris, E.F.; Mello, V.; Báo, S.N. From Nature to Innovation: The Uncharted Potential of Natural Deep Eutectic Solvents. Molecules 2023, 28, 7653. [Google Scholar] [CrossRef]
- Radošević, K.; Canak, I.; Panić, M.; Markov, K.; Cvjetko Bubalo, M.; Frece, J.; Srček, V.; Radojcic Redovnikovic, I. Antimicrobial, Cytotoxic and Antioxidative Evaluation of Natural Deep Eutectic Solvents. Environ. Sci. Pollut. Res. 2018, 25, 14188–14196. [Google Scholar] [CrossRef]
- Wen, Q.; Chen, J.-X.; Tang, Y.-L.; Wang, J.; Yang, Z. Assessing the Toxicity and Biodegradability of Deep Eutectic Solvents. Chemosphere 2015, 132, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Ćurko, N.; Gaurina Srček, V.; Cvjetko Bubalo, M.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Natural Deep Eutectic Solvents as Beneficial Extractants for Enhancement of Plant Extracts Bioactivity. LWT 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Torres-Cornejo, M.; Alvarez-Rivera, G.; Mendiola, J. Deep Eutectic Solvents for the Extraction of Bioactive Compounds from Natural Sources and Agricultural By-Products. Appl. Sci. 2021, 11, 4897. [Google Scholar] [CrossRef]
- Ivkovic, D.; Cvijetić, I.; Radoicic, A.; Stojkovic-Filipovic, J.; Trifkovic, J.; Krstic Ristivojevic, M.; Ristivojevic, P. NADES-Based Extracts of Selected Medicinal Herbs as Promising Formulations for Cosmetic Usage. Processes 2024, 12, 992. [Google Scholar] [CrossRef]
- Doldolova, K.; Bener, M.; Lalikoğlu, M.; Aşçı, Y.S.; Arat, R.; Apak, R. Optimization and Modeling of Microwave-Assisted Extraction of Curcumin and Antioxidant Compounds from Turmeric by Using Natural Deep Eutectic Solvents. Food Chem. 2021, 353, 129337. [Google Scholar] [CrossRef]
- Benvenutti, L.; Sanchez-Camargo, A.d.P.; Zielinski, A.A.F.; Ferreira, S.R.S. NADES as Potential Solvents for Anthocyanin and Pectin Extraction from Myrciaria Cauliflora Fruit By-Product: In Silico and Experimental Approaches for Solvent Selection. J. Mol. Liq. 2020, 315, 113761. [Google Scholar] [CrossRef]
- Huber, V.; Muller, L.; Degot, P.; Touraud, D.; Kunz, W. NADES-Based Surfactant-Free Microemulsions for Solubilization and Extraction of Curcumin from Curcuma longa. Food Chem. 2021, 355, 129624. [Google Scholar] [CrossRef]
- Rodsamai, T.; Chaijan, M.; Nisoa, M.; Donlao, N.; Rawdkuen, S.; Chunglok, W.; Cheong, L.-Z.; Panpipat, W. Improved Curcumin Recovery and In Vitro Biological Activity of Turmeric Extracts Using Nipa Palm Syrup– and Nipa Palm Vinegar–Based Natural Deep Eutectic Solvent (NADES) Hybridized with Microwave-Assisted Extraction. Food Bioprocess Technol. 2024, 17, 2009–2022. [Google Scholar] [CrossRef]
- Zannou, O.; Pashazadeh, H.; Ghellam, M.; Ibrahim, S.A.; Koca, I. Extraction of Anthocyanins from Borage (Echium Amoenum) Flowers Using Choline Chloride and a Glycerol-Based, Deep Eutectic Solvent: Optimization, Antioxidant Activity, and In Vitro Bioavailability. Molecules 2022, 27, 134. [Google Scholar] [CrossRef]
- Ferreira, A.S.D.; Craveiro, R.; Duarte, A.R.; Barreiros, S.; Cabrita, E.J.; Paiva, A. Effect of Water on the Structure and Dynamics of Choline Chloride/Glycerol Eutectic Systems. J. Mol. Liq. 2021, 342, 117463. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of Polyphenolic Antioxidants from Orange Peel Waste Using Deep Eutectic Solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Tarikuzzaman, M.; Sagar, V.; Wong, M.J.; Lynam, J.G. Temperature Effects on Physiochemical Characteristics of Sugar-Based Natural Deep Eutectic Solvents. Adv. Mater. Sci. Eng. 2024, 2024, 6641317. [Google Scholar] [CrossRef]
- El Achkar, T.; Fourmentin, S.; Greige-Gerges, H. Deep Eutectic Solvents: An Overview on Their Interactions with Water and Biochemical Compounds. J. Mol. Liq. 2019, 288, 111028. [Google Scholar] [CrossRef]
- Al-Risheq, D.I.M.; Nasser, M.S.; Qiblawey, H.; Hussein, I.A.; Benamor, A. Choline Chloride Based Natural Deep Eutectic Solvent for Destabilization and Separation of Stable Colloidal Dispersions. Sep. Purif. Technol. 2021, 255, 117737. [Google Scholar] [CrossRef]
- Cruz-Reyes, I.G.; Mendoza-Pérez, J.A.; Ruiz-Guerrero, R.; Medina-Velázquez, D.Y.; Zepeda-Vallejo, L.G.; Morales-Ramírez, Á.D.J. Kinetics of Zn–C Battery Leaching with Choline Chloride/Urea Natural Deep Eutectic Solvents. Recycling 2022, 7, 86. [Google Scholar] [CrossRef]
- Palos-Hernández, A.; Gutiérrez Fernández, M.Y.; Escuadra Burrieza, J.; Pérez-Iglesias, J.L.; González-Paramás, A.M. Obtaining Green Extracts Rich in Phenolic Compounds from Underexploited Food By-Products Using Natural Deep Eutectic Solvents. Opportunities and Challenges. Sustain. Chem. Pharm. 2022, 29, 100773. [Google Scholar] [CrossRef]
- Koh, Q.Q.; Kua, Y.L.; Gan, S.; Tan, K.W.; Lee, T.Z.E.; Cheng, W.K.; Lau, H.L.N. Sugar-Based Natural Deep Eutectic Solvent (NADES): Physicochemical Properties, Antimicrobial Activity, Toxicity, Biodegradability and Potential Use as Green Extraction Media for Phytonutrients. Sustain. Chem. Pharm. 2023, 35, 101218. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring Properties of Natural Deep Eutectic Solvents with Water to Facilitate Their Applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Lazović, M.Č.; Jović, M.D.; Petrović, M.; Dimkić, I.Z.; Gašić, U.M.; Milojković Opsenica, D.M.; Ristivojević, P.M.; Trifković, J.Đ. Potential Application of Green Extracts Rich in Phenolics for Innovative Functional Foods: Natural Deep Eutectic Solvents as Media for Isolation of Biocompounds from Berries. Food Funct. 2024, 15, 4122–4139. [Google Scholar] [CrossRef]
- Park, C.Y.; Lee, K.-Y.; Gul, K.; Rahman, M.S.; Kim, A.-N.; Chun, J.; Kim, H.-J.; Choi, S.-G. Phenolics and Antioxidant Activity of Aqueous Turmeric Extracts as Affected by Heating Temperature and Time. LWT 2019, 105, 149–155. [Google Scholar] [CrossRef]
- Akter, J.; Hossain, M.A.; Takara, K.; Islam, M.Z.; Hou, D.-X. Antioxidant Activity of Different Species and Varieties of Turmeric (Curcuma spp.): Isolation of Active Compounds. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 215, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Coscarella, M.; Nardi, M.; Alipieva, K.; Bonacci, S.; Popova, M.; Procopio, A.; Scarpelli, R.; Simeonov, S. Alternative Assisted Extraction Methods of Phenolic Compounds Using NaDESs. Antioxidants 2024, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef]
- Smiljanić, K.; Prodić, I.; Trifunovic, S.; Krstić Ristivojević, M.; Aćimović, M.; Stanković Jeremić, J.; Lončar, B.; Tešević, V. Multistep Approach Points to Compounds Responsible for the Biological Activity and Safety of Hydrolates from Nine Lamiaceae Medicinal Plants on Human Skin Fibroblasts. Antioxidants 2023, 12, 1988. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Benkova, M.; Soukup, O.; Marek, J. Antimicrobial Susceptibility Testing: Currently Used Methods and Devices and the near Future in Clinical Practice. J. Appl. Microbiol. 2020, 129, 806–822. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Pawar, J.S.; Akhter, N.; Lucy, I.B. Conventional Methods and Future Trends in Antimicrobial Susceptibility Testing. Saudi J. Biol. Sci. 2023, 30, 103582. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Odo, E.O.; Ikwuegbu, J.A.; Obeagu, E.I.; Chibueze, S.A.; Ochiaka, R.E. Analysis of the Antibacterial Effects of Turmeric on Particular Bacteria. Medicine 2023, 102, e36492. [Google Scholar] [CrossRef]
- Jyotirmayee, B.; Mahalik, G. A Review on Selected Pharmacological Activities of Curcuma longa L. Int. J. Food Prop. 2022, 25, 1377–1398. [Google Scholar] [CrossRef]
- Bedair, H.M.; Samir, T.M.; Mansour, F.R. Antibacterial and Antifungal Activities of Natural Deep Eutectic Solvents. Appl. Microbiol. Biotechnol. 2024, 108, 198. [Google Scholar] [CrossRef] [PubMed]
- Morais, P.; Gonçalves, F.; Ventura, S. On the Ecotoxicity of Cholinium-Based Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2015, 3, 3398–3404. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Mazur, M.; Radošević, K.; Radojčić Redovniković, I. Baker’s Yeast-Mediated Asymmetric Reduction of Ethyl 3-Oxobutanoate in Deep Eutectic Solvents. Process Biochem. 2015, 50, 1788–1792. [Google Scholar] [CrossRef]
- Greenacre, E.; Brocklehurst, T.; Waspe, C.; Wilson, D.; Wilson, P. Salmonella Enterica Serovar Typhimurium and Listeria Monocytogenes Acid Tolerance Response Induced by Organic Acids at 20 C: Optimization and Modeling. Appl. Environ. Microbiol. 2003, 69, 3945–3951. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin Whitening Agents: Medicinal Chemistry Perspective of Tyrosinase Inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Rocchitta, G.; Rozzo, C.; Pisano, M.; Fabbri, D.; Dettori, M.A.; Ruzza, P.; Honisch, C.; Dallocchio, R.; Dessì, A.; Migheli, R.; et al. Inhibitory Effect of Curcumin-Inspired Derivatives on Tyrosinase Activity and Melanogenesis. Molecules 2022, 27, 7942. [Google Scholar] [CrossRef]
- Athipornchai, A.; Niyomtham, N.; Pabuprapap, W.; Ajavakom, V.; Duca, M.; Azoulay, S.; Suksamrarn, A. Potent Tyrosinase Inhibitory Activity of Curcuminoid Analogues and Inhibition Kinetics Studies. Cosmetics 2021, 8, 35. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Overview of Skin Whitening Agents: Drugs and Cosmetic Products. Cosmetics 2016, 3, 27. [Google Scholar] [CrossRef]
- Usuki, A.; Ohashi, A.; Sato, H.; Ochiai, Y.; Ichihashi, M.; Funasaka, Y. The Inhibitory Effect of Glycolic Acid and Lactic Acid on Melanin Synthesis in Melanoma Cells. Exp. Dermatol. 2003, 12, 43–50. [Google Scholar] [CrossRef]
- Ying, T.-H.; Chen, C.-W.; Hsiao, Y.-P.; Hung, S.-J.; Chung, J.-G.; Yang, J.-H. Citric Acid Induces Cell-Cycle Arrest and Apoptosis of Human Immortalized Keratinocyte Cell Line (HaCaT) via Caspase- and Mitochondrial-Dependent Signaling Pathways. Anticancer Res. 2013, 33, 4411–4420. [Google Scholar]
- Hsiao, Y.-P.; Huang, H.-L.; Lai, W.-W.; Chung, J.-G.; Yang, J.-H. Antiproliferative Effects of Lactic Acid via the Induction of Apoptosis and Cell Cycle Arrest in a Human Keratinocyte Cell Line (HaCaT). J. Dermatol. Sci. 2009, 54, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to Its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Priyadarshi, R.; Ezati, P.; Rhim, J.-W. Curcumin and Its Uses in Active and Smart Food Packaging Applications—A Comprehensive Review. Food Chem. 2022, 375, 131885. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.M.S.; da Silva, G.H.; Barros, P.P.; Srebernich, S.M.; Shiraishi, C.T.C.; de Camargos, V.R.; Lasca, T.B. Use of Curcuma longa in Cosmetics: Extraction of Curcuminoid Pigments, Development of Formulations, and in Vitro Skin Permeation Studies. Braz. J. Pharm. Sci. 2014, 50, 885–893. [Google Scholar] [CrossRef]
- Slaček, G.; Kotnik, P.; Osmić, A.; Postružnik, V.; Knez, Ž.; Finšgar, M.; Knez Marevci, M. The Extraction Process, Separation, and Identification of Curcuminoids from Turmeric Curcuma longa. Foods 2023, 12, 4000. [Google Scholar] [CrossRef]
- Jeliński, T.; Przybyłek, M.; Cysewski, P. Natural Deep Eutectic Solvents as Agents for Improving Solubility, Stability and Delivery of Curcumin. Pharm. Res. 2019, 36, 116. [Google Scholar] [CrossRef]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant Activity and Total Phenolic Content of Selected Jordanian Plant Species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. ISBN 0076-6879. [Google Scholar]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Kifle, Z.; Enyew, E. Evaluation of In Vivo Antidiabetic, In Vitro α-Amylase Inhibitory, and In Vitro Antioxidant Activity of Leaves Crude Extract and Solvent Fractions of Bersama Abyssinica Fresen (Melianthaceae). J. Evid.-Based Integr. Med. 2020, 25, 2515690X2093582. [Google Scholar] [CrossRef]
- Stojković, D.; Gašić, U.; Uba, A.I.; Zengin, G.; Rajaković, M.; Stevanović, M.; Drakulić, D. Chemical profiling of Anthriscus cerefolium (L.) Hoffm., biological potential of the herbal extract, molecular modeling and KEGG pathway analysis. Fitoterapia 2024, 177, 106115. [Google Scholar] [CrossRef]
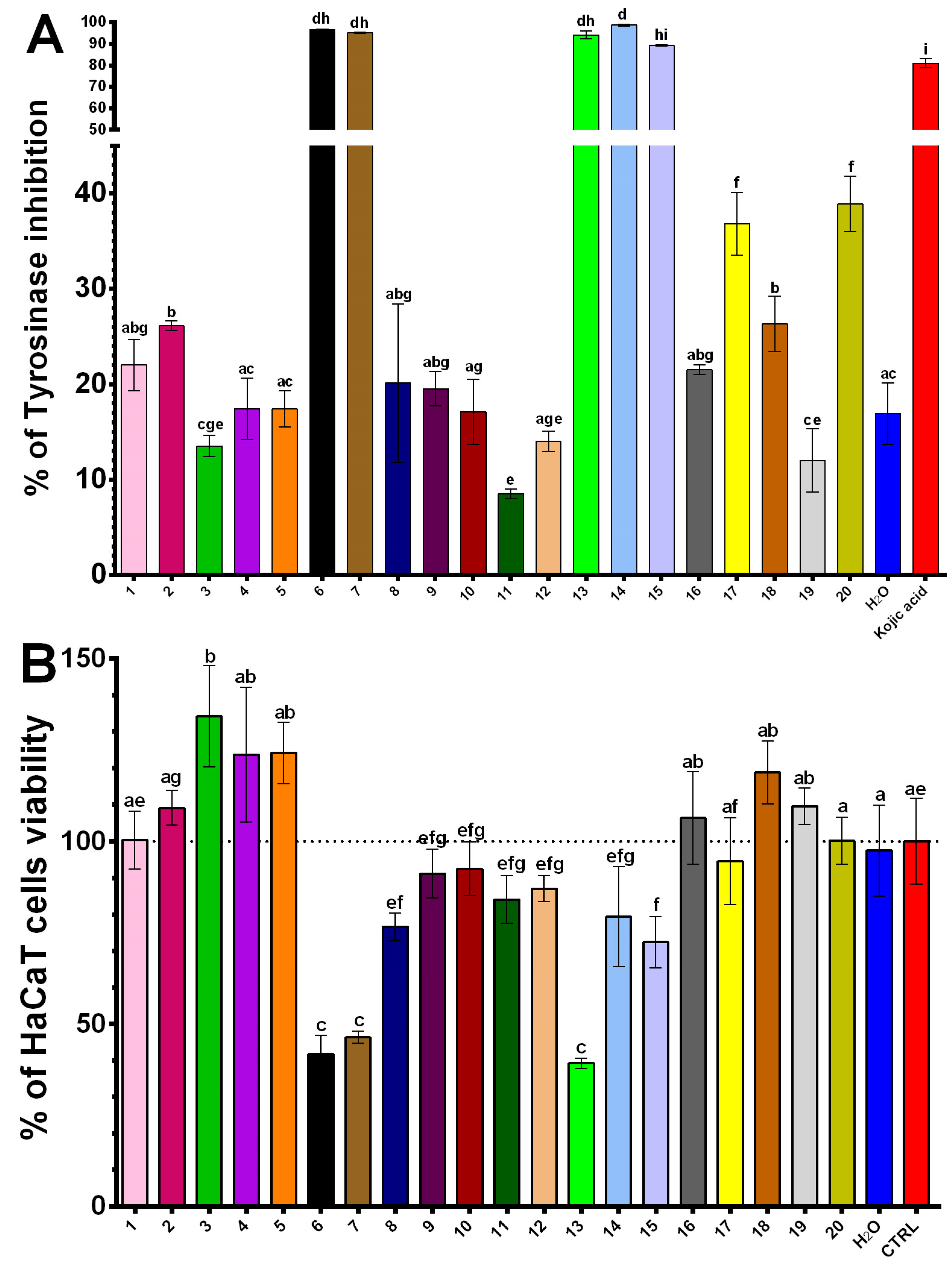
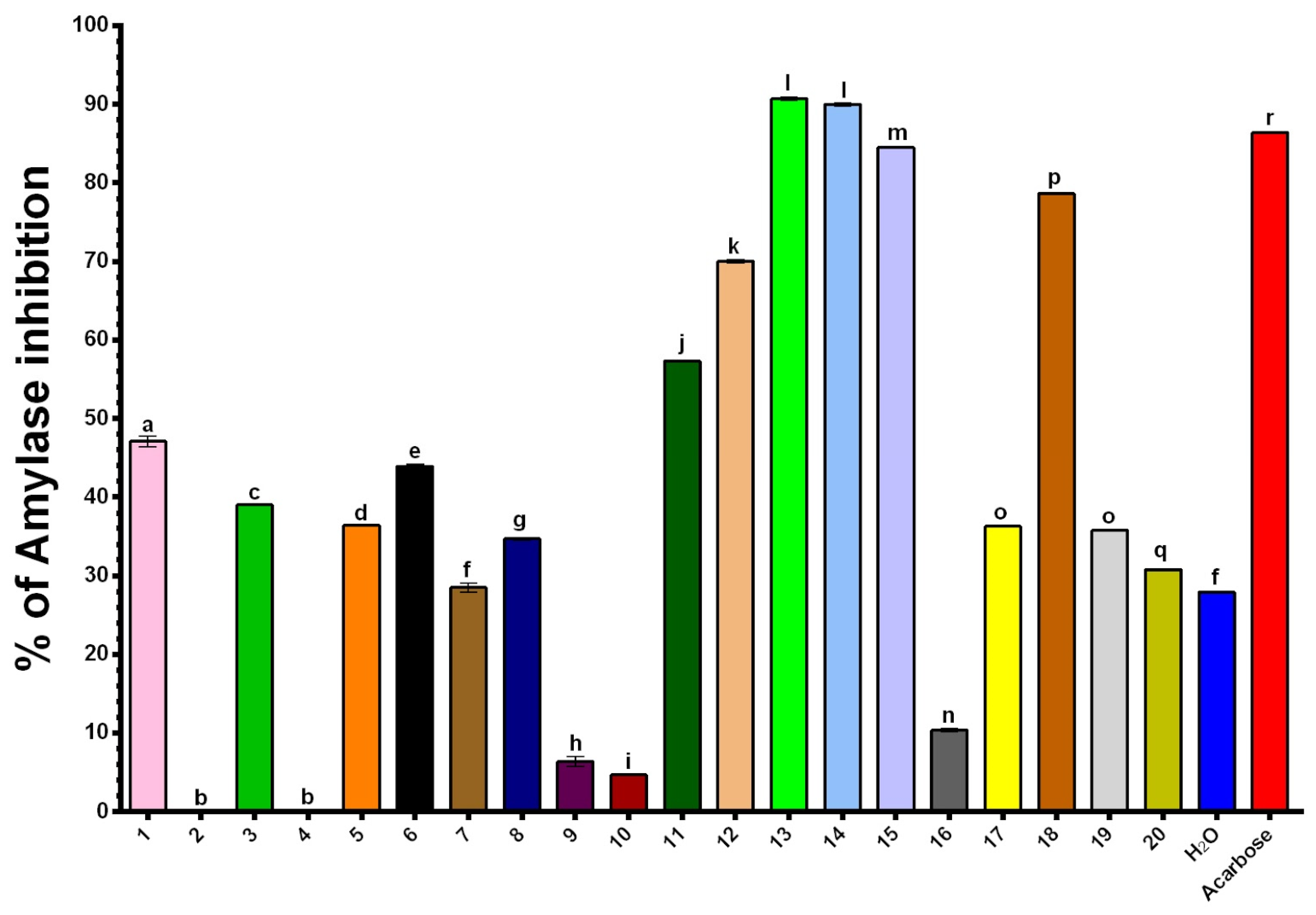
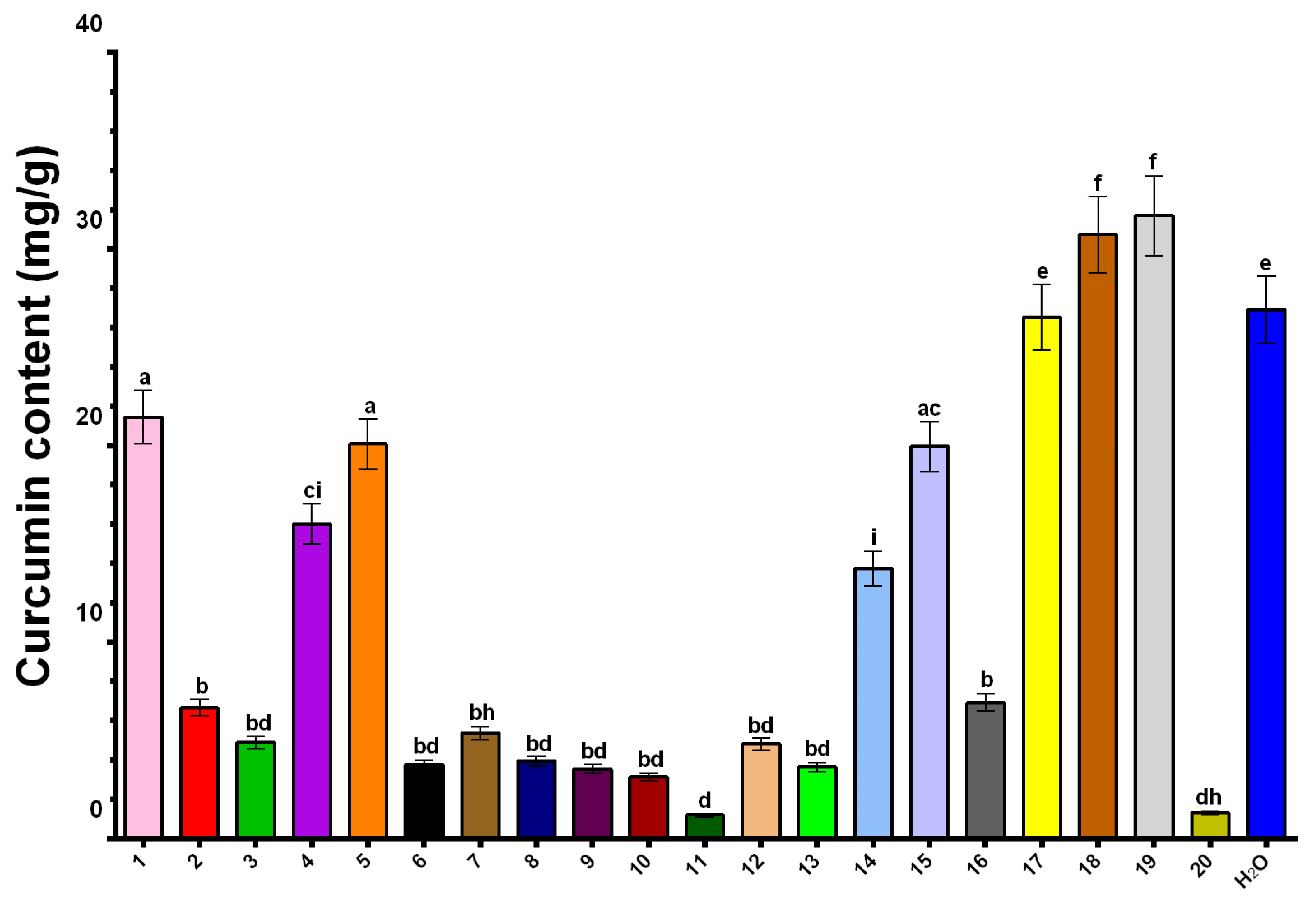
| Extract | NADES Composition | TPC (mg GAE/g) | DPPH (μmol TE/g) | ABTS (μmol TE/g) |
|---|---|---|---|---|
| NADES 1 | ChCl/Glycerol/Water 1:1:5 | 12.42 ± 0.13 a | 6.9 ± 0.5 ae | 0.49 ± 0.03 ad |
| NADES 2 | ChCl/Fructose/Water 1:1:5 | 54.77 ± 0.22 b | 8.5 ± 1.6 ae | 0.34 ± 0.02 ad |
| NADES 3 | ChCl/Glycerol/Water 1:2:5 | 13.97 ± 0.44 af | 4.88 ± 0.04 a | 4.25 ± 0.08 bf |
| NADES 4 | ChCl/Glucose/Water 1:1:3 | 6.92 ± 0.14 c | 17.4 ± 0.9 bcf | 4.20 ± 0.16 bf |
| NADES 5 | ChCl/Glycerol/Water 1:1:2 | 13.45 ± 0.36 af | 19.82 ± 1.02 cb | 4.29 ± 0.02 bf |
| NADES 6 | ChCl/Citric acid/Water 1:2:5 | 14.08 ± 0.09 af | 20.2 ± 3.2 bf | 3.94 ± 0.11 ce |
| NADES 7 | ChCl/Citric acid/Water 2:1:5 | 8.25 ± 0.10 cd | 20.4 ± 4.5 bcg | 4.18 ± 0.22 bf |
| NADES 8 | ChCl/Glycerol 1:1 | 9.41 ± 0.59 d | 7.6 ± 0.2 ae | 0.34 ± 0.23 ad |
| NADES 9 | ChCl/Glycerol 1:2 | 17.76 ± 0.17 e | 7.9 ± 2.9 ae | 4.09 ± 0.05 bf |
| NADES 10 | ChCl/Glycerol 1:3 | 14.88 ± 0.07 f | 8.6 ± 0.8 ae | 0.50 ± 0.04 ad |
| NADES 11 | Glycerol/Urea/Water 1:1:2 | 20.42 ± 0.06 g | 24.1 ± 0.6 cg | 4.74 ± 0.14 b |
| NADES 12 | ChCl/Xylitol/Water 1:1:5 | 12.74 ± 0.04 ak | 15.6 ± 3.8 bf | 1.65 ± 0.47 cd |
| NADES 13 | ChCl/Citric acid/Water 1:2:3 | 11.55 ± 0.92 k | 15.0 ± 0.5 bf | 3.45 ± 0.21 cefg |
| NADES 14 | ChCl/Lactic acid/Water 1:2:5 | 21.54 ± 0.19 gh | 16.4 ± 1.7 bf | 0.22 ± 0.05 ad |
| NADES 15 | ChCl/Citric acid/Water 1:1:5 | 15.75 ± 0.02 f | 22.1 ± 0.2 cg | 3.51 ± 0.19 cefg |
| NADES 16 | ChCl/Urea/Water 1:2:5 | 18.02 ± 0.09 e | 17.7 ± 0.2 bf | 3.00 ± 0.02 e |
| NADES 17 | ChCl/Glycerol/Citric acid/Water 0.5:2:0.5:5 | 20.36 ± 0.06 g | 30.5 ± 1.0 d | 4.62 ± 0.07 b |
| NADES 18 | ChCl/1,2-propanediol/Water 1:1:1 | 17.11 ± 0.01e | 11.7 ± 0.8 ef | 1.04 ± 0.03 d |
| NADES 19 | Glycerol/Betaine/Water 1:1:3 | 22.80 ± 0.18 hj | 11.3 ± 0.3 af | 4.35 ± 0.03 bg |
| NADES 20 | Glycerol/Lysine/Water 1:1:3 | 36.84 ± 0.13 i | 29.6 ± 0.3 d | 4.64 ± 0.03 b |
| H2O | 24.07 ± 1.68 j | 26.6 ± 0.3 dg | 4.18 ± 0.89 bg |
| Extract | NADES Composition | S. typhimurium ATCC 14028 | L. monocytogenes ATCC 13932 |
|---|---|---|---|
| NADES 1 | ChCl/Glycerol/Water 1:1:5 | – | – |
| NADES 2 | ChCl/Fructose/Water 1:1:5 | – | – |
| NADES 3 | ChCl/Glycerol/Water 1:2:5 | – | – |
| NADES 4 | ChCl/Glucose/Water 1:1:3 | 23.5 ± 1.5 | – |
| NADES 5 | ChCl/Glycerol/Water 1:1:2 | – | – |
| NADES 6 | ChCl/Citric acid/Water 1:2:5 | 27.0 ± 1.0 | 35.0 ± 1.0 |
| NADES 7 | ChCl/Citric acid/Water 2:1:5 | 24.5 ± 1.0 | 35.0 ± 1.0 |
| NADES 8 | ChCl/Glycerol 1:1 | – | – |
| NADES 9 | ChCl/Glycerol 1:2 | – | – |
| NADES 10 | ChCl/Glycerol 1:3 | – | – |
| NADES 11 | Glycerol/Urea/Water 1:1:2 | – | 11.0 ± 0.0 |
| NADES 12 | ChCl/Xylitol/Water 1:1:5 | – | 12.0 ± 0.5 |
| NADES 13 | ChCl/Citric acid/Water 1:2:3 | 25.0 ± 1.0 | 34.0 ± 2.0 |
| NADES 14 | ChCl/Lactic acid/Water 1:2:5 | – | – |
| NADES 15 | ChCl/Citric acid/Water 1:1:5 | 21.0 ± 1.5 | 35.0 ± 1.0 |
| NADES 16 | ChCl/Urea/Water 1:2:5 | – | 11.0 ± 0.0 |
| NADES 17 | ChCl/Glycerol/Citric acid/Water 0.5:2:0.5:5 | 15.0 ± 0.5 | 33.0 ± 1.0 |
| NADES 18 | ChCl/1,2-propanediol/Water 1:1:1 | – | 12.5 ± 0.5 |
| NADES 19 | Glycerol/Betaine/Water 1:1:3 | – | 12.0 ± 1.0 |
| NADES 20 | Glycerol/Lysine/Water 1:1:3 | – | – |
| H2O | – | – | |
| Streptomycin | 23.0 ± 1.0 | 26.5 ± 1.5 |
| Abbreviation | NADES Composition | Molar Ratio |
|---|---|---|
| NADES 1 | ChCl/Glycerol/Water | 1:1:5 |
| NADES 2 | ChCl/Fructose/Water | 1:1:5 |
| NADES 3 | ChCl/Glycerol/Water | 1:2:5 |
| NADES 4 | ChCl/Glucose/Water | 1:1:3 |
| NADES 5 | ChCl/Glycerol/Water | 1:1:2 |
| NADES 6 | ChCl/Citric acid/Water | 1:2:5 |
| NADES 7 | ChCl/Citric acid/Water | 2:1:5 |
| NADES 8 | ChCl/Glycerol | 1:1 |
| NADES 9 | ChCl/Glycerol | 1:2 |
| NADES 10 | ChCl/Glycerol | 1:3 |
| NADES 11 | Glycerol/urea/Water | 1:1:2 |
| NADES 12 | ChCl/Xylitol/Water | 1:1:5 |
| NADES 13 | ChCl/Citric acid/Water | 1:2:3 |
| NADES 14 | ChCl/Lactic acid/Water | 1:2:5 |
| NADES 15 | ChCl/Citric acid/Water | 1:1:5 |
| NADES 16 | ChCl/Urea/Water | 1:2:5 |
| NADES 17 | ChCl/Glycerol/Citric acid/Water | 0.5:2:0.5:5 |
| NADES 18 | ChCl/1,2-propanediol/Water | 1:1:1 |
| NADES 19 | Glycerol/Betaine/Water | 1:1:3 |
| NADES 20 | Glycerol/Lysine/Water | 1:1:3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanović, J.; Jović, M.; Trifković, J.; Smiljanić, K.; Gašić, U.; Krstić Ristivojević, M.; Ristivojević, P. Green Extraction of Bioactives from Curcuma longa Using Natural Deep Eutectic Solvents: Unlocking Antioxidative, Antimicrobial, Antidiabetic, and Skin Depigmentation Potentials. Plants 2025, 14, 163. https://doi.org/10.3390/plants14020163
Jovanović J, Jović M, Trifković J, Smiljanić K, Gašić U, Krstić Ristivojević M, Ristivojević P. Green Extraction of Bioactives from Curcuma longa Using Natural Deep Eutectic Solvents: Unlocking Antioxidative, Antimicrobial, Antidiabetic, and Skin Depigmentation Potentials. Plants. 2025; 14(2):163. https://doi.org/10.3390/plants14020163
Chicago/Turabian StyleJovanović, Jelena, Marko Jović, Jelena Trifković, Katarina Smiljanić, Uroš Gašić, Maja Krstić Ristivojević, and Petar Ristivojević. 2025. "Green Extraction of Bioactives from Curcuma longa Using Natural Deep Eutectic Solvents: Unlocking Antioxidative, Antimicrobial, Antidiabetic, and Skin Depigmentation Potentials" Plants 14, no. 2: 163. https://doi.org/10.3390/plants14020163
APA StyleJovanović, J., Jović, M., Trifković, J., Smiljanić, K., Gašić, U., Krstić Ristivojević, M., & Ristivojević, P. (2025). Green Extraction of Bioactives from Curcuma longa Using Natural Deep Eutectic Solvents: Unlocking Antioxidative, Antimicrobial, Antidiabetic, and Skin Depigmentation Potentials. Plants, 14(2), 163. https://doi.org/10.3390/plants14020163












