Assessment of the Photosynthetic Response of Potato Plants Inoculated with Rhizoctonia solani and Treated with Flesh-Colored Potato Extracts Nanoencapsulated with Solid Lipid Nanoparticles
Abstract
1. Introduction
2. Results
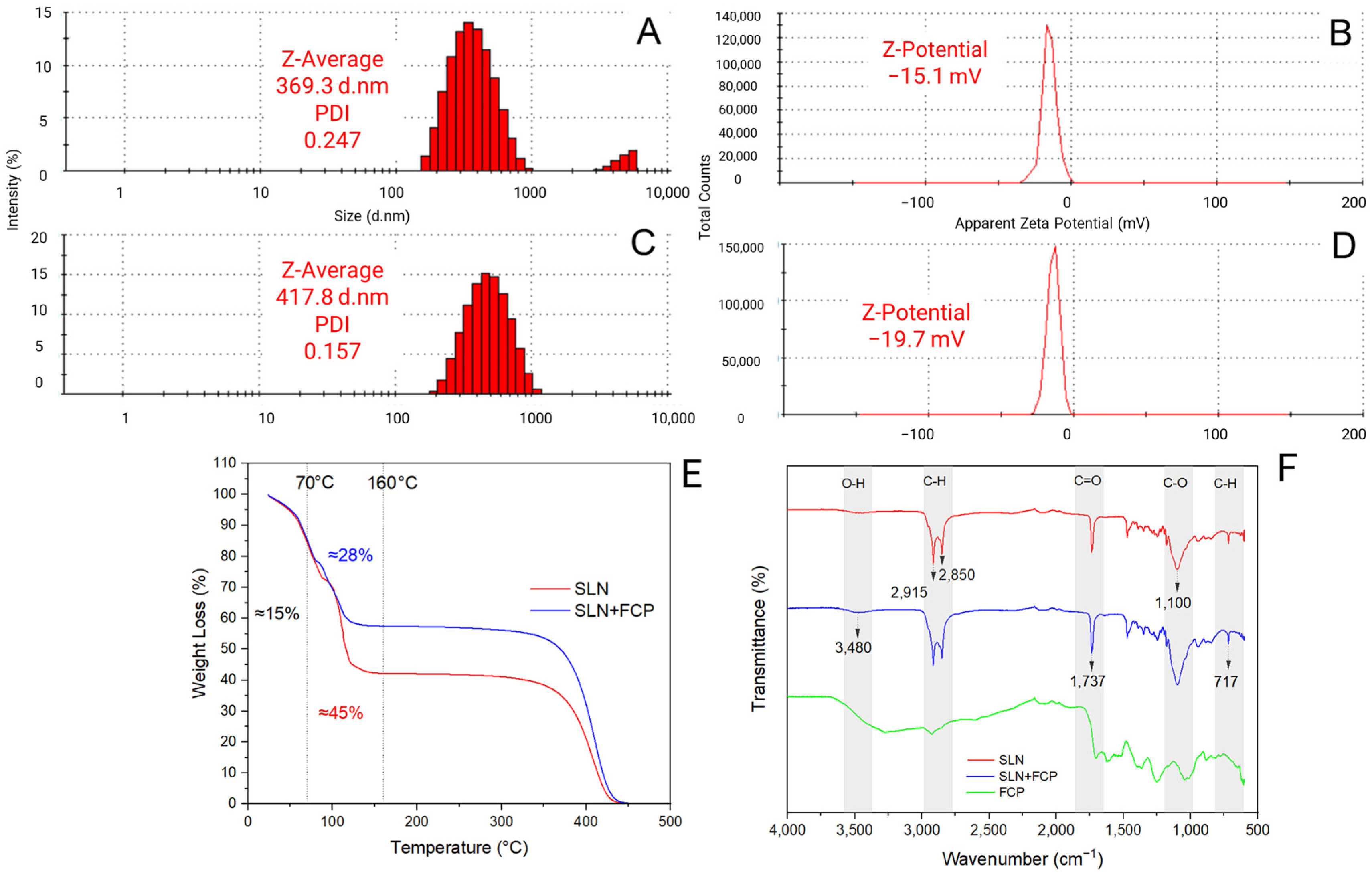
2.1. Physicochemical Characterization of Solid Lipid Nanoparticles Loaded with Flesh-Colored Potato Extract
2.2. Evaluation of Photosynthetic Response
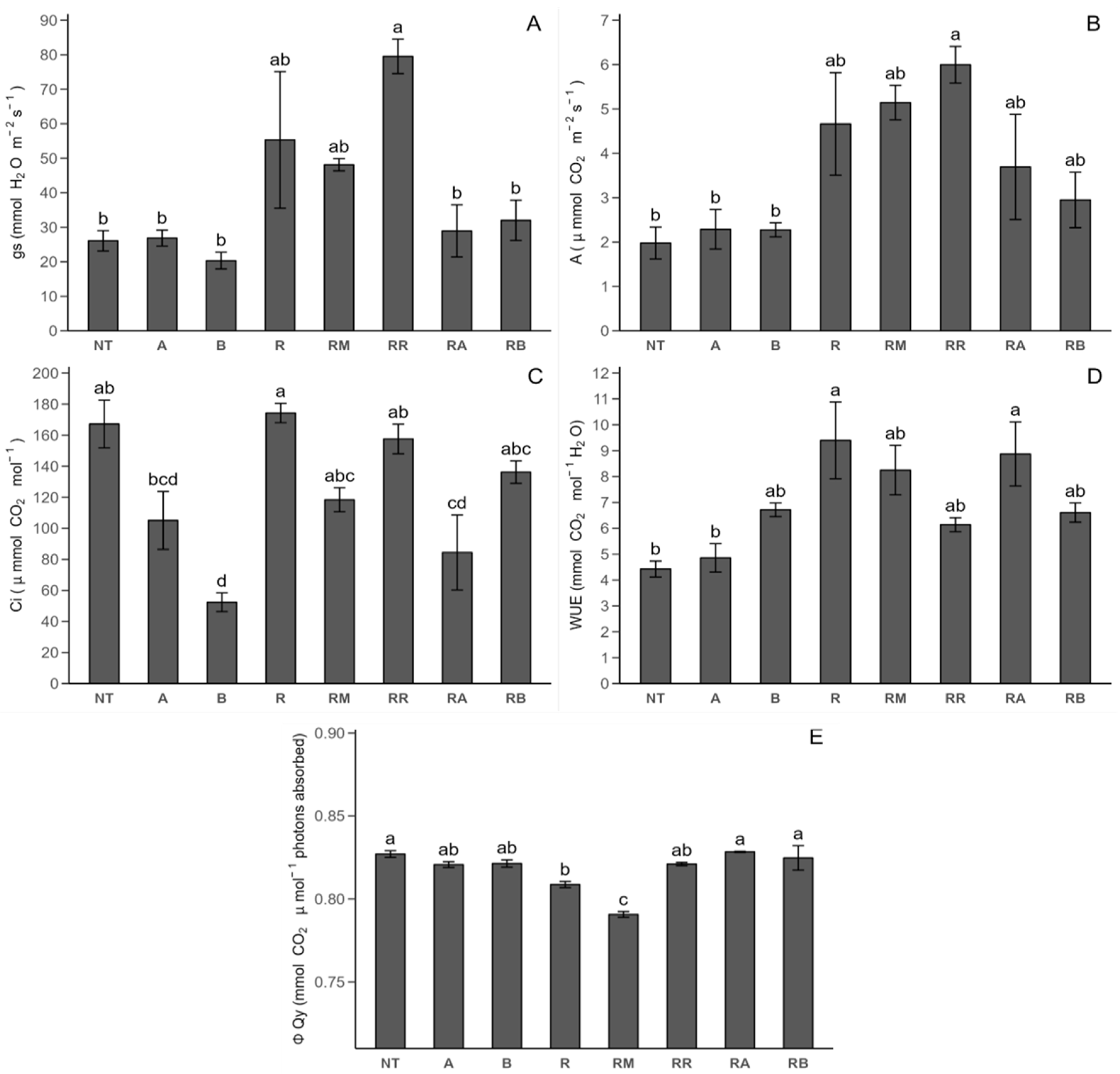
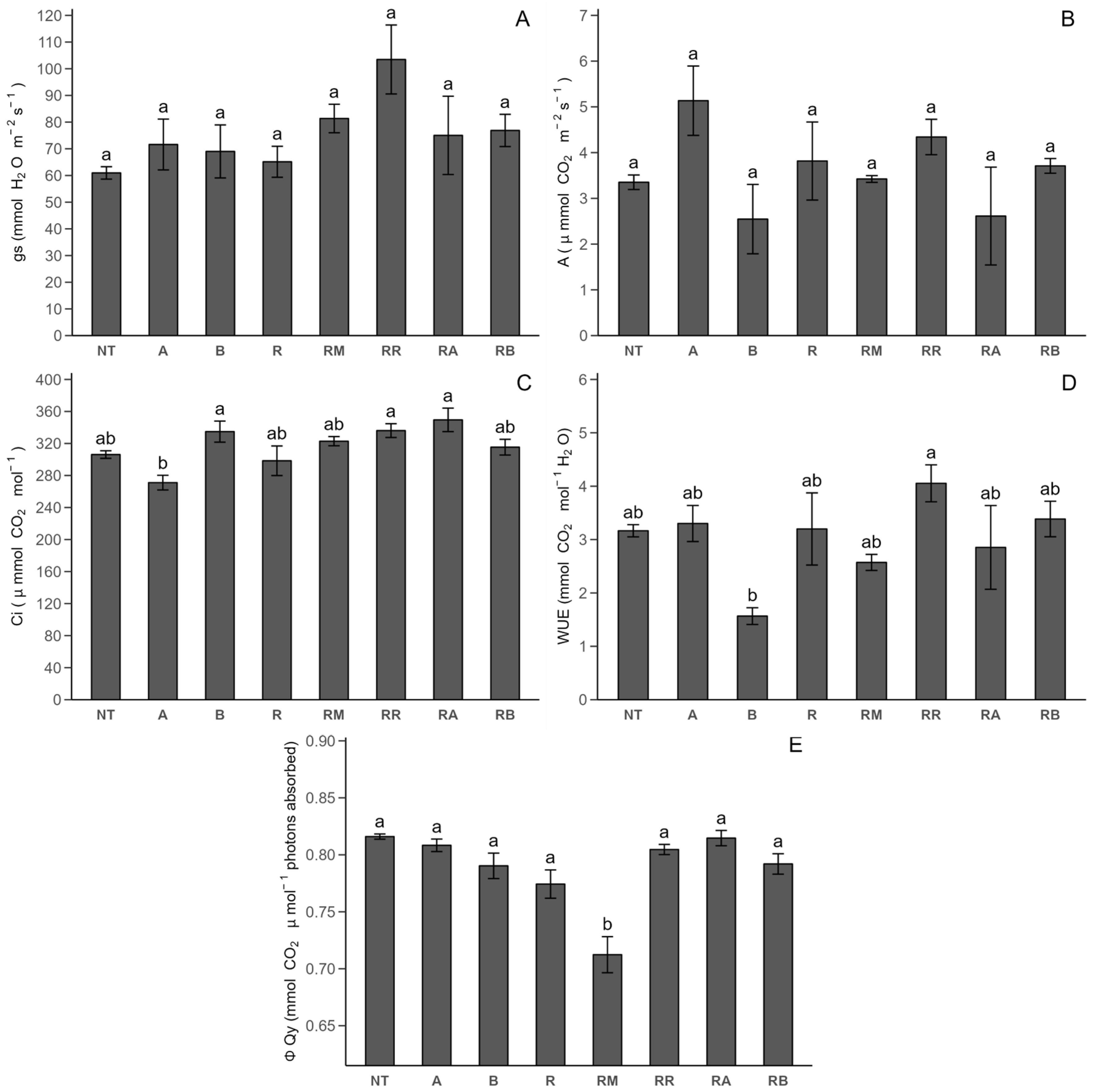
2.2.1. Photosynthetic Parameters
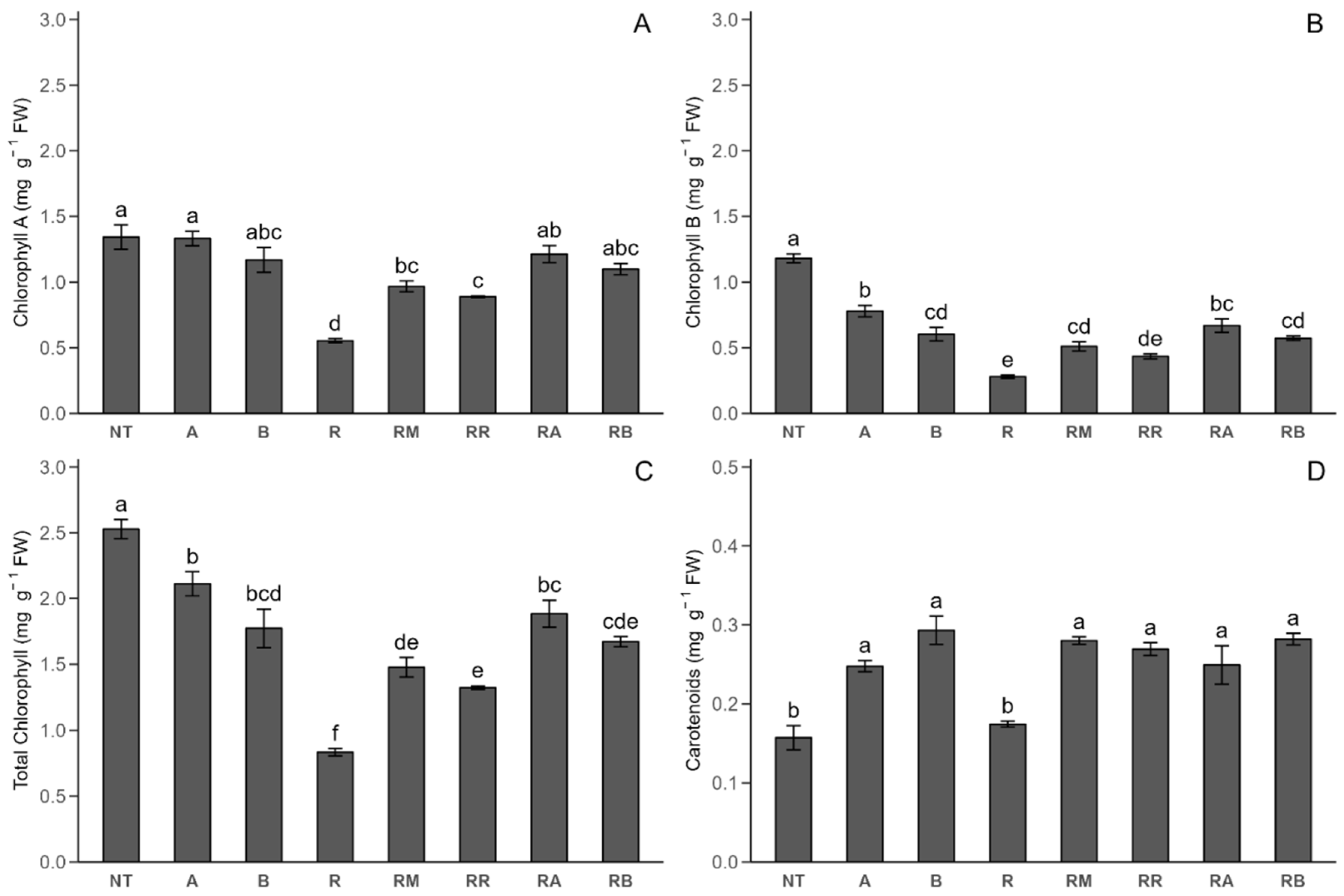
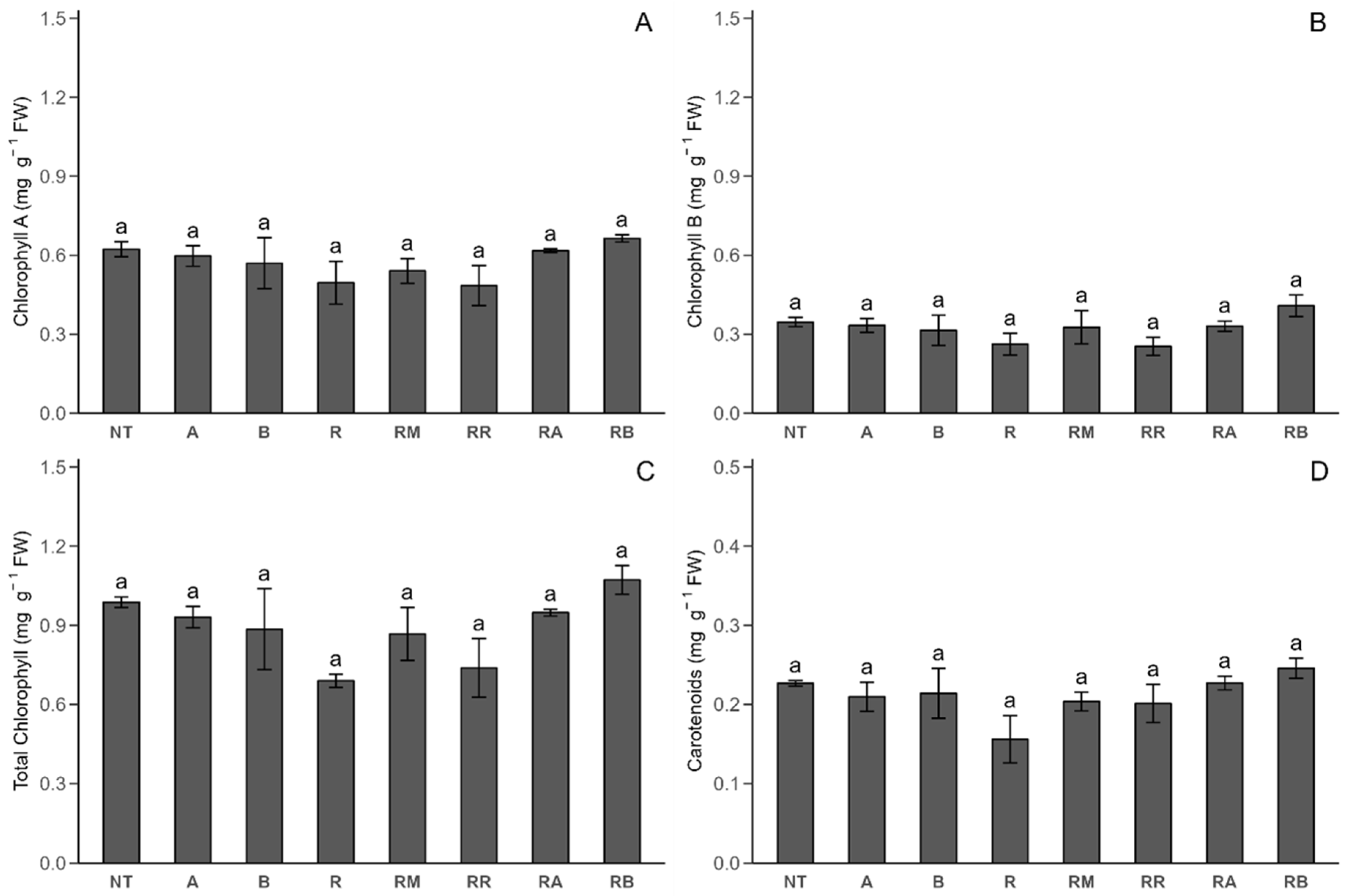
2.2.2. Photosynthetic Pigments
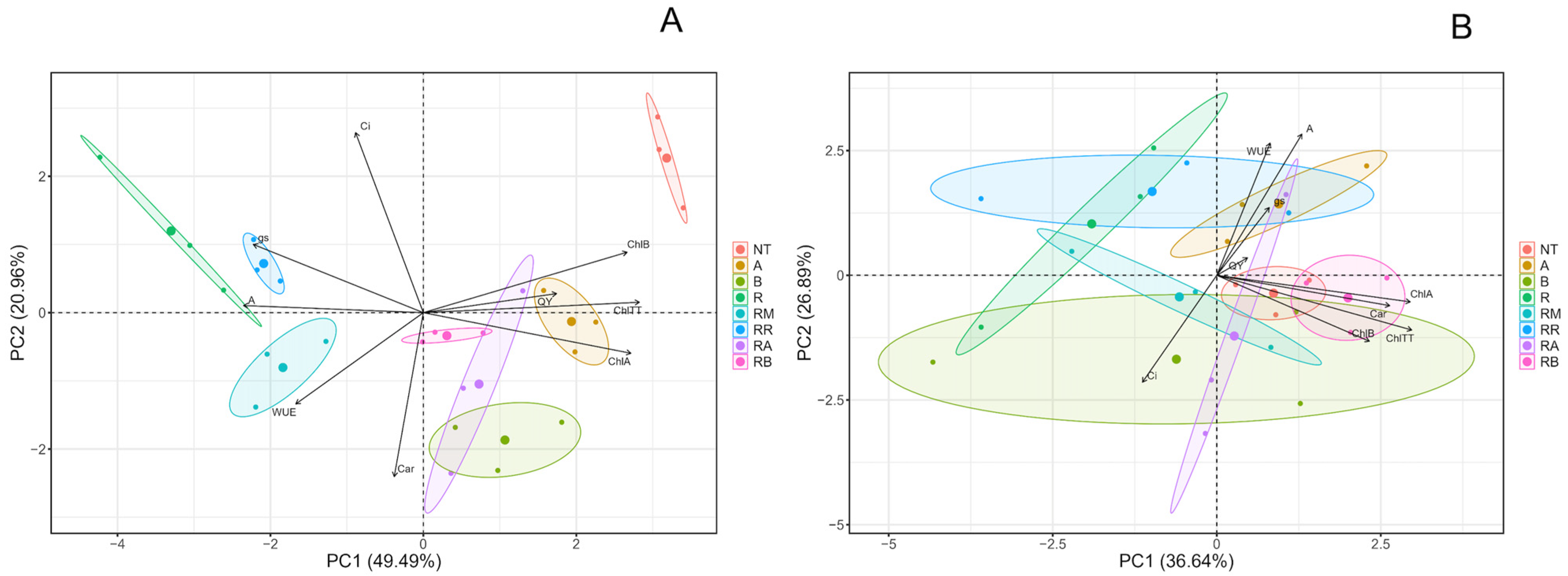
2.3. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Preparation of Flesh-Colored Potato Extract
4.3. Formulation of Solid Lipid Nanoparticles Loaded with Flesh-Colored Potato Extract
4.4. Physicochemical Characterization of Solid Lipid Nanoparticle Formulations
4.5. Greenhouse Application of the Nanoencapsulated Extract in Potato Plants Inoculated with Rhizoctonia solani
4.6. Photosynthetic Determination
4.7. Photosynthetic Pigment Contents in Leaves
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, C.; He, Y.; Zhang, W.; He, J. Potato Proteins for Technical Applications: Nutrition, Isolation, Modification and Functional Properties—A Review. Innov. Food Sci. Emerg. Technol. 2024, 91, 103533. [Google Scholar] [CrossRef]
- FAO FAOSTAT Commodities by Country/Chile. Available online: https://www.fao.org/faostat/en/#rankings/commodities_by_country (accessed on 9 October 2024).
- Abbou, M.; Chabbi, M.; Benicha, M. Assessment of Phytosanitary Practices on the Environment: Case Study Potato of Loukkos (Northwest Morocco). Environ. Monit. Assess. 2023, 195, 352. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, S.L.; Petropoulos, S.A.; Alexopoulos, A.; Heleno, S.A.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C.F.R. Potato Peels as Sources of Functional Compounds for the Food Industry: A Review. Trends Food Sci. Technol. 2020, 103, 118–129. [Google Scholar] [CrossRef]
- Hao, J.; Ashley, K. Irreplaceable Role of Amendment-Based Strategies to Enhance Health and Disease Suppression in Potato Production. Microorganisms 2021, 9, 1660. [Google Scholar] [CrossRef]
- Akber, M.A.; Fang, X. Research Progress on Diseases Caused by the Soil-Borne Fungal Pathogen Rhizoctonia solani in Alfalfa. Agronomy 2024, 14, 1483. [Google Scholar] [CrossRef]
- Ghazala, I.; Charfeddine, M.; Charfeddine, S.; Haddar, A.; Ellouz-Chaabouni, S.; Gargouri-Bouzid, R. Lipopeptides from Bacillus mojavensis I4 Confer Induced Tolerance toward Rhizoctonia solani in Potato (Solanum tuberosum). Physiol. Mol. Plant Pathol. 2022, 121, 101895. [Google Scholar] [CrossRef]
- Ajayi-Oyetunde, O.O.; Bradley, C.A. Rhizoctonia solani: Taxonomy, Population Biology and Management of Rhizoctonia Seedling Disease of Soybean. Plant Pathol. 2018, 67, 3–17. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Lu, J.; Min, F.; Guo, M.; Qi, W.; Wang, W.; Dong, X.; Mao, Y.; Hu, L.; et al. Potato Calcineurin B-like Protein CBL4, Interacting with Calcineurin B-like Protein-Interacting Protein Kinase CIPK2, Positively Regulates Plant Resistance to Stem Canker Caused by Rhizoctonia solani. Front. Microbiol. 2023, 13, 1032900. [Google Scholar] [CrossRef]
- Sedlák, P.; Sedláková, V.; Doležal, P.; Baštová, P.; Vašek, J.; Hausvater, E. Foliar Application of Fungicides Registered Against Late Blight Influences Main Potato Tuber Diseases and Key Quantitative Characteristics of Tubers. Potato Res. 2022, 65, 171–191. [Google Scholar] [CrossRef]
- Alaoui, K.; Chafik, Z.; Kharmach, E.Z. Fungicides against Mildew Case of Potato (Solanum tuberosum L.) from the Triffa Plain, Irrigated Area of Moulouya. E3S Web Conf. 2021, 240, 01004. [Google Scholar] [CrossRef]
- Gómez, F.; Bravo, C.; Ringler, I.; Santander, C.; González, F.; Viscarra, F.; Mardones, C.; Contreras, B.; Cornejo, P.; Ruiz, A. Evaluation of the Antifungal Potential of Grape Cane and Flesh-Coloured Potato Extracts against Rhizoctonia sp. in Solanum tuberosum Crops. Plants 2023, 12, 2974. [Google Scholar] [CrossRef] [PubMed]
- Ercoli, S.; Parada, J.; Bustamante, L.; Hermosín-Gutiérrez, I.; Contreras, B.; Cornejo, P.; Ruiz, A. Noticeable Quantities of Functional Compounds and Antioxidant Activities Remain after Cooking of Colored Fleshed Potatoes Native from Southern Chile. Molecules 2021, 26, 314. [Google Scholar] [CrossRef] [PubMed]
- Ercoli, S.; Cartes, J.; Cornejo, P.; Tereucán, G.; Winterhalter, P.; Contreras, B.; Ruiz, A. Stability of Phenolic Compounds, Antioxidant Activity and Colour Parameters of a Coloured Extract Obtained from Coloured-Flesh Potatoes. LWT–Food Sci. Technol. 2021, 136, 110370. [Google Scholar] [CrossRef]
- Sharma, R.J.; Gupta, R.C.; Singh, S.; Bansal, A.K.; Singh, I.P. Stability of Anthocyanins- and Anthocyanidins-Enriched Extracts, and Formulations of Fruit Pulp of Eugenia Jambolana (‘jamun’). Food Chem. 2016, 190, 808–817. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, J.; Yang, X. Deformable Nanocarriers for Enhanced Drug Delivery and Cancer Therapy. Exploration 2024, 4, 20230037. [Google Scholar] [CrossRef]
- Peng, S.; Yuan, X.; Li, H.; Wei, Y.; Zhou, B.; Ding, G.; Bai, J. Recent Progress in Nanocarrier-Based Drug Delivery Systems for Antitumour Metastasis. Eur. J. Med. Chem. 2023, 252, 115259. [Google Scholar] [CrossRef]
- Oktarina, H.; Woodhall, J.; Singleton, I. The Potential of Silver Nanoparticles to Control Rhizoctonia solani (AG3-PT) Growth in Vitro. J. Nat. 2021, 21, 1–4. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid Lipid Nanoparticles: Production, Characterization and Applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Borges, A.; de Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef]
- Hosseinpour, F.; Moravvej, G.; Modarres Awal, M.; Golmohammadzadeh, S. Insecticidal Activity of Solid Lipid Nanoparticle Loaded by Ziziphora clinopodioides Lam. against Tribolium castaneum (Herbst, 1797) (Coleoptera: Tenebrionidae). Int. J. Pest Manag. 2020, 67, 147–154. [Google Scholar] [CrossRef]
- Campos, E.; De Oliveira, J.L.; Da Silva, C.M.G.; Pascoli, M.; Pasquoto, T.; Lima, R.; Abhilash, P.C.; Fernandes Fraceto, L. Polymeric and Solid Lipid Nanoparticles for Sustained Release of Carbendazim and Tebuconazole in Agricultural Applications. Sci. Rep. 2015, 5, 13809. [Google Scholar] [CrossRef] [PubMed]
- Gikas, G.; Parlakidis, P.; Mavropoulos, T.; Vryzas, Z. Particularities of Fungicides and Factors Affecting Their Fate and Removal Efficacy: A Review. Sustainability 2022, 14, 4056. [Google Scholar] [CrossRef]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An Overlooked Pesticide Class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef]
- Semenova, N.A.; Burmistrov, D.E.; Shumeyko, S.A.; Gudkov, S.V. Fertilizers Based on Nanoparticles as Sources of Macro- and Microelements for Plant Crop Growth: A Review. Agronomy 2024, 14, 1646. [Google Scholar] [CrossRef]
- Cayún, Y.; Alarcón, S.; Tereucán, G.; Cornejo, P.; Santander, C.; Gómez, F.; Contreras, B.; Ruiz, A. Effect of Arbuscular Mycorrhizal Fungi Inoculation on the Metabolic Activity of Solanum tuberosum Plants Under Fungicide Application. J. Soil Sci. Plant Nutr. 2023, 23, 3623–3639. [Google Scholar] [CrossRef]
- Yang, H.; Luo, P. Changes in Photosynthesis Could Provide Important Insight into the Interaction between Wheat and Fungal Pathogens. Int. J. Mol. Sci. 2021, 22, 8865. [Google Scholar] [CrossRef]
- Yang, M.-H.; Zhang, L.; Xu, S.-T.; McLaughlin, N.B.; Liu, J.-H. Effect of Water Soluble Humic Acid Applied to Potato Foliage on Plant Growth, Photosynthesis Characteristics and Fresh Tuber Yield under Different Water Deficits. Sci. Rep. 2020, 10, 7854. [Google Scholar] [CrossRef]
- Yahya, M.; Saeed, N.A.; Nadeem, S.; Hamed, M.; Saleem, K. Effect of Leaf Rust Disease on Photosynthetic Rate, Chlorophyll Contents and Grain Yield of Wheat. Arch. Phytopathol. Pflanzenschutz. 2020, 53, 425–439. [Google Scholar] [CrossRef]
- Zheng, T.; Liu, N.; Wu, L.; Li, M.; Sun, H.; Zhang, Q.; Wu, J. Estimation of Chlorophyll Content in Potato Leaves Based on Spectral Red Edge Position. IFAC-PapersOnLine 2018, 51, 602–606. [Google Scholar] [CrossRef]
- Lu, Y.; Yao, J. Chloroplasts at the Crossroad of Photosynthesis, Pathogen Infection and Plant Defense. Int. J. Mol. Sci. 2018, 19, 3900. [Google Scholar] [CrossRef]
- Atiq, M. Evaluation of Antifungal Potential of Phytoextracts and Chemicals Against Root Rot of Soybean Caused by Rhizoctonia solani. Plant Prot. 2023, 7, 585–593. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.-X. Lipid Nanoparticles for Drug Delivery. Adv. Nanobiomed. Res. 2022, 2, 2100109. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret Ftir Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Fincheira, P.; Espinoza, J.; Vera, J.; Berrios, D.; Nahuelcura, J.; Ruiz, A.; Quiroz, A.; Bustamante, L.; Cornejo, P.; Tortella, G.; et al. The Impact of 2-Ketones Released from Solid Lipid Nanoparticles on Growth Modulation and Antioxidant System of Lactuca sativa. Plants 2023, 12, 3094. [Google Scholar] [CrossRef]
- Musielak, E.; Feliczak-Guzik, A.; Nowak, I. Optimization of the Conditions of Solid Lipid Nanoparticles (SLN) Synthesis. Molecules 2022, 27, 2202. [Google Scholar] [CrossRef]
- Fincheira, P.; Quiroz, A.; Medina, C.; Tortella, G.; Hermosilla, E.; Diez, M.C.; Rubilar, O. Plant Growth Induction by Volatile Organic Compound Released from Solid Lipid Nanoparticles and Nanostructured Lipid Carriers. Colloids Surf. A Physicochem. Eng. Asp. 2020, 596, 124739. [Google Scholar] [CrossRef]
- Subroto, E.; Sguizzato, M.; Andoyo, R.; Indiarto, R. Solid Lipid Nanoparticles: Review of the Current Research on Encapsulation and Delivery Systems for Active and Antioxidant Compounds. Antioxidants 2023, 12, 633. [Google Scholar] [CrossRef]
- Santander, C.; Vidal, G.; Ruiz, A.; Vidal, C.; Cornejo, P. Salinity Eustress Increases the Biosynthesis and Accumulation of Phenolic Compounds That Improve the Functional and Antioxidant Quality of Red Lettuce. Agronomy 2022, 12, 598. [Google Scholar] [CrossRef]
- Marek, J.; de Azevedo, D.; Ono, E.O.; Rodrigues, J.D.; Faria, C.M.D.R. Photosynthetic and Productive Increase in Tomato Plants Treated with Strobilurins and Carboxamides for the Control of Alternaria solani. Sci. Hortic. 2018, 242, 76–89. [Google Scholar] [CrossRef]
- Holz, T.M.; Dorneles, K.R.; Brunetto, A.E.; Segundo, J.B.M.; Delevatti, H.A.A.; Souza, G.M.; Dallagnol, L.J. Effect of Silicon and Fungicide on Photosynthetic Responses in Barley Leaves Challenged by Bipolaris sorokiniana. Physiol. Mol. Plant Pathol. 2022, 120, 101849. [Google Scholar] [CrossRef]
- Petit, A.N.; Fontaine, F.; Vatsa, P.; Clément, C.; Vaillant-Gaveau, N. Fungicide Impacts on Photosynthesis in Crop Plants. Photosynth. Res. 2012, 111, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Chanratana, M.; Kim, K.; Seshadri, S.; Sa, T. Impact of Arbuscular Mycorrhizal Fungi on Photosynthesis, Water Status, and Gas Exchange of Plants under Salt Stress–a Meta-Analysis. Front. Plant Sci. 2019, 10, 433629. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R. Role of Arbuscular Mycorrhiza in Amelioration of Salinity. In Salt Stress in Plants: Signalling, Omics and Adaptations; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2013; pp. 301–354. [Google Scholar]
- Jakl, M.; Kovač, I.; Zeljković, S.Ć.; Dytrtová, J.J. Triazole Fungicides in Soil Affect the Yield of Fruit, Green Biomass, and Phenolics Production of Solanum lycopersicum L. Food Chem. 2021, 351, 129328. [Google Scholar] [CrossRef] [PubMed]
- Al-Surhanee, A.A.; Afzal, M.; Bouqellah, N.A.; Ouf, S.A.; Muhammad, S.; Jan, M.; Kaleem, S.; Hashem, M.; Alamri, S.; Abdel Latef, A.A.H.; et al. The Antifungal Activity of Ag/CHI NPs against Rhizoctonia solani Linked with Tomato Plant Health. Plants 2021, 10, 2283. [Google Scholar] [CrossRef]
- Dias, M.C. Phytotoxicity: An Overview of the Physiological Responses of Plants Exposed to Fungicides. J. Bot. 2012, 2012, 135479. [Google Scholar] [CrossRef]
- Rajendran, U.M.; Kathirvel, E.; Narayanaswamy, A. Effects of a Fungicide, an Insecticide, and a Biopesticide on Tolypothrix scytonemoides. Pestic. Biochem. Physiol. 2007, 87, 164–171. [Google Scholar] [CrossRef]
- Julião, E.C.; Santana, M.D.; Freitas-Lopes, R.d.L.; Vieira, A.d.P.; de Carvalho, J.S.B.; Lopes, U.P. Reduction of Brown Leaf Spot and Changes in the Chlorophyll a Content Induced by Fungicides in Cassava Plants. Eur. J. Plant Pathol. 2020, 157, 433–439. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Lozowicka, B. Biochemical Compounds and Stress Markers in Lettuce upon Exposure to Pathogenic Botrytis cinerea and Fungicides Inhibiting Oxidative Phosphorylation. Planta 2022, 255, 61. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Ramírez, D.A.; Yactayo, W.; Gutiérrez, R.; Mares, V.; De Mendiburu, F.; Posadas, A.; Quiroz, R. Chlorophyll Concentration in Leaves Is an Indicator of Potato Tuber Yield in Water-Shortage Conditions. Sci. Hortic. 2014, 168, 202–209. [Google Scholar] [CrossRef]
- Steele, M.R.; Gitelson, A.A.; Rundquist, D.C. A Comparison of Two Techniques for Nondestructive Measurement of Chlorophyll Content in Grapevine Leaves. Agron. J. 2008, 100, 779–782. [Google Scholar] [CrossRef]
- Khalafallah, A.A.; Abo-Ghalia, H.H. Responses of Wheat Plants Associated with Arbuscular Mycorrhizal Fungi to Short-Term Water Stress Followed by Recovery at Three Growth Stages. J. Appl. Sci. Res. 2008, 4, 570–580. [Google Scholar]
- Arivalagan, M.; Somasundaram, R. Alteration of Photosynthetic Pigments and Antioxidant Systems in Tomato under Drought with Tebuconazole and Hexaconazole Applications. J. Sci. Agric. 2017, 1, 146. [Google Scholar] [CrossRef][Green Version]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Petelewicz, P.; Orlinski, P.M.; Baird, J.H. Effects of Fungicides on Creeping Bentgrass Health and Rooting Characteristics under Abiotic Stress. Int. Turfgrass Soc. Res. J. 2022, 14, 893–901. [Google Scholar] [CrossRef]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and Regulation of Plants Phenolics in Abiotic Stress Tolerance: An Overview. In Plant Signaling Molecules. Role and Regulation under Stressful Environments; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 157–168. [Google Scholar]
- Nasseri, M.; Golmohammadzadeh, S.; Arouiee, H.; Jaafari, M.R.; Neamati, H. Antifungal Activity of Zataria multiflora Essential Oil-Loaded Solid Lipid Nanoparticles in-Vitro Condition. Iran. J. Basic Med. Sci. 2016, 19, 1231–1237. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhang, Y.L.; Zhou, J.P.; Lv, H.X. Solid Lipid Nanoparticles Modified with Stearic Acid-Octaarginine for Oral Administration of Insulin. Int. J. Nanomed. 2012, 7, 3333–3339. [Google Scholar] [CrossRef]
- Kramm, V. (Ed.) Manual del Cultivo de la Papa en Chile [en Línea]; No. 375; Boletín INIA—Instituto de Investigaciones Agropecuarias: Chillán, Chile, 2017; Available online: https://hdl.handle.net/20.500.14001/6706 (accessed on 6 November 2023).
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Packer, L., Douce, R., Eds.; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
| Name | Potato Seed | R. solani | Fungicide | SLN + FCP | Replicates |
|---|---|---|---|---|---|
| NT | Yes | No | No | No | 6 |
| A | Yes | No | No | Dose A | 6 |
| B | Yes | No | No | Dose B | 6 |
| R | Yes | Yes | No | No | 6 |
| RM | Yes | Yes | MONCUT | No | 6 |
| RR | Yes | Yes | ReflectXtra | No | 6 |
| RA | Yes | Yes | No | Dose A | 6 |
| RB | Yes | Yes | No | Dose B | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas, S.; Fincheira, P.; González, F.; Santander, C.; Meier, S.; Santos, C.; Contreras, B.; Ruiz, A. Assessment of the Photosynthetic Response of Potato Plants Inoculated with Rhizoctonia solani and Treated with Flesh-Colored Potato Extracts Nanoencapsulated with Solid Lipid Nanoparticles. Plants 2025, 14, 156. https://doi.org/10.3390/plants14020156
Rivas S, Fincheira P, González F, Santander C, Meier S, Santos C, Contreras B, Ruiz A. Assessment of the Photosynthetic Response of Potato Plants Inoculated with Rhizoctonia solani and Treated with Flesh-Colored Potato Extracts Nanoencapsulated with Solid Lipid Nanoparticles. Plants. 2025; 14(2):156. https://doi.org/10.3390/plants14020156
Chicago/Turabian StyleRivas, Sheina, Paola Fincheira, Felipe González, Christian Santander, Sebastián Meier, Cledir Santos, Boris Contreras, and Antonieta Ruiz. 2025. "Assessment of the Photosynthetic Response of Potato Plants Inoculated with Rhizoctonia solani and Treated with Flesh-Colored Potato Extracts Nanoencapsulated with Solid Lipid Nanoparticles" Plants 14, no. 2: 156. https://doi.org/10.3390/plants14020156
APA StyleRivas, S., Fincheira, P., González, F., Santander, C., Meier, S., Santos, C., Contreras, B., & Ruiz, A. (2025). Assessment of the Photosynthetic Response of Potato Plants Inoculated with Rhizoctonia solani and Treated with Flesh-Colored Potato Extracts Nanoencapsulated with Solid Lipid Nanoparticles. Plants, 14(2), 156. https://doi.org/10.3390/plants14020156








