QTL Mapping for Leaf Rust Resistance in a Common Wheat Recombinant Inbred Line Population of Doumai/Shi4185
Abstract
1. Introduction
2. Results
2.1. Phenotypic Analysis
2.2. QTL for Leaf Rust Resistance
2.3. Additive Effect of QTL for Leaf Rust Resistance
2.4. Validation of KASP Markers
2.5. Prediction of Candidate Genes for Leaf Rust Resistance
3. Discussion
3.1. Comparison with Previously Reported QTL
3.1.1. QLr.lfnu-1BL1 and QLr.lfnu-1BL2
3.1.2. QLr.lfnu-2AL
3.1.3. QLr.lfnu-7BL1 and QLr.lfnu-7BL2
3.2. Prediction of Potential Candidate Genes for Leaf Rust Resistance
3.3. Application in Wheat Breeding
4. Methods
4.1. Plant Materials and Phenotypic Evaluation
4.2. Statistical Analyses and Linkage Mapping
4.3. Development of KASP Markers
4.4. Candidate Gene Prediction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full name |
| ABC | ATP-binding cassette |
| ANOVA | Analysis of variance |
| APR | Adult-plant resistance |
| ASR | All-stage resistance |
| BLUE | Best linear unbiased estimation |
| Hb2 | Broad-sense heritability |
| KASP | Kompetitive allele-specific PCR |
| LOD | Log of odds |
| MAS | Marker-assisted selection |
| MDS | Maximum disease severity |
| Pt | Puccinia triticina |
| PVE | Phenotypic variances explained |
| QTL | Quantitative trait loci |
| RILs | Recombinant inbred lines |
| SNP | Single-nucleotide polymorphism |
Appendix A
| Kasp Marker | Prime | Sequence |
|---|---|---|
| KASP-LR-2AL | FAM | GAAGGTGACCAAGTTCATGCTAGTCACAACCCCGCCTCA |
| HEX | GAAGGTCGGAGTCAACGGATTAGTCACAACCCCGCCTCG | |
| Common | ACATGTGCCTCCAGCTGC | |
| KASP-LR-7BL2 | FAM | GAAGGTGACCAAGTTCATGCTGCACGTCTCCTTCATTGGAT |
| HEX | GAAGGTCGGAGTCAACGGATTGCACGTCTCCTTCATTGGAC | |
| Common | CTCATCTTCTTTCAGGCCGG |
References
- Huerta-Espino, J.; Singh, R.P.; Germán, S.; McCallum, B.D.; Park, R.F.; Chen, W.Q.; Bhardwaj, S.C.; Goyeau, H. Global status of wheat leaf rust caused by Puccinia triticina. Euphytica 2011, 179, 143–160. [Google Scholar] [CrossRef]
- Li, Z.; Lan, C.; He, Z.; Singh, R.P.; Rosewarne, G.M.; Chen, X.; Xia, X. Overview and application of QTL for adult plant resistance to leaf rust and powdery mildew in wheat. Crop Sci. 2014, 54, 1907–1925. [Google Scholar] [CrossRef]
- Rehman, S.U.; Qiao, L.; Shen, T.; Hua, L.; Li, H.; Ahmad, Z.; Chen, S. Exploring the frontier of wheat rust resistance: Latest approaches, mechanisms, and novel insights. Plants 2024, 13, 2502. [Google Scholar] [CrossRef]
- Wang, N.; Tang, C.; Fan, X.; He, M.; Gan, P.; Zhang, S.; Hu, Z.; Wang, X.; Yan, T.; Shu, W.; et al. Inactivation of a wheat protein kinase gene confers broad-spectrum resistance to rust fungi. Cell 2022, 185, 2961–2974. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; William, H.M.; Huerta-Espino, J.; Rosewarne, G. Wheat rust in Asia: Meeting the challenges with old and new technologies. In Proceedings of the 4th International Crop Science Congress, Brisbane, Australia, 26 September–1 October 2004; Volume 26, pp. 1–13. [Google Scholar]
- Zhao, J.; Kang, Z. Fighting wheat rusts in China: A look back and into the future. Phytopathol. Res. 2023, 5, 6. [Google Scholar] [CrossRef]
- Basnet, B.; Juliana, P.; Bhattarai, K.; Upreti, U. A review on major rust resistance gene and amino acid changes on wheat (Triticum aestivum L). Adv. Agric. 2022, 2022, 7419326. [Google Scholar] [CrossRef]
- Panneer, S.; Sivasamy, M.; Vikas, V.K.; Bhardwaj, S.C.; Gangwar, O.P.; Peter, J.; Sivasamy, V. Stacking effective ASR and APR rust genes for multiple disease resistance in bread wheat cultivars. Crop Breed. Appl. Biotechnol. 2023, 23, e44082317. [Google Scholar]
- Sharma, D.; Avni, R.; Gutierrez-Gonzalez, J.; Kumar, R.; Sela, H.; Prusty, M.R.; Shatil-Cohen, A.; Molnár, I.; Holušová, K.; Said, M.; et al. A single NLR gene confers resistance to leaf and stripe rust in wheat. Nat. Commun. 2024, 15, 9925. [Google Scholar] [CrossRef]
- Anguelova-Merhar, V.S.; VanDer Westhuizen, A.J.; Pretorius, Z.A. β-1,3-glucanase and chitinase activities and the resistance response of wheat to leaf rust. J. Phytopathol. 2001, 149, 381–384. [Google Scholar]
- Yang, W.; Liu, D.; Li, J.; Zhang, L.; Wei, H.; Hu, X.; Zheng, Y.; He, Z.; Xia, X. Synthetic hexaploid wheat and its utilization for wheat genetic improvement in China. J. Genet. Genom. 2015, 42, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Rajaram, S.; Kronstad, W.E.; Mundt, C.C.; Singh, R.P. Associations and genetics of three components of slow rusting in leaf rust of wheat. Euphytica 1993, 68, 99–109. [Google Scholar] [CrossRef]
- Bansal, U.K.; Forrest, K.L.; Hayden, M.J.; Miah, H.; Singh, D.; Bariana, H.S. Characterisation of a new stripe rust resistance gene Yr47 and its genetic association with the leaf rust resistance gene Lr52. Theor. Appl. Genet. 2011, 122, 1461–1466. [Google Scholar] [CrossRef]
- Herrera-Foessel, S.A.; Singh, R.P.; Huerta-Espino, J.; Rosewarne, G.M.; Periyannan, S.K.; Viccars, L.; Calvo-Salazar, V.; Lan, C.; Lagudah, E.S. Lr68: A new gene conferring slow rusting resistance to leaf rust in wheat. Theor. Appl. Genet. 2012, 124, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Klymiuk, V.; Yaniv, E.; Huang, L.; Raats, D.; Fatiukha, A.; Chen, S.; Feng, L.; Frenkel, Z.; Krugman, T.; Lidzbarsky, G.; et al. Cloning of the wheat Yr15 resistance gene sheds light on the plant tandem kinase-pseudokinase family. Nat. Commun. 2018, 9, 3735. [Google Scholar] [CrossRef]
- Kolmer, J.A.; Fajolu, O.L.; Bai, B.; Chen, X.; Jin, Y. Genetics of leaf rust resistance in the soft red winter wheat cultivar AGS 2000. Plant Dis. 2021, 105, 1593–1598. [Google Scholar]
- Kolodziej, M.C.; Singla, J.; Sánchez-Martín, J.; Zbinden, H.; Šimková, H.; Karafiátová, M.; Doležel, J.; Gronnier, J.; Poretti, M.; Glauser, G.; et al. A membrane-bound ankyrin repeat protein confers race-specific leaf rust disease resistance in wheat. Nat. Commun. 2021, 12, 956. [Google Scholar] [CrossRef]
- Amo, A.; Soriano, J.M. Unravelling consensus genomic regions conferring leaf rust resistance in wheat via meta-QTL analysis. Plant Genome 2022, 15, e20185. [Google Scholar] [CrossRef]
- Li, C.; Liu, H.; Wang, J.; Pan, Q.; Wang, Y.; Wu, K.; Jia, P.; Mu, Y.; Tang, H.; Xu, Q.; et al. Characterization and fine mapping of a lesion mimic mutant (Lm5) with enhanced stripe rust and powdery mildew resistance in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2022, 135, 421–438. [Google Scholar] [CrossRef]
- Pal, N.; Jan, I.; Saini, D.K.; Kumar, K.; Kumar, A.; Sharma, P.K.; Kumar, S.; Balyan, H.S.; Gupta, P.K. Meta-QTLs for multiple disease resistance involving three rusts in common wheat (Triticum aestivum L.). Theor. Appl. Genet. 2022, 135, 2385–2405. [Google Scholar] [CrossRef]
- Soriano, J.M.; Royo, C. Dissecting the genetic architecture of leaf rust resistance in wheat by QTL meta-analysis. Phytopathology 2015, 105, 1585–1593. [Google Scholar] [CrossRef]
- Bhardwaj, S.C.; Singh, G.P.; Gangwar, O.P.; Prasad, P.; Kumar, S. Status of wheat rust research and progress in rust management-Indian context. Agronomy 2019, 9, 892. [Google Scholar] [CrossRef]
- Suenaga, K.; Singh, R.P.; Huerta-Espino, J.; William, H.M. Microsatellite markers for genes Lr34/Yr18 and other quantitative trait loci for leaf rust and stripe rust resistance in bread wheat. Phytopathology 2003, 93, 881–890. [Google Scholar] [CrossRef]
- Bariana, H.; Forrest, K.; Qureshi, N.; Miah, H.; Hayden, M.; Bansal, U. Adult plant stripe rust resistance gene Yr71 maps close to Lr24 in chromosome 3D of common wheat. Mol. Breed. 2016, 36, 98. [Google Scholar] [CrossRef]
- Chugunkova, T.V.; Pastukhova, N.L.; Pirko, Y.V.; Blume, Y.B. Genetic Basis of Resistance to Wheat Yellow Rust. Cytol. Genet. 2025, 59, 186–196. [Google Scholar] [CrossRef]
- Majeed, K.; Sufyan, M.; Abbasi, K.; Ahsan, R.; Bux, H.; Zakria, M.; Fayyaz, M.; Mirza, J.I.; Ali, M.; Rasheed, A.; et al. Stripe rust resistance in historical wheat cultivars at seedling and adult plant stages and tagging effective resistance genes using molecular markers. J. Plant Pathol. 2025, 107, 1–12. [Google Scholar] [CrossRef]
- Rasheed, A.; Liu, J.; Appels, R.; He, Z. Mobilizing Triticeae diversity from gene banks to farmer’s field. Mol. Plant 2025, 18, 566–569. [Google Scholar] [CrossRef]
- Mago, R.; Bariana, H.S.; Dundas, I.S.; Spielmeyer, W.; Lawrence, G.J.; Pryor, A.J.; Ellis, J.G. Development of PCR markers for the selection of wheat stem rust resistance genes Sr24 and Sr26 in diverse wheat germplasm. Theor. Appl. Genet. 2005, 111, 496–504. [Google Scholar] [CrossRef]
- Dyck, P.L.; Samborski, D.J. Inheritance of resistance to leaf rust and stem rust in the wheat cultivar Glenlea. Can. J. Genet. Cytol. 1974, 16, 323–332. [Google Scholar] [CrossRef]
- Singh, R.P.; Huerta-Espino, J.; Rajaram, S.; Crossa, J. Agronomic effects from chromosome translocations 7DL.7Ag and 1BL1RS in spring wheat. Crop Sci. 1998, 38, 27–33. [Google Scholar] [CrossRef]
- Marais, G.F.; Pretorius, Z.A.; Wellings, C.R.; McCallum, B.; Marais, A.S. Lr51: A new gene for leaf rust resistance in wheat. Theor. Appl. Genet. 2005, 110, 971–976. [Google Scholar]
- Koller, T.; Brunner, S.; Herren, G.; Hurni, S.; Keller, B. Pyramiding of transgenic Pm3 alleles in wheat results in improved powdery mildew resistance in the field. Theor. Appl. Genet. 2018, 131, 861–871. [Google Scholar] [CrossRef]
- Schnurbusch, T.; Paillard, S.; Schori, A.; Messmer, M.; Schachermayr, G.; Winzeler, M.; Keller, B. Dissection of quantitative and durable leaf rust resistance in Swiss winter wheat reveals a major resistance QTL in the Lr34 chromosomal region. Theor. Appl. Genet. 2004, 108, 477–484. [Google Scholar] [CrossRef]
- Li, Z.F.; Xia, X.C.; He, Z.H.; Li, X.; Zhang, L.J.; Wang, H.Y.; Meng, Q.F.; Yang, W.X.; Li, G.Q.; Liu, D.Q. Seedling and slow rusting resistance to leaf rust in Chinese wheat cultivars. Plant Dis. 2010, 94, 45–53. [Google Scholar] [CrossRef]
- Hiebert, C.W.; Thomas, J.B.; McCallum, B.D.; Humphreys, D.G.; DePauw, R.M.; Hayden, M.J.; Mago, R.; Schnippenkoetter, W.; Spielmeyer, W. An introgression on wheat chromosome 4DL in RL6077 (Thatcher6/PI 250413) confers adult plant resistance to stripe rust and leaf rust (Lr67). Theor. Appl. Genet. 2010, 121, 1083–1091. [Google Scholar] [CrossRef]
- Herrera-Foessel, S.A.; Singh, R.P.; Huerta-Espino, J.; Crossa, J.; Yuen, J.; Djurle, A. Genetic analysis of slow-rusting resistance to leaf rust in spring wheat. Theor. Appl. Genet. 2008, 117, 1029–1039. [Google Scholar]
- Kuraparthy, V.; Chhuneja, P.; Dhaliwal, H.S.; Kaur, S.; Bowden, R.L.; Gill, B.S. Characterization and mapping of cryptic alien introgression from Aegilops geniculata with new leaf rust and stripe rust resistance genes Lr57 and Yr40 in wheat. Theor. Appl. Genet. 2007, 114, 1379–1389. [Google Scholar] [CrossRef]
- William, H.M.; Singh, R.P.; Huerta-Espino, J.; Palacios, G.; Suenaga, K. Characterization of genetic loci conferring adult plant resistance to leaf rust and stripe rust in spring wheat. Genome 2006, 49, 977–990. [Google Scholar] [CrossRef]
- Lillemo, M.; Asalf, B.; Singh, R.P.; Huerta-Espino, J.; Chen, X.M.; He, Z.H.; Bjørnstad, Å. The adult plant rust resistance loci Lr34/Yr18 and Lr46/Yr29 are important determinants of partial resistance to powdery mildew in bread wheat line Saar. Theor. Appl. Genet. 2008, 116, 1155–1166. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, Z.; Li, X.; Li, M.; Xue, L.; Li, X. QTL Mapping of Adult Plant Resistance to Wheat Leaf Rust in the Xinong1163-4× Thatcher RIL Population. Agronomy 2025, 15, 1717. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, L.; Hao, C.; Pan, Y.; Guo, M.; Huang, Y.; Zhang, X. TaRLK-1B: A novel wheat gene conferring resistance to leaf rust revealed by a genome-wide association study. J. Integr. Agric. 2025, in press. [Google Scholar] [CrossRef]
- Gao, P.; Zhou, Y.; Gebrewahid, T.W.; Zhang, P.; Wang, S.; Liu, D.; Li, Z. QTL mapping for adult-plant resistance to leaf rust in Italian wheat cultivar Libellula. Plant Dis. 2024, 108, 13–19. [Google Scholar] [CrossRef]
- Li, X.; Tan, W.; Feng, J.; Yan, Q.; Tian, R.; Chen, Q.; Zhou, X. Mapping QTLs for Stripe Rust Resistance and Agronomic Traits in Chinese Winter Wheat Lantian 31 Using 15K SNP Array. Agriculture 2025, 15, 1444. [Google Scholar] [CrossRef]
- Dyck, P.L. The transfer of leaf rust resistance from Triticum turgidum ssp. dicoccoides to hexaploid wheat. Can. J. Plant Sci. 1994, 74, 671–673. [Google Scholar] [CrossRef]
- Seah, S.; Bariana, H.; Jahier, J.; Sivasithamparam, K.; Lagudah, E.S. The introgressed segment carrying rust resistance genes Yr17, Lr37 and Sr38 in wheat can be assayed by a cloned disease resistance gene-like sequence. Theor. Appl. Genet. 2001, 102, 600–605. [Google Scholar] [CrossRef]
- Hysing, S.C.; Merker, A.; Liljeroth, E.; Koebner, R.M.; Zeller, F.J.; Hsam, S.L.K. Powdery mildew resistance in 155 Nordic bread wheat cultivars and landraces. Hereditas 2007, 144, 102–119. [Google Scholar] [CrossRef]
- Boukhatem, N.; Baret, P.V.; Mingeot, D.; Jacquemin, J.M. Quantitative trait loci for resistance against yellow rust in two wheat-derived recombinant inbred line populations. Theor. Appl. Genet. 2002, 104, 111–118. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Q.; Tian, B.; He, F.; Chen, Y.; Bai, G.; Akhunova, A.; Trick, H.N.; Akhunov, E. Gene editing of the wheat homologs of TONNEAU 1-recruiting motif encoding gene affects grain shape and weight in wheat. Plant J. 2019, 100, 251–264. [Google Scholar] [CrossRef]
- McCallum, B.D.; Hiebert, C.W.; Cloutier, S.; Bakkeren, G.; Rosa, S.B.; Humphreys, D.G.; Marais, G.F.; McCartney, C.A.; Panwar, V.; Rampitsch, C.; et al. A review of wheat leaf rust research and the development of resistant cultivars in Canada. Can. J. Plant Pathol. 2016, 38, 1–18. [Google Scholar] [CrossRef]
- Li, C.; Xu, X.T.; Zhang, Y.; Liu, S.; Wu, J.; Han, D.; Bai, G. Mapping QTLs for adult-plant resistance to yellow rust in a hard winter wheat population Heyne× Lakin. Theor. Appl. Genet. 2025, 138, 192. [Google Scholar] [CrossRef]
- Maccaferri, M.; Zhang, J.; Bulli, P.; Abate, Z.; Chao, S.; Cantu, D.; Bossolini, E.; Chen, X.; Pumphrey, M.; Dubcovsky, J. A genome-wide association study of resistance to stripe rust (Puccinia striiformis f. sp. tritici) in a worldwide collection of hexaploid spring wheat (Triticum aestivum L.). G3 Genes Genomes Genet. 2015, 5, 449–465. [Google Scholar] [CrossRef]
- Patpour, M.; Hovmøller, M.S.; Rodriguez-Algaba, J.; Randazzo, B.; Villegas, D.; Shamanin, V.P.; Berlin, A.; Flath, K.; Czembor, P.; Hanzalova, A.; et al. Wheat stem rust back in Europe: Diversity, prevalence and impact on host resistance. Front. Plant Sci. 2022, 13, 882440. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jørgensen, L.N.; Hovmøller, M.S.; Huerta-Espino, J. Disease impact on wheat yield potential and prospects of genetic control. Annu. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef]
- Rosewarne, G.M.; Herrera-Foessel, S.A.; Singh, R.P.; Huerta-Espino, J.; Lan, C.X.; He, Z. Quantitative trait loci of stripe rust resistance in wheat. Theor. Appl. Genet. 2013, 126, 2427–2449. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; N’Diaye, A.; Walkowiak, S.; Nilsen, K.T.; Cory, A.T.; Haile, J.; Kutcher, H.R.; Ammar, K.; Loladze, A.; Huerta-Espino, J.; et al. Genetic analysis of resistance to stripe rust in durum wheat (Triticum turgidum L. var. durum). PLoS ONE 2018, 13, e0203283. [Google Scholar] [CrossRef]
- Lagudah, E.S.; McFadden, H.; Singh, R.P.; Huerta-Espino, J.; Bariana, H.S.; Spielmeyer, W. Molecular genetic characterization of the Lr34/Yr18 slow rusting resistance gene region in wheat. Theor. Appl. Genet. 2006, 114, 21–30. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, L.; Liu, D.; Zeng, J.; Cao, S.; Xia, X.; Yan, J.; Song, X.; He, Z.; Zhang, Y. Fine mapping and validation of a major QTL for grain weight on chromosome 5B in bread wheat. Theor. Appl. Genet. 2021, 134, 3731–3741. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, D.; Xu, X.; Ren, Y.; Gao, F.; Song, J.; Jia, A.; Hao, Y.; He, Z.; Xia, X. Fine mapping of QPm.caas-3BS, a stable QTL for adult-plant resistance to powdery mildew in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2022, 135, 1083–1099. [Google Scholar] [CrossRef]
- Rasheed, A.; Wen, W.; Gao, F.; Zhai, S.; Jin, H.; Liu, J.; Guo, Q.; Zhang, Y.; Dreisigacker, S.; Xia, X.; et al. Development and validation of KASP assays for genes underpinning key economic traits in bread wheat. Theor. Appl. Genet. 2016, 129, 1843–1860. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Hao, Y.; Xia, X.; Khan, A.; Xu, Y.; Varshney, R.K.; He, Z. Crop breeding chips and genotyping platforms: Progress, challenges, and perspectives. Mol. Plant 2017, 10, 1047–1064. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Y.; Yuan, S.; Yue, J.; Li, Y.; Liao, X.; Ying, S.; Liu, Z.; Bai, J.; Zhang, L. The new function of superoxide dismutase (SOD) in Triticum aestivum L.: Promote early flowering. Agric. Commun. 2023, 1, 100007. [Google Scholar]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef]
- Wen, W.; He, Z.; Gao, F.; Liu, J.; Jin, H.; Zhai, S.; Qu, Y.; Xia, X. A high-density consensus map of common wheat integrating four mapping populations sffcanned by the 90K SNP array. Front. Plant Sci. 2017, 8, 1389. [Google Scholar] [CrossRef] [PubMed]

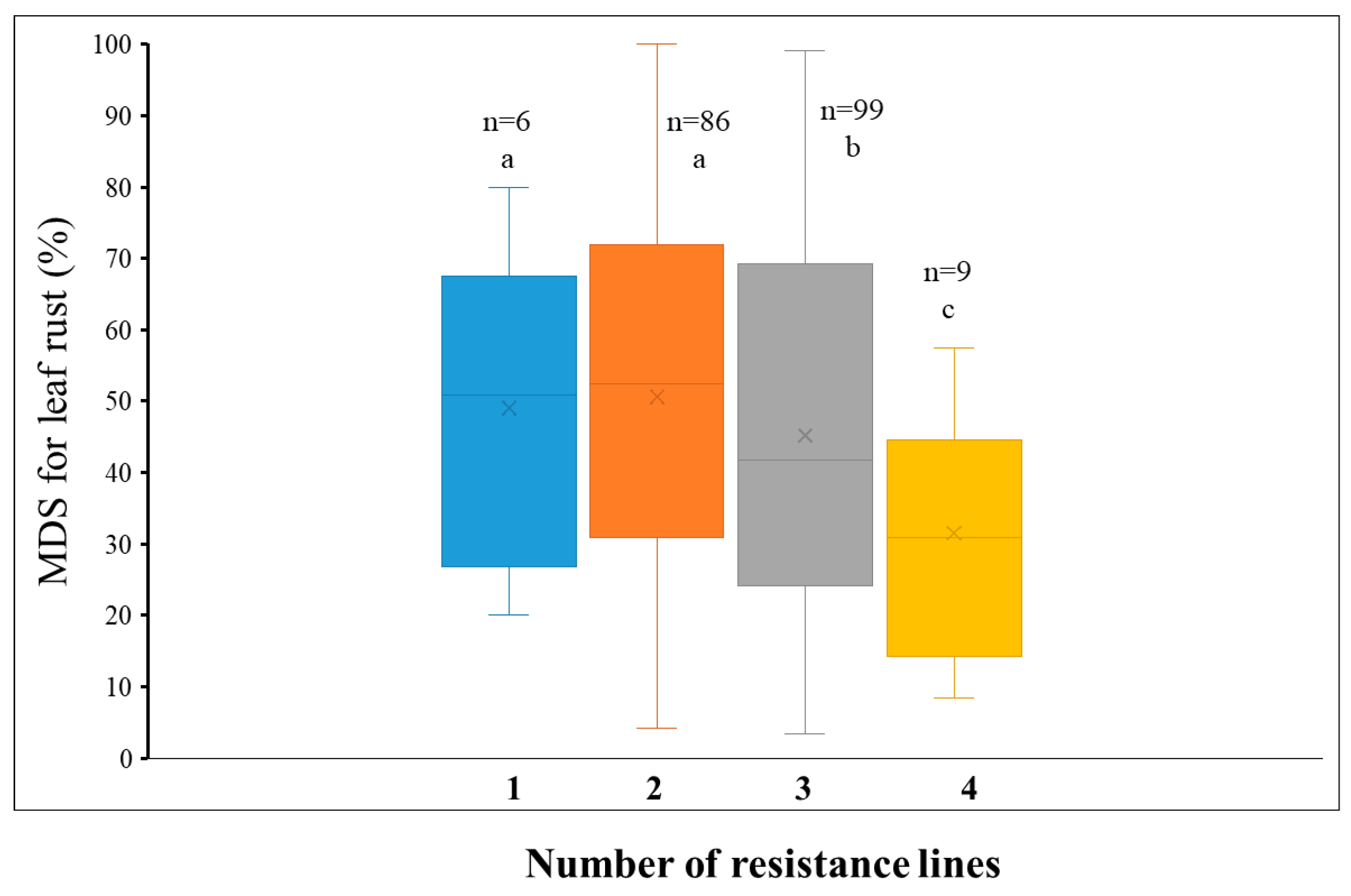
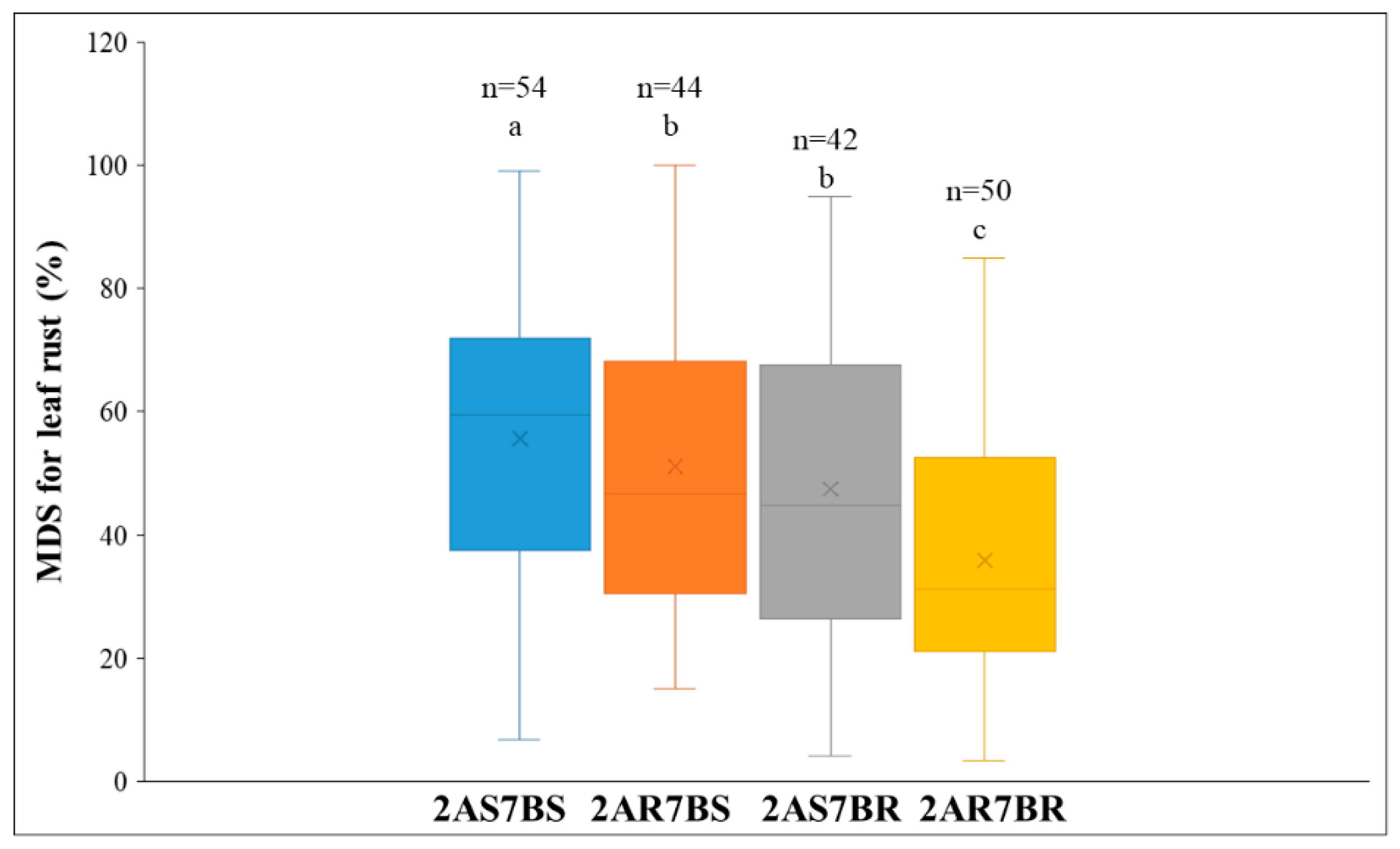

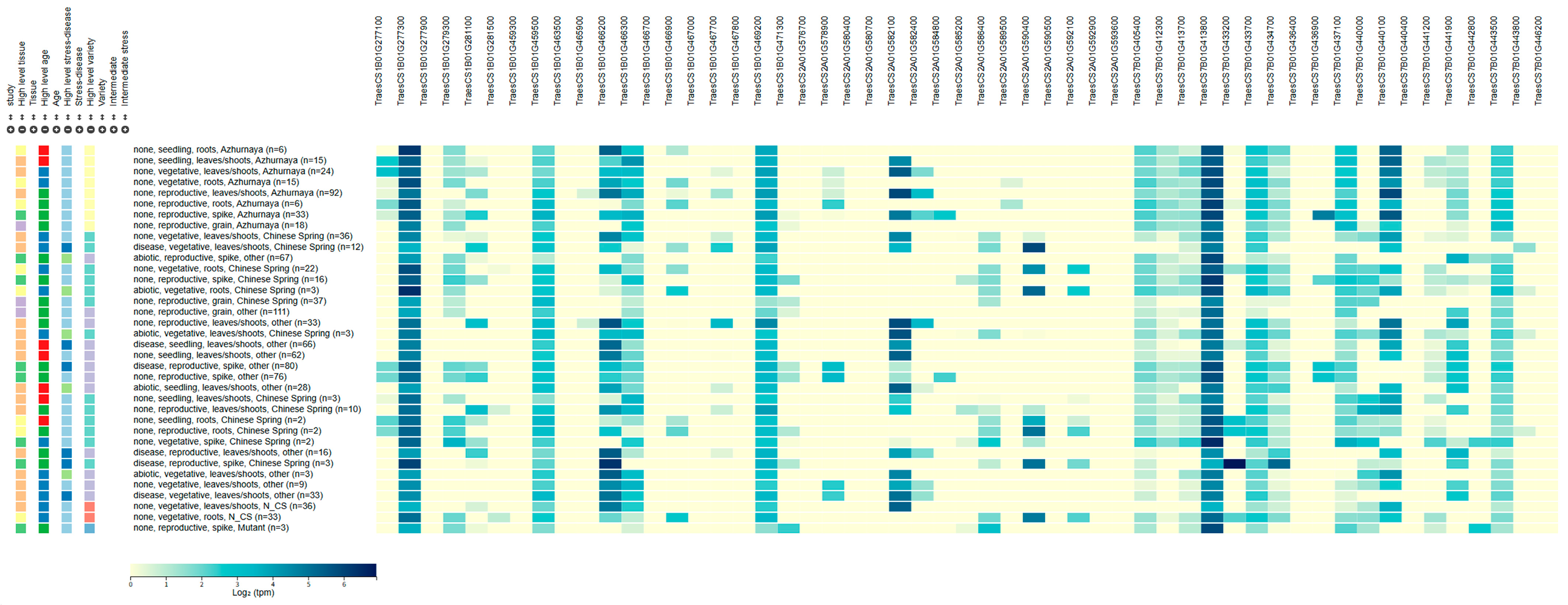
| Environment | Doumai | Shi4185 | Mean MDS (%) | Range of MDS (%) | Standard Deviation | Coefficient of Variation |
|---|---|---|---|---|---|---|
| Xinxiang2021 | 48.0 | 38.0 | 43.3 | 0–98 | 33.2 | 0.8 |
| Xinxiang2022 | 52.0 | 32.0 | 53.8 | 0–95 | 28.8 | 0.5 |
| Zhengzhou2021 | 50.0 | 35.0 | 52.2 | 0–96 | 33.8 | 0.6 |
| Zhengzhou2022 | 22.0 | 18.0 | 34.9 | 1–98 | 28.3 | 0.8 |
| QTL | Chromosome | Marker Interval | Physical Interval (Mb) | LOD | PVE (%) a | Additive | Environment b |
|---|---|---|---|---|---|---|---|
| QLr.lfnu-1BL1 | 1B | tplb0033h08_101~ D_contig03023_692 | 484.2–487.6 | 2.92–3.56 | 6.24–7.15 | −7.82–9.04 | E1, E3 |
| QLr.lfnu-1BL2 | 1B | Tdurum_contig84791_198~ Ex_c1058_1537 | 674.0–678.3 | 3.38–3.98 | 6.85–8.67 | −8.58–9.97 | E1, E4 |
| QLr.lfnu-2AL | 2A | wsnp_JD_c289_450995~ Excalibur_c40335_198 | 772.9–779.9 | 1.84–1.96 | 6.75–6.98 | 7.57–8.56 | E3, E4 |
| QLr.lfnu-7BL1 | 7B | BobWhite_c7208_88~ IACX8294 | 675.7–681.8 | 2.71–3.54 | 4.54–8.91 | 6.75–8.57 | E1, E3 |
| QLr.lfnu-7BL2 | 7B | tplb0058p02_2806~ BobWhite_rep_c53128_119 | 701.3–708.0 | 2.41–3.55 | 4.97–7.41 | 7.53–9.20 | E2, E4 |
| QTL | KASP Marker Name | Genotype | No. of Lines | Maximum Disease Severity (%) | p-Value |
|---|---|---|---|---|---|
| QLr.lfnu-2AL | KASP-LR-2AL | AA | 43 | 54.1 | 0.049 |
| GG | 99 | 47.9 | |||
| QLr.lfnu-7BL2 | KASP-LR-7BL2 | TT | 68 | 53.1 | 0.007 |
| CC | 82 | 47.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, W.; Wang, R.; Zhao, N.; Zhang, X.; Zhu, S.; Liu, J. QTL Mapping for Leaf Rust Resistance in a Common Wheat Recombinant Inbred Line Population of Doumai/Shi4185. Plants 2025, 14, 3113. https://doi.org/10.3390/plants14193113
Wang Y, Li W, Wang R, Zhao N, Zhang X, Zhu S, Liu J. QTL Mapping for Leaf Rust Resistance in a Common Wheat Recombinant Inbred Line Population of Doumai/Shi4185. Plants. 2025; 14(19):3113. https://doi.org/10.3390/plants14193113
Chicago/Turabian StyleWang, Yamei, Wenjing Li, Rui Wang, Nannan Zhao, Xinye Zhang, Shu Zhu, and Jindong Liu. 2025. "QTL Mapping for Leaf Rust Resistance in a Common Wheat Recombinant Inbred Line Population of Doumai/Shi4185" Plants 14, no. 19: 3113. https://doi.org/10.3390/plants14193113
APA StyleWang, Y., Li, W., Wang, R., Zhao, N., Zhang, X., Zhu, S., & Liu, J. (2025). QTL Mapping for Leaf Rust Resistance in a Common Wheat Recombinant Inbred Line Population of Doumai/Shi4185. Plants, 14(19), 3113. https://doi.org/10.3390/plants14193113






