Physiological and Transcriptional Responses of Sorghum Seedlings Under Alkali Stress
Abstract
1. Introduction
2. Results
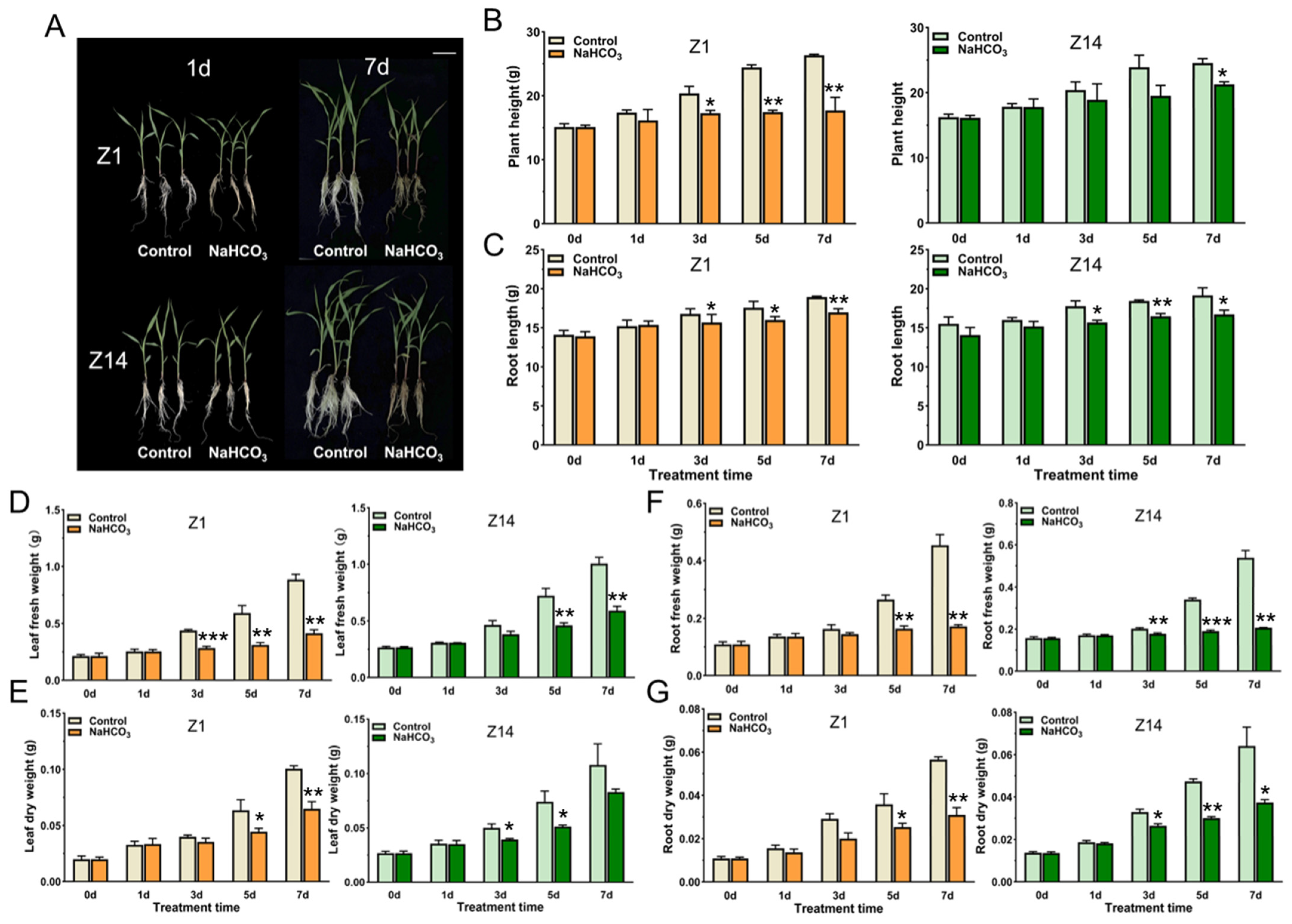
2.1. Responses of Sorghum Seedlings of Different Genotypes to Alkaline Stress
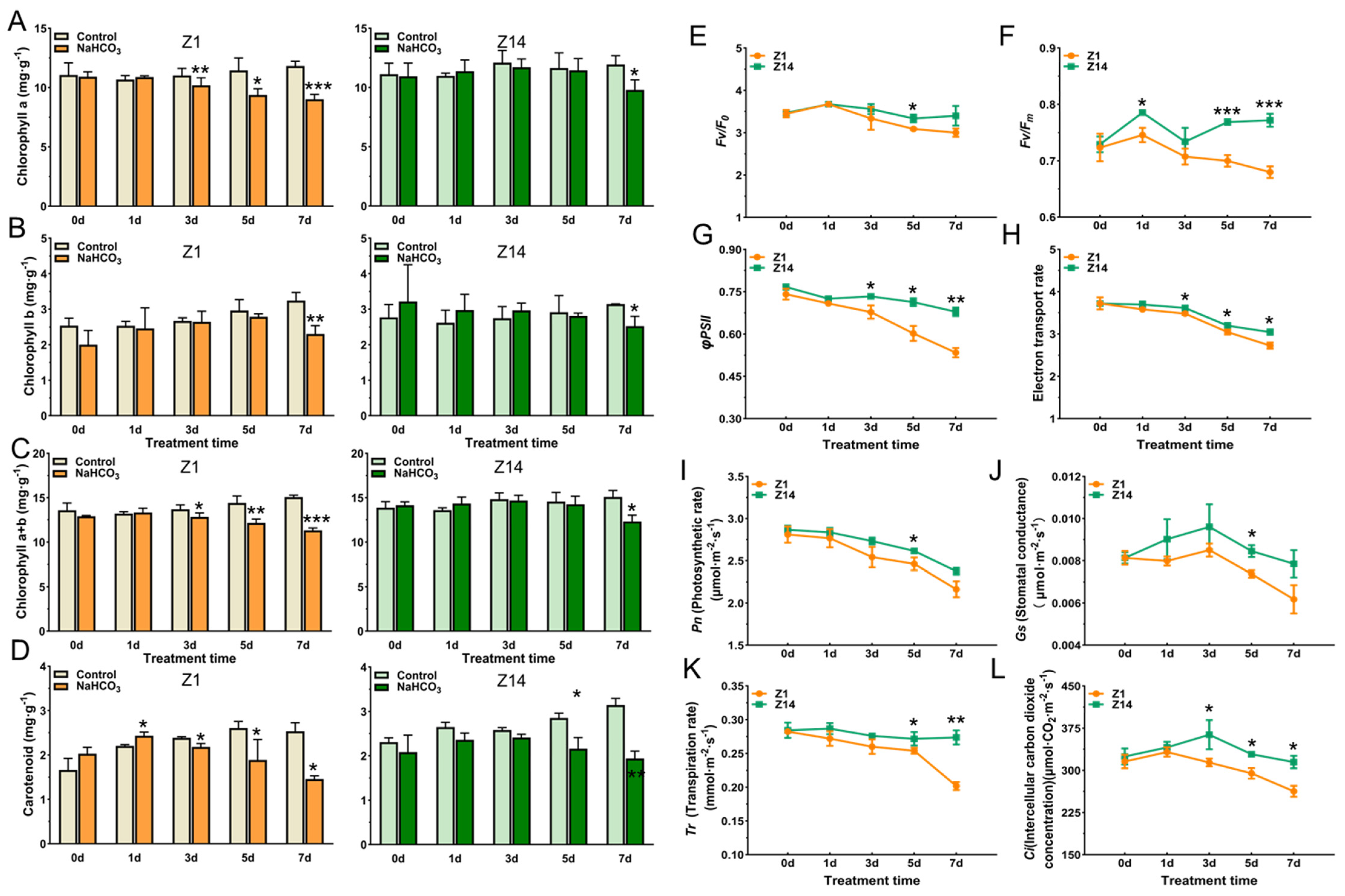
2.2. Effects of Chlorophyll, Carotenoids, and Photosynthetic Fluorescence Parameters of Sorghum Seedlings Under Alkali Stress
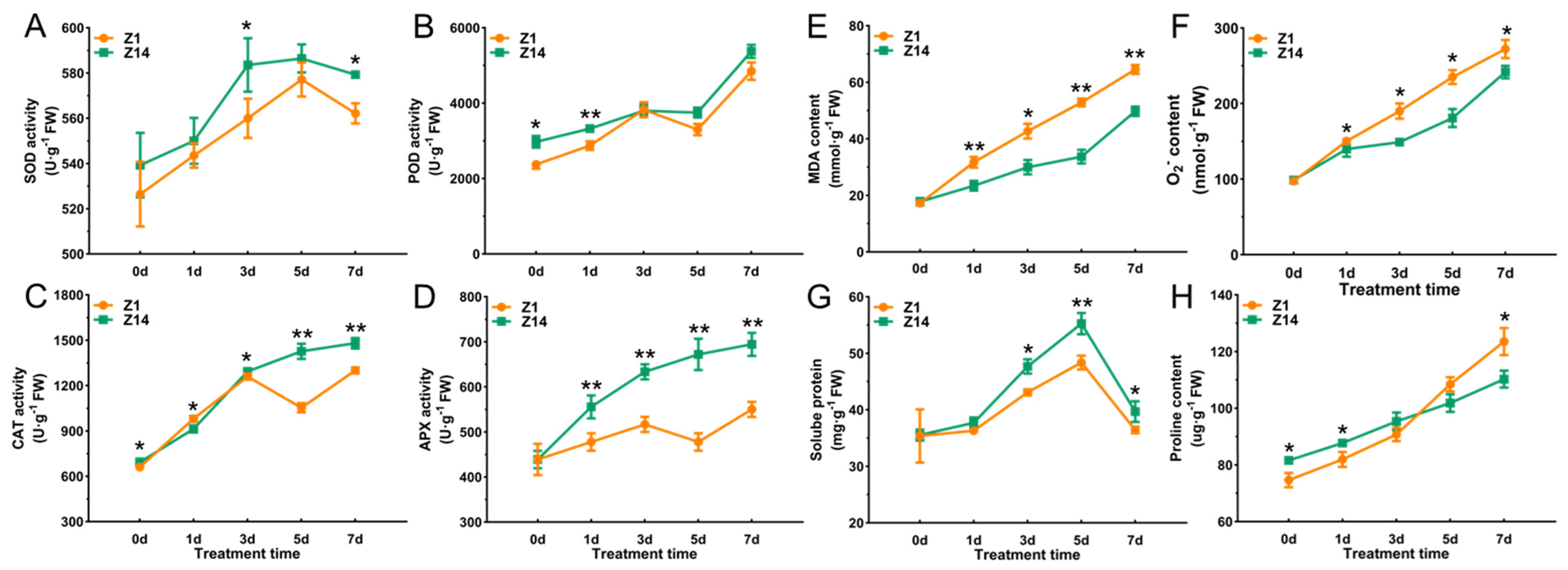
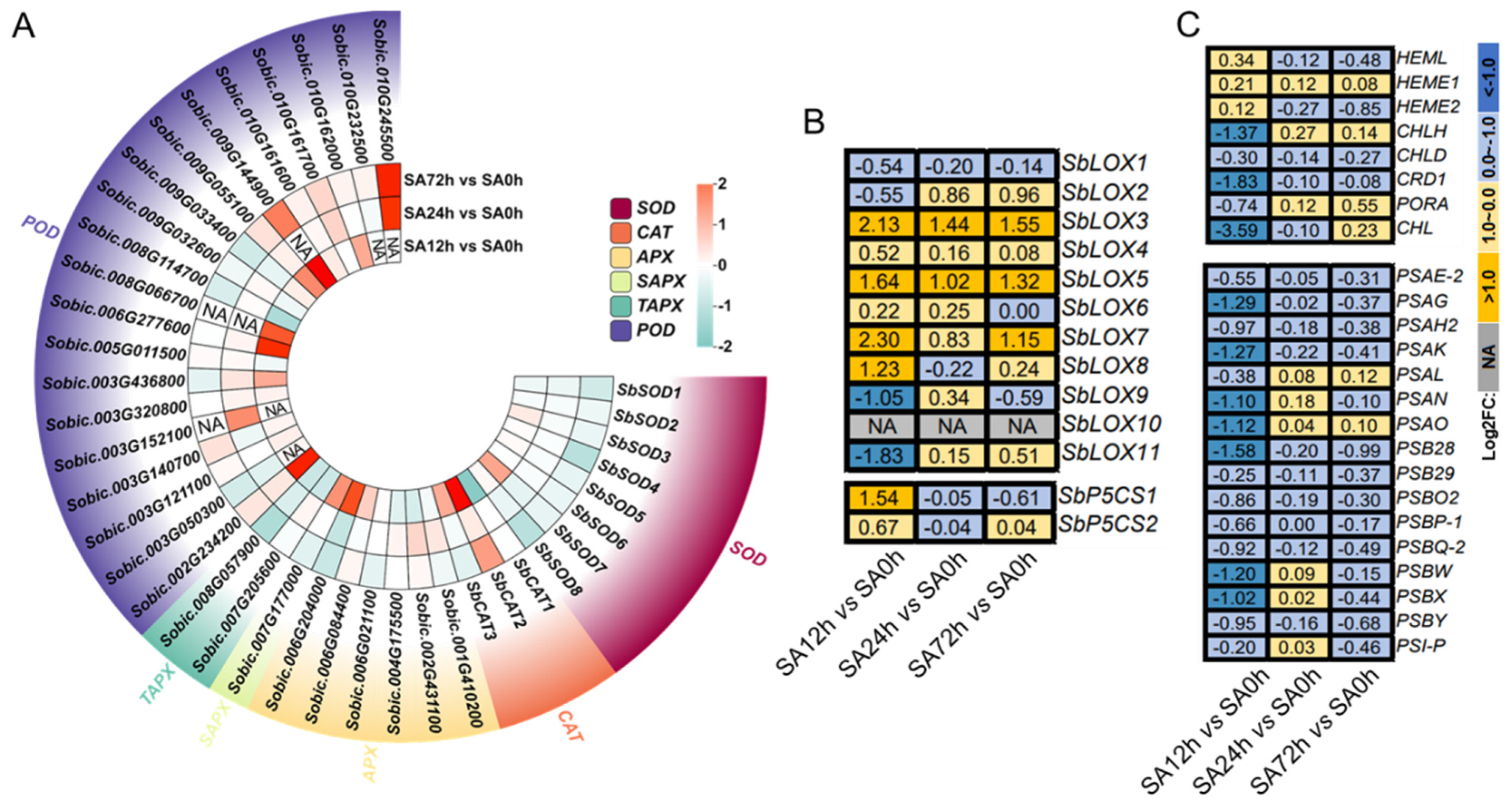
2.3. Response of the Antioxidant System of Sorghum Seedling Leaves Under Alkali Stress
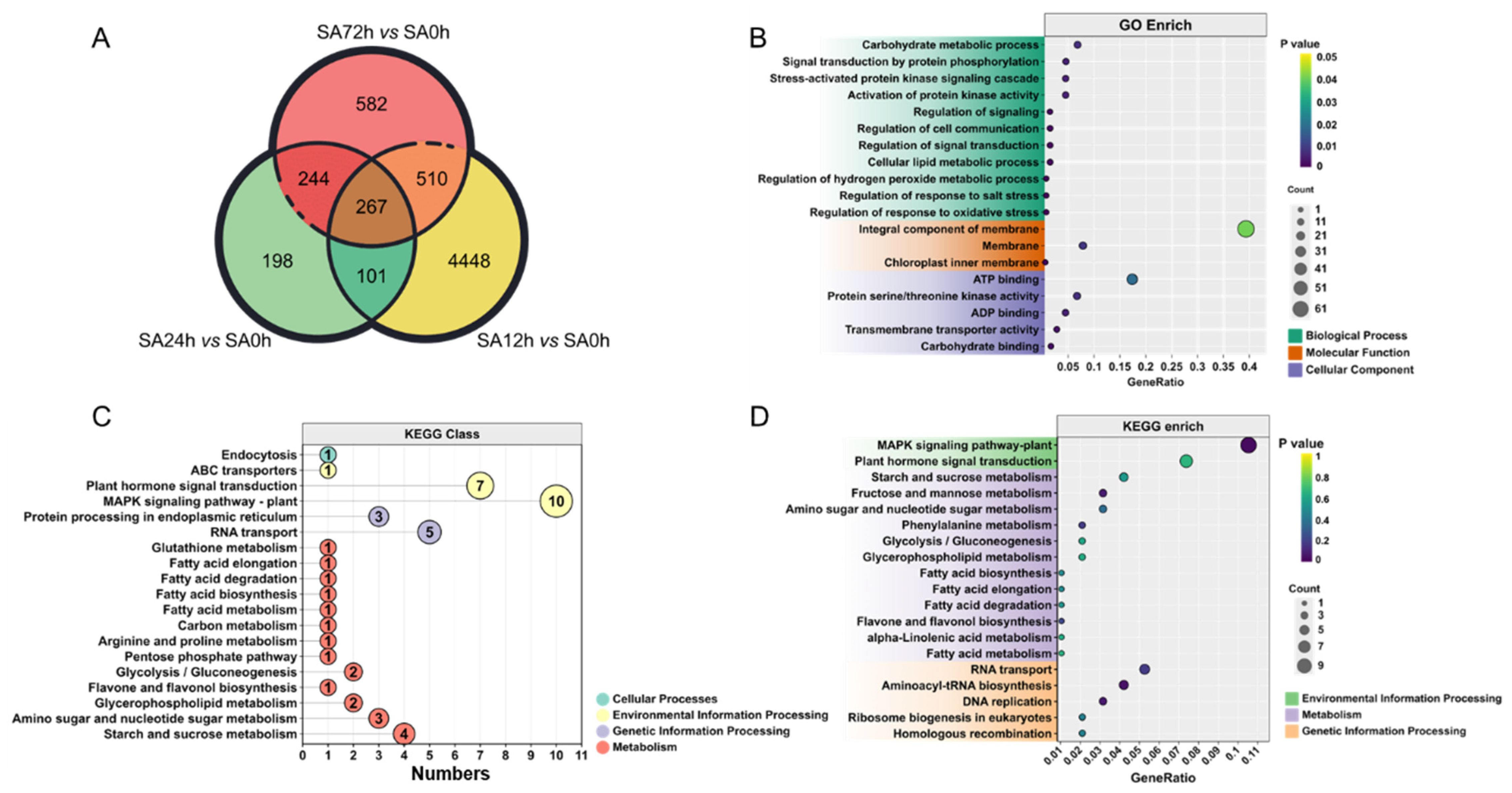
2.4. Differentially Expressed Genes in Leaves of Z14 Sorghum Variety Under Alkali Stress
2.5. Enrichment of DEGs in Z14 Sorghum Seedlings Under Alkali Stress
2.6. Analysis of Differentially Expressed Transcription Factors in Z14 Sorghum Leaves Under Alkali Stress
2.7. Expression Profiles of DEGs Involved in Physiological Responses in Z14 Sorghum Seedlings Under Alkali Stress
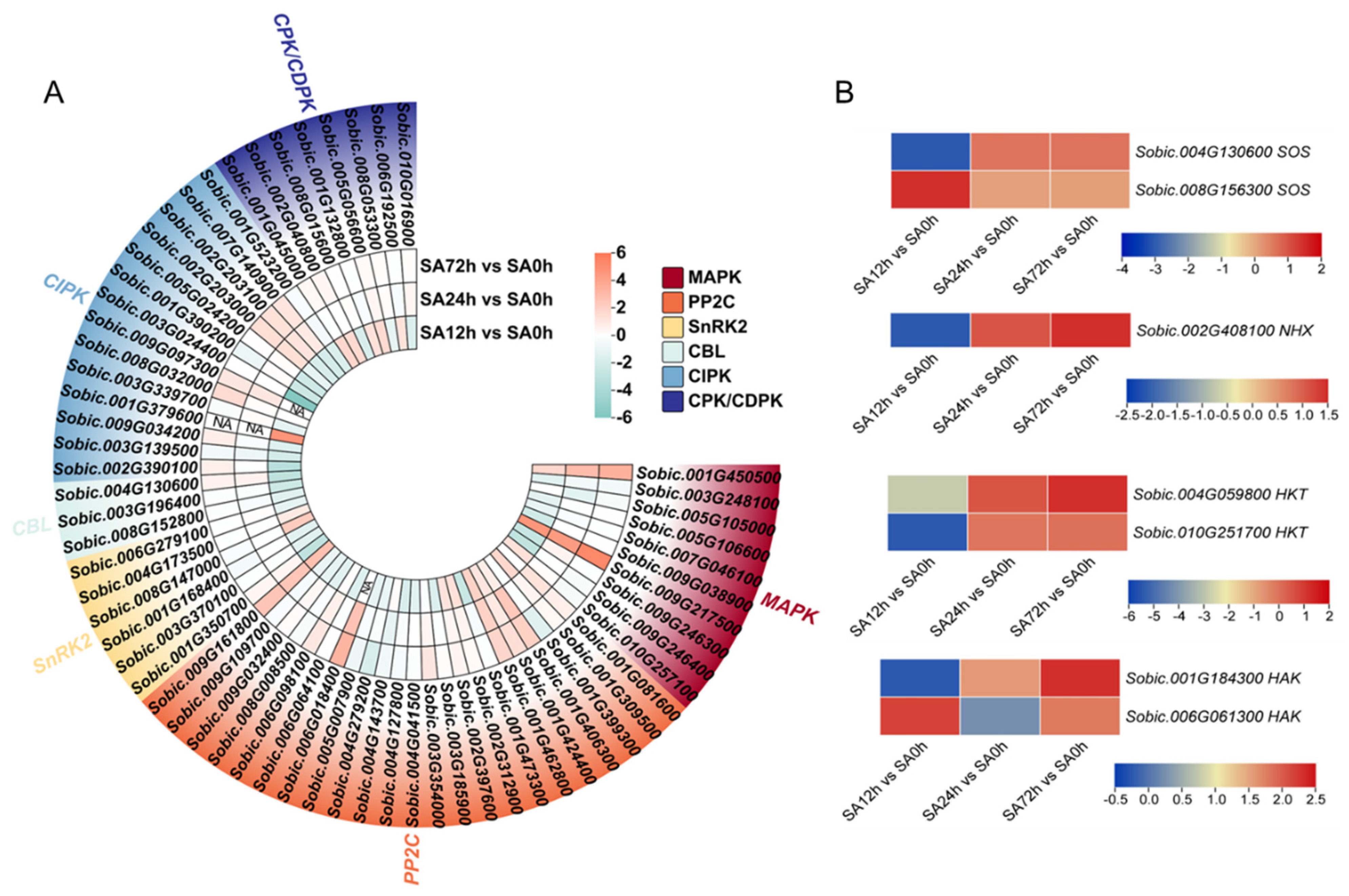
2.8. Expression Profiles of DEGs Involved in Stress Signaling in Sorghum Seedlings Z14 Under Alkali Stress
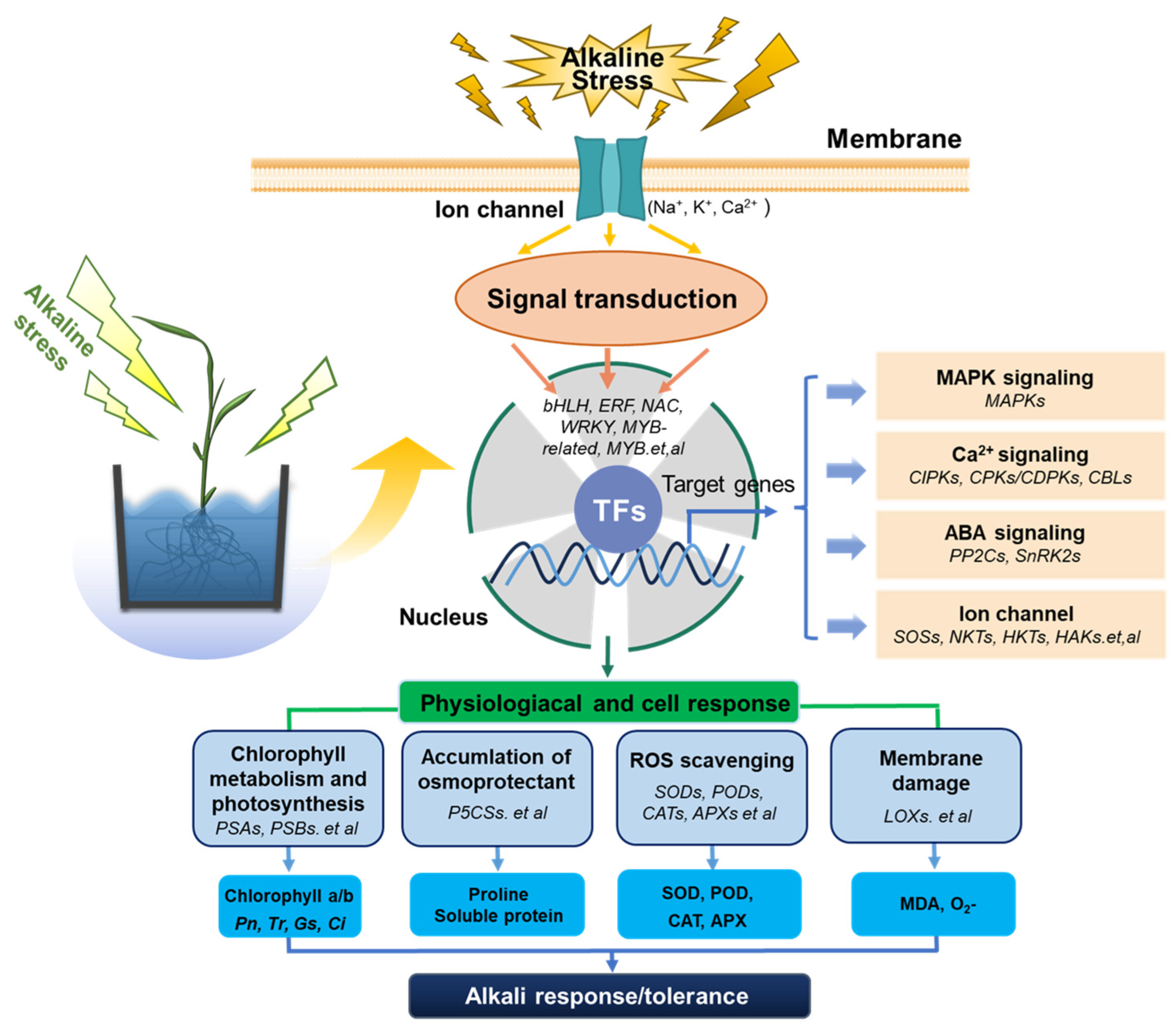
3. Discussion
4. Materials and Methods
4.1. Materials and Treatments
4.2. Determination of Physiological Parameters
4.3. Transcriptome Analysis
4.4. Quantitative Real-Time PCR
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef]
- Fang, S.B.; Tu, W.R.; Mu, L.; Sun, Z.L.; Hu, Q.Y.; Yang, Y. Saline alkali water desalination project in Southern Xinjiang of China: A review of desalination planning, desalination schemes and economic analysis. Renew. Sustain. Energy Rev. 2019, 113, 109268. [Google Scholar] [CrossRef]
- Feng, L.; Li, Q.; Zhou, D.; Jia, M.; Liu, Z.; Hou, Z.; Ren, Q.; Ji, S.; Sang, S.; Lu, S.; et al. B. subtilis CNBG-PGPR-1 induces methionine to regulate ethylene pathway and ROS scavenging for improving salt tolerance of tomato. Plant J. 2024, 117, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.L.; Gao, Z.W.; Gao, Y.; Liu, G.F.; Sheng, L.X.; Wang, D.L. Eco-physiological characteristics of alfalfa seedlings in response to various mixed salt-alkaline stresses. J. Integr. Plant Biol. 2008, 50, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S. Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Li, J.; Yang, Y.; Jiang, C.; Guo, Y. Designing salt stress-resilient crops: Current progress and future challenges. J. Integr. Plant Biol. 2024, 66, 303–329. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Wang, H.; Li, Y.; Yang, Y.; Xu, Y.; Fan, X.; Guo, Z.; Han, Y.; Lin, X. Physiological mechanisms and core genes in response to saline-alkali stress in foxtail millet (Setaria italica L.). Biomolecules 2025, 15, 859. [Google Scholar] [CrossRef]
- Li, N.; Cao, B.; Chen, Z.; Xu, K. Root morphology ion absorption and antioxidative defense system of two Chinese cabbage cultivars (Brassica rapa L.) reveal the different adaptation mechanisms to salt and alkali stress. Protoplasma 2022, 259, 385–398. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; He, F.; Li, M.; Zi, Y.; Long, R.; Zhao, G.; Zhu, L.; Hong, L.; Wang, S.; et al. Multi-omics integrative analysis provided new insights into alkaline stress in alfalfa. Plant Physiol. Biochem. 2024, 215, 109048. [Google Scholar] [CrossRef]
- Ma, L.; Lian, Y.; Li, S.; Fahim, A.M.; Hou, X.; Liu, L.; Pu, Y.; Yang, G.; Wang, W.; Wu, J.; et al. Integrated transcriptome and metabolome analysis revealed molecular regulatory mechanism of saline-alkali stress tolerance and identified bHLH142 in winter rapeseed (Brassica rapa). Int. J. Biol. Macromol. 2025, 295, 139542. [Google Scholar] [CrossRef]
- Ling, L.; An, Y.; Wang, D.; Tang, L.; Du, B.; Shu, Y.; Bai, Y.; Guo, C. Proteomic analysis reveals responsive mechanisms for saline-alkali stress in alfalfa. Plant Physiol. Biochem. 2022, 170, 146–159. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, X.; Wan, W.; Zhu, X.; Shi, M.; Zhang, L.; Yang, F.; Jin, S. MYB4 in Lilium pumilum affects plant saline-alkaline tolerance. Plant Signal Behav. 2024, 19, 2370724. [Google Scholar] [CrossRef]
- Ketehouli, T.; Zhou, Y.G.; Dai, S.Y.; Carther, K.F.I.; Sun, D.Q.; Li, Y.; Nguyen, Q.V.H.; Xu, H.; Wang, F.W.; Liu, W.C.; et al. A soybean calcineurin B-like protein-interacting protein kinase, GmPKS4, regulates plant responses to salt and alkali stresses. J. Plant Physiol. 2021, 256, 153331. [Google Scholar] [CrossRef] [PubMed]
- Swigonová, Z.; Lai, J.; Ma, J.; Ramakrishna, W.; Llaca, V.; Bennetzen, J.L.; Messing, J. Close split of sorghum and maize genome progenitors. Genome Res. 2004, 14, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.N.; Thomas, J.B.; Dahlberg, J.; Rhee, S.Y.; Mortimer, J.C. Progress and challenges in sorghum biotechnology, a multipurpose feedstock for the bioeconomy. J. Exp. Bot. 2022, 73, 646–664. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into plant salt stress signaling and tolerance. J. Genet. Genom. 2024, 51, 16–34. [Google Scholar] [CrossRef]
- Cui, Z.; Hao, F.; Dong, X.; Gao, Y.; Yao, B.; Wang, Y.; Zhang, Y.; Lin, G. Integrated physiological, transcriptomic and metabolomic analyses reveal ROS regulatory mechanisms in two castor bean varieties under alkaline stress. Plant Physiol. Biochem. 2025, 220, 109518. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, F.; Xie, P.; Sun, S.; Qiao, X.; Tang, S.; Chen, C.; Yang, S.; Mei, C.; Yang, D.; et al. A Gγ protein regulates alkaline sensitivity in crops. Science 2023, 379, eade8416. [Google Scholar] [CrossRef]
- Gu, R.; Wan, Z.Q.; Tang, F.; Liu, X.T.; Yang, Y.T.; Shi, F.L. Physiological and transcriptomic analysis of salt tolerant Glaux maritima grown under high saline condition. Front. Plant Sci. 2023, 14, 1173191. [Google Scholar] [CrossRef]
- Guo, R.; Yang, Z.; Li, F.; Yan, C.; Zhong, X.; Liu, Q.; Xia, X.; Li, H.; Zhao, L. Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum) to salt and alkali stress. BMC Plant Biol. 2015, 15, 170. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Wang, F.; Wu, H.; Yang, M.; Xu, W.; Zhao, W.; Qiu, R.; Kang, N.; Cui, G. Unveiling salt tolerance mechanisms and hub genes in alfalfa (Medicago sativa L.) through transcriptomic and WGCNA analysis. Plants 2024, 13, 3141. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhu, C.; Li, B.; Ning, W.; Yin, J. Combining physiology and transcriptome to reveal mechanisms of hosta ‘Golden Cadet’ in response to alkali stress. Plants 2025, 14, 593. [Google Scholar] [CrossRef]
- Yang, L.; Jin, Y.; Huang, W.; Sun, Q.; Liu, F.; Huang, X. Full-length transcriptome sequences of ephemeral plant Arabidopsis pumila provides insight into gene expression dynamics during continuous salt stress. BMC Genom. 2018, 19, 717. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Lai, J.; Liu, Y.; Song, M.; Li, F.; Gong, Q. Integrated biochemical, transcriptomic and metabolomic analyses provide insight into heat stress response in Yangtze sturgeon (Acipenser dabryanus). Ecotoxicol. Environ. Saf. 2023, 249, 114366. [Google Scholar] [CrossRef] [PubMed]
- Harun, S.; Abdullah-Zawawi, M.R.; Goh, H.H.; Mohamed-Hussein, Z.A. A comprehensive gene inventory for glucosinolate biosynthetic pathway in Arabidopsis thaliana. J. Agric. Food Chem. 2020, 68, 7281–7297. [Google Scholar] [CrossRef]
- Debbarma, J.; Sarki, Y.N.; Saikia, B.; Boruah, H.P.D.; Singha, D.L.; Chikkaputtaiah, C. Ethylene response factor (ERF) family proteins in abiotic stresses and CRISPR-Cas9 genome editing of ERFs for multiple abiotic stress tolerance in crop plants: A review. Mol. Biotechnol. 2019, 61, 153–172. [Google Scholar] [CrossRef]
- Ng, D.W.; Abeysinghe, J.K.; Kamali, M. Regulating the Regulators: The control of transcription factors in plant defense signaling. Int. J. Mol. Sci. 2018, 19, 3737. [Google Scholar] [CrossRef]
- Seo, E.; Choi, D.; Choi. Functional studies of transcription factors involved in plant defenses in the genomics era. Brief. Funct. Genom. 2015, 14, 260–267. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, P.; Zhou, T.; Wu, Y.; Yuan, M.; Zhang, X.; Liu, Y. Genome-wide characterization and expression analysis of the bHLH gene family in response to abiotic stresses in Zingiber officinale Roscoe. BMC Genom. 2025, 26, 143. [Google Scholar] [CrossRef]
- Verma, D.; Jalmi, S.K.; Bhagat, P.K.; Verma, N.; Sinha, A.K. A bHLH transcription factor, MYC2, imparts salt intolerance by regulating proline biosynthesis in Arabidopsis. FEBS J. 2020, 287, 2560–2576. [Google Scholar] [CrossRef]
- Michael, D.P.J.; Liu, Q.; Yin, Y.; Wei, X.; Lu, J.; Rehman, F.U.; Temitope, A.; Qian, B.; Xia, H.; Han, J.; et al. LcTprxII overexpression enhances physiological and biochemical effects in maize under alkaline (Na2CO3) stress. Plants 2025, 14, 1467. [Google Scholar] [CrossRef]
- Yu, Y.; Duan, X.; Ding, X.; Chen, C.; Zhu, D.; Yin, K.; Cao, L.; Song, X.; Zhu, P.; Li, Q.; et al. A novel AP2/ERF family transcription factor from Glycine soja, GsERF71, is a DNA binding protein that positively regulates alkaline stress tolerance in Arabidopsis. Plant Mol. Biol. 2017, 94, 509–530. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, T.; Li, J.; Chen, Z.; Guo, B.; An, X. Genome-wide analysis of the MYB-related transcription factor family and associated responses to abiotic stressors in Populus. Int. J. Biol. Macromol. 2021, 191, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, Y.; Wang, L.; Hu, P.; Wang, Y.; Jia, Y.; Zhang, C.; Zhang, Y.; Zhang, Y.; Wang, C.; et al. Expression of the MYB transcription factor gene BplMYB46 affects abiotic stress tolerance and secondary cell wall deposition in Betula platyphylla. Plant Biotechnol. J. 2017, 15, 107–121. [Google Scholar] [CrossRef]
- Mahajan, S.; Pandey, G.K.; Tuteja, N. Calcium- and salt-stress signaling in plants: Shedding light on SOS pathway. Arch. Biochem. Biophys. 2008, 471, 146–158. [Google Scholar] [CrossRef]
- Hao, F.; Cui, Z.; Dong, X.; Gao, Y.; Wang, R.; Zhang, H.; Lin, G. Exogenous calcium enhances castor tolerance to saline-alkaline stress by regulating antioxidant enzyme activity and activating Ca2+ and ROS signaling crosstalk. Int. J. Mol. Sci. 2024, 25, 12717. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, B.; Zhao, Y.; Chu, M.; Yu, H.; Gao, D.; Wang, J.; Li, Z.; Liu, S.; Li, Y.; et al. Physiological and Transcriptional Responses of Sorghum Seedlings Under Alkali Stress. Plants 2025, 14, 3106. https://doi.org/10.3390/plants14193106
Liu X, Wang B, Zhao Y, Chu M, Yu H, Gao D, Wang J, Li Z, Liu S, Li Y, et al. Physiological and Transcriptional Responses of Sorghum Seedlings Under Alkali Stress. Plants. 2025; 14(19):3106. https://doi.org/10.3390/plants14193106
Chicago/Turabian StyleLiu, Xinyu, Bo Wang, Yiyu Zhao, Min Chu, Han Yu, Di Gao, Jiaheng Wang, Ziqi Li, Sibei Liu, Yuhan Li, and et al. 2025. "Physiological and Transcriptional Responses of Sorghum Seedlings Under Alkali Stress" Plants 14, no. 19: 3106. https://doi.org/10.3390/plants14193106
APA StyleLiu, X., Wang, B., Zhao, Y., Chu, M., Yu, H., Gao, D., Wang, J., Li, Z., Liu, S., Li, Y., Wei, Y., Wei, J., & Xu, J. (2025). Physiological and Transcriptional Responses of Sorghum Seedlings Under Alkali Stress. Plants, 14(19), 3106. https://doi.org/10.3390/plants14193106






