Effects of Different Rootstocks on Graft Compatibility, Growth, Yield, and Fruit Quality of Table Grape ‘Fengguang’
Abstract
1. Introduction
2. Results
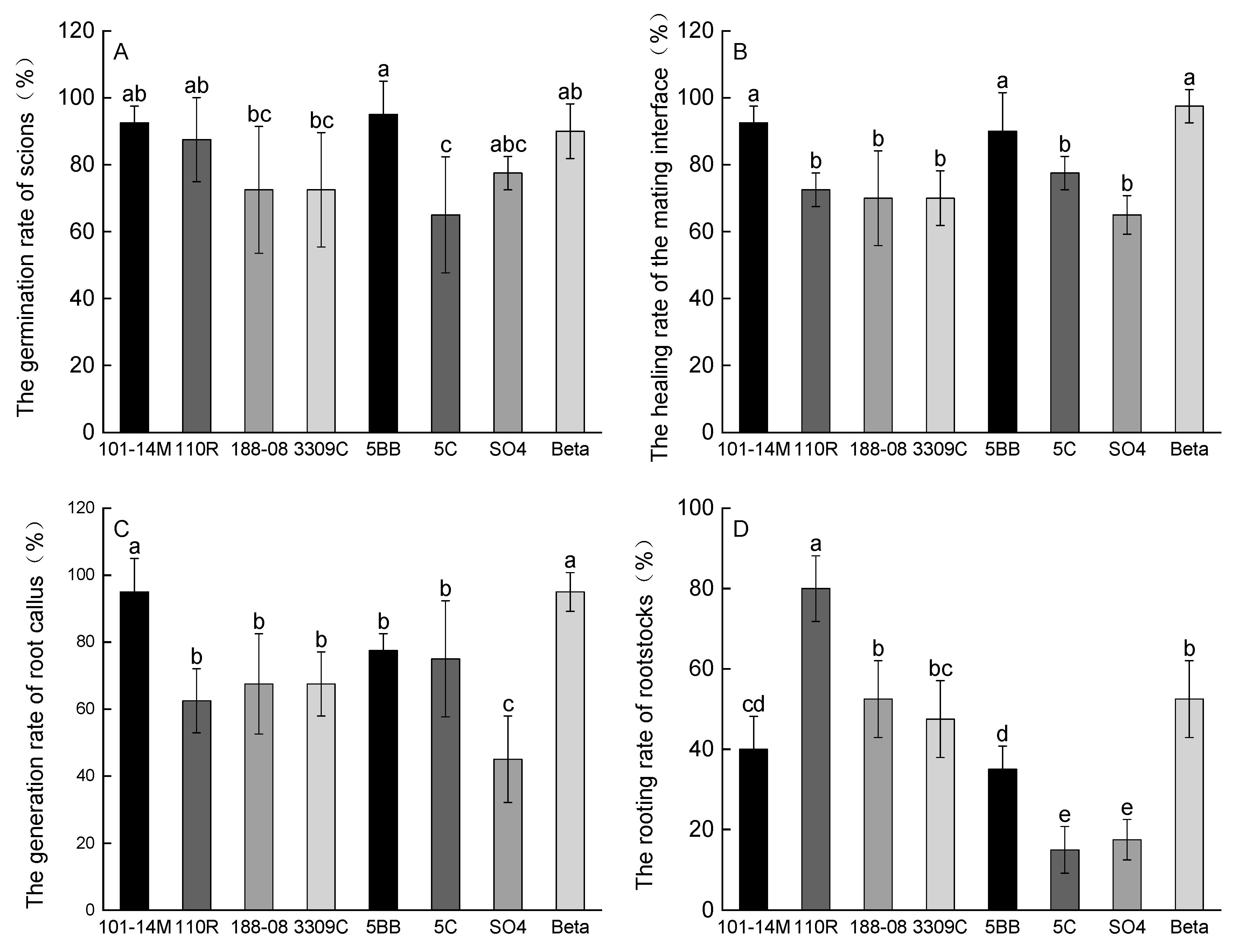
2.1. Graft Compatibility Indicators
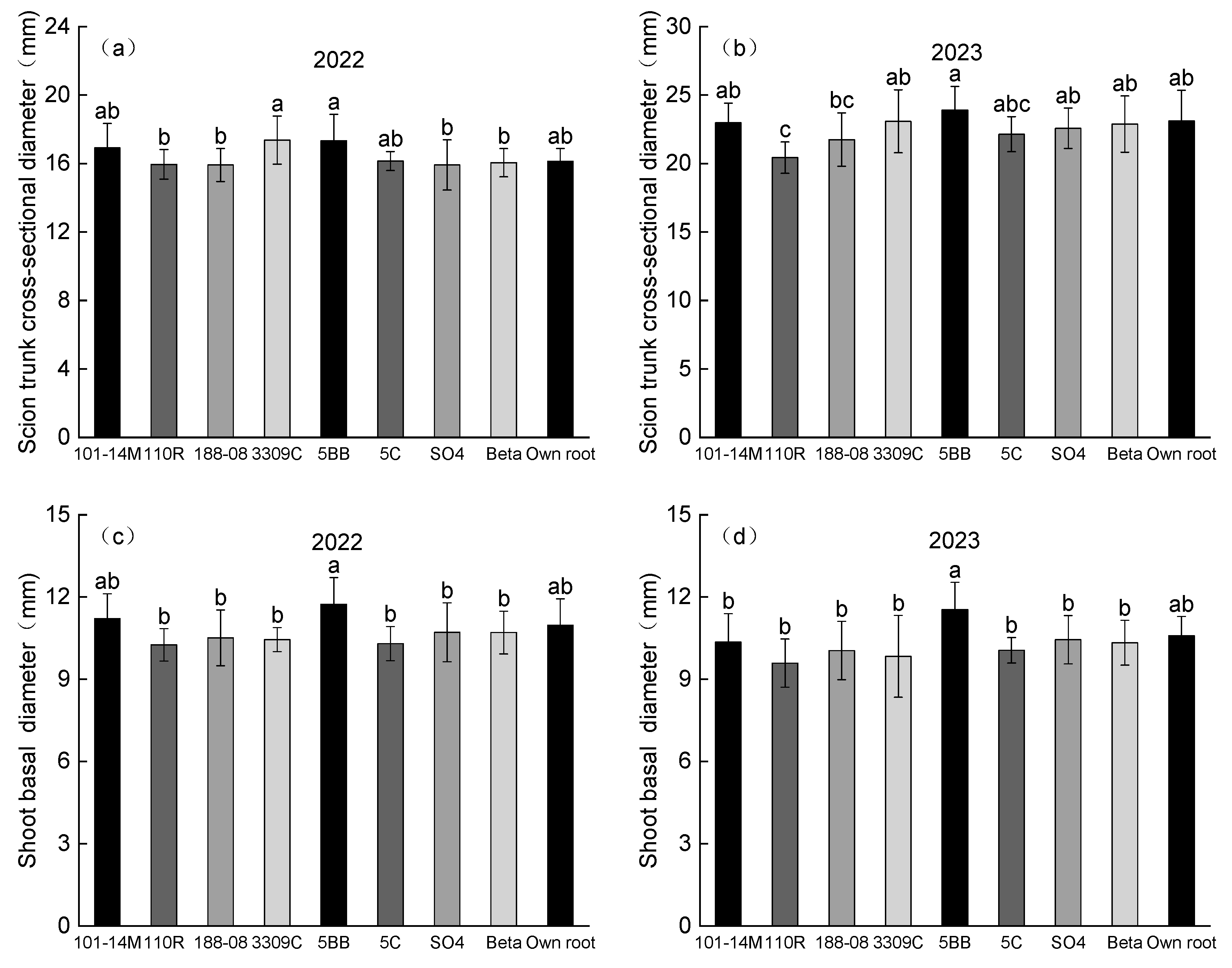
2.2. Vine Growth
2.3. Yield and Appearance Attributes of Cluster and Berry
2.4. Physical and Chemical Indicators
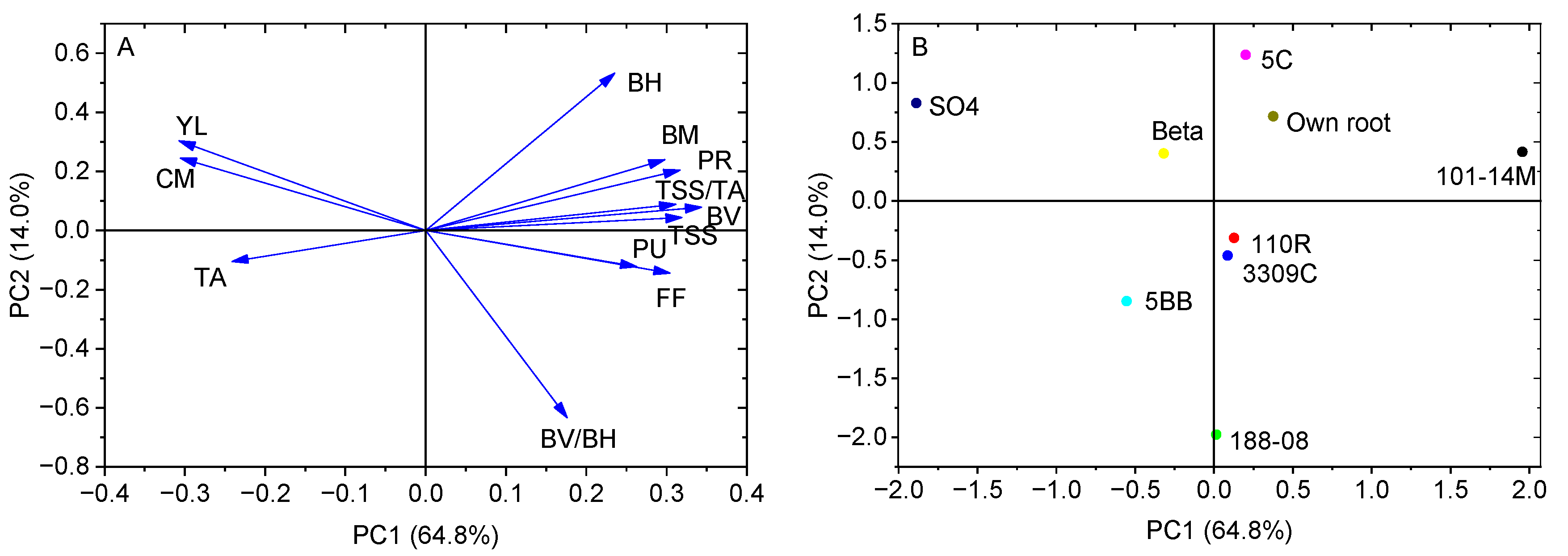
2.5. Principal Component Analysis
2.6. Comprehensive Evaluation of Yield and Berry Quality
3. Discussion
4. Materials and Methods
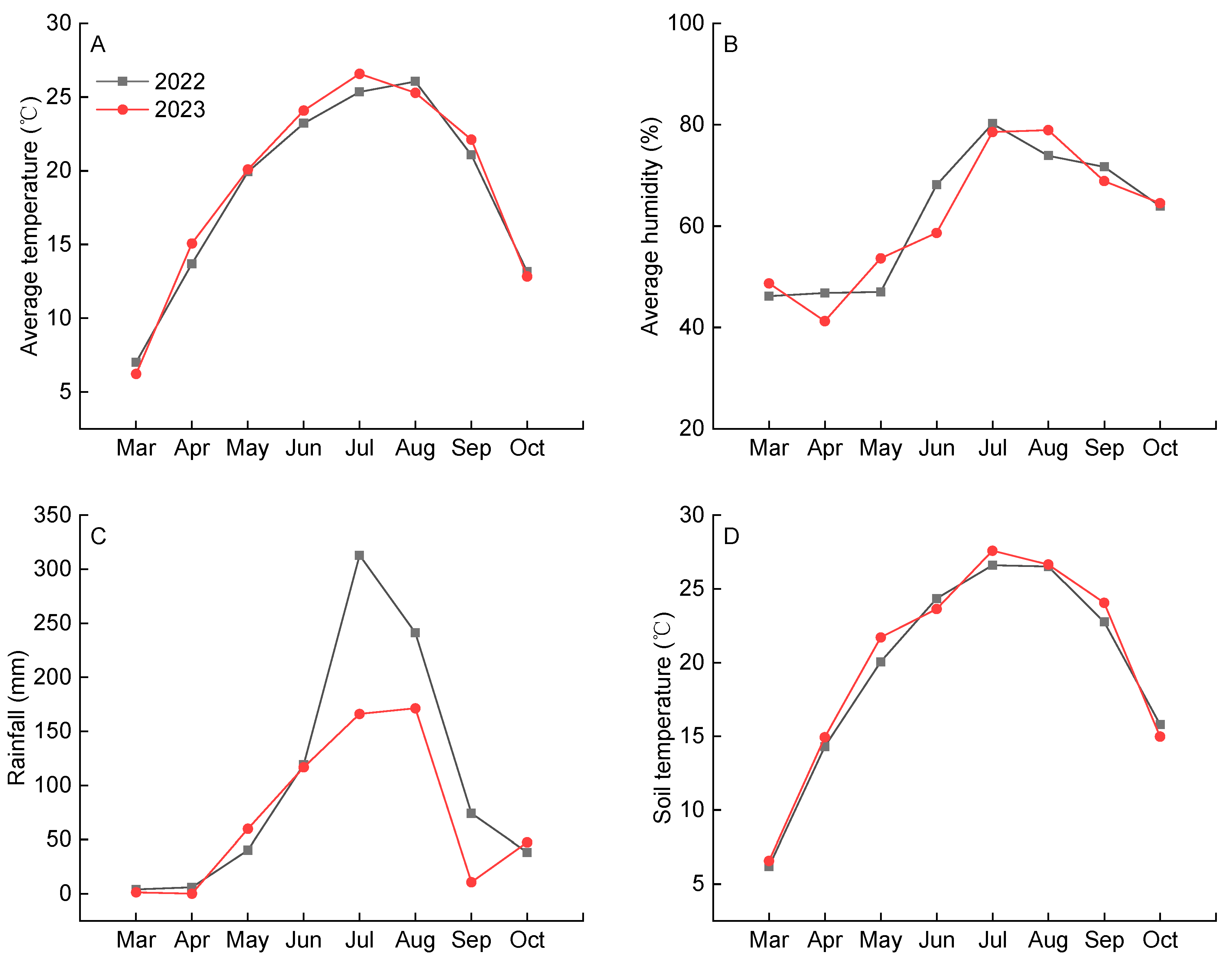
4.1. Experimental Site and Growth Conditions
4.2. Plant Materials, Experimental Design and Vine Management
4.3. Affinity Metrics for Grafting
4.4. Growth Parameters
4.5. Yield Calculation
4.6. Fruit Quality Parameters
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, X.T.; Sun, D.; Wu, M.H.; Li, H.Q.; Liu, F.Q.; He, F.; Pan, Q.H.; Wang, J. Influence of cluster positions in the canopy and row orientation on the flavonoid and volatile compound profiles in Vitis vinifera L. Cabernet franc and Chardonnay berries. Food Res. Int. 2021, 143, 110306. [Google Scholar] [CrossRef]
- Goulioti, E.; Jeffery, D.W.; Gialouris, P.L.; Nastou, E.; Dasenaki, M.; Kontoudakis, N.; Thomaidis, N.; Kotseridis, Y. Effect of terroir on aroma and sensory characteristics of Xinomavro (Vitis vinifera L. cv.) red wines from different Greek protected designations of origin. Food Chem. 2025, 486, 144507. [Google Scholar] [CrossRef]
- Fu, J.; Wang, X.; Fu, Z.; Mu, W. Assessment of consumers’ perception and cognition toward table grape consumption in China. Br. Food J. 2014, 116, 611–628. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, J.; Xu, X.; Perl, A.; Chen, S.; Ma, H. Adoption of table grape cultivars: An attribute preference study on Chinese grape growers. Sci. Hortic. 2017, 216, 66–75. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, J.; Zhang, Z.; Yao, M. Study on price fluctuation characteristics and influencing factors of table grape in China. North. Hortic. 2024, 48, 136–144. [Google Scholar]
- Oliveira, C.R.S.d.; Mendonca Junior, A.F.d.; Leão, P.C.d.S. Rootstock effects on fruit yield and quality of ‘BRS Tainá’ seedless table grape in semi-arid tropical conditions. Plants 2024, 13, 2314. [Google Scholar] [CrossRef]
- Flor, L.; Toro, G.; Carriquí, M.; Buesa, I.; Sabater, A.; Medrano, H.; Escalona, J.M. Impact of severe water stress on drought resistance mechanisms and hydraulic vulnerability segmentation in grapevine: The role of rootstock. J. Exp. Bot. 2025, 76, 3141–3157. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Nai, G.; Yan, H.; Sun, P.; Bao, J.; Li, Z.; Chen, G.; Zhang, J.; Wang, J.; Ma, L.; et al. The mechanism of micrografting with salt-tolerant rootstock in improving the salt tolerance of Scion in Vitis vinifera. Physiol. Plant. 2025, 177, e70403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhong, H.; Zhou, X.; Pan, M.; Xu, J.; Liu, M.; Wang, M.; Liu, G.; Xu, T.; Wang, Y.; et al. Grafting with rootstocks promotes phenolic compound accumulation in grape berry skin during development based on integrative multi-omics analysis. Hortic. Res. 2022, 9, uhac055. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, S.; Fevereiro, P.; Kragler, F.; Pina, A. Plant grafting and graft incompatibility: A review from the grapevine perspective. Sci. Hortic. 2022, 299, 111019. [Google Scholar] [CrossRef]
- Feng, M.; Augstein, F.; Kareem, A.; Melnyk, C.W. Plant grafting: Molecular mechanisms and applications. Mol. Plant 2024, 17, 75–91. [Google Scholar] [CrossRef]
- Wang, L.; Liao, Y.; Liu, Q.; Zhao, T.; Jia, L.; Chen, Z. Advances in understanding the graft healing mechanism: A review of factors and regulatory pathways. Hortic. Res. 2024, 11, uhae175. [Google Scholar] [CrossRef]
- Guo, Z.; Sun, Y.; Liu, C.; Han, B.; Li, M.; Yin, Y.; Jia, N.; Yuan, J.; Zhao, S. A new good quality and large berry grape cultivar Fengguang. J. Fruit Sci. 2021, 38, 1396–1398. [Google Scholar]
- Wang, Y.; Chen, W.K.; Gao, X.T.; He, L.; Yang, X.H.; He, F.; Duan, C.Q.; Wang, J. Rootstock-mediated effects on Cabernet Sauvignon performance: Vine Growth, berry ripening, flavonoids, and aromatic profiles. Int. J. Mol. Sci. 2019, 20, 401. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, Y.; Lu, H.C.; Yang, H.Y.; Li, H.Q.; Gao, X.T.; Pei, X.X.; He, F.; Duan, C.Q.; Wang, J. The combined influence of rootstock and vintage climate on the grape and wine flavonoids of Vitis vinifera L. cv. Cabernet Sauvignon in eastern China. Front. Plant Sci. 2022, 13, 978497. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gamboa, G.; Gómez-Plaza, E.; Bautista-Ortín, A.B.; Garde-Cerdán, T.; Moreno-Simunovic, Y.; Martínez-Gil, A.M. Rootstock effects on grape anthocyanins, skin and seed proanthocyanidins and wine color and phenolic compounds from Vitis vinifera L. Merlot grapevines. J. Sci. Food Agric. 2019, 99, 2846–2854. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, H.; Li, Y.; Du, T.; Jia, J.; Xi, Z. The expression of aroma components and related genes in Merlot and Marselan scion–rootstock grape and wine. Foods 2022, 11, 2777. [Google Scholar] [CrossRef]
- Carrasco-Quiroz, M.; Martínez-Gil, A.M.; Gutiérrez-Gamboa, G.; Moreno-Simunovic, Y. Effect of rootstocks on volatile composition of Merlot wines. J. Sci. Food Agric. 2020, 100, 3517–3524. [Google Scholar] [CrossRef]
- Li, M.; Yan, X.; Guo, Z.; Jia, N.; Yuan, J.; Han, B.; Yin, Y.; Sun, Y.; Liu, C.; Zhao, S. Rootstock influence on vegetative growth, yield, and fruit quality of ‘Petit Verdot’. Eur. J. Hortic. Sci. 2019, 84, 343–349. [Google Scholar] [CrossRef]
- Li, M.; Guo, Z.; Jia, N.; Yuan, J.; Han, B.; Yin, Y.; Sun, Y.; Liu, C.; Zhao, S. Evaluation of eight rootstocks on the growth and berry quality of ‘Marselan’ grapevines. Sci. Hortic. 2019, 248, 58–61. [Google Scholar] [CrossRef]
- Gu, Y.; Fan, X.; Jiang, K.; Liu, P.; Chang, H.; Andom, O.; Cheng, J.; Li, Z. Omics analysis of ‘Shine Muscat’ grape grafted on different rootstocks in response to cadmium stress. Sci. Total Environ. 2024, 936, 173472. [Google Scholar] [CrossRef]
- Chen, H.; Li, C.; Li, Y.; Wang, X.; Xi, Z. Effects of cultivars as rootstocks on the expression of aroma components and related genes in Shine Muscat grape. Eur. Food Res. Technol. 2024, 250, 1043–1059. [Google Scholar] [CrossRef]
- Jin, Z.X.; Sun, T.Y.; Sun, H.; Yue, Q.Y.; Yao, Y.X. Modifications of ‘Summer Black’ grape berry quality as affected by the different rootstocks. Sci. Hortic. 2016, 210, 130–137. [Google Scholar] [CrossRef]
- Yin, Y.; Jia, N.; Li, M.; Liu, C.; Yuan, J.; Han, B.; Sun, Y.; Zhao, S.; Guo, Z. Rootstocks induce shifts in tree vigor, yield and berry quality of ‘Summer Black’ grapevines. Eur. J. Hortic. Sci. 2021, 86, 41–48. [Google Scholar] [CrossRef]
- Shahda, M. Effect of rootstocks on growth, yield and fruit quality of Red Globe grape. Ann. Agric. Sci. Moshtohor 2016, 54, 339–344. [Google Scholar] [CrossRef]
- Ibacache, A.; Albornoz, F.; Zurita-Silva, A. Yield responses in Flame seedless, Thompson seedless and Red Globe table grape cultivars are differentially modified by rootstocks under semi arid conditions. Sci. Hortic. 2016, 204, 25–32. [Google Scholar] [CrossRef]
- Chou, M.; Li, K. Rootstock and seasonal variations affect anthocyanin accumulation and quality traits of ‘Kyoho‘ grape berries in subtropical double cropping system. Vitis 2014, 53, 193–199. [Google Scholar]
- Motosugi, H.; Yamamoto, Y.; Naruo, T.; Yamaguchi, D. Growth and fruit quality of ‘Kyoho’ grapevines grafted on autotetraploid rootstocks. J. Jpn. Soc. Hortic. Sci. 2007, 76, 271–278. [Google Scholar] [CrossRef]
- Han, X.; Xing, T.; Wang, J.; He, F. Comparison of branch graft compatibilities and growth differences between different combinations of grapes. Sino-Overseas Grapevine Wine 2022, 29, 1–7. [Google Scholar]
- LaI, N.; Ramteke, V.; Diwan, G.; Singh, P.; Sahu, N.; Hota, D.; Maneesha, S.R.; Meena, N.K.; Sharma, K.M.; Shiurkar, G.B.; et al. Physiological, biochemical, and molecular responses during grafting in horticultural crops. Plant Mol. Biol. Rep. 2025, 43, 1–22. [Google Scholar] [CrossRef]
- Reynolds, A.G.; Wardle, D.A. Rootstocks Impact vine performance and fruit composition of grapes in British Columbia. HortTechnology 2001, 11, 419–427. [Google Scholar] [CrossRef]
- Jogaiah, S.; Sharma, A.K.; Adsule, P.G. Rootstock influence on the biochemical composition and polyphenol oxidase activity of ‘Thompson Seedless’ grapes and raisins. Int. J. Fruit Sci. 2014, 14, 133–146. [Google Scholar] [CrossRef]
- Chen, Y.; Fei, Y.; Howell, K.; Chen, D.; Clingeleffer, P.; Zhang, P. Rootstocks for grapevines now and into the future: Selection of rootstocks based on drought tolerance, soil nutrient availability, and soil pH. Aust. J. Grape Wine Res. 2024, 2024, 6704238. [Google Scholar] [CrossRef]
- Li, M.; Yin, Y.; Jia, N.; Han, B.; Sun, Y.; Liu, C.; Zeng, Q.; Guo, Y.; Liu, S.; Guo, Z. Rootstock-induced impact on vine growth, yield, berry quality, and aroma profiles of ‘Chunguang’ in northern China. Eur. J. Hortic. Sci. 2023, 88, 1–8. [Google Scholar] [CrossRef]
- Jin, Z.; Sun, H.; Sun, T.; Wang, Q.; Yao, Y. Modifications of ‘Gold Finger’ grape berry quality as affected by the different rootstocks. J. Agric. Food Chem. 2016, 64, 4189–4197. [Google Scholar] [CrossRef]
- Tandonnet, J.P.; Cookson, S.J.; Vivin, P.; Ollat, N. Scion genotype controls biomass allocation and root development in grafted grapevine. Aust. J. Grape Wine Res. 2009, 16, 290–300. [Google Scholar] [CrossRef]
- Harris, Z.N.; Pratt, J.E.; Kovacs, L.G.; Klein, L.L.; Kwasniewski, M.T.; Londo, J.P.; Wu, A.S.; Miller, A.J. Grapevine scion gene expression is driven by rootstock and environment interaction. BMC Plant Biol. 2023, 23, 211. [Google Scholar] [CrossRef]
- Lu, L.; Delrot, S.; Liang, Z. From acidity to sweetness: A comprehensive review of carbon accumulation in grape berries. Mol. Hortic. 2024, 4, 22. [Google Scholar] [CrossRef]
- Du, Y.; Wang, Z.; Sun, Q.; Zhai, H.; Wang, Z. Evaluation on grape phylloxera resistance in several grape varieties and rootstocks. Acta Entomol. Sin. 2008, 51, 33–39. [Google Scholar]
- Fu, Q.; Tan, Y.; Zhai, H.; Du, Y. Evaluation of salt resistance mechanisms of grapevine hybrid rootstocks. Sci. Hortic. 2019, 243, 148–158. [Google Scholar] [CrossRef]
- Lupo, Y.; Schlisser, A.; Dong, S.; Rachmilevitch, S.; Fait, A.; Lazarovitch, N. Root system response to salt stress in grapevines (Vitis spp.): A link between root structure and salt exclusion. Plant Sci. 2022, 325, 111460. [Google Scholar] [CrossRef]
- Carbonneau, A. The early selection of grapevine rootstocks for resistance to drought conditions. Am. J. Enol. Vitic. 1985, 36, 195. [Google Scholar] [CrossRef]
- Gao, Z.; Zhai, H.; Zang, X.; Zhu, H.; Du, Y. Using differential thermal analysis to analyze grape buds cold hardiness of 8 rootstocks and 6 cultivars. Acta Hortic. Sinica 2014, 41, 17–25. [Google Scholar]
- Hou, P.; Zhang, J.; Zhang, X. Fuzzy comprehensive evaluation for selecting mini watermelon cultivars. J. Sci. Food Agric. 2010, 90, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Li, Y.; Zhao, S.; Ding, W.; Hui, N.; Li, H. Effect of dark period lighting regulation on cucumber seedling morphology and comprehensive evaluation analysis and comprehensive evaluation. Trans. Chin. Soc. Agric. Eng. 2014, 30, 201–208. [Google Scholar]
| Year | Rootstock | Yield (kg/vine) | Cluster Mass (g) | Berry Mass (g) | BV 1 (mm) | BH 2 (mm) | BV/BH |
|---|---|---|---|---|---|---|---|
| 2022 | 101-14M | 4.31 ± 0.08 e 3 | 529.53 ± 10.32 c | 12.32 ± 0.15 a | 31.60 ± 1.86 a | 27.00 ± 2.11 a | 1.17 ± 0.05 abc |
| 110R | 4.98 ± 0.19 b | 547.27 ± 20.53 bc | 11.48 ± 0.09 c | 29.85 ± 2.21 bc | 25.20 ± 1.46 abc | 1.18 ± 0.06 ab | |
| 188-08 | 4.71 ± 0.06 cd | 540.53 ± 7.33 bc | 11.02 ± 0.04 f | 28.84 ± 1.85 bc | 24.21 ± 1.54 c | 1.19 ± 0.09 a | |
| 3309C | 4.81 ± 0.27 bcd | 526.77 ± 29.10 c | 10.73 ± 0.09 g | 28.24 ± 1.58 c | 25.00 ± 1.69 bc | 1.13 ± 0.04 bc | |
| 5BB | 4.65 ± 0.13 d | 559.27 ± 14.82 b | 11.13 ± 0.05 ef | 29.10 ± 1.99 bc | 25.07 ± 1.74 bc | 1.16 ± 0.06 abc | |
| 5C | 4.91 ± 0.05 bc | 539.23 ± 5.59 bc | 11.86 ± 0.07 b | 30.22 ± 1.24 ab | 26.61 ± 2.00 ab | 1.14 ± 0.06 abc | |
| SO4 | 5.47 ± 0.02 a | 585.67 ± 1.86 a | 10.52 ± 0.12 h | 28.09 ± 2.60 c | 25.23 ± 2.43 abc | 1.11 ± 0.06 c | |
| Beta | 5.04 ± 0.06 b | 561.93 ± 6.71 ab | 11.20 ± 0.03 de | 29.51 ± 1.62 bc | 25.88 ± 1.32 abc | 1.14 ± 0.05 abc | |
| Own root | 4.81 ± 0.05 bcd | 557.13 ± 4.92 b | 11.32 ± 0.04 d | 29.71 ± 1.26 bc | 26.34 ± 1.52 ab | 1.13 ± 0.05 bc | |
| 2023 | 101-14M | 4.59 ± 0.23 d | 551.30 ± 28.05 c | 11.95 ± 0.10 a | 29.94 ± 1.94 a | 25.97 ± 1.79 a | 1.16 ± 0.12 ab |
| 110R | 5.04 ± 0.22 bc | 569.87 ± 25.12 bc | 11.35 ± 0.07 c | 27.83 ± 1.85 cd | 24.50 ± 1.48 b | 1.14 ± 0.07 ab | |
| 188-08 | 4.80 ± 0.10 cd | 560.92 ± 11.37 bc | 10.93 ± 0.10 e | 29.29 ± 1.79 abc | 24.45 ± 1.14 b | 1.20 ± 0.04 a | |
| 3309C | 4.99 ± 0.15 bc | 560.40 ± 16.65 bc | 10.67 ± 0.09 f | 29.60 ± 1.69 ab | 25.53 ± 1.35 ab | 1.16 ± 0.06 ab | |
| 5BB | 4.87 ± 0.08 bc | 589.47 ± 9.78 ab | 10.97 ± 0.07 e | 28.22 ± 2.10 bcd | 24.66 ± 1.20 b | 1.15 ± 0.04 ab | |
| 5C | 5.15 ± 0.12 b | 578.30 ± 13.00 abc | 11.74 ± 0.08 b | 28.23 ± 1.71 bcd | 25.06 ± 1.19 ab | 1.13 ± 0.04 b | |
| SO4 | 5.60 ± 0.16 a | 607.90 ± 17.95 a | 10.39 ± 0.11 g | 27.28 ± 1.51 d | 24.40 ± 1.66 b | 1.12 ± 0.05 b | |
| Beta | 5.11 ± 0.09 b | 578.97 ± 9.52 abc | 11.15 ± 0.06 d | 29.11 ± 1.18 abc | 25.53 ± 0.88 ab | 1.14 ± 0.05 ab | |
| Own root | 4.94 ± 0.14bc | 576.70 ± 16.23 abc | 11.22 ± 0.03 cd | 29.34 ± 0.67 abc | 25.45 ± 0.61 ab | 1.15 ± 0.04 ab |
| Year | Rootstock | Pulling Resistance (N) | Pressure Resistance (N) | Flesh Firmness (kg/cm2) | TSS 1 (%) | TA 2 (%) | TSS/TA |
|---|---|---|---|---|---|---|---|
| 2022 | 101-14M | 4.84 ± 0.26 a 3 | 19.40 ± 0.61 a | 0.70 ± 0.03 a | 17.57 ± 0.35 a | 0.72 ± 0.02 bc | 24.51 ± 0.07 a |
| 110R | 4.79 ± 0.14 ab | 18.08 ± 0.36 cde | 0.54 ± 0.02 e | 17.13 ± 0.15 bc | 0.73 ± 0.02 ab | 23.37 ± 0.27 b | |
| 188-08 | 4.62 ± 0.18 bc | 17.86 ± 0.45 de | 0.62 ± 0.02 b | 17.03 ± 0.21 bc | 0.73 ± 0.02 abc | 23.44 ± 0.29 b | |
| 3309C | 4.83 ± 0.20 a | 17.77 ± 0.29 e | 0.62 ± 0.02 b | 16.80 ± 0.30 cd | 0.70 ± 0.02 c | 23.89 ± 0.41 ab | |
| 5BB | 4.77 ± 0.14 abc | 18.37 ± 0.27 c | 0.61 ± 0.02 bc | 16.40 ± 0.36 de | 0.75 ± 0.02 a | 21.97 ± 0.28 c | |
| 5C | 4.61 ± 0.10 c | 19.19 ± 0.38 a | 0.56 ± 0.01 d | 16.73 ± 0.21 cd | 0.70 ± 0.02 c | 23.80 ± 0.41 ab | |
| SO4 | 4.30 ± 0.18 d | 17.24 ± 0.30 f | 0.50 ± 0.03 f | 16.27 ± 0.21 e | 0.74 ± 0.02 ab | 22.10 ± 0.80 c | |
| Beta | 4.64 ± 0.15 bc | 18.16 ± 0.26 cd | 0.62 ± 0.02 b | 16.87 ± 0.12 c | 0.75 ± 0.01 a | 22.59 ± 0.33 c | |
| Own root | 4.72 ± 0.15 abc | 18.73 ± 0.42 b | 0.59 ± 0.02 c | 17.40 ± 0.10 ab | 0.71 ± 0.01 bc | 24.40 ± 0.10 a | |
| 2023 | 101-14M | 4.82 ± 0.24 a | 21.01 ± 1.14 a | 0.79 ± 0.05 a | 18.03 ± 0.12 a | 0.71 ± 0.02 c | 25.29 ± 0.69 a |
| 110R | 4.78 ± 0.17 a | 18.80 ± 0.79 b | 0.56 ± 0.03 cd | 17.20 ± 0.10 cde | 0.70 ± 0.02 c | 24.70 ± 0.46 ab | |
| 188-08 | 4.38 ± 0.19 d | 17.64 ± 0.32 d | 0.61 ± 0.02 b | 17.30 ± 0.17 cd | 0.73 ± 0.02 abc | 23.82 ± 0.45 bc | |
| 3309C | 4.77 ± 0.13 ab | 17.32± 0.20 de | 0.56 ± 0.03 cd | 17.43 ± 0.21 bc | 0.72 ± 0.03 bc | 24.11 ± 0.68 b | |
| 5BB | 4.74 ± 0.15 ab | 17.29 ± 0.23 de | 0.58 ± 0.02 bc | 16.90 ± 0.10 f | 0.75 ± 0.02 ab | 22.44 ± 0.38 d | |
| 5C | 4.54 ± 0.13 c | 18.22 ± 0.30 c | 0.54 ± 0.02 d | 17.13 ± 0.06 def | 0.71 ± 0.02 c | 24.14 ± 0.76 b | |
| SO4 | 4.20 ± 0.16 e | 17.06 ± 0.31 e | 0.48 ± 0.04 e | 16.93 ± 0.15 ef | 0.76 ± 0.03 a | 22.30 ± 0.60 d | |
| Beta | 4.61 ± 0.15 bc | 17.62 ± 0.24 d | 0.58 ± 0.02 bc | 17.20 ± 0.10 cde | 0.75 ± 0.01 ab | 22.94 ± 0.39 cd | |
| Own root | 4.66 ± 0.13 abc | 17.64 ± 0.28 d | 0.56 ± 0.02 cd | 17.67 ± 0.21 b | 0.72 ± 0.02 bc | 24.43 ± 0.23 ab |
| Rootstock | YL | CM | BM | BV | BH | BV/BH | PU | PR | FF | TSS | TA | TSS/TA | Comprehensive Evaluation Value | Rank |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 101-14M | 0.03 | 0.03 | 0.08 | 0.08 | 0.08 | 0.06 | 0.08 | 0.07 | 0.07 | 0.07 | 0.04 | 0.07 | 0.76 | 1 |
| 110R | 0.06 | 0.05 | 0.06 | 0.05 | 0.04 | 0.06 | 0.07 | 0.05 | 0.04 | 0.05 | 0.04 | 0.06 | 0.61 | 4 |
| 188-08 | 0.05 | 0.04 | 0.04 | 0.05 | 0.03 | 0.08 | 0.05 | 0.04 | 0.05 | 0.05 | 0.05 | 0.05 | 0.58 | 7 |
| 3309C | 0.05 | 0.03 | 0.04 | 0.05 | 0.05 | 0.05 | 0.07 | 0.04 | 0.05 | 0.05 | 0.04 | 0.06 | 0.57 | 8 |
| 5BB | 0.05 | 0.06 | 0.05 | 0.04 | 0.04 | 0.05 | 0.07 | 0.04 | 0.05 | 0.03 | 0.08 | 0.03 | 0.59 | 6 |
| 5C | 0.06 | 0.05 | 0.07 | 0.05 | 0.06 | 0.04 | 0.06 | 0.05 | 0.04 | 0.04 | 0.03 | 0.06 | 0.60 | 5 |
| SO4 | 0.08 | 0.08 | 0.03 | 0.03 | 0.04 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.07 | 0.03 | 0.51 | 9 |
| Beta | 0.06 | 0.06 | 0.05 | 0.05 | 0.06 | 0.04 | 0.06 | 0.04 | 0.05 | 0.05 | 0.07 | 0.04 | 0.63 | 3 |
| Own root | 0.05 | 0.05 | 0.05 | 0.06 | 0.06 | 0.05 | 0.07 | 0.05 | 0.04 | 0.06 | 0.04 | 0.06 | 0.65 | 2 |
| Depth | Organic Matter (%) | Total N(%) | Available P (mg/kg) | Available K (mg/kg) | Available Ca (mg/kg) | Available Mg (mg/kg) |
|---|---|---|---|---|---|---|
| 0–20 cm | 3.08 | 0.19 | 1.84 | 2.13 | 3.28 | 3.62 |
| 20–40 cm | 2.63 | 0.12 | 1.05 | 1.94 | 2.04 | 3.57 |
| 40–60 cm | 1.66 | 0.13 | 0.93 | 1.85 | 2.51 | 3.52 |
| Rootstocks | Phylloxera | Salinity | Drought | Coldness |
|---|---|---|---|---|
| 101-14M | HR | R | S | R |
| 110R | HR | MR | HR | LR |
| 188-08 | – | LR | MR | – |
| 3309C | HR | MR | – | MR |
| 5BB | HR | MR | S | HR |
| 5C | HR | MR | – | MR |
| SO4 | HR | S | R | HR |
| Beta | S | MR | S | HR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, N.; Li, M.; Liu, C.; Han, B.; Sun, Y.; Han, S.; Wang, X.; Yin, Y. Effects of Different Rootstocks on Graft Compatibility, Growth, Yield, and Fruit Quality of Table Grape ‘Fengguang’. Plants 2025, 14, 3098. https://doi.org/10.3390/plants14193098
Jia N, Li M, Liu C, Han B, Sun Y, Han S, Wang X, Yin Y. Effects of Different Rootstocks on Graft Compatibility, Growth, Yield, and Fruit Quality of Table Grape ‘Fengguang’. Plants. 2025; 14(19):3098. https://doi.org/10.3390/plants14193098
Chicago/Turabian StyleJia, Nan, Minmin Li, Changjiang Liu, Bin Han, Yan Sun, Shuli Han, Xinyu Wang, and Yonggang Yin. 2025. "Effects of Different Rootstocks on Graft Compatibility, Growth, Yield, and Fruit Quality of Table Grape ‘Fengguang’" Plants 14, no. 19: 3098. https://doi.org/10.3390/plants14193098
APA StyleJia, N., Li, M., Liu, C., Han, B., Sun, Y., Han, S., Wang, X., & Yin, Y. (2025). Effects of Different Rootstocks on Graft Compatibility, Growth, Yield, and Fruit Quality of Table Grape ‘Fengguang’. Plants, 14(19), 3098. https://doi.org/10.3390/plants14193098







