Efficient Production of Vigorous Scions by Optimizing Leaf Retention in Passiflora edulis
Abstract
1. Introduction
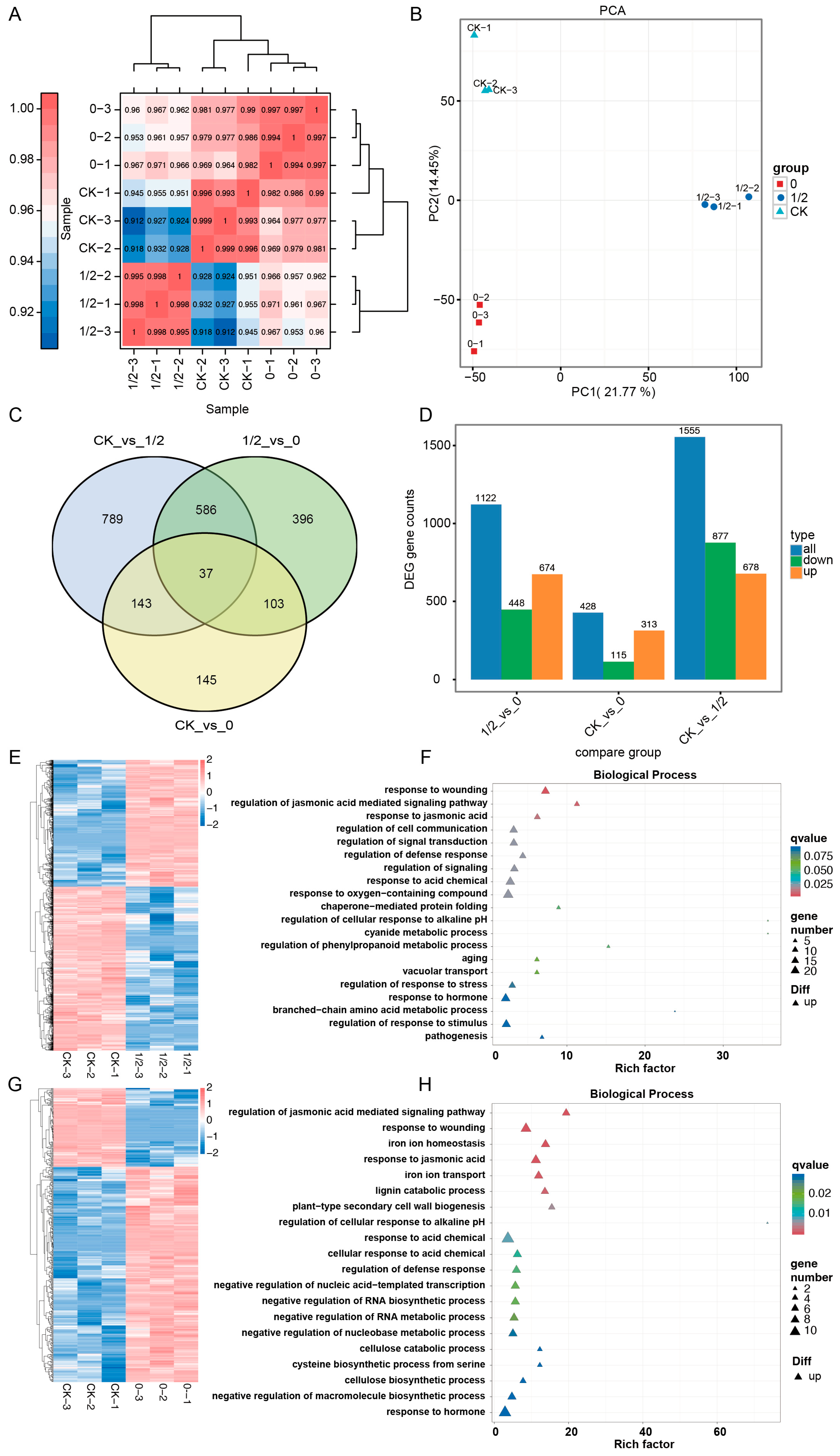
2. Results
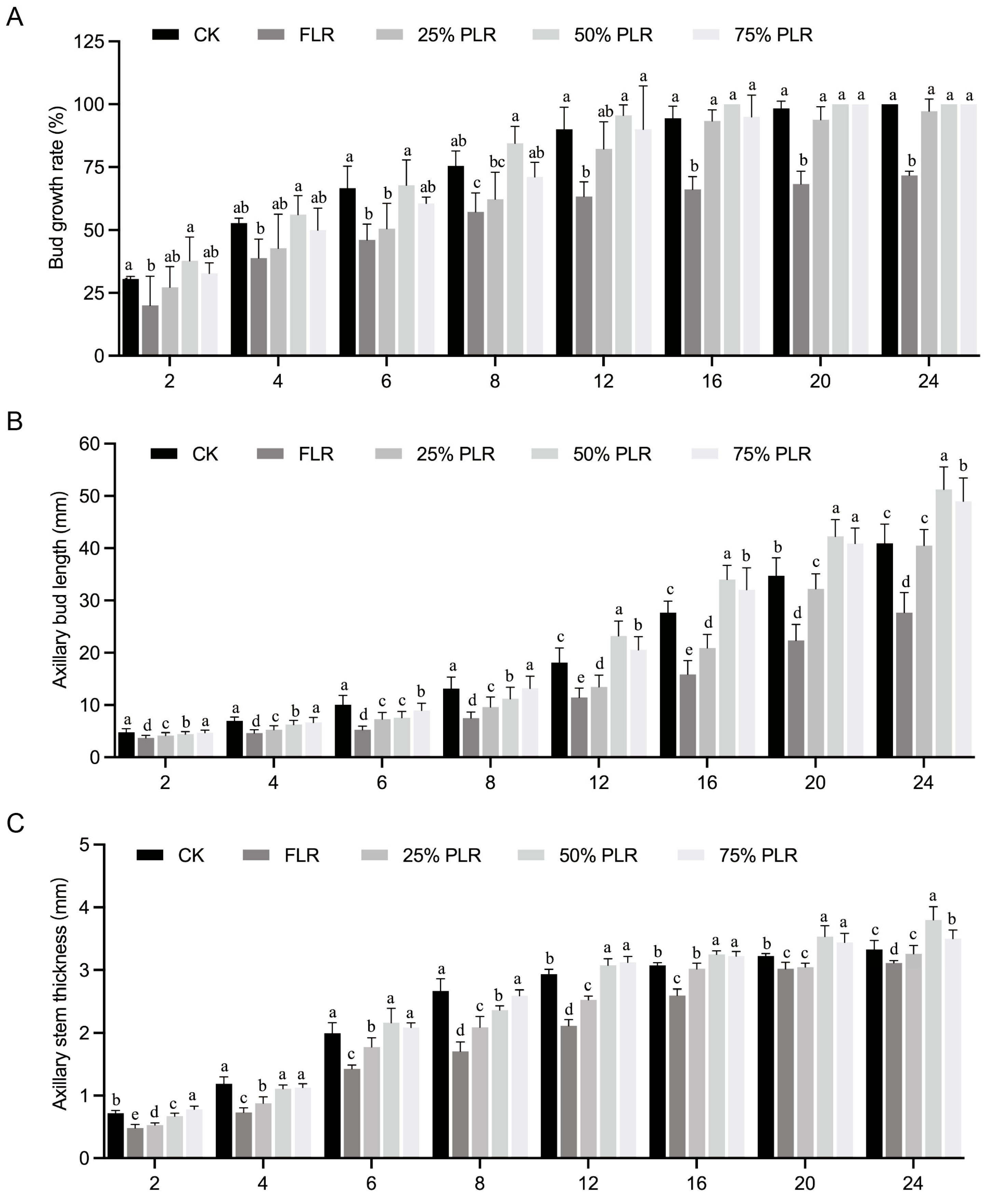
2.1. Effects of Differential Leaf Retention on Axillary Bud Development in P. edulis
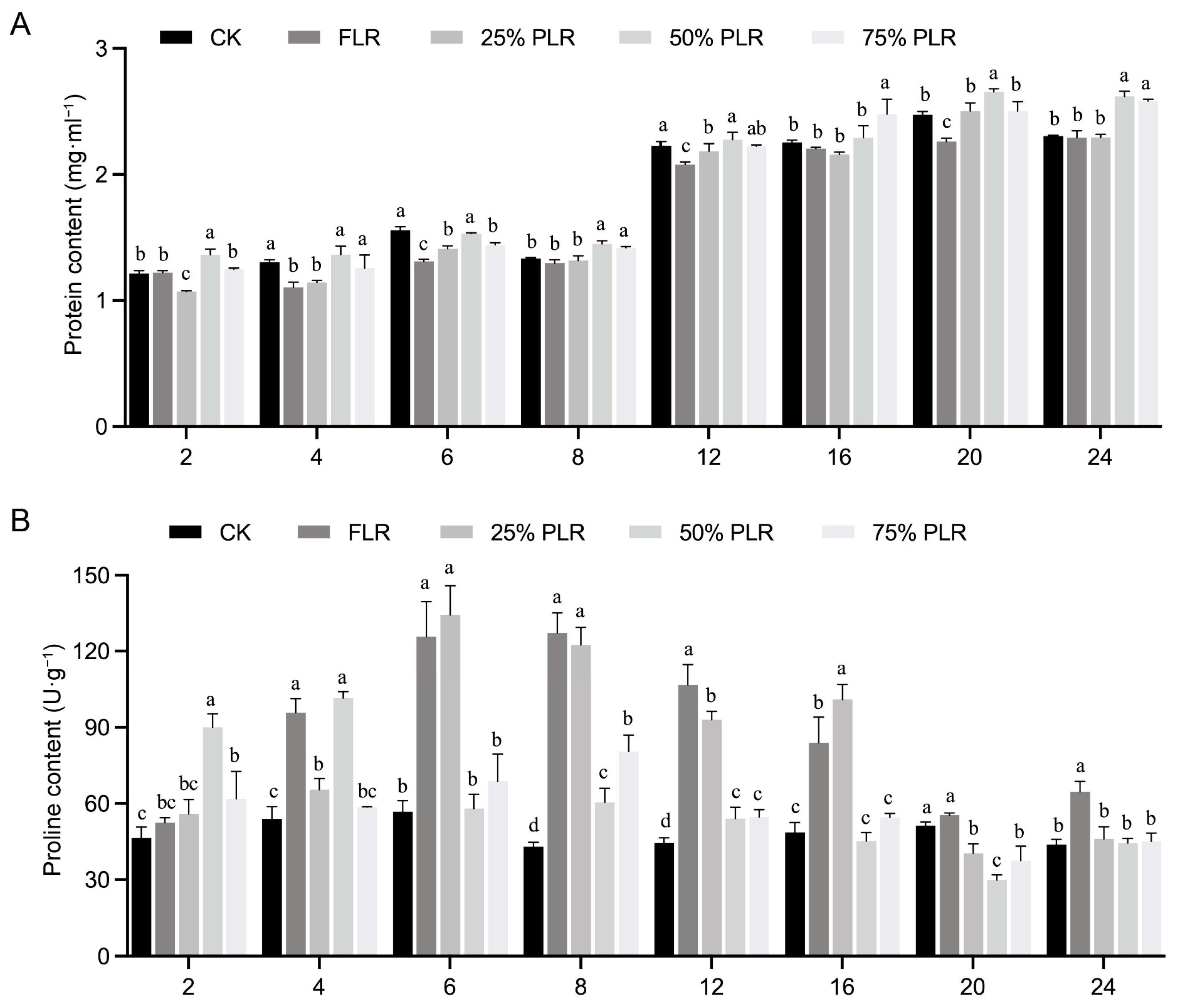
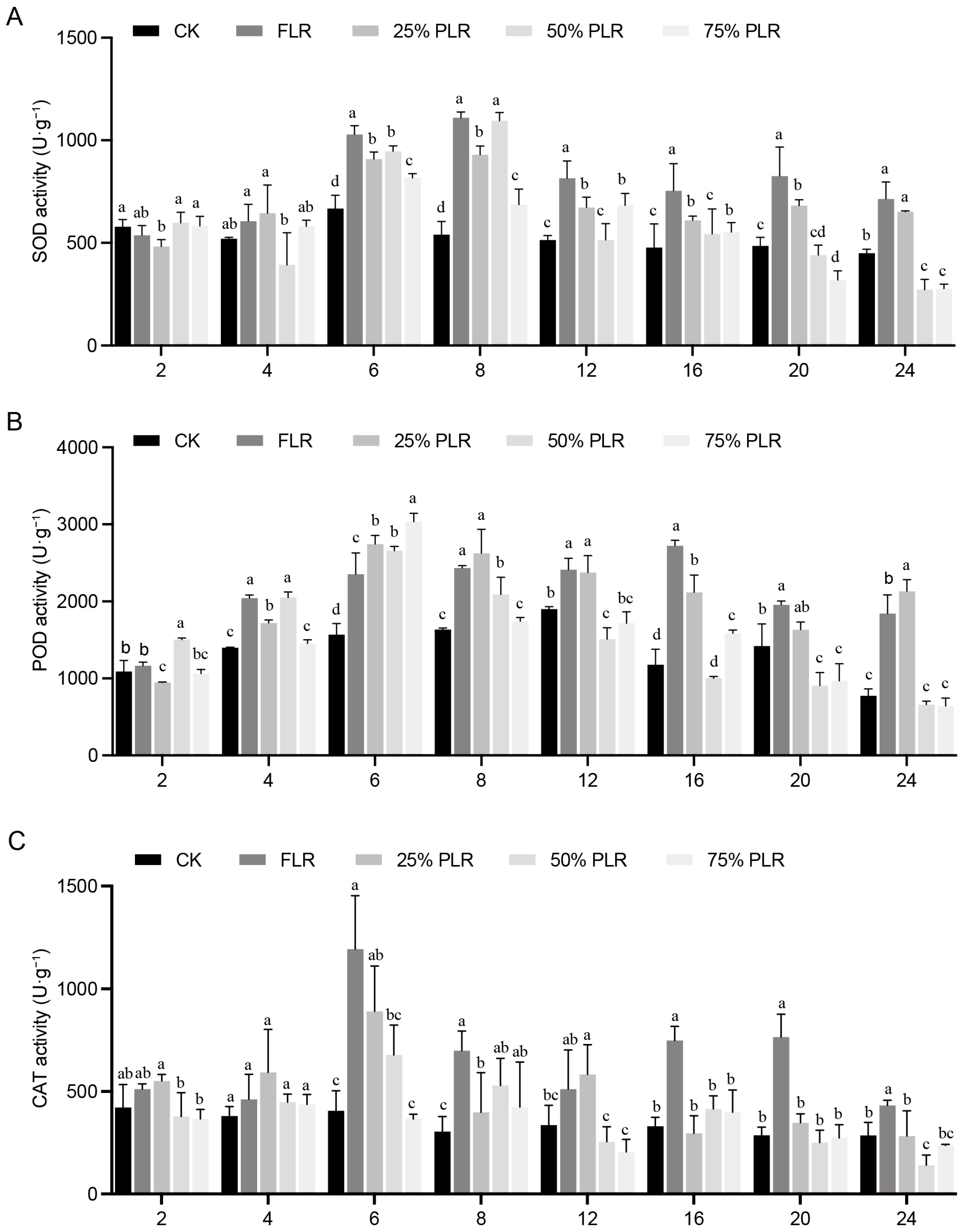
2.2. Oxidative Stress Response to Differential Leaf Retention in Axillary Buds
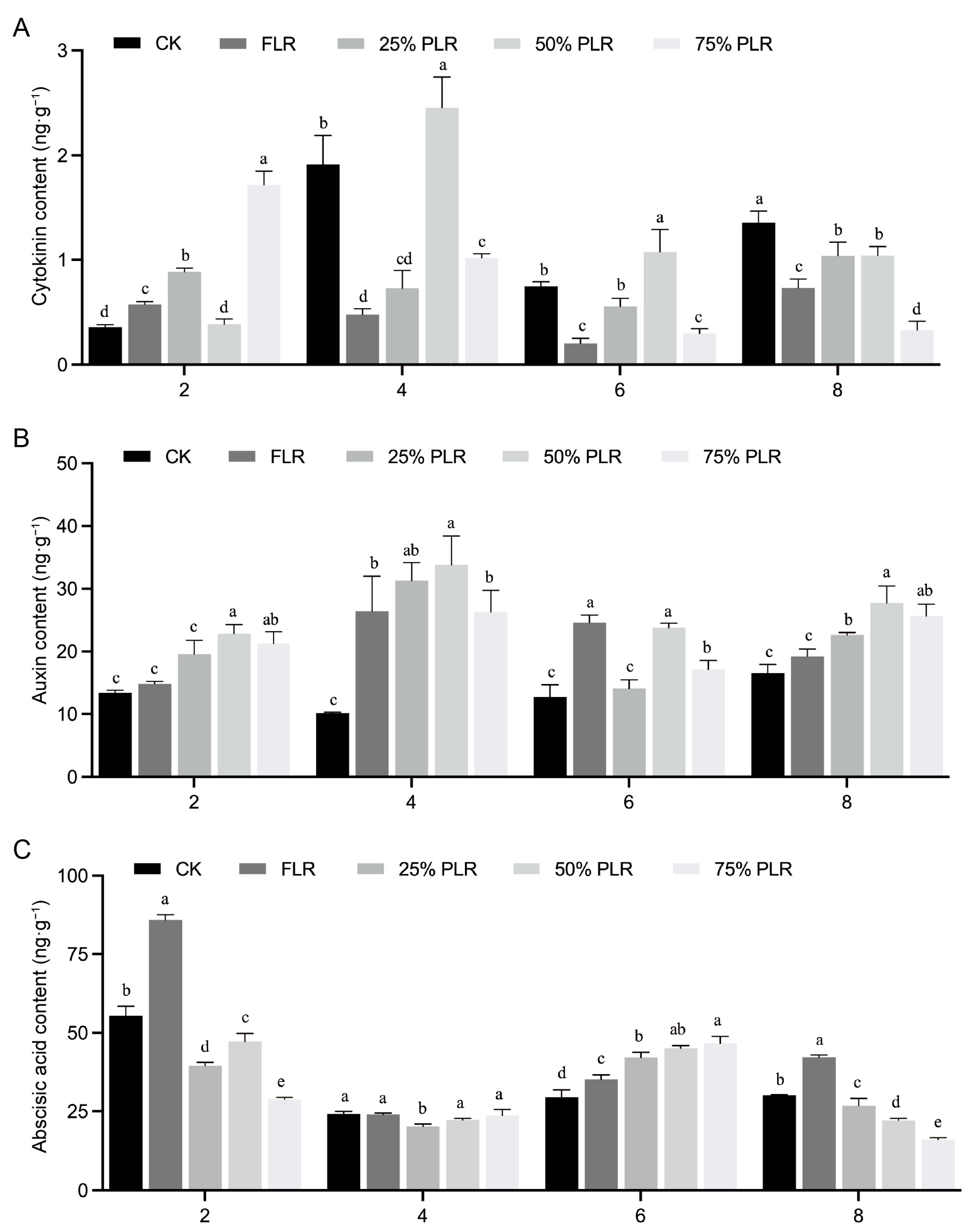
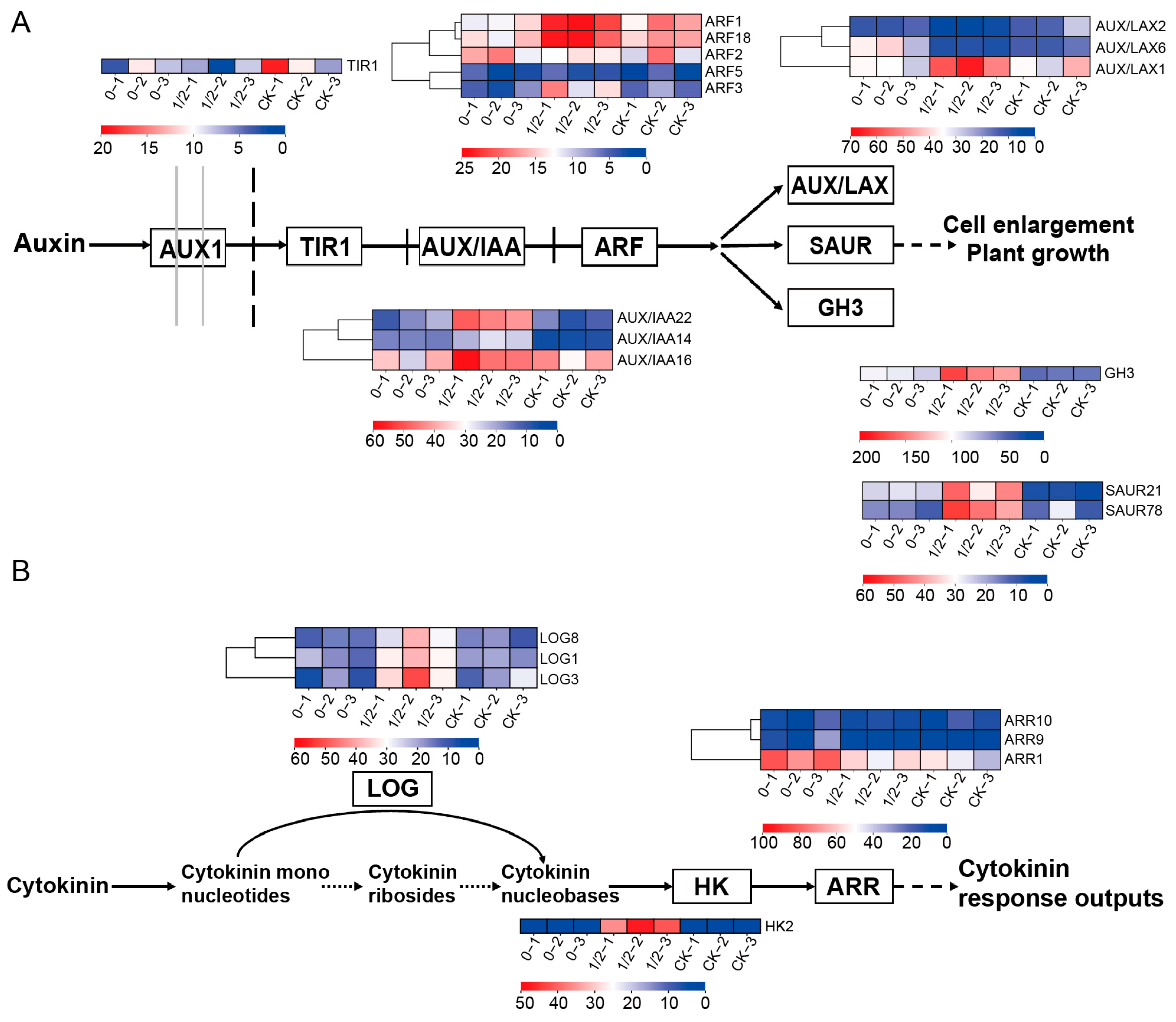
2.3. Hormonal Dynamics Response to Differential Leaf Retention in Axillary Buds
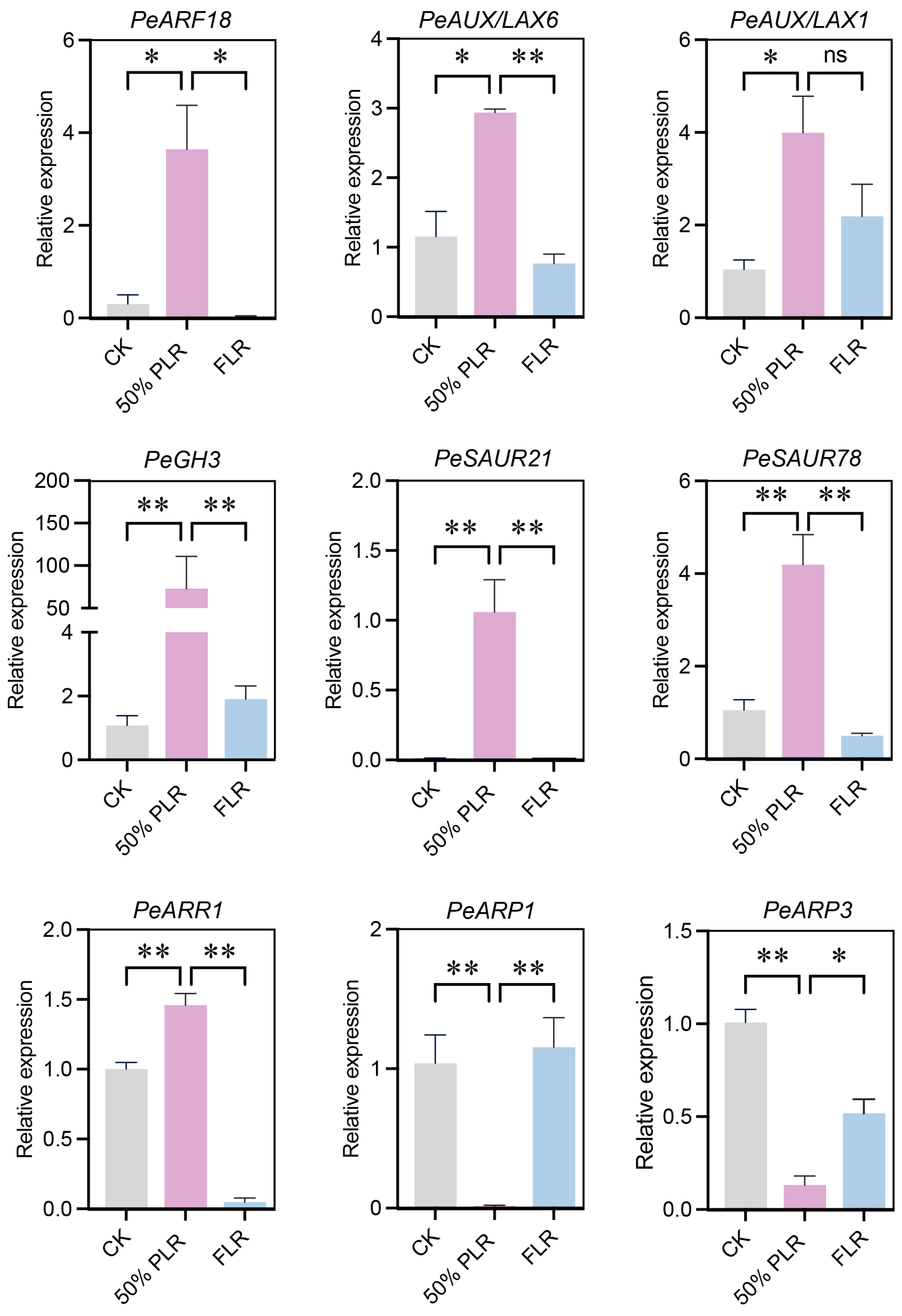
2.4. Phytohormonal Regulatory Mechanisms Underlying Axillary Bud Outgrowth
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Measurements
4.3. Plant Hormone Components Assay
4.4. Assays for Antioxidant Enzyme Activity and Proline Accumulation Analysis
4.5. RNA Extraction, RNA Sequencing, and qRT-PCR
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Castillo, N.; Ambachew, D.; Melgarejo, L.; Blair, M. Morphological and Agronomic Variability Among Cultivars, Landraces, and Genebank Accessions of Purple Passion Fruit, Passiflora edulis f. Edulis. HortScience 2020, 55, 768–777. [Google Scholar] [CrossRef]
- Posadas, B.; Stafne, E.; Blare, T.; Downey, L.; Anderson, J.; Crane, J.; Gazis, R.; Faber, B.; Stockton, D.G.; Carrillo, D.; et al. Grower and Operational Characteristics of US Passion Fruit Farmers. Technol. Hortic. 2023, 3, 25. [Google Scholar] [CrossRef]
- Correia, A.; Alexandre, R.; Pfenning, L.; Cabanez, P.; Ferreira, A.; Ferreira, M.; De Lima, P.; De Mello, T.; Otoni, W.; Lopes, J. Passiflora Mucronata, a Passion Fruit Wild Species Resistant to Fusariosis and a Potential Rootstock for Commercial Varieties. Sci. Hortic. 2022, 302, 111174. [Google Scholar] [CrossRef]
- Ribeiro, L.; Nery, L.; Vieira, L.; Mercadante-Simões, M. Histological Study of Micrografting in Passionfruit. Plant Cell Tissue Organ Cult. PCTOC 2015, 123, 173–181. [Google Scholar] [CrossRef]
- Hieu, T.; Phong, T.; Khai, H.; Mai, N.; Cuong, D.; Luan, V.; Tung, H.; Nam, N.; Tan Nhut, D. Efficient Production of Vigorous Passion Fruit Rootstock for in Vitro Grafting. Plant Cell Tissue Organ Cult. 2022, 148, 635–648. [Google Scholar] [CrossRef]
- Nahar, A.; Choudhury, M.; Rahim, M. Effect of Scion Defoliation and Stock Leaf Retention on Growth of Grafted Lime (Cv. Bau Lime-1). Asian J. Med. Biol. Res. 2018, 4, 44–48. [Google Scholar] [CrossRef]
- Lopez, M.; Hurtado-Salazar, A.; Ocampo, J.; Silva, D.; Ceballos-Aguirre, N. Evaluation of Purple Passion Fruit Grafted onto a Fusarium Wilt-Tolerant Rootstock. Pesqui. Agropecuária Bras. 2023, 58, e03011. [Google Scholar] [CrossRef]
- Palliotti, A.; Gatti, M.; Poni, S. Early Leaf Removal to Improve Vineyard Efficiency: Gas Exchange, Source-to-Sink Balance, and Reserve Storage Responses. Am. J. Enol. Vitic. 2011, 62, 219–228. [Google Scholar] [CrossRef]
- Li, Y.; Liu, D.; Luo, Y.; Abbas Khan, M.; Mahmood Alam, S.; Liu, Y.-Z. Transcriptome Analysis Reveals the Key Network of Axillary Bud Outgrowth Modulated by Topping in Citrus. Gene 2024, 926, 148623. [Google Scholar] [CrossRef]
- Hooijdonk, B.; Woolley, D.; Warrington, I.; Tustin, S. Rootstocks Modify Scion Architecture, Endogenous Hormones, and Root Growth of Newly Grafted ‘Royal Gala’ Apple Trees. J. Am. Soc. Hortic. Sci. 2011, 136, 93–102. [Google Scholar] [CrossRef]
- Van Hooijdonk, B.M.; Woolley, D.J.; Warrington, I.J.; Tustin, D.S. Initial Alteration of Shoot Architecture by Dwarfing Apple Rootstocks Involves Shoot/Root/Shoot Signalling Between Auxins, Gibberellins and Cytokinins. Acta Hortic. 2011, 85, 857–863. [Google Scholar] [CrossRef]
- Tan, M.; Li, G.; Chen, X.; Xing, L.; Ma, J.; Zhang, D.; Ge, H.; Han, M.; Sha, G.; An, N. Role of Cytokinin, Strigolactone, and Auxin Export on Outgrowth of Axillary Buds in Apple. Front. Plant Sci. 2019, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, W.; Mao, J.; Yang, S.; Li, W.; Ma, Z.; Zhao, X.; Chen, B. Effects of Exogenous Growth Regulators and Bud Picking on Grafting of Grapevine Hard Branches. Sci. Hortic. 2020, 264, 109186. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Wang, Y.; Zou, J.; Lin, S.; Chen, M.; Miao, P.; Jia, X.; Cheng, P.; Pang, X.; et al. Transcriptomic Analysis of the Effect of Pruning on Growth, Quality, and Yield of Wuyi Rock Tea. Plants 2023, 12, 3625. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhang, Q.; Shi, L.; Wang, Y.; Li, M.; Chen, Y.; Zhang, M.; Chen, J.; Chen, M.; Jia, X.; et al. Joint Analysis of Transcriptome and Hormone Metabolome on the Mechanism of Pruning Effect on Tea Tree (Camellia sinensis) Growth. Ind. Crops Prod. 2024, 218, 118929. [Google Scholar] [CrossRef]
- Montesinos, Á.; Maldera, F.; Thorp, G.T.; Rubio-Cabetas, M.J. Scion–Rootstock Combination Determines Pruning Responses in Young Almond Trees. HortScience 2024, 59, 1–7. [Google Scholar] [CrossRef]
- Hayat, F.; Iqbal, S.; Coulibaly, D.; Razzaq, M.K.; Nawaz, M.A.; Jiang, W.; Shi, T.; Gao, Z.; Hayat, F.; Iqbal, S.; et al. An Insight into Dwarfing Mechanism Contribution of Scionrootstock Interactions toward Fruit Crop Improvement. Fruit Res. 2021, 1, 3. [Google Scholar] [CrossRef]
- Cheng, Y.; Liang, C.; Qiu, Z.; Zhou, S.; Liu, J.; Yang, Y.; Wang, R.; Yin, J.; Ma, C.; Cui, Z.; et al. Jasmonic Acid Negatively Regulates Branch Growth in Pear. Front. Plant Sci. 2023, 14, 1105521. [Google Scholar] [CrossRef]
- Öztürk, A. The Effects of Different Rootstocks on the Graft Success and Stion Development of Some Pear Cultivars. Int. J. Fruit Sci. 2021, 21, 932–944. [Google Scholar] [CrossRef]
- Salem, M.A.; Yoshida, T.; Perez de Souza, L.; Alseekh, S.; Bajdzienko, K.; Fernie, A.R.; Giavalisco, P. An Improved Extraction Method Enables the Comprehensive Analysis of Lipids, Proteins, Metabolites and Phytohormones from a Single Sample of Leaf Tissue under Water-Deficit Stress. Plant J. 2020, 103, 1614–1632. [Google Scholar] [CrossRef]
- Tang, Y.; Qu, Z.; Lei, J.; He, R.; Adelson, D.L.; Zhu, Y.; Yang, Z.; Wang, D. The Long Noncoding RNA FRILAIR Regulates Strawberry Fruit Ripening by Functioning as a Noncanonical Target Mimic. PLoS Genet. 2021, 17, e1009461. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Huang, D.; Zhang, S.; Wang, W.; Ma, F.; Wu, B.; Xu, Y.; Xu, B.; Chen, D.; Zou, M.; et al. Chromosome-Scale Genome Assembly Provides Insights into the Evolution and Flavor Synthesis of Passion Fruit (Passiflora edulis Sims). Hortic. Res. 2021, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, P.; Chen, J.; Li, L.; Tang, L.; Huang, W.; Wei, X.; Xu, J. The Accumulation Pattern of Sugars and Organic Acids in Diverse Fruit Pulps Coordinates Sweet-Sour Taste of Passion Fruit. LWT 2025, 225, 117948. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Tang, Y.; Lai, J.; Li, L.; Zhou, P.; Yu, D.; Tang, L.; Xu, J. Efficient Production of Vigorous Scions by Optimizing Leaf Retention in Passiflora edulis. Plants 2025, 14, 2483. https://doi.org/10.3390/plants14162483
Wei X, Tang Y, Lai J, Li L, Zhou P, Yu D, Tang L, Xu J. Efficient Production of Vigorous Scions by Optimizing Leaf Retention in Passiflora edulis. Plants. 2025; 14(16):2483. https://doi.org/10.3390/plants14162483
Chicago/Turabian StyleWei, Xiuqing, Yajun Tang, Jianglong Lai, Liang Li, Ping Zhou, Dong Yu, Limei Tang, and Jiahui Xu. 2025. "Efficient Production of Vigorous Scions by Optimizing Leaf Retention in Passiflora edulis" Plants 14, no. 16: 2483. https://doi.org/10.3390/plants14162483
APA StyleWei, X., Tang, Y., Lai, J., Li, L., Zhou, P., Yu, D., Tang, L., & Xu, J. (2025). Efficient Production of Vigorous Scions by Optimizing Leaf Retention in Passiflora edulis. Plants, 14(16), 2483. https://doi.org/10.3390/plants14162483





