A Methodological Approach for Evaluating the Genotypic Variation for Physiological Adaptation of Potato Wild Relatives for Heat Tolerance Breeding
Abstract
1. Introduction
2. Results
2.1. Genetic and Environmental Influences on Potato Traits Under Contrasting Conditions
2.2. Heat Stress Affects the Physiological and Tuber Trait Genetic Variability
2.3. Physiological Trait Association with Heat Stress
2.4. Wild Potato Genotypes Acclimatize to High-Temperature Stress at Tuberization Stage
2.5. Principal Components Analysis
2.6. Ranking of Wild Potato Genotypes Based on Their Heat Tolerance
2.7. Clustering of Wild Potato Accession Based on the Similarity Observed by the Comprehensive Values
3. Discussion
4. Materials and Methods
4.1. Plant Material and HS Application
4.2. Measurement of Photosynthetic and Chlorophyll Fluorescence Parameters
4.3. Measurement of Pigment Traits
4.4. Measurement of Tuber Traits
4.5. Measurement of Biomass
4.6. Genotypic Values Prediction
4.7. Heat Tolerance Analysis
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Levy, D.; Veilleux, R.E. Adaptation of Potato to High Temperatures and Salinity—A Review. Am. J. Potato Res. 2007, 84, 487–506. [Google Scholar] [CrossRef]
- Hijmans, R.J. The Effect of Climate Change on Global Potato Production. Am. J. Potato Res. 2003, 80, 271–279. [Google Scholar] [CrossRef]
- Lutaladio, N.; Castaldi, L. Potato: The Hidden Treasure. J. Food Compos. Anal. 2009, 22, 491–493. [Google Scholar] [CrossRef]
- Devaux, A.; Kromann, P.; Ortiz, O. Potatoes for Sustainable Global Food Security. Potato Res. 2014, 57, 185–199. [Google Scholar] [CrossRef]
- CIP. CIP Annual Report 2021. From Lab to Field to Scale: Demand-Driven Solutions for Food Systems Transformation; International Potato Center (CIP): Lima, Peru, 2022. [Google Scholar]
- Jansky, S.H.; Dempewolf, H.; Camadro, E.L.; Simon, R.; Zimnoch-Guzowska, E.; Bisognin, D.A.; Bonierbale, M. A Case for Crop Wild Relative Preservation and Use in Potato. Crop Sci. 2013, 53, 746–754. [Google Scholar] [CrossRef]
- Spooner, D.M.; Bamberg, J.B. Potato Genetic Resources: Sources of Resistance and Systematics. Am. Potato J. 1994, 71, 325–337. [Google Scholar] [CrossRef]
- Bashir, I.; Nicolao, R.; Heiden, G. Wild Potatoes: A Genetic Reservoir for Potato Breeding. In Wild Germplasm for Genetic Improvement in Crop Plants; Elsevier: Amsterdam, The Netherlands, 2021; pp. 215–240. [Google Scholar]
- Hawkes, J.G. The Potato: Evolution, Biodiversity and Genetic Resources; Belhaven Press: London, UK, 1990; ISBN 9781852930455. [Google Scholar]
- Bethke, P.C.; Halterman, D.A.; Jansky, S.H. Potato Germplasm Enhancement Enters the Genomics Era. Agronomy 2019, 9, 575. [Google Scholar] [CrossRef]
- Zamir, D. Improving Plant Breeding with Exotic Genetic Libraries. Nat. Rev. Genet. 2001, 2, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Lazaridi, E.; Kapazoglou, A.; Gerakari, M.; Kleftogianni, K.; Passa, K.; Sarri, E.; Papasotiropoulos, V.; Tani, E.; Bebeli, P.J. Crop Landraces and Indigenous Varieties: A Valuable Source of Genes for Plant Breeding. Plants 2024, 13, 758. [Google Scholar] [CrossRef]
- Reynolds, M.; Atkin, O.K.; Bennett, M.; Cooper, M.; Dodd, I.C.; Foulkes, M.J.; Frohberg, C.; Hammer, G.; Henderson, I.R.; Huang, B.; et al. Addressing Research Bottlenecks to Crop Productivity. Trends Plant Sci. 2021, 26, 607–630. [Google Scholar] [CrossRef]
- Havux, M.; Tardy, F.; Ravenel, J.; Chanu, D.; Parot, P. Thylakoid Membrane Stability to Heat Stress Studied by Flash Spectroscopic Measurements of the Electrochromic Shift in Intact Potato Leaves: Influence of the Xanthophyll Content. Plant. Cell Environ. 1996, 19, 1359–1368. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Hays, D.; Chapman, S. Breeding for Adaptation to Heat and Drought Stress. In Climate Change and Crop Production; CABI: Wallingford, CT, USA, 2010; pp. 71–91. [Google Scholar]
- Prashar, A.; Yildiz, J.; McNicol, J.W.; Bryan, G.J.; Jones, H.G. Infra-Red Thermography for High Throughput Field Phenotyping in Solanum tuberosum. PLoS ONE 2013, 8, e65816. [Google Scholar] [CrossRef]
- Tang, R.; Niu, S.; Zhang, G.; Chen, G.; Haroon, M.; Yang, Q.; Rajora, O.P.; Li, X.-Q. Physiological and Growth Responses of Potato Cultivars to Heat Stress. Botany 2018, 96, 897–912. [Google Scholar] [CrossRef]
- Zhou, J.; Li, K.; Li, Y.; Li, M.; Guo, H. Responses of Aerial and Belowground Parts of Different Potato (Solanum tuberosum L.) Cultivars to Heat Stress. Plants 2023, 12, 818. [Google Scholar] [CrossRef]
- Fang, G.; Yang, S.; Ruan, B.; Ye, G.; He, M.; Su, W.; Zhou, Y.; Wang, J.; Yang, S. Research Progress on Physiological, Biochemical, and Molecular Mechanisms of Potato in Response to Drought and High Temperature. Horticulturae 2024, 10, 827. [Google Scholar] [CrossRef]
- Yan, W.; Tinker, N.A. Biplot Analysis of Multi-Environment Trial Data: Principles and Applications. Can. J. Plant Sci. 2006, 86, 623–645. [Google Scholar] [CrossRef]
- Crossa, J.; Pérez-Rodríguez, P.; Cuevas, J.; Montesinos-López, O.; Jarquín, D.; de los Campos, G.; Burgueño, J.; González-Camacho, J.M.; Pérez-Elizalde, S.; Beyene, Y.; et al. Genomic Selection in Plant Breeding: Methods, Models, and Perspectives. Trends Plant Sci. 2017, 22, 961–975. [Google Scholar] [CrossRef]
- Piepho, H.P.; Möhring, J.; Melchinger, A.E.; Büchse, A. BLUP for Phenotypic Selection in Plant Breeding and Variety Testing. Euphytica 2008, 161, 209–228. [Google Scholar] [CrossRef]
- Sood, S.; Bhardwaj, V.; Kaushik, S.K.; Sharma, S. Prediction Based on Estimated Breeding Values Using Genealogy for Tuber Yield and Late Blight Resistance in Auto-Tetraploid Potato (Solanum tuberosum L.). Heliyon 2020, 6, e05624. [Google Scholar] [CrossRef] [PubMed]
- Barbiero, N.Z.; Moro, G.L.J.; de Oliveira Bernardes, C.; de Arruda, V.C.; de Oliveira Moulin Carias, C.M.; Guilhen, J.H.S.; Altoé, S.C.; de Oliveira, E.J.; da Silva Ferreira, M.F.; Posse, S.C.P.; et al. Selection Index and Prediction of Genetic Values in Cassava via Reml/Blup. Euphytica 2024, 220, 41. [Google Scholar] [CrossRef]
- Vilela de Resende, M.D.; Duarte, J.B. Precision And Quality Control in Variety Trials. Pesqui. Agropecuária Trop. 2007, 37, 182. [Google Scholar]
- Xu, Y.; Liu, X.; Fu, J.; Wang, H.; Wang, J.; Huang, C.; Prasanna, B.M.; Olsen, M.S.; Wang, G.; Zhang, A. Enhancing Genetic Gain through Genomic Selection: From Livestock to Plants. Plant Commun. 2020, 1, 100005. [Google Scholar] [CrossRef]
- Bashir, I.; Nicolao, R.; Haerter, J.A.; de Brito, G.G.; Castro, C.M.; Heiden, G. Phenotyping Wild Potatoes for Photosynthesis Associated Traits Under Heat Stress. Am. J. Potato Res. 2025, 102, 33–50. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A. Physiological Characteristics of Cassava Tolerance to Prolonged Drought in the Tropics: Implications for Breeding Cultivars Adapted to Seasonally Dry and Semiarid Environments. Brazilian J. Plant Physiol. 2007, 19, 257–286. [Google Scholar] [CrossRef]
- Bashir, I.; Nardino, M.; Castro, C.M.; Heiden, G. Genotypic Response and Selection of Potato Germplasm Under Heat Stress. Potato Res. 2023, 66, 85–104. [Google Scholar] [CrossRef]
- Kumar, S.; Thakur, P.; Kaushal, N.; Malik, J.A.; Gaur, P.; Nayyar, H. Effect of Varying High Temperatures during Reproductive Growth on Reproductive Function, Oxidative Stress and Seed Yield in Chickpea Genotypes Differing in Heat Sensitivity. Arch. Agron. Soil Sci. 2013, 59, 823–843. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Murty, M.V.R.; Quick, W.P. Rice Responses to Rising Temperatures—Challenges, Perspectives and Future Directions. Plant. Cell Environ. 2015, 38, 1686–1698. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Pisipati, S.R.; Mutava, R.N.; Tuinstra, M.R. Sensitivity of Grain Sorghum to High Temperature Stress during Reproductive Development. Crop Sci. 2008, 48, 1911–1917. [Google Scholar] [CrossRef]
- Cairns, J.E.; Crossa, J.; Zaidi, P.H.; Grudloyma, P.; Sanchez, C.; Araus, J.L.; Thaitad, S.; Makumbi, D.; Magorokosho, C.; Bänziger, M.; et al. Identification of Drought, Heat, and Combined Drought and Heat Tolerant Donors in Maize. Crop Sci. 2013, 53, 1335–1346. [Google Scholar] [CrossRef]
- Sato, S.; Kamiyama, M.; Iwata, T.; Makita, N.; Furukawa, H.; Ikeda, H. Moderate Increase of Mean Daily Temperature Adversely Affects Fruit Set of Lycopersicon esculentum by Disrupting Specific Physiological Processes in Male Reproductive Development. Ann. Bot. 2006, 97, 731–738. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, H.; Kong, L.; Li, X.; Chen, Y.; Wang, S.; Liu, B. Multivariate Analysis Compares and Evaluates Heat Tolerance of Potato Germplasm. Plants 2024, 13, 142. [Google Scholar] [CrossRef]
- Wang, G.Y.; Ahmad, S.; Wang, Y.; Wang, B.W.; Huang, J.H.; Jahan, M.S.; Zhou, X.B.; Shi, C.Q. Multivariate Analysis Compares and Evaluates Drought and Flooding Tolerances of Maize Germplasm. Plant Physiol. 2023, 193, 339–355. [Google Scholar] [CrossRef]
- Neri, P.; Gu, L.; Song, Y. The Effect of Temperature on Photosystem II Efficiency across Plant Functional Types and Climate. Biogeosciences 2024, 21, 2731–2758. [Google Scholar] [CrossRef]
- Adewumi, A.S.; Agre, P.A.; Asare, P.A.; Adu, M.O.; Taah, K.J.; Mondo, J.M.; Akaba, S. Exploring the Bush Yam (Dioscorea praehensilis Benth) as a Source of Agronomic and Quality Trait Genes in White Guinea Yam (Dioscorea rotundata Poir) Breeding. Agronomy 2021, 12, 55. [Google Scholar] [CrossRef]
- Darkwa, K.; Olasanmi, B.; Asiedu, R.; Asfaw, A. Review of Empirical and Emerging Breeding Methods and Tools for Yam (Dioscorea spp.) Improvement: Status and Prospects. Plant Breed. 2020, 139, 474–497. [Google Scholar] [CrossRef]
- Adigoun-Akotegnon, F.A.; Adoukonou-Sagbadja, H.; Fadinan, C.; Tchougourou, A.; Agassounon-Tchibozo, M.; Ahanhanzo, C. Diversity, Distribution and Ethnobotanical Importance of Cultivated and Wild African Trifoliate Yam [Dioscorea dumetorum (Kunth) Pax] in Benin. Genet. Resour. Crop Evol. 2019, 66, 659–683. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Resende, M.D.V. de Software Selegen-REML/BLUP: A Useful Tool for Plant Breeding. Crop Breed. Appl. Biotechnol. 2016, 16, 330–339. [Google Scholar] [CrossRef]
- Tian, Z.; Yang, Y.; Wang, F. A Comprehensive Evaluation of Heat Tolerance in Nine Cultivars of Marigold. Hortic. Environ. Biotechnol. 2015, 56, 749–755. [Google Scholar] [CrossRef]
- R Core Team, R. R Core Team R: A Language and Environment for Statistical Computing 2021; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–8. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. Corrplot: Visualization of a Correlation Matrix; CRAN Contrib. Packag.; R Foundation for Statistical Computing: Vienna, Austria, 2010. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
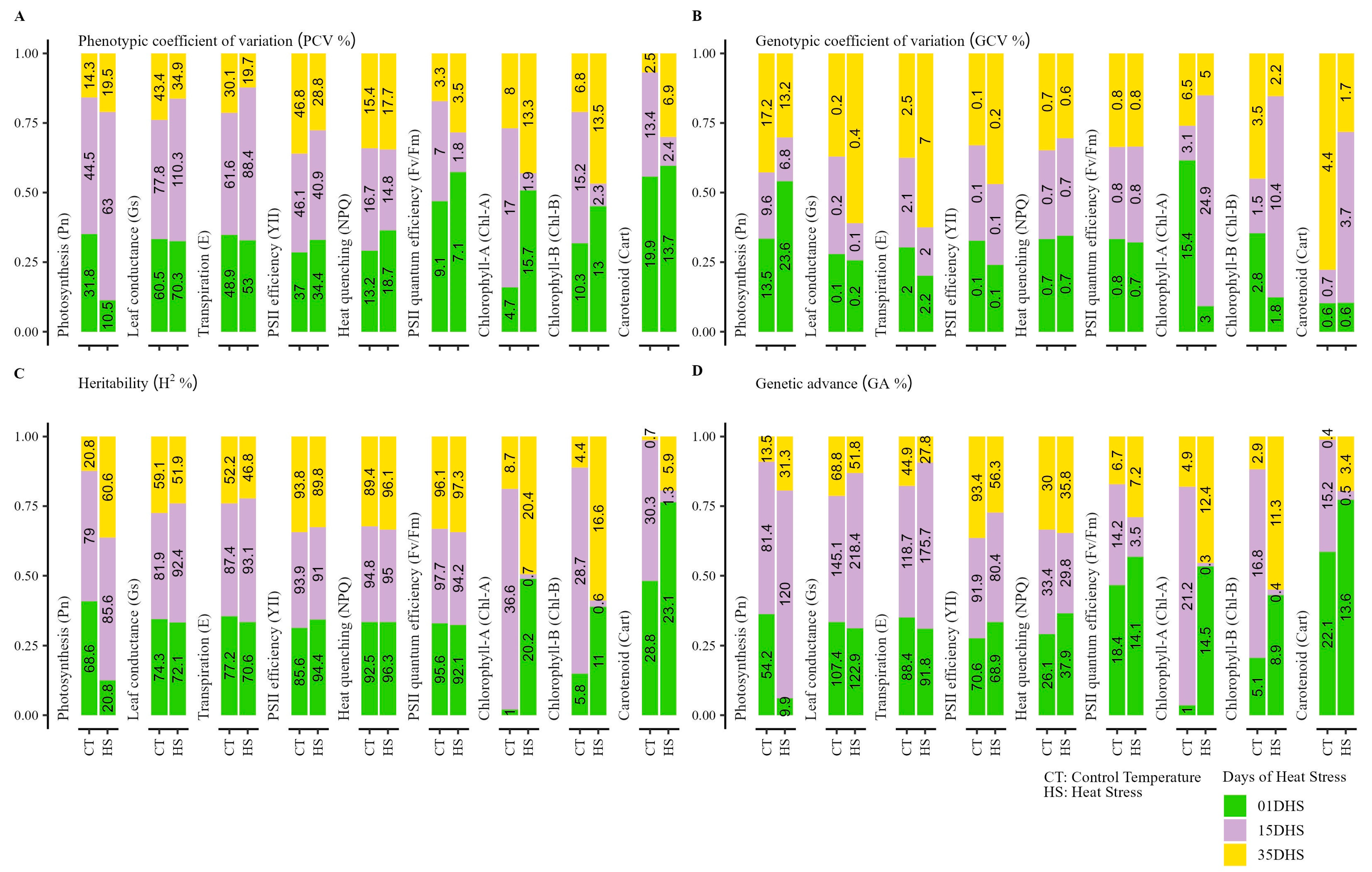

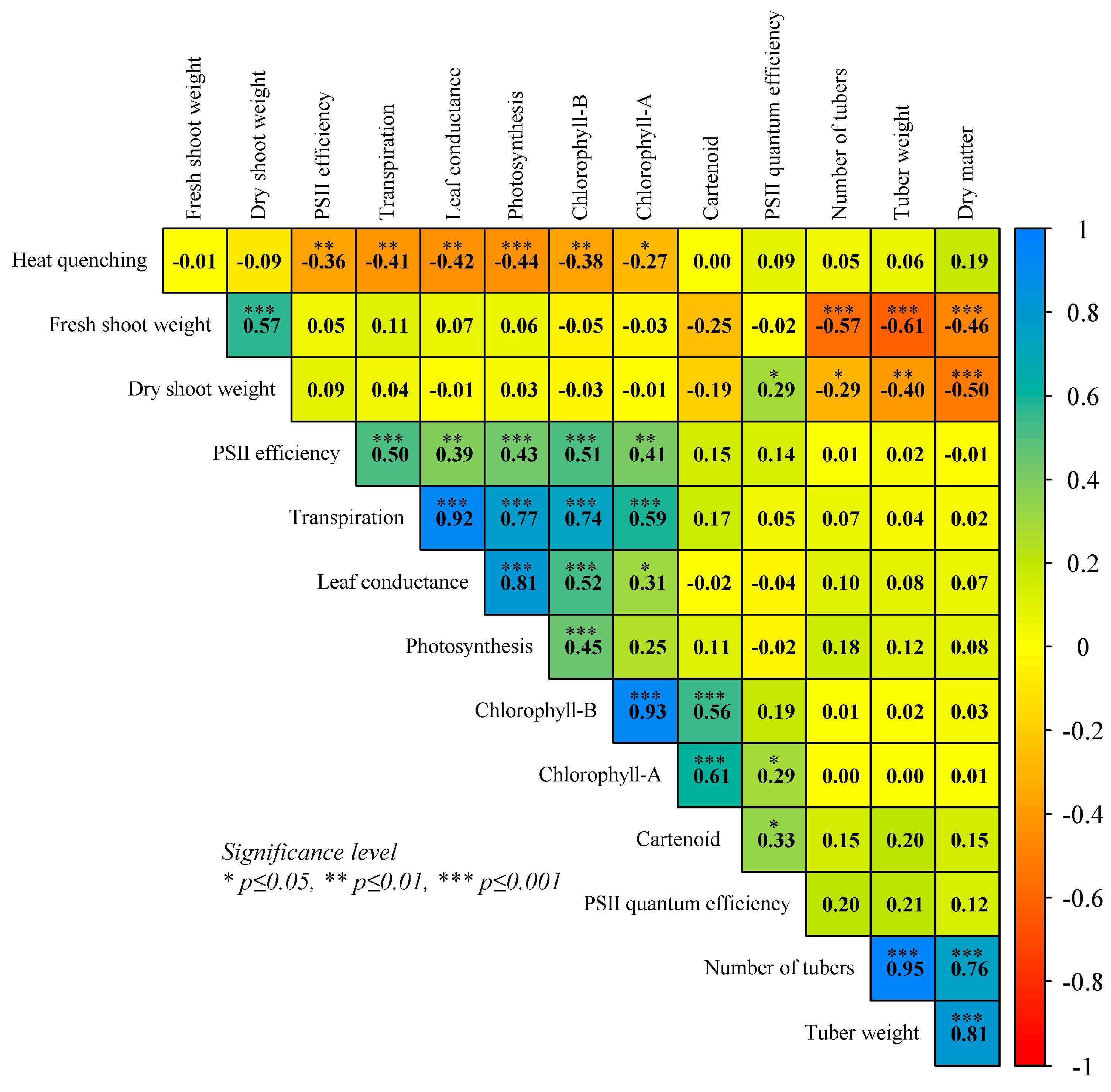
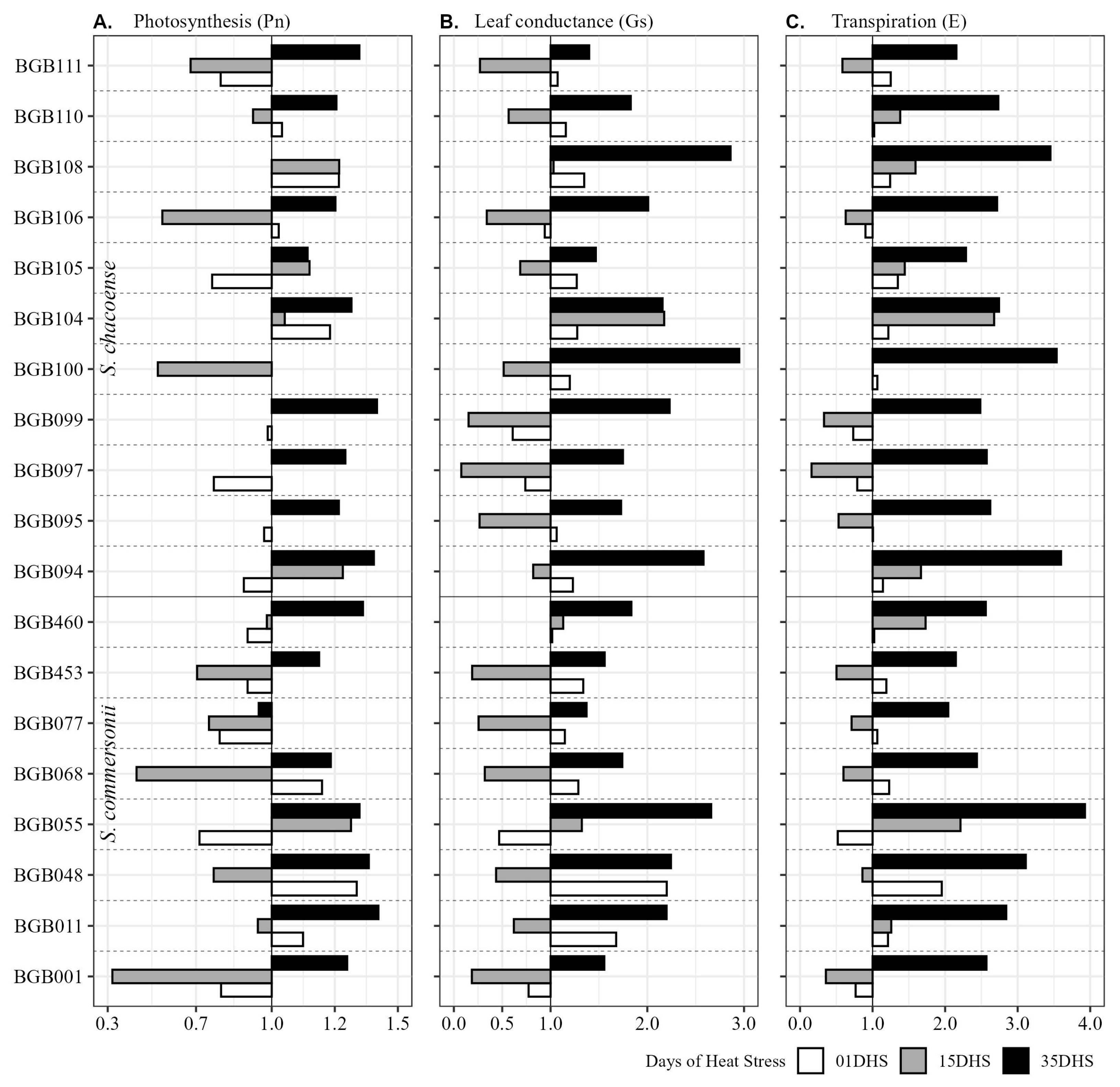
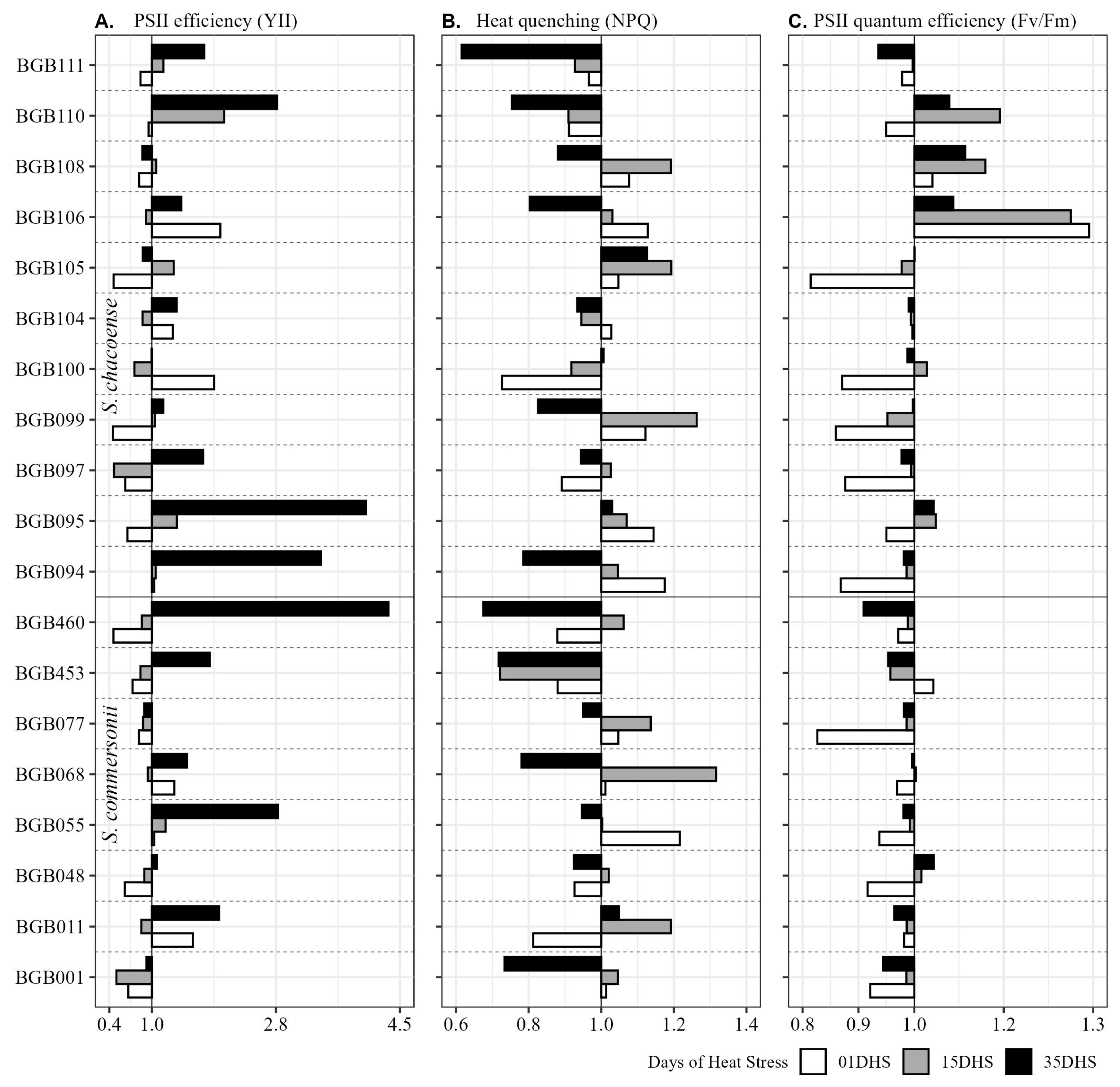

| Trait | Temp. | BLUP Variation | ± sd | HTC | G | T | G × T | GCV % | PCV % | H2 | GA% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FSW (g) | CT | 144.9–284.6 | 211.6 ± 36.8 | 0.83 | *** | ** | ns | 316.0 | 21.7 | 44.9 | 30.0 |
| HS | 109.3–247.2 | 176.3 ± 36.4 | 273.0 | 22.0 | 41.7 | 29.2 | |||||
| DSW (g) | CT | 26.1–37.8 | 32.1 ± 3.1 | 0.97 | *** | ns | ns | 62.0 | 10.8 | 26.7 | 11.5 |
| HS | 25.0–36.8 | 31.0 ± 3.1 | 44.8 | 13.2 | 47.9 | 18.8 | |||||
| NT | CT | 3.1–40.2 | 17.8 ± 11.1 | 0.56 | *** | ** | ns | 23.0 | 68.1 | 59.8 | 108.5 |
| HS | 0.1–33.4 | 12.5 ± 10.2 | 13.4 | 92.9 | 85.8 | 177.2 | |||||
| TW (g) | CT | 15.4–158.8 | 69.9 ± 48.1 | 0.29 | *** | *** | ns | 82.5 | 81.5 | 71.8 | 142.2 |
| HS | 7.6–122.2 | 39.4 ± 42.7 | 41.5 | 100.0 | 89.9 | 195.3 | |||||
| DM (%) | CT | 3.2–30.4 | 20.9 ± 8.5 | 0.76 | *** | * | ns | 22.4 | 47.1 | 86.9 | 90.4 |
| HS | 0.7–27.1 | 17.9 ± 8.5 | 41.5 | 100.0 | 89.9 | 195.3 |
| Trait | Temp. | 1 DHS | 15 DHS | 35 DHS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BLUP Variation | Mean ± SD | t-Test | HTC Mean | BLUP Variation | Mean ± SD | t-Test | HTC Mean | BLUP Variation | Mean ± SD | t-Test | HTC Mean | ||
| Pn | CT | 5.24–15.91 | 11.16 ± 2.70 | ns | 1.02 | 1.94–13.12 | 8.49 ± 3.50 | ** | 0.77 | 5.56–10.00 | 7.84 ± 1.10 | *** | 1.32 |
| HS | 8.29–13.18 | 10.75 ± 1.40 | 1.23–12.40 | 6.29 ± 3.60 | 7.82–13.23 | 10.24 ± 1.3 | |||||||
| Gs | CT | 0.02–0.29 | 0.12 ± 0.10 | ns | 1.15 | 0.02–0.39 | 0.16 ± 0.10 | ** | 0.60 | 0.06–0.27 | 0.14 ± 0.10 | *** | 2.01 |
| HS | 0.03–0.33 | 0.13 ± 0.10 | 0.01–0.28 | 0.08 ± 0.10 | 0.15–0.47 | 0.27 ± 0.10 | |||||||
| E | CT | 0.45–3.40 | 1.78 ± 0.80 | ns | 1.09 | 0.30–3.92 | 2.00 ± 1.20 | ns | 1.06 | 0.87–2.64 | 1.8 ± 0.50 | *** | 2.77 |
| HS | 0.46–3.62 | 1.88 ± 0.90 | 0.29–5.45 | 1.88 ± 1.60 | 3.44–6.42 | 4.77 ± 0.70 | |||||||
| YII | CT | 0.03–0.19 | 0.11 ± 0.04 | ns | 1.02 | 0.03–0.21 | 0.12 ± 0.05 | ns | 1.03 | 0.02–0.19 | 0.12 ± 0.10 | *** | 2.42 |
| HS | 0.05–0.22 | 0.09 ± 0.03 | 0.06–0.19 | 0.11 ± 0.04 | 0.08–0.28 | 0.18 ± 0.10 | |||||||
| NPQ | CT | 0.45–0.85 | 0.67 ± 0.10 | ns | 1.01 | 0.42–0.90 | 0.65 ± 0.10 | ns | 1.04 | 0.50–0.91 | 0.69 ± 0.10 | *** | 0.86 |
| HS | 0.40–0.85 | 0.67 ± 0.10 | 0.43–0.82 | 0.67 ± 0.10 | 0.36–0.74 | 0.59 ± 0.10 | |||||||
| Fv/Fm | CT | 0.50–0.79 | 0.75 ± 0.10 | ** | 0.95 | 0.59–0.78 | 0.75 ± 0.10 | ns | 1.02 | 0.71–0.80 | 0.76 ± 0.02 | ns | 0.99 |
| HS | 0.59–0.78 | 0.70 ± 0.10 | 0.73–0.78 | 0.76 ± 0.01 | 0.69–0.79 | 0.75 ± 0.03 | |||||||
| Chl-A | CT | 1.47–1.60 | 1.52 ± 0.03 | *** | 0.90 | 1.62–2.19 | 1.89 ± 0.20 | *** | 1.09 | 1.68–2.18 | 1.92 ± 0.20 | *** | 1.17 |
| HS | 1.33–1.44 | 1.37 ± 0.02 | 1.79–2.35 | 2.05 ± 0.20 | 1.99–2.50 | 2.24 ± 0.20 | |||||||
| Chl-B | CT | 0.61–0.73 | 0.66 ± 0.03 | *** | 0.89 | 0.74–0.88 | 0.82 ± 0.04 | *** | 1.02 | 0.59–0.84 | 0.73 ± 0.10 | *** | 1.23 |
| HS | 0.56–0.67 | 0.59 ± 0.02 | 0.76–0.90 | 0.83 ± 0.04 | 0.75–1.00 | 0.90 ± 0.10 | |||||||
| Cart | CT | 0.25–0.43 | 0.31 ± 0.04 | ns | 0.98 | 0.33–0.43 | 0.38 ± 0.03 | *** | 1.11 | 0.36–0.40 | 0.38 ± 0.01 | *** | 1.09 |
| HS | 0.25–0.37 | 0.30 ± 0.03 | 0.37–0.47 | 0.42 ± 0.03 | 0.40–0.44 | 0.42 ± 0.01 | |||||||
| Index | 1 DHS | 15 DHS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | Total Weight | PC1 | PC2 | PC3 | PC4 | Total Weight | |
| Photosynthesis | 0.61 | 0.40 | 0.46 | 0.03 | −0.30 | 0.19 | −0.34 | 0.82 | 0.23 | −0.09 | 0.09 |
| Leaf conductance | 0.22 | 0.80 | 0.33 | −0.34 | 0.14 | 0.17 | −0.29 | 0.81 | 0.11 | −0.37 | 0.05 |
| Transpiration | 0.23 | 0.80 | 0.21 | −0.37 | 0.31 | 0.17 | −0.32 | 0.88 | 0.12 | −0.28 | 0.07 |
| PSII efficiency | 0.30 | 0.12 | 0.49 | 0.66 | −0.31 | 0.16 | 0.09 | 0.56 | −0.31 | 0.47 | 0.11 |
| Heat quenching | −0.10 | −0.59 | −0.07 | 0.03 | 0.65 | −0.04 | 0.36 | −0.04 | 0.09 | −0.40 | 0.03 |
| PSII quantum efficiency | 0.14 | −0.20 | 0.68 | 0.46 | 0.24 | 0.14 | 0.25 | 0.31 | −0.11 | 0.69 | 0.16 |
| Chlorophyll-A | 0.68 | −0.53 | 0.40 | −0.22 | −0.02 | 0.07 | −0.04 | 0.02 | 0.93 | 0.32 | 0.17 |
| Chlorophyll-B | 0.62 | −0.50 | 0.41 | −0.40 | 0.01 | 0.05 | 0.19 | 0.07 | 0.77 | −0.03 | 0.16 |
| Carotenoid | 0.77 | −0.46 | 0.19 | −0.26 | −0.02 | 0.07 | 0.13 | 0.04 | 0.87 | 0.32 | 0.20 |
| Fresh shoot weight | −0.67 | 0.21 | 0.47 | 0.04 | 0.12 | −0.02 | −0.81 | 0.10 | −0.17 | −0.02 | −0.15 |
| Dry shoot weight | −0.53 | 0.06 | 0.49 | 0.11 | 0.50 | 0.03 | −0.62 | 0.20 | −0.33 | 0.56 | −0.06 |
| Number of tubers | 0.76 | 0.20 | −0.33 | 0.33 | 0.26 | 0.18 | 0.80 | 0.44 | −0.29 | 0.06 | 0.17 |
| Fresh tuber weight | 0.82 | 0.13 | −0.34 | 0.29 | 0.26 | 0.17 | 0.87 | 0.34 | −0.19 | 0.03 | 0.18 |
| Dry matter | 0.77 | 0.35 | −0.28 | 0.09 | 0.22 | 0.18 | 0.85 | 0.26 | −0.01 | −0.09 | 0.18 |
| Eigenvalue | 4.61 | 2.82 | 2.18 | 1.38 | 1.23 | 3.74 | 2.95 | 2.67 | 1.60 | ||
| CR (%) | 32.95 | 20.13 | 15.55 | 9.89 | 8.77 | 26.69 | 21.11 | 19.04 | 11.42 | ||
| CRR (%) | 32.95 | 53.08 | 68.63 | 78.52 | 87.29 | 26.69 | 47.80 | 66.84 | 78.26 | ||
| Weight | 0.38 | 0.23 | 0.18 | 0.11 | 0.10 | 0.34 | 0.27 | 0.24 | 0.15 | ||
| 35 DHS | |||||||||||
| Photosynthesis | 0.53 | 0.25 | 0.69 | 0.00 | −0.31 | 0.19 | |||||
| Leaf conductance | 0.66 | 0.50 | 0.47 | −0.11 | −0.05 | 0.23 | |||||
| Transpiration | 0.51 | 0.64 | 0.48 | −0.07 | 0.16 | 0.25 | |||||
| PSII efficiency | −0.23 | 0.05 | 0.48 | −0.11 | 0.68 | 0.07 | |||||
| Heat quenching | 0.45 | 0.30 | −0.53 | −0.18 | 0.25 | 0.07 | |||||
| PSII quantum efficiency | 0.63 | 0.05 | −0.13 | 0.65 | 0.12 | 0.18 | |||||
| Chlorophyll-A | −0.75 | −0.40 | 0.37 | 0.12 | 0.03 | −0.14 | |||||
| Chlorophyll-B | −0.50 | −0.52 | 0.41 | 0.02 | 0.39 | −0.08 | |||||
| Carotenoid | −0.77 | −0.14 | 0.32 | 0.16 | −0.35 | −0.14 | |||||
| Fresh shoot weight | −0.45 | 0.63 | −0.15 | 0.16 | 0.26 | 0.02 | |||||
| Dry shoot weight | −0.41 | 0.43 | −0.03 | 0.77 | 0.06 | 0.06 | |||||
| Number of tubers | 0.76 | −0.48 | 0.20 | 0.28 | −0.01 | 0.13 | |||||
| Fresh tuber weight | 0.80 | −0.49 | 0.13 | 0.15 | 0.01 | 0.11 | |||||
| Dry matter | 0.66 | −0.59 | −0.14 | 0.04 | 0.23 | 0.05 | |||||
| Eigenvalue | 5.07 | 2.68 | 1.96 | 1.25 | 1.06 | ||||||
| CR (%) | 36.25 | 19.16 | 13.97 | 8.93 | 7.58 | ||||||
| CRR (%) | 36.25 | 55.40 | 69.37 | 78.31 | 85.89 | ||||||
| Weight | 0.42 | 0.22 | 0.16 | 0.10 | 0.09 | ||||||
| Genotype | 1 DHS | 15 DHS | 35 DHS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCEV Value | Rank | F Value | Rank | HCEV Value | Rank | F Value | Rank | HCEV Value | Rank | F Value | Rank | |
| BGB001 | 0.33 | 18 | −1.68 | 18 | 0.20 | 19 | −1.95 | 19 | 0.26 | 17 | 0.23 | 6 |
| BGB011 | 0.62 | 7 | 0.49 | 7 | 0.63 | 3 | 1.00 | 3 | 0.42 | 11 | −0.08 | 12 |
| BGB048 | 0.72 | 2 | 1.24 | 2 | 0.59 | 5 | 0.68 | 5 | 0.53 | 5 | 0.19 | 8 |
| BGB055 | 0.29 | 19 | −2.08 | 19 | 0.34 | 17 | −1.14 | 17 | 0.56 | 3 | 1.08 | 1 |
| BGB068 | 0.65 | 6 | 0.73 | 6 | 0.43 | 14 | −0.34 | 13 | 0.36 | 13 | −0.29 | 15 |
| BGB077 | 0.42 | 17 | −0.88 | 16 | 0.47 | 10 | −0.08 | 11 | 0.18 | 19 | −0.83 | 19 |
| BGB094 | 0.57 | 10 | 0.08 | 10 | 0.62 | 4 | 0.94 | 4 | 0.54 | 4 | 0.36 | 3 |
| BGB095 | 0.55 | 11 | −0.06 | 12 | 0.45 | 12 | −0.29 | 12 | 0.46 | 10 | 0.22 | 7 |
| BGB097 | 0.47 | 14 | −0.39 | 13 | 0.57 | 7 | 0.68 | 6 | 0.41 | 12 | −0.77 | 18 |
| BGB099 | 0.51 | 12 | 0.01 | 11 | 0.46 | 11 | 0.02 | 10 | 0.48 | 9 | −0.55 | 17 |
| BGB100 | 0.72 | 3 | 1.30 | 1 | 0.37 | 16 | −0.72 | 15 | 0.62 | 2 | 0.29 | 5 |
| BGB104 | 0.67 | 5 | 0.81 | 5 | 0.57 | 8 | 0.55 | 7 | 0.53 | 6 | −0.18 | 13 |
| BGB105 | 0.50 | 13 | −0.58 | 14 | 0.38 | 15 | −0.72 | 16 | 0.36 | 14 | −0.27 | 14 |
| BGB106 | 0.73 | 1 | 1.07 | 4 | 0.58 | 6 | 0.53 | 8 | 0.51 | 7 | 0.06 | 11 |
| BGB108 | 0.71 | 4 | 1.15 | 3 | 0.68 | 2 | 1.30 | 1 | 0.74 | 1 | 0.52 | 2 |
| BGB110 | 0.61 | 8 | 0.38 | 8 | 0.70 | 1 | 1.30 | 2 | 0.48 | 8 | 0.09 | 10 |
| BGB111 | 0.58 | 9 | 0.14 | 9 | 0.53 | 9 | 0.28 | 9 | 0.34 | 15 | −0.50 | 16 |
| BGB453 | 0.44 | 15 | −0.92 | 17 | 0.27 | 18 | −1.61 | 18 | 0.26 | 18 | 0.11 | 9 |
| BGB460 | 0.44 | 16 | −0.81 | 15 | 0.43 | 13 | −0.42 | 14 | 0.28 | 16 | 0.31 | 4 |
| Chamber-1: Control | Chamber 2: Heat Stress | ||||
|---|---|---|---|---|---|
| Time | Temperature °C | Humidity % | Time | Temperature °C | Humidity % |
| 00:00–04:00 | 19 | 65 | 23:00–01:00 | 27 | 65 |
| 04:00–06:00 | 15 | 65 | 01:00–04:00 | 26 | 65 |
| 06:00–09:00 | 14 | 65 | 04:00–06:00 | 25 | 65 |
| 09:00–10:00 | 16 | 50 | 06:00–09:00 | 24 | 50 |
| 10:00–11:00 | 19 | 50 | 09:00–11:00 | 27 | 50 |
| 11:00–12:00 | 23 | 50 | 11:00–12:00 | 30 | 50 |
| 12:00–14:00 | 25 | 50 | 12:00–14:00 | 31 | 50 |
| 14:00–18:00 | 27 | 50 | 14:00–18:00 | 34 | 50 |
| 18:00–21:00 | 26 | 50 | 18:00–21:00 | 31 | 50 |
| 21:00–00:00 | 23 | 65 | 21:00–23:00 | 28 | 65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashir, I.; Nicolao, R.; Shimoia, E.P.; do Amarante, L.; Castro, C.M.; Heiden, G. A Methodological Approach for Evaluating the Genotypic Variation for Physiological Adaptation of Potato Wild Relatives for Heat Tolerance Breeding. Plants 2025, 14, 3096. https://doi.org/10.3390/plants14193096
Bashir I, Nicolao R, Shimoia EP, do Amarante L, Castro CM, Heiden G. A Methodological Approach for Evaluating the Genotypic Variation for Physiological Adaptation of Potato Wild Relatives for Heat Tolerance Breeding. Plants. 2025; 14(19):3096. https://doi.org/10.3390/plants14193096
Chicago/Turabian StyleBashir, Ikram, Rodrigo Nicolao, Eduardo Pereira Shimoia, Luciano do Amarante, Caroline Marques Castro, and Gustavo Heiden. 2025. "A Methodological Approach for Evaluating the Genotypic Variation for Physiological Adaptation of Potato Wild Relatives for Heat Tolerance Breeding" Plants 14, no. 19: 3096. https://doi.org/10.3390/plants14193096
APA StyleBashir, I., Nicolao, R., Shimoia, E. P., do Amarante, L., Castro, C. M., & Heiden, G. (2025). A Methodological Approach for Evaluating the Genotypic Variation for Physiological Adaptation of Potato Wild Relatives for Heat Tolerance Breeding. Plants, 14(19), 3096. https://doi.org/10.3390/plants14193096










