Examining the “Night Break” Method in Cannabis sativa Horticulture: Vegetative Daily Light Integral Affects Yield of Extractable Biomass in C. sativa
Abstract
1. Introduction
2. Methods
2.1. Vegetative Phase
2.2. Flowering Phase
2.3. Measurement of PPFD and Calculation of DLI
2.4. Phenotypic Analysis
2.5. Chemotypic Analysis
2.6. Statistical Analysis
3. Results
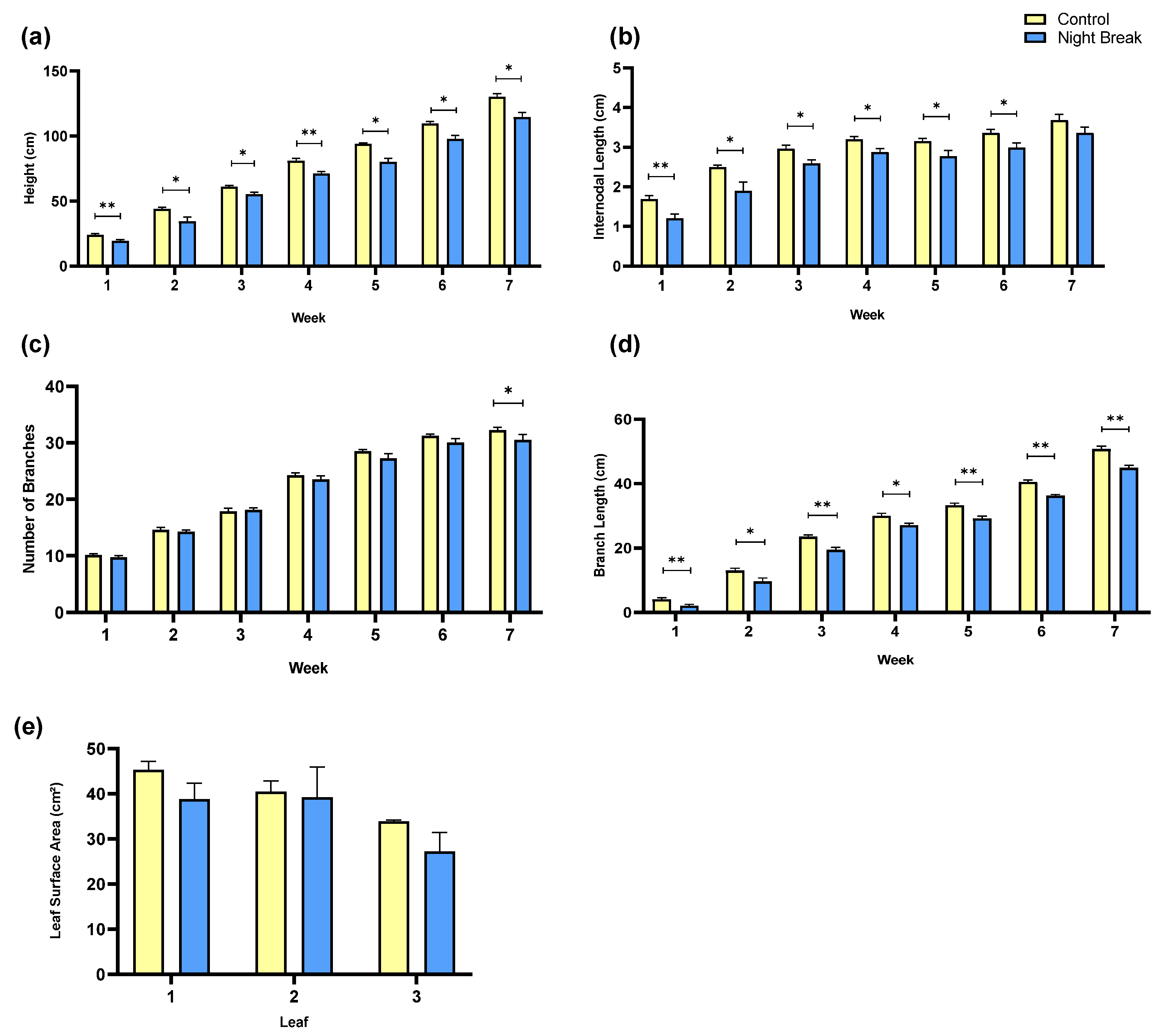
3.1. Morphological Development
3.2. Vegetative and Floral Tissue Biomass
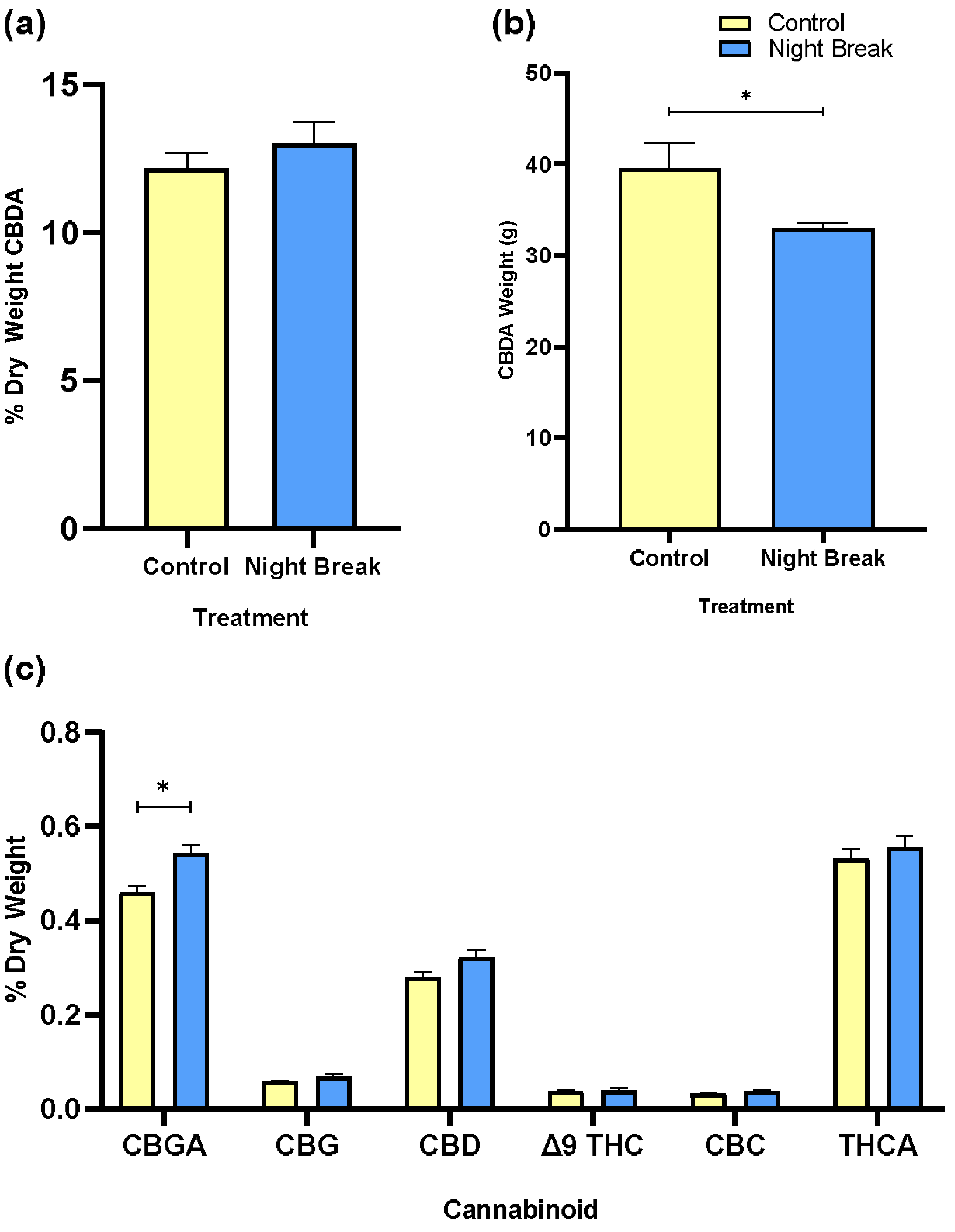
3.3. Cannabinoid Composition and Yield
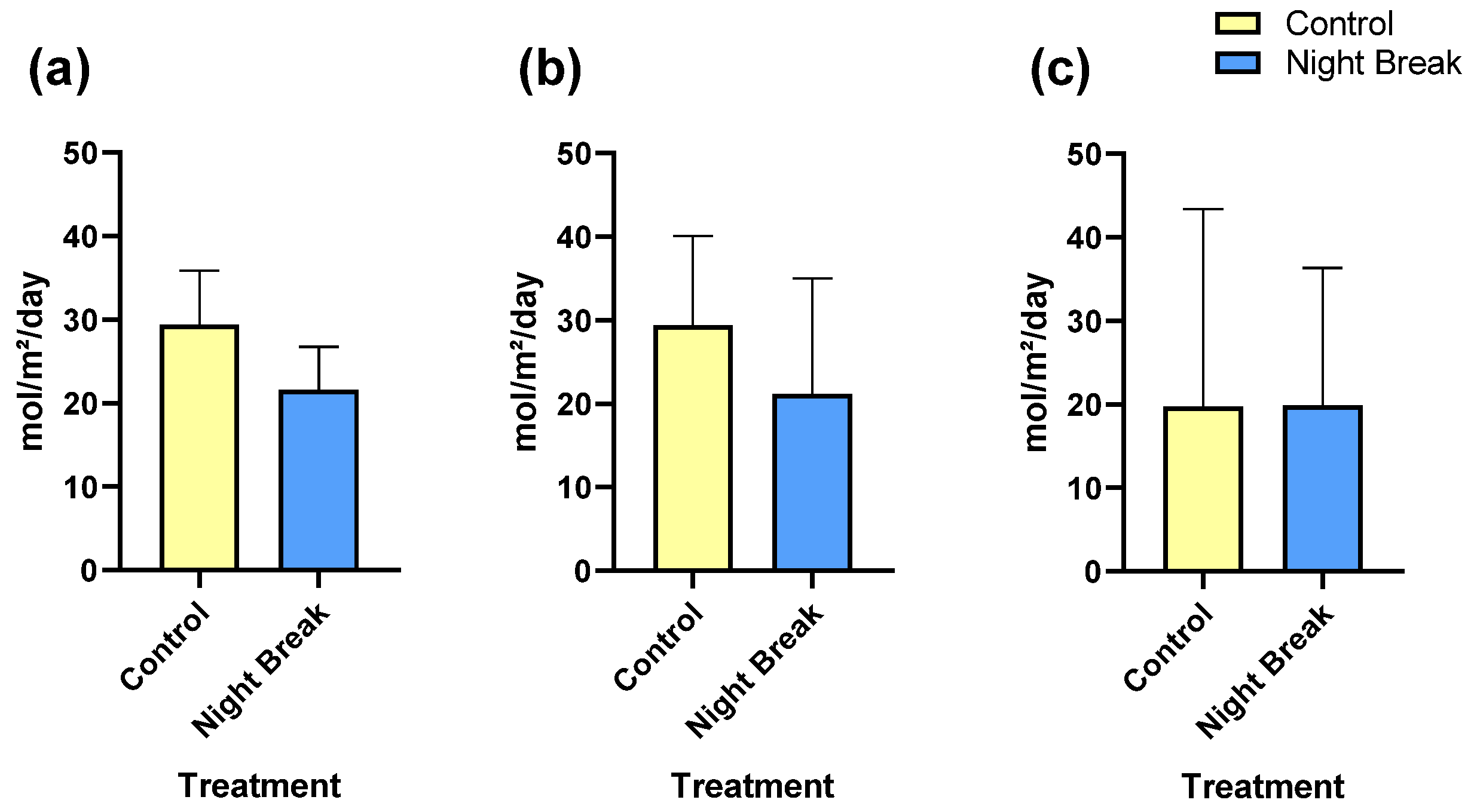
3.4. Floral and Vegetative DLI
3.5. Yield, Electricity Use, and Economic Outcomes
4. Discussion
4.1. Light Intensity, Spectrum, and Photoperiod Considerations
4.2. Night Break and Alternative Lighting Approaches
4.3. Economic Implications of the Night Break Method
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| CBC | cannabichromene |
| CBD | cannabidiol |
| CBDA | cannabidiolic acid (carboxylated) |
| CBG | cannabigerol |
| CBGA | cannabigerolic acid (carboxylated) |
| C. sativa | Cannabis sativa |
| DE | daylength extension |
| Δ9 THC | delta-9-tetrahydrocannabinol |
| DLI | daily light integral |
| HPLC | high performance liquid chromatography |
| HPS | high pressure sodium |
| LED | light emitting diode |
| NB | night break |
| PAR | photosynthetically active radiation |
| PPFD | photosynthetic photon flux density |
| SD | short day |
| SEM | standard error of the mean |
| THCA | tetrahydrocannabinolic acid |
References
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Placido, D.F.; Lee, C.C. Potential of Industrial Hemp for Phytoremediation of Heavy Metals. Plants 2022, 11, 595. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jaeger, K.E.; Wigge, P.A. FT protein acts as a long-range signal in Arabidopsis. Curr. Biol. 2007, 17, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Hempton, A.K.; Imaizumi, T. Photoperiodic flowering in Arabidopsis: Multilayered regulatory mechanisms of CONSTANS and the florigen FLOWERING LOCUS T. Plant Commun. 2023, 4, 100552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haiden, S.R.; Johnson, N.; Berkowitz, G.A. Transcriptomic analysis of CDL-gated photoperiodic flowering mechanisms in can-nabis and their responsiveness to R: FR ratios in controlled environment agriculture. Sci. Rep. 2025, 15, 17628. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corbesier, L.; Vincent, C.; Jang, S.; Fornara, F.; Fan, Q.; Searle, I.; Giakountis, A.; Farrona, S.; Gissot, L.; Turnbull, C.; et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 2007, 316, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, A.; Schmid, M. Regulation of flowering time: All roads lead to Rome. Cell. Mol. Life Sci. 2011, 68, 2013–2037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thomas, B. Light signals and flowering. J. Exp. Bot. 2006, 57, 3387–3393. [Google Scholar] [CrossRef]
- Li, J.; Li, G.; Wang, H.; Wang Deng, X. Phytochrome signaling mechanisms. Arab. Book 2011, 9, e0148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Casal, J.J. Phytochromes, cryptochromes, phototropin: Photoreceptor interactions in plants. Photochem. Photobiol. 2000, 71, 1–11. [Google Scholar] [CrossRef]
- Kendrick, R.E.; Kronenberg, G.H.M. Photomorphogenesis in Plants, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994. [Google Scholar]
- Valverde, F.; Mouradov, A.; Soppe, W.; Ravenscroft, D.; Samach, A.; Coupland, G. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 2004, 303, 1003–1006. [Google Scholar] [CrossRef]
- Kusuma, P.; Westmoreland, F.M.; Zhen, S.; Bugbee, B. Photons from NIR LEDs can delay flowering in short-day soybean and Cannabis: Implications for phytochrome activity. PLoS ONE 2021, 16, e0255232. [Google Scholar] [CrossRef]
- Whipker, B.E.; Cockson, P.; Smith, J.T. Night interruption lighting equally effective as daylength extension in retaining the vegetative state of Cannabis mother plants. Crop Forage Turfgrass Manag. 2020, 6, e20001. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, H.J.; Kim, K.S. Night interruption promotes vegetative growth and flowering of Cymbidium. Sci. Hortic. 2011, 130, 887–893. [Google Scholar] [CrossRef]
- Kjaer, K.H.; Ottosen, C. Growth of Chrysanthemum in Response to Supplemental Light Provided by Irregular Light Breaks during the Night. J. Am. Soc. Hortic. Sci. 2011, 136, 3–9. [Google Scholar] [CrossRef]
- Rodriguez-Morrison, V.; Llewellyn, D.; Zheng, Y. Cannabis yield, potency, and leaf photosynthesis respond dif-ferently to increasing light levels in an indoor environment. Front. Plant Sci. 2021, 12, 646020. [Google Scholar] [CrossRef]
- Kang, Y.; Wu, Q.; Pan, G.; Yang, H.; Li, J.; Yang, X.; Zhong, M. High daily light integral positively regulates pho-tosynthetic capacity through mediating nitrogen partitioning and leaf anatomical characteristics in flowering Chinese cabbage. Sci. Hortic. 2023, 316, 112715. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Injamum-Ul-Hoque, M.; Shaffique, S.; Ayoobi, A.; Rahman, M.A.; Rahman, M.M.; Choi, H.W. Il-luminating Cannabis sativa L.: The power of light in enhancing C. sativa growth and secondary metabolite production. Plants 2024, 13, 2774. [Google Scholar] [CrossRef]
- Moher, M.; Jones, M.; Zheng, Y. Photoperiodic Response of In Vitro Cannabis sativa Plants. HortScience 2020, 56, 108–113. [Google Scholar] [CrossRef]
- Runkle, E.S.; Heins, R.D. Specific functions of red and far-red light in flowering. J. Am. Soc. Hortic. Sci. 2001, 126, 304–308. [Google Scholar]
- Chandra, S.; Lata, H.; Khan, I.A.; ElSohly, M.A. Photosynthetic response of Cannabis sativa L. to variations in photosynthetic photon flux densities, temperature and CO2 conditions. Physiol. Mol. Biol. Plants 2008, 12, 299–306. [Google Scholar] [CrossRef]
- Anderson Carlos, J.R.; Rosas-Anderson Pablo, J. Leafscan, Version 1.3.21 [Mobile application software]; Carlos J. R. Anderson (publisher): USA. 2017. Available online: https://itunes.apple.com/app/id1254892230 (accessed on 12 March 2024).
- Apicella, P.V.; Sands, L.B.; Ma, Y.; Berkowitz, G.A. Delineating genetic regulation of cannabinoid biosynthesis during female flower development in Cannabis sativa. Plant Direct 2022, 6, e412. [Google Scholar] [CrossRef]
- Collado, C.E.; Hwang, S.J.; Hernández, R. Supplemental greenhouse lighting increased the water use efficiency, crop growth, and cutting production in Cannabis sativa. Front. Plant Sci. 2024, 15, 1371702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, J.; Collado, C.E.; Lam, V.P.; Hernández, R. Flowering Response of Cannabis Sativa L. ‘Suver Haze’ Under Varying Daylength-Extension Light Intensities and Durations. Horticulturae 2023, 9, 526. [Google Scholar] [CrossRef]
- Elkins, C.; van Iersel, M.W. Longer Photoperiods with the Same Daily Light Integral Increase Daily Electron Transport through Photosystem II in Lettuce. Plants 2020, 9, 1172. [Google Scholar] [CrossRef]
- Garrido, J.; Corral, C.; García-Valverde, M.T.; Hidalgo-García, J.; Ferreiro-Vera, C.; Martínez-Quesada, J.J. Subcanopy and Inter-Canopy Supplemental Light Enhances and Standardizes Yields in Medicinal Cannabis (Cannabis sativa L.). Plants 2025, 14, 1469. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration. Electricity Monthly Update Table and Graph. Available online: https://www.eia.gov/electricity/monthly/epm_table_grapher.php?t=epmt_5_6_a (accessed on 20 May 2024).
- Bogue, E.; Chen, D. Cannabis Law and Price Comparisons with Certain Northeast States (Report No. 2025-R-0045). Connecticut General Assembly, Office of Legislative Research. Available online: https://www.cga.ct.gov/2025/rpt/pdf/2025-R-0045.pdf (accessed on 28 February 2025).

| Treatment | Flower Weight (g) | Stem Weight (g) | Leaf Weight (g) | Total Biomass (g) |
|---|---|---|---|---|
| Control | 1295 * | 1087 * | 568 | 2951 * |
| Experimental | 1015 | 912 | 479 | 2406 |
| Treatment | Electricity Usage (kWh) | Cost ($) |
|---|---|---|
| Control | 540 | 88.61 |
| Experimental | 390 | 63.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grover, E.F.; Haiden, S.R.; Berkowitz, G.A. Examining the “Night Break” Method in Cannabis sativa Horticulture: Vegetative Daily Light Integral Affects Yield of Extractable Biomass in C. sativa. Plants 2025, 14, 3095. https://doi.org/10.3390/plants14193095
Grover EF, Haiden SR, Berkowitz GA. Examining the “Night Break” Method in Cannabis sativa Horticulture: Vegetative Daily Light Integral Affects Yield of Extractable Biomass in C. sativa. Plants. 2025; 14(19):3095. https://doi.org/10.3390/plants14193095
Chicago/Turabian StyleGrover, Evan F., Samuel R. Haiden, and Gerald A. Berkowitz. 2025. "Examining the “Night Break” Method in Cannabis sativa Horticulture: Vegetative Daily Light Integral Affects Yield of Extractable Biomass in C. sativa" Plants 14, no. 19: 3095. https://doi.org/10.3390/plants14193095
APA StyleGrover, E. F., Haiden, S. R., & Berkowitz, G. A. (2025). Examining the “Night Break” Method in Cannabis sativa Horticulture: Vegetative Daily Light Integral Affects Yield of Extractable Biomass in C. sativa. Plants, 14(19), 3095. https://doi.org/10.3390/plants14193095







