Genomic and Functional Characterization of Acetolactate Synthase (ALS) Genes in Stress Adaptation of the Noxious Weed Amaranthus palmeri
Abstract
1. Introduction
2. Results
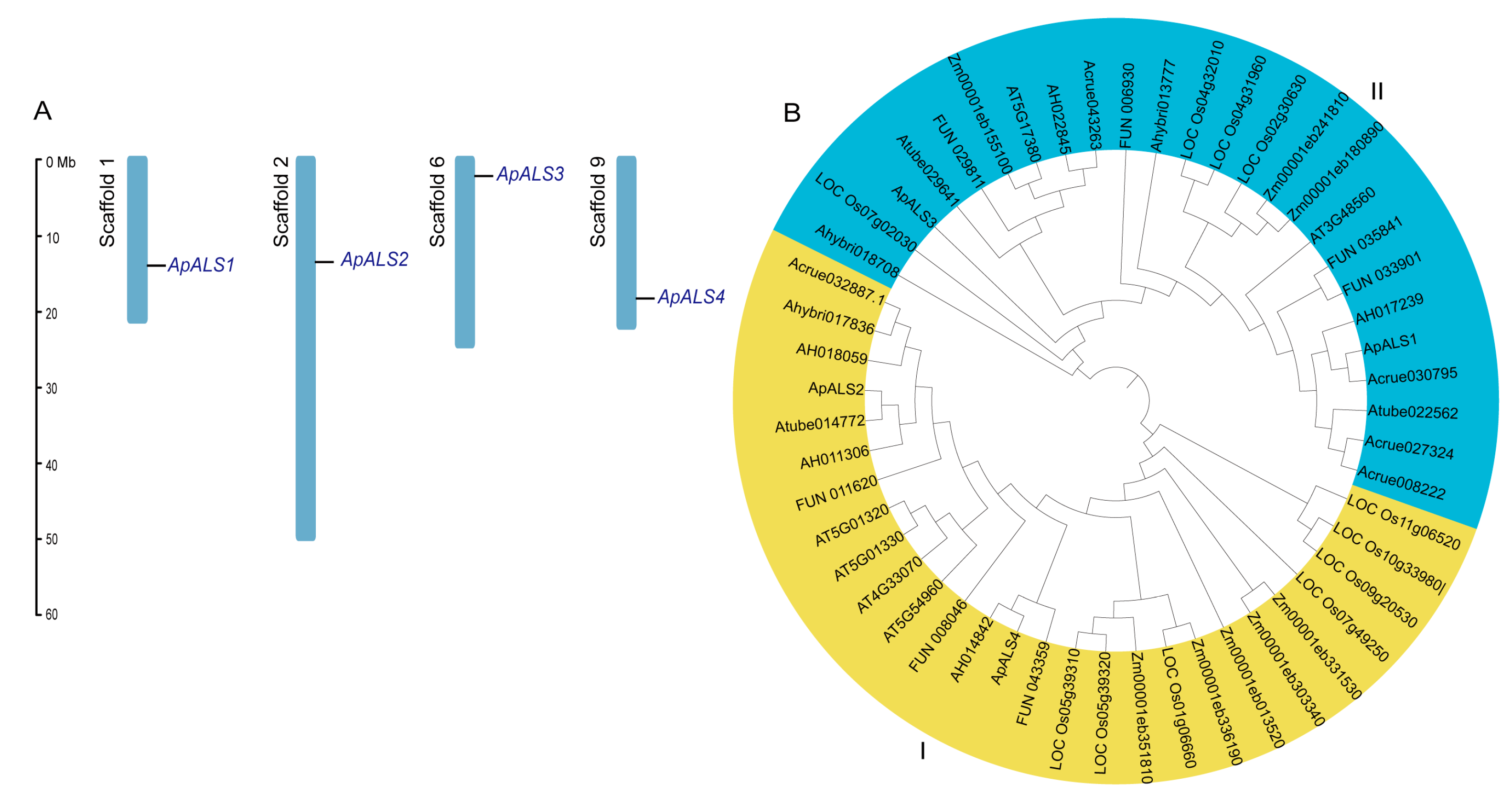
2.1. Identification, Chromosome Mapping, and Phylogenetic Analyses of ApALS Proteins
2.2. Protein Features of ApALS Proteins
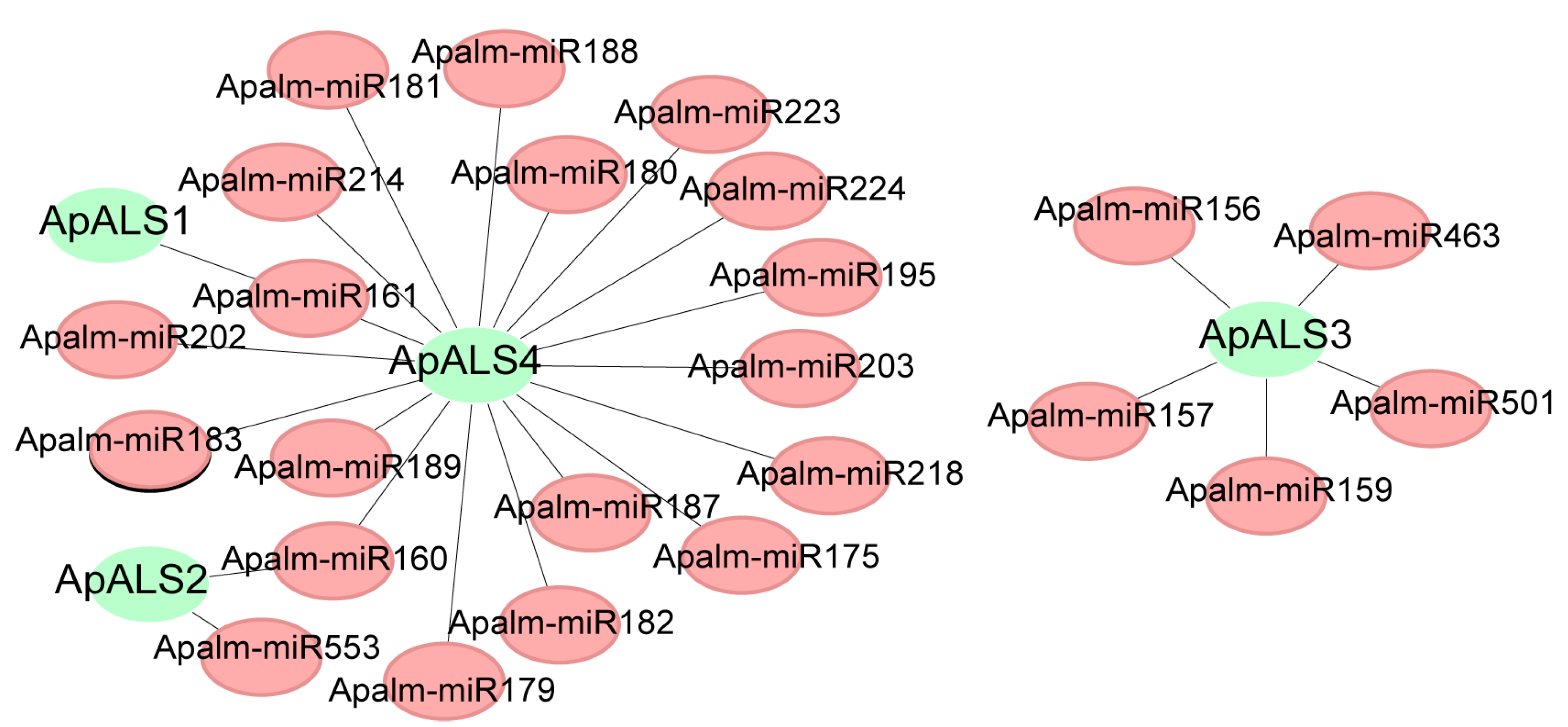
2.3. Gene Structure, Conserved Motifs, Cis-Acting Elements, and miRNA Targets of ApALSs
2.4. ApALS Expression Analysis Under Different Treatments
2.5. Subcellular Localization Analysis of ApALS Genes
2.6. Role of the AtALS1 in Imazethapyr Resistance
3. Discussion
4. Materials and Methods
4.1. Identification and Sequence Analysis of ApALS Proteins
4.2. Physicochemical Properties of ApALS Proteins
4.3. Chromosomal Distribution of ApALSs
4.4. Phylogenetic Analysis of ApALSs
4.5. Gene Structure, Motif, Cis-Acting Element, and miRNA-ApALSs Targets Analyses
4.6. Plant Materials and Growth Conditions
4.7. Stress Treatment Conditions of A. palmeri
4.8. Real-Time Quantitative PCR (RT-qPCR) Analysis
4.9. Subcellular Localization Analysis of ApALSs
4.10. Herbicide Resistance Assay in Mutant Plants
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ward, S.M.; Webster, T.M.; Steckel, L.E. Palmer amaranth (Amaranthus palmeri): A review. Weed Technol. 2013, 27, 12–27. [Google Scholar] [CrossRef]
- Bensch, C.N.; Horak, M.J.; Peterson, D. Interference of redroot pigweed (Amaranthus retroflexus), Palmer amaranth (A. palmeri), and common waterhemp (A. rudis) in soybean. Weed Sci. 2003, 51, 37–43. [Google Scholar] [CrossRef]
- King, T.A.; Norsworthy, J.K.; Butts, T.R.; Fernandes, S.B.; Drescher, G.L.; Avent, T.H. Effect of Palmer amaranth (Amaranthus palmeri) time of emergence on furrow-irrigated rice yields and weed seed production. Weed Sci. 2025, 73, e21. [Google Scholar] [CrossRef]
- Shimono, A.; Kanbe, H.; Nakamura, S.; Ueno, S.; Yamashita, J.; Asai, M. Initial invasion of glyphosate-resistant Amaranthus palmeri around grain-import ports in Japan. Plants People Planet 2022, 2, 640–648. [Google Scholar] [CrossRef]
- Culpepper, A.S.; Grey, T.L.; Vencill, W.K.; Kichler, J.M.; Webster, T.M.; Brown, S.M.; Yolk, A.C.; Davis, J.W.; Hanna, W.W. Glyphosate-resistant palmer amaranth (Amaranthus palmeri) confirmed in Georgia. Weed Sci. 2006, 54, 620–626. [Google Scholar] [CrossRef]
- Heap, I. The International Survey of Resistant Weeds. 2025. Available online: http://www.weedscience.com (accessed on 14 May 2025).
- Li, Z.Y. Amaranthus palmeri—A newly naturalized species of Amaranthus in China. Chin. Bull. Bot. 2003, 38, 734–735. [Google Scholar]
- Ji, M.J. Resistance mechanisms of Amaranthus palmeri to imazethapyr. Chin. Acad. Agric. Sci. 2022. [Google Scholar] [CrossRef]
- Tan, S.; Evans, R.R.; Dahmer, M.L.; Singh, B.K.; Shaner, D.L. Imidazolinone-tolerant crops: History, current status and future. Pest Manag. Sci. Former. Pestic. Sci. 2005, 61, 246–257. [Google Scholar] [CrossRef]
- Duggleby, R.G.; McCourt, J.A.; Guddat, L.W. Structure and mechanism of inhibition of plant acetohydroxyacid synthase. Plant Physiol. Biochem. 2008, 46, 309–324. [Google Scholar] [CrossRef]
- Liu, D.; Yu, S.; Ji, B.; Peng, Q.; Gao, J.; Zhang, J.; Guo, Y.; Hu, M. Molecular Mechanisms of Herbicide Resistance in Rapeseed: Current Status and Future Prospects for Resistant Germplasm Development. Int. J. Mol. Sci. 2025, 26, 8292. [Google Scholar] [CrossRef]
- Chipman, D.; Barak, Z.; Schloss, J.V. Biosynthesis of 2-aceto-2-hydroxy acids: Acetolactate synthases and acetohydroxyacid synthases. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1998, 1385, 401–419. [Google Scholar] [CrossRef]
- Singh, B.K.; Shaner, D.L. Biosynthesis of branched chain amino acids: From test tube to field. Plant Cell 1995, 7, 935. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Chen, L.; Deng, X.W.; Tang, X. Development of herbicide resistance genes and their application in rice. Crop J. 2022, 10, 26–35. [Google Scholar] [CrossRef]
- Zhao, L.; Deng, L.; Zhang, Q.; Jing, X.; Ma, M.; Yi, B.; Wen, J.; Ma, C.; Tu, J.; Fu, T.; et al. Autophagy contributes to sulfonylurea herbicide tolerance via GCN2-independent regulation of amino acid homeostasis. Autophagy 2018, 14, 702–714. [Google Scholar] [CrossRef]
- Herbicide Resistance Action Committee (HRAC). Global Herbicide Mode of Action Classification; Retrieved from HRAC Global Herbicide MoA Classification Tool; Herbicide Resistance Action Committee: Corvallis, OR, USA, 2024. [Google Scholar]
- Mazur, B.J.; Falco, S.C. The development of herbicide resistance crops. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 441–470. [Google Scholar] [CrossRef]
- Ren, H.L. Overview of acetolactate synthase and ALS gene research. Chin. Agric. Sci. Bull. 2016, 32, 37–42. [Google Scholar]
- Heap, I. Global perspective of herbicide-resistant weeds. Pest Manag. Sci. 2014, 70, 1306–1315. [Google Scholar] [CrossRef]
- Mallory-Smith, C.A.; Thill, D.C.; Dial, M.J. Identification of sulfonylurea herbicide-resistant prickly lettuce (Lactuca serriola). Weed Technol. A J. Weed Sci. Soc. Am. 1990, 4, 163–168. [Google Scholar] [CrossRef]
- Jander, G.; Baerson, S.R.; Hudak, J.A.; Gonzalez, K.A.; Gruys, K.J.; Last, R.L. Ethylmethanesulfonate saturation mutagenesis in Arabidopsis to determine frequency of herbicide resistance. Plant Physiol. 2003, 131, 139–146. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: www.weedscience.org (accessed on 24 September 2025).
- Yu, C.S.; Chen, Y.C.; Lu, C.H.; Hwang, J.K. Prediction of protein subcellular localization. Proteins Struct. Funct. Bioinform. 2006, 64, 643–651. [Google Scholar] [CrossRef]
- Hu, M.L.; Long, W.H.; Gao, J.Q.; Chen, F.; Zhou, X.Y.; Zhang, W.; Chen, S.; Zhang, J.F.; Pu, H.M. Subcellular locating, prokaryotic expression and enzymatic activity analysis of rapeseed acetolactate synthase gene BnALS3. Chin. J. Oil Crop Sci. 2018, 40, 309–317. [Google Scholar]
- Chen, B. Functional Characterization of a Trichome-Specific Highly Expressed Acetolactate Synthase Gene in Glycolipid Biosynthesis in Tobacco; Chinese Academy of Agricultural Sciences: Beijing, China, 2020. [Google Scholar]
- Pan, W.; Zhu, Y.; Li, P.; Li, Z.; Xu, C.; Jin, M.; Tang, X. Natural and artificial evolution of acetolactate synthase for crop breeding. Crop J. 2025. [Google Scholar] [CrossRef]
- Mayya, V.K.; Duchaine, T.F. Ciphers and executioners: How 3′-untranslated regions determine the fate of messenger RNAs. Front. Genet. 2019, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Lytle, J.R.; Yario, T.A.; Steitz, J.A. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′ UTR as in the 3′ UTR. Proc. Natl. Acad. Sci. USA 2007, 104, 9667–9672. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Zeng, J.; Yun, Z.; Liu, Y.; Qu, H.; Jiang, Y.; Duan, X.; Xia, R. Micro RNA 528, a hub regulator modulating ROS homeostasis via targeting of a diverse set of genes encoding copper-containing proteins in monocots. New Phytol. 2020, 225, 385–399. [Google Scholar] [CrossRef]
- Yin, J.; Wang, L.; Zhao, J.; Li, Y.; Huang, R.; Jiang, X.; Zhou, X.; Zhu, X.; He, Y.; He, Y. Genome-wide characterization of the C2H2 zinc-finger genes in Cucumis sativus and functional analyses of four CsZFPs in response to stresses. BMC Plant Biol. 2020, 20, 359. [Google Scholar] [CrossRef]
- Sang, Y.M.; Liu, Q.F.; Lee, J.; Ma, W.; McVey, D.S.; Blecha, F. Expansion of amphibian intronless interferons revises the paradigm for interferon evolution and functional diversity. Sci. Rep. 2016, 6, 29072. [Google Scholar] [CrossRef]
- Sheshadri, S.A.; Nishanth, M.J.; Simon, B. Stress-mediated cis-element transcription factor interactions interconnecting primary and specialized metabolism in planta. Front. Plant Sci. 2016, 7, 1725. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, H.; Zou, Y.; Qiu, J.; Wen, Y.; Xu, D. Are nutrient stresses associated with enantioselectivity of the chiral herbicide imazethapyr in Arabidopsis thaliana? J. Agric. Food Chem. 2015, 63, 10209–10217. [Google Scholar] [CrossRef]
- Fassiano, A.V.; March, H.; Santos, M.; Juarez, A.B.; Carmen, M.D.; Molina, R.D. Toxicological effects of active and inert ingredients of imazethapyr formulation Verosil® against Scenedesmus vacuolatus (Chlorophyta). Environ. Sci. Pollut. Res. 2022, 29, 31384–31399. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Z.; Zhang, N.; Liu, J.; Tian, Z.; Sun, C. Transcriptomic and metabolomic analysis provides insight into imazethapyr toxicity to non-target plants. Environ. Sci. Pollut. Res. 2024, 31, 28368–28378. [Google Scholar] [CrossRef] [PubMed]
- Zabalza, A.; Royuela, M. Inducing hypoxic stress responses by herbicides that inhibit amino acid biosynthesis. In Low-Oxygen Stress in Plants: Oxygen Sensing and Adaptive Responses to Hypoxia; Springer: Vienna, Austria, 2013; pp. 381–394. [Google Scholar] [CrossRef]
- Wang, M.J.; Xuan, N.X.; Wu, J. Molecular docking and molecular dynamics simulations of acetolactate synthase with inhibitors ZJ0777 and CIE. J. Zhejiang Univ. (Sci. Ed.) 2015, 42, 709–713+725. [Google Scholar]
- Wang, J.; Du, Y.; Zhang, L.; Deng, Y.; Wang, T.; Wang, S.; Ji, M. Pro-197-Ser mutation combinations in acetolactate synthase (ALS) homoeologous genes affect ALS inhibitor herbicide resistance levels in Monochoria korsakowii. Pest Manag. Sci. 2025, 81, 1894–1902. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Li, S.; Ren, B.; Yan, F.; Spetz, C.; Li, X.; Zhou, X.; Zhou, H. Base-editing-mediated artificial evolution of OsALS1 in Planta to develop novel herbicide-tolerant rice germplasms. Mol. Plant 2020, 13, 565–572. [Google Scholar] [CrossRef]
- Molin, W.T.; Nandula, V.K.; Wright, A.A.; Bond, J.A. Transfer and expression of ALS inhibitor resistance from Palmer amaranth (Amaranthus palmeri) to an A. spinosus × A. palmeri hybrid. Weed Sci. 2016, 64, 240–247. [Google Scholar] [CrossRef]
- Takano, H.K.; Beffa, R.; Preston, C.; Westra, P.; Dayan, F.E. Reactive oxygen species trigger the fast action of glufosinate. Planta 2019, 249, 1837–1849. [Google Scholar] [CrossRef]
- Priess, G.L.; Norsworthy, J.K.; Godara, N.; Mauromoustakos, A.; Butts, T.R.; Roberts, T.L.; Barber, T. Confirmation of glufosinate-resistant Palmer amaranth and response to other herbicides. Weed Technol. 2022, 36, 368–372. [Google Scholar] [CrossRef]
- Wu, J.; Chen, C.; Xian, G.Y.; Liu, D.; Lin, L.; Yin, S.; Sun, Q.; Fang, Y.; Zhang, H.; Wang, Y. Engineering herbicide resistant oilseed rape by CRISPR/Cas9-mediated cytosine base-editing. Plant Biotechnol. J. 2020, 18, 1857–1859. [Google Scholar] [CrossRef]
- Dweikat, I.M.; Gelli, M.; Bernards, M.; Martin, A.; Jhala, A. Mutations in the acetolactate synthase (ALS) enzyme affect shattercane (Sorghum bicolor) response to ALS-inhibiting herbicides. Hereditas 2023, 160, 28. [Google Scholar] [CrossRef]
- Sen, M.K.; Hamouzová, K.; Roy, A.; Soukup, J. Transposable element-driven evolution of herbicide resistance in plants. J. Exp. Bot. 2025, 76, 1495–1499. [Google Scholar] [CrossRef]
- Tranel, P.J.; Wright, T.R. Resistance of weeds to ALS-inhibiting herbicides: What have we learned? Weed Sci. 2002, 50, 700–712. [Google Scholar] [CrossRef]
- Yang, S.H.; Kim, E.; Park, H.; Koo, Y. Selection of the high efficient sgRNA for CRISPR-Cas9 to edit herbicide related genes, PDS, ALS, and EPSPS in tomato. Appl. Biol. Chem. 2022, 65, 13. [Google Scholar] [CrossRef]
- Wen, S.Z. Gene Editing and Transgenic Overexpression of Arabidopsis acetolactate Synthase (AtALS) for Herbicide Resistance Analysis. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2019. [Google Scholar] [CrossRef]
- Qiao, D.; Wang, J.; Lu, M.H.; Xin, C.; Chai, Y.; Jiang, Y.; Sun, W.; Cao, Z.; Guo, S.; Wang, C.X.; et al. Optimized prime editing efficiently generates heritable mutations in maize. J. Integr. Plant Biol. 2023, 65, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.X.; Li, D.; Wang, C.Y. Development of herbicide-resistant sorghum through site-directed mutagenesis of maize acetolactate synthase (ALS) gene. Mol. Plant Breed. 2017, 15, 4563–4572. [Google Scholar] [CrossRef]
- Xiao, Y.H. Reference Gene Mining in Cyperus esculentus and Functional Characterization of Herbicide Target Genes ALS and EPSPS. Master’s Thesis, South-Central Minzu University, Wuhan, China, 2023. [Google Scholar] [CrossRef]
- He, Q.Y.; Guo, G.; Wang, C.; Xy, Y.; Zhang, L.; Qi, J.; Xu, J.; Zhang, L. Identification and expression pattern analysis of kenaf (Hibiscus cannabinus) acetolactate synthase (ALS) genes. Mol. Plant Breed. 2022. Available online: http://kns.cnki.net/kcms/detail/46.1068.S.20220216.1554.019.html (accessed on 12 April 2025).
- Singh, A.; Mahato, A.K.; Maurya, A.; Rajkumar, S.; Singh, A.K.; Bhardwaj, R.; Kaushik, S.K.; Kumar, S.; Gupta, V.; Singh, K. Amaranth Genomic Resource Database: An integrated database resource of Amaranth genes and genomics. Front. Plant Sci. 2023, 14, 1203855. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Berkman, S.J.; Roscoe, E.M.; Bourret, J.C. Comparing self-directed methods for training staff to create graphs using Graphpad Prism. J. Appl. Behav. Anal. 2019, 52, 188–204. [Google Scholar] [CrossRef]
- Ge, Z.; Jing, Y.; Zhu, J.; Yang, L.E.; Lu, S.; Deng, Y. APE1 localizes to chloroplast stromules and interacts with ATI1 in Arabidopsis. Plant Signal. Behav. 2025, 20, 2511830. [Google Scholar] [CrossRef]
- Rehman, U.; Saad, A.A.; Bhat, M.A.; Masood, A.; Kanth, R.H.; Saxena, A.; Mir, A.H.; Wani, F.J.; Bhat, M.A. Imazethapyr as post-emergent herbicide in common-bean (Phaseolus vulgaris L.) under rainfed temperate condition of Kashmir, India. Indian J. Weed Sci. 2023, 55, 425–430. [Google Scholar] [CrossRef]
- Ma, C.; Pei, Z.Q.; Bai, X.; Feng, J.Y.; Zhang, L.; Fan, J.R.; Wang, J.; Zhang, T.G.; Zheng, S. Involvement of NO and Ca2+ in the enhancement of cold tolerance induced by melatonin in winter turnip rape (Brassica rapa L.). Plant Physiol. Biochem. 2022, 190, 262–276. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Song, M.; Bimpong, D.; Wang, F.; Chen, W.; Ma, D.; Du, L. Genomic and Functional Characterization of Acetolactate Synthase (ALS) Genes in Stress Adaptation of the Noxious Weed Amaranthus palmeri. Plants 2025, 14, 3088. https://doi.org/10.3390/plants14193088
Ren J, Song M, Bimpong D, Wang F, Chen W, Ma D, Du L. Genomic and Functional Characterization of Acetolactate Synthase (ALS) Genes in Stress Adaptation of the Noxious Weed Amaranthus palmeri. Plants. 2025; 14(19):3088. https://doi.org/10.3390/plants14193088
Chicago/Turabian StyleRen, Jiao, Mengyuan Song, Daniel Bimpong, Fulian Wang, Wang Chen, Dongfang Ma, and Linfeng Du. 2025. "Genomic and Functional Characterization of Acetolactate Synthase (ALS) Genes in Stress Adaptation of the Noxious Weed Amaranthus palmeri" Plants 14, no. 19: 3088. https://doi.org/10.3390/plants14193088
APA StyleRen, J., Song, M., Bimpong, D., Wang, F., Chen, W., Ma, D., & Du, L. (2025). Genomic and Functional Characterization of Acetolactate Synthase (ALS) Genes in Stress Adaptation of the Noxious Weed Amaranthus palmeri. Plants, 14(19), 3088. https://doi.org/10.3390/plants14193088





