Cucumber Green Mottle Mosaic Virus Decreases Chlorophyll a Content in Cucurbit Crops by Upregulating the Key Gene in Chlorophyll Catabolic Pathway, Chlorophyllase 1
Abstract
1. Introduction
2. Results
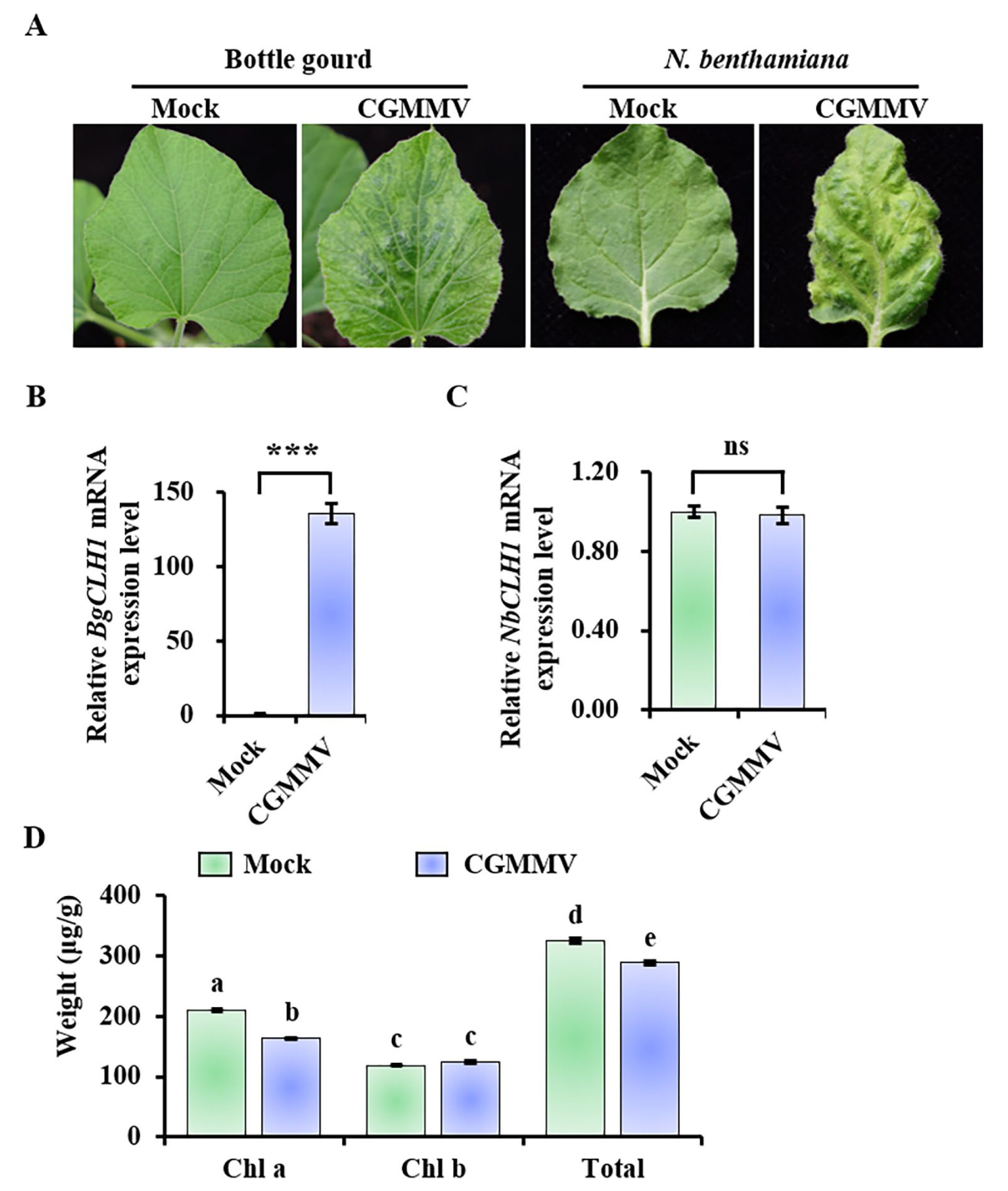
2.1. CGMMV Infection Significantly Induced the Expression of BgCLH1 in Bottle Gourd, but Not in N. benthamiana
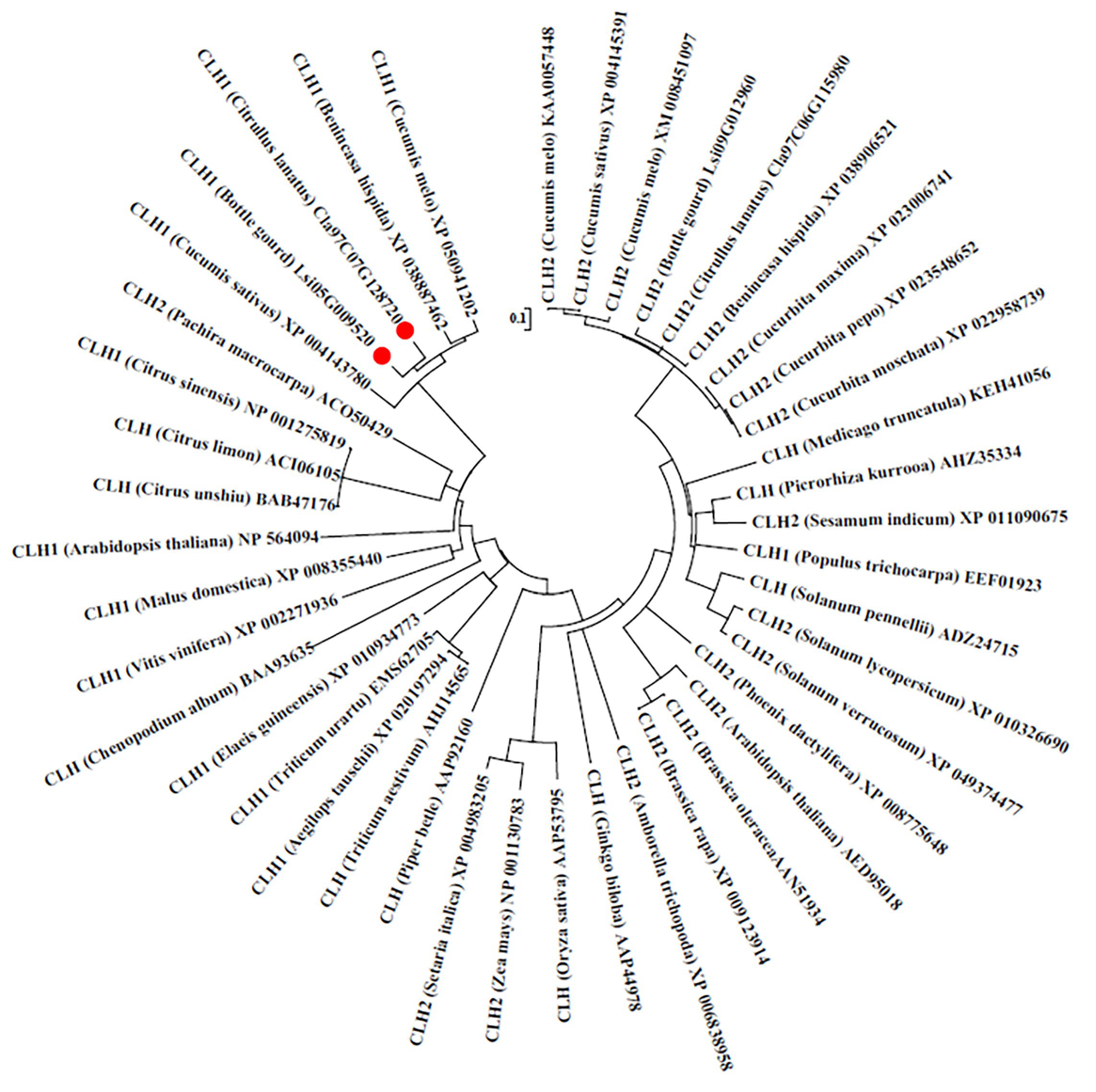
2.2. Phylogenetic Analysis of BgCLH1
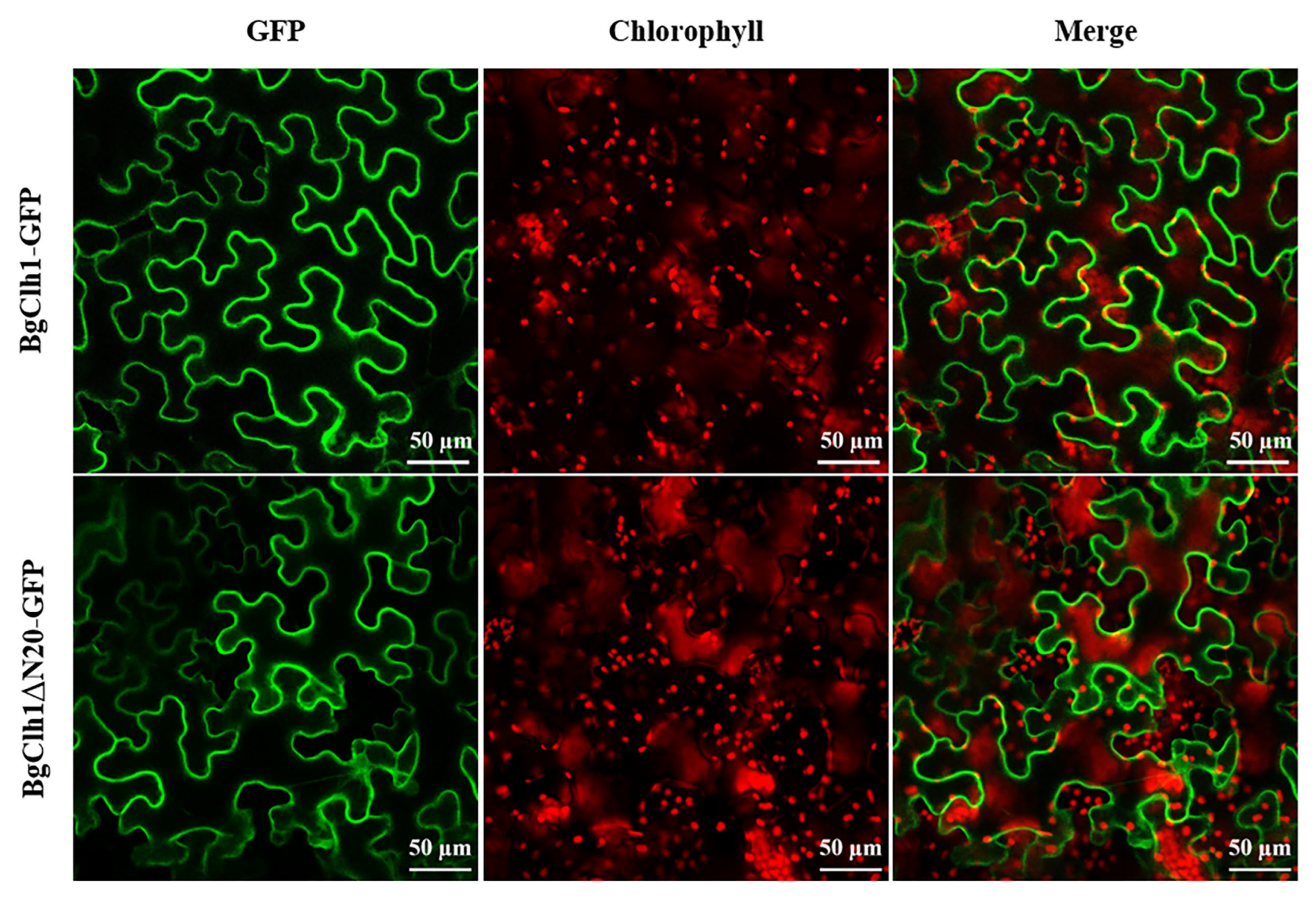
2.3. Subcellular Localization of BgCLH1
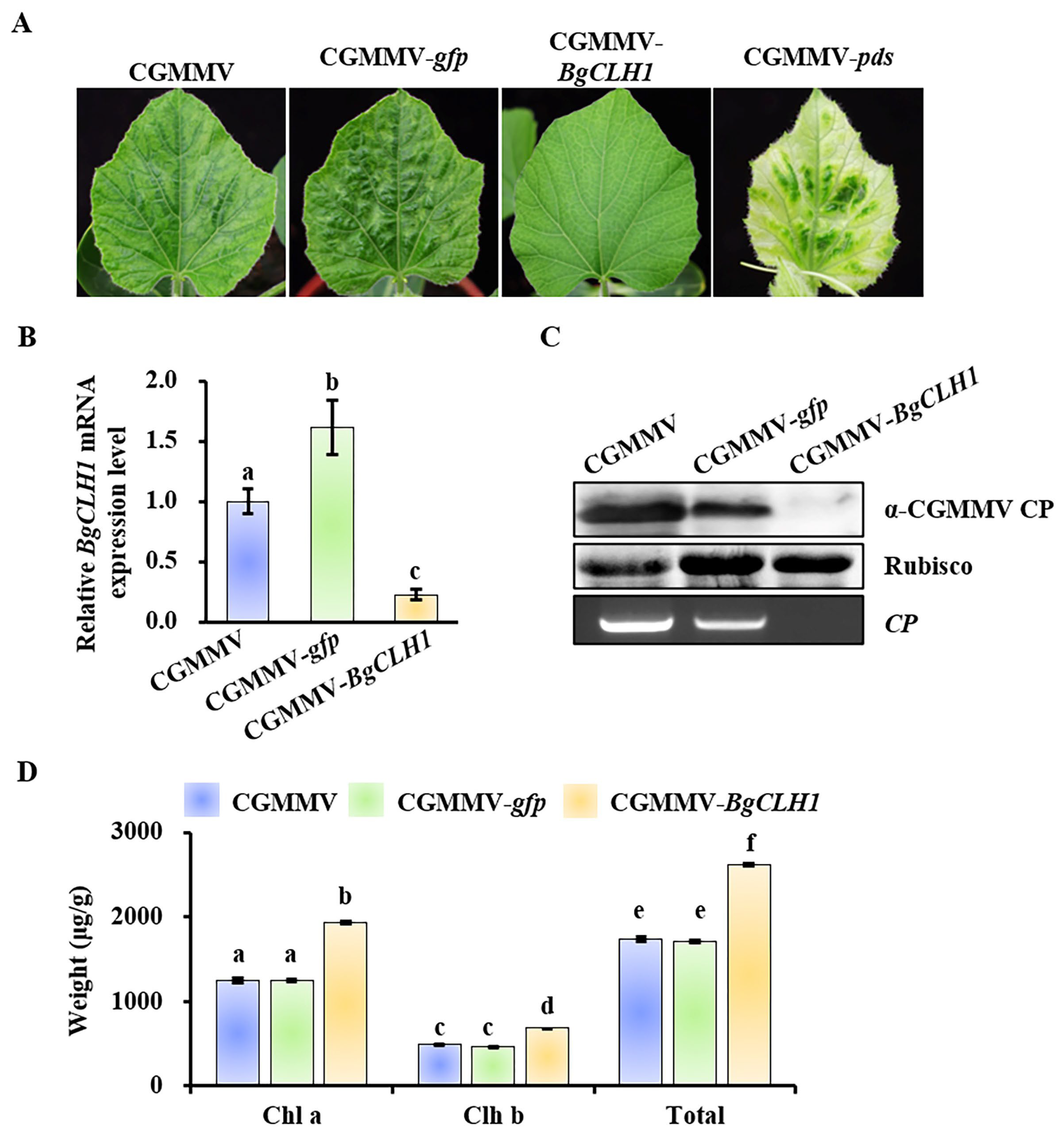
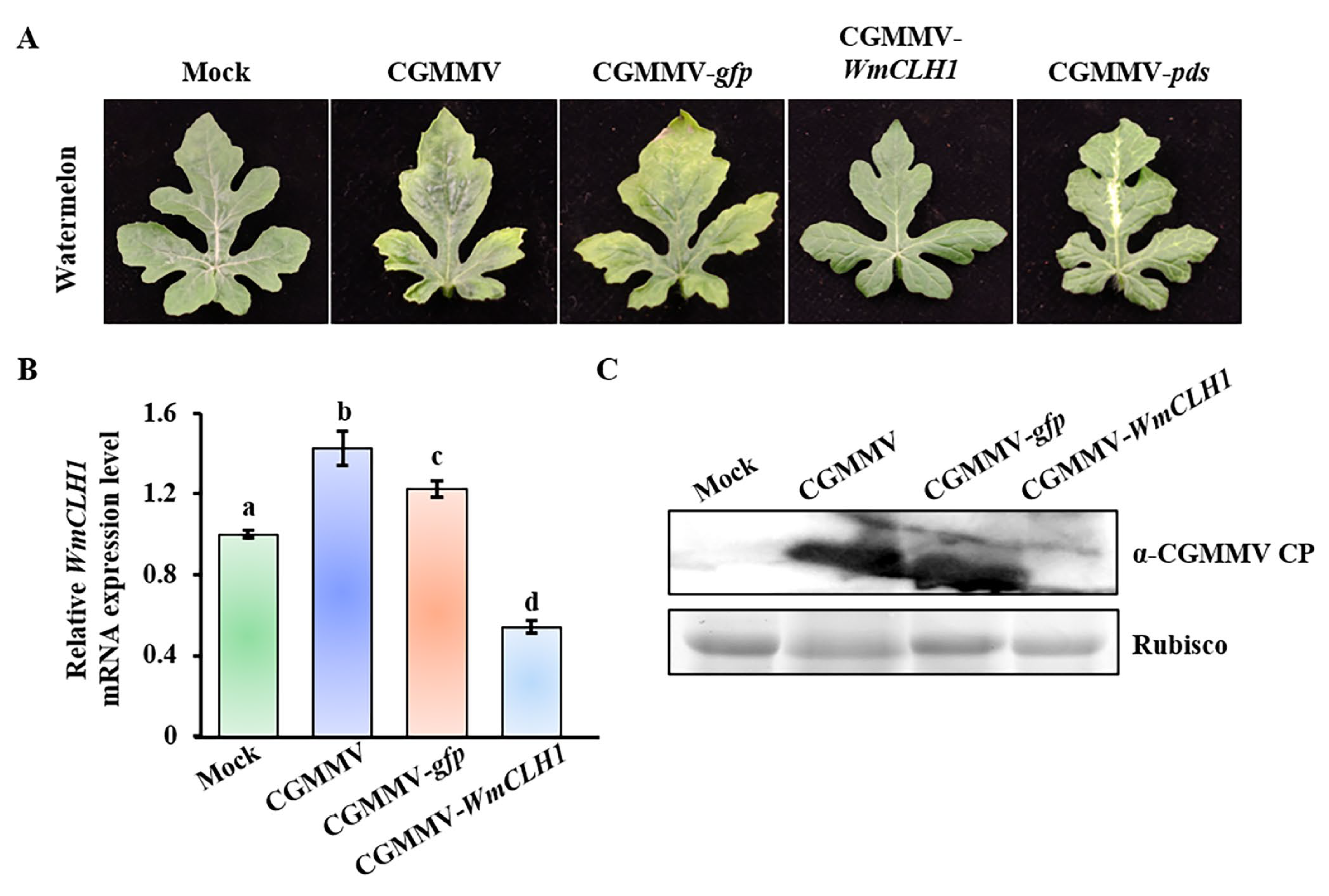
2.4. Down-Regulation of BgCLH1 Expression Reduced Viral Infection and Increased Chlorophyll Accumulation
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions
4.2. Virus Inoculation and Detection
4.3. Real-Time PCR Analysis
4.4. Plasmid Construction
4.5. Chlorophyll Measurement
4.6. Phylogenetic Tree Construction
4.7. Confocal Microscopy
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, T.; Zhou, T. Unraveling the mechanisms of virus-induced symptom development in plants. Plants 2023, 12, 2830. [Google Scholar] [CrossRef]
- Li, Y.; Cui, H.; Cui, X.; Wang, A. The altered photosynthetic machinery during compatible virus infection. Curr. Opin. Virol. 2016, 17, 19–24. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Chakraborty, S. Chloroplast: The Trojan horse in plant–virus interaction. Mol. Plant Pathol. 2017, 19, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Medina-Puche, L.; Tan, H.; Dogra, V.; Wu, M.; Rosas-Diaz, T.; Wang, L.; Ding, X.; Zhang, D.; Fu, X.; Kim, C.; et al. A defense pathway linking plasma membrane and chloroplasts and co-opted by pathogens. Cell 2020, 182, 1109–1124.e25. [Google Scholar] [CrossRef] [PubMed]
- Dombrovsky, A.; Tran-Nguyen, L.T.T.; Jones, R.A.C. Cucumber green mottle mosaic virus: Rapidly increasing global distribution, etiology, epidemiology, and management. Annu. Rev. Phytopathol. 2017, 55, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Nong, Y.; Farooq, T.; Tang, Y.F.; She, X.M.; Yu, L.; Lan, G.B.; Zhou, X.P.; He, Z.F. Small RNA deep sequencing reveals the presence of multiple viral infections in cucurbit crops in Guangdong, China. J. Integr. Agric. 2022, 21, 1389–1400. [Google Scholar] [CrossRef]
- Liu, M.; Liang, Z.; Aranda, M.A.; Hong, N.; Liu, L.; Kang, B.; Gu, Q. A cucumber green mottle mosaic virus vector for virus-induced gene silencing in cucurbit plants. Plant Methods 2020, 16, 9. [Google Scholar] [CrossRef]
- Li, Z.G.; Tang, Y.F.; Lan, G.B.; Yu, L.; Ding, S.W.; She, X.M.; He, Z.F. Transcriptome and metabolome analyses reveal that jasmonic acids may facilitate the infection of cucumber green mottle mosaic virus in bottle gourd. Int. J. Mol. Sci. 2023, 24, 16566. [Google Scholar] [CrossRef]
- Kong, L.; Wu, J.; Lu, L.; Xu, Y.; Zhou, X. Interaction between rice stripe virus disease-specific protein and host PsbP enhances virus symptoms. Molecular Plant 2014, 7, 691–708. [Google Scholar] [CrossRef]
- Smith, N.A.; Eamens, A.L.; Wang, M.-B. Viral small interfering RNAs target host genes to mediate disease symptoms in plants. PLoS Pathog. 2011, 7, e1002022. [Google Scholar] [CrossRef]
- Lehto, K.; Tikkanen, M.; Hiriart, J.B.; Paakkarinen, V.; Aro, E.M. Depletion of the photosystem II core complex in mature tobacco leaves infected by the flavum strain of tobacco mosaic virus. Mol. Plant-Microbe Interact. 2003, 16, 1135–1144. [Google Scholar] [CrossRef]
- Padmanabhan, M.S.; Goregaoker, S.P.; Golem, S.; Shiferaw, H.; Culver, J.N. Interaction of the tobacco mosaic virus replicase protein with the Aux/IAA protein PAP1/IAA26 is associated with disease development. J. Virol. 2005, 79, 2549–2558. [Google Scholar] [CrossRef]
- Harpaz-Saad, S.; Azoulay, T.; Arazi, T.; Ben-Yaakov, E.; Mett, A.; Shiboleth, Y.M.; Hortensteiner, S.; Gidoni, D.; Gal-On, A.; Goldschmidt, E.E.; et al. Chlorophyllase is a rate-limiting enzyme in chlorophyll catabolism and is posttranslationally regulated. Plant Cell 2007, 19, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Tanaka, R. Chlorophyll metabolism. Curr. Opin. Plant Biol. 2006, 9, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Mayer, H. Untersuchungen über die Chlorophyllase. Planta 1930, 11, 294–330. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Ohta, H.; Okawa, K.; Iwamatsu, A.; Shimada, H.; Masuda, T.; Takamiya, K. Cloning of chlorophyllase, the key enzyme in chlorophyll degradation: Finding of a lipase motif and the induction by methyl jasmonate. Proc. Natl. Acad. Sci. USA 1999, 96, 15362–15367. [Google Scholar] [CrossRef]
- Jacob-Wilk, D.; Holland, D.; Goldschmidt, E.E.; Riov, J.; Eyal, Y. Chlorophyll breakdown by chlorophyllase: Isolation and functional expression of the Chlase1 gene from ethylene-treated citrus fruit and its regulation during development. Plant J. 1999, 20, 653–661. [Google Scholar] [CrossRef]
- Benedetti, C.E.; Arruda, P. Altering the expression of the chlorophyllase gene ATHCOR1 in transgenic Arabidopsis caused changes in the chlorophyll-to-chlorophyllide ratio. Plant Physiol. 2002, 128, 1255–1263. [Google Scholar] [CrossRef]
- McFeeters, R.F. Substrate specificity of chlorophyllase. Plant Physiol. 1975, 55, 377–381. [Google Scholar] [CrossRef]
- Srivastava, A.; Tiwari, C. Influence of cucumber green mottle mosaic virus on chlorophyllase activity of Cucumis sativus (Linn.). J. Living World 1996, 3, 38–40. [Google Scholar]
- Bailiss, K.W. Infection of cucumber cotyledons by cucumber mosaic virus and the participation of chlorophyllase in the development of chlorotic lesions. Ann. Bot. 1970, 34, 647–655. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Bi, H.; Zhang, P. Why mosaic? Gene expression profiling of African cassava mosaic virus-infected cassava reveals the effect of chlorophyll degradation on symptom development. J. Integr. Plant Biol. 2014, 56, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, K.I.; Tsuchiya, T.; Ohta, H. Degradation pathway(s) of chlorophyll: What has gene cloning revealed? Trends Plant Sci. 2000, 5, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Y.; Khan, I.; Jiao, Q.S.; Zada, A.; Jia, T. Chlorophyllase, a common plant hydrolase enzyme with a long history, is still a puzzle. Genes 2021, 12, 1871. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Makita, S.; Schelbert, S.; Sano, S.; Ochiai, M.; Tsuchiya, T.; Hasegawa, S.F.; Hortensteiner, S.; Tanaka, A.; Tanaka, R. Reexamination of chlorophyllase function implies its involvement in defense against chewing herbivores. Plant Physiol. 2015, 167, 660–670. [Google Scholar] [CrossRef]
- Hu, X.; Jia, T.; Hortensteiner, S.; Tanaka, A.; Tanaka, R. Subcellular localization of chlorophyllase2 reveals it is not involved in chlorophyll degradation during senescence in Arabidopsis thaliana. Plant Sci. 2020, 290, 110314. [Google Scholar] [CrossRef]
- Chou, Y.L.; Ko, C.Y.; Yen, C.C.; Chen, L.F.; Shaw, J.F. A novel recombinant chlorophyllase1 from Chlamydomonas reinhardtii for the production of chlorophyllide derivatives. J. Agric. Food Chem. 2015, 63, 9496–9503. [Google Scholar] [CrossRef]
- Kariola, T.; Brader, G.; Li, J.; Palva, E.T. Chlorophyllase 1, a damage control enzyme, affects the balance between defense pathways in plants. Plant Cell 2005, 17, 282–294. [Google Scholar] [CrossRef]
- Li, Z.; Du, Z.; Tang, Y.; She, X.; Wang, X.; Zhu, Y.; Yu, L.; Lan, G.; He, Z. C4, the pathogenic determinant of tomato leaf curl Guangdong virus, may suppress post-transcriptional gene silencing by interacting with BAM1 protein. Front. Microbiol. 2020, 11, 851. [Google Scholar] [CrossRef]
- Goodin, M.M.; Dietzgen, R.G.; Schichnes, D.; Ruzin, S.; Jackson, A.O. pGD vectors: Versatile tools for the expression of green and red fluorescent protein fusions in agroinfiltrated plant leaves. Plant J. 2002, 31, 375–383. [Google Scholar] [CrossRef]
- Fan, H.; Sun, H.; Wang, Y.; Zhang, Y.; Wang, X.; Li, D.; Yu, J.; Han, C. Deep sequencing–based transcriptome profiling reveals comprehensive insights into the responses of Nicotiana benthamiana to beet necrotic yellow vein virus infections containing or lacking RNA4. PLoS ONE 2014, 9, e85284. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Zheng, X.; Ye, J.; Huang, Y.; Chen, H.; Mei, X.; Xie, Z.; Cao, L.; Zeng, Y.; Larkin, R.M.; et al. Regulation of carotenoid and chlorophyll pools in hesperidia, anatomically unique fruits found only in Citrus. Plant Physiol. 2021, 187, 829–845. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Tang, Y.; Lan, G.; Yu, L.; Ding, S.; He, Z.; She, X. Cucumber Green Mottle Mosaic Virus Decreases Chlorophyll a Content in Cucurbit Crops by Upregulating the Key Gene in Chlorophyll Catabolic Pathway, Chlorophyllase 1. Plants 2025, 14, 3086. https://doi.org/10.3390/plants14193086
Li Z, Tang Y, Lan G, Yu L, Ding S, He Z, She X. Cucumber Green Mottle Mosaic Virus Decreases Chlorophyll a Content in Cucurbit Crops by Upregulating the Key Gene in Chlorophyll Catabolic Pathway, Chlorophyllase 1. Plants. 2025; 14(19):3086. https://doi.org/10.3390/plants14193086
Chicago/Turabian StyleLi, Zhenggang, Yafei Tang, Guobing Lan, Lin Yu, Shanwen Ding, Zifu He, and Xiaoman She. 2025. "Cucumber Green Mottle Mosaic Virus Decreases Chlorophyll a Content in Cucurbit Crops by Upregulating the Key Gene in Chlorophyll Catabolic Pathway, Chlorophyllase 1" Plants 14, no. 19: 3086. https://doi.org/10.3390/plants14193086
APA StyleLi, Z., Tang, Y., Lan, G., Yu, L., Ding, S., He, Z., & She, X. (2025). Cucumber Green Mottle Mosaic Virus Decreases Chlorophyll a Content in Cucurbit Crops by Upregulating the Key Gene in Chlorophyll Catabolic Pathway, Chlorophyllase 1. Plants, 14(19), 3086. https://doi.org/10.3390/plants14193086




