Turning Susceptibility into Strength: A New Era of Durable Resistance in Plants Through Genome Editing
Abstract
1. Introduction
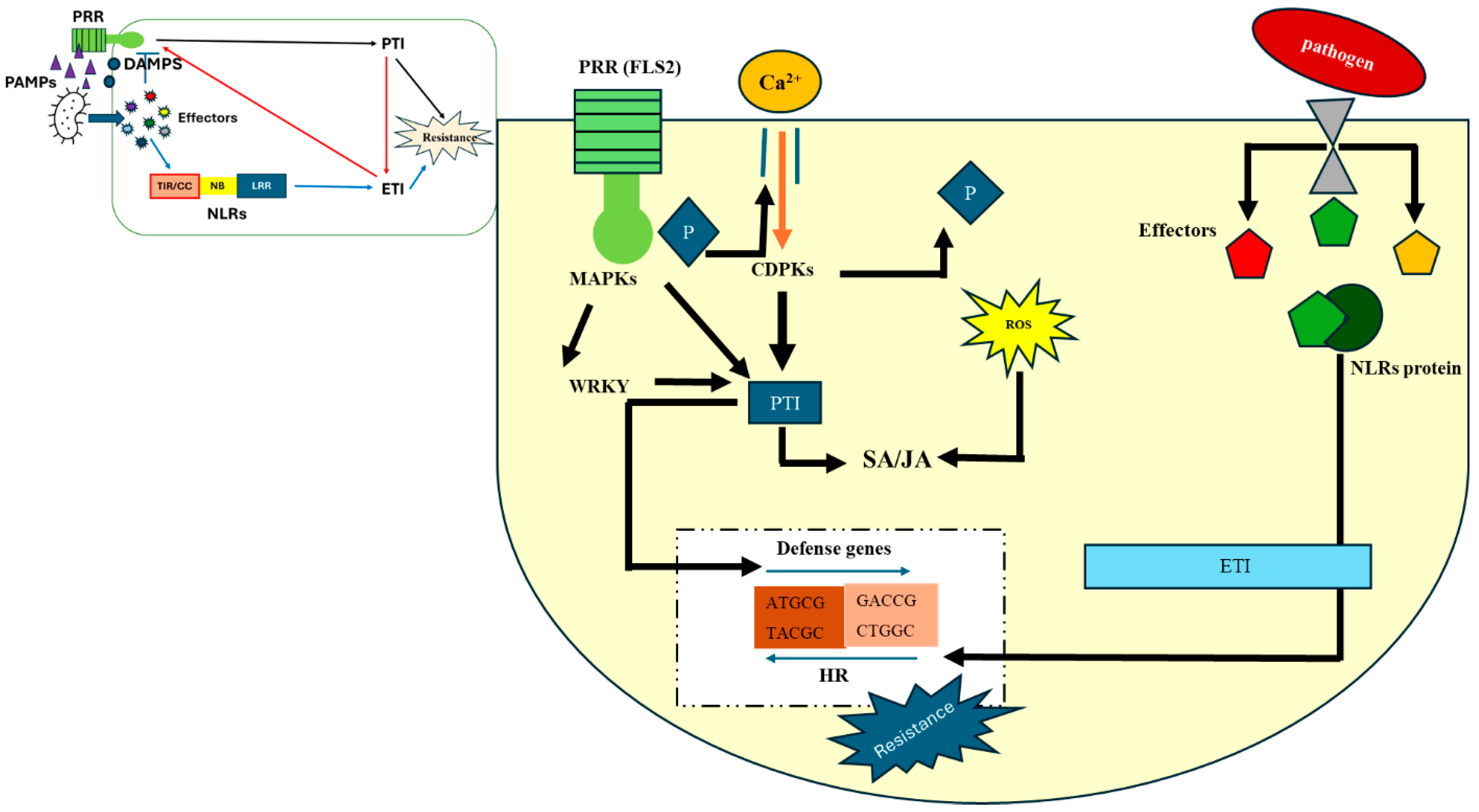
2. Defense Mechanism in Plants Against Biotic Stress
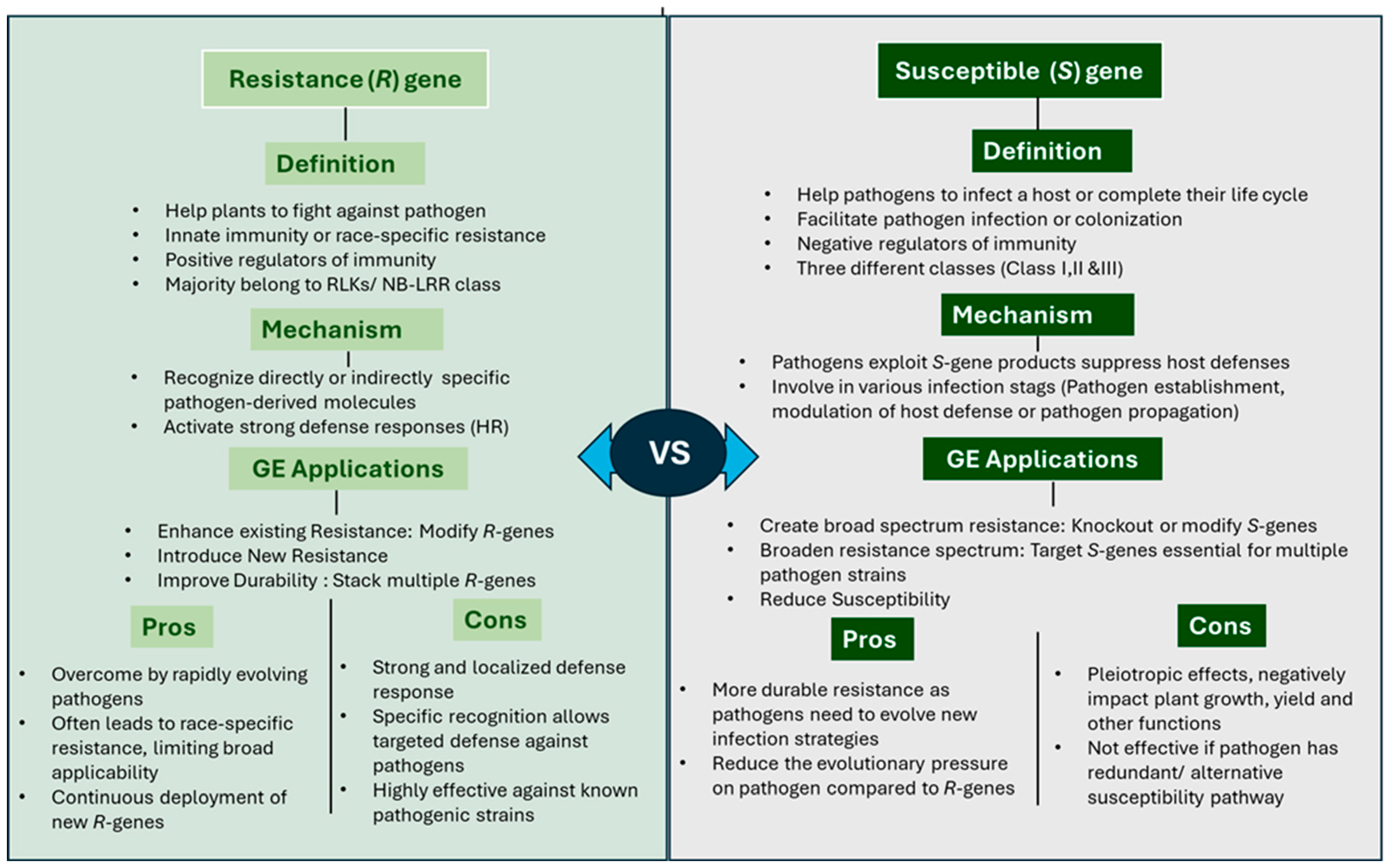
3. R-Genes vs. S-Genes
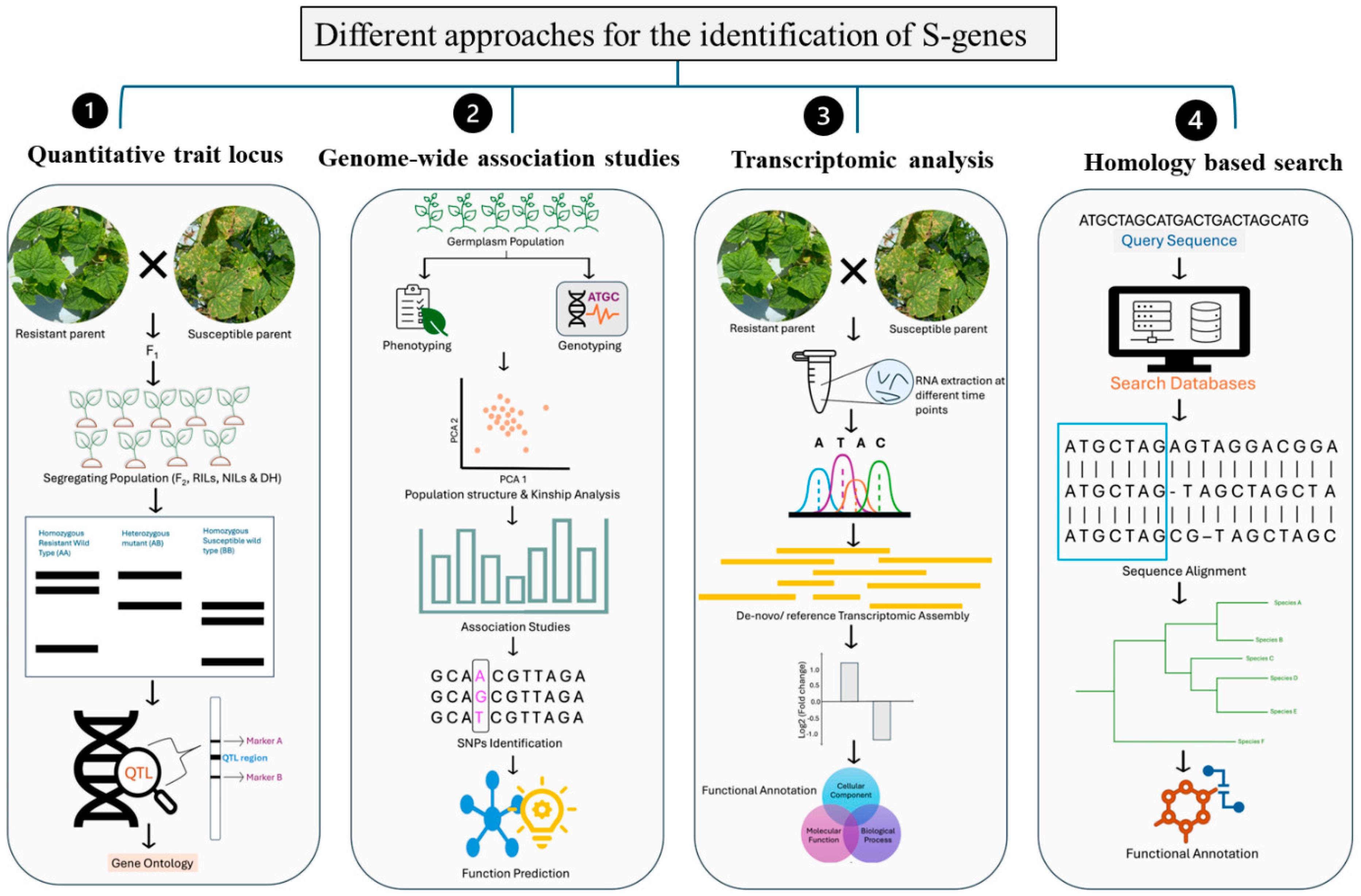
4. Methods for Identification of S-Genes in Plants
4.1. Quantitative Trait Locus (QTL)
4.2. Genome-Wide Association Studies (GWASs)
4.3. Transcriptomic Analysis
4.4. Homology-Based Search
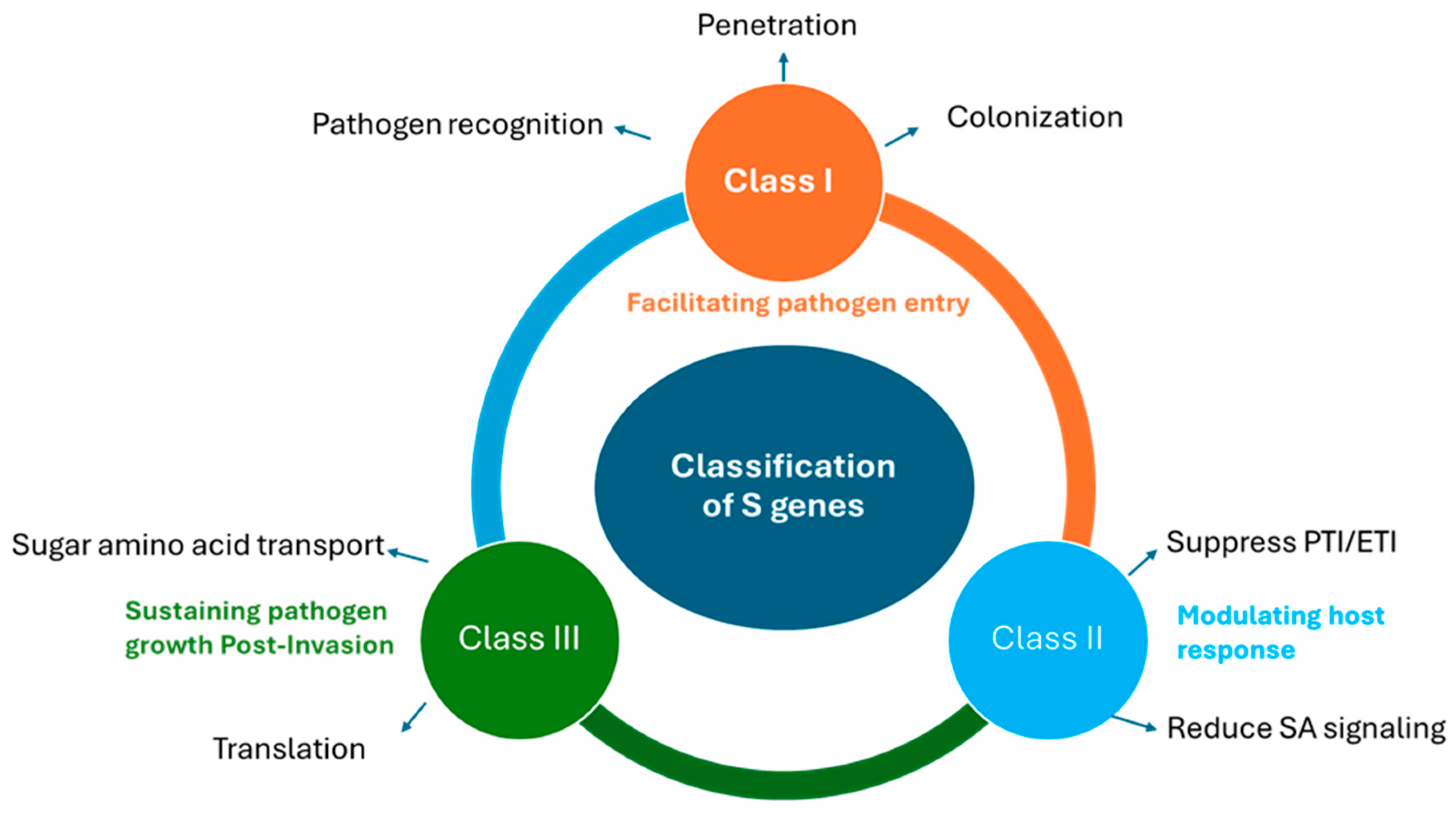
5. Classification and Mechanisms of S-Genes
5.1. Facilitating Host Recognition and Entry
5.2. Modulating Host Immune Responses
5.3. Sustaining Pathogen Growth Post-Invasion
6. Introgression of S-Gene for Durable Resistance
Classical Breeding Approaches
7. Targeting S-Genes for Durable Resistance
7.1. Targeting eIF4 Genes
7.2. Targeting Mlo Genes
7.3. Targeting SWEET Family Genes
7.4. Targeting Other S-Genes
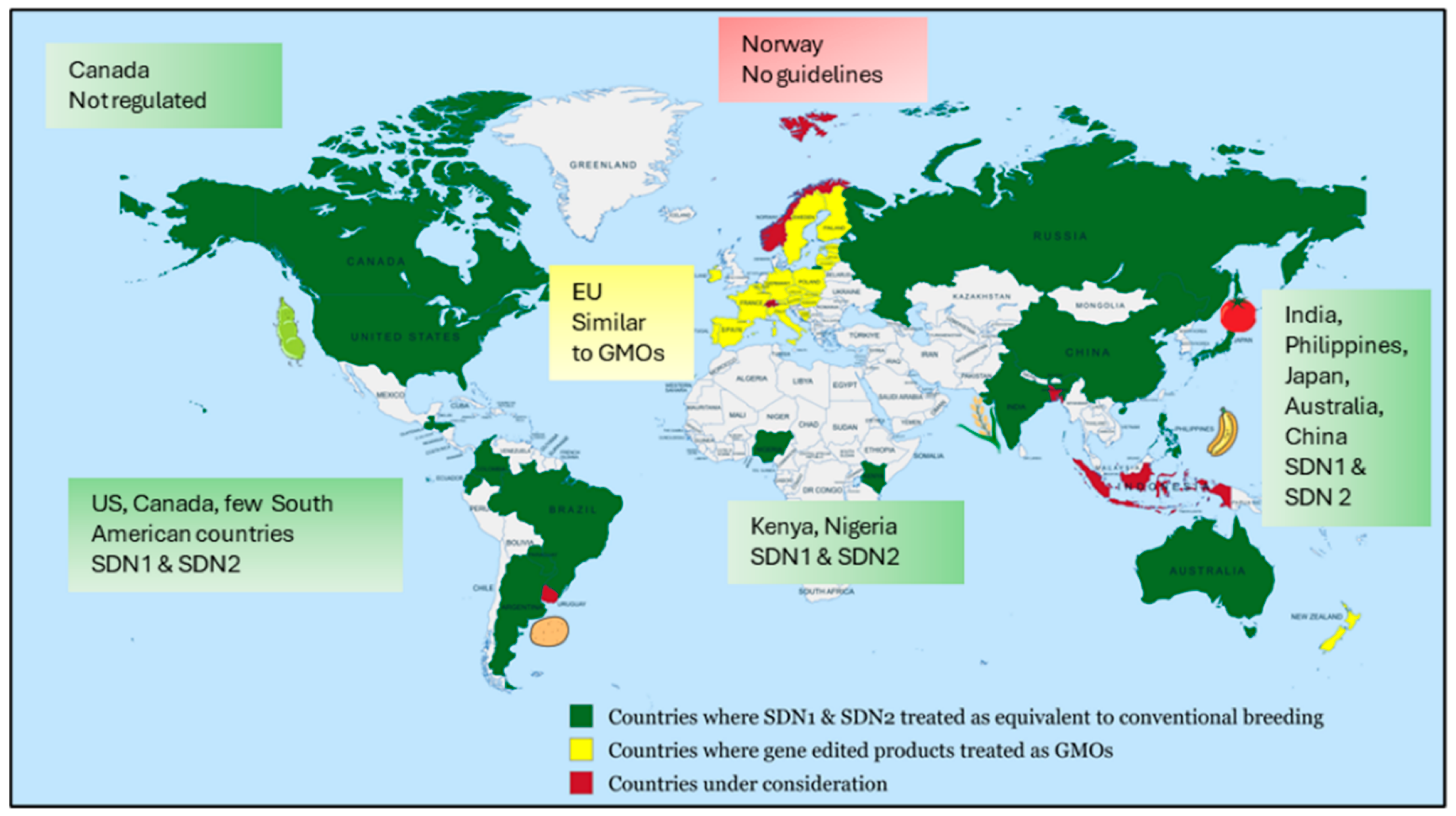
8. Global Policies and Regulatory Approaches for GE Development and Commercialization
9. Challenges and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- FAO. How to Feed the World in 2050; Food and Agriculture Organization: Rome, Italy, 2009. [Google Scholar]
- Zayan, S.A. Impact of climate change on plant diseases and IPM strategies. In Plant Diseases—Current Threats and Management Trends; IntechOpen: London, UK, 2019. [Google Scholar]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Couto, D.; Zipfel, C. Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 2016, 16, 537–552. [Google Scholar] [CrossRef]
- Jones, N.T.; Gilbert, B. Biotic forcing: The push–pull of plant ranges. Plant Ecol. 2016, 217, 1331–1344. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kourelis, J.; Van der Hoorn, R.A.L. Defended to the nines: 25 years of resistance gene cloning identifies nine mechanisms for R protein function. Plant Cell 2018, 30, 285–299. [Google Scholar] [CrossRef]
- Dangl, J.L.; Horvath, D.M.; Staskawicz, B.J. Pivoting the plant immune system from dissection to deployment. Science 2013, 341, 746–751. [Google Scholar] [CrossRef] [PubMed]
- van Schie, C.N.; Takken, F.L.W. Susceptibility genes 101: How to be a good host. Annu. Rev. Phytopathol. 2014, 52, 551–581. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.S.E.A.; Mukhtar, M.S.; Mansoor, S. Genome editing: Targeting susceptibility genes for plant disease resistance. Trends Biotechnol. 2018, 36, 898–906. [Google Scholar] [CrossRef]
- Vogel, J.P.; Raab, T.K.; Schiff, C.; Somerville, S.C. PMR6, a pectate lyase-like gene required for powdery mildew susceptibility in Arabidopsis. Plant Cell 2002, 14, 2095–2106. [Google Scholar] [CrossRef]
- Eckardt, N.A. Plant disease susceptibility genes? Plant Cell 2002, 14, 1983–1986. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Zhong, Y.; Yan, H.; Yuanda, L.; Jiang, B.; Zhong, G. Exploration of susceptible genes with clustered regularly interspaced short palindromic repeats–tissue-specific knockout (CRISPR-TSKO) to enhance host resistance. Crit. Rev. Plant Sci. 2020, 39, 387–417. [Google Scholar] [CrossRef]
- Das, A.; Sharma, N.; Prasad, M. CRISPR/Cas9: A novel weapon in the arsenal to combat plant diseases. Front. Plant Sci. 2019, 9, 2008. [Google Scholar] [CrossRef] [PubMed]
- Freisleben, R.; Lein, A. Über die Auffindung einer mehltauresistenten Mutante nach Röntgenbestrahlung einer anfälligen reinen Linie von Sommergerste. Naturwissenschaften 1942, 30, 608. [Google Scholar] [CrossRef]
- Kusch, S.; Panstruga, R. mlo-based resistance: An apparently universal “weapon” to defeat powdery mildew disease. Mol. Plant Microbe Interact. 2017, 30, 179–189. [Google Scholar] [CrossRef]
- Humphry, M.; Reinstädler, A.; Ivanov, S.; Bisseling, T.; Panstruga, R. Durable broad-spectrum powdery mildew resistance in pea er1 plants is conferred by natural loss-of-function mutations in PsMLO1. Mol. Plant Pathol. 2011, 12, 866–878. [Google Scholar] [CrossRef]
- Pavan, S.; Schiavulli, A.; Appiano, M.; Marcotrigiano, A.R.; Cillo, F.; Visser, R.G.; Bai, Y.; Lotti, C.; Ricciardi, L. Pea powdery mildew er1 resistance is associated with loss-of-function mutations at a MLO homologous locus. Theor. Appl. Genet. 2011, 123, 1425–1431. [Google Scholar] [CrossRef]
- Zheng, Z.; Nonomura, T.; Appiano, M.; Pavan, S.; Matsuda, Y.; Toyoda, H.; Wolters, A.M.A.; Visser, R.G.; Bai, Y. Loss of function in Mlo orthologs reduces susceptibility of pepper and tomato to powdery mildew disease caused by Leveillula taurica. PLoS ONE 2013, 8, e70723. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef]
- Pessina, S.; Angeli, D.; Martens, S.; Visser, R.G.F.; Bai, Y.; Salamini, F.; Velasco, R.; Schouten, H.J.; Malnoy, M. The knock-down of the expression of MdMLO19 reduces susceptibility to powdery mildew (Podosphaera leucotricha) in apple (Malus domestica). Plant Biotechnol. J. 2016, 14, 2033–2044. [Google Scholar] [CrossRef]
- Acevedo-Garcia, J.; Spencer, D.; Thieron, H.; Reinstädler, A.; Hammond-Kosack, K.; Phillips, A.L.; Panstruga, R. mlo-based powdery mildew resistance in hexaploid bread wheat generated by a non-transgenic TILLING approach. Plant Biotechnol. J. 2017, 15, 367–378. [Google Scholar] [CrossRef]
- Consonni, C.; Humphry, M.E.; Hartmann, H.A.; Livaja, M.; Durner, J.; Westphal, L.; Vogel, J.P.; Lipka, V.; Kemmerling, B.; Schulze-Lefert, P. Conserved requirement for a plant host cell protein in powdery mildew pathogenesis. Nat. Genet. 2006, 38, 716–720. [Google Scholar] [CrossRef]
- Berg, J.A.; Appiano, M.; Santillán Martínez, M.; Hermans, F.W.K.; Vriezen, W.H.; Visser, R.G.F.; Bai, Y.; Schouten, H.J. A transposable element insertion in the susceptibility gene CsaMLO8 results in hypocotyl resistance to powdery mildew in cucumber. BMC Plant Biol. 2015, 15, 243. [Google Scholar] [CrossRef]
- Iovieno, P.; Andolfo, G.; Schiavulli, A.; Catalano, D.; Ricciardi, L.; Frusciante, L.; Pavan, S. Structure, evolution and functional inference on the MLO gene family in three cultivated Cucurbitaceae spp. BMC Genom. 2015, 16, 1112. [Google Scholar] [CrossRef] [PubMed]
- Porterfield, R.; Meru, G. Candidate Susceptibility Genes for Powdery and Downy Mildew in Watermelon and Squash. J. Phylogenet. Evol. Biol. 2017, 5, 186. [Google Scholar] [CrossRef]
- Sonenberg, N.; Morgan, M.A.; Merrick, W.C.; Shatkin, A.J. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5’-terminal cap in mRNA. Proc. Natl. Acad. Sci. USA 1978, 75, 4843–4847. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Krishnaswamy, S. Eukaryotic translation initiation factor 4E-mediated recessive resistance to plant viruses and its utility in crop improvement. Mol. Plant Pathol. 2012, 13, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Charron, C.; Nicolaï, M.; Gallois, J.L.; Robaglia, C.; Moury, B.; Palloix, A.; Caranta, C. Natural variation and functional analyses provide evidence for co-evolution between plant eIF4E and potyviral VPg. Plant J. 2008, 54, 56–68. [Google Scholar] [CrossRef]
- Sun, J.; Li, N.; Oh, K.S.; Dutta, B.; Vayttaden, S.J.; Lin, B.; Fraser, I.D. Comprehensive RNAi-based screening of human and mouse TLR pathways identifies species-specific preferences in signaling protein use. Sci. Signal. 2016, 9, ra3. [Google Scholar] [CrossRef]
- Sun, Q.; Lin, L.; Liu, D.; Wu, D.; Fang, Y.; Wu, J.; Wang, Y. CRISPR/Cas9-mediated multiplex genome editing of the BnWRKY11 and BnWRKY70 genes in Brassica napus L. Int. J. Mol. Sci. 2018, 19, 2716. [Google Scholar] [CrossRef]
- Li, T.; Liu, B.; Spalding, M.H.; Weeks, D.P.; Yang, B. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat. Biotechnol. 2012, 30, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.S.E.A.; Mahas, A.; Vanderschuren, H.; Mahfouz, M.M. Engineering crops of the future: CRISPR approaches to develop climate-resilient and disease-resistant plants. Genome Biol. 2020, 21, 289. [Google Scholar] [CrossRef]
- Tyagi, S.; Kumar, R.; Kumar, V.; Won, S.Y.; Shukla, P. Engineering disease resistant plants through CRISPR-Cas9 technology. GM Crops Food 2021, 12, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Boller, T.; He, S.Y. Innate immunity in plants: An arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science 2009, 324, 742–744. [Google Scholar] [CrossRef]
- Tanaka, K.; Heil, M. Damage-associated molecular patterns (DAMPs) in plant innate immunity: Applying the danger model and evolutionary perspectives. Annu. Rev. Phytopathol. 2021, 59, 53–75. [Google Scholar] [CrossRef]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Speth, E.B.; Lee, Y.N.; He, S.Y. Pathogen virulence factors as molecular probes of basic plant cellular functions. Curr. Opin. Plant Biol. 2007, 10, 580–586. [Google Scholar] [CrossRef]
- Nuernberger, T.; Kemmerling, B. PAMP-triggered basal immunity in plants. Adv. Bot. Res. 2009, 51, 823937. [Google Scholar]
- Bentham, A.R.; De la Concepcion, J.C.; Mukhi, N.; Zdrzałek, R.R.; Draeger, M.; Gorenkin, D.; Hughes, R.K.; Banfield, M.J. A molecular roadmap to the plant immune system. J. Biol. Chem. 2020, 295, 14916–14935. [Google Scholar] [CrossRef]
- Xie, S.S.; Duan, C.G. Epigenetic regulation of plant immunity: From chromatin codes to plant disease resistance. aBIOTECH 2023, 4, 124–139. [Google Scholar] [CrossRef]
- Kourelis, J.; Adachi, H. Activation and regulation of NLR immune receptor networks. Plant Cell Physiol. 2022, 63, 1366–1377. [Google Scholar] [CrossRef]
- Chiang, Y.; Coaker, G. Effector triggered immunity: NLR immune perception and downstream defense responses. Arab. Book 2015, 13, e0183. [Google Scholar] [CrossRef]
- Deng, Y.; Ning, Y.; Yang, D.L.; Zhai, K.; Wang, G.L.; He, Z. Molecular basis of disease resistance and perspectives on breeding strategies for resistance improvement in crops. Mol. Plant 2020, 13, 1402–1419. [Google Scholar] [CrossRef]
- Dangl, J.L.; Jones, J.D. Plant pathogens and integrated defence responses to infection. Nature 2001, 411, 826–833. [Google Scholar] [CrossRef]
- Langner, T.; Kamoun, S.; Belhaj, K. CRISPR Crops: Plant genome editing toward disease resistance. Annu. Rev. Phytopathol. 2018, 56, 479–512. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vallet, A.; Fouché, S.; Fudal, I.; Hartmann, F.E.; Soyer, J.L.; Tellier, A.; Croll, D. The genome biology of effector gene evolution in filamentous plant pathogens. Annu. Rev. Phytopathol. 2018, 56, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Dong, O.X.; Ronald, P.C. Genetic engineering for disease resistance in plants: Recent progress and future perspectives. Plant Physiol. 2019, 180, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Amezrou, R.; Audéon, C.; Compain, J.; Gélisse, S.; Ducasse, A.; Saintenac, C.; Lapalu, N. A secreted protease-like protein in Zymoseptoria tritici is responsible for avirulence on Stb9 resistance gene in wheat. PLoS Pathog. 2023, 19, e1011376. [Google Scholar] [CrossRef]
- Veillet, F.; Durand, M.; Kroj, T.; Cesari, S.; Gallois, J.L. Precision breeding made real with CRISPR: Illustration through genetic resistance to pathogens. Plant Commun. 2020, 1, 100027. [Google Scholar] [CrossRef]
- van Wersch, S.; Tian, L.; Hoy, R.; Li, X. Plant NLRs: The whistleblowers of plant immunity. Plant Commun. 2020, 1, 100016. [Google Scholar] [CrossRef]
- Giannakopoulou, A.; Steele, J.F.; Segretin, M.E.; Bozkurt, T.O.; Zhou, J.; Robatzek, S. Tomato I2 immune receptor can be engineered to confer partial resistance to the oomycete Phytophthora infestans in addition to the fungus Fusarium oxysporum. Mol. Plant Microbe Interact. 2015, 28, 1316–1329. [Google Scholar] [CrossRef]
- Andolfo, G.; Iovieno, P.; Frusciante, L.; Ercolano, M.R. Genome-editing technologies for enhancing plant disease resistance. Front. Plant Sci. 2016, 7, 1813. [Google Scholar] [CrossRef]
- Jacob, P.; Avni, A.; Bendahmane, A. Translational research: Exploring and creating genetic diversity. Trends Plant Sci. 2018, 23, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Kapos, P.; Devendrakumar, K.T.; Li, X. Plant NLRs: From discovery to application. Plant Sci. 2019, 279, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, H.; Szurek, B.; Van den Ackerveken, G. Stop helping pathogens: Engineering plant susceptibility genes for durable resistance. Curr. Opin. Biotechnol. 2021, 70, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Gaj, T.; Sirk, S.J.; Shui, S.L.; Liu, J. Genome-editing technologies: Principles and applications. Cold Spring Harb. Perspect. Biol. 2016, 8, a023754. [Google Scholar] [CrossRef]
- Sufyan, M.; Daraz, U.; Hyder, S.; Zulfiqar, U.; Iqbal, R.; Eldin, S.M.; Rafiq, F. An overview of genome engineering in plants, including its scope, technologies, progress and grand challenges. Funct. Integr. Genom. 2023, 23, 119. [Google Scholar] [CrossRef]
- Choudry, M.W.; Riaz, R.; Nawaz, P.; Ashraf, M.; Ijaz, B.; Bakhsh, A. CRISPR-Cas9 mediated understanding of plants’ abiotic stress-responsive genes to combat changing climatic patterns. Funct. Integr. Genom. 2024, 24, 132. [Google Scholar] [CrossRef]
- Pavan, S.; Jacobsen, E.; Visser, R.G.; Bai, Y. Loss of susceptibility as a novel breeding strategy for durable and broad-spectrum resistance. Mol. Breed. 2010, 25, 1–12. [Google Scholar] [CrossRef]
- Engelhardt, S.; Stam, R.; Hückelhoven, R. Good riddance? Breaking disease susceptibility in the era of new breeding technologies. Agronomy 2018, 8, 114. [Google Scholar] [CrossRef]
- Lou, X.-Y.; Ma, J.Z.; Yang, M.C.K.; Zhu, J.; Liu, P.-Y.; Deng, H.-W.; Elston, R.C.; Li, M.D. Improvement of mapping accuracy by unifying linkage and association analysis. Genetics 2006, 172, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, S.; Meng, X.; Chai, Z.; Wang, D.; Yuan, Y.; Chen, K.; Jiang, L.; Li, J.; Gao, C. Generating broad-spectrum tolerance to ALS-inhibiting herbicides in rice by base editing. Sci. China Life Sci. 2021, 64, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Henning, J.A.; Townsend, M.S.; Gent, D.H. QTL mapping of powdery mildew susceptibility in hop (Humulus lupulus L.). Euphytica 2011, 180, 411–420. [Google Scholar] [CrossRef]
- Santos, C.; Polanco, C.; Rubiales, D.; Vaz Patto, M.C. The MLO1 powdery mildew susceptibility gene in Lathyrus species: The power of high-density linkage maps in comparative mapping and synteny analysis. Plant Genome 2021, 14, e20090. [Google Scholar] [CrossRef]
- Alseekh, S.; Kostova, D.; Bulut, M.; Fernie, A.R. Genome-wide association studies: Assessing trait characteristics in model and crop plants. Cell. Mol. Life Sci. 2021, 78, 5743–5754. [Google Scholar] [CrossRef]
- Tibbs Cortes, L.; Zhang, Z.; Yu, J. Status and prospects of genome-wide association studies in plants. Plant Genome 2021, 14, e20077. [Google Scholar] [CrossRef]
- van de Bunt, M.; Cortes, A.; IGAS Consortium; Brown, M.A.; Morris, A.P.; McCarthy, M.I. Evaluating the performance of fine-mapping strategies at common variant GWAS loci. PLoS Genet. 2015, 11, e1005535. [Google Scholar] [CrossRef]
- Deng, X.; Huang, D.; Wang, Y.; An, H.; Bai, D.; Wang, X.; Niu, S.; Song, X. Genome-wide association study of salicylic acid provides genetic insights for tea plant selective breeding. Hortic. Res. 2025, 12, uhae362. [Google Scholar] [CrossRef]
- Chittem, K.; Yajima, W.R.; Goswami, R.S.; del Río Mendoza, L.E. Transcriptome analysis of the plant pathogen Sclerotinia sclerotiorum interaction with resistant and susceptible canola (Brassica napus) lines. PLoS ONE 2020, 15, e0229844. [Google Scholar] [CrossRef]
- Cao, Y.; Zhou, H.; Zhou, X.; Li, F. Control of plant viruses by CRISPR/Cas system-mediated adaptive immunity. Front. Microbiol. 2020, 11, 593700. [Google Scholar] [CrossRef]
- Ju, X.; Li, F.; Li, J.; Wu, C.; Xiang, G.; Zhao, X.; Nan, Y.; Zhao, D.; Ding, Q. Genome-wide transcriptomic analysis of highly virulent African swine fever virus infection reveals complex and unique virus host interaction. Vet. Microbiol. 2021, 261, 109211. [Google Scholar] [CrossRef]
- Meng, H.; Sun, M.; Jiang, Z.; Liu, Y.; Sun, Y.; Liu, D.; Jiang, C. Comparative transcriptome analysis reveals resistant and susceptible genes in tobacco cultivars in response to infection by Phytophthora nicotianae. Sci. Rep. 2021, 11, 809. [Google Scholar] [CrossRef]
- Schouten, H.J.; Krauskopf, J.; Visser, R.G.F. Identification of candidate genes required for susceptibility to powdery or downy mildew in cucumber. Euphytica 2014, 200, 475–486. [Google Scholar] [CrossRef]
- Tian, D.; Wang, P.; Tang, B.; Teng, X.; Li, C.; Liu, X.; Zou, D.; Song, S.; Zhang, Z. GWAS Atlas: A curated resource of genome-wide variant-trait associations in plants and animals. Nucleic Acids Res. 2020, 48, D927–D932. [Google Scholar] [CrossRef] [PubMed]
- Zahid, G.; Kaçar, Y.A.; Dönmez, D.; Küden, A.; Giordani, T. Perspectives and recent progress of genome-wide association studies (GWAS) in fruits. Mol. Biol. Rep. 2022, 49, 5341–5352. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K. GWAS for genetics of complex quantitative traits: Genome to pangenome and SNPs to SVs and k-mers. BioEssays 2021, 43, 2100109. [Google Scholar] [CrossRef]
- Morales, J.; Pujar, S.; Loveland, J.E.; Astashyn, A.; Bennett, R.; Berry, A.; Cox, E. A joint NCBI and EMBL-EBI transcript set for clinical genomics and research. Nature 2022, 604, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, K.K.; Khan, S.; Herath, V.; Brooks, S.; Mortimer, P.E.; Nadir, S.; Hyde, K.D.; Xu, J. Genome wide identification of the MLO gene family associated with powdery mildew resistance in rubber trees (Hevea brasiliensis). Trop. Plant Biol. 2020, 13, 331–342. [Google Scholar] [CrossRef]
- Pessina, S.; Pavan, S.; Catalano, D.; Gallotta, A.; Visser, R.G.F.; Bai, Y.; Malnoy, M. Characterization of the MLO gene family in Rosaceae and gene expression analysis in Malus domestica. BMC Genom. 2014, 15, 618. [Google Scholar] [CrossRef]
- Calle Garcia, J.; Guadagno, A.; Paytuvi-Gallart, A.; Saera-Vila, A.; Amoroso, C.G.; D’Esposito, D.; Andolfo, G.; Aiese Cigliano, R.; Sanseverino, W.; Ercolano, M.R. PRGdb 4.0: An updated database dedicated to genes involved in plant disease resistance process. Nucleic Acids Res. 2022, 50, D1483–D1490. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Wang, J.; Kwok, D.; Cui, F.; Zhang, Z.; Zhao, D.; Li, M.J.; Zou, Q. webTWAS: A resource for disease candidate susceptibility genes identified by transcriptome-wide association study. Nucleic Acids Res. 2022, 50, D1123–D1130. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Remmert, M.; Biegert, A.; Hauser, A.; Söding, J. HHblits: Lightning-fast iterative protein sequence searching by HMM-HMM alignment. Nat. Methods 2012, 9, 173–175. [Google Scholar] [CrossRef]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Emanuelsson, O.; Brunak, S.; von Heijne, G.; Nielsen, H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protoc. 2007, 2, 953–971. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, P.; Luo, J.; Jiang, Y. Secreted protein prediction system combining CJ-SPHMM, TMHMM, and PSORT. Mamm. Genome 2003, 14, 859–865. [Google Scholar] [CrossRef]
- Sperschneider, J.; Catanzariti, A.M.; DeBoer, K.; Petre, B.; Gardiner, D.M.; Singh, K.B.; Taylor, J.M. LOCALIZER: Subcellular localization prediction of both plant and effector proteins in the plant cell. Sci. Rep. 2017, 7, 44598. [Google Scholar] [CrossRef]
- Sperschneider, J.; Dodds, P.N.; Singh, K.B.; Taylor, J.M. ApoplastP: Prediction of effectors and plant proteins in the apoplast using machine learning. New Phytol. 2018, 217, 1764–1778. [Google Scholar] [CrossRef]
- Gupta, A.; Sankararamakrishnan, R. dbSWEET: An integrated resource for SWEET superfamily to understand, analyze and predict the function of sugar transporters in prokaryotes and eukaryotes. J. Mol. Biol. 2018, 430, 2203–2211. [Google Scholar] [CrossRef] [PubMed]
- Sperschneider, J.; Dodds, P.N. EffectorP 3.0: Prediction of apoplastic and cytoplasmic effectors in fungi and oomycetes. Mol. Plant Microbe Interact. 2021, 34, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Kulmanov, M.; Khan, M.A.; Hoehndorf, R. DeepGO: Predicting protein functions from sequence and interactions using a deep ontology-aware classifier, Bioinformatics. Bioinformatics 2018, 34, 660–668. [Google Scholar] [CrossRef]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010, 38, W214–W220. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Blum, M.; Andreeva, A.; Florentino, L.C.; Chuguransky, S.R.; Grego, T.; Hobbs, E.; Pinto, B.L. InterPro: The protein sequence classification resource in 2025. Nucleic Acids Res. 2025, 53, D444–D456. [Google Scholar] [CrossRef]
- Carreón-Anguiano, K.G.; Islas-Flores, I.; Vega-Arreguín, J.; Sáenz-Carbonell, L.; Canto-Canché, B. EffHunter: A tool for prediction of effector protein candidates in fungal proteomic databases. Biomolecules 2020, 10, 712. [Google Scholar] [CrossRef]
- Bülow, L.; Schindler, M.; Choi, C.; Hehl, R. PathoPlant: A database on plant-pathogen interactions. Silico Biol. 2004, 4, 529–536. [Google Scholar] [CrossRef]
- Lei, C.; Zhou, K.; Zheng, J.; Zhao, M.; Huang, Y.; He, H.; Zhang, Z. Arapathogen2.0: An improved prediction of plant–pathogen protein–protein interactions empowered by the natural language processing technique. J. Proteome Res. 2023, 23, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Ammari, M.G.; Gresham, C.R.; McCarthy, F.M.; Nanduri, B. HPIDB 2.0: A curated database for host–pathogen interactions. Database 2016, 2016, baw103. [Google Scholar] [CrossRef]
- Urban, M.; Cuzick, A.; Seager, J.; Wood, V.; Rutherford, K.; Venkatesh, S.Y.; Hammond-Kosack, K.E. PHI-base in 2022: A multi-species phenotype database for pathogen–host interactions. Nucleic Acids Res. 2022, 50, D837–D847. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Bolser, D.M.; Staines, D.M.; Perry, E.; Kersey, P.J. Ensembl Plants: Integrating tools for visualizing, mining, and analyzing plant genomic data. In Plant Genomics Databases; Van Dijk, A., Ed.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1533, pp. 1–31. [Google Scholar] [CrossRef]
- Reiser, L.; Bakker, E.; Subramaniam, S.; Chen, X.; Sawant, S.; Khosa, K.; Prithvi, T.; Berardini, T.Z. The Arabidopsis Information Resource in 2024. Genetics 2024, 227, iyae027. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Stoeckert, C.J., Jr.; Roos, D.S. OrthoMCL: Identification of Ortholog Groups for Eukaryotic Genomes. Genome Res. 2003, 13, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed]
- Hansjakob, A.; Riederer, M.; Hildebrandt, U. Wax matters: Absence of very-long-chain aldehydes from the leaf cuticular wax of the glossy11 mutant of maize compromises the prepenetration processes of Blumeria graminis. Plant Pathol. 2011, 60, 1151–1161. [Google Scholar] [CrossRef]
- Wang, E.; Schornack, S.; Marsh, J.F.; Gobbato, E.; Schwessinger, B.; Eastmond, P. A common signaling process that promotes mycorrhizal and oomycete colonization of plants. Curr. Biol. 2012, 22, 2242–2246. [Google Scholar] [CrossRef]
- Uppalapati, S.R.; Ishiga, Y.; Doraiswamy, V.; Bedair, M.; Mittal, S.; Chen, J.; Nakashima, J.; Tang, Y.; Tadege, M.; Ratet, P. Loss of abaxial leaf epicuticular wax in Medicago truncatula irg1/palm1 mutants results in reduced spore differentiation of anthracnose and nonhost rust pathogens. Plant Cell 2012, 24, 353–370. [Google Scholar] [CrossRef]
- Chassot, C.; Nawrath, C.; Metraux, J.P. The cuticle: Not only a barrier for plant defence—A novel defence syndrome in plants with cuticular defects. Plant Signal Behav. 2008, 3, 142–144. [Google Scholar] [CrossRef]
- L’Haridon, F.; Besson-Bard, A.; Binda, M.; Serrano, M.; Abou-Mansour, E. A permeable cuticle is associated with the release of reactive oxygen species and induction of innate immunity. PLoS Pathog. 2011, 7, e1002148. [Google Scholar] [CrossRef]
- Zhu, Y.; Nam, J.; Carpita, N.C.; Matthysse, A.G.; Gelvin, S.B. Agrobacterium-mediated root transformation is inhibited by mutation of an Arabidopsis cellulose synthase-like gene. Plant Physiol. 2003, 133, 1000–1010. [Google Scholar] [CrossRef]
- Zhu, C.; Perry, S.E. Control of expression and autoregulation of AGL15, a member of the MADS-box family. Plant J. 2005, 41, 583–594. [Google Scholar] [CrossRef]
- Desclos-Theveniau, M.; Arnaud, D.; Huang, T.Y.; Lin, G.J.C.; Chen, W.Y.; Lin, Y.C.; Zimmerli, L. The Arabidopsis lectin receptor kinase LecRK-V. 5 represses stomatal immunity induced by Pseudomonas syringae pv. tomato DC3000. PLoS Pathog. 2012, 8, e1002513. [Google Scholar] [CrossRef]
- Sawinski, K.; Mersmann, S.; Robatzek, S.; Böhmer, M. Guarding the green: Pathways to stomatal immunity. Mol. Plant-Microbe Interact. 2013, 26, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Elmore, J.M.; Coaker, G. Investigating the functions of the RIN4 protein complex during plant innate immune responses. Plant Signal. Behav. 2009, 4, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Eichmann, R.; Bischof, M.; Weis, C.; Shaw, J.; Lacomme, C.; Schweizer, P.; Duchkov, D.; Hensel, G.; Kumlehn, J.; Hückelhoven, R. BAX INHIBITOR-1 is required for full susceptibility of barley to powdery mildew. Mol. Plant Microbe Interact. 2010, 23, 1217–1227. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.S. A guide to genome engineering with programmable nucleases. Nat. Rev. Genet. 2014, 15, 321–334. [Google Scholar] [CrossRef]
- Schultheiss, H.; Dechert, C.; Kogel, K.H.; Huckelhoven, R. A small GTP-binding host protein is required for entry of powdery mildew fungus into epidermal cells of barley. Plant Physiol. 2002, 128, 1447–1454. [Google Scholar] [CrossRef]
- Poraty-Gavra, L.; Zimmermann, P.; Haigis, S.; Bednarek, P.; Hazak, O.; Stelmakh, O.R.; Sadot, E.; Schulze-Lefert, P.; Gruissem, W.; Yalovsky, S. The Arabidopsis Rho of plants GTPase AtROP6 functions in developmental and pathogen response pathways. Plant Physiol. 2013, 161, 1172–1188. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shiotani, K.; Togashi, T.; Miki, D.; Aoyama, M.; Wong, H.L.; Kawasaki, T.; Shimamoto, K. Analysis of the Rac/Rop small GTPase family in rice: Expression, subcellular localization and role in disease resistance. Plant Cell Physiol. 2010, 51, 585–595. [Google Scholar] [CrossRef]
- Jung, Y.H.; Agrawal, G.K.; Rakwal, R.; Kim, J.A.; Lee, M.O.; Choi, P.G.; Kim, Y.J. Functional characterization of OsRacB GTPase–a potentially negative regulator of basal disease resistance in rice. Plant Physiol. Biochem. 2006, 44, 68–77. [Google Scholar] [CrossRef]
- Kessler, S.A.; Shimosato-Asano, H.; Keinath, N.F.; Wuest, S.E.; Ingram, G. Conserved molecular components for pollen tube reception and fungal invasion. Science 2010, 330, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, D.; Naumann, M.; Falter, C.; Zwikowics, C.; Jamrow, T.; Manisseri, C.; Somerville, S.C.; Voigt, C.A. Elevated early callose deposition results in complete penetration resistance to powdery mildew in Arabidopsis. Plant Physiol. 2013, 161, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Liu, H.; Qiu, D.; Zhou, Y.; Li, X.; Xu, C.; Wang, S. A pair of allelic WRKY genes play opposite roles in rice-bacteria interactions. Plant Physiol. 2009, 151, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Pirrello, C.; Malacarne, G.; Moretto, M.; Lenzi, L.; Perazzolli, M.; Zeilmaker, T.; Van den Ackerveken, G.; Pilati, S.; Moser, C.; Giacomelli, L. Grapevine DMR6-1 is a candidate gene for susceptibility to downy mildew. Biomolecules 2022, 12, 182. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The impact of microbes in the orchestration of plants’ resistance to biotic stress: A disease management approach. Appl. Microbiol. Biotechnol. 2019, 103, 9–25. [Google Scholar] [CrossRef]
- Guerrero, J.; Regedanz, E.; Lu, L.; Ruan, J.; Bisaro, D.M.; Sunter, G. Manipulation of the plant host by the geminivirus AC2/C2 protein, a central player in the infection cycle. Front. Plant Sci. 2020, 11, 59. [Google Scholar] [CrossRef]
- Ghosh, D.; Malavika, M.; Chakraborty, S. Impact of viral silencing suppressors on plant viral synergism: A global agro-economic concern. Appl. Microbiol. Biotechnol. 2021, 105, 6301–6313. [Google Scholar] [CrossRef]
- van Damme, M.; Zeilmaker, T.; Elberse, J.; Andel, A.; de Sain-van der Velden, M.; van den Ackerveken, G. Downy mildew resistance in Arabidopsis by mutation of HOMOSERINE KINASE. Plant Cell 2009, 21, 2179–2189. [Google Scholar] [CrossRef]
- Stuthman, D.D.; Leonard, K.J.; Miller-Garvin, J. Breeding crops for durable resistance to disease. Adv. Agron. 2007, 95, 319–367. [Google Scholar]
- Gao, X.; Brodhagen, M.; Isakeit, T.; Brown, S.H.; Göbel, C.; Betran, J.; Feussner, I.; Keller, N.P.; Kolomiets, M.V. Inactivation of the lipoxygenase ZmLOX3 increases susceptibility of maize to Aspergillus spp. Mol. Plant Microbe Interact. 2009, 22, 222–231. [Google Scholar] [CrossRef]
- Pathuri, I.P.; Reitberger, I.E.; Hückelhoven, R.; Proels, R.K. Alcohol dehydrogenase 1 of barley modulates susceptibility to the parasitic fungus Blumeria graminis f. sp. hordei. J. Exp. Bot. 2011, 62, 3449–3457. [Google Scholar] [CrossRef]
- Vogel, J.P.; Raab, T.K.; Somerville, C.R.; Somerville, S.C. Mutations in PMR5 result in powdery mildew resistance and altered cell wall composition. Plant J. 2004, 40, 968–978. [Google Scholar] [CrossRef]
- Callot, C.; Gallois, J.L. Pyramiding resistances based on translation initiation factors in Arabidopsis is impaired by male gametophyte lethality. Plant Signal. Behav. 2014, 9, e27940. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Numaga, T.; Ohshima, K.; Yano, M.; Ohsawa, R. Arabidopsis TOBAMOVIRUS MULTIPLICATION (TOM) 2 locus encodes a transmembrane protein that interacts with TOM1. EMBO J. 2003, 22, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, T.; Imai, T.; Satoh, R.; Kawashima, A.; Takahashi, M. Complete inhibition of tobamovirus multiplication by simultaneous mutations in two homologous host genes. J. Virol. 2002, 76, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, T.; Ohta, T.; Takahashi, M.; Meshi, T.; Schmidt, R. TOM1, an Arabidopsis gene required for efficient multiplication of a tobamovirus, encodes a putative transmembrane protein. Proc. Natl. Acad. Sci. USA 2000, 97, 10107–10112. [Google Scholar] [CrossRef] [PubMed]
- Solomon, P.S.; Lowe, R.G.; Tan, K.C.; Waters, O.D.; Oliver, R.P. Stagonospora nodorum: Cause of Stagonospora nodorum blotch of wheat. Mol. Plant Pathol. 2006, 7, 147–156. [Google Scholar] [CrossRef]
- Cowger, C.; Ward, B.; Brown-Guedira, G.; Brown, J. Role of effector-sensitivity gene interactions and durability of quantitative resistance to Septoria nodorum blotch in eastern U.S. wheat. Front. Plant Sci. 2020, 11, 155. [Google Scholar] [CrossRef]
- Kariyawasam, G.; Nelson, A.C.; Williams, S.; Solomon, P.S.; Faris, J.D.; Friesen, T.L. The necrotrophic pathogen Parastagonospora nodorum is a master manipulator of wheat defense. Mol. Plant Microbe Interact. 2023, 36, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Faris, J.D.; Meinhardt, S.W.; Ali, S.; Rasmussen, J.B.; Friesen, T.L. Genetic and physical mapping of a gene conditioning sensitivity in wheat to a partially purified host-selective toxin produced by Stagonospora nodorum. Phytopathology 2004, 94, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Z.; Faris, J.D.; Oliver, R.P.; Syme, R.; McDonald, M.C.; A McDonald, B.; Solomon, P.S.; Lu, S.; Shelver, W.L.; et al. The cysteine-rich necrotrophic effector SnTox1 produced by Stagonospora nodorum triggers susceptibility of wheat lines harboring Snn1. PLoS Pathog. 2012, 8, e1002467. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Y.; Kim, Y.M.; Faris, J.D.; Shelver, W.L.; de Wit, P.J.G.M.; Xu, S.S.; Friesen, T.L. SnTox1, a Parastagonospora nodorum necrotrophic effector, is a dual-function protein that facilitates infection while protecting from wheat-produced chitinases. New Phytol. 2016, 211, 1052–1064. [Google Scholar] [CrossRef]
- Shi, G.; Friesen, T.L.; Saini, J.; Xu, S.S.; Rasmussen, J.B.; Faris, J.D. The wheat Snn7 gene confers susceptibility on recognition of the Parastagonospora nodorum necrotrophic effector SnTox7. Plant Genome 2015, 8, 1–10. [Google Scholar] [CrossRef]
- Liu, Z.; Faris, J.D.; Oliver, R.P.; Tan, K.C.; Solomon, P.S.; McDonald, M.C.; McDonald, B.A.; Nunez, A.; Lu, S.; Rasmussen, J.B.; et al. SnTox3 acts in effector-triggered susceptibility to induce disease on wheat carrying the Snn3 gene. PLoS Pathog. 2009, 5, e1000581. [Google Scholar] [CrossRef]
- Friesen, T.L.; Stukenbrock, E.H.; Liu, Z.; Meinhardt, S.; Ling, H.; Faris, J.D.; Rasmussen, J.B.; Solomon, P.S.; McDonald, B.A.; Oliver, R.P. Emergence of a new disease as a result of interspecific virulence gene transfer. Nature 2006, 38, 953–956. [Google Scholar] [CrossRef]
- Friesen, T.L.; Meinhardt, S.W.; Faris, J.D. The Stagonospora nodorum–wheat pathosystem involves multiple proteinaceous host-selective toxins and corresponding host sensitivity genes that interact in an inverse gene-for-gene manner. Plant J. 2007, 51, 681–692. [Google Scholar] [CrossRef]
- Abeysekara, N.S.; Friesen, T.L.; Keller, B.; Faris, J.D. Identification and characterization of a novel host-toxin interaction in the wheat–Stagonospora nodorum pathosystem. Theor. Appl. Genet. 2009, 120, 117–126. [Google Scholar] [CrossRef]
- Gao, Y.; Faris, J.D.; Liu, Z.; Kim, Y.M.; Syme, R.A.; Oliver, R.P.; Xu, S.S.; Friesen, T.L. Identification and characterization of the SnTox6-Snn6 interaction in the Parastagonospora nodorum–wheat pathosystem. Mol. Plant Microbe Interact. 2015, 28, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Smith, D.L.; Kabbage, M.; Roth, M.G. Effectors of plant necrotrophic fungi. Front. Plant Sci. 2021, 12, 687713. [Google Scholar] [CrossRef]
- Peng, Z.; Hu, Y.; Zhang, J.; Huguet-Tapia, J.C.; Block, A.K.; Park, S.; Sapkota, S.; Liu, Z.; Liu, S.; White, F.F. Xanthomonas translucens commandeers the host rate-limiting step in ABA biosynthesis for disease susceptibility. Proc. Natl. Acad. Sci. USA 2019, 116, 20938–20946. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.M.A.; Khojasteh, M.; Wang, Q.; Haq, F.; Xu, X.; Li, Y.; Zou, L.; Osdaghi, E.; Chen, G. Comparative transcriptomic analysis of wheat cultivars in response to Xanthomonas translucens pv. cerealis and its T2SS, T3SS, and TALEs deficient strains. Phytopathology 2023, 113, 2073–2082. [Google Scholar]
- Lorang, J.; Kidarsa, T.; Bradford, C.S.; Gilbert, B.; Curtis, M.; Tzeng, S.C.; Maier, C.S.; Wolpert, T.J. Tricking the guard: Exploiting plant defense for disease susceptibility. Science 2012, 338, 659–662. [Google Scholar] [CrossRef]
- Smýkal, P.; Šafářová, D.; Navrátil, M.; Dostalová, R. Marker assisted pea breeding: eIF4E allele specific markers to pea seed-borne mosaic virus (PSbMV) resistance. Mol. Breed. 2010, 26, 425–438. [Google Scholar]
- Cook, A.A. A mutation for resistance to potato virus Y in pepper. Proc. Am. Soc. Hortic. Sci. 1961, 78, 550–552. [Google Scholar]
- Moury, B.; Verdin, E. Viruses of pepper crops in the Mediterranean basin: A remarkable stasis. Adv. Virus Res. 2012, 84, 127–162. [Google Scholar]
- Ayme, V.; Souche, S.; Caranta, C.; Jacquemond, M.; Chadoeuf, J.; Palloix, A.; Moury, B. Different mutations in the genome-linked protein VPg of Potato virus Y confer virulence on the pvr23 resistance in pepper. Mol. Plant Microbe Interact. 2006, 19, 557–563. [Google Scholar] [CrossRef]
- Zlobin, N.; Taranov, V. Plant eIF4E isoforms as factors of susceptibility and resistance to potyviruses. Front. Plant Sci. 2023, 14, 1041868. [Google Scholar] [CrossRef] [PubMed]
- Masuta, C.; Nishimura, M.; Morishita, H.; Hataya, T. A single amino acid change in viral genome associated protein of potato virus Y correlates with resistance breaking in ‘Virgin A Mutant’ tobacco. Phytopathology 1999, 89, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Truniger, V.; Aranda, M.A. Recessive resistance to plant viruses. Adv. Virus Res. 2009, 75, 119–231. [Google Scholar]
- Wittmann, S.; Chatel, H.; Fortin, M.G.; Laliberté, J.F. Interaction of the viral protein genome linked of turnip mosaic potyvirus with the translational eukaryotic initiation factor (iso) 4E of Arabidopsis thaliana using the yeast two-hybrid system. Virology 1997, 234, 84–92. [Google Scholar] [CrossRef]
- Ruffel, S.; Dussault, M.H.; Palloix, A.; Moury, B.; Bendahmane, A.; Robaglia, C.; Caranta, C. A natural recessive resistance gene against potato virus Y in pepper corresponds to the eukaryotic initiation factor 4E (eIF4E). Plant J. 2002, 32, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Nicaise, V.; German-Retana, S.; Sanjuán, R.; Dubrana, M.-P.; Mazier, M.; Maisonneuve, B.; Candresse, T.; Caranta, C.; LeGall, O. The eukaryotic translation initiation factor 4E controls lettuce susceptibility to the potyvirus lettuce mosaic virus. Plant Physiol. 2003, 132, 1272–1282. [Google Scholar] [CrossRef]
- Gao, Z.; Johansen, E.; Eyers, S.; Thomas, C.L.; Ellis, T.H.N.; Maule, A.J. The potyvirus recessive resistance gene, sbm1, identifies a novel role for eIF4E in cell-to-cell trafficking. Plant J. 2004, 40, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Stein, N.; Perovic, D.; Kumlehn, J.; Pellio, B.; Stracke, S.; Streng, S.; Ordon, F.; Graner, A. The eukaryotic translation initiation factor 4E confers multiallelic recessive Bymovirus resistance in Hordeum vulgare (L.). Plant J. 2005, 42, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Razzak, A.N.A.S.; Guiraud, T.; Peypelut, M.; Walter, J.; Houvenaghel, M.C.; Candresse, T.; Le Gall, O.; German-Retana, S. Involvement of the cylindrical inclusion (CI) protein in the overcoming of an eIF4E-mediated resistance against Lettuce mosaic potyvirus. Mol. Plant Pathol. 2009, 10, 109–113. [Google Scholar] [CrossRef]
- Nakahara, K.S.; Shimada, R.; Choi, S.H.; Yamamoto, H.; Shao, J.; Uyeda, I. Involvement of the P1 cistron in overcoming eIF4E-mediated recessive resistance against Clover yellow vein virus in pea. Mol. Plant Microbe Interact. 2010, 23, 1460–1469. [Google Scholar] [CrossRef]
- Chandrasekaran, J.; Brumin, M.; Wolf, D.; Leibman, D.; Klap, C.; Pearlsman, M.; Sherman, A.; Arazi, T.; Gal-On, A. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol. Plant Pathol. 2016, 17, 1140–1153. [Google Scholar] [CrossRef]
- Pyott, D.E.; Sheehan, E.; Molnar, A. Engineering of CRISPR/Cas9-mediated potyvirus resistance in transgene-free Arabidopsis plants. Mol. Plant Pathol. 2016, 17, 1276–1288. [Google Scholar] [CrossRef]
- Macovei, A.; Sevilla, N.R.; Cantos, C.; Jonson, G.B.; Slamet-Loedin, I.; Čermák, T.; Voytas, D.F.; Choi, I.R.; Chadha-Mohanty, P. Novel alleles of rice eIF4G generated by CRISPR/Cas9-targeted mutagenesis confer resistance to rice tungro spherical virus. Plant Biotechnol. J. 2018, 16, 1918–1927. [Google Scholar] [CrossRef]
- Coutinho de Oliveira, L.; Volpon, L.; Rahardjo, A.K.; Osborne, M.J.; Culjkovic-Kraljacic, B.; Trahan, C.; Oeffinger, M.; Kwok, B.H.; Borden, K.L.B. Structural studies of the eIF4E–VPg complex reveal a direct competition for capped RNA: Implications for translation. Proc. Natl. Acad. Sci. USA 2019, 116, 24056–24065. [Google Scholar] [CrossRef] [PubMed]
- Bastet, A.; Zafirov, D.; Giovinazzo, N.; Guyon-Debast, A.; Nogué, F.; Robaglia, C.; Gallois, J.L. Mimicking natural polymorphism in eIF4E by CRISPR-Cas9 base editing is associated with resistance to potyviruses. Plant Biotechnol. J. 2019, 17, 1736–1750. [Google Scholar] [CrossRef]
- Atarashi, H.; Jayasinghe, W.H.; Kwon, J.; Kim, H.; Taninaka, Y.; Igarashi, M.; Ito, K.; Yamada, T.; Masuta, C.; Nakahara, K.S. Artificially edited alleles of the eukaryotic translation initiation factor 4E1 gene differentially reduce susceptibility to cucumber mosaic virus and potato virus Y in tomato. Front. Microbiol. 2020, 11, 564310. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Venkatesh, J.; Lee, J.H.; Kim, J.; Lee, H.E. Genome editing of eIF4E1 in tomato confers resistance to pepper mottle virus. Front. Plant Sci. 2020, 11, 1098. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, K.; Thenault, C.; Nogué, F.; Perrot, L.; Mazier, M.; Gallois, J.L. CRISPR-based knock-out of eIF4E2 in a cherry tomato background successfully recapitulates resistance to pepper veinal mottle virus. Plant Sci. 2022, 316, 111160. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.A.; Lin, Z.D.; Moll, T.; Chauhan, R.D.; Hayden, L.; Renninger, K. Simultaneous CRISPR/Cas9-mediated editing of cassava eIF4E isoforms nCBP-1 and nCBP-2 reduces cassava brown streak disease symptom severity and incidence. Plant Biotechnol. J. 2019, 17, 421–434. [Google Scholar] [CrossRef]
- Noureen, A.; Khan, M.Z.; Amin, I.; Zainab, T.; Mansoor, S. CRISPR/Cas9-mediated targeting of susceptibility factor eIF4E-enhanced resistance against potato virus Y. Front. Genet. 2022, 13, 922019. [Google Scholar] [CrossRef]
- Shirazi Parsa, H.; Sabet, M.S.; Moieni, A.; Shojaeiyan, A.; Dogimont, C.; Boualem, A.; Bendahmane, A. CRISPR/Cas9-mediated cytosine base editing using an improved transformation procedure in melon (Cucumis melo L.). Int. J. Mol. Sci. 2023, 24, 11189. [Google Scholar] [CrossRef] [PubMed]
- Pechar, G.S.; Donaire, L.; Gosalvez, B.; García-Almodovar, C.; Sánchez-Pina, M.A.; Truniger, V.; Aranda, M.A. Editing melon EIF4E associates with virus resistance and male sterility. Plant Biotechnol. J. 2022, 20, 2006–2022. [Google Scholar] [CrossRef]
- Fidan, H.; Calis, O.; Ari, E.; Atasayar, A.; Sarikaya, P.; Tek, M.I.; Izmirli, A.; Oz, Y.; Firat, G. Knockout of eIF4E using CRISPR/Cas9 for large-scale production of resistant cucumber cultivar against WMV, ZYMV, and PRSV. Front. Plant Sci. 2023, 14, 1143813. [Google Scholar] [CrossRef]
- Li, M.; Qiu, Y.; Zhu, D.; Xu, X.; Tian, S.; Wang, J.; Yu, Y. Editing eIF4E in the watermelon genome using CRISPR/Cas9 technology confers resistance to ZYMV. Int. J. Mol. Sci. 2024, 25, 11468. [Google Scholar] [CrossRef]
- Jørgensen, I.H. Discovery, characterization and exploitation of Mlo powdery mildew resistance in barley. Euphytica 1992, 63, 141–152. [Google Scholar] [CrossRef]
- Tek, M.I.; Calis, O.; Fidan, H.; Shah, M.D.; Celik, S.; Wani, S.H. CRISPR/Cas9-based Mlo-mediated resistance against Podosphaera xanthii in cucumber (Cucumis sativus L.). Front. Plant Sci. 2022, 13, 1081506. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, D.; Zhang, Y.; Deng, M.; Chen, Y.; Lv, B.; Li, B.; Lei, Y.; Wang, Y.; Zhao, L. Genome-edited powdery mildew resistance in wheat without growth penalties. Nature 2022, 602, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.Y.; Guo, Y.; Cheng, Y.; Hu, Y.; Xiao, S.; Wang, Y. CRISPR/Cas9-mediated mutagenesis of VvMLO3 results in enhanced resistance to powdery mildew in grapevine (Vitis vinifera). Hortic. Res. 2020, 7, 116. [Google Scholar] [CrossRef]
- Shnaider, Y.; Elad, Y.; Rav-David, D.; Pashkovsky, E.; Leibman, D.; Kravchik, M.; Shtarkman-Cohen, M.; Gal-On, A.; Spiegelman, Z. Development of powdery mildew resistance in cucumber using CRISPR/Cas9-mediated mutagenesis of CsaMLO8. Phytopathology 2023, 113, 786–790. [Google Scholar] [CrossRef]
- Nekrasov, V.; Wang, C.; Win, J.; Lanz, C.; Weigel, D.; Kamoun, S. Rapid generation of a transgene-free powdery mildew resistant tomato by genome deletion. Sci. Rep. 2017, 7, 482. [Google Scholar] [CrossRef]
- Pramanik, D.; Shelake, R.M.; Park, J.; Kim, M.J.; Hwang, I.; Park, Y.; Kim, J.Y. CRISPR/Cas9-mediated generation of pathogen-resistant tomato against tomato yellow leaf curl virus and powdery mildew. Int. J. Mol. Sci. 2021, 22, 1878. [Google Scholar] [CrossRef]
- Bui, T.P.; Le, H.; Ta, D.T. Enhancing powdery mildew resistance in soybean by targeted mutation of MLO genes using the CRISPR/Cas9 system. BMC Plant Biol. 2023, 23, 533. [Google Scholar] [CrossRef]
- Antony, G.; Zhou, J.; Huang, S.; Li, T.; Liu, B.; White, F.; Yang, B. Rice xa13 recessive resistance to bacterial blight is defeated by induction of the disease susceptibility gene Os-11N3. Plant Cell 2010, 22, 3864–3876. [Google Scholar] [CrossRef] [PubMed]
- Oliva, R.; Ji, C.; Atienza-Grande, G.; Huguet-Tapia, J.C.; Perez-Quintero, A.; Li, T.; Eom, J.S.; Li, C.; Nguyen, H.; Liu, B.; et al. Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat. Biotechnol. 2019, 37, 1344–1350. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, X.; Gong, Q.; Li, Z.; Li, Y.; Wang, S. Engineering broad-spectrum bacterial blight resistance by simultaneously disrupting variable TALE-binding elements of multiple susceptibility genes in rice. Mol. Plant 2019, 12, 1434–1446. [Google Scholar] [CrossRef]
- Zafar, K.; Khan, M.Z.; Amin, I.; Mukhtar, Z.; Yasmin, S.; Arif, M.; Ejaz, K.; Mansoor, S. Precise CRISPR-Cas9 mediated genome editing in Super Basmati rice for resistance against bacterial blight by targeting the major susceptibility gene. Front. Plant Sci. 2020, 11, 575. [Google Scholar] [CrossRef]
- Zeng, X.; Luo, Y.; Vu, N.T.Q. CRISPR/Cas9-mediated mutation of OsSWEET14 in rice cv. Zhonghua11 confers resistance to Xanthomonas oryzae pv. oryzae without yield penalty. BMC Plant Biol. 2020, 20, 313. [Google Scholar]
- Aji, C.M.; Parjanto; Ahmad, Y. Evaluation of Agronomic Performance of Mutant Rice Lines of Mentik Wangi Variety (Oryza sativa L.) Resulting from OSSWEET11 Gene Editing. Int. J. Des. Nat. Ecodyn. 2025, 20, 289–298. [Google Scholar] [CrossRef]
- Li, C.; Liu, B.; Dong, H.; Yang, B. Enhancing resistance to bacterial blight in rice using CRISPR-based base editing technology. Crop J. 2025, 13, 115–124. [Google Scholar]
- Thomazella, P.D.; Brail, Q.; Dahlbeck, D.; Staskawicz, B. CRISPR-Cas9 mediated mutagenesis of a DMR6 ortholog in tomato confers broad-spectrum disease resistance. bioRxiv 2016. [Google Scholar] [CrossRef]
- Frye, C.A.; Tang, D.; Innes, R.W. Negative regulation of defense responses in plants by a conserved MAPKK kinase. Proc. Natl. Acad. Sci. USA 2001, 98, 373–378. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, Y.; Wu, G.; Zou, S.; Chen, Y.; Gao, C.; Tang, D. Simultaneous modification of three homoeologs of TaEDR1 by genome editing enhances powdery mildew resistance in wheat. Plant J. 2017, 91, 714–724. [Google Scholar] [CrossRef]
- Pompili, V.; Dalla Costa, L.; Piazza, S.; Pindo, M.; Malnoy, M. Reduced fire blight susceptibility in apple cultivars using a high-efficiency CRISPR/Cas9-FLP/FRT-based gene editing system. Plant Biotechnol. J. 2019, 18, 845–858. [Google Scholar] [CrossRef]
- Santillán Martínez, M.I.; Bracuto, V.; Koseoglou, E.; Appiano, M.; Jacobsen, E.; Visser, R.G.F.; Wolters, A.M.A.; Bai, Y. CRISPR/Cas9-targeted mutagenesis of the tomato susceptibility gene PMR4 for resistance against powdery mildew. BMC Plant Biol. 2020, 20, 497. [Google Scholar] [CrossRef]
- Kieu, N.P.; Lenman, M.; Wang, E.S.; Petersen, B.L.; Andreasson, E. Mutations introduced in susceptibility genes through CRISPR/Cas9 genome editing confer increased late blight resistance in potatoes. Sci. Rep. 2021, 11, 4487. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Yang, Y.; Schmid, M.; Wang, Y. miRNA mediated regulation and interaction between plants and pathogens. Int. J. Mol. Sci. 2021, 22, 2913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Anarjan, M.B.; Win, K.T.; Begum, S.; Lee, S. QTL-seq analysis of powdery mildew resistance in a Korean cucumber inbred line. Theor. Appl. Genet. 2021, 134, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Uddin, S.; Macoy, D.M.; Shin, G.I.; Jeong, S.Y.; Ali, I.; Hwang, J.W.; Ji, M.G.; Lee, S.C.; Park, J.H. Nucleoredoxin gene SINRX1 negatively regulates tomato immunity by activating SA signaling pathway. Plant Physiol. Biochem. 2023, 200, 107804. [Google Scholar] [CrossRef]
- Azad, M.F.; Dawar, P.; Esim, N.; Rock, C.D. Role of miRNAs in sucrose stress response, reactive oxygen species, and anthocyanin biosynthesis in Arabidopsis thaliana. Front. Plant Sci. 2023, 14, 1278320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Waseem, M.; Zeng, Z.; Xu, J.; Chen, C.; Liu, Y.; Zhai, J.; Xia, R. MicroRNA482/2118, a miRNA superfamily essential for both disease resistance and plant development. New Phytol. 2022, 233, 2047–2057. [Google Scholar] [CrossRef]
- Bigini, V.; Camerlengo, F.; Botticella, E.; Sestili, F.; Savatin, D.V. Biotechnological resources to increase disease-resistance by improving plant immunity: A sustainable approach to save cereal crop production. Plants 2021, 10, 1146. [Google Scholar] [CrossRef]
- Bi, W.; Liu, J.; Li, Y.; He, Z.; Chen, Y.; Zhao, T.; Liang, X.; Wang, X.; Meng, X.; Dou, D.; et al. CRISPR/Cas9-guided editing of a novel susceptibility gene in potato improves Phytophthora resistance without growth penalty. Plant Biotechnol. J. 2024, 22, 4–6. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, B.; Cao, Y.; Zhang, Y.; Song, H.; Huang, C.; Sun, T.; Long, C.; Liao, J.; Zhuo, K. CRISPR/Cas9-mediated mutagenesis of the susceptibility gene OsHPP04 in rice confers enhanced resistance to rice root-knot nematode. Front. Plant Sci. 2023, 14, 1134653. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, J.N.; Ntui, V.O.; Shah, T.; Tripathi, L. CRISPR/Cas9-mediated editing of DMR6 orthologue in banana (Musa spp.) confers enhanced resistance to bacterial disease. Plant Biotechnol. J. 2021, 19, 1291–1293. [Google Scholar] [CrossRef]
- Ma, M.; Yang, L.; Hu, Z.; Mo, C.; Geng, S.; Zhao, X.; He, Q.; Xiao, L.; Lu, L.; Wang, D. Multiplex gene editing reveals cucumber Mildew Resistance Locus O family roles in powdery mildew resistance. Plant Physiol. 2024, 195, 1069–1088. [Google Scholar] [CrossRef]
- Giacomelli, L.; Zeilmaker, T.; Giovannini, O.; Salvagnin, U.; Masuero, D.; Franceschi, P.; Vrhovsek, U.; Scintilla, S.; Rouppe van der Voort, J.; Moser, C. Simultaneous editing of two DMR6 genes in grapevine results in reduced susceptibility to downy mildew. Front. Plant Sci. 2023, 14, 1242240. [Google Scholar] [CrossRef]
- Djennane, S.; Gersch, S.; Le-Bohec, F.; Piron, M.-C.; Baltenweck, R.; Lemaire, O.; Merdinoglu, D.; Hugueney, P.; Nogué, F.; Mestre, P. CRISPR/Cas9 editing of Downy mildew resistant 6 (DMR6-1) in grapevine leads to reduced susceptibility to Plasmopara viticola. J. Exp. Bot. 2023, 75, 2100–2112. [Google Scholar] [CrossRef]
- Nizan, S.; Amitzur, A.; Dahan-Meir, T.; Benichou, J.I.; Bar-Ziv, A.; Perl-Treves, R. Mutagenesis of the melon Prv gene by CRISPR/Cas9 breaks papaya ringspot virus resistance and generates an autoimmune allele with constitutive defense responses. J. Exp. Bot. 2023, 74, 4579–4596. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Nazarain-Firouzabadi, F.; Ismaili, A.; Ahmadvand, R.; Poormazaheri, H. CRISPR/Cas StNRL1 gene knockout increases resistance to late blight and susceptibility to early blight in potato. Front. Plant Sci. 2024, 14, 1278127. [Google Scholar] [CrossRef]
- Ye, W.; Hossain, R.; Pröbsting, M.; Ali, A.A.M.; Han, L. Knock-out of BnHva22c reduces the susceptibility of Brassica napus to infection with the fungal pathogen Verticillium longisporum. Crop J. 2024, 12, 503–514. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Y.; Gao, L.; Xie, M.; Zhang, X.; Zeng, L.; Liu, J.; Liu, Y.; Zhang, Y.; Tong, C.; et al. Genome editing of RECEPTOR-LIKE KINASE 902 confers resistance to necrotrophic fungal pathogens in Brassica napus without growth penalties. Plant Biotechnol. J. 2024, 22, 538–540. [Google Scholar] [CrossRef]
- Hasley, J.A.R.; Navet, N.; Tian, M. CRISPR/Cas9-mediated mutagenesis of sweet basil candidate susceptibility gene ObDMR6 enhances downy mildew resistance. PLoS ONE 2021, 16, e0253245. [Google Scholar] [CrossRef] [PubMed]
- Ortigosa, A.; Gimenez-Ibanez, S.; Leonhardt, N.; Solano, R. Design of a bacterial speck resistant tomato by CRISPR/Cas9-mediated editing of SlJAZ2. Plant Biotechnol. J. 2019, 17, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Meng, J.; He, X.; Zhang, Y.; Liu, Y.; Zhang, C.; Qi, H.; Luan, Y. Editing mir482b and mir482c simultaneously by CRISPR/Cas9 enhanced tomato resistance to Phytophthora infestans. Phytopathology 2021, 111, 1008–1016. [Google Scholar] [CrossRef]
- Thomazella, D.P.; Seong, K.; Mackelprang, R.; Dahlbeck, D.; Geng, Y.; Gill, U.S.; Qi, T.; Pham, J.; Giuseppe, P.; Lee, C.Y. Loss of function of a DMR6 ortholog in tomato confers broad-spectrum disease resistance. Proc. Natl. Acad. Sci. USA 2021, 118, e2026152118. [Google Scholar] [CrossRef]
- Aalders, T.R.; de Sain, M.; Gawehns, F.; Oudejans, N.; Jak, Y.D.; Dekker, H.L.; Takken, F.L. Specific members of the TOPLESS family are susceptibility genes for Fusarium wilt in tomato and Arabidopsis. Plant Biotechnol. J. 2024, 22, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Blanvillain-Baufum, S.M.R.; Sol, M.; Auguy, F.; Doucoure, H.; Szurek, B.; Meynard, D.; Portefaix, M.; Cunnac, S.; Guiderdoni, E.; Boch, J.; et al. Targeted promoter editing for rice resistance to Xanthomonas oryzae pv. oryzae reveals differential activities for SWEET14-inducing TAL effectors. Plant Biotechnol. J. 2017, 15, 306–317. [Google Scholar] [CrossRef]
- Zhou, J.; Peng, Z.; Long, J.; Sosso, D.; Liu, B.; Eom, J.S.; Huang, S.; Liu, S.; Vera Cruz, C.; Frommer, W.B.; et al. Gene targeting by the TAL effector PthXo2 reveals cryptic resistance gene for bacterial blight of rice. Plant J. 2015, 82, 632–643. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, X.; Li, Y.; Liu, L.; Wang, Q.; Wang, Y. Tal6b/AvrXa27A, a hidden TALE targeting the susceptibility gene OsSWEET11a and the resistance gene Xa27 in rice. Plant Commun. 2024, 5, 100721. [Google Scholar] [CrossRef]
- Sha, G.; Sun, P.; Kong, X.; Han, X.; Sun, Q.; Fouillen, L. Genome editing of a rice CDP-DAG synthase confers multipathogen resistance. Nature 2023, 618, 1017–1023. [Google Scholar] [CrossRef]
- Song, H.; Lin, B.; Huang, Q.; Sun, L.; Chen, J.; Hu, L.; Zhuo, K.; Liao, J. The Meloidogyne graminicola effector MgMO289 targets a novel copper metallochaperone to suppress immunity in rice. J. Exp. Bot. 2021, 72, 5638–5655. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Shi, X.; He, F.; Wang, D.; Xiao, N.; Fang, H.; Wang, R.; Zhang, F.; Wang, M.; Li, A. Engineering broad-spectrum disease-resistant rice by editing multiple susceptibility genes. J. Integr. Plant Biol. 2021, 63, 1639–1648. [Google Scholar] [CrossRef]
- Wang, F.; Wang, C.; Liu, P.; Lei, C.; Hao, W.; Gao, Y.; Zhao, K. Enhanced rice blast resistance by CRISPR/Cas9-targeted mutagenesis of the ERF transcription factor gene OsERF922. PLoS ONE 2016, 11, e0154027. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.; Chen, S.; Lei, T.; Xu, L.; He, Y.; Wu, L.; Yao, L.; Zou, X. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol. J. 2017, 15, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Q.; Yang, X.; Xu, J.; Liu, G.; Yao, X.; Lou, L. CRISPR/Cas9-mediated mutagenesis of Clpsk1 in watermelon to confer resistance to Fusarium oxysporum f. sp. niveum. Plant Cell Rep. 2020, 39, 589–595. [Google Scholar] [CrossRef]
- Wang, N.; Fan, X.; He, M.; Hu, Z.; Tang, C.; Zhang, S.; Wang, X. Transcriptional repression of TaNOX10 by TaWRKY19 compromises ROS generation and enhances wheat susceptibility to stripe rust. Plant Cell 2022, 34, 1784–1803. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, H.; Guo, M.; Pan, Y.; Hao, C.; Hou, J.; Li, T. Knockout of GRAIN WIDTH2 has a dual effect on enhancing leaf rust resistance and increasing grain weight in wheat. Plant Biotechnol. J. 2024, 22, 2007. [Google Scholar] [CrossRef]
- El-Mounadi, K.; Morales-Floriano, M.L.; Garcia-Ruiz, H. Principles, applications, and biosafety of plant genome editing using CRISPR-Cas9. Front. Plant Sci. 2020, 11, 56. [Google Scholar] [CrossRef]
- Plan, D.; Van den Eede, G. The EU Legislation on GMOs, An Overview; Publications Office of the European Union: Luxembourg, 2010. [Google Scholar]
- Callaway, E. EU law deals blow to CRISPR crops. Nature 2018, 560, 16. [Google Scholar] [CrossRef]
- Singh, D.J.K.; Mat Jalaluddin, N.S.; Sanan-Mishra, N.; Harikrishna, J.A. Genetic modification in Malaysia and India: Current regulatory framework and the special case of non-transformative RNAi in agriculture. Plant Cell Rep. 2019, 38, 1449–1463. [Google Scholar] [CrossRef]
- Waltz, E. Gene-edited CRISPR mushroom escapes US regulation. Nature 2016, 532, 293. [Google Scholar] [CrossRef]
- Fister, A.S.; Landherr, L.; Maximova, S.N.; Guiltinan, M.J. Transient expression of CRISPR/Cas9 machinery targeting TcNPR3 enhances defense response in Theobroma cacao. Front. Plant Sci. 2018, 9, 268. [Google Scholar] [CrossRef]
- Lee, S.-W. Current status on the modification of the scope for GMO regulation on the gene edited plants with no remnants of inserted foreign DNA fragments. J. Plant Biotechnol. 2019, 46, 137–142. [Google Scholar] [CrossRef]
- Buchholzer, M.; Frommer, W.B. An increasing number of countries regulate genome editing in crops. New Phytol. 2023, 237, 12–15. [Google Scholar] [CrossRef]
- Bhatta, B.P.; Malla, S. Improving horticultural crops via CRISPR/Cas9: Current successes and prospects. Plants 2020, 9, 1360. [Google Scholar] [CrossRef] [PubMed]
- Rukavtsova, E.B.; Zakharchenko, N.S.; Lebedev, V.G.; Shestibratov, K.A. CRISPR-Cas genome editing for horticultural crops improvement: Advantages and prospects. Horticulturae 2023, 9, 38. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; He, Y.; Li, S.; Yan, L.; Li, Y.; Zhu, Z.; Xia, L. Plant base editing and prime editing: The current status and future perspectives. J. Integr. Plant Biol. 2023, 65, 444–467. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Cao, Q.; Li, Y.; He, M.; Liu, X. Advances in cis-element- and natural variation-mediated transcriptional regulation and applications in gene editing of major crops. J. Exp. Bot. 2023, 74, 5441–5457. [Google Scholar] [CrossRef]
| Database /Software | Domain Name | Uses | Framework & Model Type | References |
|---|---|---|---|---|
| PRGdb 4.0 | http://prgdb.org/prgdb4/ | To predict resistance genes and investigate gene expression under specific plant–pathogen conditions | - | García et al., 2022 [84] |
| webTWAS | http://www.webtwas.net/#/ | A database of potential disease S-genes identified through transcriptome-wide association studies. | - | Ca et al., 2022 [85] |
| HMMER | www.hmmer.org | General profile-HMM database search and alignment tool | - | Finn et al., 2011 [86] |
| PSIBLAST | https://www.ebi.ac.uk/jdispatcher/sss/psiblast | Progressive BLAST algorithm employing PSSMs | - | Altschul et al., 1997 [87] |
| Hhblits | http://www.github.com/soedinglab/hh-suite | Identification of distant sequence homologs | - | Remmert et al., 2012 [88] |
| SignalP | http://www.cbs.dtu.dk/services/SignalP | Prediction of secreted proteins by detecting N-terminal signal peptides, a common initial step for effector prediction | - | Teufel et al., 2022 [89] |
| TargetP | http://www.cbs.dtu.dk/services/TargetP | General prediction of protein subcellular or extracellular localization | - | Emanuelsson et al., 2007 [90] |
| TMHMM | http://www.cbs.dtu.dk/services/TMHMM | Prediction of transmembrane domains in proteins | - | Chen 2003 [91] |
| LOCALIZER | https://localizer.csiro.au | Prediction of localization within host plant cells | - | Sperschneider et al., 2017 [92] |
| ApoplastP | https://apoplastp.csiro.au | Prediction of proteins localized in the plant apoplast | - | Sperschneider et al., 2018 [93] |
| dbSWEET | https://ngdc.cncb.ac.cn/databasecommons/database/id/6133 | SWEET transporter prediction | Python/HTML | Gupta et al., 2018 [94] |
| EffectorP | https://effectorp.csiro.au/ | Predict fungal and oomycete effectors and classify localization | Naïve Bayes + C4.5 Decision Trees (Ensemble) | Sperschneider et al., 2021 [95] |
| DeepGO | https://deepgo.cbrc.kaust.edu.sa/deepgo/ | Predicts protein function to prioritize S-gene candidates | Convolutional Neural Networks (CNNs) and Deep Neural Networks (DNN) | Maxat et al., 2018 [96] |
| GeneMANIA | http://genemania.org | Predict gene function using network topology, co-expression, and orthologous features | Label propagation and adaptive network weighting | Warde-Farley et al., 2010 [97] |
| BLAST | https://blast.ncbi.nlm.nih.gov/Blast.cgi | S-gene (homologous) identification | Alignment-based | Altschul et al., 1990 [98] |
| InterProScan | https://www.ebi.ac.uk/interpro/search/sequence/ | Identification of S-genes based on protein domains or families | Rule-based domain annotation | Blum et al., 2025 [99] |
| Effhunter | https://github.com/GisCarreon/EffHunter_v.1.0 | Fungal effector prediction | Rule-driven pipeline | Carreón-Anguiano et al., 2020 [100] |
| Pathoplant | https://ngdc.cncb.ac.cn/databasecommons/database/id/1500 | Reference for Plant-pathogen interaction | Relational Database | Bülow et al., 2004 [101] |
| InterSPPI-AraPathogen2.0 | http://zzdlab.com/intersppi/arapathogen/ | Predict Arabidopsis-pathogen interaction | XGBoost (Extreme Gradient Boosting) | Lei et al., 2023 [102] |
| HPIDB3.0 | https://cales.arizona.edu/hpidb/ | Host–pathogen protein Interaction | Relational Database | Ammari et al., 2016 [103] |
| PHIbase | http://www.phi-base.org/ | Host–pathogen interaction database | Curated database | Urban et al. 2022 [104] |
| Phytozome | https://phytozome-next.jgi.doe.gov | Predict gene families and evolutionary relationships | Distance- and rule-based comparative genomics database | Goodstein et al., 2012 [105] |
| Plant Ensembl | https://plants.ensembl.org/ | Gene sequences and orthologs | Rest & Perl API | Bolser et al., 2017 [106] |
| TAIR (The Arabidopsis Information Resource) | https://www.arabidopsis.org | Reference for model species to identify orthologs | Curated resources | Reiser et al., 2024 [107] |
| Plant Resistance Gene Database (PRGdb) | http://www.prgdb.org/prgdb4/ | Predicts primarily R-genes, but useful for cross-referencing S-gene candidates | Domain scoring and rule-based thresholds | García et al., 2021 [84] |
| OrthoMCL | https://orthomcl.org | Gene family clustering and ortholog identification | Markov Cluster Algorithm (MCL) | Li et al., 2003 [108] |
| Gene Expression Omnibus (GEO) | https://www.ncbi.nlm.nih.gov/geo/ | Identify differentially expressed genes as S-gene candidates | Expression repository | Barrett et al., 2013 [109] |
| Crop | S-Gene | Disease | Associated Pathogen | Function of S-Gene | Result/Outcome | Reference |
|---|---|---|---|---|---|---|
| Arabidopsis thaliana | elF(iso)4E | Viral disease | Yellow mosaic virus (YMV) | Recessive resistance alleles against potyviruses | Induced mutation in elF(iso)4E imparts complete resistance | Pyott et al., 2016 [173] |
| Banana (Musa acuminate) | MusaDMR6 | Xanthomonas wilt | Xanthomonas campestris pv. musacearum | Salicylic acid degradation (SA-5-hydroxylase), suppresses host immunity | Improved resistance with no detrimental impact on plant | Tripathi et al., 2021 [215] |
| Cassava (Manihot esculenta) | nCBP1 and nCBP2 | Viral resistance | Cassava brown streak virus (CBSV) | Susceptibility factor | Resilience to viral disease | Gomez et al., 2019 [180] |
| Cucumber (Cucumis sativus L.) | CsaMLO1, CsaMLO8, CsaMLO11 | Powdery mildew (PM) | Podosphaera xanthii | Negative regulator of pre- (CsaMLO8) and post-invasive (CsaMLO1, CsaMLO11) defense | CsaMLO8 loss-of-function conferred highest penetration resistance; with CsaMLO1 and CsaMLO11 double mutations seemed good candidates for HR-based resistance against PM pathogen | Tek et al., 2022 [187], Ma et al., 2024 [216] |
| elF4E | Viral diseases | Potyviruses | Encodes a translation initiation factor that interacts with potyviral VPg proteins for viral infection. | Loss-of-function mutations disrupt viral replication, conferring resistance. Homozygous elF4E_1DEL and elF4E_1-3DEL mutants showed complete resistance to watermelon mosaic virus (WMV), papaya ringspot virus (PRSV), and zucchini yellow mosaic virus (ZYMV) | Chandrasekaran et al., 2016 [172] Fidan et al., 2023 [184] | |
| Grapevine (Vitis vinifera) | VvDMR6-1 and VvDMR6-2 | Downy mildew (DM) | Plasmopara viticola | Negative regulator of plant immunity | Reduced severity, simultaneous editing of both genes is required for reduced susceptibility | Giacomelli et al., 2023 [217] Djennane et al., 2023 [218] |
| Melon (Cucumis melo) | elF4E | Viral disease | Ringspot mosaic virus-W (RMV-W) | Encodes a cap-binding protein essential for translation initiation. It acts as a proviral factor by facilitating viral RNA translation and in sexual development | Loss-of-function mutations confer virus resistance but also cause male sterility | Shirazi et al., 2023 [182] Pechar et al., 2022 [183] |
| Prv | Viral disease | PRSV-W | NLR resistance gene required for PRSV immunity | Knockout mutants lost PRSV resistance; one allele (prvΔ154) exhibited an autoimmune dwarf phenotype suppressed at high temperatures | Nizan et al., 2023 [219] | |
| Potato (Solanum tuberosum) | StNRL1 | Late blight | Phytophthora infestans | Forms a protein complex with the P. infestans effector, leading to degradation of SWAP70, a positive regulator of cell death | Knockdown enhanced resistance to P. infestans but increased susceptibility to Alternaria alternata, suggesting a dual role in defense | Norouzi et al., 2024 [220] |
| eIF4E | Virus resistance | Potato virus Y (PVY) | Translation initiation factor required by PVY via VPg interaction | Knockout of eIF4E conferred broad-spectrum resistance to PVY with significantly delayed/reduced virus titer, and no adverse growth or developmental abnormalities | Noureen et al., 2022 [181] | |
| StPM1 | Late blight | Phytophthora infestans | Encodes a plasma membrane protein that interacts with StRbohC, promoting its degradation and negatively regulating reactive oxygen species (ROS) production | Knockout mutants exhibited enhanced resistance to P. infestans without growth penalties; increased expression of defense-related genes. | Bi et al., 2024 [213] | |
| Rapeseed (Brassica napus) | BnHva22c | Stem striping | Verticillium longisporum | Susceptibility factor | Improved resistance | Ye et al., 2024 [221] |
| BnaA05.RLK902 | Stem rot disease (SRD), grey mold disease (GMD) | Sclerotinia sclerotiorum, Botrytis cinera | Plasma membrane RLK negatively regulating necrotrophic immunity | Resistance to both diseases; no growth trade-off | Zhao et al., 2024 [222] | |
| BnWRKY70 | Sclerotinia stem rot | Sclerotinia sclerotiorum | Transcription factor; negatively regulates defense response against S. sclerotiorum | Knockouts showed enhanced resistance; overexpression increased susceptibility | Sun et al., 2018 [33] | |
| Sweet basil (Ocimum basilicum) | ObDMR6 | DM | Hyaloperonospora arabidopsidis | SA-5-hydroxylase homolog that degrades salicylic acid, lowering host immunity and promoting pathogen growth | Knockout of ObDMR6 led to enhanced resistance with reduced sporangia and pathogen biomass | Hasley et al., 2021 [223] |
| Tomato (Solanum lycopersicum) | SlMlo1 | PM | Oidium neolycopersici | Susceptibility factor for fungal PM | Knockout of SlMlo1 conferred complete resistance to PM | Pramanik et al., 2021 [192] |
| SlPMR4 | PM | Oidium neolycopersici (On) | Encodes callose synthase at fungal penetration site, which is exploited by pathogen | Disrupting SlPMR4 reduces susceptibility by increasing HR | Santillán Martínez et al., 2020 [205] | |
| SlJAZ2 | Bacterial speck | Pseudomonas syringae pv. Tomato (Pto) DC300 | Encodes coronatine co-receptor in stomatal guard cell that facilitates pathogen colonization | Knockout resulted in increased resistance | Ortigosa et al., 2019 [224] | |
| SlNRX1 | Bacterial speck | Alternaria brassicicola and Pseudomonas syringae pv. maculicola | Mutation boosting the salicylic pathway for immunity | Enhances plant immunity by negatively modulating the expression of the gene | Cha et al., 2023 [209] | |
| miR482b, miR482c | Late blight | Phytophthora infestans | Repress NBS-LRR defense gene transcripts | CRISPR knockout of miR482b + miR482c conferred resistance to P. infestans. Double mutants showed stronger resistance than miR482b alone | Hong et al., 2021 [225] | |
| eIF4E1 | Virus resistance | Pepper mottle virus (PepMoV) | Translation initiation factor (eIF4E1) is hijacked by potyviruses via VPg interaction to facilitate viral translation | Knockout conferred strong resistance to PepMoV, with no resistance to tobacco etch virus (TEV) and no adverse growth defects | Atarashi et al., 2020 [177] Yoon et al., 2020 [178] | |
| SlDMR6-1 | Resistance to bacterial, oomycete, and fungal pathogens | Pseudomonas syringae, Xanthomonas gardneri, Xanthomonas perforans, Phytophthora capsici, Pseudoidium neolycopersici | Encodes a 2-oxoglutarate Fe (II)-dependent dioxygenase that acts as a salicylic acid (SA) 5-hydroxylase, converting SA to 2,5-dihydroxybenzoic acid | Knockout of the tomato susceptibility gene SlDMR6-1 significantly enhances resistance against a broad range of pathogens, including bacteria, oomycetes, and fungi | Thomazella et al., 2021 [226] | |
| eIF4E2 | Virus resistance | Pepper veinal mottle virus (PVMV) | Loss-of-function prevents PVMV from hijacking the cap-binding complex | Knockout of eIF4E2 conferred resistance to PVMV | Kuroiwa et al., 2022 [179] | |
| TPL1, TPL2 | Fusarium wilt | Fusarium oxysporum | Encode transcriptional co-repressors that interact with the fungal SIX8 effector, promoting disease susceptibility | TPL1 knockout reduced susceptibility; TPL1/TPL2 double knockout provided higher resistance | Aadlers et al., 2023 [227] | |
| Rice (Oryza sativa) | OsSWEET14, Os SWEET11, OsSWEET13 | Bacterial leaf blight | Xanthomonas oryzae pv. oryzae (Xoo) | Encodes a sugar transporter that is used by Xoo TALE (transcription-ativator-like effectors) (e.g., AvrXa7, PthXo3/2, TalC, Tal5) for nutrient acquisition, promoting pathogen virulence | Mutations in TALE-binding elements (EBEs) prevent pathogen-induced expression and confer resistance | Blanvillain-Baufume et al., 2016 [228] Zhou et al., 2015 [229] Olivia et al., 2019 [195] Zafer et al., 2020 [197] Aji et al., 2025 [199]; Li et al., 2025 [200] |
| EBETal6b of OsSWEET11a | Bacterial blight | Xanthomonas oryzae pv. oryzae | Sugar transporter | Rapid resistance response that blocked disease development | Xu et al., 2024 [230] | |
| RBL1Δ12 | Rice blast | Magnaporthe oryzae | Associated with effector secretion and fungal infection | Confers broad-spectrum disease resistance | Sha et al., 2023 [231] | |
| OsHPP04 | Root-knot nematode | Meloidogyne graminicola | Act as a negative regulator of host immunity against rice root-knot nematode (Meloidogyne graminicola) | Enhance resistance with no adverse effect on main agronomic traits | Huang et al., 2023 [214] Song et al., 2021 [232] | |
| Pi21 and Bsr-d1 | Rice blast | Magnaporthe oryzae | Suppresses basal defense mechanisms | The triple mutant had much higher resistance to both M. oryzae and Xoo than the single mutants | Tao et al., 2021 [233] | |
| Xa5 | Bacterial blight | Xanthomonas oryzae pv. oryzae | Susceptibility factor for disease | Triple mutant with higher resistance | Tao et al., 2021 [233] | |
| eIF4G | RTD (Rice tungro disease) | Rice tungro spherical virus (RTSV) | Translation initiation factor exploited by viral RNA for protein synthesis and infection | Resistance to RTSV without growth penalty | Macovei et al., 2018 [174] Cao et al., 2020 [74] | |
| OsERF922 | Rice blast | Magnaporthe oryzae | Encodes an ERF transcription factor that negatively regulates plant defense | Reduced blast lesion formation without affecting agronomic traits; heritable resistance in T1 and T2 generations | Wang et al., 2016 [234] | |
| Wanjincheng orange (Citrus sinensis Osbeck) | CsLOB1 | Citrus canker | Zucchini yellow mosaic virus (ZYMV) | Encodes a transcription factor activated by Xcc effector PthA4, promoting disease development | Promoter editing of CsLOB1 enhanced resistance; no symptoms in some mutants | Peng et al., 2017 [235] |
| Watermelon (Citrullus lanatus) | Clpsk1 | Fusarium wilt | Fusarium oxysporum f. sp. Niveum (FON) | Encodes the precursor of phytosulfokine (PSK), a peptide hormone that negatively regulates plant immunity | Loss-of-function mutations enhance resistance to FON | Zhang et al., 2020 [236] |
| CleIF4E1 | Viral disease | ZYMV, Cucumber green mottled mosaic virus (CGMMV) | Major recessive factors for many viruses (especially potyviruses) | Mutant line exhibited resistance to ZYMV but not CGMMV, with developmental defects and reduced yield | Li et al., 2024 [185] | |
| Wheat (Triticum aestivum) | TaMLO-A1, TaMLO-B1, TaMLO-D1 | PM | Blumeria graminis f. sp. tritici (Bgt) | MLO proteins negatively regulate defense | Triple-mutant plants exhibited heritable, broad-spectrum resistance to PM | Li et al., 2022 [188] |
| SlMlo1 | PM | Blumeria graminis f. sp. tritici (Bgt) | MLO proteins negatively regulate defense | Complete resistance to PM | Nekrasov et al. 2017 [191] Pramanik et al., 2021 [192] | |
| TaEDR1 (homoeo alleles in A, B, and D subgenomes) | PM | Blumeria graminis f. sp. tritici (Bgt) | Negative regulator of PM resistance | Triple-mutant Taedr1 wheat plants showed resistance to powdery mildew with no off-target mutations or pleiotropic effects | Zhang et al., 2017 [203] | |
| TaWRKY19 | Stripe rust | Puccinia striiformis f. sp. tritici (Pst) | WRKY transcription factor; negative regulator of plant immune response | Knockout resulted in strong resistance to stripe rust | Wang et al., 2022 [237] | |
| TaGW2 | Leaf rust | Puccinia triticina Eriksson (Pt) | E3 ubiquitin ligase; negative regulator of wheat grain width and weight | Knockout led to resistance to leaf rust with increased grain width and weight | Liu et al., 2024 [238] | |
| TaPslPK1 | Stripe rust | Puccinia striiformis f. sp. tritici (Pst) | Encodes a receptor-like cytoplasmic kinase targeted by the fungal effector PsSpg1, promoting virulence by phosphorylating TaCBF1d and modulating gene expression | Knockout of TaPsIPK1 conferred broad-spectrum resistance against Pst without affecting agronomic traits in field tests | Wang et al., 2022 [237] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakur, S.; Kaur, S.; Adhikari, S.; Sabharwal, P.; Fu, Y.; Meru, G. Turning Susceptibility into Strength: A New Era of Durable Resistance in Plants Through Genome Editing. Plants 2025, 14, 3080. https://doi.org/10.3390/plants14193080
Thakur S, Kaur S, Adhikari S, Sabharwal P, Fu Y, Meru G. Turning Susceptibility into Strength: A New Era of Durable Resistance in Plants Through Genome Editing. Plants. 2025; 14(19):3080. https://doi.org/10.3390/plants14193080
Chicago/Turabian StyleThakur, Shallu, Simranjot Kaur, Sudeep Adhikari, Prerna Sabharwal, Yuqing Fu, and Geoffrey Meru. 2025. "Turning Susceptibility into Strength: A New Era of Durable Resistance in Plants Through Genome Editing" Plants 14, no. 19: 3080. https://doi.org/10.3390/plants14193080
APA StyleThakur, S., Kaur, S., Adhikari, S., Sabharwal, P., Fu, Y., & Meru, G. (2025). Turning Susceptibility into Strength: A New Era of Durable Resistance in Plants Through Genome Editing. Plants, 14(19), 3080. https://doi.org/10.3390/plants14193080







