Transcriptomic Insights into the Effects of Inoculation Density in Areca catechu Tissue Culture
Abstract
1. Introduction
2. Results
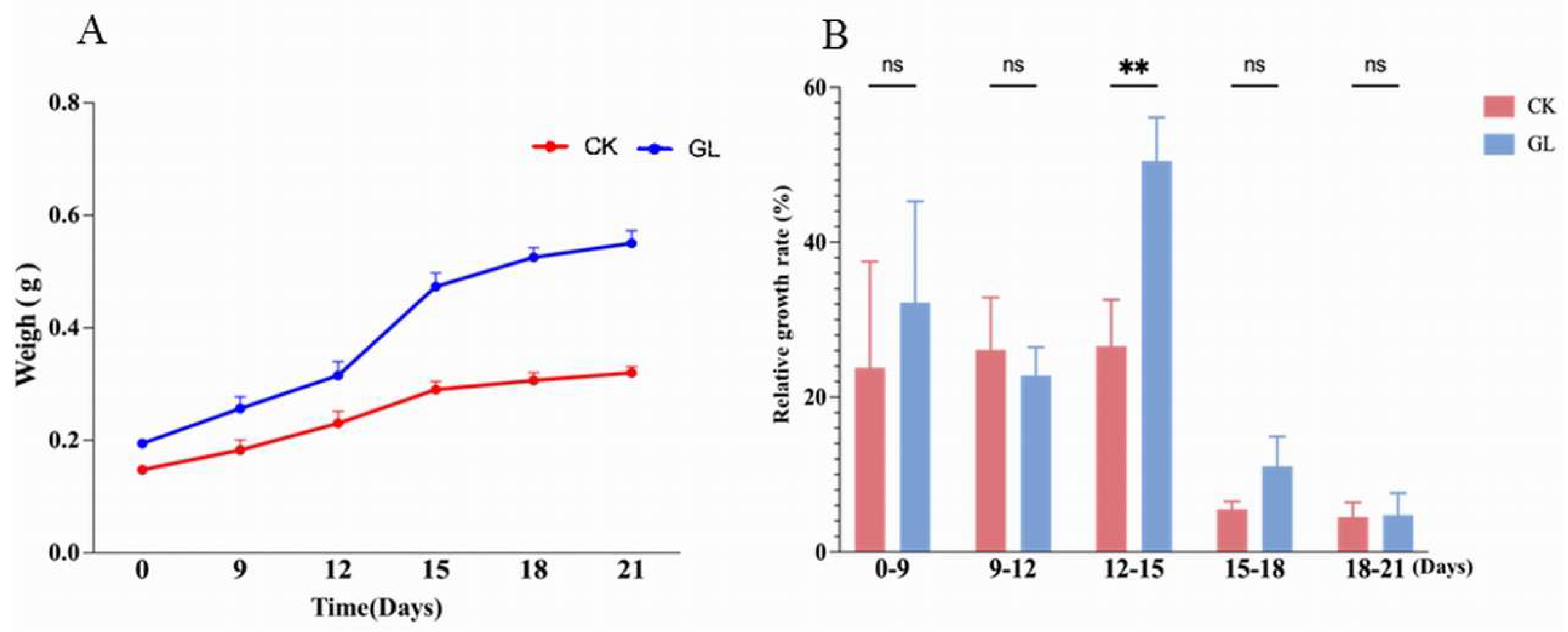
2.1. Effect of Inoculation Density on Embryogenic Callus Proliferation
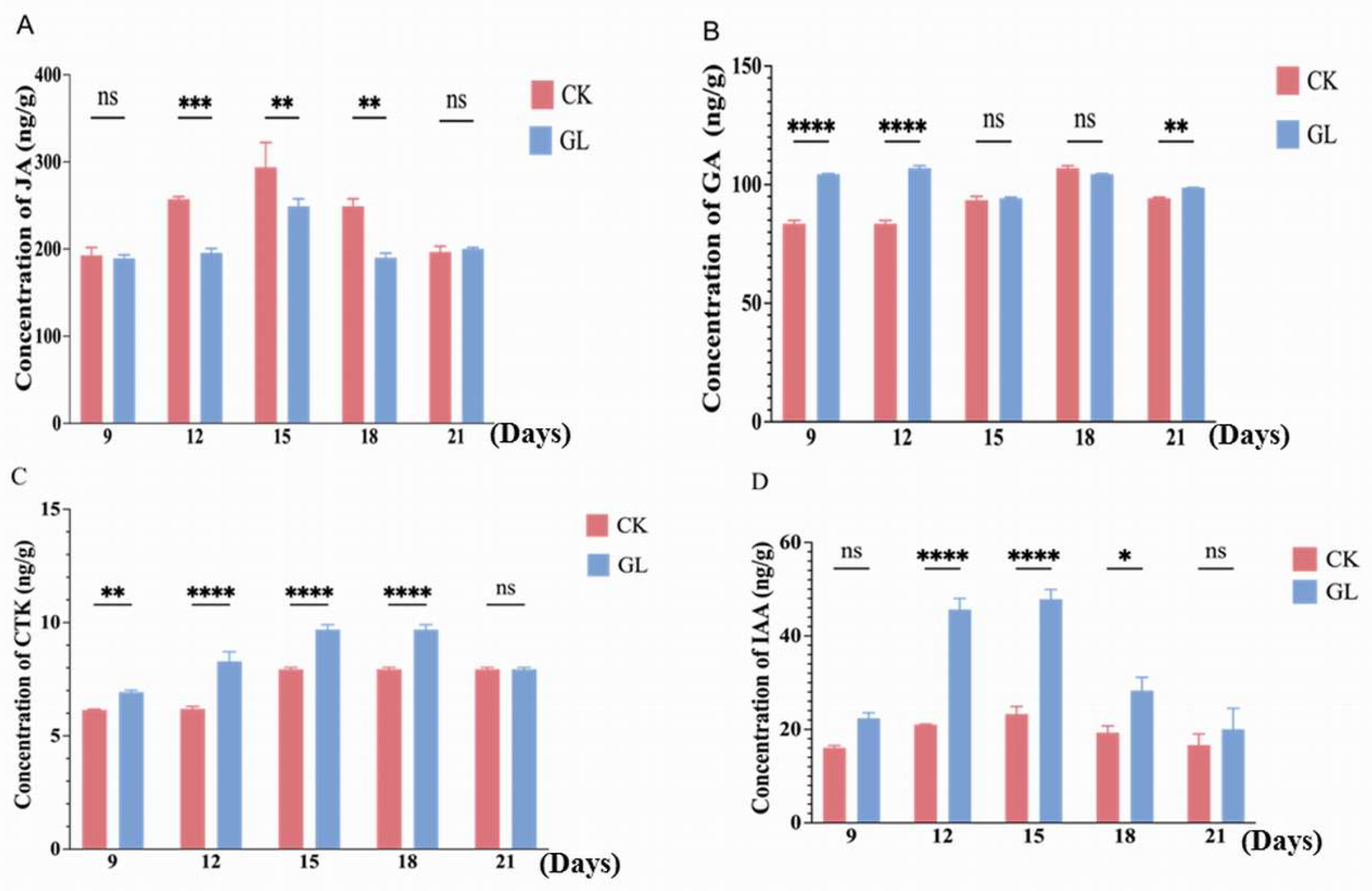
2.2. Analysis of Endogenous Hormone Content
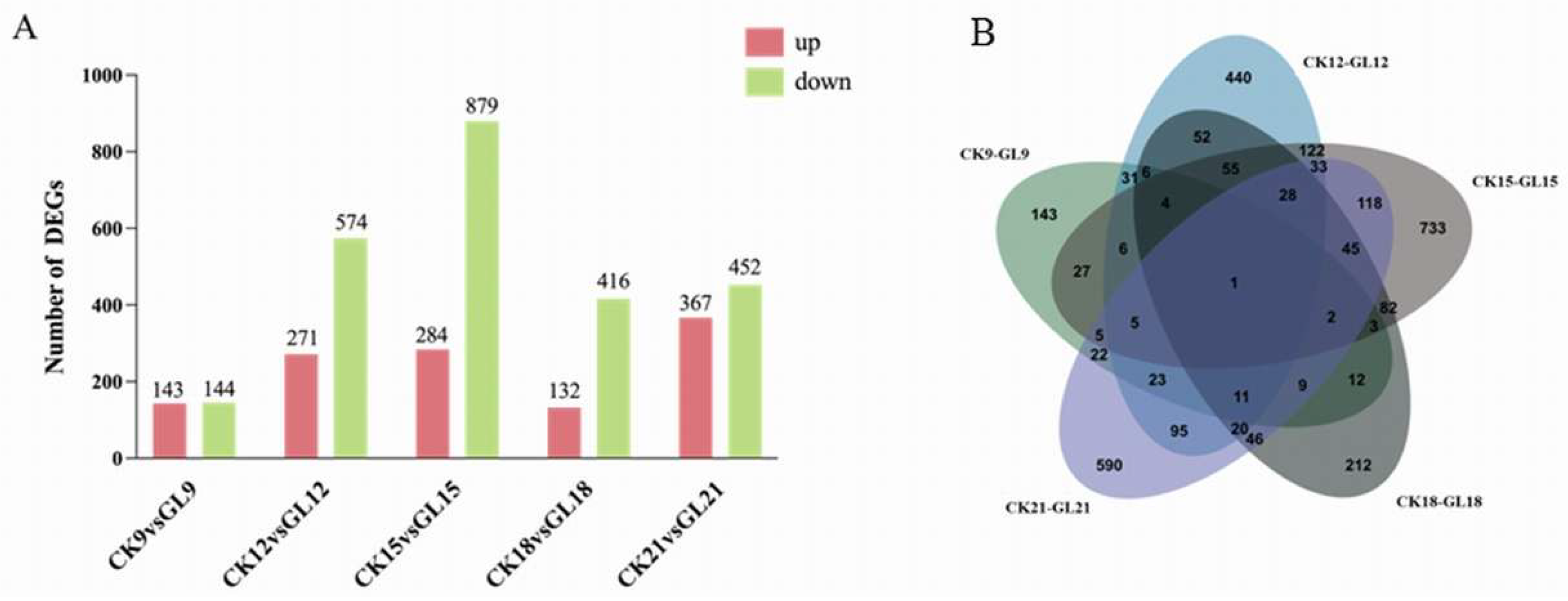
2.3. Analysis of Differentially Expressed Genes (DEGs)
2.4. KEGG Pathway Enrichment Analysis
2.5. Transcription Factor (TF) Analysis
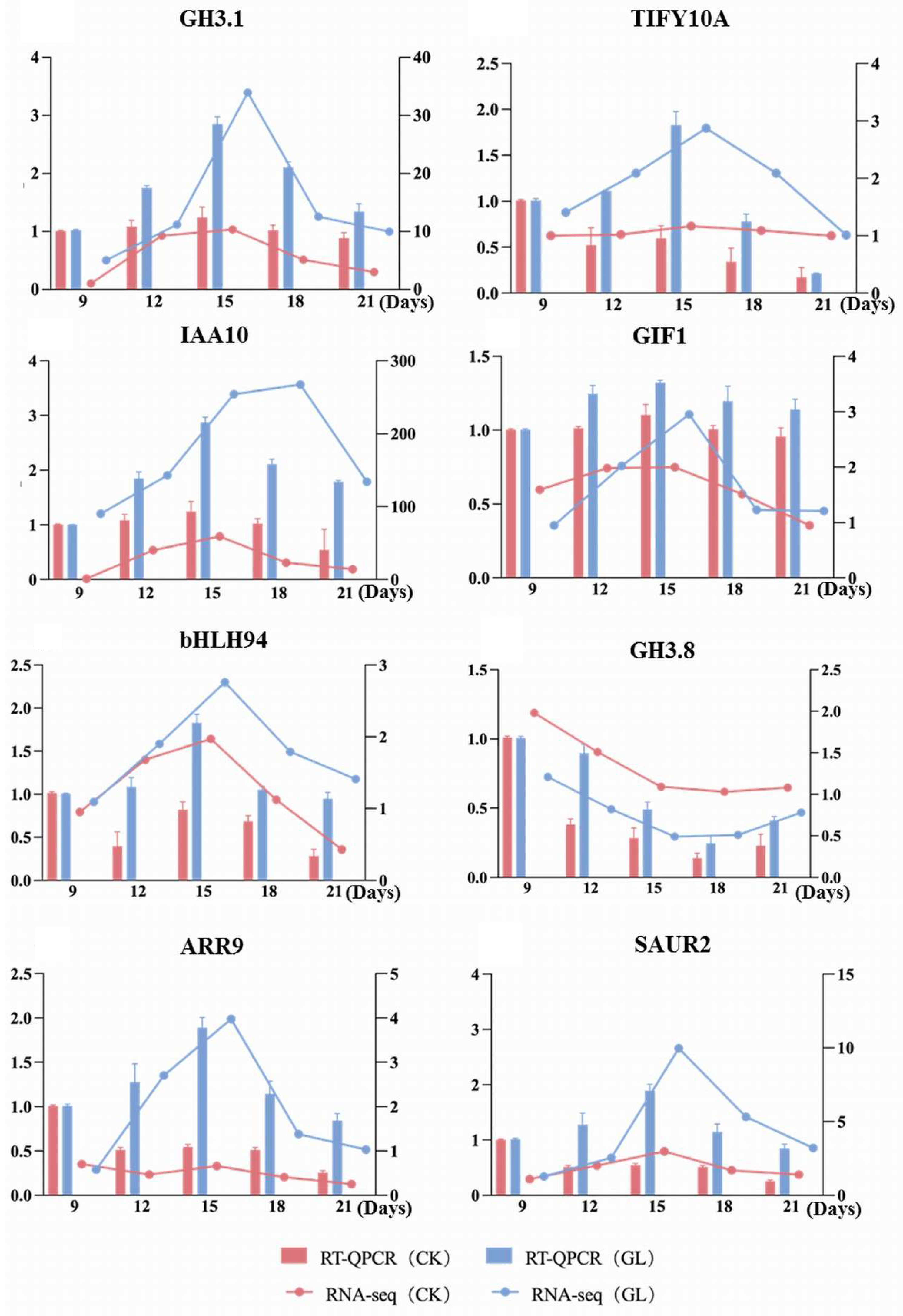
2.6. qRT-PCR Analysis
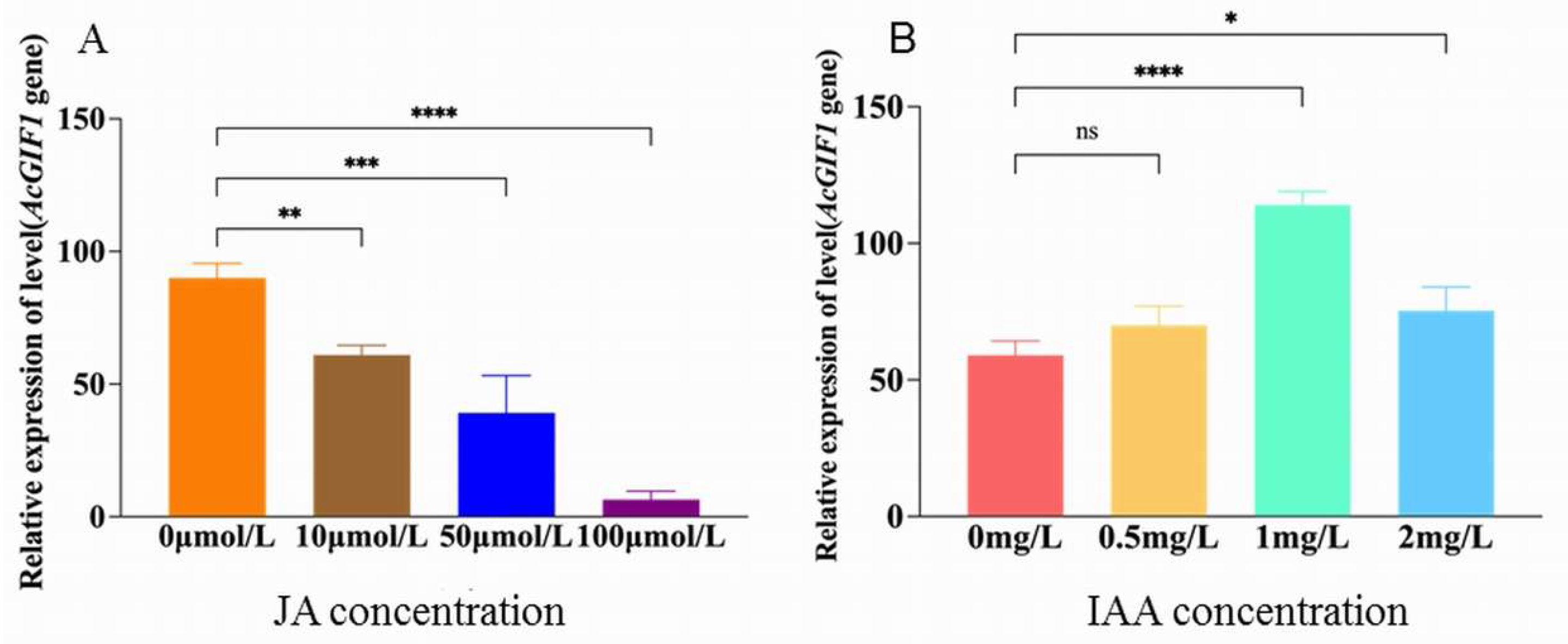
2.7. Effect of Jasmonic Acid on AcGIF1 Expression
2.8. Effect of Indole-3-Acetic Acid (IAA) on AcGIF1 Expression
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Embryogenic Callus Inoculation
4.3. RNA Extraction
4.4. Transcriptome Sequencing and Data Analysis
4.5. Identification and Analysis of Differentially Expressed Genes (DEGs)
4.6. Measurement of Endogenous Hormone Levels
4.7. Quantitative Real-Time PCR (qRT-PCR)
4.8. Exogenous JA and IAA Treatment Experiment
4.9. Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parthasarathy, V.A.; Karun, A.; Rajesh, M.K. Biotechnology of Coconut. J. Hortic. Sci. 2007, 2, 1–12. [Google Scholar] [CrossRef]
- Salehi, B.; Konovalov, D.A.; Fru, P.; Kapewangolo, P.; Peron, G.; Ksenija, M.S.; Cardoso, S.M.; Pereira, O.R.; Nigam, M.; Nicola, S.; et al. Areca catechu-From farm to food and biomedical applications. Phytother. Res. 2020, 34, 2140–2158. [Google Scholar] [CrossRef] [PubMed]
- Amudhan, M.S.; Begum, V.H.; Hebbar, K.B. A review on phytochemical and pharmacological potential of Areca catechu L. Seed Int. J. Pharm. Sci. Res. 2012. [Google Scholar] [CrossRef][Green Version]
- Dai, J.; Chen, X.; Yun, Y.; Wang, Y.; Yu, J. Transcriptome Data Assembly and Gene Function Annotation of Areca catechu L. Flower Fruit. Mol. Plant Breed. 2019, 17, 1814–1826. [Google Scholar][Green Version]
- Lei, J.; Gao, Y.; Khan, W.U.; Li, H.; Ma, X.; Wang, D.; Tang, Y.; Liu, Z. First Report of Grammothele lineata Causing Stem Rot of Areca catechu in Hainan Province, China. Plant Dis. 2021, 105, 2240. [Google Scholar] [CrossRef]
- Zhou, G.; Yin, H.; Chen, F.; Wang, Y.; Gao, Q.; Yang, F.; He, C.; Zhang, L.; Wan, Y. The genome of Areca catechu provides insights into sex determination of monoecious plants. New Phytol. 2022, 236, 2327–2343. [Google Scholar] [CrossRef]
- Wang, H.C.; Chen, J.T.; Chang, W.C. Somatic embryogenesis and plant regeneration from leaf, root and stem-derived callus cultures of Areca catechu. Biol. Plant. 2006, 50, 279–282. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, R.; Zhang, H.; Cao, X.; Li, Z.; Zhang, Z.; Zhai, J.; Huang, X. Prevalence of Yellow Leaf Disease (YLD) and its Associated Areca Palm Velarivirus 1 (APV1) in Betel Palm (Areca catechu) Plantations in Hainan, China. Plant Dis. 2020, 104, 2556–2562. [Google Scholar] [CrossRef]
- Zheng, Y.S.; Zhao, J.; Yang, X.Q.; Zou, J.X.; Li, D.D. Molecular mechanism of the effect of PEG and UV stress on the metabolism of arecoline in Areca catechu callus. Sci. Hortic. 2023, 321, 112299. [Google Scholar] [CrossRef]
- Haque, R.M.; Kuo, Y.H.; Lambein, F.; Hussain, M. Effect of environmental factors on the biosynthesis of the neuro-excitatory amino acid beta-ODAP (beta-N-oxalyl-L-alpha,beta-diaminopropionic acid) in callus tissue of Lathyrus sativus. Food Chem. Toxicol. 2011, 49, 583–588. [Google Scholar] [CrossRef]
- Konan, K.E.; Durand-Gasselin, T.; Kouadio, Y.J.; Flori, A.; Rival, A.; Duval, Y.; Pannetier, C. In vitro conservation of oil palm somatic embryos for 20 years on a hormone-free culture medium: Characteristics of the embryogenic cultures, derived plantlets and adult palms. Plant Cell Rep. 2010, 29, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Suranthran, P.; Sinniah, U.R.; Subramaniam, S.; Aziz, M.A.; Romzi, N.; Gantait, S. Effect of plant growth regulators and activated charcoal on in vitro growth and development of oil palm (Elaeis guineensis Jacq. var. Dura) zygotic embryo. Afr. J. Biotechnol. 2011, 10, 10600–10606. [Google Scholar]
- Wang, H.C.; Chen, J.T.; Chang, W.C. Morphogenetic routes of long-term embryogenic callus culture of Areca catechu. Biol. Plant. 2010, 54, 1–5. [Google Scholar] [CrossRef]
- Takayanagi, N.; Mukai, M.; Sugiyama, M.; Ohtani, M. Transcriptional regulation of cell proliferation competence-associated Arabidopsis genes, CDKA;1, RID1 and SRD2, by phytohormones in tissue culture. Plant Biotechnol. 2022, 39, 329–333. [Google Scholar] [CrossRef]
- Liu, Z.; Li, N.; Zhang, Y.; Li, Y. Transcriptional repression of GIF1 by the KIX-PPD-MYC repressor complex controls seed size in Arabidopsis. Nat. Commun. 2020, 11, 1846. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Cui, D.; Du, Y.; Zhang, D.; Sun, W.; Du, H.; Zhang, Z. GIF1 controls ear inflorescence architecture and floral development by regulating key genes in hormone biosynthesis and meristem determinacy in maize. BMC Plant Biol. 2022, 22, 127. [Google Scholar] [CrossRef]
- Sha, G.; Cheng, J.; Wang, X.; Xue, Q.; Zhang, H.; Zhai, R.; Yang, C.; Wang, Z.; Xu, L. PbbHLH137 interacts with PbGIF1 to regulate pear fruit development by promoting cell expansion to increase fruit size. Physiol. Plant 2024, 176, e14451. [Google Scholar] [CrossRef]
- Li, J.; Pan, W.; Zhang, S.; Ma, G.; Li, A.; Zhang, H.; Liu, L. A rapid and highly efficient sorghum transformation strategy using GRF4-GIF1/ternary vector system. Plant J. 2024, 117, 1604–1613. [Google Scholar] [CrossRef]
- Wei, S.; Chen, Y.; Hou, J.; Yang, Y.; Yin, T. Aux/IAA and ARF Gene Families in Salix suchowensis: Identification, Evolution, and Dynamic Transcriptome Profiling During the Plant Growth Process. Front. Plant Sci. 2021, 12, 666310. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Aslam, M.; Jakada, B.H.; Qin, Y.; Cai, H. The Glycine-Rich Domain Protein GRDP2 Regulates Ovule Development via the Auxin Pathway in Arabidopsis. Front. Plant Sci. 2021, 12, 698487. [Google Scholar] [CrossRef]
- Delfin, J.C.; Kanno, Y.; Seo, M.; Kitaoka, N.; Matsuura, H.; Tohge, T.; Shimizu, T. AtGH3.10 is another jasmonic acid-amido synthetase in Arabidopsis thaliana. Plant J. 2022, 110, 1082–1096. [Google Scholar] [CrossRef] [PubMed]
- Seifu, Y.W.; Pukysova, V.; Rydza, N.; Bilanovicova, V.; Zwiewka, M.; Sedláček, M.; Nodzyński, T. Mapping the membrane orientation of auxin homeostasis regulators PIN5 and PIN8 in Arabidopsis thaliana root cells reveals their divergent topology. Plant Methods 2024, 20, 84. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Huang, J.; Zheng, C.; Cai, C.; Wang, Q.; Wu, C.-A. Salt and methyl jasmonate aggravate growth inhibition and senescence in Arabidopsis seedlings via the JA signaling pathway. Plant Sci. 2017, 261, 1–9. [Google Scholar] [CrossRef]
- Tamogami, S.; Rakwal, R.; Agrawal, G.K. Interplant communication: Airborne methyl jasmonate is essentially converted into JA and JA-Ile activating jasmonate signaling pathway and VOCs emission. Biochem. Biophys. Res. Commun. 2008, 376, 723–727. [Google Scholar] [CrossRef]
- Cheng, Y.; Liang, C.; Qiu, Z.; Zhou, S.; Liu, J.; Yang, Y.; Wang, R.; Yin, J.; Ma, C.; Cui, Z.; et al. Jasmonic acid negatively regulates branch growth in pear. Front. Plant Sci. 2023, 14, 1105521. [Google Scholar] [CrossRef]
- Wang, Y.C.; Wang, N.; Xu, H.F.; Jiang, S.H.; Fang, H.C.; Su, M.-Y.; Zhang, Z.-Y.; Zhang, T.-L.; Chen, X.-S. Auxin regulates anthocyanin biosynthesis through the Aux/IAA-ARF signaling pathway in apple. Hortic. Res. 2018, 5, 59. [Google Scholar] [CrossRef] [PubMed]



| Primere | Sequence (5′-3′) |
|---|---|
| Ac-Actin-F | TTCCAGCCTTCGCTCATT |
| Ac-Actin-R | CCTCCACCACTAAGCACAATG |
| bHLH94-F | GTAGGCGGAGCCATTGATTT |
| bHLH94-R | GCTGCTGGAGTGTCCTCTTCT |
| GIF1-F | GGCCCTCGGTATATGCAACA |
| GIF1-R | CAGCAAGCAGCACTGCATAG |
| IAA10-F | TGCCGTTGGGTCCACTAATC |
| IAA10-R | CCTCAAATGCTGCAGTCACG |
| TIFY10A-F | TCAGCAACTGCAGCAAGAGT |
| TIFY10A-R | CGACAGCCAACCTTTGATGC |
| GH3.8-F | GCTCATGGACTACGCCATCT |
| GH3.8-R | GTCGTCTCCACCCACTTCAG |
| SAUR2-F | CATATCGGTGTGCTTGCTGC |
| SAUR2-R | GGCAAGTACCACAAAGCTGC |
| ARR9-F | ACCGTGTCTTAGCTGTGGATG |
| ARR9-R | GCAGTTCCTGGGTCTACAGG |
| GH3.17-F | GTGATGAGCCAATTCGTGCC |
| GH3.17-R | ACTACAAGAGCCGCCACTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Li, Y.; Liu, Z.; Zheng, Y.; Zou, J.; Li, D. Transcriptomic Insights into the Effects of Inoculation Density in Areca catechu Tissue Culture. Plants 2025, 14, 3073. https://doi.org/10.3390/plants14193073
Yan J, Li Y, Liu Z, Zheng Y, Zou J, Li D. Transcriptomic Insights into the Effects of Inoculation Density in Areca catechu Tissue Culture. Plants. 2025; 14(19):3073. https://doi.org/10.3390/plants14193073
Chicago/Turabian StyleYan, Jinqi, Yu Li, Zijia Liu, Yusheng Zheng, Jixin Zou, and Dongdong Li. 2025. "Transcriptomic Insights into the Effects of Inoculation Density in Areca catechu Tissue Culture" Plants 14, no. 19: 3073. https://doi.org/10.3390/plants14193073
APA StyleYan, J., Li, Y., Liu, Z., Zheng, Y., Zou, J., & Li, D. (2025). Transcriptomic Insights into the Effects of Inoculation Density in Areca catechu Tissue Culture. Plants, 14(19), 3073. https://doi.org/10.3390/plants14193073






