Phyto-Algal Consortia as a Complementary System for Wastewater Treatment and Biorefinery
Abstract
1. Introduction
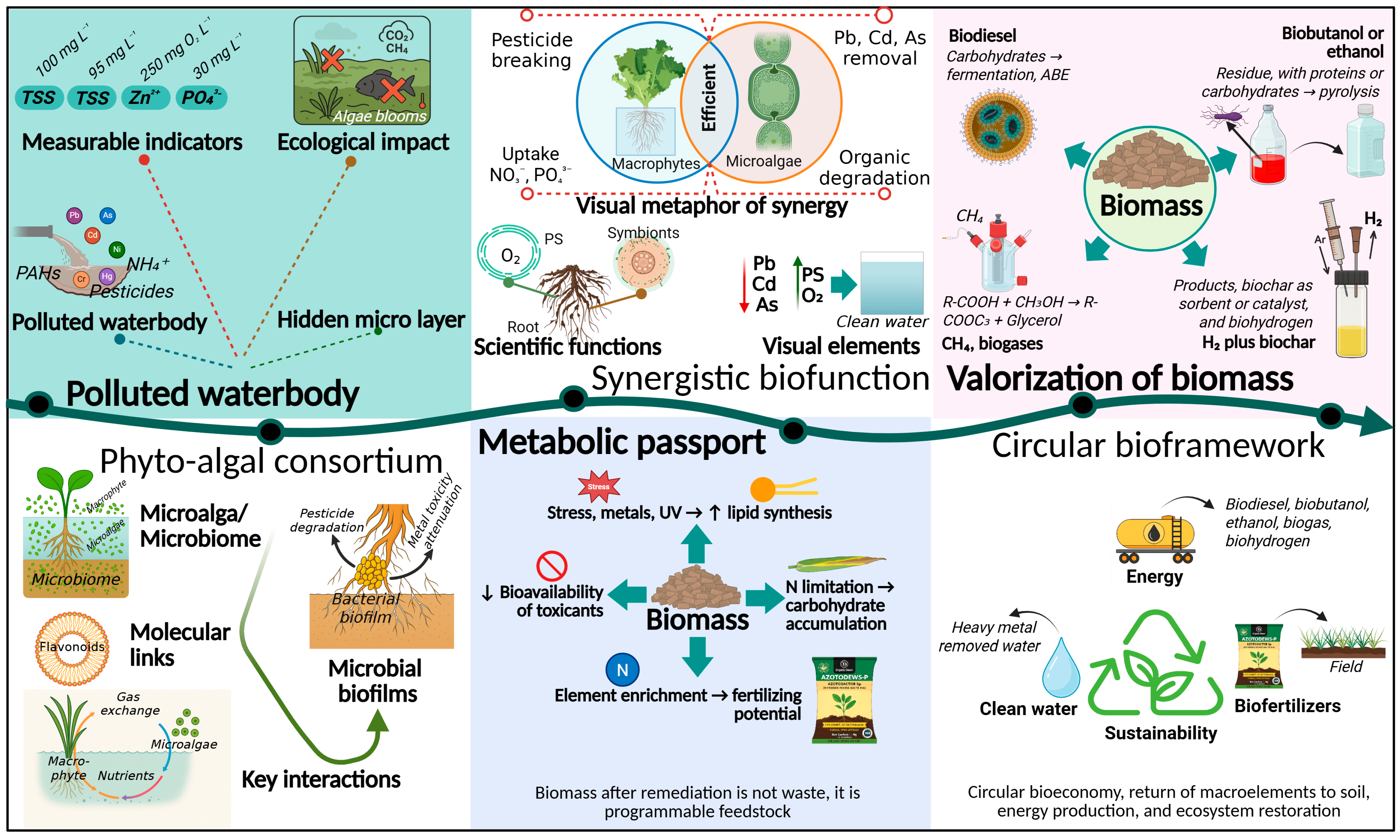
2. Functional Ecologies of Phyto-Algal Consortia
3. The Metabolic Fates of Phyto-Algal Consortia Biomass
3.1. Biotransformation of Pollutants in Hybrid Phyto-Algal Systems
3.2. Integrated Platform of Phytoremediation and Biorefinery Within a Circular Bioeconomy Framework
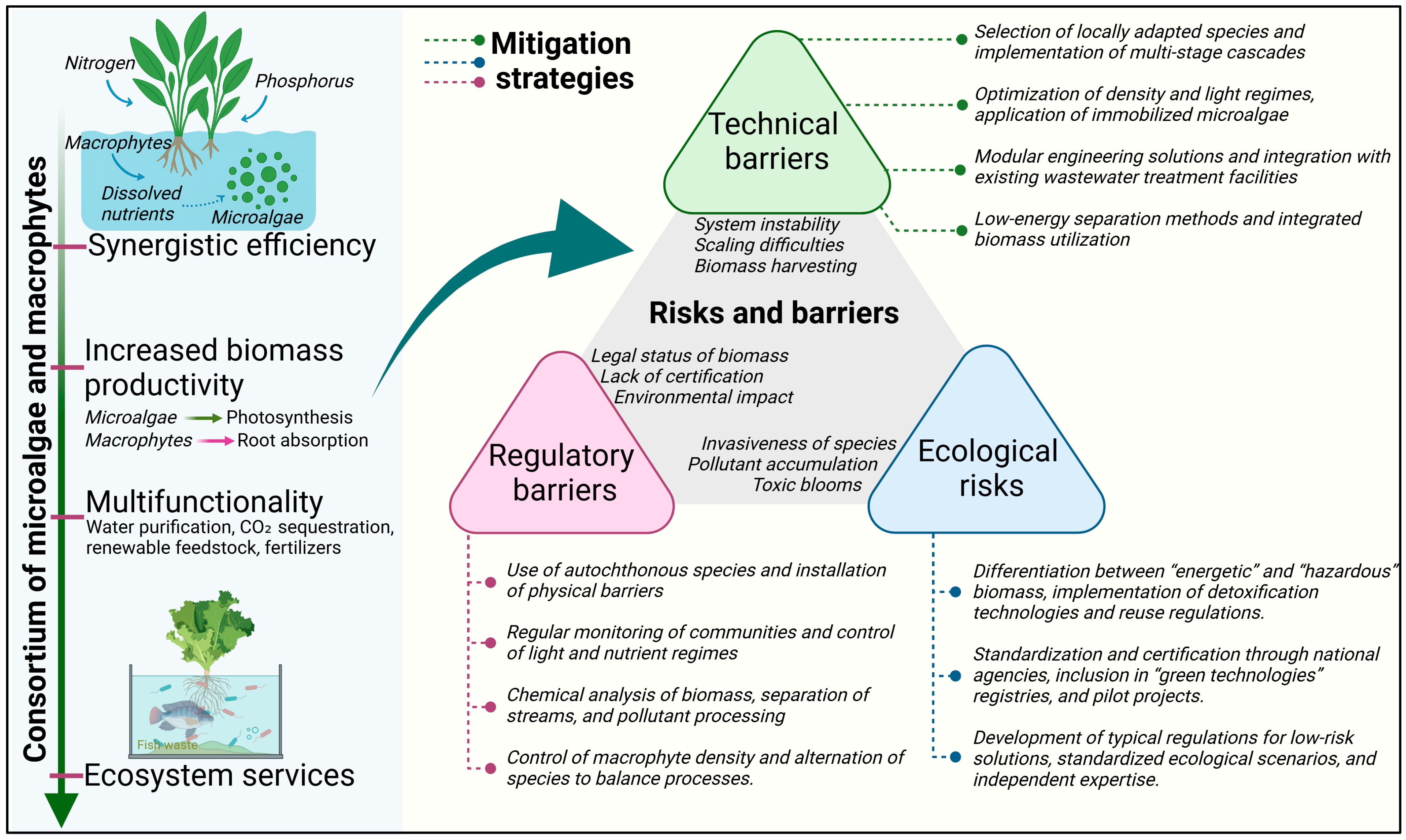
4. Challenges and Opportunities of Phyto-Algal Consortia
4.1. Contemporary Engineering Solutions and Scale-Up Pathways
4.2. Barriers to Scale-Up and Enabling Measures
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CBE HGT | circular bioeconomics horizontal gene transfer |
| HRAP | high-rate algal pond |
| HRT | hydraulic retention time |
| LCA | life cycle assessment |
| MP | metabolic passport (of biomass) |
| MA | microalgae |
| PBR | photobioreactor |
| SDGs | United Nations Sustainable Development Goals |
| SOD | superoxide dismutase |
| MDA | malondialdehyde |
| TCA | tricarboxylic acid cycle |
| TEA | techno-economic analysis |
References
- Daripa, A.; Malav, L.C.; Yadav, D.K.; Chattaraj, S. Metal Contamination in Water Resources Due to Various Anthropogenic Activities. In Metals in Water; Elsevier: Amsterdam, The Netherlands, 2023; pp. 111–127. [Google Scholar] [CrossRef]
- Goala, M.; Bachheti, A.; Kumar, V. A Comprehensive Review of Recent Advances in Phytoremediation of Wastewaters Using Azolla Species. 3 Biotech 2025, 15, 238. [Google Scholar] [CrossRef] [PubMed]
- Ogidi, O.I.; Akpan, U.M. Aquatic Biodiversity Loss: Impacts of Pollution and Anthropogenic Activities and Strategies for Conservation. In Sustainable Development and Biodiversity; Springer Nature: Singapore, 2022; pp. 421–448. [Google Scholar] [CrossRef]
- Akhtar, N.; Ishak, M.I.S.; Bhawani, S.A.; Umar, K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Sayanthan, S.; Hasan, H.A.; Abdullah, S.R.S. Floating Aquatic Macrophytes in Wastewater Treatment: Toward a Circular Economy. Water 2024, 16, 870. [Google Scholar] [CrossRef]
- Chen, N.; Usman, M. Energy Use, Energy Depletion, and Environmental Degradation: Exploitation of Natural Resources. Nat. Resour. Forum 2025. [Google Scholar] [CrossRef]
- Mahapatra, S.; Kumar, D.; Singh, B.; Sachan, P.K. Biofuels and Their Sources of Production: A Review on Cleaner Sustainable Alternative against Conventional Fuel, in the Framework of the Food and Energy Nexus. Energy Nexus 2021, 4, 100036. [Google Scholar] [CrossRef]
- Rehman, A.; Alam, M.M.; Ozturk, I.; Alvarado, R.; Murshed, M.; Işık, C.; Ma, H. Globalization and Renewable Energy Use: How Are They Contributing to Upsurge the CO2 Emissions? A Global Perspective. Environ. Sci. Pollut. Res. 2022, 30, 9699–9712. [Google Scholar] [CrossRef]
- Pandey, K.D.; Singh, S.K.; Shukla, L.; Rai, V.K.; Singh, R.P.; Yadav, P.; Gupta, R.K.; Singh, P.K.; Kaushalendra, N.; Kumar, A. Mechanistic Approaches and Factors Regulating Microalgae Mediated Heavy Metal Remediation from the Aquatic Ecosystem. In Advances in Chemical Pollution, Environmental Management and Protection; Elsevier: Amsterdam, The Netherlands, 2022; pp. 285–299. [Google Scholar] [CrossRef]
- Ahmad, F.; Manefield, M. Photosystem Modulation and Extracellular Silicification in Green Microalgae: Key Strategies for Lead Tolerance and Removal. Heliyon 2024, 10, e36366. [Google Scholar] [CrossRef]
- Kirbayeva, D.K.; Shayakhmetova, A.Y.; Kossalbayev, B.D.; Sadvakasova, A.K.; Bauenova, M.O. Harnessing MicroRNAs and CRISPR to Enhance Biofuel Production in Microalgae. Int. J. Hydrogen Energy 2025, 157, 150399. [Google Scholar] [CrossRef]
- Abdi, G.; Barwant, M.M.; Behera, M.; Singh, L. Phyco-Application for the Treatment of Industrial Wastewater. In Wastewater Treatment Through Nature-Based Solutions; Dwivedi, N., Dwivedi, S., Mishra, R.K., Eds.; Springer Nature: Singapore, 2025; pp. 139–156. [Google Scholar] [CrossRef]
- Anand, S.; Bharti, S.K.; Dviwedi, N.; Barman, S.C.; Kumar, N. Macrophytes for the Reclamation of Degraded Waterbodies with Potential for Bioenergy Production; Springer: Singapore, 2017; pp. 333–351. [Google Scholar] [CrossRef]
- Walters, C.; Steyn, M.; Ndlela, L.; Nocanda, X.; Moloi, M.; Oberholster, P. Phycoremediation of Industrial Wastewater: Review of Algae Consortia. Int. J. Environ. Sci. Technol. 2024, 22, 6209–6224. [Google Scholar] [CrossRef]
- Sameena, P.P.; Janeeshma, E.; Sarath, N.G.; Puthur, J.T. Phytoremediation and Phycoremediation: A Sustainable Solution for Wastewater Treatment; Springer: Cham, Switzerland, 2022; pp. 171–191. [Google Scholar] [CrossRef]
- Ahila, K.G.; Ravindran, B.; Muthunarayanan, V.; Nguyen, D.D.; Nguyen, X.C.; Chang, S.W.; Nguyen, V.K.; Thamaraiselvi, C. Phytoremediation Potential of Freshwater Macrophytes for Treating Dye-Containing Wastewater. Sustainability 2020, 13, 329. [Google Scholar] [CrossRef]
- Mandal, R.N.; Bera, P. Macrophytes Used as Multifaceted Benefits Including Feeding, Bioremediation, and Symbiosis in Freshwater Aquaculture—A Review. Rev. Aquac. 2024, 17, e12983. [Google Scholar] [CrossRef]
- Justin, L.D.; Olukanni, D.O.; Babaremu, K.O. Performance Assessment of Local Aquatic Macrophytes for Domestic Wastewater Treatment in Nigerian Communities: A Review. Heliyon 2022, 8, e10093. [Google Scholar] [CrossRef]
- Singh, P.; Singh, G.; Singh, A.; Mishra, V.K.; Shukla, R. Macrophytes for Utilization in Constructed Wetland as Efficient Species for Phytoremediation of Emerging Contaminants from Wastewater. Wetlands 2024, 44, 22. [Google Scholar] [CrossRef]
- Anyanwu, I.N.; Okeke, C.S.; Nwankwo, S.C.; Nwachukwu, M.O.; Michael, M.O.; Opara, V.C.; Anorue, C.O.; Azuama, O.C.; Oti, P.O.; Ekechukwu, L.E.; et al. Aquatic Macrophytes (Spirogyra porticalis and Nymphaea, L.) as Substrates for Biofuel Production: Potentials and Challenges. Sci. Afr. 2022, 18, e01412. [Google Scholar] [CrossRef]
- Sayago, U.F.C.; Gómez-Caicedo, M.I.; Mercado Suárez, Á.L. Design of a Sustainable System for Wastewater Treatment and Generation of Biofuels Based on the Biomass of the Aquatic Plant Eichhornia crassipes. Sci. Rep. 2024, 14, 11068. [Google Scholar] [CrossRef]
- Romanowska-Duda, Z.; Piotrowski, K.; Szufa, S.; Sklodowska, M.; Naliwajski, M.; Emmanouil, C.; Kungolos, A.; Zorpas, A.A. Valorization of Spirodela polyrrhiza Biomass for the Production of Biofuels for Distributed Energy. Sci. Rep. 2023, 13, 16533. [Google Scholar] [CrossRef]
- Fang, W.-T.; Hsu, C.-H.; LePage, B. Bioremediation and Biofuel Production Using Microalgae. In Wetlands for Remediation in the Tropics; De Magalhães, T.L., Otte, M.L., Eds.; Wetlands: Ecology, Conservation and Management; Springer: Cham, Switzerland, 2023; Volume 9, pp. 155–174. [Google Scholar] [CrossRef]
- Yazvenko, S. Phycoremediation Using Freshwater Algae and Safe Ecology. In Sustainable Remediation for Pollution and Climate Resilience; Abdel Latef, A.A.H., Zayed, E.M., Omar, A.A., Eds.; Springer Nature: Singapore, 2025; pp. 261–292. [Google Scholar] [CrossRef]
- Kaloudas, D.; Pavlova, N.; Penchovsky, R. Phycoremediation of Wastewater by Microalgae: A Review. Environ. Chem. Lett. 2021, 19, 2905–2920. [Google Scholar] [CrossRef]
- Rude, K.; Yothers, C.; Barzee, T.J.; Kutney, S.; Zhang, R.; Franz, A. Growth Potential of Microalgae on Ammonia-Rich Anaerobic Digester Effluent for Wastewater Remediation. Algal Res. 2022, 62, 102613. [Google Scholar] [CrossRef]
- Ashraf, N.; Ahmad, F.; Lu, Y. Synergy between Microalgae and Microbiome in Polluted Waters. Trends Microbiol. 2023, 31, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Sadvakasova, A.K.; Bauenova, M.O.; Kossalbayev, B.D.; Zayadan, B.K.; Huang, Z.; Wang, J.; Balouch, H.; Alharby, H.F.; Chang, J.-S.; Allakhverdiev, S.I. Synthetic Algocyanobacterial Consortium as an Alternative to Chemical Fertilizers. Environ. Res. 2023, 233, 116418. [Google Scholar] [CrossRef]
- Soo, A.; Kim, J.; Shon, H.K. Technologies for the Wastewater Circular Economy—A Review. Desalin. Water Treat. 2024, 317, 100205. [Google Scholar] [CrossRef]
- Gil, M.F.; Azzara, N.; Fassolari, M.; Berón, C.M.; Battaglia, M.E. Hormone Released by the Microalgae Neochloris aquatica and Alkalinization Influence Growth of Terrestrial and Floating Aquatic Plants. Plant Physiol. Biochem. 2023, 197, 107635. [Google Scholar] [CrossRef]
- Jayaprada, N.V.T.; Wewalwela, J.J.; Galahitigama, G.A.H.; Pandipperuma, P.A.N.P. Microbial Interactions with Aquatic Plants. In Current Status of Fresh Water Microbiology; Soni, R., Suyal, D.C., Morales-Oyervides, L., Sungh Chauhan, J., Eds.; Springer Nature: Singapore, 2023; pp. 135–160. [Google Scholar] [CrossRef]
- Nygymetova, A.M.; Sadvakasova, A.K.; Zaletova, D.E.; Kossalbayev, B.D.; Bauenova, M.O.; Wang, J.; Huang, Z.; Sarsekeyeva, F.K.; Kirbayeva, D.K.; Allakhverdiev, S.I. Development and Transfer of Microbial Agrobiotechnologies in Contrasting Agrosystems: Experience of Kazakhstan and China. Plants 2025, 14, 2208. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, R.S.A.; Fusaro, T.; Marques, R.Z.; Brito, J.C.M.; Juneau, P.; Gomes, M.P. The Use of Aquatic Macrophytes as a Nature-Based Solution to Prevent Ciprofloxacin Deleterious Effects on Microalgae. Water 2023, 15, 2143. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W.; Zhao, S. Biology, Ecology and Management of Aquatic Macrophytes and Algae (Volume I). Biology 2025, 14, 246. [Google Scholar] [CrossRef]
- Fallahi, A.; Rezvani, F.; Asgharnejad, H.; Khorshidi Nazloo, E.; Hajinajaf, N.; Higgins, B. Interactions of Microalgae-Bacteria Consortia for Nutrient Removal from Wastewater: A Review. Chemosphere 2021, 272, 129878. [Google Scholar] [CrossRef] [PubMed]
- Malla, M.A.; Ansari, F.A.; Bux, F.; Kumari, S. Re-Vitalizing Wastewater: Nutrient Recovery and Carbon Capture through Microbe-Algae Synergy Using Omics-Biology. Environ. Res. 2024, 259, 119439. [Google Scholar] [CrossRef]
- Govaert, L.; Fronhofer, E.A.; Lion, S.; Eizaguirre, C.; Bonte, D.; Egas, M.; Hendry, A.P.; Martins, A.D.B.; Melián, C.J.; Raeymaekers, J.A.M.; et al. Eco-Evolutionary Feedbacks—Theoretical Models and Perspectives. arXiv 2018, arXiv:1806.07633. [Google Scholar] [CrossRef]
- Zhou, S.; Li, W.; He, S. Microalgal Diversity Enhances Water Purification Efficiency in Experimental Microcosms. Front. Ecol. Evol. 2023, 11, 1125743. [Google Scholar] [CrossRef]
- Li, B.; Xing, P.; Wu, Q.L. Synergistic Alleviation of Lake Eutrophication and Carbon Emission by Macrophyte Restoration. Innovation 2025, 6, 101044. [Google Scholar] [CrossRef]
- Koehl, M.A.R.; Daniel, T.L. Hydrodynamic Interactions Between Macroalgae and Their Epibionts. Front. Mar. Sci. 2022, 9, 872960. [Google Scholar] [CrossRef]
- Huang, R.; Liu, W.; Su, J.; Li, S.; Wang, L.; Jeppesen, E.; Zhang, W. Keystone Microalgae Species Determine the Removal Efficiency of Sulfamethoxazole: A Case Study of Chlorella pyrenoidosa and Microalgae Consortia. Front. Plant Sci. 2023, 14, 1193668. [Google Scholar] [CrossRef]
- García-Jiménez, B.; Torres-Bacete, J.; Nogales, J. Metabolic Modelling Approaches for Describing and Engineering Microbial Communities. Comput. Struct. Biotechnol. J. 2021, 19, 226–246. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, Y.; Dai, L.; Yang, A.; Qiao, J. Assembly of Functional Microbial Ecosystems: From Molecular Circuits to Communities. FEMS Microbiol. Rev. 2024, 48, fuae026. [Google Scholar] [CrossRef]
- Omarjee, A.; Taljaard, S.; Adams, J.B.; Chetty, A. The Influence of Macrophytes on Diurnal pH Variability in Subtropical Estuaries: A Mesocosm Study. Estuar. Coast. Shelf Sci. 2025, 312, 109047. [Google Scholar] [CrossRef]
- Martinsen, K.T.; Zak, N.B.; Baastrup-Spohr, L.; Kragh, T.; Sand-Jensen, K. Ecosystem Metabolism and Gradients of Temperature, Oxygen and Dissolved Inorganic Carbon in the Littoral Zone of a Macrophyte-Dominated Lake. J. Geophys. Res. Biogeosci. 2022, 127, e2022JG007193. [Google Scholar] [CrossRef]
- Andersen, M.R.; Kragh, T.; Sand-Jensen, K. Extreme Diel Dissolved Oxygen and Carbon Cycles in Shallow Vegetated Lakes. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171427. [Google Scholar] [CrossRef] [PubMed]
- Semple, A.; Pandhal, J. Engineering the Phycosphere: Fundamental Concepts and Tools for the Bottom-up Design of Microalgal-Bacterial Consortia. Appl. Phycol. 2024, 6, 21–51. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, X.; Zhao, M.; Zheng, X.; Gu, J.; Wang, Z.; Fan, C.; Gu, W. The Status of Research on the Root Exudates of Submerged Plants and Their Effects on Aquatic Organisms. Water 2024, 16, 1920. [Google Scholar] [CrossRef]
- Xia, C.; Yu, D.; Wang, Z.; Xie, D. Stoichiometry Patterns of Leaf Carbon, Nitrogen and Phosphorous in Aquatic Macrophytes in Eastern China. Ecol. Eng. 2014, 70, 406–413. [Google Scholar] [CrossRef]
- Sunda, W.G. Feedback Interactions between Trace Metal Nutrients and Phytoplankton in the Ocean. Front. Microbiol. 2012, 3, 204. [Google Scholar] [CrossRef]
- Tejido-Nuñez, Y.; Aymerich, E.; Sancho, L.; Refardt, D. Co-Cultivation of Microalgae in Aquaculture Water: Interactions, Growth and Nutrient Removal Efficiency at Laboratory- and Pilot-Scale. Algal Res. 2020, 49, 101940. [Google Scholar] [CrossRef]
- Passarge, J.; Hol, S.; Escher, M.; Huisman, J. Competition for Nutrients and Light: Stable Coexistence, Alternative Stable States, or Competitive Exclusion? Ecol. Monogr. 2006, 76, 57–72. [Google Scholar] [CrossRef]
- Abdelaal, M.; Mashaly, I.A.; Srour, D.S.; Dakhil, M.A.; El-Liethy, M.A.; El-Keblawy, A.; El-Barougy, R.F.; Halmy, M.W.A.; El-Sherbeny, G.A. Phytoremediation Perspectives of Seven Aquatic Macrophytes for Removal of Heavy Metals from Polluted Drains in the Nile Delta of Egypt. Biology 2021, 10, 560. [Google Scholar] [CrossRef]
- Rezania, S.; Taib, S.M.; Md Din, M.F.; Dahalan, F.A.; Kamyab, H. Comprehensive Review on Phytotechnology: Heavy Metals Removal by Diverse Aquatic Plants Species from Wastewater. J. Hazard. Mater. 2016, 318, 587–599. [Google Scholar] [CrossRef]
- Hu, H.; Hong, Y. Algal-Bloom Control by Allelopathy of Aquatic Macrophytes—A Review. Front. Environ. Sci. Eng. China 2008, 2, 421–438. [Google Scholar] [CrossRef]
- Nezbrytska, I.; Usenko, O.; Konovets, I.; Leontieva, T.; Abramiuk, I.; Goncharova, M.; Bilous, O. Potential Use of Aquatic Vascular Plants to Control Cyanobacterial Blooms: A Review. Water 2022, 14, 1727. [Google Scholar] [CrossRef]
- Lee, S.M.; Ryu, C.M. Algae as New Kids in the Beneficial Plant Microbiome. Front. Plant Sci. 2021, 12, 599742. [Google Scholar] [CrossRef] [PubMed]
- Wijewardene, L.; Wu, N.; Fohrer, N.; Riis, T. Epiphytic Biofilms in Freshwater and Interactions with Macrophytes: Current Understanding and Future Directions. Aquat. Bot. 2022, 176, 103467. [Google Scholar] [CrossRef]
- Chen, J.; Zang, Y.; Yang, Z.; Qu, T.; Sun, T.; Liang, S.; Zhu, M.; Wang, Y.; Tang, X. Composition and Functional Diversity of Epiphytic Bacterial and Fungal Communities on Marine Macrophytes in an Intertidal Zone. Front. Microbiol. 2022, 13, 839465. [Google Scholar] [CrossRef]
- Wu, X.; Kong, L.; Pan, J.; Feng, Y.; Liu, S. Metagenomic Approaches to Explore the Quorum Sensing-Mediated Interactions Between Algae and Bacteria in Sequence Membrane Photo-Bioreactors. Front. Bioeng. Biotechnol. 2022, 10, 851376. [Google Scholar] [CrossRef]
- Cheng, X.; Li, X.; Tong, M.; Wu, J.; Chan, L.L.; Cai, Z.; Zhou, J. Indole-3-Acetic Acid as a Cross-Talking Molecule in Algal-Bacterial Interactions and a Potential Driving Force in Algal Bloom Formation. Front. Microbiol. 2023, 14, 1236925. [Google Scholar] [CrossRef]
- Nef, C.; Dittami, S.; Kaas, R.; Briand, E.; Noël, C.; Mairet, F.; Garnier, M. Sharing Vitamin B12 between Bacteria and Microalgae Does Not Systematically Occur: Case Study of the Haptophyte Tisochrysis Lutea. Microorganisms 2022, 10, 1337. [Google Scholar] [CrossRef]
- Tsoi, R.; Dai, Z.; You, L. Emerging Strategies for Engineering Microbial Communities. Biotechnol. Adv. 2019, 37, 107372. [Google Scholar] [CrossRef]
- Wang, J.; Aghajani Delavar, M. Modelling Phytoremediation: Concepts, Methods, Challenges and Perspectives. Soil Environ. Health 2024, 2, 100062. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.-J.; Varjani, S.; Lam, S.S.; Allakhverdiev, S.I.; Chang, J.-S. Microalgae-Based Wastewater Treatment—Microalgae-Bacteria Consortia, Multi-Omics Approaches and Algal Stress Response. Sci. Total Environ. 2022, 845, 157110. [Google Scholar] [CrossRef] [PubMed]
- Vancaester, E.; Depuydt, T.; Osuna-Cruz, C.M.; Vandepoele, K.; Battistuzzi, F.U. Comprehensive and Functional Analysis of Horizontal Gene Transfer Events in Diatoms. Mol. Biol. Evol. 2020, 37, 3243–3257. [Google Scholar] [CrossRef]
- Goyal, A. Horizontal Gene Transfer Drives the Evolution of Dependencies in Bacteria. iScience 2022, 25, 104312. [Google Scholar] [CrossRef]
- Ross, I.L.; Le, H.P.; Budiman, S.; Xiong, D.; Hemker, F.; Millen, E.A.; Oey, M.; Hankamer, B. A Cyclical Marker System Enables Indefinite Series of Oligonucleotide-Directed Gene Editing in Chlamydomonas Reinhardtii. Plant Physiol. 2024, 196, 2330–2345. [Google Scholar] [CrossRef] [PubMed]
- Nievergelt, A.P.; Diener, D.R.; Bogdanova, A.; Brown, T.; Pigino, G. Efficient Precision Editing of Endogenous Chlamydomonas Reinhardtii Genes with CRISPR-Cas. Cell Rep. Methods 2023, 3, 100562. [Google Scholar] [CrossRef]
- Battarra, C.; Angstenberger, M.; Bassi, R.; Dall’Osto, L. Efficient DNA-Free Co-Targeting of Nuclear Genes in Chlamydomonas Reinhardtii. Biol. Direct 2024, 19, 108. [Google Scholar] [CrossRef]
- Dhokane, D.; Kancharla, N.; Savarimuthu, A.; Bhadra, B.; Bandyopadhyay, A.; Dasgupta, S. Genome Editing in Chlamydomonas Reinhardtii Using Cas9-gRNA Ribonucleoprotein Complex: A Step-by-Step Guide. Methods Mol. Biol. 2023, 2653, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; You, Y.; Xue, L.; Hao, B. Algal Coexistence Created Jointly by Neutral Competitor and Asymmetrical Competitors in Shallow Aquatic Ecosystems. Ecol. Indic. 2025, 173, 113447. [Google Scholar] [CrossRef]
- Miao, L.; Wang, C.; Adyel, T.M.; Zhao, J.; Yan, N.; Wu, J.; Hou, J. Periphytic Biofilm Formation on Natural and Artificial Substrates: Comparison of Microbial Compositions, Interactions, and Functions. Front. Microbiol. 2021, 12, 684903. [Google Scholar] [CrossRef]
- He, T.; Chen, Y.; Wang, Y.; Peng, Z.; Mou, Y.; Wang, L. Responses of Microbial Community to Heterogeneous Dissolved Organic Nitrogen Constituents in the Hyporheic Zones of Treated Sewage–Dominated Rivers. Microb. Ecol. 2025, 88, 71. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, O.; Wagner, B.; Derlon, N.; Tlili, A. Synthetic Periphyton as a Model System to Understand Species Dynamics in Complex Microbial Freshwater Communities. npj Biofilms Microbiomes 2022, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Sadvakasova, A.K.; Kossalbayev, B.D.; Zaletova, D.; Bauenova, M.O.; Huang, Z.; Zharmukhamedov, S.K.; Shabala, S.; Allakhverdiev, S.I. Combating Phytopathogens by Integration of Metagenomics and Phototrophic Biotechnologies: Toward Sustainable Agricultural Practices. Crit. Rev. Plant Sci. 2025, 44, 70–87. [Google Scholar] [CrossRef]
- Kossalbayev, B.D.; Wei, M.; Wang, J.; Pang, Y.; Lv, M.; Sadvakasova, A.K.; Bauenova, M.O.; Zhang, X.; Zhao, W.; Xu, S.; et al. Growth Promotion of Synthetic Microbial Communities Influenced by the Function, Diversity and Interactions of Their Constituent Strains and Soil Types. World J. Microbiol. Biotechnol. 2025, 41, 181. [Google Scholar] [CrossRef]
- Patel, V.K.; Sahoo, N.K.; Patel, A.K.; Rout, P.K.; Naik, S.N.; Kalra, A. Exploring Microalgae Consortia for Biomass Production: A Synthetic Ecological Engineering Approach Towards Sustainable Production of Biofuel Feedstock. In Algal Biofuels; Gupta, S.K., Malik, A., Bux, F., Eds.; Springer: Cham, Switzerland, 2017; pp. 109–126. [Google Scholar] [CrossRef]
- Bang Truong, H.; Nguyen, T.H.T.; Ba Tran, Q.; Son Lam, V.; Thao Nguyen Nguyen, T.; Cuong Nguyen, X. Algae-Constructed Wetland Integrated System for Wastewater Treatment: A Review. Bioresour. Technol. 2024, 406, 131003. [Google Scholar] [CrossRef]
- Goh, P.S.; Ahmad, N.A.; Lim, J.W.; Liang, Y.Y.; Kang, H.S.; Ismail, A.F.; Arthanareeswaran, G. Microalgae-Enabled Wastewater Remediation and Nutrient Recovery through Membrane Photobioreactors: Recent Achievements and Future Perspective. Membranes 2022, 12, 1094. [Google Scholar] [CrossRef]
- Ciempiel, W.; Czemierska, M.; Wiącek, D.; Szymańska, M.; Jarosz-Wilkołazka, A.; Krzemińska, I. Lead Biosorption and Chemical Composition of Extracellular Polymeric Substances Isolated from Mixotrophic Microalgal Cultures. Sci. Rep. 2025, 15, 9093. [Google Scholar] [CrossRef]
- Soto-Ramírez, R.; Lobos, M.-G.; Córdova, O.; Poirrier, P.; Chamy, R. Effect of Growth Conditions on Cell Wall Composition and Cadmium Adsorption in Chlorella vulgaris: A New Approach to Biosorption Research. J. Hazard. Mater. 2021, 411, 125059. [Google Scholar] [CrossRef]
- Mahlangu, D.; Mphahlele, K.; De Paola, F.; Mthombeni, N.H. Microalgae-Mediated Biosorption for Effective Heavy Metals Removal from Wastewater: A Review. Water 2024, 16, 718. [Google Scholar] [CrossRef]
- Balzano, S.; Sardo, A.; Blasio, M.; Chahine, T.B.; Dell’Anno, F.; Sansone, C.; Brunet, C. Microalgal Metallothioneins and Phytochelatins and Their Potential Use in Bioremediation. Front. Microbiol. 2020, 11, 517. [Google Scholar] [CrossRef]
- Sattayawat, P.; Yunus, I.S.; Noirungsee, N.; Mukjang, N.; Pathom-Aree, W.; Pekkoh, J.; Pumas, C. Synthetic Biology-Based Approaches for Microalgal Bio-Removal of Heavy Metals from Wastewater Effluents. Front. Environ. Sci. 2021, 9, 778260. [Google Scholar] [CrossRef]
- Gaignard, C.; Zissis, G.; Buso, D. Influence of Different Abiotic Factors on Lipid Production by Microalgae—A Review. OCL 2021, 28, 57. [Google Scholar] [CrossRef]
- Yu, B.S.; Pyo, S.; Lee, J.; Han, K. Microalgae: A Multifaceted Catalyst for Sustainable Solutions in Renewable Energy, Food Security, and Environmental Management. Microb. Cell Fact. 2024, 23, 308. [Google Scholar] [CrossRef]
- Calatrava, V.; Gonzalez-Ballester, D.; Dubini, A. Microalgae for Bioremediation: Advances, Challenges, and Public Perception on Genetic Engineering. BMC Plant Biol. 2024, 24, 1261. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M.M.; El-Kassas, H.Y.; Ali, S.S. Microalgae-Based Bioremediation of Refractory Pollutants: An Approach towards Environmental Sustainability. Microb. Cell Fact. 2025, 24, 19. [Google Scholar] [CrossRef] [PubMed]
- Abinandan, S.; Venkateswarlu, K.; Megharaj, M. Phenotypic Changes in Microalgae at Acidic pH Mediate Their Tolerance to Higher Concentrations of Transition Metals. Curr. Res. Microb. Sci. 2021, 2, 100081. [Google Scholar] [CrossRef]
- Bashir, K.M.I.; Lee, H.-J.; Mansoor, S.; Jahn, A.; Cho, M.-G. The Effect of Chromium on Photosynthesis and Lipid Accumulation in Two Chlorophyte Microalgae. Energies 2021, 14, 2260. [Google Scholar] [CrossRef]
- Miazek, K.; Iwanek, W.; Remacle, C.; Richel, A.; Goffin, D. Effect of Metals, Metalloids and Metallic Nanoparticles on Microalgae Growth and Industrial Product Biosynthesis: A Review. Int. J. Mol. Sci. 2015, 16, 23929–23969. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Jenkins, R.O.; Haris, P.I. Extending the Geographic Reach of the Water Hyacinth Plant in Removal of Heavy Metals from a Temperate Northern Hemisphere River. Sci. Rep. 2018, 8, 11071. [Google Scholar] [CrossRef] [PubMed]
- Montes-Rocha, J.A.; Diaz-Torres, R.D.C.; Alonso-Castro, A.J.; Ilizaliturri-Hernández, C.A.; Carrizales-Yáñez, L.; Carranza-Álvarez, C. Determination and Removal of Potentially Toxic Elements by Phragmites australis (Cav.) Trin. ex Steud. (Poaceae) in the Valles River, San Luis Potosí (Central Mexico). Plants 2024, 14, 33. [Google Scholar] [CrossRef]
- Pang, Y.L.; Quek, Y.Y.; Lim, S.; Shuit, S.H. Review on Phytoremediation Potential of Floating Aquatic Plants for Heavy Metals: A Promising Approach. Sustainability 2023, 15, 1290. [Google Scholar] [CrossRef]
- Chitimus, D.; Nedeff, V.; Mosnegutu, E.; Barsan, N.; Irimia, O.; Nedeff, F. Studies on the Accumulation, Translocation, and Enrichment Capacity of Soils and the Plant Species Phragmites australis (Common Reed) with Heavy Metals. Sustainability 2023, 15, 8729. [Google Scholar] [CrossRef]
- Lim, Z.S.; Wong, C.-Y.; Ahmad, S.A.; Puasa, N.A.; Phang, L.Y.; Shaharuddin, N.A.; Merican, F.; Convey, P.; Zulkharnain, A.; Shaari, H.; et al. Harnessing Diesel-Degrading Potential of an Antarctic Microalga from Greenwich Island and Its Physiological Adaptation. Biology 2023, 12, 1142. [Google Scholar] [CrossRef]
- Kyratzopoulou, E.; Kyzaki, N.; Malletzidou, L.; Nerantzis, E.; Kazakis, N.A. The Efficiency of Chlorella vulgaris in Heavy Metal Removal: A Comparative Study of Mono- and Multi-Component Metal Systems. Clean Technol. 2025, 7, 35. [Google Scholar] [CrossRef]
- Kariyawasam, T.; Petkovich, M.; Vriens, B. Diclofenac Degradation by Immobilized Chlamydomonas reinhardtii and Scenedesmus obliquus. MicrobiologyOpen 2024, 13, e70013. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, J.; Xia, A.; Huang, Y.; Zhu, X.; Zhu, X.; Liao, Q. Microalgae Cultivation for Antibiotic Oxytetracycline Wastewater Treatment. Environ. Res. 2022, 214, 113850. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, F.; Li, H.; Zhao, S.; Liu, Q.; Lin, Y.; Wang, G.; Wu, J. Biodegradation of Phenol by Isochrysis galbana Screened from Eight Species of Marine Microalgae: Growth Kinetic Models, Enzyme Analysis and Biodegradation Pathway. J. Appl. Phycol. 2019, 31, 445–455. [Google Scholar] [CrossRef]
- Azizi, S.; Bayat, B.; Tayebati, H.; Hashemi, A.; Pajoum Shariati, F. Nitrate and Phosphate Removal from Treated Wastewater by Chlorella vulgaris under Various Light Regimes within Membrane Flat Plate Photobioreactor. Environ. Prog. Sustain. Energy 2021, 40, e13519. [Google Scholar] [CrossRef]
- Castellanos-Estupiñan, M.; Carrillo-Botello, A.; Rozo-Granados, L.; Becerra-Moreno, D.; García-Martínez, J.; Urbina-Suarez, N.; López-Barrera, G.; Barajas-Solano, A.; Bryan, S.; Zuorro, A. Removal of Nutrients and Pesticides from Agricultural Runoff Using Microalgae and Cyanobacteria. Water 2022, 14, 558. [Google Scholar] [CrossRef]
- Hayyat, M.U.; Nawaz, R.; Irfan, A.; Al-Hussain, S.A.; Aziz, M.; Siddiq, Z.; Ahmad, S.; Zaki, M.E.A. Evaluating the Phytoremediation Potential of Eichhornia crassipes for the Removal of Cr and Li from Synthetic Polluted Water. Int. J. Environ. Res. Public Health 2023, 20, 3512. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Aslam, A.; Qadeer, A.; Javied, S.; Nisar, N.; Hassan, N.; Hussain, A.; Ali, B.; Iqbal, R.; Chaudhary, T.; et al. Domestic Wastewater Treatment by Pistia stratiotes in Constructed Wetland. Sci. Rep. 2024, 14, 7553. [Google Scholar] [CrossRef]
- Fitrisyah, M.R.; Fauzi, A.M.; Yani, M. Bioremediation of Petroleum Contaminated Water Using Oil Spill Dispersant and Lemna minor in Laboratory-Scale Constructed Wetland. Wetl. Ecol. Manag. 2025, 33, 51. [Google Scholar] [CrossRef]
- Guedes-Alonso, R.; Herrera-Melián, J.A.; Sánchez-Suárez, F.; Díaz-Mendoza, V.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Removal of Pharmaceuticals in a Macrophyte Pond-Constructed Wetland System and the Effect of a Low Effluent Recirculation. Water 2022, 14, 2340. [Google Scholar] [CrossRef]
- Herrera-Melián, J.A.; Guedes-Alonso, R.; Tite-Lezcano, J.C.; Santiago, D.E.; Ranieri, E.; Alonso-Bilbao, I. The Effect of Effluent Recirculation in a Full-Scale Constructed Wetland System. Sustainability 2023, 15, 4310. [Google Scholar] [CrossRef]
- Pavlidis, G.; Zotou, I.; Karasali, H.; Marousopoulou, A.; Bariamis, G.; Nalbantis, I.; Tsihrintzis, V.A. Experiments on Pilot-Scale Constructed Floating Wetlands Efficiency in Removing Agrochemicals. Toxics 2022, 10, 790. [Google Scholar] [CrossRef]
- O’Mahoney, R.; Coughlan, N.E.; Walsh, É.; Jansen, M.A.K. Cultivation of Lemna minor on Industry-Derived, Anaerobically Digested, Dairy Processing Wastewater. Plants 2022, 11, 3027. [Google Scholar] [CrossRef]
- Kotoula, D.; Iliopoulou, A.; Irakleous-Palaiologou, E.; Gatidou, G.; Aloupi, M.; Antonopoulou, P.; Fountoulakis, M.S.; Stasinakis, A.S. Municipal Wastewater Treatment by Combining in Series Microalgae Chlorella sorokiniana and Macrophyte Lemna minor: Preliminary Results. J. Clean. Prod. 2020, 271, 122704. [Google Scholar] [CrossRef]
- Krishnaswamy, V.G. Combined Treatment of Synthetic Textile Effluent Using Mixed Azo Dye by Phyto and Phycoremediation. Int. J. Phytoremediat. 2021, 23, 924–936. [Google Scholar] [CrossRef]
- Condori, M.A.M.; Pachapuma, K.A.M.; Chana, M.P.G.; Huillca, O.Q.; Llayqui, N.E.V.; López-Rosales, L.; García-Camacho, F. An Environmentally Sustainable Approach for Raw Whey Treatment through Sequential Cultivation of Macrophytes and Microalgae. Appl. Sci. 2024, 14, 8139. [Google Scholar] [CrossRef]
- Xinjie, W.; Xin, N.; Qilu, C.; Ligen, X.; Yuhua, Z.; Qifa, Z. Vetiver and Dictyosphaerium Sp. Co-Culture for the Removal of Nutrients and Ecological Inactivation of Pathogens in Swine Wastewater. J. Adv. Res. 2019, 20, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Akmukhanova, N.R.; Zayadan, B.K.; Sadvakasova, A.K.; Bolatkhan, K.; Bauenova, M.O. Consortium of Higher Aquatic Plants and Microalgae Designed to Purify Sewage of Heavy Metal Ions. Russ. J. Plant Physiol. 2018, 65, 143–149. [Google Scholar] [CrossRef]
- Braglia, R.; Rugnini, L.; Malizia, S.; Scuderi, F.; Redi, E.L.; Canini, A.; Bruno, L. Exploiting the Potential in Water Cleanup from Metals and Nutrients of Desmodesmus Sp. and Ampelodesmos mauritanicus. Plants 2021, 10, 1461. [Google Scholar] [CrossRef]
- Song, Y.; Hu, Z.; Yang, X.; An, Y.; Lu, Y. Synergistic Microalgae–Duckweed Systems for Enhanced Aquaculture Wastewater Treatment, Biomass Recovery, and CO2 Sequestration: A Novel Approach for Sustainable Resource Recovery. Environ. Res. 2025, 274, 121271. [Google Scholar] [CrossRef]
- Bao, C.; Cao, Y.; Zhao, L.; Li, X.; Zhang, J.; Mao, C. Biofuel Production from Phytoremediated Biomass via Various Conversion Routes: A Review. Energies 2025, 18, 822. [Google Scholar] [CrossRef]
- Su, M.; Dell’Orto, M.; Scaglia, B.; D’Imporzano, G.; Bani, A.; Adani, F. Growth Performance, Biochemical Composition and Nutrient Recovery Ability of Twelve Microalgae Consortia Isolated from Various Local Organic Wastes Grown on Nano-Filtered Pig Slurry. Molecules 2022, 27, 422. [Google Scholar] [CrossRef]
- Maranho, L.T.; Gomes, M.P. Morphophysiological Adaptations of Aquatic Macrophytes in Wetland-Based Sewage Treatment Systems: Strategies for Resilience and Efficiency under Environmental Stress. Plants 2024, 13, 2870. [Google Scholar] [CrossRef]
- Yadav, K.; Vasistha, S.; Nawkarkar, P.; Kumar, S.; Rai, M.P. Algal Biorefinery Culminating Multiple Value-Added Products: Recent Advances, Emerging Trends, Opportunities, and Challenges. 3 Biotech 2022, 12, 244. [Google Scholar] [CrossRef]
- Díaz, V.; Leyva-Díaz, J.C.; Almécija, M.C.; Poyatos, J.M.; Del Mar Muñío, M.; Martín-Pascual, J. Microalgae Bioreactor for Nutrient Removal and Resource Recovery from Wastewater in the Paradigm of Circular Economy. Bioresour. Technol. 2022, 363, 127968. [Google Scholar] [CrossRef]
- Ali, S.S.; El-Sheekh, M.; Manni, A.; Ruiz, H.A.; Elsamahy, T.; Sun, J.; Schagerl, M. Microalgae-Mediated Wastewater Treatment for Biofuels Production: A Comprehensive Review. Microbiol. Res. 2022, 265, 127187. [Google Scholar] [CrossRef]
- Geng, Y.; Shaukat, A.; Azhar, W.; Raza, Q.-U.-A.; Tahir, A.; Abideen, M.Z.U.; Zia, M.A.B.; Bashir, M.A.; Rehim, A. Microalgal Biorefineries: A Systematic Review of Technological Trade-Offs and Innovation Pathways. Biotechnol. Biofuels Bioprod. 2025, 18, 93. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Ashraf, N.; Deng, X.; Yin, D. Crystallizing bacterial extracellular protein reveals paths of silver mineralization for recovery. J. Environ. Chem. Eng. 2025, 13, 119215. [Google Scholar] [CrossRef]
- Greeshma, K.; Kim, H.-S.; Ramanan, R. The Emerging Potential of Natural and Synthetic Algae-Based Microbiomes for Heavy Metal Removal and Recovery from Wastewaters. Environ. Res. 2022, 215, 114238. [Google Scholar] [CrossRef]
- Okeke, E.S.; Ejeromedoghene, O.; Okoye, C.O.; Ezeorba, T.P.C.; Nyaruaba, R.; Ikechukwu, C.K.; Oladipo, A.; Orege, J.I. Microalgae Biorefinery: An Integrated Route for the Sustainable Production of High-Value-Added Products. Energy Convers. Manag. X 2022, 16, 100323. [Google Scholar] [CrossRef]
- De Morais, E.G.; Da Silveira, J.T.; Schüler, L.M.; De Freitas, B.C.B.; Costa, J.A.V.; De Morais, M.G.; Ferrer, I.; Barreira, L. Biomass Valorization via Pyrolysis in Microalgae-Based Wastewater Treatment: Challenges and Opportunities for a Circular Bioeconomy. J. Appl. Phycol. 2023, 35, 2689–2708. [Google Scholar] [CrossRef]
- Harun, I.; Pushiri, H.; Amirul-Aiman, A.J.; Zulkeflee, Z. Invasive Water Hyacinth: Ecology, Impacts and Prospects for the Rural Economy. Plants 2021, 10, 1613. [Google Scholar] [CrossRef]
- Ejileugha, C.; Onyegbule, U.O.; Osuoha, J.O. Use of Additives in Composting Promotes Passivation and Reduction in Bioavailability of Heavy Metals (HMs) in Compost. Rev. Environ. Contam. 2024, 262, 2. [Google Scholar] [CrossRef]
- Gezahegn, A.; Selassie, Y.G.; Agegnehu, G.; Addisu, S.; Mihretie, F.A.; Kohira, Y.; Lewoyehu, M.; Sato, S. Sustainable Weed Management and Soil Enrichment with Water Hyacinth Composting and Mineral Fertilizer Integration. Environ. Chall. 2024, 16, 101007. [Google Scholar] [CrossRef]
- Ortíz-Sánchez, E.; Guillén-Garcés, R.A.; Morales-Arrieta, S.; Ugochukwu Okoye, P.; Olvera-Vargas, H.; Sebastian, P.J.; Arias, D.M. Cultivation of Carbohydrate-Rich Microalgae with Great Settling Properties Using Cooling Tower Wastewater. Environ. Sci. Pollut. Res. 2023, 31, 38999–39014. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Figueroa-Torres, G.M.; Wan Mahmood, W.M.A.; Pittman, J.K.; Theodoropoulos, C. Microalgal Biomass as a Biorefinery Platform for Biobutanol and Biodiesel Production. Biochem. Eng. J. 2020, 153, 107396. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, S.; Choi, H.-G.; Han, S.J. Co-Production of Biodiesel and Bioethanol Using Psychrophilic Microalga Chlamydomonas sp. KNM0029C Isolated from Arctic Sea Ice. Biotechnol. Biofuels 2020, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Onay, M. Investigation of Biobutanol Efficiency of Chlorella sp. Cultivated in Municipal Wastewater. J. Geosci. Environ. Prot. 2018, 6, 40–50. [Google Scholar] [CrossRef]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K.; et al. Microbiological Insights into Anaerobic Digestion for Biogas, Hydrogen or Volatile Fatty Acids (VFAs): A Review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef]
- Czekała, W.; Jasiński, T.; Grzelak, M.; Witaszek, K.; Dach, J. Biogas Plant Operation: Digestate as the Valuable Product. Energies 2022, 15, 8275. [Google Scholar] [CrossRef]
- Eusébio, A.; Santos, C.A.; Marques, I.P. Anaerobic Digestion of Microalga Chlorella protothecoides and Metagenomic Analysis of Reddish-Colored Digestate. Appl. Sci. 2023, 13, 3325. [Google Scholar] [CrossRef]
- Tonon, G.; Magnus, B.S.; Mohedano, R.A.; Leite, W.R.M.; da Costa, R.H.R.; Filho, P.B. Pre-Treatment of Duckweed Biomass, Obtained from Wastewater Treatment Ponds, for Biogas Production. Waste Biomass Valor. 2017, 8, 2363–2369. [Google Scholar] [CrossRef]
- Kaur, M.; Srikanth, S.; Kumar, M.; Sachdeva, S.; Puri, S.K. An integrated approach for efficient conversion of Lemna minor to biogas. Energy Convers. Manag. 2019, 180, 25–35. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Bolognesi, S.; Bernardi, G.; Callegari, A.; Dondi, D.; Capodaglio, A.G. Biochar Production from Sewage Sludge and Microalgae Mixtures: Properties, Sustainability and Possible Role in Circular Economy. Biomass Conv. Bioref. 2021, 11, 289–299. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, F.; Wang, H.; Ho, S.-H. The Magic of Algae-Based Biochar: Advantages, Preparation, and Applications. Bioengineered 2023, 14, 2252157. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Chen, W.-H.; Kamyab, H.; Kumar, G.; Al-Muhtaseb, A.H.; Ngamcharussrivichai, C. Cultivation of Microalgae Chlorella sp. in Municipal Sewage for Biofuel Production and Utilization of Biochar Derived from Residue for the Conversion of Hematite Iron Ore (Fe2O3) to Iron (Fe)—Integrated Algal Biorefinery. Energy 2019, 189, 116128. [Google Scholar] [CrossRef]
- Kamshybayeva, G.; Kossalbayev, B.; Huang, Z.; Bauenova, M.O.; Allakhverdiev, S.I. Hydrogen Production with a Newly Discovered Cyanobacteria Strain. BIO Web Conf. 2024, 100, 02022. [Google Scholar] [CrossRef]
- Giang, T.T.; Lunprom, S.; Liao, Q.; Reungsang, A.; Salakkam, A. Improvement of Hydrogen Production from Chlorella sp. Biomass by Acid-Thermal Pretreatment. PeerJ 2019, 7, e6637. [Google Scholar] [CrossRef] [PubMed]
- Satheesh, S.; Pugazhendi, A.; Al-Mur, B.A.; Balasubramani, R. Biohydrogen Production Coupled with Wastewater Treatment Using Selected Microalgae. Chemosphere 2023, 334, 138932. [Google Scholar] [CrossRef]
- Leong, Y.K.; Su, R.-H.; Varjani, S.; Lee, D.-J.; Chang, J.-S. Integrated Biohydrogen Production and Dairy Manure Wastewater Treatment via a Microalgae Platform. Int. J. Hydrogen Energy 2024, 52, 404–417. [Google Scholar] [CrossRef]
- Nowicka, B. Heavy Metal–Induced Stress in Eukaryotic Algae—Mechanisms of Heavy Metal Toxicity and Tolerance with Particular Emphasis on Oxidative Stress in Exposed Cells and the Role of Antioxidant Response. Environ. Sci. Pollut. Res. 2022, 29, 16860–16911. [Google Scholar] [CrossRef]
- Longo, S.; Cellura, M.; Luu, L.Q.; Nguyen, T.Q.; Rincione, R.; Guarino, F. Circular Economy and Life Cycle Thinking Applied to the Biomass Supply Chain: A Review. Renew. Energy 2024, 220, 119598. [Google Scholar] [CrossRef]
- Nour, A.H.; Bin Mokaizh, A.A.; Alazaiza, M.Y.D.; Bashir, M.J.K.; Mustafa, S.E. Innovative Strategies for Microalgae-Based Bioproduct Extraction in Biorefineries: Current Trends and Future Solutions Integrating Wastewater Treatment. Sustainability 2024, 16, 10565. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Ahmad, A.; Said, N.S.M.; Imron, M.F.; Abdullah, S.R.S.; Othman, A.R.; Purwanti, I.F.; Abu Hasan, H. Macrophytes as Wastewater Treatment Agents: Nutrient Uptake and Potential of Produced Biomass Utilization toward Circular Economy Initiatives. Sci. Total Environ. 2021, 790, 148219. [Google Scholar] [CrossRef]
- Do Nascimento Filho, S.L.; Gama, W.A.; Do Nascimento Moura, A. Effect of the Structural Complexity of Aquatic Macrophytes on Epiphytic Algal, Macroinvertebrates, and Their Interspecific Relationships. Aquat. Sci. 2021, 83, 57. [Google Scholar] [CrossRef]
- Thomaz, S.M. Ecosystem Services Provided by Freshwater Macrophytes. Hydrobiologia 2023, 850, 2757–2777. [Google Scholar] [CrossRef]
- Khellaf, N.; Djelal, H.; Amrane, A. An Overview of the Valorization of Aquatic Plants in Effluent Depuration through Phytoremediation Processes. Appl. Microbiol. 2022, 2, 309–318. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, W.; Yang, J.; Zhao, X.; Chen, Y.; Yao, L.; Hou, H. Enhanced Biomass Production and Pollutant Removal by Duckweed in Mixotrophic Conditions. Bioresour. Technol. 2020, 317, 124029. [Google Scholar] [CrossRef]
- Amulya, K.; Morris, S.; Lens, P.N.L. Aquatic Biomass as Sustainable Feedstock for Biorefineries. Biofuels Bioprod. Bioref. 2023, 17, 1012–1029. [Google Scholar] [CrossRef]
- Mohanty, M. Remediation of Heavy Metals by Different Aquatic Macrophytes. In Aquatic Macrophytes: Ecology, Functions and Services; Kumar, S., Bauddh, K., Singh, R., Kumar, N., Kumar, R., Eds.; Springer Nature: Singapore, 2023; pp. 207–219. [Google Scholar] [CrossRef]
- Ali, H.H.; Fayed, M.I.A.; Lazim, I.I. Use of Aquatic Plants in Removing Pollutants and Treating the Wastewater: A Review. J. Glob. Innov. Agric. Sci. 2022, 10, 61–70. [Google Scholar] [CrossRef]
- Ali, S.S.; Hassan, L.H.S.; El-Sheekh, M. Microalgae-Mediated Bioremediation: Current Trends and Opportunities—A Review. Arch. Microbiol. 2024, 206, 343. [Google Scholar] [CrossRef]
- Allakhverdiev, E.S.; Kossalbayev, B.D.; Sadvakasova, A.K.; Bauenova, M.O.; Belkozhayev, A.M.; Rodnenkov, O.V.; Martynyuk, T.V.; Maksimov, G.V.; Allakhverdiev, S.I. Spectral Insights: Navigating the Frontiers of Biomedical and Microbiological Exploration with Raman Spectroscopy. J. Photochem. Photobiol. B Biol. 2024, 252, 112870. [Google Scholar] [CrossRef]
- Geremia, E.; Ripa, M.; Catone, C.M.; Ulgiati, S. A Review about Microalgae Wastewater Treatment for Bioremediation and Biomass Production—A New Challenge for Europe. Environments 2021, 8, 136. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Manickam, P. Phycoremediation of Industrial Wastewater: Challenges and Prospects. In Bioremediation for Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2021; pp. 99–123. [Google Scholar] [CrossRef]
- Moghiseh, Z.; Almasi, F. Metabolic Activity and Pathway Study of Emerging Contaminants Biodegradation Using a Photo-Bioelectrochemical System: A Review. 3 Biotech 2025, 15, 173. [Google Scholar] [CrossRef]
- Kumar, N.; Sharma, G.; Chandel, H.; Shyam, K.; Thakur, S.; Vaswani, P.; Saxena, G. Microalgae in Wastewater Treatment and Biofuel Production: Recent Advances, Challenges, and Future Prospects. In Omics Insights in Environmental Bioremediation; Springer Nature: Singapore, 2022; pp. 237–271. [Google Scholar] [CrossRef]
- Al-Jabri, H.; Das, P.; Khan, S.; Thaher, M.; AbdulQuadir, M. Treatment of Wastewaters by Microalgae and the Potential Applications of the Produced Biomass—A Review. Water 2020, 13, 27. [Google Scholar] [CrossRef]
- Kalwani, M.; Devi, A.; Patil, K.; Kumari, A.; Dalvi, V.; Malik, A.; Tyagi, A.; Shukla, P.; Pabbi, S. Microalgae-Mediated Wastewater Treatment and Enrichment of Wastewater-Cultivated Biomass for Biofuel Production. In Expanding Horizon of Cyanobacterial Biology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 259–281. [Google Scholar] [CrossRef]
- Olabi, A.; Shehata, N.; Sayed, E.T.; Rodriguez, C.; Anyanwu, R.C.; Russell, C.; Abdelkareem, M.A. Role of Microalgae in Achieving Sustainable Development Goals and Circular Economy. Sci. Total Environ. 2023, 854, 158689. [Google Scholar] [CrossRef]
- Ubando, A.T.; Ng, E.A.S.; Chen, W.-H.; Culaba, A.B.; Kwon, E.E. Life Cycle Assessment of Microalgal Biorefinery: A State-of-the-Art Review. Bioresour. Technol. 2022, 360, 127615. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Zhang, J.; Liu, B.; Xue, H.; Wu, L.; Li, Z. Knowledge Mapping of High-Rate Algal Ponds Research. Water 2023, 15, 1916. [Google Scholar] [CrossRef]
- Lugo, A.; Xu, X.; Abeysiriwardana-Arachchige, I.S.A.; Bandara, G.C.L.; Nirmalakhandan, N.; Xu, P. Techno-Economic Assessment of a Novel Algal-Membrane System versus Conventional Wastewater Treatment and Advanced Potable Reuse Processes: Part II. J. Environ. Manag. 2023, 331, 117189. [Google Scholar] [CrossRef]
- Li, M.; Waite, A.; Wang, S. Piloting Experience of ROTEC’s Flow Reversal RO (FRRO) for 90% Recovery in Brackish Water Desalination. Desalination 2024, 576, 117348. [Google Scholar] [CrossRef]
- Liang, Y.Y. Role of Spacers in Osmotic Membrane Desalination: Advances, Challenges, Practical and Artificial Intelligence-Driven Solutions. Process Saf. Environ. Prot. 2025, 201, 107587. [Google Scholar] [CrossRef]
- Abu Hasan, H.; Muhamad, M.H.; Ji, B.; Nazairi, N.A.; Jiat, K.W.; Sim, S.I.S.W.A.; Poh, A.F.M.S. Revolutionizing Wastewater Treatment with Microalgae: Unveiling Resource Recovery, Mechanisms, Challenges, and Future Possibilities. Ecol. Eng. 2023, 197, 107117. [Google Scholar] [CrossRef]
- Liberti, D.; Pinheiro, F.; Simões, B.; Varela, J.; Barreira, L. Beyond Bioremediation: The Untapped Potential of Microalgae in Wastewater Treatment. Water 2024, 16, 2710. [Google Scholar] [CrossRef]
- Hosny, S.; Elshobary, M.E.; El-Sheekh, M.M. Unleashing the Power of Microalgae: A Pioneering Path to Sustainability and Achieving the Sustainable Development Goals. Environ. Sci. Pollut. Res. 2025, 32, 17312–17342. [Google Scholar] [CrossRef]
- Júnior, J.C.A.B.; de Almeida Silva, M.C.; Hoyos, N.L.M.; Monteggia, L.O. Evaluation of UASB Effluent Post-Treatment in Pilot-Scale by Microalgae HRP and Macrophytes Pond for Nutrient Recovery. J. Clean. Prod. 2022, 357, 131951. [Google Scholar] [CrossRef]
- Li, X.; Wu, S.; Yang, C.; Zeng, G. Microalgal and Duckweed Based Constructed Wetlands for Swine Wastewater Treatment: A Review. Bioresour. Technol. 2020, 318, 123858. [Google Scholar] [CrossRef]
- Khalid, Z.; Alam, S.N.; Singh, B.; Guldhe, A. Prospects of Carbon Capture and Carbon Sequestration Using Microalgae and Macrophytes. In Algae and Aquatic Macrophytes in Cities; Elsevier: Amsterdam, The Netherlands, 2022; pp. 119–134. [Google Scholar] [CrossRef]
- Krzywonos, M.; Romanowska-Duda, Z.; Seruga, P.; Messyasz, B.; Mec, S. The Use of Plants from the Lemnaceae Family for Biofuel Production—A Bibliometric and In-Depth Content Analysis. Energies 2023, 16, 2058. [Google Scholar] [CrossRef]
- Thakur, T.K.; Barya, M.P.; Dutta, J.; Mukherjee, P.; Thakur, A.; Swamy, S.L.; Anderson, J.T. Integrated Phytobial Remediation of Dissolved Pollutants from Domestic Wastewater through Constructed Wetlands: An Interactive Macrophyte–Microbe-Based Green and Low-Cost Decontamination Technology with Prospective Resource Recovery. Water 2023, 15, 3877. [Google Scholar] [CrossRef]
| Pollutant/Feedstock | Consortium Composition | Removal Efficiency (Approx.) | Key Mechanisms and Notes | Ref. |
|---|---|---|---|---|
| Municipal wastewater (COD, NH4–N, TKN, PO4–P) | Chlorella sorokiniana (UTEX 1230) + Lemna minor | COD—99%; NH4–N—90%; TKN—88%; PO4–P—91% | Sequential reactors: microalgae grow on raw influent and remove carbon and phosphate; duckweed polishes ammonium/TKN; hetero/mixotrophic uptake, nitrification–denitrification, NH3 stripping, Ca/Mg-phosphate precipitation; stable operation with high biomass yields | [111] |
| Textile wastewater (azo dyes; COD, BOD5, TDS) | Pistia stratiotes/ Chlorella vulgaris | Pistia: decolorization 84; COD—61; BOD5—71.9; TDS—72. Chlorella: decolorization 99 (batch)/74 (scale-up); phytotoxicity ↓ (seed germination 80% vs. 30% untreated) | Pistia: adsorption/biosorption on roots and biofilms, enzymatic azo-group reduction; rhizosphere co-biodegradation. Chlorella: biosorption + intracellular enzymatic breakdown; photosynthetically induced coagulation/precipitation at elevated pH | [112] |
| Cheese-making wastewater | Lemna minor (PBR-1) + Chlorella sp. or Scenedesmus sp. (PBR-2) | COD—60%; NO3-N—90–100%; PO4-P/SO42−: partial at RW—30% → complete at TRW—10% after PBR-2 | Two-stage phyto-algal system: duckweed removes suspended solids and assimilates N/P; microalgae grow on treated effluent and assimilate residual nutrients/organics; sequential design improves carbohydrate/lipid content in biomass and facilitates harvesting | [113] |
| Swine wastewater (NH4–N, P, pathogens) | Vetiveria zizanioides + Dictyosphaerium sp. | NH4–N ↓ to 5 mg L−1 in 13 d (vs. ≥34 days in single-culture controls); P ↓ to 2 mg L−1; DO > 10 mg L−1; Escherichia spp. eliminated | Algal photosynthesis supplies O2 and ROS (pathogen inactivation); plant root respiration acidifies medium and reduces NH3 toxicity; algae deplete HCO3−; joint C/N/P uptake accelerates removal; synergistic growth | [114] |
| Mixed heavy metals + nutrients | Pistia stratiotes/Elodea canadensis + Ankistrodesmus sp., Chlorella vulgaris | Cd—89%; Zn—93%; Cu—82%; Pb—90%; BOD5—93%; nutrients up to 98% | Biosorption/bioaccumulation by plant roots and algal cells; adhesion of algae to macrophyte roots ↑contact/retention; microbial mineralization and co-precipitation; selective metal uptake (Zn > Cu > Cd > Pb) | [115] |
| Excess nutrients and heavy metals (Cu, Ni) | Desmodesmus sp. + Ampelodesmos mauritanicus | N removal: 70% (no metals); 59% (with Ni); <7% (with Cu or Cu/Ni mix). P removal: 90% (no metals); 36–39% (with Ni or Cu); 13% (Ni/Cu mix). Metals: Cu—74%; Ni—85% (single); Cu—59% (mix); Ni in mix ≪ 85% | Rapid adsorption on algal cell walls—intracellular accumulation; nutrient uptake by algae and plant roots; competitive binding favors Cu over Ni; root-zone phytoremediation complements algal removal | [116] |
| Aquaculture wastewater (NO3–N, NH4–N, TN, TP, COD) | Chlorella sp. + Spirodela polyrhiza | NO3-N—91%; NH4-N—99%; TP—100%; TN—92%; COD—95% | Direct N/P uptake by microalgae and duckweed; photosynthetic oxygenation enhances aerobic mineralization; microbial nitrification–denitrification; phosphate sorption/co-precipitation; functionally diverse consortium yields feed/bioenergy-ready biomass | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balouch, H.; Sadvakasova, A.K.; Kossalbayev, B.D.; Bauenova, M.O.; Zaletova, D.E.; Kumarbekuly, S.; Kirbayeva, D.K. Phyto-Algal Consortia as a Complementary System for Wastewater Treatment and Biorefinery. Plants 2025, 14, 3069. https://doi.org/10.3390/plants14193069
Balouch H, Sadvakasova AK, Kossalbayev BD, Bauenova MO, Zaletova DE, Kumarbekuly S, Kirbayeva DK. Phyto-Algal Consortia as a Complementary System for Wastewater Treatment and Biorefinery. Plants. 2025; 14(19):3069. https://doi.org/10.3390/plants14193069
Chicago/Turabian StyleBalouch, Huma, Assemgul K. Sadvakasova, Bekzhan D. Kossalbayev, Meruyert O. Bauenova, Dilnaz E. Zaletova, Sanat Kumarbekuly, and Dariga K. Kirbayeva. 2025. "Phyto-Algal Consortia as a Complementary System for Wastewater Treatment and Biorefinery" Plants 14, no. 19: 3069. https://doi.org/10.3390/plants14193069
APA StyleBalouch, H., Sadvakasova, A. K., Kossalbayev, B. D., Bauenova, M. O., Zaletova, D. E., Kumarbekuly, S., & Kirbayeva, D. K. (2025). Phyto-Algal Consortia as a Complementary System for Wastewater Treatment and Biorefinery. Plants, 14(19), 3069. https://doi.org/10.3390/plants14193069








