Bacillus subtilis Strain TCX1 Isolated from Ambrosia artemisiifolia: Enhancing Cucumber Growth and Biocontrol Against Cucumber Fusarium Wilt
Abstract
1. Introduction
2. Results
2.1. Isolation and Screening of Antifungal Endophytic Bacteria from Ambrosia artemisiifolia
2.1.1. Isolation and Identification of Endophytic Bacteria
2.1.2. Detection and Screening of Potent Antifungal Endophytes from Isolated Strains
2.2. Plant Growth-Promoting Functions of Antifungal Bacillus subtilis Strain TCX1
2.3. Bacillus subtilis Strain TCX1 Demonstrated Stable Colonization in Cucumber Seedlings
2.4. Enhanced Biocontrol of Cucumber Fusarium Wilt by Bacillus subtilis Strain TCX1
2.5. Damaging Activity of Bacillus subtilis Strain TCX1 Against FOC
2.5.1. Bacillus subtilis Strain TCX1 Exhibits Hydrolase Activity and High Likelihood of Producing Lipopeptides
2.5.2. Bacillus subtilis Strain TCX1 Impaired the Cell Membrane of FOC
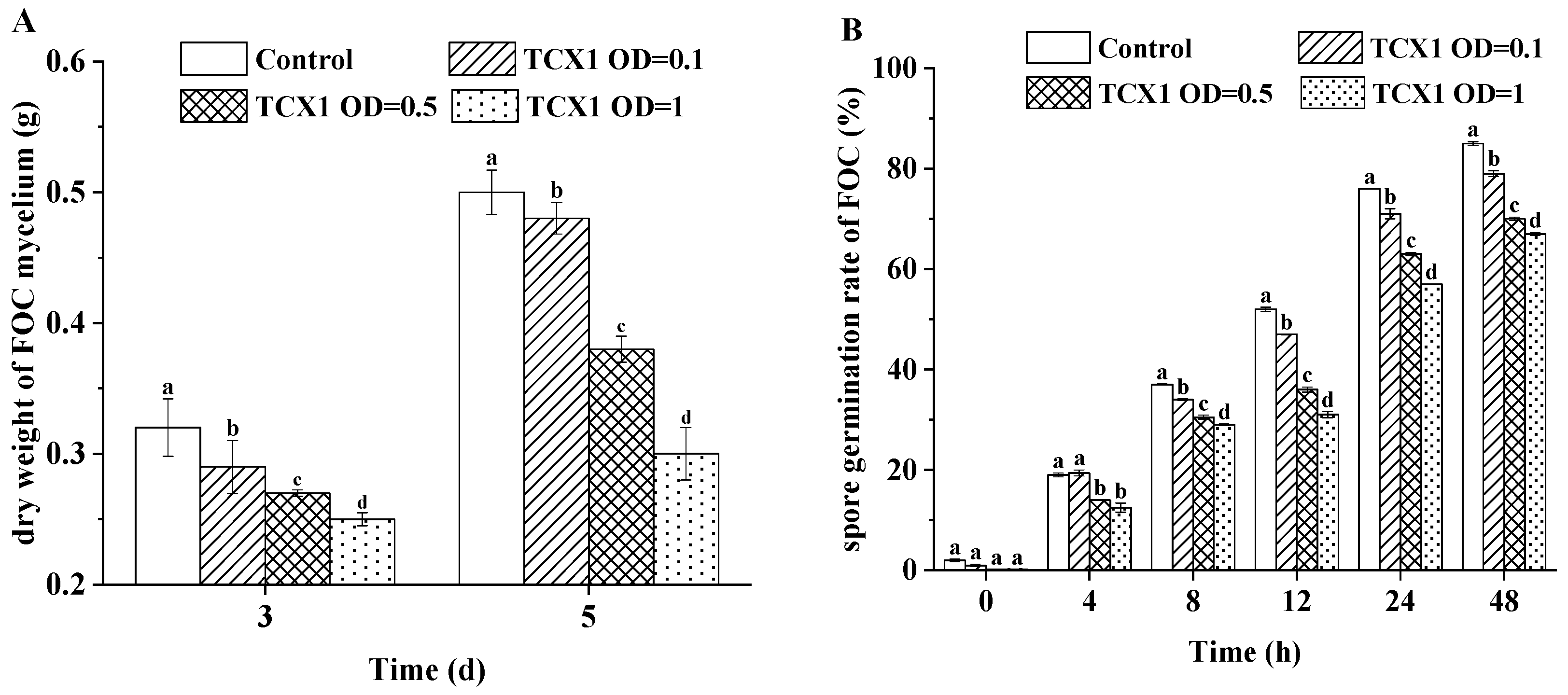
2.5.3. Bacillus subtilis Strain TCX1 Impeded the Growth of FOC Mycelium and the Germination of FOC Spores
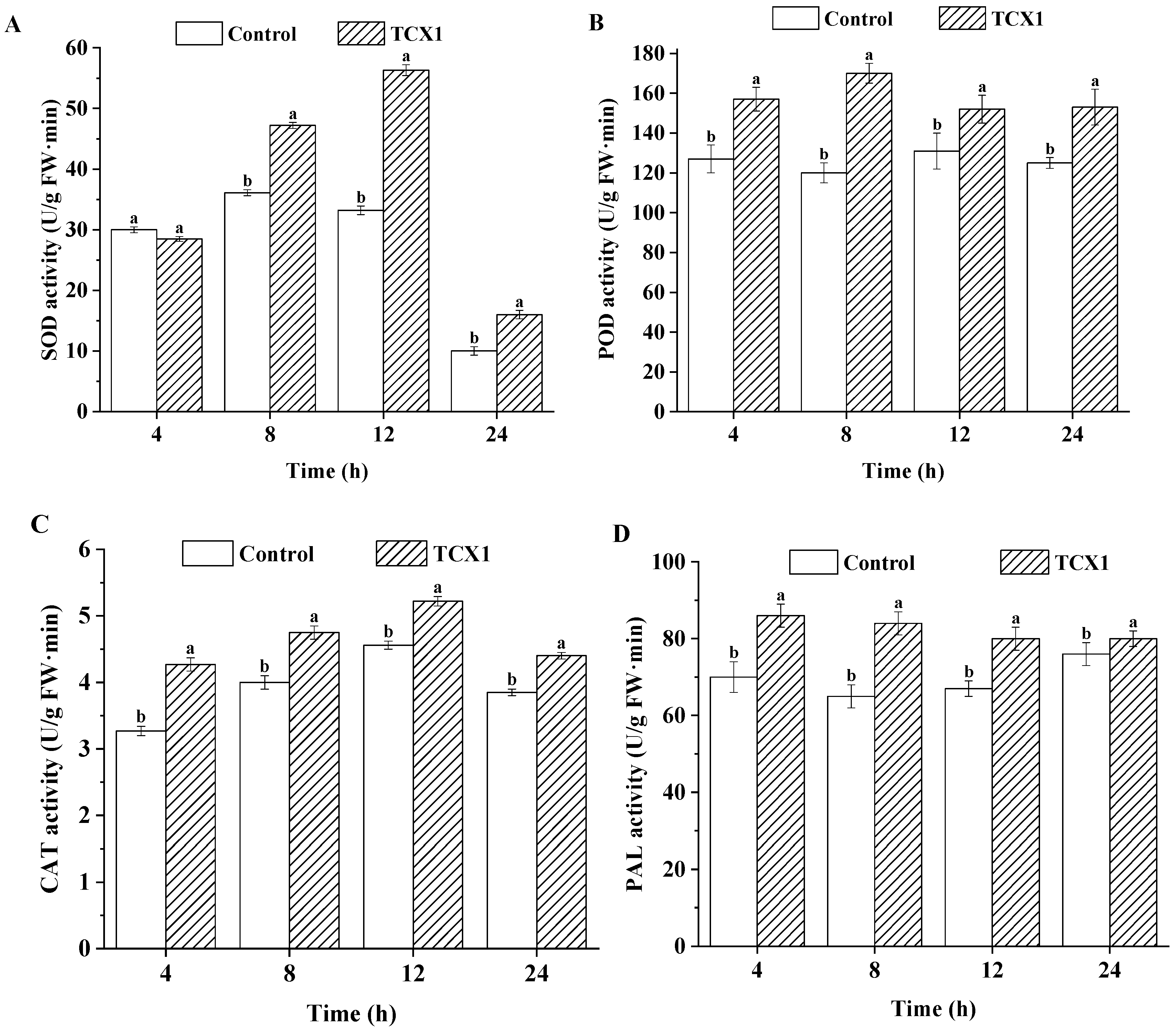
2.5.4. FOC Upregulated Metabolic Enzymes to Counteract the Detrimental Effects of Bacillus subtilis strain TCX1
2.6. Bacillus subtilis Strain TCX1 Induces Cucumber Tolerance to FOC
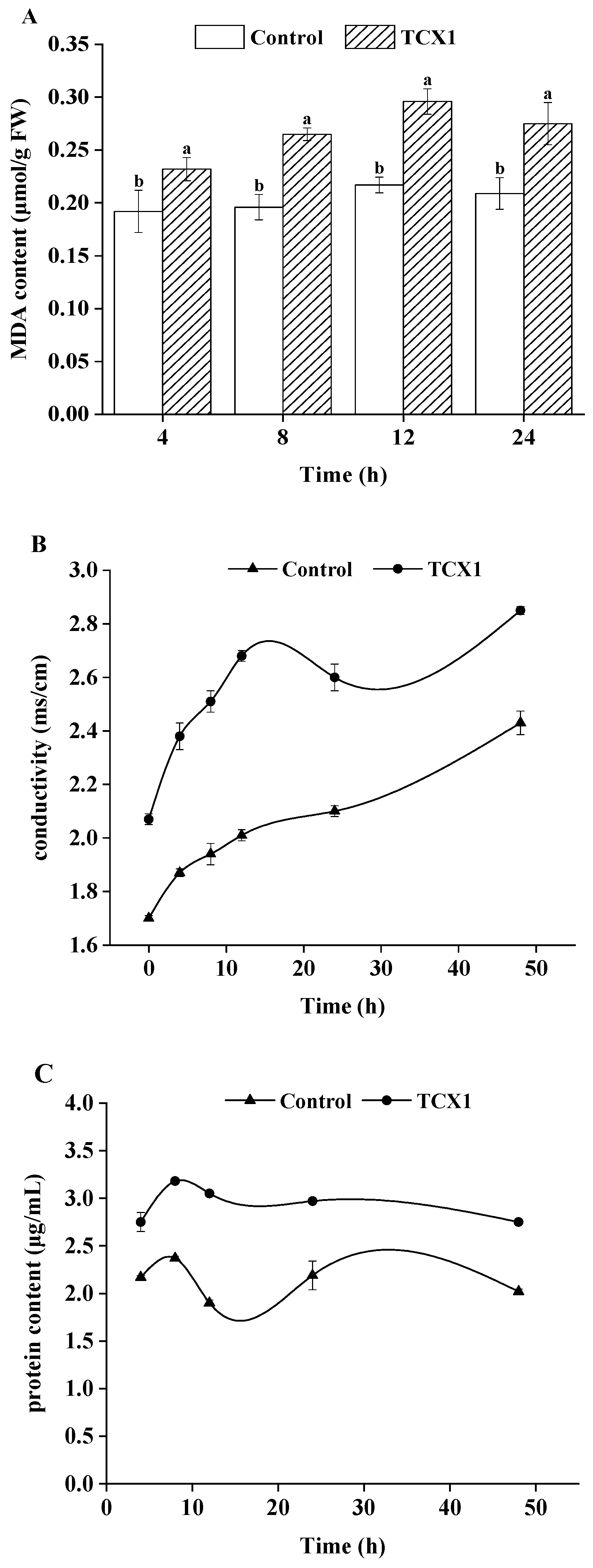
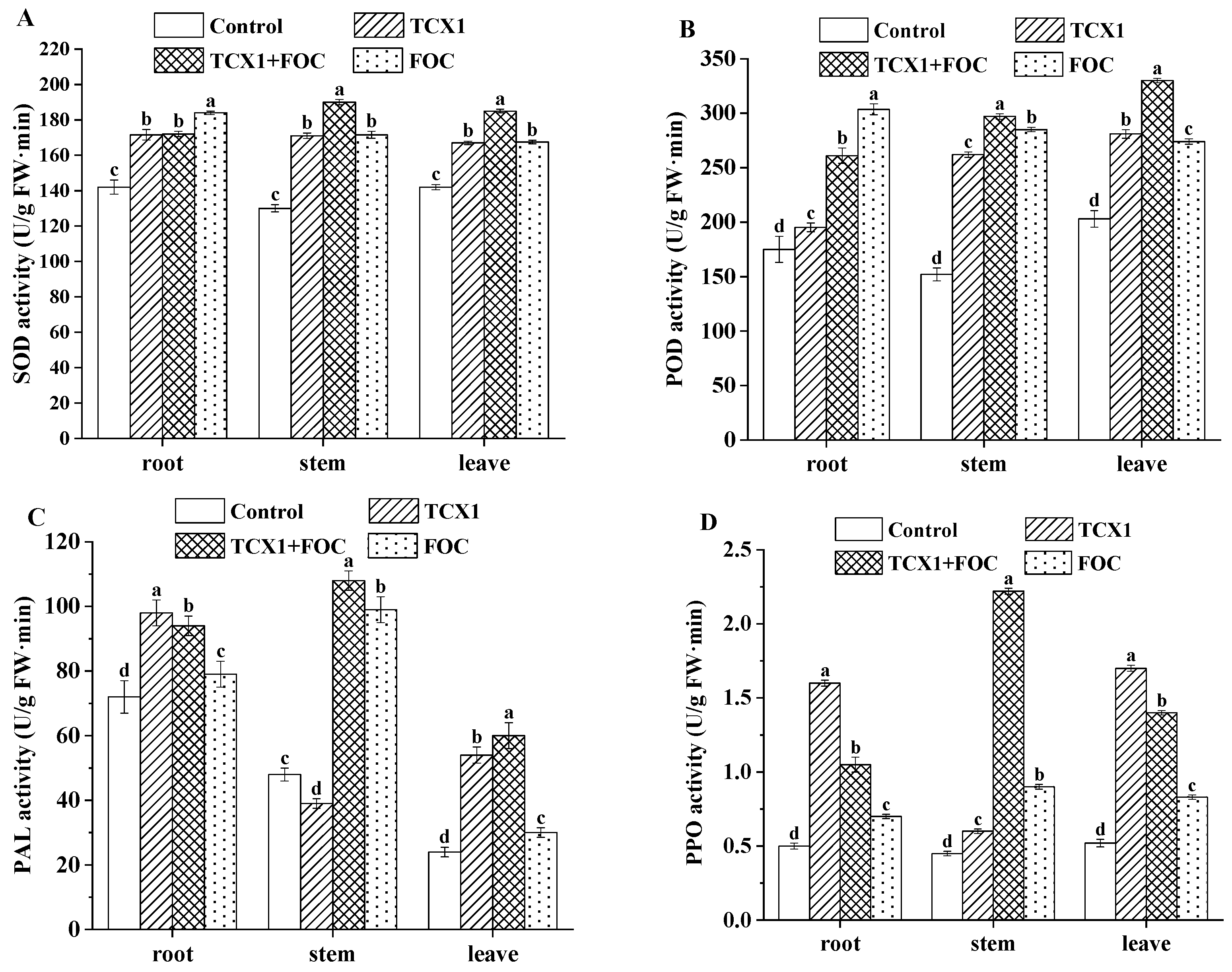
2.6.1. Bacillus subtilis Strain TCX1 Improved Plant Antioxidants Expression in Cucumber
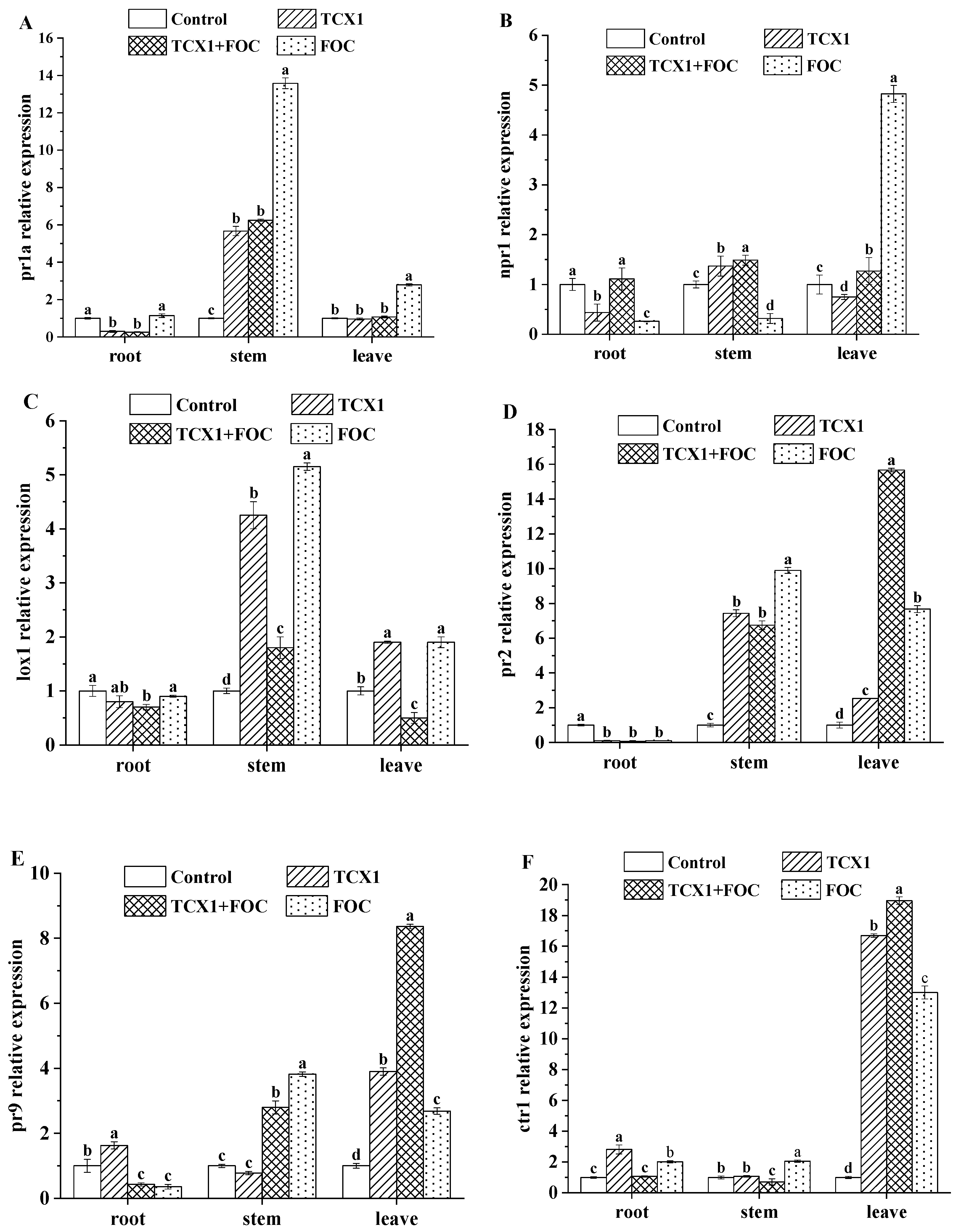
2.6.2. Bacillus subtilis Strain TCX1 Triggered the Induction of ISR and SAR in Cucumber
3. Discussion
4. Materials and Methods
4.1. Endophytic Bacteria Isolation
4.2. Identification of the Endophytic Bacteria by 16S rRNA Sequencing
4.3. Plate Confrontation Method
4.4. Biological Characteristics of the Strain
4.4.1. Phosphate Solubilization
4.4.2. Indole Acetic Acid (IAA) Production
4.4.3. ACC Deaminase Activity
4.4.4. Enzyme Activity Assay of Endophytic Bacteria
4.5. Assessment of Colonization Ability of Bacillus subtilis TCX1
4.6. Greenhouse Pot Experiment
4.7. FOC Mycelium Growth and Spore Germination Assays
4.7.1. Mycelial Growth Inhibition Assay
4.7.2. Spore Germination Inhibition Assay
4.8. FOC Mycelia Membrane Stability Analysis
4.8.1. Assessment of Mycelial Membrane Permeability
4.8.2. Measurement of MDA Content
4.8.3. Determination of Cellular Protein Content
4.9. Determination of Antioxidant Enzyme Activities
4.9.1. Antioxidant Enzyme Activities in FOC Co-Cultured with TCX1
4.9.2. Analysis of Antioxidant Enzyme Activities in Cucumber Seedlings
4.10. Quantitative Real-Time PCR
4.11. Data Processing and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| FOC | Fusarium oxysporum f. sp. cucumerinum |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| POD | Peroxidase |
| PAL | Phenylalnine ammonialyase |
| PPO | Polyphenol oxidase |
| CFU | Colony-forming units |
| OD | Optical density |
| NCBI | National center for biotechnology information |
| BLAST | Basic local alignment search tool |
| MEGA | Molecular evolutionary genetics analysis |
| PDA | Potato dextrose agar medium |
| PBS | Phosphate-buffered saline |
| Rif | Rifampicin |
| IAA | indole-3-acetic acid |
| ACC | 1-amino-cyclopropane-1-carboxylicacid |
| MDA | Malondialdehyde |
| SAR | systemic acquired resistance |
| ISR | Induced systemic resistance |
| PCR | Polymerase chain reaction |
| ROS | reactive oxygen species |
| RSM | response surface methodology |
References
- Krishna, T.P.A.; Veeramuthu, D.; Maharajan, T.; Soosaimanickam, M. The era of plant breeding: Conventional breeding to genomics-assisted breeding for crop improvement. Curr. Genom. 2023, 23, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zhang, J.; Mu, Z.; Wang, Y.; Wen, C.; Wu, T.; Yu, C.; Li, Z.; Wang, H. Recent progress on the molecular breeding of Cucumis sativus L. in China. Theor. Appl. Genet. 2020, 133, 1777–1790. [Google Scholar] [CrossRef]
- He, Y.; Wei, M.; Yan, Y.; Yu, C.; Cheng, S.; Sun, Y.; Zhu, X.; Wei, L.; Wang, H.; Miao, L. Research advances in genetic mechanisms of major cucumber diseases resistance. Front. Plant Sci. 2022, 19, 862486. [Google Scholar] [CrossRef]
- Cohen, R.; Orgil, G.; Burger, Y.; Saar, U.; Elkabetz, M.; Tadmor, Y.; Edelstein, M.; Belausov, E.; Maymon, M.; Freeman, S. Differences in the responses of melon accessions to Fusarium root and stem rot and their colonization by Fusarium oxysporum f. sp radicis-cucumerinum. Plant Pathol. 2015, 64, 655–663. [Google Scholar] [CrossRef]
- Zhao, X.; Hou, D.; Xu, J.; Wang, K.; Hu, Z. Antagonistic activity of fungal strains against Fusarium crown rot. Plants 2022, 19, 255. [Google Scholar] [CrossRef]
- Lal, D.; Dev, D.; Kumari, S.; Pandey, S.; Aparna; Sharma, N.; Nandni, S.; Jha, R.K.; Singh, A. Fusarium wilt pandemic: Current understanding and molecular perspectives. Funct. Integr. Genom. 2024, 24, 41. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.R. Fusarium oxysporum and the Fusarium wilt syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef]
- Raza, W.; Ling, N.; Zhang, R.; Huang, Q.; Xu, Y.; Shen, Q. Success evaluation of the biological control of Fusarium wilts of cucumber, banana, and tomato since 2000 and future research strategies. Crit. Rev. Biotechnol. 2017, 37, 202–212. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Xu, C.; Liu, K.; Bi, H.; Ai, X. H2O2 functions as a downstream signal of IAA to mediate H2S-induced chilling tolerance in cucumber. Int. J. Mol. Sci. 2021, 29, 12910. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Ali, M.S.; Islam, M.N.; Rahman, M.M.; Hasan, M.M.; Baek, K.H. Endophyte mediated biocontrol mechanisms of phytopathogens in agriculture. Res. Microbiol. 2024, 175, 104229. [Google Scholar] [CrossRef]
- Christian, N.; Perlin, M.H. Plant-endophyte communication: Scaling from molecular mechanisms to ecological outcomes. Mycologia 2024, 116, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Abadi, V.A.J.M.; Sepehri, M.; Rahmani, H.A.; Dolatabad, H.K.; Shamshiripour, M.; Khatabi, B. Diversity and abundance of culturable nitrogen-fixing bacteria in the phyllosphere of maize. J. Appl. Microbiol. 2021, 131, 898–912. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Zhang, Y.; Zhou, B.; Wang, Q.; Gu, J.; Liu, G.; Qin, Z.; Li, Z. Changes in antibiotic resistance genes and mobile genetic elements during cattle manure composting after inoculation with Bacillus subtilis. Bioresour. Technol. 2019, 292, 122011. [Google Scholar] [CrossRef]
- Ahemad, M. Phosphate-solubilizing bacteria-assisted phytoremediation of metalliferous soils: A review. 3 Biotech 2015, 5, 111–121. [Google Scholar] [CrossRef]
- Zubair, M.; Hanif, A.; Farzand, A.; Sheikh, T.M.M.; Khan, A.R.; Suleman, M.; Ayaz, M.; Gao, X. Genetic Screening and expression analysis of Psychrophilic Bacillus spp. Reveal their potential to alleviate cold stress and modulate phytohormones in wheat. Microorganisms 2019, 10, 337. [Google Scholar] [CrossRef]
- Gowtham, H.G.; Singh, B.; Murali, M.; Shilpa, N.; Prasad, M.; Aiyaz, M. Induction of drought tolerance in tomato upon the application of ACC deaminase producing plant growth promoting rhizobacterium Bacillus subtilis Rhizo SF 48. Microbiol. Res. 2020, 25, 126422. [Google Scholar]
- Kiesewalter, H.T.; Lozano-Andrade, C.N.; Wibowo, M.; Strube, M.L.; Maróti, G.; Snyder, D.; Jørgensen, T.S.; Larsen, T.O.; Cooper, V.S.; Weber, T.; et al. Genomic and Chemical Diversity of Bacillus subtilis secondary metabolites against plant pathogenic fungi. mSystems 2021, 23, 00770-20. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hao, G.; Zhou, M.; Chen, M.; Ling, H.; Shang, Y. Secondary metabolites of Bacillus subtilis L2 show antiviral activity against pseudorabies virus. Front. Microbiol. 2023, 30, 1277782. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, X.; Wu, X.; Liu, W.; Liu, J. Identification of Gonatophragmium mori Causing Mulberry Zonate Leaf spot disease and characterization of their biological enemies in Guangxi, China. Plant Dis. 2024, 108, 162–174. [Google Scholar] [CrossRef]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 10, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; VanWees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, Z.; Fan, J.; Liang, Y.; Bernier, M.C.; Gao, Y.; Zhao, L.; Opiyo, S.O.; Xia, Y. Bacillus proteolyticus OSUB18 triggers induced systemic resistance against bacterial and fungal pathogens in Arabidopsis. Front. Plant Sci. 2023, 14, 1078100. [Google Scholar] [CrossRef]
- Duan, Y.; Han, M.; Grimm, M.; Schierstaedt, J.; Imani, J.; Cardinale, M.; Le Jean, M.; Nesme, J.; Sørensen, S.J.; Schikora, A. Hordeum vulgare differentiates its response to beneficial bacteria. BMC Plant Biol. 2023, 23, 460. [Google Scholar] [CrossRef] [PubMed]
- Jinal, N.H.; Amaresan, N. Evaluation of biocontrol Bacillus species on plant growth promotion and systemic-induced resistant potential against bacterial and fungal wilt-causing pathogens. Arch. Microbiol. 2020, 202, 1785–1794. [Google Scholar] [CrossRef]
- Yánez-Mendizábal, V.; Falconí, C.E. Bacillus subtilis CtpxS2-1 induces systemic resistance against anthracnose in Andean lupin by lipopeptide production. Biotechnol. Lett. 2021, 43, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.; Anand, V.; Kumar, S.; Verma, I.; Anshu, A.; Pandey, I.A.; Kumar, M.; Behera, S.K.; Srivastava, S.; Singh, P.C. Bacillus subtilis NBRI-W9 simultaneously activates SAR and ISR against Fusarium chlamydosporum NBRI-FOL7 to increase wilt resistance in tomato. J. Appl. Microbiol. 2024, 135, lxae013. [Google Scholar] [CrossRef]
- Montagnani, C.; Gentili, R.; Smith, M.; Guarino, M.F.; Citterio, S. The worldwide spread, success, and impact of ragweed (Ambrosia spp.). Crit. Rev. Plant Sci. 2017, 36, 139–178. [Google Scholar] [CrossRef]
- Kamran, M.; Imran, Q.M.; Ahmed, M.B.; Falak, N.; Khatoon, A.; Yun, B.W. Endophyte-mediated stress tolerance in plants: A sustainable strategy to enhance resilience and assist crop improvement. Cells 2022, 19, 3292. [Google Scholar] [CrossRef]
- Kushwaha, P.; Kashyap, P.L.; Bhardwaj, A.K.; Kuppusamy, P.; Srivastava, A.K.; Tiwari, R.K. Bacterial endophyte mediated plant tolerance to salinity: Growth responses and mechanisms of action. World J. Microbiol. Biotechnol. 2020, 29, 26. [Google Scholar] [CrossRef]
- Khan, A.L.; Hamayun, M.; Kang, S.M.; Kim, Y.H.; Jung, H.Y.; Lee, J.H.; Lee, I.J. Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: An example of Paecilomyces formosus LHL10. BMC Microbiol. 2012, 12, 3. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Yadav, R.; Ramakrishna, W. Bacillus subtilis impact on plant growth, soil health and environment: Dr. Jekyll and Mr. Hyde. J. Appl. Microbiol. 2022, 132, 3543–3562. [Google Scholar] [CrossRef]
- Sharma, M.; Sood, G.; Chauhan, A. Assessment of plant growth promotion potential of endophytic bacterium B. subtilis KU21 isolated from Rosmarinus officinalis. Curr. Microbiol. 2024, 4, 207. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.S.; Mrnka, L.; Lovecká, P.; Frantík, T.; Fenclová, M.; Demnerová, K.; Vosátka, M. Bacterial and fungal endophyte communities in healthy and diseased oilseed rape and their potential for biocontrol of Sclerotinia and Phoma disease. Sci. Rep. 2021, 15, 3810. [Google Scholar] [CrossRef]
- Jensen, C.N.G.; Pang, J.K.Y.; Gottardi, M.; Kračun, S.K.; Svendsen, B.A.; Nielsen, K.F.; Kovács, Á.T.; Moelbak, L.; Fimognari, L.; Husted, S.; et al. Bacillus subtilis promotes plant phosphorus (P) acquisition through P solubilization and stimulation of root and root hair growth. Physiol. Plant 2024, 176, 14338. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira-Paiva, C.A.; Bini, D.; de Sousa, S.M.; Ribeiro, V.P.; Dos Santos, F.C.; de Paula Lana, U.G.; de Souza, F.F.; Gomes, E.A.; Marriel, I.E. Inoculation with Bacillus megaterium CNPMS B119 and Bacillus subtilis CNPMS B2084 improve P-acquisition and maize yield in Brazil. Front. Microbiol. 2024, 26, 1426166. [Google Scholar] [CrossRef]
- Blake, C.; Christensen, M.N.; Kovács, Á.T. Molecular aspects of plant growth promotion and protection by Bacillus subtilis. Mol. Plant Microbe Interact. 2021, 34, 15–25. [Google Scholar] [CrossRef]
- Desoignies, N.; Schramme, F.; Ongena, M.; Legrève, A. Systemic resistance induced by Bacillus lipopeptides in Beta vulgaris reduces infection by the rhizomania disease vector Polymyxa betae. Mol. Plant Pathol. 2013, 14, 416–421. [Google Scholar] [CrossRef]
- Romero, D.; de Vicente, A.; Rakotoaly, R.H.; Dufour, S.E.; Veening, J.W.; Arrebola, E.; Cazorla, F.M.; Kuipers, O.P.; Paquot, M.; Pérez-García, A. The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca. Mol. Plant Microbe Interact. 2007, 20, 430–440. [Google Scholar] [CrossRef]
- Gao, P.; Qin, J.; Li, D.; Zhou, S. Inhibitory effect and possible mechanism of a Pseudomonas strain QBA5 against gray mold on tomato leaves and fruits caused by Botrytis cinerea. PLoS ONE 2018, 10, 0190932. [Google Scholar] [CrossRef]
- Yi, Y.J.; Yin, Y.N.; Yang, Y.A.; Liang, Y.Q.; Shan, Y.T.; Zhang, C.F.; Zhang, Y.R.; Liang, Z.P. Antagonistic activity and mechanism of Bacillus subtilis XZ16-1 suppression of wheat powdery mildew and growth promotion of Wheat. Phytopathology 2022, 112, 2476–2485. [Google Scholar] [CrossRef]
- Rashad, Y.M.; Abdalla, S.A.; Sleem, M.M. Endophytic Bacillus subtilis SR22 triggers defense responses in tomato against rhizoctonia root rot. Plants 2022, 11, 2051. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Fathi Abd Allah, E. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Chowdappa, P.; Kumar, S.M.; Lakshmi, M.J.; Upreti, K. Growth stimulation and induction of systemic resistance in tomato against early and late blight by Bacillus subtilis OTPB1 or Trichoderma harzianum OTPB3. Biol. Control 2013, 65, 109–117. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, S.H.; Balaraju, K.; Park, K.S.; Nam, K.W.; Park, J.W.; Park, K. Growth promotion and induced disease suppression of four vegetable crops by a selected plant growth-promoting rhizobacteria (PGPR) strain Bacillus subtilis 21–1 under two different soil conditions. Acta Physiol. Plant 2014, 36, 1353–1362. [Google Scholar] [CrossRef]
- Yarullina, L.; Kalatskaja, J.; Tsvetkov, V.; Burkhanova, G.; Yalouskaya, N.; Rybinskaya, K.; Zaikina, E.; Cherepanova, E.; Hileuskaya, K.; Nikalaichuk, V. The influence of chitosan derivatives in combination with Bacillus subtilis bacteria on the development of systemic resistance in potato plants with viral infection and drought. Plants 2024, 13, 2210. [Google Scholar] [CrossRef]
- Wang, S.; Ji, B.; Su, X.; Li, H.; Dong, C.; Chen, S.; Zhu, Y.; Feng, W. Isolation of endophytic bacteria from Rehmannia glutinosa Libosch and their potential to promote plant growth. J. Gen. Appl. Microbiol. 2020, 66, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.S.; Lovecká, P.; Mrnka, L.; Vychodilová, A.; Strejček, M.; Fenclová, M.; Demnerová, K. Distinct communities of poplar endophytes on an un-polluted and a risk element-polluted site and their plant growth-promoting potential in vitro. Microb. Ecol. 2018, 75, 955–969. [Google Scholar] [CrossRef]
- Liu, Y.H.; Guo, J.W.; Li, L.; Asem, M.D.; Zhang, Y.G. Endophytic bacteria associated with endangered plant Ferula sinkiangensis K. M. Shen in an arid land: Diversity and plant growth-promoting traits. J. Arid Land 2017, 9, 432–445. [Google Scholar] [CrossRef]
- Płociniczak, T.; Sinkkonen, A.; Romantschuk, M.; Sułowicz, S.; Piotrowska-Seget, Z. Rhizospheric bacterial strain Brevibacterium casei MH8a colonizes plant tissues and enhances Cd, Zn, Cu phytoextraction by white mustard. Front. Plant Sci. 2016, 16, 101. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sha, Y.; Wu, D. Surfactant induces ROS-mediated cell membrane permeabilization for the enhancement of mannatide production. Process. Biochem. 2020, 91, 172–180. [Google Scholar] [CrossRef]
- Li, R.P.; Zhang, H.Y.; Liu, W.M.; Zheng, X. Biocontrol of postharvest gray and blue mold decay of apples with Rhodotorula mucilaginosa and possible mechanisms of action. Int. J. Food. Microbiol. 2011, 146, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Kavian, S.; Safarzadeh, S.; Yasrebi, J. Zinc improves growth and antioxidant enzyme activity in Aloe vera plant under salt stress. S. Afr. J. Bot. 2022, 147, 1221–1229. [Google Scholar] [CrossRef]
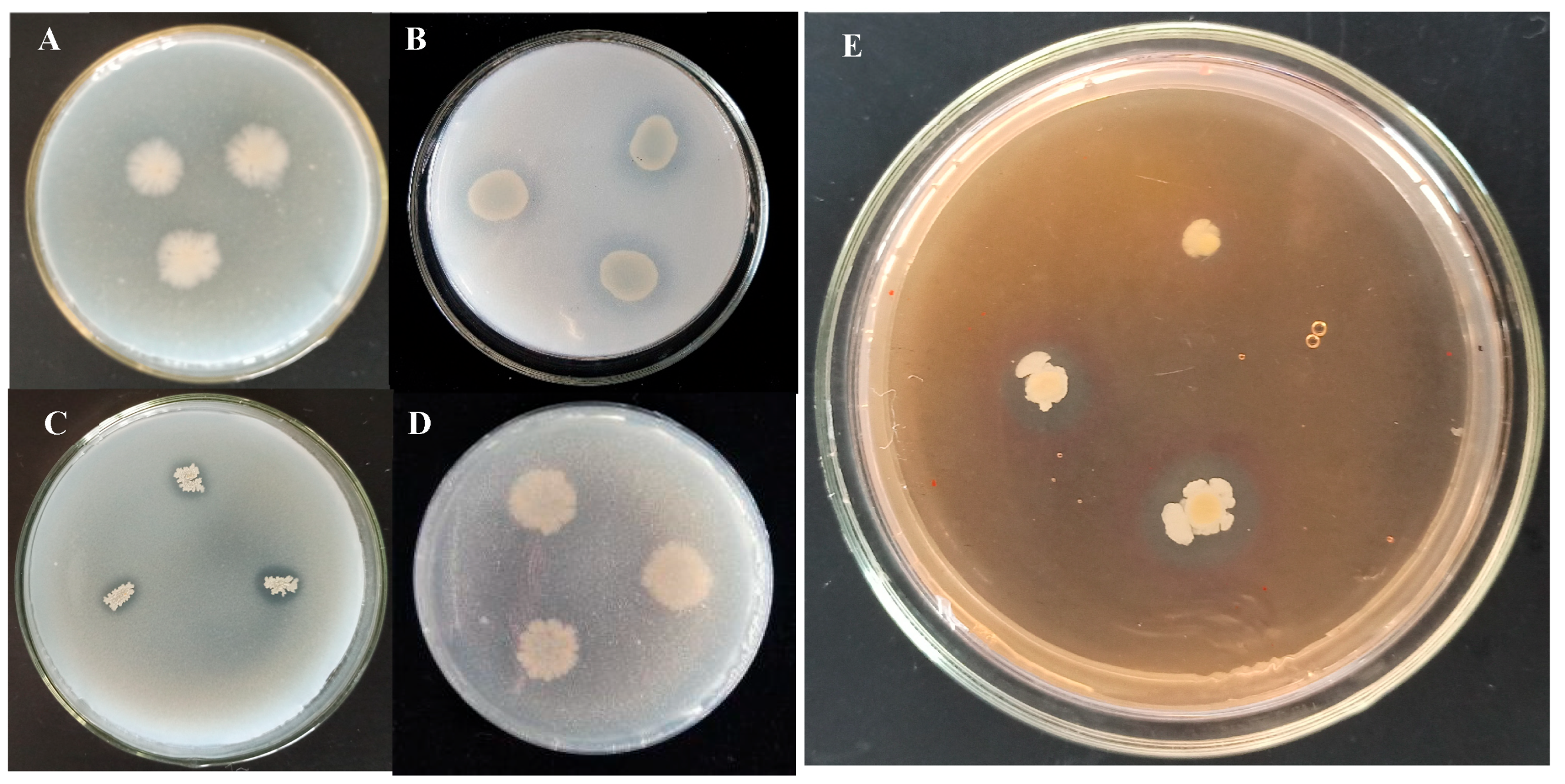
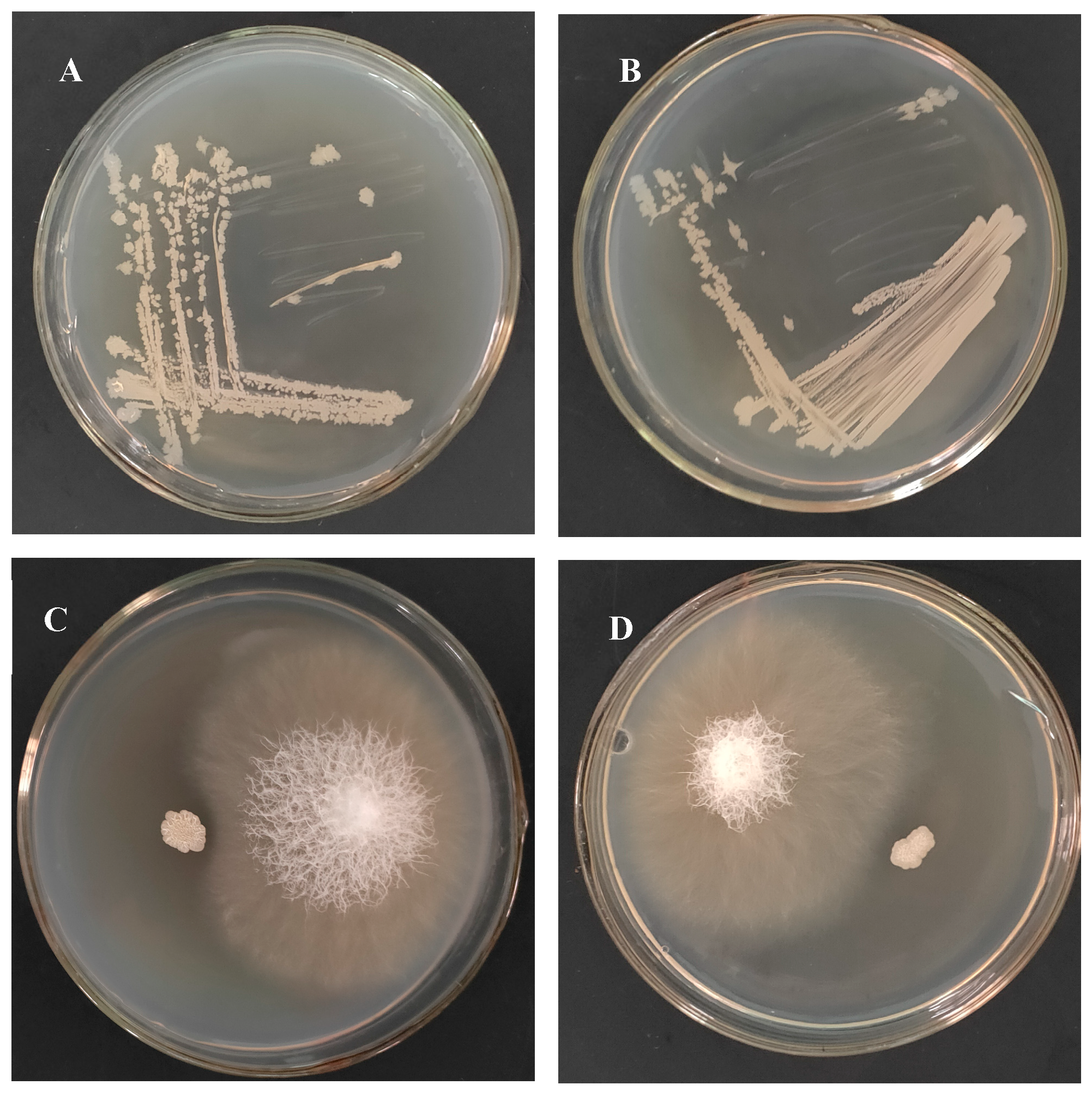

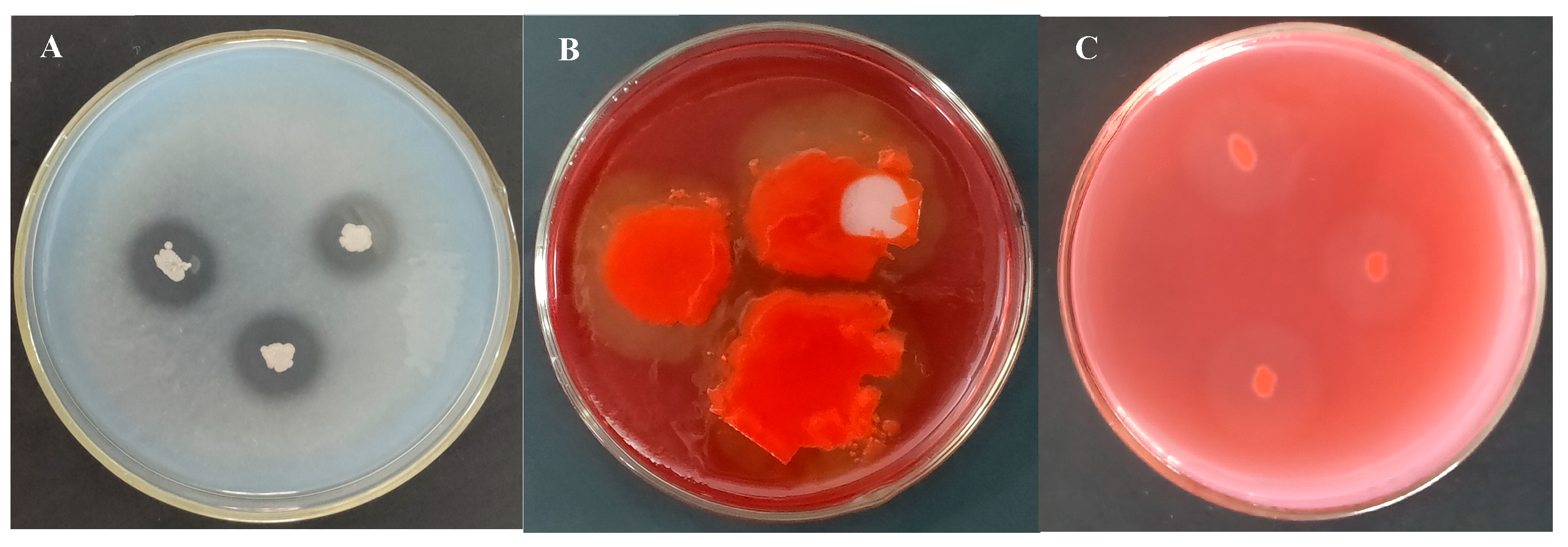
| Isolates | Sites | Closest NCBI Match/Species with GenBank Accession Number | Percentage of Identity (%) |
|---|---|---|---|
| TCX1 | Root | Bacillus subtilis strain Md1-37 (MF581443.1) | 99.65 |
| TCX2 | Leave | Pseudomonas fluorescens strain YG-1 (MN585724.1) | 99.86 |
| TCX3 | Stem | Xanthomonas campestris strain JCT-42 (MF285891.1) | 99.79 |
| TCX4 | Root | Lysinibacillus fusiformis strain BH45 (KY910256.1) | 99.72 |
| TCX5 | Root | Pseudomonas aeruginosa strain M4 (MT180543.1) | 100 |
| TCX6 | Stem | Bacillus cereus strain SRG14 (MK743993.1) | 99.93 |
| TCX7 | Root | Enterobacter hormaechei strain SRG9 (MK743988.1) | 99.58 |
| TCX8 | Root | Aeromonas taiwanensis strain Ichip_2-1 (MW48741 6.1) | 99.93 |
| TCX9 | Stem | Curtobacterium flaccumfaciens pv. flaccumfaciens strain x-2 (HQ713508.1) | 99.86 |
| TCX10 | Root | Delftia sp. BN-HKY2 (HQ731449.1) | 99.79 |
| TCX11 | Root | Bacillus sp. strain 6063(MT393628.1) | 99.72 |
| TCX12 | Stem | Bacillus safensis strain JCT-42 (MH820175.1) | 99.66 |
| TCX13 | Root | Bacillus safensis strain KLV20 (MT634636.1) | 100 |
| TCX14 | Root | Bacillus pumilus strain ACCC04398 (MZ067892.1) | 99.79 |
| Endophytic Bacterium | Inhibition Rate (%) | ||||
|---|---|---|---|---|---|
| TCX1 | TCX6 | TCX7 | TCX12 | TCX14 | |
| Acremonium strictum | – | 27 | – | – | 28.1 |
| Fusarium graminearum | 66.9 | 28.3 | – | 29.7 | 35.0 |
| Cercospora zeae-maydis | 57.6 | 26.0 | – | 31.7 | 27.2 |
| Phytophthora capsici Leonian | 53.7 | 41.4 | 37.3 | – | – |
| Sclerotinia sclerotiorum | 77.0 | 70.0 | 36.1 | 62.5 | – |
| Bipolaris zeicola | 54.8 | 63.0 | 23.2 | 27.9 | 31.3 |
| Fusarium oxysporum f. sp. cucumerinum | 86.0 | 29.6 | 21.0 | 45.9 | 32.8 |
| Trichothecium roseum | 52.1 | 33.3 | – | 74.9 | 67.8 |
| Pythium aphanidermatum | 45.0 | 34.0 | 30.3 | – | 36.7 |
| Fusarium oxysporum f. sp. melonis | 71.7 | 36.0 | 28.2 | 53.4 | – |
| Fusarium culmorum | 49.5 | 25.6 | 27.0 | 24.3 | 26.0 |
| Botrytis cinerea Pers | 54.0 | – | – | 38.5 | 34.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Zhu, M.; Zhao, Y.; Yi, E.; Zhang, J.; Wang, Z.; Wang, C.; Yu, C.; Ma, L. Bacillus subtilis Strain TCX1 Isolated from Ambrosia artemisiifolia: Enhancing Cucumber Growth and Biocontrol Against Cucumber Fusarium Wilt. Plants 2025, 14, 3068. https://doi.org/10.3390/plants14193068
Dong Y, Zhu M, Zhao Y, Yi E, Zhang J, Wang Z, Wang C, Yu C, Ma L. Bacillus subtilis Strain TCX1 Isolated from Ambrosia artemisiifolia: Enhancing Cucumber Growth and Biocontrol Against Cucumber Fusarium Wilt. Plants. 2025; 14(19):3068. https://doi.org/10.3390/plants14193068
Chicago/Turabian StyleDong, Yuzhu, Mengzhuo Zhu, Yingwen Zhao, Enjing Yi, Jing Zhang, Ze Wang, Chenxi Wang, Cuimei Yu, and Lianju Ma. 2025. "Bacillus subtilis Strain TCX1 Isolated from Ambrosia artemisiifolia: Enhancing Cucumber Growth and Biocontrol Against Cucumber Fusarium Wilt" Plants 14, no. 19: 3068. https://doi.org/10.3390/plants14193068
APA StyleDong, Y., Zhu, M., Zhao, Y., Yi, E., Zhang, J., Wang, Z., Wang, C., Yu, C., & Ma, L. (2025). Bacillus subtilis Strain TCX1 Isolated from Ambrosia artemisiifolia: Enhancing Cucumber Growth and Biocontrol Against Cucumber Fusarium Wilt. Plants, 14(19), 3068. https://doi.org/10.3390/plants14193068





