Development and Application of an In Vitro Pollen Viability Assay for Comparative Safety Assessment of Transgenic Alfalfa (Medicago sativa L.)
Abstract
1. Introduction
2. Results
2.1. Microscopic Observation of Pollen Morphology
2.2. Screening of the Initial Medium
2.3. Further Optimization of Pollen Germination Medium
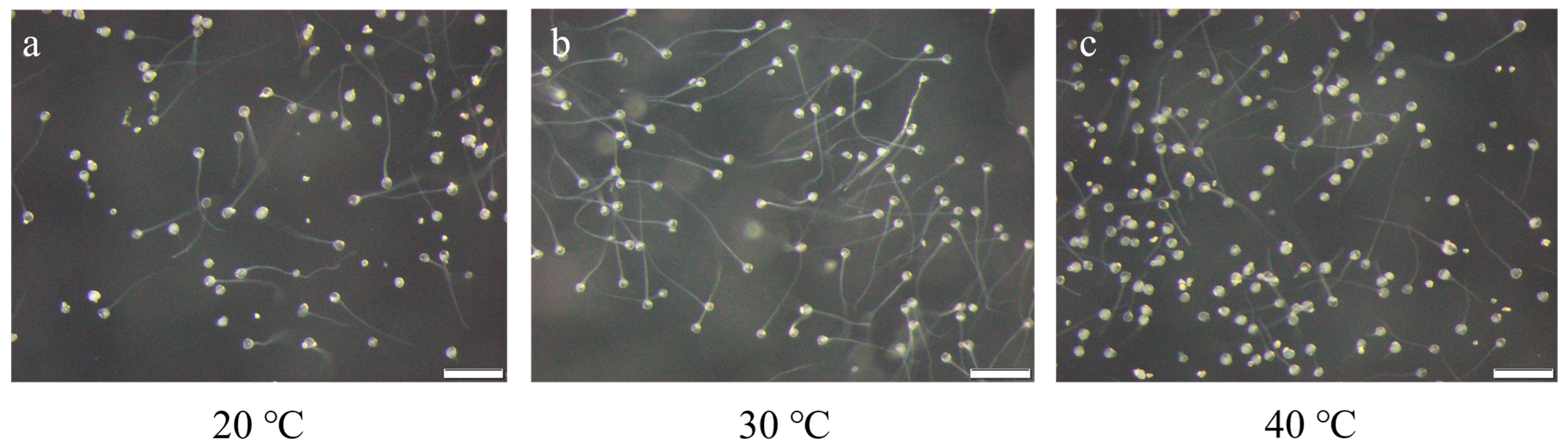
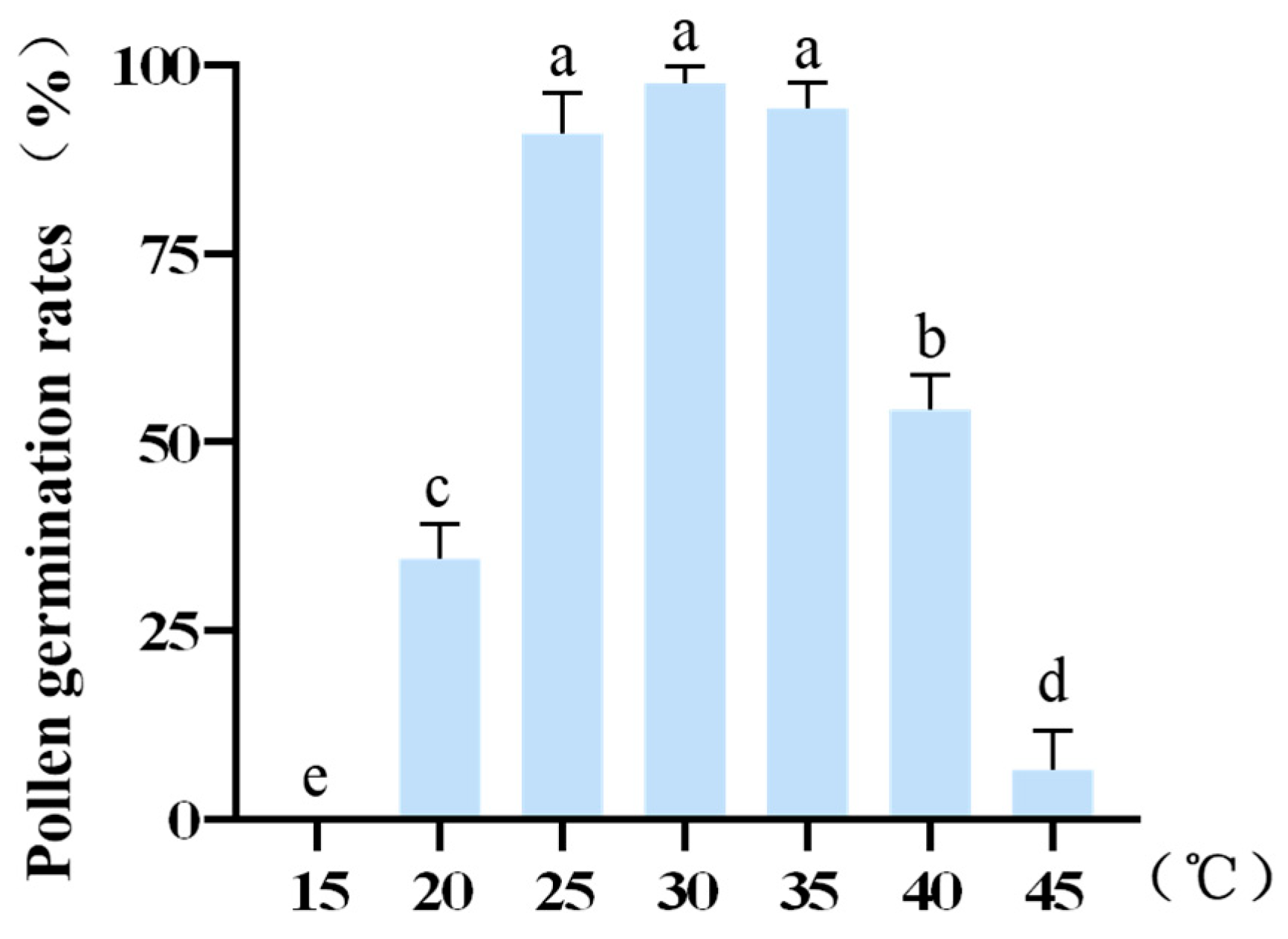
2.4. Effect of Temperature Treatment on Pollen Viability
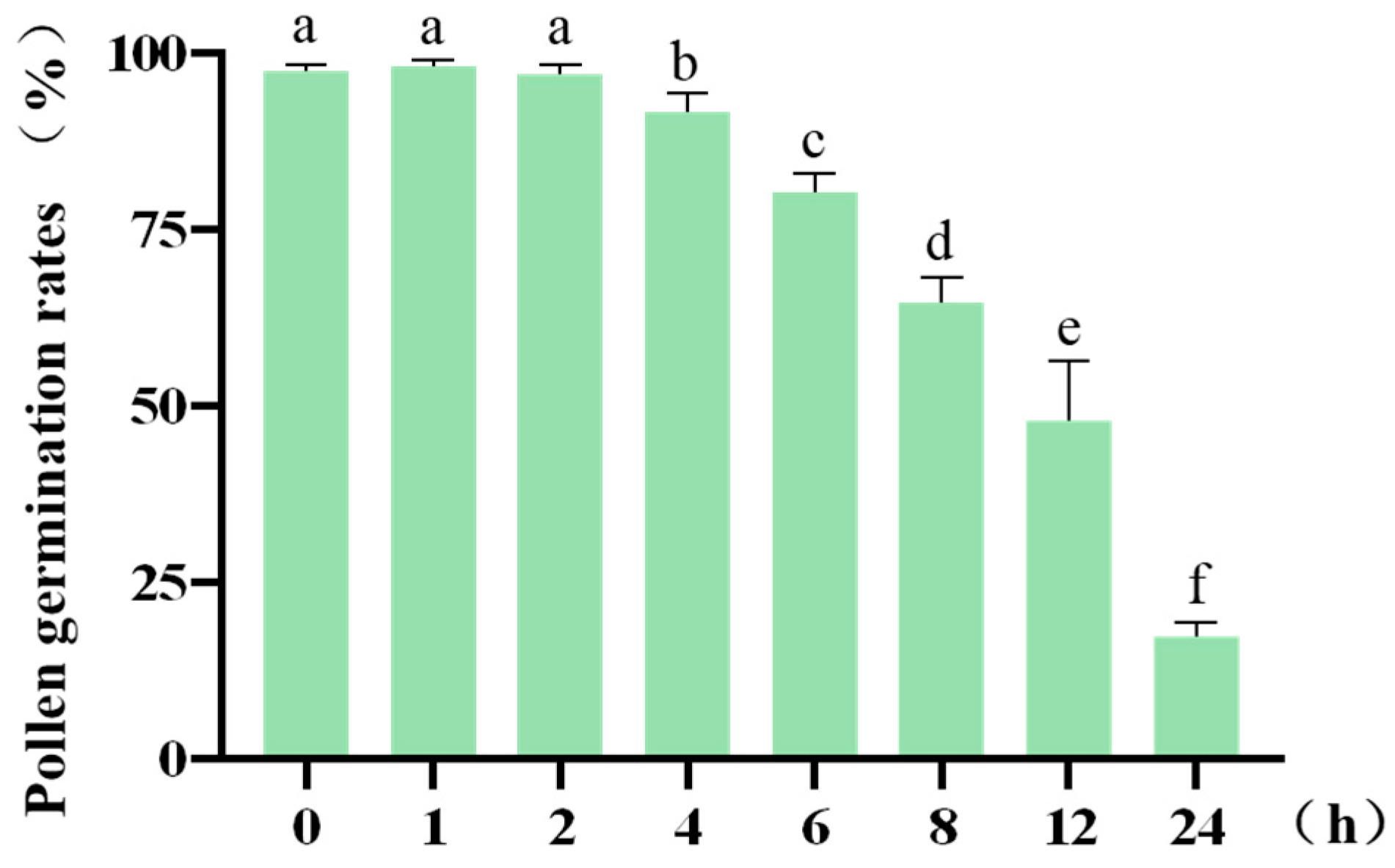
2.5. Effect of In Vitro Duration on Pollen Viability
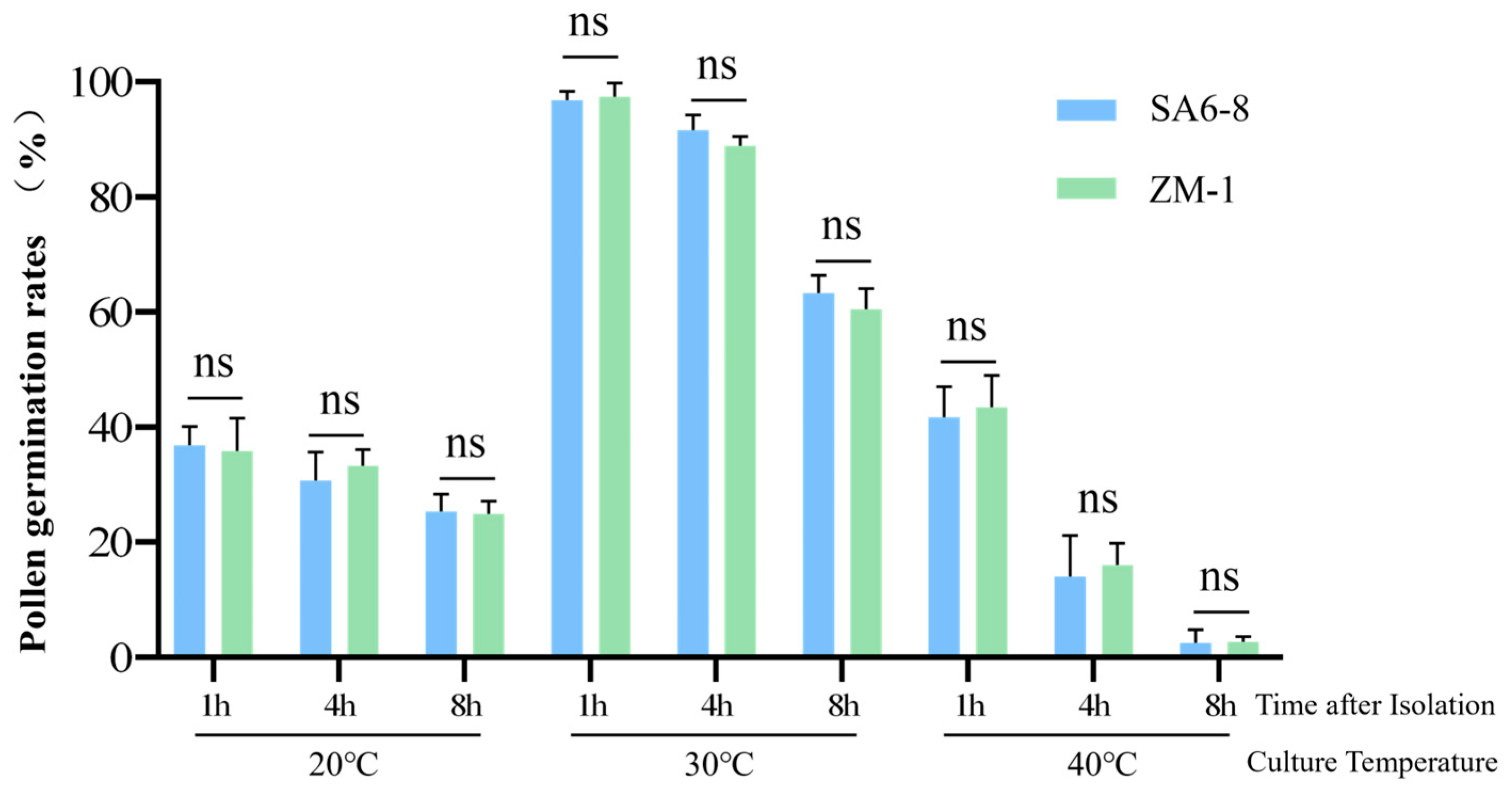
2.6. The Transgenic Event in SA6-8 Did Not Affect Pollen Viability Relative to ZM-1 Controls
3. Discussion
4. Materials and Methods
4.1. Plant Material and Pollen Collection
4.2. Plant Material and Pollen Collection
4.3. Preparation and Optimization of Culture Media
4.4. Temperature Treatment of Pollen
4.5. Pollen In Vitro Treatment
4.6. Pollen Germination
4.7. Statistical Analysis
4.8. Microscopic Examination of Pollen Morphology
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| H3BO3 | Boric acid |
| CaCl2 | Directory of open access journals |
| KH2PO4 | Monopotassium phosphate |
| MgSO4 | Magnesium sulfate |
| GM | Genetically modified |
| GMOs | Genetically modified organisms |
| PNG | Portable network graphics |
Appendix A

| Treatment | Germination Rate % | ||
|---|---|---|---|
| M1 | 71.67 | 68.85 | 73.44 |
| M2 | 88.33 | 83.61 | 87.30 |
| M3 | 92.19 | 93.65 | 98.44 |
| M4 | 90.00 | 88.89 | 92.31 |
| M5 | 79.69 | 82.26 | 81.82 |
| M6 | 43.33 | 34.43 | 31.75 |
| M7 | 0.00 | 0.00 | 0.00 |
| Treatment | Germination Rate % | ||
|---|---|---|---|
| M8 | 63.49 | 69.23 | 71.21 |
| M9 | 73.85 | 81.82 | 81.25 |
| M10 | 73.33 | 65.08 | 67.69 |
| M11 | 78.33 | 81.54 | 83.08 |
| M12 | 93.33 | 91.94 | 95.16 |
| M13 | 85.48 | 87.50 | 87.88 |
| M14 | 48.44 | 51.52 | 54.69 |
| M15 | 56.45 | 55.00 | 52.38 |
| M16 | 58.33 | 57.14 | 60.00 |
| Treatment | Germination Rate % | ||
|---|---|---|---|
| M17 | 91.67 | 90.77 | 92.42 |
| M18 | 96.67 | 98.06 | 100.00 |
| M19 | 95.00 | 93.65 | 93.85 |
| M20 | 93.33 | 95.31 | 93.94 |
| M21 | 92.19 | 92.19 | 93.94 |
| Temperature (°C) | Germination Rate % | ||
|---|---|---|---|
| 15 | 0.00 | 0.00 | 0.00 |
| 20 | 37.10 | 29.23 | 37.31 |
| 25 | 85.07 | 92.42 | 95.38 |
| 30 | 95.08 | 98.51 | 99.12 |
| 35 | 97.01 | 90.63 | 95.45 |
| 40 | 49.23 | 58.46 | 55.22 |
| 45 | 1.67 | 6.06 | 11.94 |
| Post-Excision Time (min) | Germination Rate % | ||
|---|---|---|---|
| 0 | 98.44 | 96.77 | 97.01 |
| 1 | 98.51 | 98.48 | 96.88 |
| 2 | 95.52 | 98.39 | 96.97 |
| 4 | 88.71 | 92.06 | 93.94 |
| 6 | 79.03 | 78.46 | 83.33 |
| 8 | 64.52 | 68.25 | 61.19 |
| 12 | 44.44 | 57.58 | 41.79 |
| 24 | 17.19 | 19.40 | 15.63 |
| Plant Material | Temperature (°C) | Post-Excision Time (min) | Germination Rate % | ||
|---|---|---|---|---|---|
| SA6-8 | 20 | 1 | 40.30 | 33.85 | 36.51 |
| ZM-1 | 20 | 1 | 34.92 | 41.94 | 30.65 |
| SA6-8 | 20 | 4 | 25.00 | 32.84 | 34.33 |
| ZM-1 | 20 | 4 | 36.36 | 32.81 | 30.77 |
| SA6-8 | 20 | 8 | 21.88 | 26.98 | 27.27 |
| ZM-1 | 20 | 8 | 22.39 | 25.81 | 26.56 |
| SA6-8 | 30 | 1 | 96.77 | 95.31 | 98.39 |
| ZM-1 | 30 | 1 | 95.52 | 96.97 | 100.00 |
| SA6-8 | 30 | 4 | 88.71 | 94.03 | 92.06 |
| ZM-1 | 30 | 4 | 90.32 | 89.23 | 87.10 |
| SA6-8 | 30 | 8 | 62.69 | 66.67 | 60.61 |
| ZM-1 | 30 | 8 | 60.66 | 63.93 | 56.72 |
| SA6-8 | 40 | 1 | 36.36 | 46.97 | 41.79 |
| ZM-1 | 40 | 1 | 45.45 | 37.31 | 47.69 |
| SA6-8 | 40 | 4 | 5.97 | 16.42 | 19.70 |
| ZM-1 | 40 | 4 | 20.00 | 15.63 | 12.50 |
| SA6-8 | 40 | 8 | 3.08 | 4.48 | 0.00 |
| ZM-1 | 40 | 8 | 1.59 | 3.33 | 3.17 |
References
- Lubieniechi, S.A.; Van Eenennaam, A.L.; Smyth, S.J. Regulation of animal and plant agricultural biotechnology. Trends Biotechnol. 2025, 43, 511–521. [Google Scholar] [CrossRef]
- Li, S.Y.; Li, P.C.; Li, X.Y.; Wen, N.; Wang, Y.X.; Lu, W.; Lin, M.; Lang, Z.H. In maize, co-expression of GAT and GR79-EPSPS provides high glyphosate resistance, along with low glyphosate residues. Abiotech 2023, 4, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Lv, W.J.; Yue, Q.; Wen, N.; Wang, Y.X.; Lang, Z.H.; Xu, W.; Li, S.Y. Utilizing the Fungal Bicistronic System for Multi-Gene Expression to Generate Insect-Resistant and Herbicide-Tolerant Maize. Int. J. Mol. Sci. 2024, 25, 3408. [Google Scholar] [CrossRef]
- Hu, H.H.; Dai, M.Q.; Yao, J.L.; Xiao, B.Z.; Li, X.H.; Zhang, Q.F.; Xiong, L.Z. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc. Natl. Acad. Sci. USA 2006, 103, 12987–12992. [Google Scholar] [CrossRef]
- Aung, B.; Gruber, M.Y.; Amyot, L.; Omari, K.; Bertrand, A.; Hannoufa, A. MicroRNA156 as a promising tool for alfalfa improvement. Plant Biotechnol. J. 2015, 13, 779–790. [Google Scholar] [CrossRef]
- Jin, Y.; Kristkova, Z.S.; Wesseler, J. Welfare impacts of China’s regulatory change toward genome-edited crops. Trends Biotechnol. 2025. [Google Scholar] [CrossRef]
- Ye, Q.Y.; Zhou, C.N.; Lin, H.; Luo, D.; Jain, D.; Chai, M.F.; Lu, Z.C.; Liu, Z.P.; Roy, S.; Dong, J.L.; et al. Medicago2035: Genomes, functional genomics and molecular breeding. Mol. Plant 2025, 18, 219–244. [Google Scholar] [CrossRef]
- Barros, J.; Templet, S.; Dixon, R.A. Development and commercialization of reduced lignin alfalfa. Curr. Opin. Biotechnol. 2019, 56, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.P.; Qiu, R.M.; Wang, K.X.; Liu, Y.R.; Zhang, W.J. Enhancing alfalfa resistance to Spodoptera herbivory by sequestering microRNA396 expression. Plant Cell Rep. 2023, 42, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, K.; Wang, S.P.; Zhu, J.H.; Wang, X.J.; Hong, J.; Wang, Z. MsCYP71 is a positive regulator for drought resistance in alfalfa. Plant Physiol. Biochem. 2023, 203, 107999. [Google Scholar] [CrossRef]
- Luo, D.; Liu, J.; Wu, Y.G.; Zhang, X.; Zhou, Q.; Fang, L.F.; Liu, Z.P. NUCLEAR TRANSPORT FACTOR 2-LIKE improves drought tolerance by modulating leaf water loss in alfalfa (Medicago sativa L.). Plant J. 2022, 112, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.X.; Yan, J.N.; Tanvir, R.; Li, L.; Liu, Y.R.; Zhang, W.J. Improved forage quality and biomass yield of alfalfa (Medicago sativa L.) by Arabidopsis QQS orphan gene. Curr. Plant Biol. 2023, 35–36, 100295. [Google Scholar] [CrossRef]
- Lin, S.; Niu, Y.; Medina, C.A.; Yu, L.X. Two linked resistance genes function divergently in defence against Verticillium Wilt in Alfalfa. Plant Biotechnol. J. 2022, 20, 619–621. [Google Scholar] [CrossRef]
- Peng, W.X.; Cai, W.Q.; Pan, J.Y.; Su, X.R.; Dou, L.R. Molecular Mechanisms of Alfalfa Response to Abiotic Stresses. Plants 2025, 14, 487. [Google Scholar] [CrossRef]
- Dai, R.; Chen, Q.; Wang, X.Y.; Huo, X.W.; Li, J.W.; An, J.B.; Mi, F.G.; Shi, F.L.; Zhang, Z.Q. Overexpression MsJAR1 gene increased lateral branches and plant height in alfalfa (Medicago sativa L.). Sci. Rep. 2025, 15, 16956. [Google Scholar] [CrossRef]
- Gou, J.Q.; Debnath, S.; Sun, L.; Flanagan, A.; Tang, Y.H.; Jiang, Q.Z.; Wen, J.Q.; Wang, Z.Y. From model to crop: Functional characterization of SPL8 in M.truncatula led to genetic improvement of biomass yield and abiotic stress tolerance in alfalfa. Plant Biotechnol. J. 2018, 16, 951–962. [Google Scholar] [CrossRef]
- Meng, Y.Y.; Zhang, W.W.; Wang, Z.M.; Yuan, F.; Guo, S.D.; Lin, H.; Niu, L.F. Co-expression of GR79 EPSPS and GAT generates high glyphosate-resistant alfalfa with low glyphosate residues. Abiotech 2023, 4, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Wolabu, T.W.; Mahmood, K.; Jerez, I.T.; Cong, L.L.; Yun, J.F.; Udvardi, M.; Tadege, M.; Wang, Z.Y.; Wen, J.Q. Multiplex CRISPR/Cas9-mediated mutagenesis of alfalfa FLOWERING LOCUS Ta1 (MsFTa1) leads to delayed flowering time with improved forage biomass yield and quality. Plant Biotechnol. J. 2023, 21, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Son, E.; Lim, S.S. Consumer Acceptance of Gene-Edited versus Genetically Modified Foods in Korea. Int. J. Environ. Res. Public Health 2021, 18, 3805. [Google Scholar] [CrossRef]
- Noack, F.; Engist, D.; Gantois, J.; Gaur, V.; Hyjazie, B.F.; Larsen, A.; M’Gonigle, L.K.; Missirian, A.; Qaim, M.; Sargent, R.D.; et al. Environmental impacts of genetically modified crops. Science 2024, 385, eado9340. [Google Scholar] [CrossRef]
- Ludlow, K.; Falck-Zepeda, J.; Smyth, S.J. Risk-appropriate, science-based innovation regulations are important. Trends Biotechnol. 2025, 43, 502–510. [Google Scholar] [CrossRef]
- Tsatsakis, A.M.; Nawaz, M.A.; Tutelyan, V.A.; Golokhvast, K.S.; Kalantzi, O.I.; Chung, D.H.; Kang, S.J.; Coleman, M.D.; Tyshko, N.; Yang, S.H.; et al. Impact on environment, ecosystem, diversity and health from culturing and using GMOs as feed and food. Food Chem. Toxicol. 2017, 107, 108–121. [Google Scholar] [CrossRef]
- Tsatsakis, A.M.; Nawaz, M.A.; Kouretas, D.; Balias, G.; Savolainen, K.; Tutelyan, V.A.; Golokhvast, K.S.; Lee, J.D.; Yang, S.H.; Chung, G. Environmental impacts of genetically modified plants: A review. Environ. Res. 2017, 156, 818–833. [Google Scholar] [CrossRef]
- Lu, B.R.; Yang, C. Gene flow from genetically modified rice to its wild relatives: Assessing potential ecological consequences. Biotechnol. Adv. 2009, 27, 1083–1091. [Google Scholar] [CrossRef]
- Song, Z.P.; Lu, B.R.; Zhu, Y.G.; Chen, J.K. Gene flow from cultivated rice to the wild species Oryza rufipogon under experimental field conditions. New Phytol. 2003, 157, 657–665. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, Q.L.; Liu, Q.S.; Meissle, M.; Yang, Y.; Wang, Y.N.; Hua, H.X.; Chen, X.P.; Peng, Y.F.; Romeis, J. Bt rice in China—Focusing the nontarget risk assessment. Plant Biotechnol. J. 2017, 15, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, R.S.; Peltier, A.; Hufford, M.B.; Oudin, E.; Saulnier, J.; Paul, L.; Knudsen, J.T.; Herren, H.R.; Gepts, P. Long-distance pollen flow assessment through evaluation of pollinator foraging range suggests transgene escape distances. Proc. Natl. Acad. Sci. USA 2008, 105, 13456–13461. [Google Scholar] [CrossRef]
- Weng, Z.K.; Deng, Y.T.; Tang, F.; Zhao, L.K.; Zhao, L.X.; Wang, Y.; Dai, X.B.; Zhou, Z.L.; Cao, Q.H. Screening and optimisation of in vitro pollen germination medium for sweetpotato (Ipomoea batatas). Plant Methods 2023, 19, 93. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Gao, W.Y.; Zhou, Y.Z.; Guo, H.Y. Optimization of In Vitro Germination and storage of Armeniaca sibirica Pollen. Sci. Hortic. 2022, 304, 111309. [Google Scholar] [CrossRef]
- Hirsche, J.; Fernández, J.M.G.; Stabentheiner, E.; Grosskinsky, D.K.; Roitsch, T. Differential Effects of Carbohydrates on Arabidopsis Pollen Germination. Plant Cell Physiol. 2017, 58, 691–701. [Google Scholar] [CrossRef]
- Shi, R.R.; Shi, F.L.; Wang, S.S. Influence of Sucrose, Boric Acid and Calcium Chloride on in vitro Pollen Germination and Tube Elongation of Alfalfa. Legume Res. 2023, 46, 1192–1198. [Google Scholar] [CrossRef]
- Chen, S.Q.; Wang, Z.; Liu, M.X.; Xie, Z.W.; Wang, H.H. Pollen Grain Germination and Pollen Tube Growth in Pistil of Rice. Rice Sci. 2008, 15, 125–130. [Google Scholar] [CrossRef]
- Wang, Q.L.; Lu, L.D.; Wu, X.Q.; Li, Y.Q.; Lin, J.X. Boron influences pollen germination and pollen tube growth in Picea meyeri. Tree Physiol. 2003, 23, 345–351. [Google Scholar] [CrossRef]
- Castillo, S.E.; Tovar, J.C.; Shamin, A.; Gutirerrez, J.; Pearson, P.; Gehan, M.A. A protocol for Chenopodium quinoa pollen germination. Plant Methods 2022, 18, 65. [Google Scholar] [CrossRef]
- Jia, W.Q.; Wang, Y.L.; Mi, Z.R.; Wang, Z.; He, S.L.; Kong, D.Z. Optimization of culture medium for in vitro germination and storage conditions of Exochorda racemosa pollen. Front. Plant Sci. 2022, 13, 994214. [Google Scholar] [CrossRef]
- Zhao, R.; Hu, X.; Yuan, D.Y.; Masabni, J.; Xiong, H.; Zou, F. Orthogonal test design for optimizing culture medium for in vitro pollen germination of interspecific oil tea hybrids. An. Da Acad. Bras. De Cienc. 2021, 93, e20190431. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.; Kothari, S.L. Influence of potassium dihydrogen phosphate on callus induction and plant regeneration in rice (Oryza sativa L.). Cereal Res. Commun. 2005, 33, 553–560. [Google Scholar] [CrossRef]
- Appiah, E.A.; Balla-Kovács, A.; Ocwa, A.; Csajbók, J.; Kutasy, E. Enhancing Alfalfa (Medicago sativa L.) Productivity: Exploring the Significance of Potassium Nutrition. Agronomy 2024, 14, 1806. [Google Scholar] [CrossRef]
- Boavida, L.C.; McCormick, S. Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J. 2007, 52, 570–582. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Montserrat, M.; Hormaza, J.I. In vitro pollen germination in avocado (Persea americana Mill.): Optimization of the method and effect of temperature. Sci. Hortic. 2011, 130, 152–156. [Google Scholar] [CrossRef]
- Kumari, M.; Prasad, A.; Rahman, L.U.; Mathur, A.K.; Mathur, A. In vitro germination, storage and microscopic studies of pollen grains of four Ocimum species. Ind. Crops Prod. 2022, 177, 114445. [Google Scholar] [CrossRef]
- Alexander, L.W. Optimizing pollen germination and pollen viability estimates for Hydrangea macrophylla, Dichroa febrifuga, and their hybrids. Sci. Hortic. 2019, 246, 244–250. [Google Scholar] [CrossRef]
- Announcement No. 423 of the Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Available online: https://www.moa.gov.cn/govpublic/ncpzlaq/202107/t20210714_6371842.htm (accessed on 26 September 2025).


| Treatment | Sucrose (g/L) | H3BO3 (mg/L) | CaCl2 (mg/L) | Germination Rate (%) |
|---|---|---|---|---|
| M1 | 50 | 200 | 60 | 71.32 ± 2.31 |
| M2 | 75 | 200 | 60 | 86.41 ± 2.49 |
| M3 | 100 | 200 | 60 | 94.76 ± 3.27 |
| M4 | 125 | 200 | 60 | 90.4 ± 1.74 |
| M5 | 150 | 200 | 60 | 81.25 ± 1.37 |
| M6 | 200 | 200 | 60 | 36.5 ± 6.07 |
| M7 | 400 | 200 | 60 | 0 ± 0 |
| Treatment | Sucrose (g/L) | H3BO3 (mg/L) | CaCl2 (mg/L) | Germination Rate (%) |
|---|---|---|---|---|
| M8 | 100 | 100 | 30 | 67.98 ± 4.01 |
| M9 | 100 | 100 | 60 | 78.97 ± 4.45 |
| M10 | 100 | 100 | 90 | 68.7 ± 4.22 |
| M11 | 100 | 200 | 30 | 80.98 ± 2.42 |
| M12 | 100 | 200 | 60 | 93.48 ± 1.62 |
| M13 | 100 | 200 | 90 | 86.95 ± 1.29 |
| M14 | 100 | 300 | 30 | 51.55 ± 3.13 |
| M15 | 100 | 300 | 60 | 54.61 ± 2.06 |
| M16 | 100 | 300 | 90 | 58.49 ± 1.44 |
| Treatment | Sucrose (g/L) | H3BO3 (mg/L) | CaCl2 (mg/L) | KH2PO4 (mg/L) | MgSO4 (mg/L) | Germination Rate (%) |
|---|---|---|---|---|---|---|
| M17 | 175 | 200 | 60 | 0 | 0 | 91.62 ± 0.83 |
| M18 | 175 | 200 | 60 | 10 | 10 | 98.24 ± 1.67 |
| M19 | 175 | 200 | 60 | 10 | 20 | 94.17 ± 0.73 |
| M20 | 175 | 200 | 60 | 20 | 10 | 94.2 ± 1.01 |
| M21 | 175 | 200 | 60 | 20 | 20 | 92.77 ± 1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhang, X.; Yang, J.; Guo, D.; Wang, X.; Wang, Z. Development and Application of an In Vitro Pollen Viability Assay for Comparative Safety Assessment of Transgenic Alfalfa (Medicago sativa L.). Plants 2025, 14, 3070. https://doi.org/10.3390/plants14193070
Chen Y, Zhang X, Yang J, Guo D, Wang X, Wang Z. Development and Application of an In Vitro Pollen Viability Assay for Comparative Safety Assessment of Transgenic Alfalfa (Medicago sativa L.). Plants. 2025; 14(19):3070. https://doi.org/10.3390/plants14193070
Chicago/Turabian StyleChen, Yuxiao, Xiaochun Zhang, Jiangtao Yang, Diandian Guo, Xujing Wang, and Zhixing Wang. 2025. "Development and Application of an In Vitro Pollen Viability Assay for Comparative Safety Assessment of Transgenic Alfalfa (Medicago sativa L.)" Plants 14, no. 19: 3070. https://doi.org/10.3390/plants14193070
APA StyleChen, Y., Zhang, X., Yang, J., Guo, D., Wang, X., & Wang, Z. (2025). Development and Application of an In Vitro Pollen Viability Assay for Comparative Safety Assessment of Transgenic Alfalfa (Medicago sativa L.). Plants, 14(19), 3070. https://doi.org/10.3390/plants14193070





