Evaluation of the Insect Resistance Efficacy of Transgenic Maize LD05 in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Bt Corn and Non-Bt Corn
2.2. Bioassays Using Plant Tissues
2.3. Field Trials
2.4. Statistics and Analysis
3. Results
3.1. Bioassay Analysis of LD05 Tissue on Different Lepidopteran Larvae
3.2. Insect Resistance Identification of Transgenic Maize LD05 in Harbin
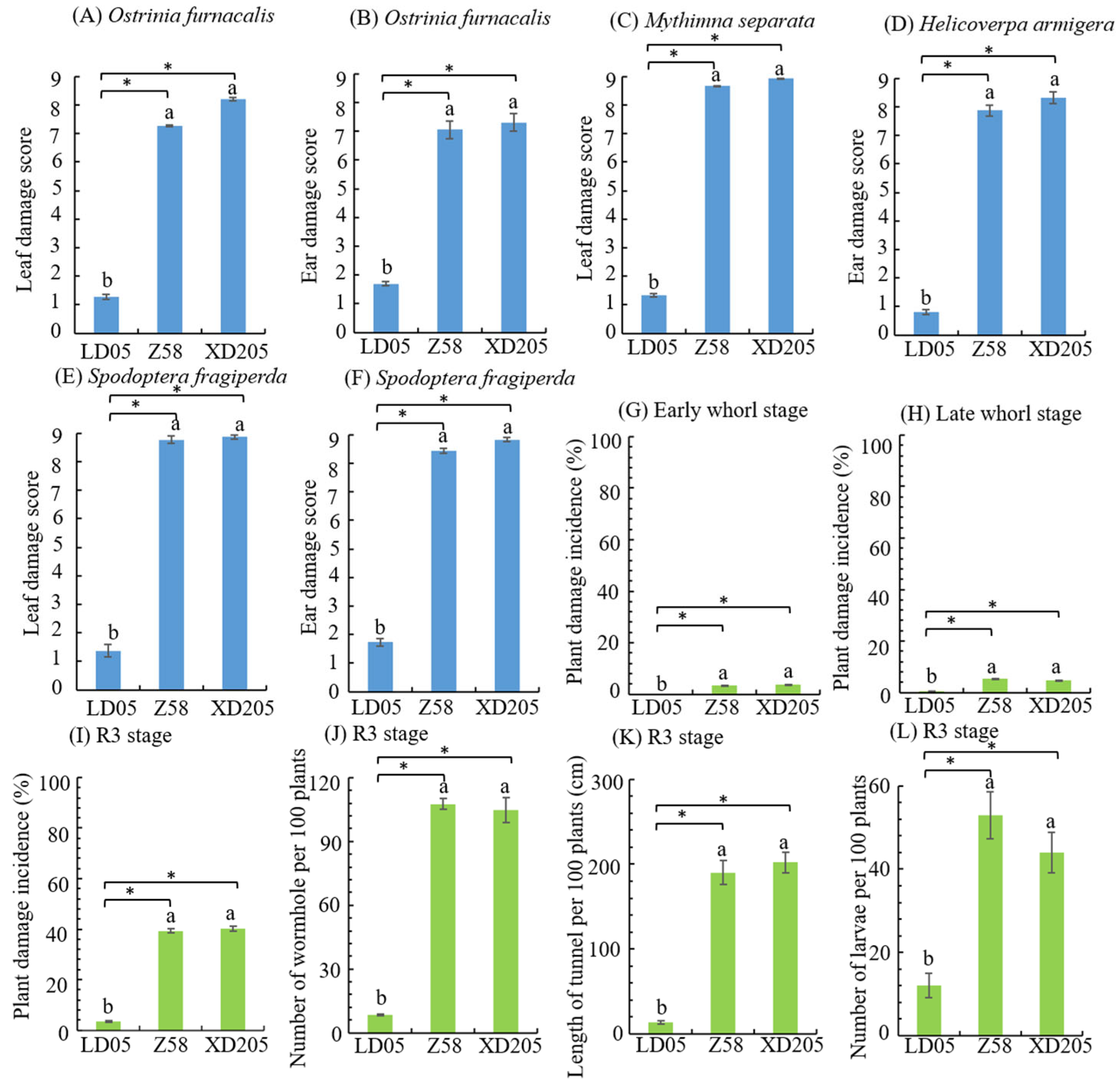
3.3. Insect Resistance Identification of Transgenic Maize LD05 in Shihezi
3.4. Insect Resistance Identification of Transgenic Maize LD05 in JINAN
3.5. Insect Resistance Identification of Transgenic Maize LD05 in Chongqing
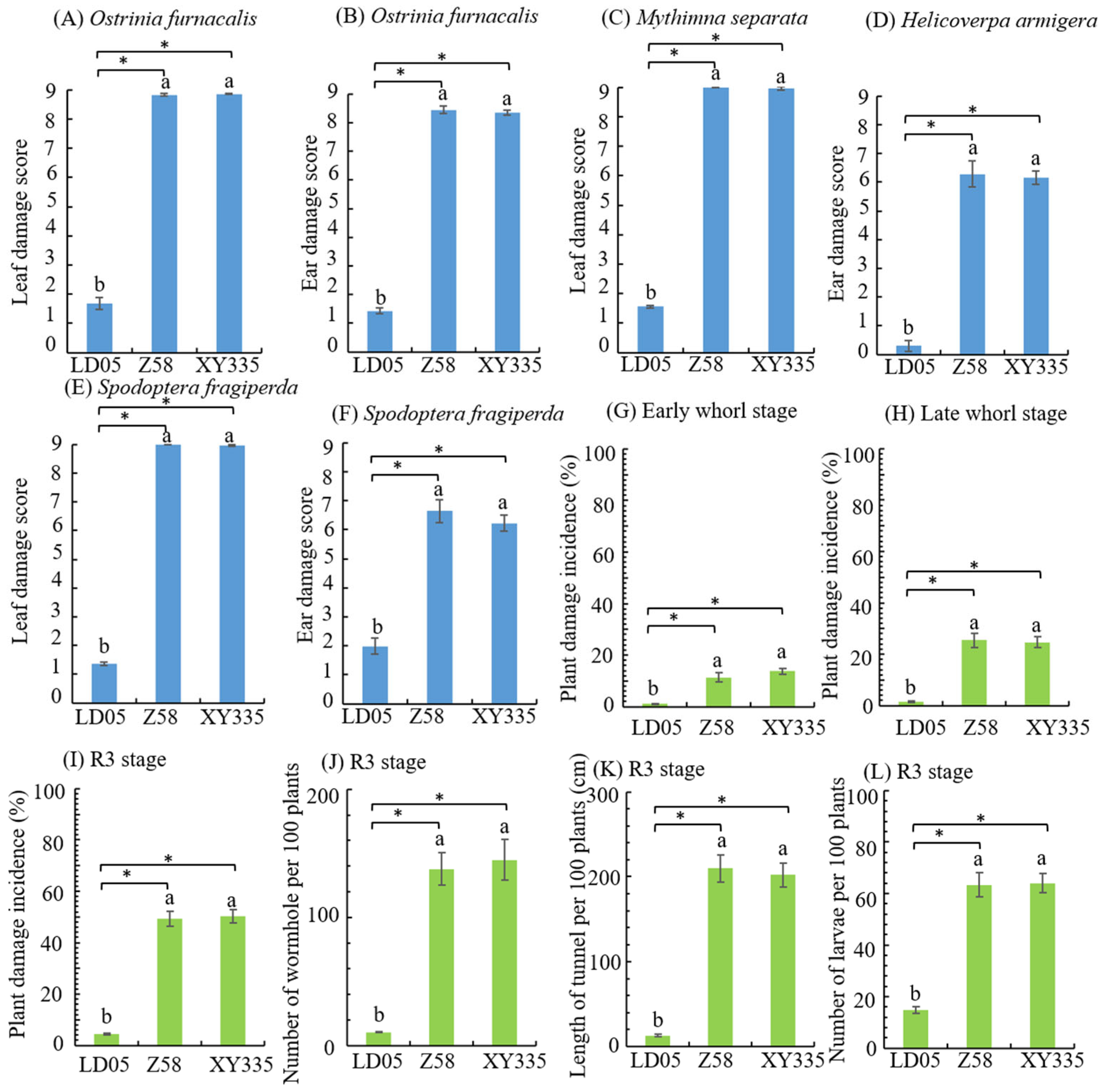
3.6. Insect Resistance Identification of Transgenic Maize LD05 in Guangzhou
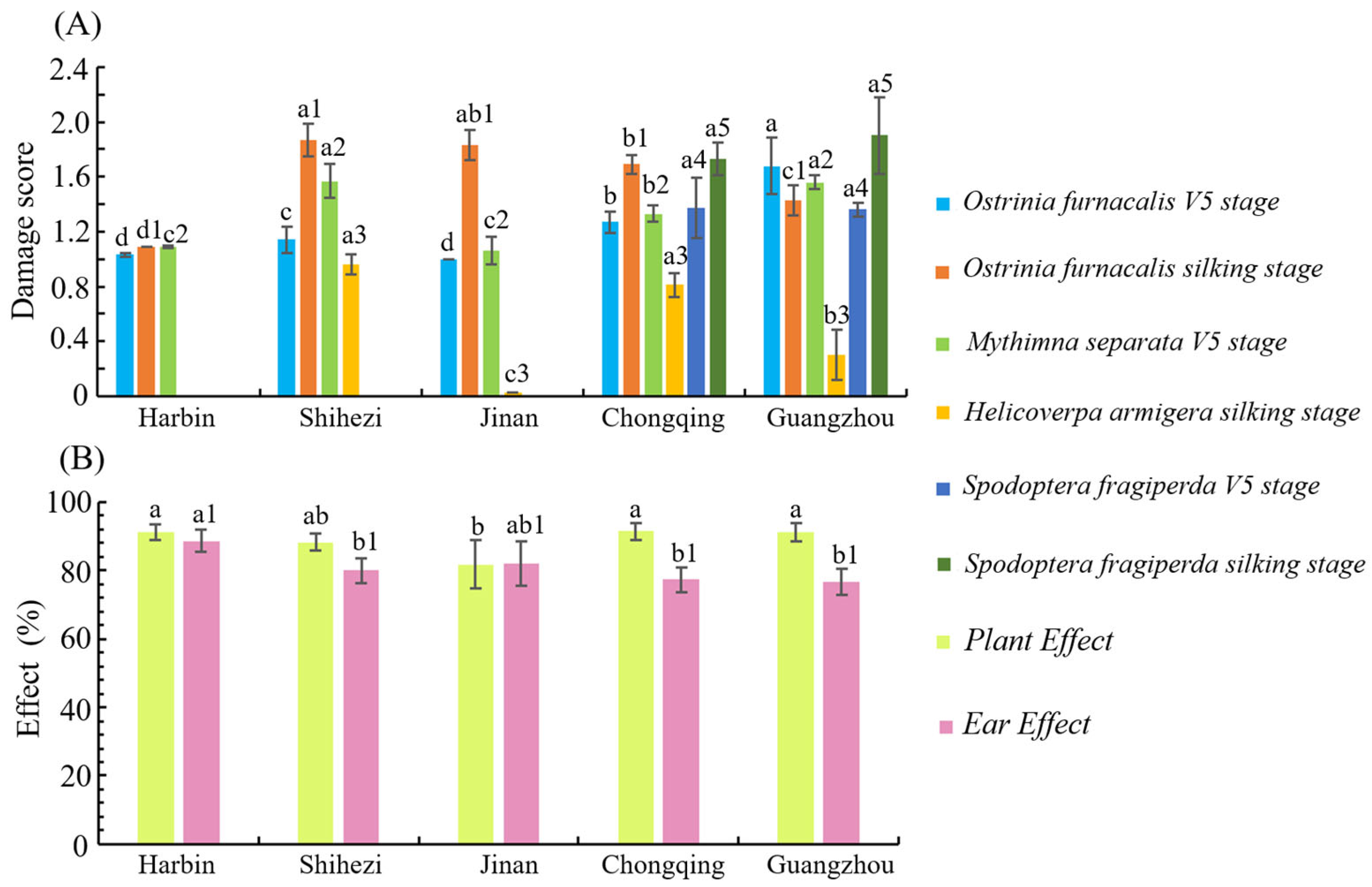
3.7. Efficacy of Transgenic Maize LD05 Against Lepidopteran Pests in Different Experimental Sites
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anderson, J.A.; Ellsworth, P.C.; Faria, J.C.; Head, G.P.; Owen, M.D.K.; Pilcher, C.D.; Shelton, A.M.; Meissle, M. Genetically engineered crops: Importance of diversified integrated pest management for agricultural sustainability. Front. Bioeng. Biotechnol. 2019, 7, 24. [Google Scholar] [CrossRef]
- Brookes, G. Genetically modified (GM) crop use 1996–2020: Environmental impacts associated with pesticide use CHANGE. GM Crops Food 2022, 13, 262–289. [Google Scholar] [CrossRef] [PubMed]
- Brookes, G. Farm income and production impacts from the use of genetically modified (GM) crop technology 1996–2020. GM Crops Food 2022, 13, 171–195. [Google Scholar] [CrossRef]
- Li, T.; Li, W. Research progress on the evolution of maize spatial pattern and its influencing factors in China. Chin. J. Agric. Re-Sour Reg. Plan. 2021, 42, 87–95. (In Chinese) [Google Scholar]
- Wang, H.; Lv, S.; Zhao, R.; Liang, P.; Zhang, S.; Gao, X.; Zhang, L.; Gu, S. Establishment of the relative susceptible baselines of Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae to commonly used insecticides. Acta Entomol. Sin. 2021, 64, 1427–1432. [Google Scholar]
- ISAAA. Global Status of Commercialized Biotech/GM Crops in 2019 (ISAAA Brief No. 55); ISAAA: Ithaca, NY, USA, 2019. [Google Scholar]
- Li, G.; Ji, T.; Zhao, S.; Feng, H.; Wu, K. High-dose assessment of transgenic insect-resistant maize events against major lepidopteran pests in China. Plants 2022, 11, 3125. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Li, F.; Hao, C.; Sun, H.; Yang, S.; Jiao, Y.; Lu, X. Impact of insect-resistant transgenic maize 2A-7 on diversity and dynamics of bacterial communities in rhizosphere soil. Plants 2023, 12, 2046. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, X.; Liu, D.; Sun, X.; Li, G.; Wu, K. Performance of the domestic Bt corn event expressing pyramided Cry1Ab and Vip3Aa19 against the invasive Spodoptera frugiperda (J. E. Smith) in China. Pest. Manag. Sci. 2023, 79, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Van Rensburg, J.B.J. First report of field resistance by the stem borer, Busseola fusca (Fuller) to Bt-transgenic maize. S. Afr. J. Plant Soil 2007, 24, 147–151. [Google Scholar] [CrossRef]
- Omoto, C.; Bernardi, O.; Salmeron, E.; Sorgatto, R.J.; Dourado, P.M.; Crivellari, A.; Carvalho, R.A.; Willse, A.; Martinelli, S.; Head, G.P. Field-evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest. Manag. Sci. 2016, 72, 1727–1736. [Google Scholar] [CrossRef]
- Chandrasena, D.I.; Signorini, A.M.; Abratti, G.; Storer, N.P.; Olaciregui, M.L.; Alves, A.P.; Pilcher, C.D. Characterization of field-evolved resistance to Bacillus thuringiensis-derived Cry1F—endotoxin in Spodoptera frugiperda populations from Argentina. Pest. Manag. Sci. 2018, 74, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Guo, J.; Brown, S.; Head, G.P.; Price, P.A.; Paula-Moraes, S.; Ni, X.; Dimase, M.; Huang, F. Field-evolved resistance of Helicoverpa zea (Boddie) to transgenic maize expressing pyramided Cry1A.105/Cry2Ab2 proteins in northeast Louisiana, the United States. J. Invertebr. Pathol. 2019, 163, 11–20. [Google Scholar] [CrossRef]
- Bates, S.L.; Zhao, J.Z.; Roush, R.T.; Shelton, A.M. Insect resistance management in GM crops: Past, present and future. Nat. Biotechnol. 2005, 23, 57–62. [Google Scholar] [CrossRef]
- Wang, Y.; He, K.; Wang, Z. Evolution of resistance to transgenic Bacillus thuringiensis maize in pest insects and a strategy for managing this. Chin. J. Appl. Entomol. 2019, 56, 12–23. (In Chinese) [Google Scholar]
- Chen, M.; Shelton, A.; Ye, G.Y. Insect-resistant genetically modified rice in China: From research to commercialization. Annu. Rev. Entomol. 2010, 56, 81–101. [Google Scholar] [CrossRef]
- Yue, R.; Li, W.; Ding, Z.; Meng, Z. Molecular Characteristics and Resistance Evaluation of Transgenic Maize LD05 with Stacked Insect and Herbicide Resistance Traits. Sci. Agric. Sin. 2025, 58, 1269–1283. (In Chinese) [Google Scholar]
- Wang, Z.Y.; Wang, X.M. Current status and management strategies for corn pests and diseases in China. Plant Prot. 2019, 45, 1–11. (in Chinese). [Google Scholar]
- Li, G.; Wu, K. Commercial strategy of transgenic insect-resistant maize in China. J. Plant Prot. 2022, 49, 17–32. (In Chinese) [Google Scholar]
- China Ministry of Agriculture. Announcement No.953-10.1-2007. Environmental Safety Testing of Genetically Modified Plants and Their Products—Insect-Resistant Corn—Part 1: Insect Resistance; Ministry of Agriculture and Rural Affairs of the People’s Republic of China (MARAPRC): Beijing, China, 2007.
- Zhang, B.; Yang, Y.; Zhou, X.; Shen, P.; Peng, Y.; Li, Y. A laboratory assessment of the potential effect of Cry1Ab/Cry2Aj-containing Bt maize pollen on Folsomia candida by toxicological and biochemical analyses. Environ. Pollut. 2017, 222, 94–100. [Google Scholar] [CrossRef]
- Liu, X.; Bai, S.; Wang, Z.; Wang, Y.; Wang, Q.; He, K. Expression of cry1Ab/cry1Ac fusion protein in transgenic Cry1Ab/Cry1Ac maize and its insecticidal effect on Ostrinia furnacalis. Acta Entomol. Sin. 2020, 63, 1201–1206. [Google Scholar]
- Zhang, D.; Wu, K. The bioassay of Chinese domestic Bt-Cry1Ab and Bt-(Cry1Ab+Vip3Aa) maize against the Fall armyworm, Spodoptera frugiperda. Plant Prot. 2019, 45, 54–60. (In Chinese) [Google Scholar]
- He, L.; Zhao, S.; Gao, X.; Wu, K. Ovipositional responses of Spodoptera frugiperda on host plants provide a basis for using Bt-transgenic maize as trap crop in China. J. Integr. Agric. 2020, 19, 2–12. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, D.; Li, D.; Zhao, S.; Wang, C.; Xiao, Y. Expression profiles of Cry1Ab protein and its insecticidal efficacy against the invasive Fall armyworm for Chinese domestic GM maize DBN9936. J. Integr. Agric. 2021, 20, 792–803. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, D.; Zhao, S.; Wu, K. Susceptibilities of the invasive Fall armyworm (Spodoptera frugiperda) to the insecticidal proteins of Bt maize in China. Toxins 2022, 14, 507. [Google Scholar] [CrossRef]
- Székács, A.; Lauber, É.; Juracsek, J.; Darvas, B. Cry1Ab toxin production of MON810 transgenic maize. Environ. Toxicol. Chem. 2010, 29, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ji, T.; Sun, X.; Jiang, Y.; Wu, K.; Feng, H. Susceptibility evaluation of invaded Spodoptera frugiperda population in Yunnan province to five Bt proteins. Plant Prot. 2019, 45, 15–20. (In Chinese) [Google Scholar]
- Gao, J.; Yang, L.; Liu, Y.; Wu, T.; Mao, J.; Lu, H.; Han, M.; Li, X.; Zhang, Z. Research progress on occurrence and control of Ostrinia furnacalis in Asia. China Plant Prot. 2024, 44, 22–28+40. (In Chinese) [Google Scholar]
- Gassmann, A.J.; Carrière, Y.; Tabashnik, B.E. Fitness costs of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 2009, 54, 147–163. [Google Scholar] [CrossRef]
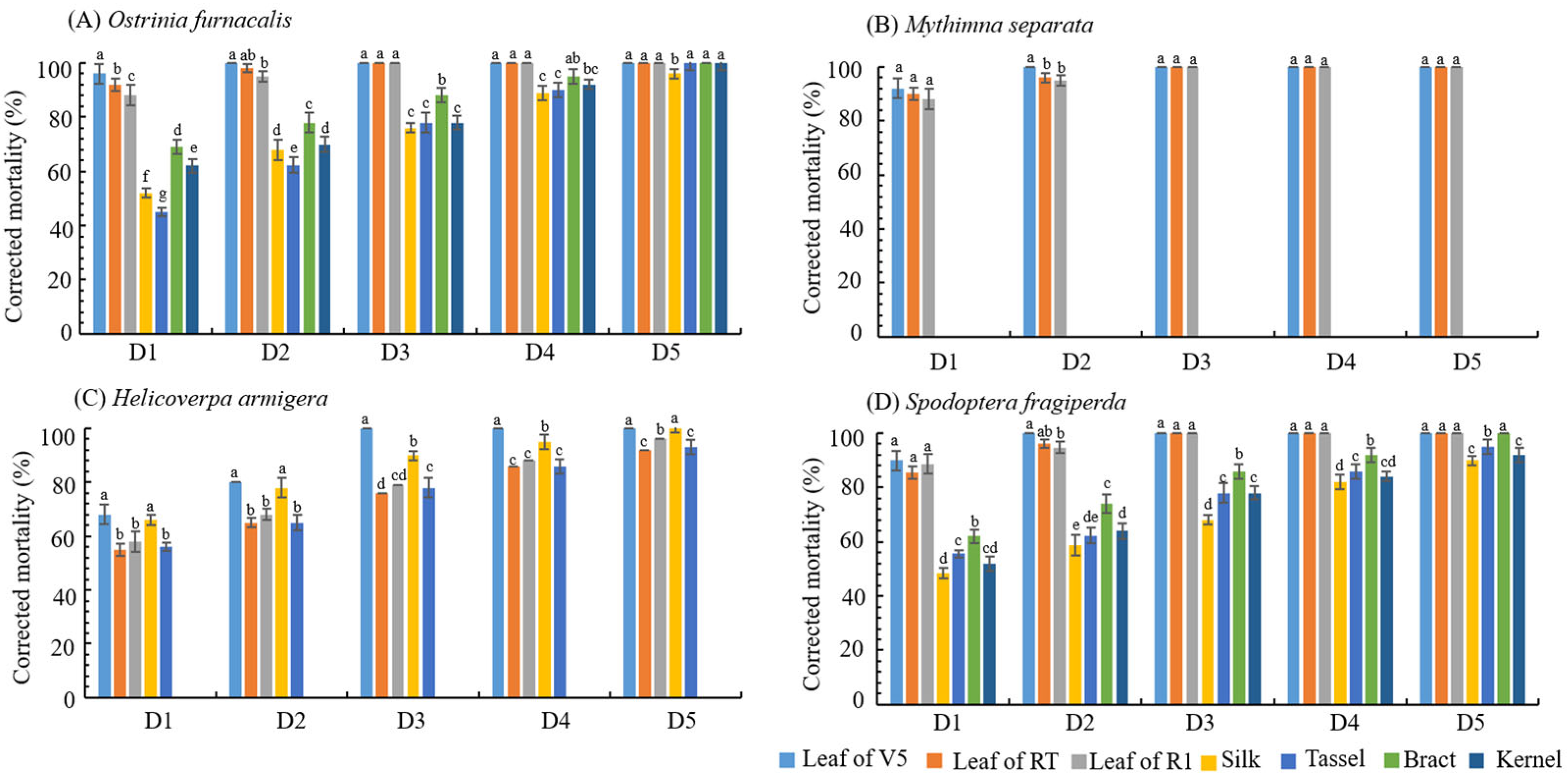
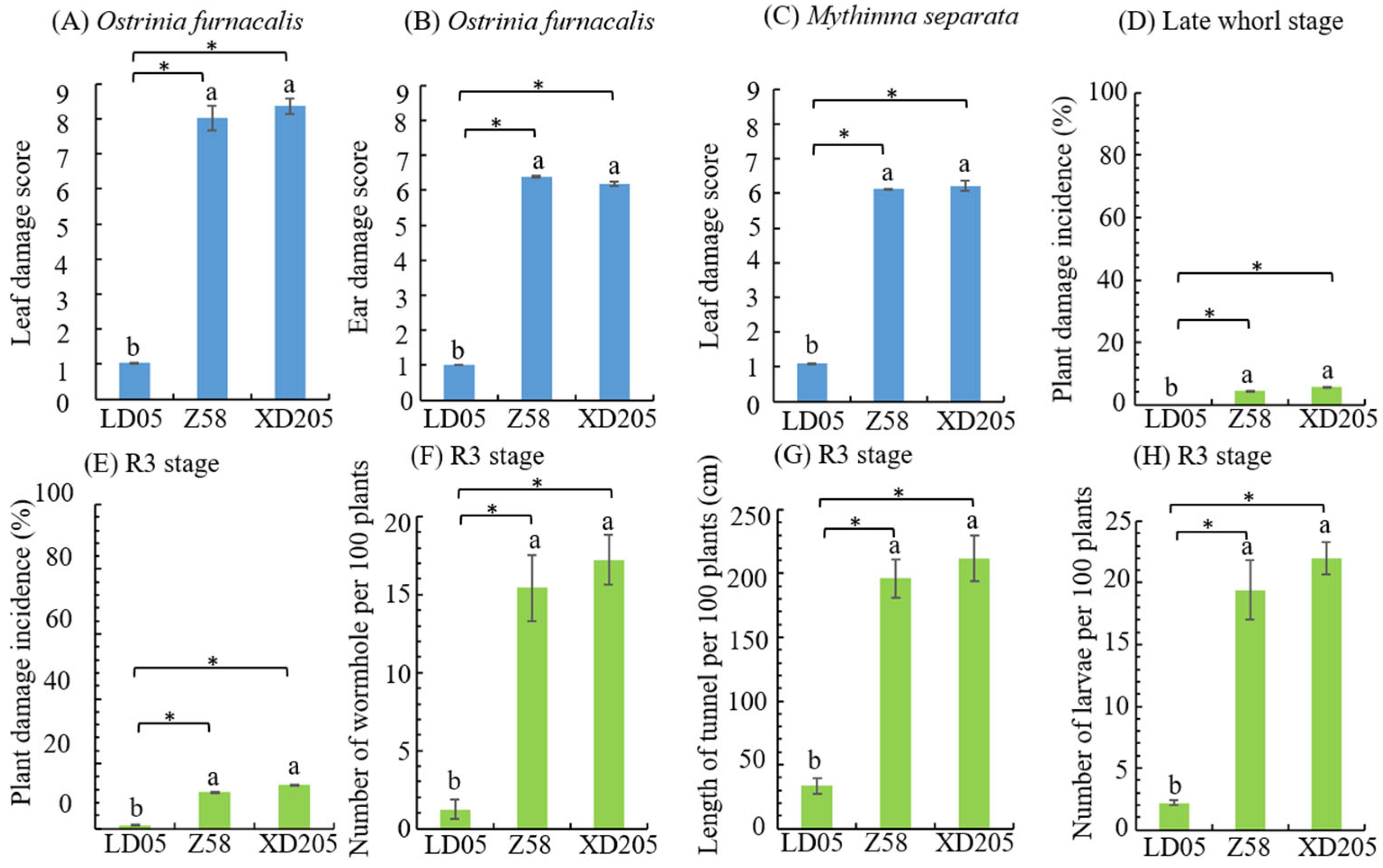
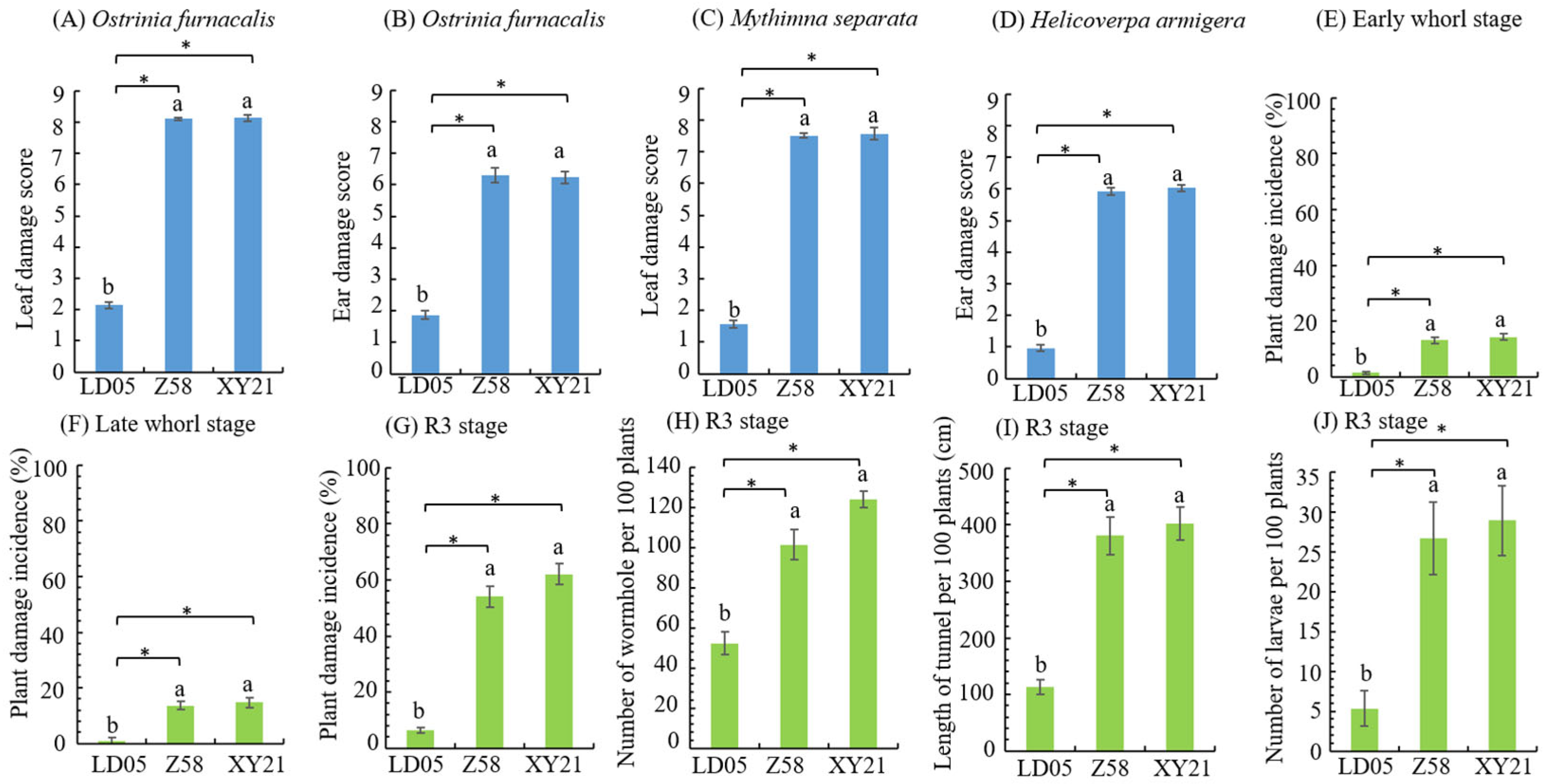
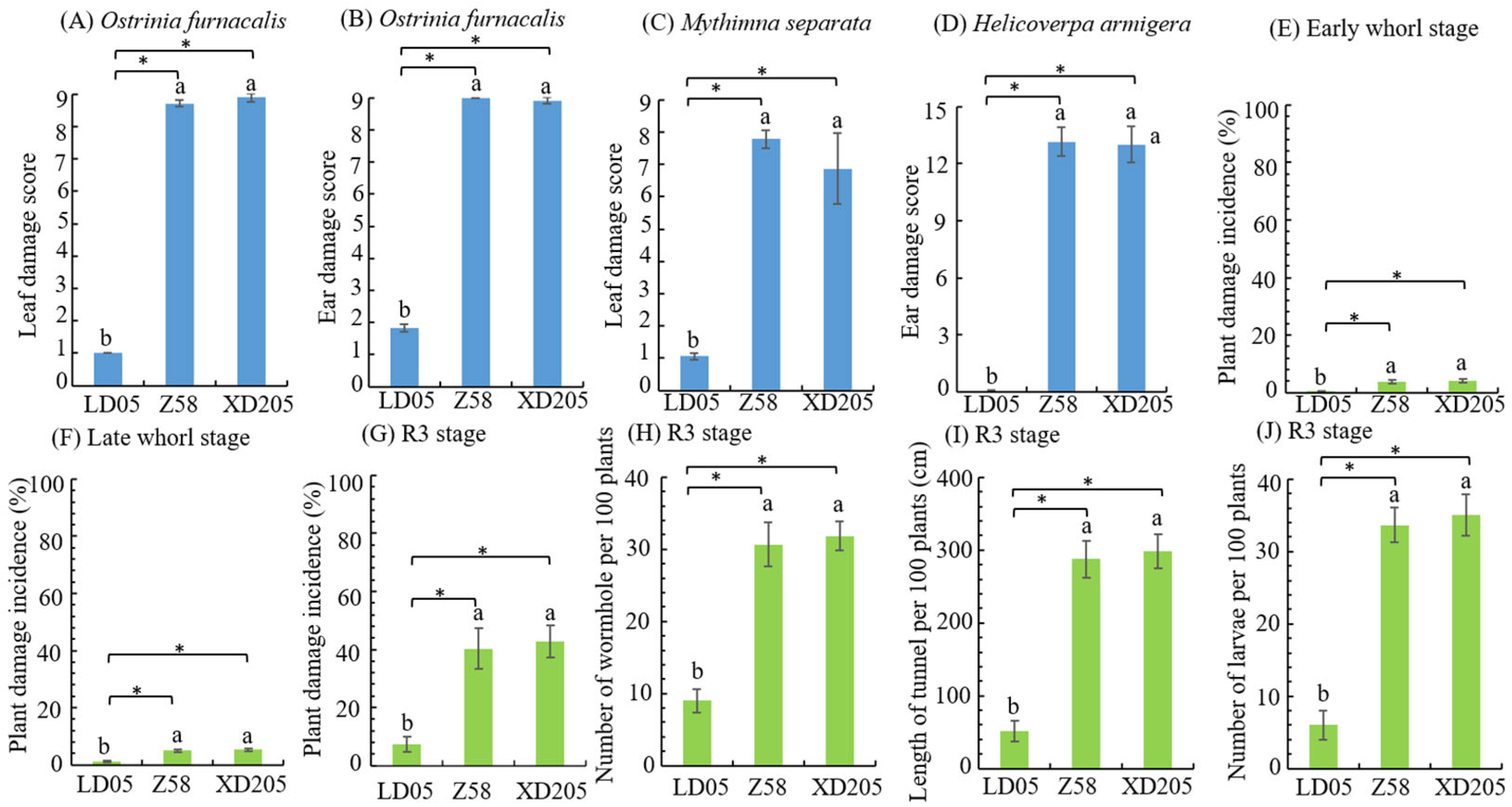
| Resistance Level | Larval Corrected Mortality Rate (y) % | |
|---|---|---|
| Test insects | O. furnacalis | M. separata/H. armigera/S. frugiperda |
| High resistance | y ≥ 95 | y ≥ 90 |
| Resistance | 95 > y ≥ 60 | 90 > y ≥ 60 |
| Medium resistance | 60 > y ≥ 40 | 60 > y ≥ 40 |
| Sense | 40 > y | 40 > y |
| Leaf Feeding Level | Description of Symptoms of O. furnacalis | Description of Symptoms of M. separata | Description of Symptoms of S. frugiperda |
|---|---|---|---|
| 1 | Only a few individual leaves have 1–2 wormholes with a diameter of ≤1 mm | The leaves were not damaged, or only had needle-like (≤1 mm) wormholes on the leaves | Leaves were not damaged or only a few pinholes on the leaves were damaged |
| 2 | Only a few individual leaves have 3 to 6 wormholes with a diameter of no more than 1 mm | Only a few insect holes of the size of bullet holes (≤5 mm) are found on individual leaves | There are pinhole damage symptoms and circular point-like semi-transparent film window holes have appeared |
| 3 | There are more than 7 wormholes with a diameter of ≤1 mm on a few leaves | A few leaves have wormholes the size of bullet holes (≤5 mm) | There are a few small (5 to 10 cm) slender semi-transparent film window holes on the leaves |
| 4 | There are 1 to 2 wormholes with a diameter of no more than 2 mm on individual leaves | Notches (≤10 mm) on individual leaves | There are a few large (10–30 cm) membrane window holes on the leaves |
| 5 | A few leaves have 3 to 6 wormholes with a diameter of no more than 2 mm | A few leaves have notches (≤10 mm) | Large film window holes appear in blocks on the leaves, and the holes are less than one-third of the leaf area |
| 6 | There are more than 7 wormholes with a diameter of no more than 2 mm on some leaves | There are notches (≤10 mm) on some leaves | The area of window holes or openings on the leaves is large and accounts for about one-third of the total leaf area |
| 7 | A few leaves have 1 to 2 wormholes with a diameter greater than 2 mm | Some leaves were partially eaten, and a few leaves had large notches (≤10 mm) | The area of window holes or openings on the leaves is large and accounts for about half of the total leaf area |
| 8 | There are 3 to 6 wormholes with a diameter greater than 2 mm on some leaves | A few leaves were eaten, and some leaves had large notches (≤10 mm). | The leaves survived and the entire leaf contained large window holes or holes that were damaged |
| 9 | Most of the leaves have more than 7 wormholes with a diameter greater than 2 mm | Most of the leaves are eaten | The leaves were completely destroyed and almost all of the leaf surfaces were damaged |
| Pest Damage Level | Average Leaf-Eating Grades of O. furnacalis During V5 Stage | Average Leaf-Eating Grades of M. separata and S. frugiperda During V5 Stage | Resistance |
|---|---|---|---|
| 1 | 1.0–2.9 | 1.0–2.0 | High resistance (HR) |
| 3 | 3.0–4.9 | 2.1–4.0 | Resistance® |
| 5 | 5.0–6.9 | 4.1–6.0 | Medium resistance (MR) |
| 7 | 7.0–8.9 | 6.1–8.0 | Sense (S) |
| 9 | 9.0 | 8.1–9.0 | High sense (HS) |
| Damage Level of Female Ear | Description of Symptoms of O. furnacalis | Description of Symptoms of H. armigera | Description of Symptoms of S. frugiperda |
|---|---|---|---|
| 0 | - | The ear is not harmed | - |
| 1 | The ear is not harmed | Only the silk was damaged | There is no breakage or only slight breakage in the silk, and the tips of the ear are not damaged |
| 2 | The damage rate of silk is less than 50% | The ear tip was damaged 1 cm | - |
| 3 | Damage rate of most filaments ≥50%; live larvae have been observed, and no more than second-instar | The ear tip was damaged 2 cm | The tip of the ear was slightly damaged, and the affected area was within 5% of the ear area |
| 4 | The ear tip was damaged ≤1 cm, or live larvae have been observed, and no more than third -instar | The ear tip was damaged 3 cm | - |
| 5 | The ear tip was damaged ≤2 cm, or live larvae have been observed, and no more than fourth -instar, the tunnel length less than 2 cm | The ear tip was damaged 4 cm | The affected area of ear accounted for 6–10% of ear area |
| 6 | The ear tip was damaged ≤3 cm, or live larvae have been observed, and more than fourth -instar, the tunnel length less than 4 cm | The ear tip was damaged 5 cm | - |
| 7 | The ear tip was damaged ≤4 cm, the tunnel length less than 6 cm | The ear tip was damaged 6 cm | The affected area of ear accounted for 11%~30% of ear area |
| 8 | The ear tip was damaged ≤5 cm, the tunnel length less than 8 cm | The ear tip was damaged 7 cm | - |
| 9 | The ear tip was damaged >5 cm, the tunnel length more than 8 cm | The ear tip was damaged 8 cm | The affected area of ear accounted for more than 30% of ear area |
| Mean Value of Damage to Ear | Resistance Type |
|---|---|
| 1.0–2.0 | High resistance (HR) |
| 2.1–3.0 | Resistance (R) |
| 3.1–5.0 | Medium resistance (MR) |
| 5.1–7.0 | Sense (S) |
| ≥7.1 | High sense (HS) |
| Time | O. furnacalis | M. separata | H. armigera | S. frugiperda | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | H | P | df | H | P | df | H | P | df | H | P | |
| DAY1 | 6 | 19.636 | 0.003 | 2 | 2.497 | 0.287 | 4 | 11.046 | 0.026 | 6 | 18.487 | 0.005 |
| DAY2 | 6 | 19.274 | 0.004 | 2 | 6.058 | 0.048 | 4 | 11.533 | 0.021 | 6 | 18.628 | 0.005 |
| DAY3 | 6 | 18.312 | 0.005 | 2 | 0 | 1 | 4 | 12.722 | 0.013 | 6 | 19.422 | 0.004 |
| DAY4 | 6 | 17.176 | 0.009 | 2 | 0 | 1 | 4 | 12.470 | 0.014 | 6 | 18.911 | 0.004 |
| DAY5 | 6 | 19.860 | 0.003 | 2 | 0 | 1 | 4 | 12.771 | 0.012 | 6 | 19.253 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Hou, X.; Zhang, H.; Yang, X.; Ding, Z.; Yue, R. Evaluation of the Insect Resistance Efficacy of Transgenic Maize LD05 in China. Plants 2025, 14, 3051. https://doi.org/10.3390/plants14193051
Li W, Hou X, Zhang H, Yang X, Ding Z, Yue R. Evaluation of the Insect Resistance Efficacy of Transgenic Maize LD05 in China. Plants. 2025; 14(19):3051. https://doi.org/10.3390/plants14193051
Chicago/Turabian StyleLi, Wenlan, Xinwei Hou, Hua Zhang, Xiaoyan Yang, Zhaohua Ding, and Runqing Yue. 2025. "Evaluation of the Insect Resistance Efficacy of Transgenic Maize LD05 in China" Plants 14, no. 19: 3051. https://doi.org/10.3390/plants14193051
APA StyleLi, W., Hou, X., Zhang, H., Yang, X., Ding, Z., & Yue, R. (2025). Evaluation of the Insect Resistance Efficacy of Transgenic Maize LD05 in China. Plants, 14(19), 3051. https://doi.org/10.3390/plants14193051





