In Vitro Propagation of Endemic Kazakh Tulips: Effects of Temperature and Growth Regulators
Abstract
1. Introduction
2. Results
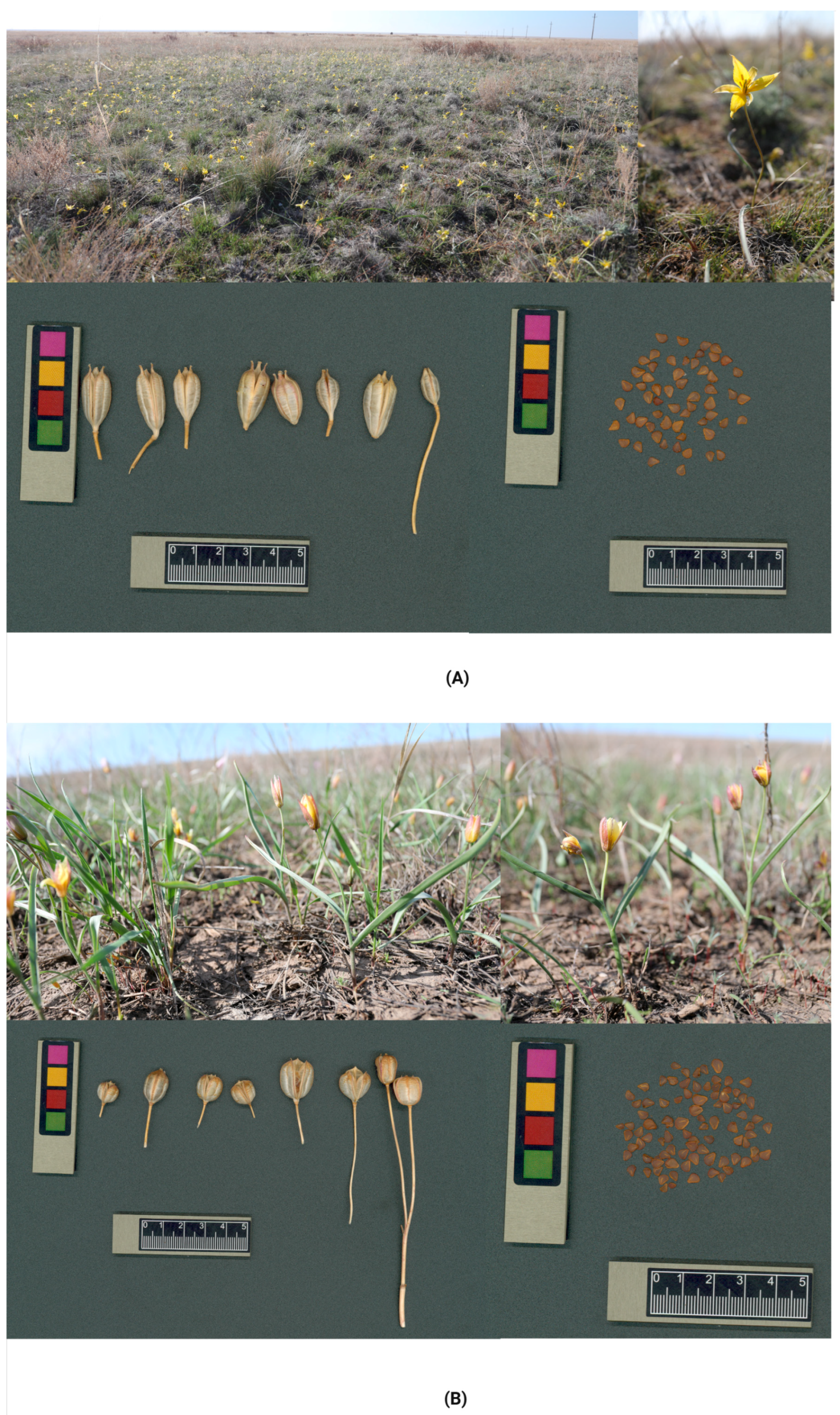
2.1. Population Status of T. auliekolica and T. turgaica
2.2. Introduction to In Vitro Culture
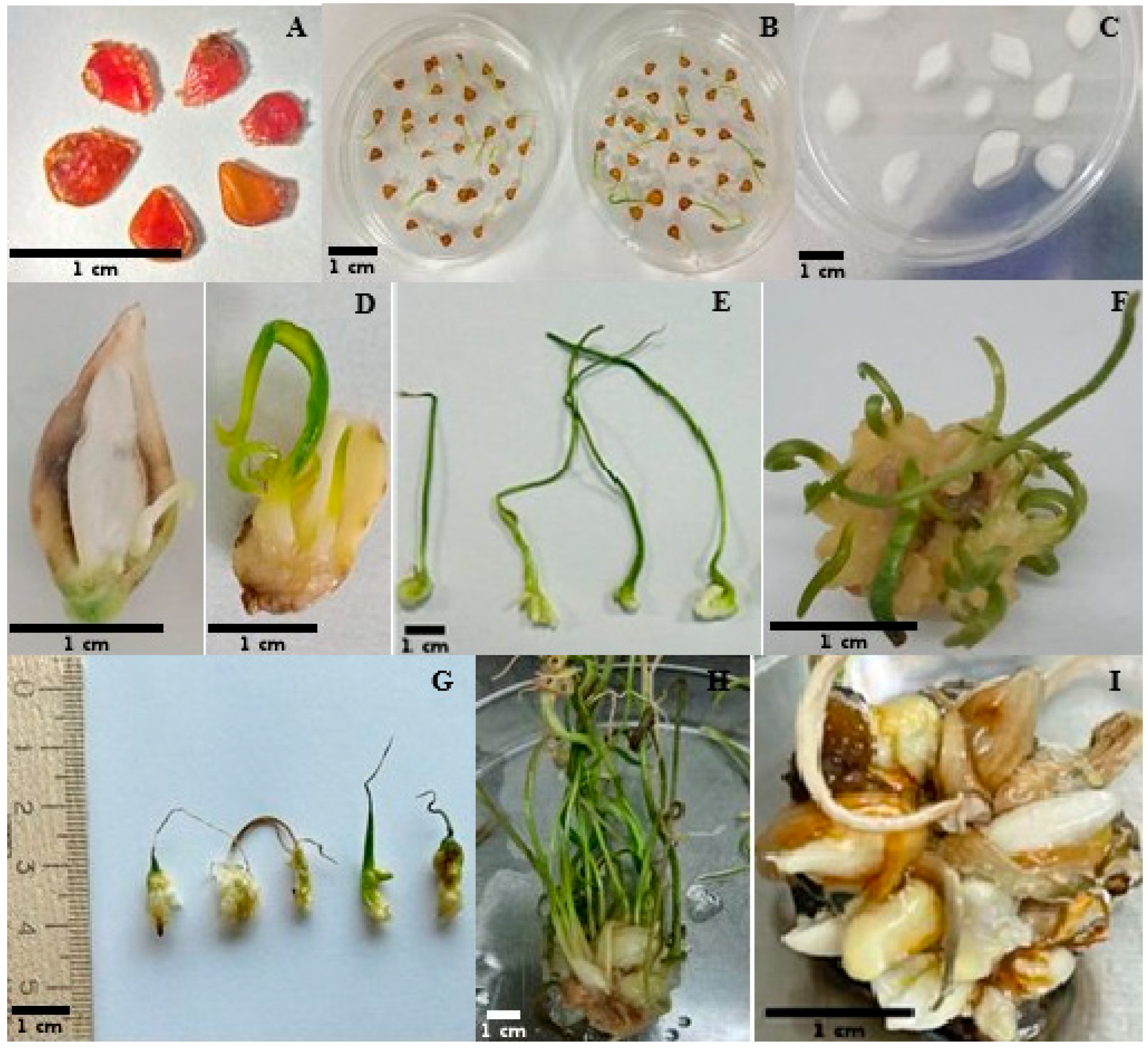
2.2.1. Establishment of Sterile Seedlings from T. auliekolica and T. turgaica Seeds
2.2.2. Microshoot Induction from T. auliekolica and T. turgaica Bulbs
2.3. Micropropagation
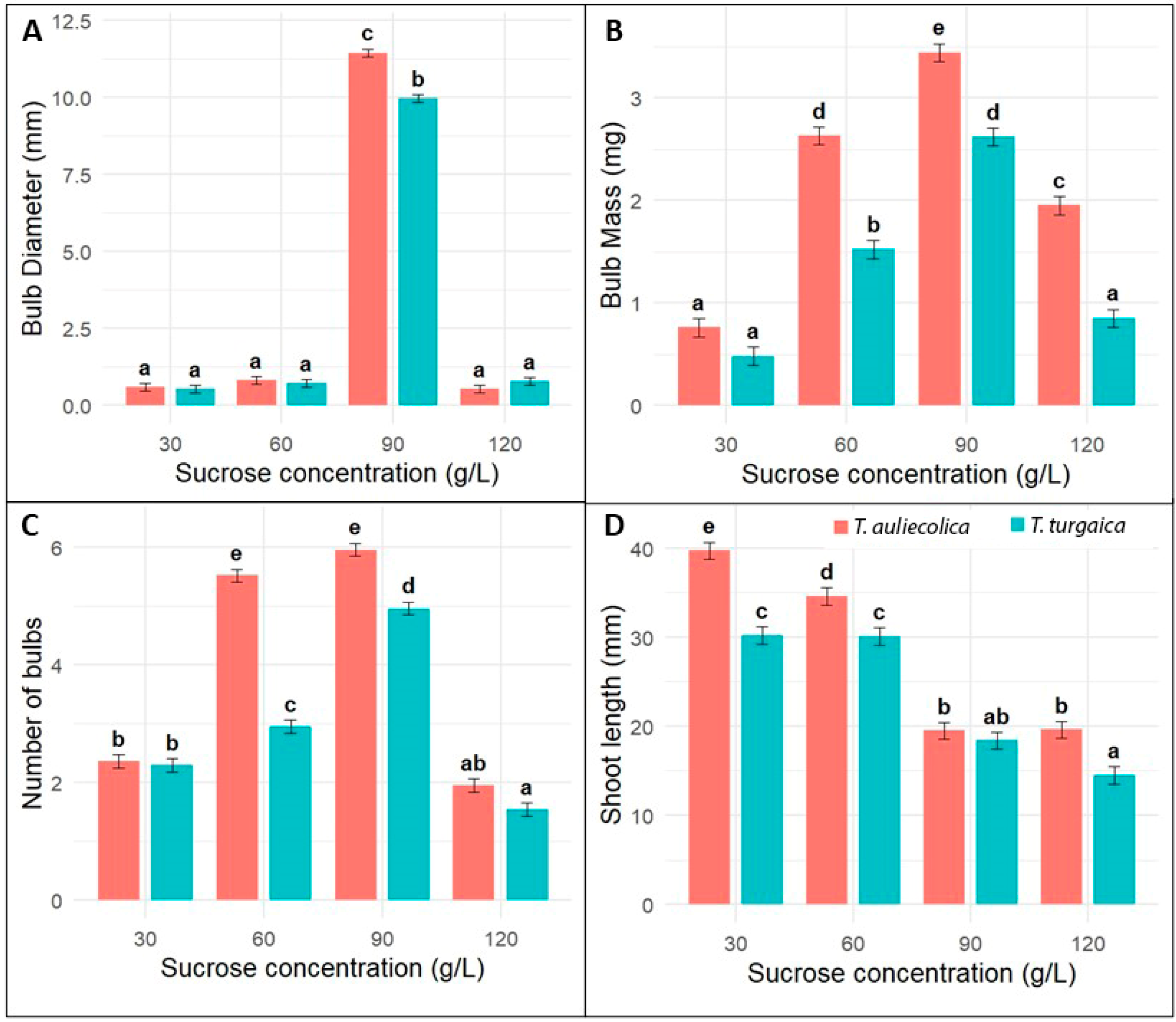
2.3.1. Seedling Development from Seeds
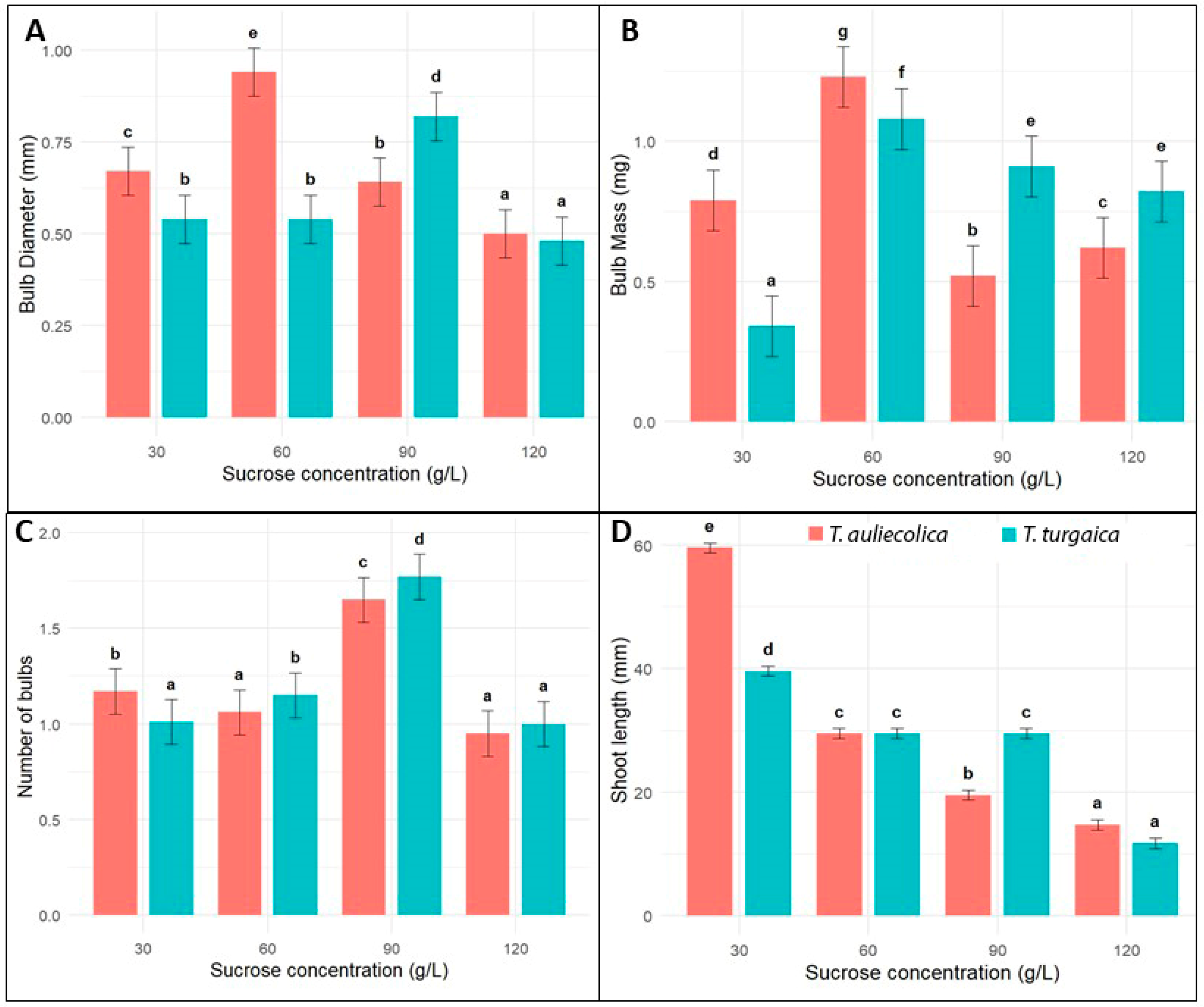
2.3.2. Microshoot Development from Bulbs
2.4. Microbulb Formation
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Tulip Plant Morphology
4.3. Determination of Seed Viability
4.4. Seed and Bulb Stratification Under In Vitro Conditions
4.5. Seed Germination Under In Vitro Conditions
4.6. Establishment of Aseptic Culture
4.7. Microbulb Induction and Development
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2iP | N6-(2-isopentenyl)adenine |
| ABA | Abscisic acid |
| ANOVA | Analysis of variance |
| BAP | Linear dichroism |
| GA3 | Gibberellic acid |
| HSD | Honestly significant difference |
| IBA | Indole-3-butyric acid |
| mT | Meta-topolin |
| NAA | 1-naphthylacetic acid |
| T50 | Time to 50% seed germination |
| TDZ | Thidiazuron |
| TTC | 2,3,5-triphenyltetrazolium chloride |
References
- Kubentayev, S.A.; Alibekov, D.T.; Perezhogin, Y.V.; Lazkov, G.A.; Kupriyanov, A.N.; Ebel, A.L.; Izbastina, K.S.; Borodulina, O.V.; Kubentayeva, B.B. Revised checklist of endemic vascular plants of Kazakhstan. PhytoKeys 2024, 238, 241–279. [Google Scholar] [CrossRef] [PubMed]
- Ivashchenko, A.A. Tulips and Other Bulbous Plants of Kazakhstan; Two Capitals: Almaty, Kazakhstan, 2005; p. 192. [Google Scholar]
- Ivashchenko, A.A.; Belyalov, O.V. Kazakhstan Is the Birthplace of Tulips; Atamura: Almaty, Kazakhstan, 2019; p. 368. (In Russian) [Google Scholar]
- Kubentayev, S.A.; Baasanmunkh, S.; Alibekov, D.T.; Tojibaev, K.S.; Nyamgerel, N.; Ivashchenko, A.A.; Tsegmed, Z.; Epiktetov, V.G.; Sitpayeva, G.T.; Izbastina, K.S.; et al. Revisiting the genus Tulipa (Liliaceae) in Kazakhstan, the country with the richest tulip diversity worldwide. PhytoKeys 2024, 250, 95–163. [Google Scholar] [CrossRef]
- Perezhogin, Y.V. New species of tulips from Northern Kazakhstan. Bot. Z. 2013, 98, 1558–1563. (In Russian) [Google Scholar]
- Chen, T.; Bao, A.; Jiapaer, G.; Guo, H.; Zheng, G.; Jiang, L.; Chang, C.; Tuerhanjiang, L. Disentangling the relative impacts of climate change and human activities on arid and semiarid grasslands in Central Asia during 1982–2015. Sci. Total Environ. 2019, 653, 1311–1325. [Google Scholar] [CrossRef]
- Pearson, R.G.; Stanton, J.C.; Shoemaker, K.T.; Aiello-Lammens, M.E.; Ersts, P.J.; Horning, N.; Fordham, D.A.; Raxworthy, C.J.; Ryu, H.Y.; McNees, J.; et al. Life history and spatial traits predict extinction risk due to climate change. Nature Clim. Change 2014, 4, 217–221. [Google Scholar] [CrossRef]
- Lughadha, E.N.; Bachman, S.P.; Leão, T.C.C.; Forest, F.; Halley, J.M.; Moat, J.; Acedo, C.; Bacon, K.L.; Brewer, R.F.A.; Gâteblé, G.; et al. Extinction risk and threats to plants and fungi. Plants People Planet 2020, 2, 389–408. [Google Scholar] [CrossRef]
- Wilson, B.; Dolotbakov, A.; Burgess, B.J.; Clubbe, C.; Lazkov, G.; Shalpykov, K.; Ganybaeva, M.; Sultangaziev, O.; Brockington, S.F. Central Asian wild tulip conservation requires a regional approach, especially in the face of climate change. Biodivers. Conserv. 2021, 30, 1705–1730. [Google Scholar] [CrossRef]
- de Groot, J.J.; Zonneveld, B.J.M. Two new tulip species from the Altai Mountains, Kazakhstan. Int. Rock Gard. 2020, 122, 3–16. [Google Scholar]
- Bobo-Pinilla, J.; Salmerón-Sánchez, E.; Mota, J.F.; Peñas, J. Genetic conservation strategies of endemic plants from edaphic habitat islands: The case of Jacobaea auricula (Asteraceae). J. Nat. Conserv. 2021, 61, 126004. [Google Scholar] [CrossRef]
- Coelho, N.; Gonçalves, S.; Romano, A. Endemic plant species conservation: Biotechnological approaches. Plants 2020, 9, 345. [Google Scholar] [CrossRef]
- Faria, D.V.; Simão, M.J.; Cipriano, R.; Werner, E.T.; Soares, T.C.B.; Aoyama, E.M.; Lima-Gontijo, A.B.P. In vitro morphogenesis and micropropagation of Aechmea ramosa var. ramosa Mart. ex Schult. f. (Bromeliaceae) from leaf explants. In Vitro Cell. Dev. Biol.-Plant 2018, 54, 530–536. [Google Scholar] [CrossRef]
- Regalado, J.J.; Carmona-Martín, E.; López-Granero, M.; Jiménez-Araujo, A.; Castro, P.; Encina, C.L. Micropropagation of Asparagus macrorrhizus, a Spanish endemic species in extreme extinction risk. Plant Cell Tiss. Organ. Cult. 2018, 132, 573–578. [Google Scholar] [CrossRef]
- Alexopoulos, A.A.; Mavrommati, E.; Kartsonas, E.; Petropoulos, S.A. Effect of temperature and sucrose on in vitro seed germination and bulblet production of Pancratium maritimum L. Agronomy 2022, 12, 2786. [Google Scholar] [CrossRef]
- Sochacki, D.; Marciniak, P.; Ciesielska, M.; Zaród, J.; Sutrisno. The influence of selected plant growth regulators and carbohydrates on in vitro shoot multiplication and bulbing of the tulip (Tulipa L.). Plants 2023, 12, 1134. [Google Scholar] [CrossRef]
- Hatzilazarou, S.; Pipinis, E.; Kostas, S.; Stagiopoulou, R.; Gitsa, K.; Dariotis, E.; Avramakis, M.; Samartza, I.; Plastiras, I.; Kriemadi, E.; et al. Influence of temperature on seed germination of five wild-growing Tulipa species of Greece associated with their ecological profiles: Implications for conservation and cultivation. Plants 2023, 12, 1574. [Google Scholar] [CrossRef]
- Maślanka, M. Effect of light spectrum, sucrose concentration, and 6-benzyl-aminopurine on in vitro adventitious bulb formation in Tulipa tarda. Agronomy 2025, 15, 642. [Google Scholar] [CrossRef]
- Pipinis, E.; Hatzilazarou, S.; Kostas, S.; Stagiopoulou, R.; Gitsa, K.; Dariotis, E.; Samartza, I.; Plastiras, I.; Kriemadi, E.; Bareka, P.; et al. Effect of temperature on breaking of morphophysiological dormancy and seed germination leading to bulblet production in two endemic tulip species from Greece. Plants 2023, 12, 1859. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Specht, C.E.; Keller, E.R.J. Temperature requirements for seed germination in species of the genus Allium L. Genet. Resour. Crop Evol. 1997, 44, 509–517. [Google Scholar] [CrossRef]
- Stepanova, A.Y.; Gladkov, E.A.; Tereshonok, D.V.; Selimov, R.N.; Goncharova, E.N.; Solov’eva, A.I. The effect of low positive temperatures on the formation of secondary metabolites in Rhodiola quadrifida (Pall.) Fisch. et C.A. Mey. In vitro cultures. Processes 2023, 11, 28. [Google Scholar] [CrossRef]
- Kozoni, M.; Samartza, I.; Pipinis, E.; Kostas, S.; Anestis, I.; Karapatzak, E.; Bareka, P.; Hatzilazarou, S.; Tsoktouridis, G.; Krigas, N. Dormancy release and seed germination in Tulipa saxatilis (Liliaceae) coupled with effects of fertilization schemes for bulblet development from seedlings. Horticulturae 2024, 10, 820. [Google Scholar] [CrossRef]
- Klupczyńska, E.A.; Pawłowski, T.A. Regulation of seed dormancy and germination mechanisms in a changing environment. Int. J. Mol. Sci. 2021, 22, 1357. [Google Scholar] [CrossRef]
- IPCC. Annex IV: Contributors to the Working Group II Contribution to the IPCC Sixth Assessment Report. In Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; pp. 2939–2963. [Google Scholar] [CrossRef]
- Vandelook, F.; Van Assche, J.A. Temperature conditions control embryo growth and seed germination of Corydalis solida (L.) Clairv., A temperate forest spring geophyte. Plant Biol. 2009, 11, 899–906. [Google Scholar] [CrossRef]
- Ritchie, S.; Gilroy, S. Gibberellins: Regulating genes and germination. New Phytol. 1998, 140, 363–383. [Google Scholar] [CrossRef]
- Gupta, R.; Chakrabarty, S.K. Gibberellic acid in plant: Still a mystery unresolved. Plant Signal Behav. 2013, 8, e25504. [Google Scholar] [CrossRef] [PubMed]
- Balaguera-López, H.E.; Cárdenas-Hernández, J.F.; Álvarez-Herrera, J.G. Effect of gibberellic acid (GA3) on seed germination and growth of tomato (Solanum lycopersicum L.). Acta Hortic. 2009, 821, 141–148. [Google Scholar] [CrossRef]
- Badino, M. Gametocidal effects of gibberellic acid (GA3, GA4+7) on common onion (Allium cepa L.). Acta Hortic. 1981, 111, 79–88. [Google Scholar] [CrossRef]
- Tang, N.; Jia, R.; Yin, J.; Wang, Y.; Tang, D. Effects of cold treatments on seedling emergence and growth of Lilium davidii var. unicolor bulblets. Hortscience 2021, 56, 1119–1124. [Google Scholar] [CrossRef]
- Vishal, B.; Kumar, P.P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef]
- Maślanka, M.; Bach, A. Induction of bulb organogenesis in in vitro cultures of tarda tulip (Tulipa tarda Stapf.) from seed-derived explants. In Vitro Cell Dev. Biol. Plant 2014, 50, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Famelaer, I.; Ennik, E.; Eikelboom, W.; Van Tuyl, J.M.; Creemers-Molenaar, J. The initiation of callus and regeneration from callus culture of Tulipa gesneriana. Plant Cell Tiss. Organ. Cult. 1996, 47, 51–58. [Google Scholar] [CrossRef]
- Yasemin, S.; Beruto, M. A review on flower bulb micropropagation: Challenges and opportunities. Horticulturae 2024, 10, 284. [Google Scholar] [CrossRef]
- Yasemin, S.; Koksal, N.; Buyukalaca, S. Indirect organogenesis and in vitro bulb formation of Pancratium maritimum. Plant Cell Tiss. Organ. Cult. 2023, 154, 713–727. [Google Scholar] [CrossRef]
- Langens-Gerrits, M.; Kuijpers, A.-M.; De Klerk, G.-J.; Croes, A. Contribution of explant carbohydrate reserves and sucrose in the medium to bulb growth of lily regenerated on scale segments in vitro. Physiol. Plant 2003, 117, 245–255. [Google Scholar] [CrossRef]
- Podwyszyńska, M. In vitro tetraploid induction in tulip (Tulipa gesneriana L.). Acta Hortic. 2012, 961, 391–396. [Google Scholar] [CrossRef]
- Sanlewski, M.; Kawa-Miszczak, L. Hormonal control of growth and development of tulips. Acta Hortic. 1992, 325, 43–54. [Google Scholar] [CrossRef]
- Miao, Y.; Zhu, Z.; Guo, Q.; Yang, X.; Liu, L.; Sun, Y.; Wang, C. Dynamic changes in carbohydrate metabolism and endogenous hormones during Tulipa edulis stolon development into a new bulb. J. Plant Biol. 2016, 59, 121–132. [Google Scholar] [CrossRef]
- Maślanka, M.; Bach, A.; Janowiak, F. Endogenous ABA content in relation to maturation of somatic embryos in Tulipa (L.) ‘Apeldoorn’ cultures. Acta Physiol. Plant 2016, 38, 270. [Google Scholar] [CrossRef]
- Bukhari, N.A.W.; Siddique, I.; Perveen, K. Preculturing effect of thidiazuron on in vitro shoot multiplication and micropropagation round in Capparis decidua (Forsk.) an important multipurpose plant. Acta Biol. Hung. 2016, 67, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.R.; Anis, M. Role of TDZ in the quick regeneration of multiple shoots from nodal explant of Vitex trifolia L.—An important medicinal plant. Appl. Biochem. Biotechnol. 2012, 168, 957–966. [Google Scholar] [CrossRef]
- Naz, R.; Anis, M.; Aref, I.M. Assessment of the potentiality of TDZ on multiple shoot induction in Bauhinia tomentosa L., a woody legume. Acta Biol. Hung. 2012, 63, 474–482. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Sochacki, D. Micropropagation of tulip: Production of virus-free stock plants. Methods Mol. Biol. 2010, 589, 243–256. [Google Scholar] [CrossRef]
- Spíchal, L.; Rakova, N.Y.; Riefler, M.; Mizuno, T.; Romanov, G.A.; Strnad, M.; Schmülling, T. Two cytokinin receptors of Arabidopsis thaliana, CRE1/AHK4 and AHK3, differ in their ligand specificity in a bacterial assay. Plant Cell Physiol. 2004, 45, 1299–1305. [Google Scholar] [CrossRef]
- Kou, Y.; Yuan, C.; Zhao, Q.; Liu, G.; Nie, J.; Ma, Z.; Cheng, C.; Teixeira da Silva, J.A.; Zhao, L. Thidiazuron triggers morphogenesis in Rosa canina L. protocorm-like bodies by changing incipient cell fate. Front. Plant Sci. 2016, 7, 557. [Google Scholar] [CrossRef]
- Grąbkowska, R.; Sitarek, P.; Wysokińska, H. Influence of thidiazuron (TDZ) pretreatment of shoot tips on shoot multiplication and ex vitro acclimatization of Harpagophytum procumbens. Acta Physiol. Plant. 2014, 36, 1661–1672. [Google Scholar] [CrossRef][Green Version]
- Singh, P.; Dwivedi, P. Two-stage culture procedure using thidiazuron for efficient micropropagation of Stevia rebaudiana, an anti-diabetic medicinal herb. 3 Biotech 2014, 4, 431–437. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Nurmansyah, N.Y.; Naidoo, Y.; Teixeira da Silva, J.A. Thidiazuron-induced abnormalities in plant tissue cultures. Plant Cell Rep. 2018, 37, 1451–1470. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Marasek-Ciolakowska, A. Micropropagation of tulip via somatic embryogenesis. Agronomy 2020, 10, 1857. [Google Scholar] [CrossRef]
- Shaheen, A.; Dewir, Y.H.; Kher, M.; Khan, M.; El-Banna, A.N.; Alaizari, A. Synergistic effect of benzylaminopurine and meta-Topolin combination for micropropagation of gerbera ‘Pink Melody’. Ciênc. Agrotec. 2022, 46, e017521. [Google Scholar] [CrossRef]
- Reshi, Z.A.; Husain, F.M.; Khanam, M.N.; Javed, S.B. Effect of meta-Topolin on morphological, physiochemical, and molecular dynamics during in vitro regeneration of Salix tetrasperma Roxb. BMC Plant Biol. 2025, 25, 121. [Google Scholar] [CrossRef] [PubMed]
- Khanam, M.N.; Javed, S.B.; Ahmad, N.; Singh, N. Substitution of Benzyladenine with Meta-Topolin Improved Shoot Regeneration and Rooting in Wedelia chinensis (Osbeck) Merr. and Quantitative Estimation of Flavonoids. In Propagation and Genetic Manipulation of Plants; Siddique, I., Ed.; Springer: Singapore, 2021; pp. 139–151. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, S.; Banerjee, M. Comparative analysis of the effect of 6-benzylaminopurin vs. meta-Topolin on in vitro regeneration, chlorophyll and protein contents in winter cherry Withania somnifera. Plant Cell Tiss. Organ. Cult. 2024, 158, 43. [Google Scholar] [CrossRef]
- Lata, H.; Chandra, S.; Techen, N.; Khan, I.A.; ElSohly, M.A. In vitro mass propagation of Cannabis sativa L.: A protocol refinement using novel aromatic cytokinin meta-topolin and the assessment of eco-physiological, biochemical and genetic fidelity of micropropagated plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 18–26. [Google Scholar] [CrossRef]
- Marković, M.; Trifunović Momčilov, M.; Uzelac, B.; Jevremović, S.; Subotić, A. Bulb dormancy in vitro—Fritillaria meleagris: Initiation, release and physiological parameters. Plants 2021, 10, 902. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; Klerk, G.J.D. The Components of Plant Tissue Culture Media II: Organic Additions, Osmotic and pH Effects, and Support Systems. In Plant Propagation by Tissue Culture; George, E.F., Hall, M.A., Klerk, G.J.D., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 115–173. [Google Scholar] [CrossRef]
- Morin, A.; Maurousset, L.; Vriet, C.; Lemoine, R.; Doidy, J.; Pourtau, N. Carbon fluxes and environmental interactions during legume development, with a specific focus on Pisum sativum. Physiol. Plant 2022, 174, e13729. [Google Scholar] [CrossRef]
- Azeri, F.N.; Öztürk, G. Microbulb and plantlet formation of a native bulbous flower, Lilium monodelphum M. Bieb, var. Armenum, through tissue culture propagation. Biotechnol. Rep. 2021, 32, e00665. [Google Scholar] [CrossRef]
- Ludwig-Müller, J. Indole-3-butyric acid in plant growth and development. Plant Growth Regul. 2000, 32, 219–230. [Google Scholar] [CrossRef]
- Lawson, J.D.; Bridges, W.C.; Adelberg, J.W. IBA delivery technique and media salts affected in vitro rooting and acclimatization of eight Prunus genotypes. Plants 2023, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Pernisová, M.; Klíma, P.; Horák, J.; Válková, M.; Malbeck, J.; Soucek, P.; Reichman, P.; Hoyerová, K.; Dubová, J.; Friml, J.; et al. Cytokinins modulate auxin-induced organogenesis in plants via regulation of the auxin efflux. Proc. Natl. Acad. Sci. USA 2009, 106, 3609–3614. [Google Scholar] [CrossRef] [PubMed]
- Raspor, M.; Motyka, V.; Kaleri, A.R.; Ninković, S.; Tubić, L.; Cingel, A.; Ćosić, T. Integrating the roles for cytokinin and auxin in de novo shoot organogenesis: From hormone uptake to signaling outputs. Int. J. Mol. Sci. 2021, 22, 8554. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, Y.; Li, S.; Zhang, W.; Yin, C.; Lin, Y. Regulation of phytohormones on the growth and development of plant root hair. Front. Plant Sci. 2022, 13, 865302. [Google Scholar] [CrossRef]
- PlantNet. Plant Identification and Information Website. Available online: https://plantnet.org/en/ (accessed on 11 September 2025).
- Tagimanova, D.; Raiser, O.; Danilova, A.; Turzhanova, A.; Khapilina, O. Micropropagation of rare endemic species Allium microdictyon Prokh. threatened in Kazakhstani Altai. Horticulturae 2024, 10, 943. [Google Scholar] [CrossRef]
- Coolbear, P.; Francis, A.; Grierson, D. The effect of low temperature pre-sowing treatment on the germination performance and membrane integrity of artificially aged tomato seeds. J. Exp. Bot. 1984, 35, 1609–1617. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]

| Seed Germination Rate (%) | |||
|---|---|---|---|
| Temperature Regime (°C) | Media Type | ||
| Distilled Water (Control) | ½MS | ½MS + 10 mg L−1 GA3 | |
| 4 | 58.0 ± 0.02 cd | 63.0 ± 0.02 de | 72.0 ± 0.01 f |
| 10 | 48.0 ± 0.03 b | 51.0 ± 0.02 b | 54.0 ± 0.01 bc |
| 20 | 0.0 ± 0 a | 0.0 ± 0 a | 5.0 ± 0.01 a |
| 4/10 | 62.0 ± 0.02 de | 68.0 ± 0.02 ef | 88.0 ± 0.02 g |
| 10/20 | 50.0 ± 0.02 b | 52.0 ± 0.02 bc | 57.0 ± 0.02 cd |
| Seed Germination Rate (%) | |||
|---|---|---|---|
| Temperature (°C) | Media Type | ||
| Distilled Water (Control) | ½MS | ½MS + 10 mg L−1 GA3 | |
| 4 | 12.0 ± 0.02 b | 15.0 ± 0.03 b | 30.0 ± 0.02 c |
| 10 | 15.0 ± 0.03 b | 35.0 ± 0.04 cd | 65.0 ± 0.04 e |
| 20 | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a |
| 4/10 | 20.0 ± 0.02 b | 20.0 ± 0.02 b | 35.0 ± 0.03 cd |
| 10/20 | 20.0 ± 0.02 b | 40.0 ± 0.02 d | 69.0 ± 0.01 e |
| Species | Number of Explants | Days to Shoot Formation | Shoot Formation Frequency (%) | Number of Shoots per Explant |
|---|---|---|---|---|
| T. auliekolica | 200 | 22.5 ± 1.5 | 95.7 ± 2.1 | 2.8 ± 0.3 |
| T. turgaica | 230 | 32.6 ± 1.5 | 91.3 ± 2.0 | 1.7 ± 0.3 |
| p-value | <0.001 | <0.001 | <0.001 |
| Culture Medium | Length of Shoots (cm) | |
|---|---|---|
| T. auliekolica | T. turgaica | |
| MS (control) | 2.6 ± 0.8 a | 1.6 ± 0.7 a |
| MSP1 | 3.5 ± 0.8 ab | 3.2 ± 1.2 ab |
| MSP2 | 4.7 ± 1.5 bc | 3.9 ± 1.0 b |
| MSP3 | 11.8 ± 1.5 d | 8.8 ± 1.9 c |
| MSP4 | 5.6 ± 1.0 c | 4.5 ±1.4 b |
| MSP5 | 3.5 ± 0.12 ab | 3.7 ± 1.2 b |
| MSP6 | 4.5 ± 1.4 bc | 4.8 ± 1.3 b |
| Culture Medium | T. auliekolica | T. turgaica | ||
|---|---|---|---|---|
| Number of Shoots/Explant | Shoot Length (cm) | Number of Shoots/Explant | Shoot Length (cm) | |
| MS (control) | 2.0 ± 0.7 a | 1.7 ± 0.8 a | 1.3 ± 0.7 a | 1.6 ± 0.7 ab |
| MSP1 | 5.0 ± 1.1 c | 2.4 ± 1.2 c | 3.5 ± 1.2 bc | 2.1 ± 0.7 ab |
| MSP2 | 2.5 ± 0.8 ab | 1.8 ± 0.8 ab | 2.2 ± 0.8 ab | 1.3 ± 0.7 a |
| MSP3 | 9.6 ± 3.3 d | 7.7 ± 1.3 d | 3.7 ± 1.1 c | 4.6 ± 0.8 c |
| MSP4 | 4.9 ± 1.2 c | 2.7 ± 1.2 c | 3.4 ± 1.2 abc | 2.2 ± 1.1 ab |
| MSP5 | 5.7 ± 1.8 c | 2.5 ± 1.3 c | 4.2 ± 1.5 abc | 2.5 ± 0.5 b |
| MSP6 | 4.3 ± 0.7 bc | 2.4 ± 1.2 bc | 3.4 ± 1.4 abc | 1.9 ± 0.9 ab |
| Culture Medium | Number of De Novo Microshoot Formation/Explant | |||||
|---|---|---|---|---|---|---|
| T. auliekolica | T. turgaica | |||||
| First Subculture | Second Subculture | Third Subculture | First Subculture | Second Subculture | Third Subculture | |
| MSP1 | 2.11 ± 0.6 abcd | 1.89 ± 0.6 abc | 1.0 ± 0.9 a | 1.79 ± 0.7 abcd | 1.51 ± 0.5 abc | 1.22 ± 0.3 ab |
| MSP2 | 1.32 ± 0.4 ab | 1.13 ± 0.3 abc | 1.0 ± 0.4 a | 1.51 ± 0.5 abc | 1.23 ± 0.4 ab | 1.0 ± 0.2 ab |
| MSP3 | 4.54 ± 1.4 g | 3.97 ± 1.3 fg | 3.4 ± 1.1 efg | 3.97 ± 1.3 f | 3.78 ± 1.2 f | 3.02 ± 1.0 ef |
| MSP4 | 2.46 ± 0.8 bscde | 2.08 ± 0.7 abcd | 1.89 ± 0.6 abc | 1.98 ±0.5 abcde | 1.51 ±0.5 abc | 0.95 ± 0.3 a |
| MSP5 | 3.54 ± 1.0 efg | 3.21 ± 1.0 def | 3.02 ± 0.9 cdef | 3.02 ± 0.9 ef | 2.65 ± 0.8 de | 2.46 ± 0.7 cde |
| MSP6 | 2.56 ± 0.7 cde | 1.98 ± 0.7 abcd | 1.8 ± 0.4 abc | 2.51 ± 0.5 cde | 2.03 ± 0.6 bcde | 1.51 ± 0.5 abc |
| Culture Medium | T. auliekolica | T. turgaica | |||
|---|---|---|---|---|---|
| Auxin (mg/L) | Microbulbs per Explant | Bulb Weight (mg) | Microbulbs per Explant | Bulb Weight (mg) | |
| MSB1 | IAA 0.5 | 4.50 ± 1.6 ab | 2.15 ± 0.8 b | 4.20 ± 1.3 ca | 1.52 ± 0.3 ca |
| MSB2 | IAA 1.0 | 4.47 ± 1.4 ab | 1.49 ± 0.6 ab | 3.85 ± 1.1 bca | 1.45 ± 0.3 bca |
| MSB3 | IAA 2.0 | 3.46 ± 0.8 a | 1.02 ± 0.5 a | 1.98 ± 0.7 a | 0.88 ± 0.3 a |
| MSB4 | IBA 0.5 | 7.69 ± 1.4 ca | 3.74 ± 0.4 ca | 6.18 ± 0.6 cb | 2.95 ± 0.3 cb |
| MSB5 | IBA 1.0 | 5.38 ± 1.5 b | 1.90 ± 0.4 b | 4.45 ± 0.7 ca | 1.06 ± 0.4 ab |
| MSB6 | IBA 2.0 | 3.80 ± 1.3 ab | 1.54 ± 0.3 ab | 2.93 ± 0.5 ab | 0.85 ± 0.2 a |
| Culture Medium | Growth Regulators (mg/L) | |||||
|---|---|---|---|---|---|---|
| BAP | TDZ | NAA | IBA | mT | 2iP | |
| MSP1 | 5.0 | 0.1 | 0.1 | |||
| MSP2 | 5.0 | 0.1 | 0.1 | |||
| MSP3 | 5.0 | 0.1 | 0.1 | |||
| MSP4 | 5.0 | 0.1 | 0.1 | |||
| MSP5 | 5.0 | 0.1 | 0.1 | |||
| MSP6 | 5.0 | 0.1 | 0.1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagimanova, D.; Raiser, O.; Kubentayeva, B.; Nagmetova, G.; Turzhanova, A.; Khapilina, O. In Vitro Propagation of Endemic Kazakh Tulips: Effects of Temperature and Growth Regulators. Plants 2025, 14, 3014. https://doi.org/10.3390/plants14193014
Tagimanova D, Raiser O, Kubentayeva B, Nagmetova G, Turzhanova A, Khapilina O. In Vitro Propagation of Endemic Kazakh Tulips: Effects of Temperature and Growth Regulators. Plants. 2025; 14(19):3014. https://doi.org/10.3390/plants14193014
Chicago/Turabian StyleTagimanova, Damelya, Olesya Raiser, Balsulu Kubentayeva, Gulden Nagmetova, Ainur Turzhanova, and Oxana Khapilina. 2025. "In Vitro Propagation of Endemic Kazakh Tulips: Effects of Temperature and Growth Regulators" Plants 14, no. 19: 3014. https://doi.org/10.3390/plants14193014
APA StyleTagimanova, D., Raiser, O., Kubentayeva, B., Nagmetova, G., Turzhanova, A., & Khapilina, O. (2025). In Vitro Propagation of Endemic Kazakh Tulips: Effects of Temperature and Growth Regulators. Plants, 14(19), 3014. https://doi.org/10.3390/plants14193014







