Stoma Detection in Soybean Leaves and Rust Resistance Analysis
Abstract
1. Introduction
- (1)
- A dataset of soybean leaf stomata was constructed, comprising healthy and rust-infected leaves from three varieties. The dataset contained 1800 RGB images with 25,396 labeled stomata instances.
- (2)
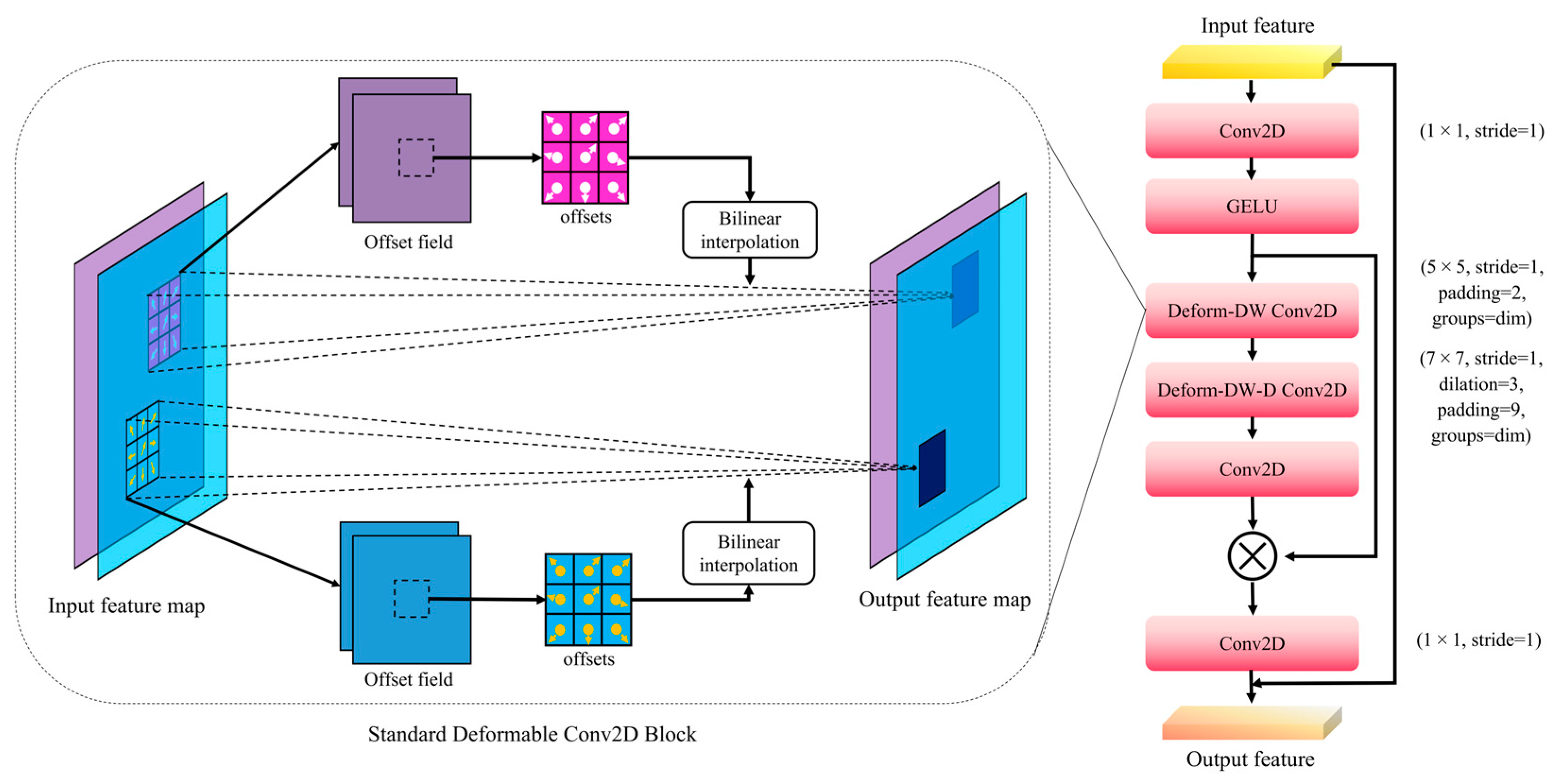
- The SPPF module of the YOLOv8 backbone was enhanced via the Large and Separable Kernel Attention (LSKA) mechanism. LSKA weighted multi-scale feature maps, resulting in enhanced adaptation to variations in multi-scale feature maps.
- (3)
- Deformable Large Kernel Attention (DLKA) was introduced into the C2f module of the Neck, adaptively capturing irregular shapes and sizes. This improved SS-YOLO’s robustness and detection performance in small object detection, complex backgrounds, and low-contrast images.
- (4)
- Based on the detection results, phenotypic parameters like the stomatal length, width, number, density, area, and stomata index of soybean leaves were automatically extracted. The relationship between stomatal characteristics and disease resistance in different soybean varieties and at different disease stages was analyzed, demonstrating the potential impact of stomatal characteristics on disease resistance.
2. Materials and Methods
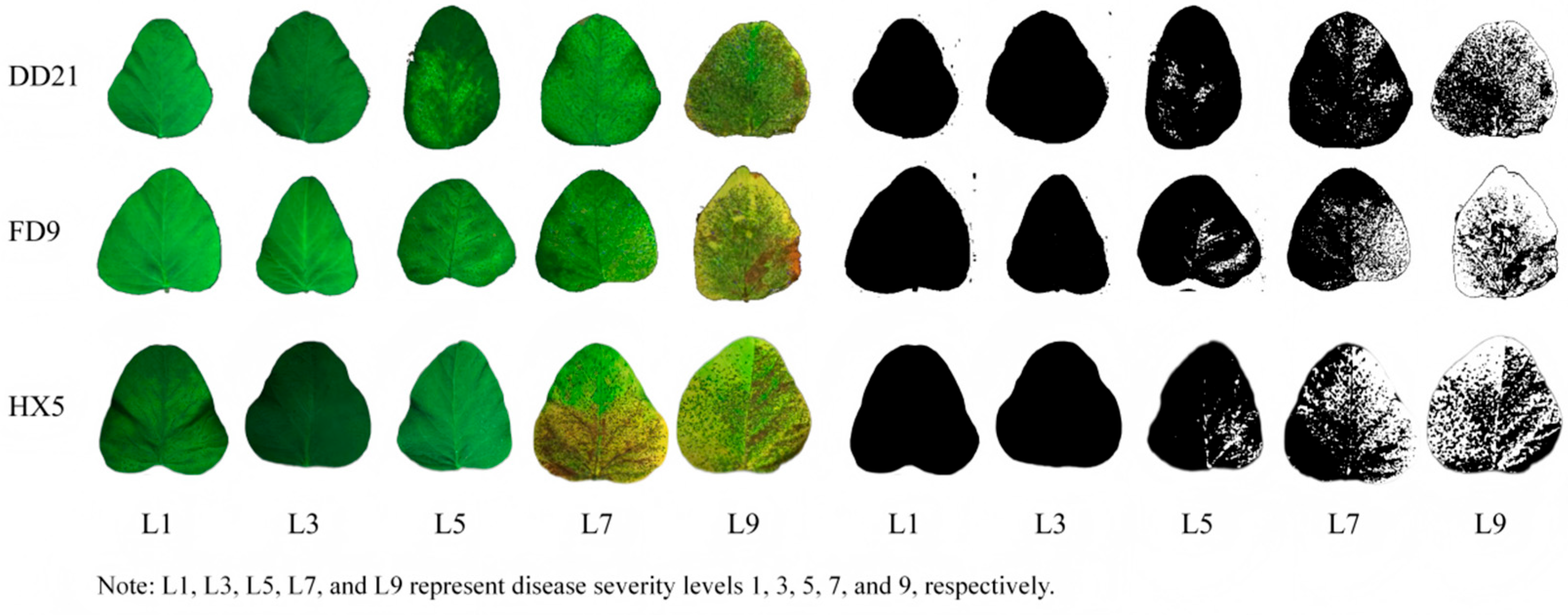
2.1. Plant Materials
- Plant Materials and Growth Conditions
- Pathogen and Inoculum Preparation
- Inoculation and Disease Management
- Preliminary Evaluation of Rust Resistance
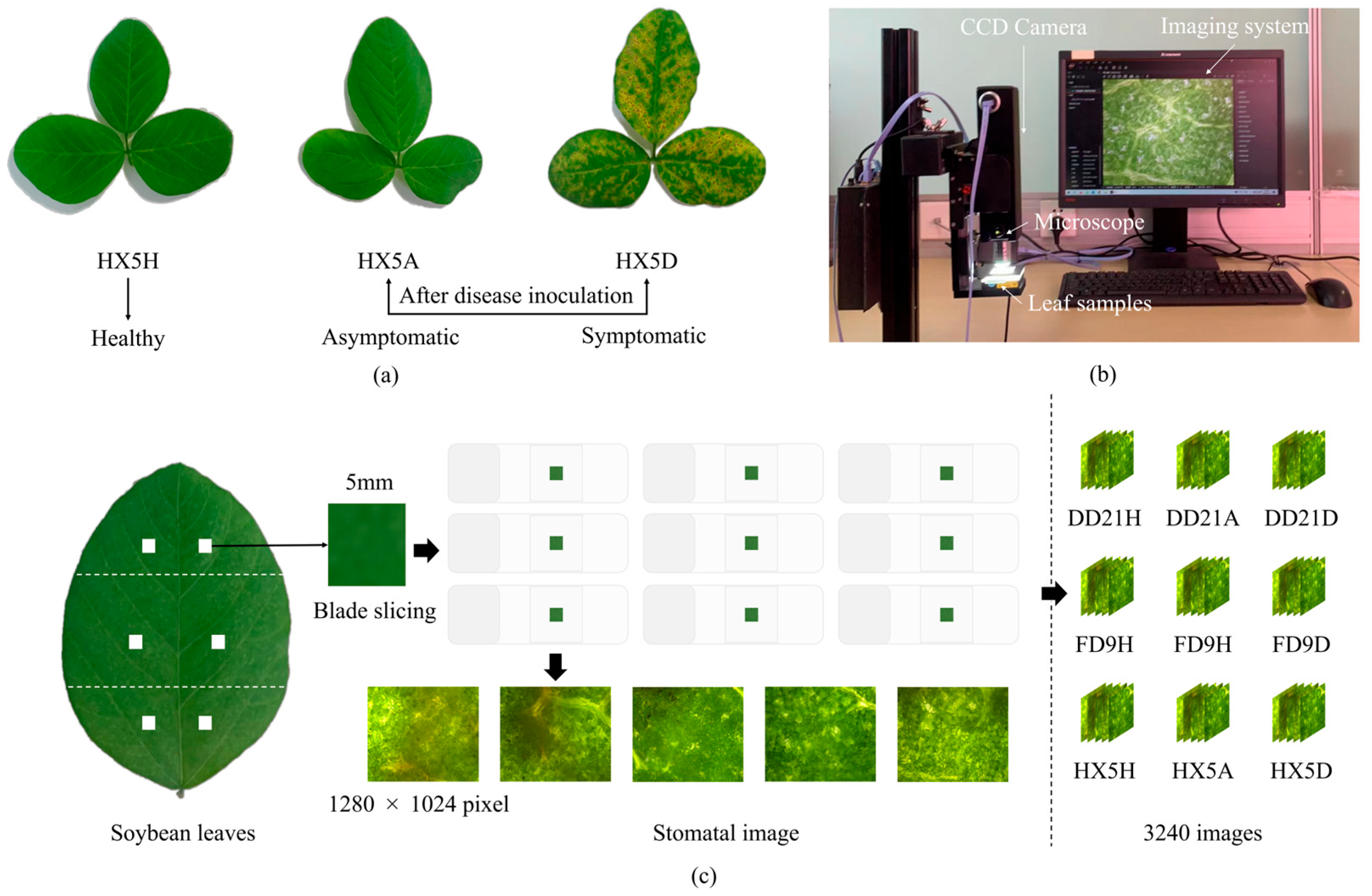
2.2. Collection Equipment and Methods
2.3. Dataset Construction
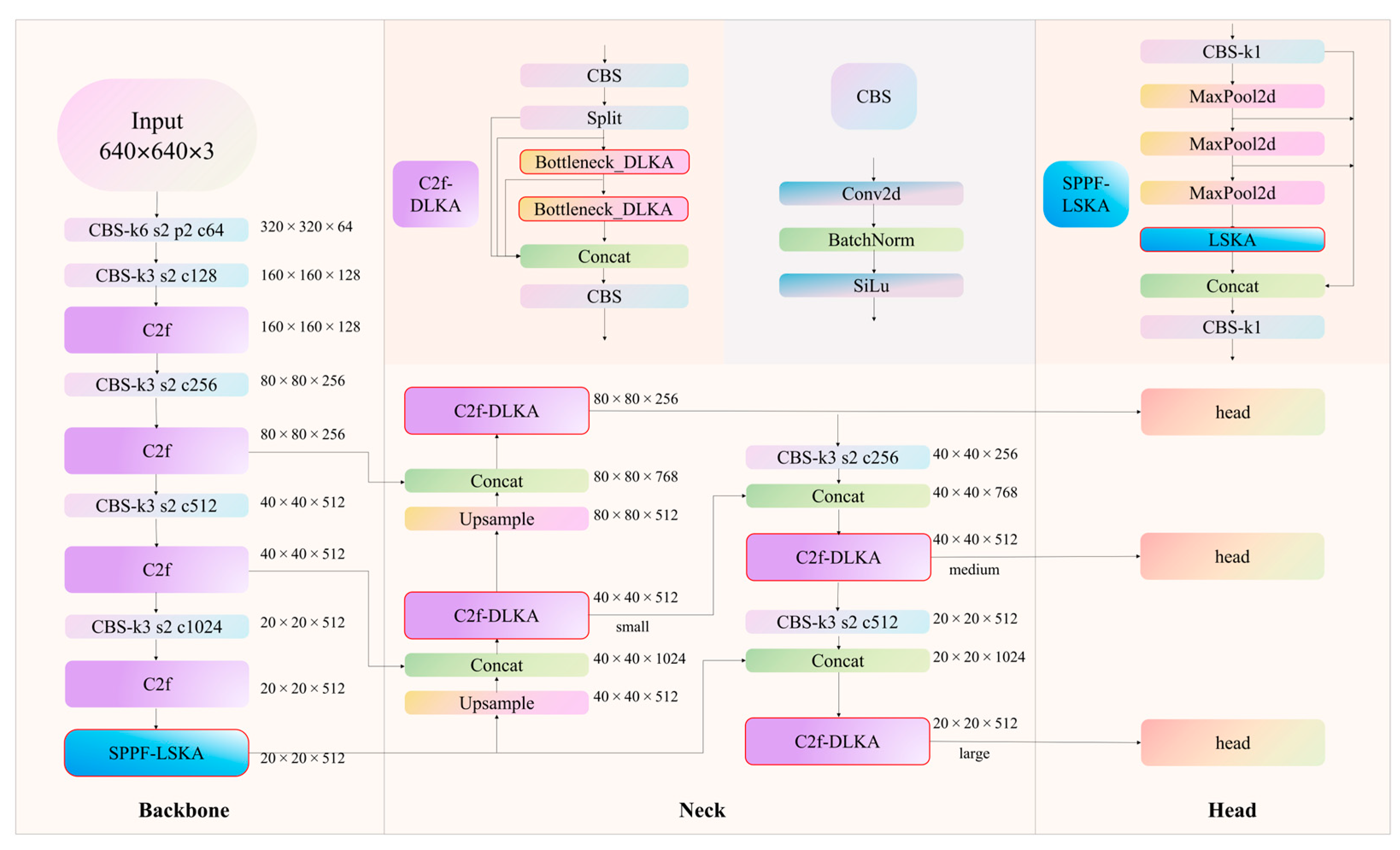
2.4. Stoma Detection Model SS-YOLO
2.5. SPPF-LSKA Module
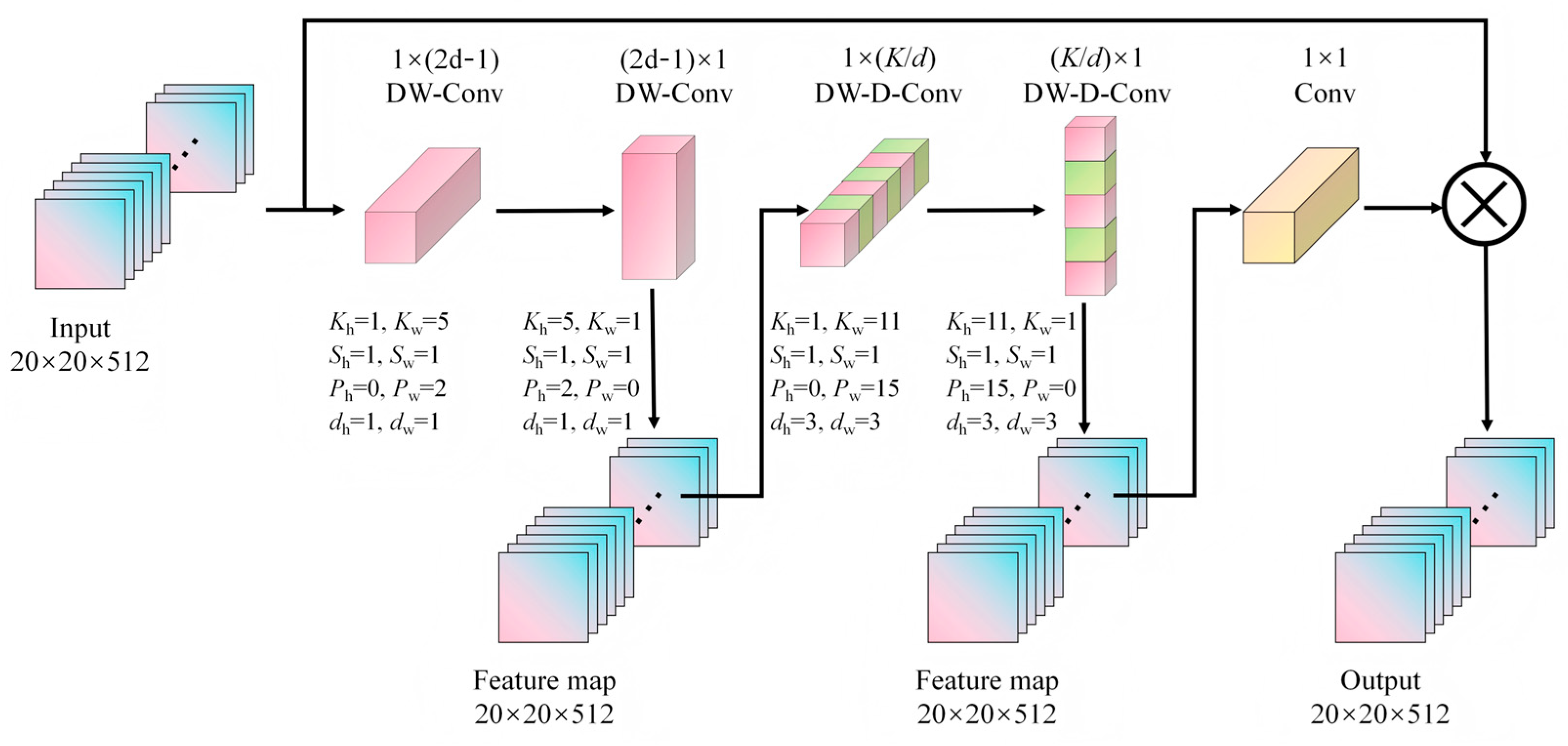
2.6. C2f-DLKA Module
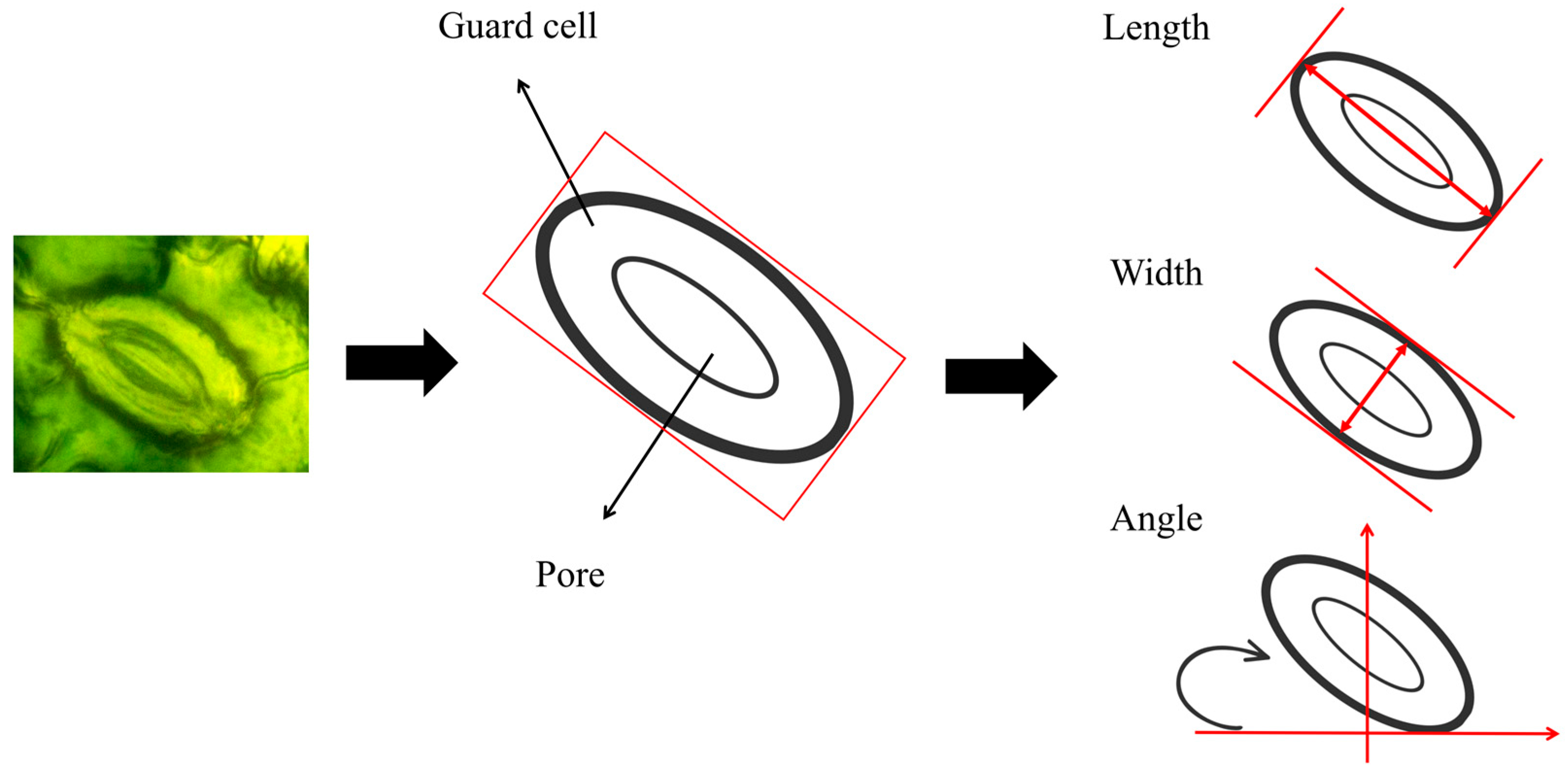
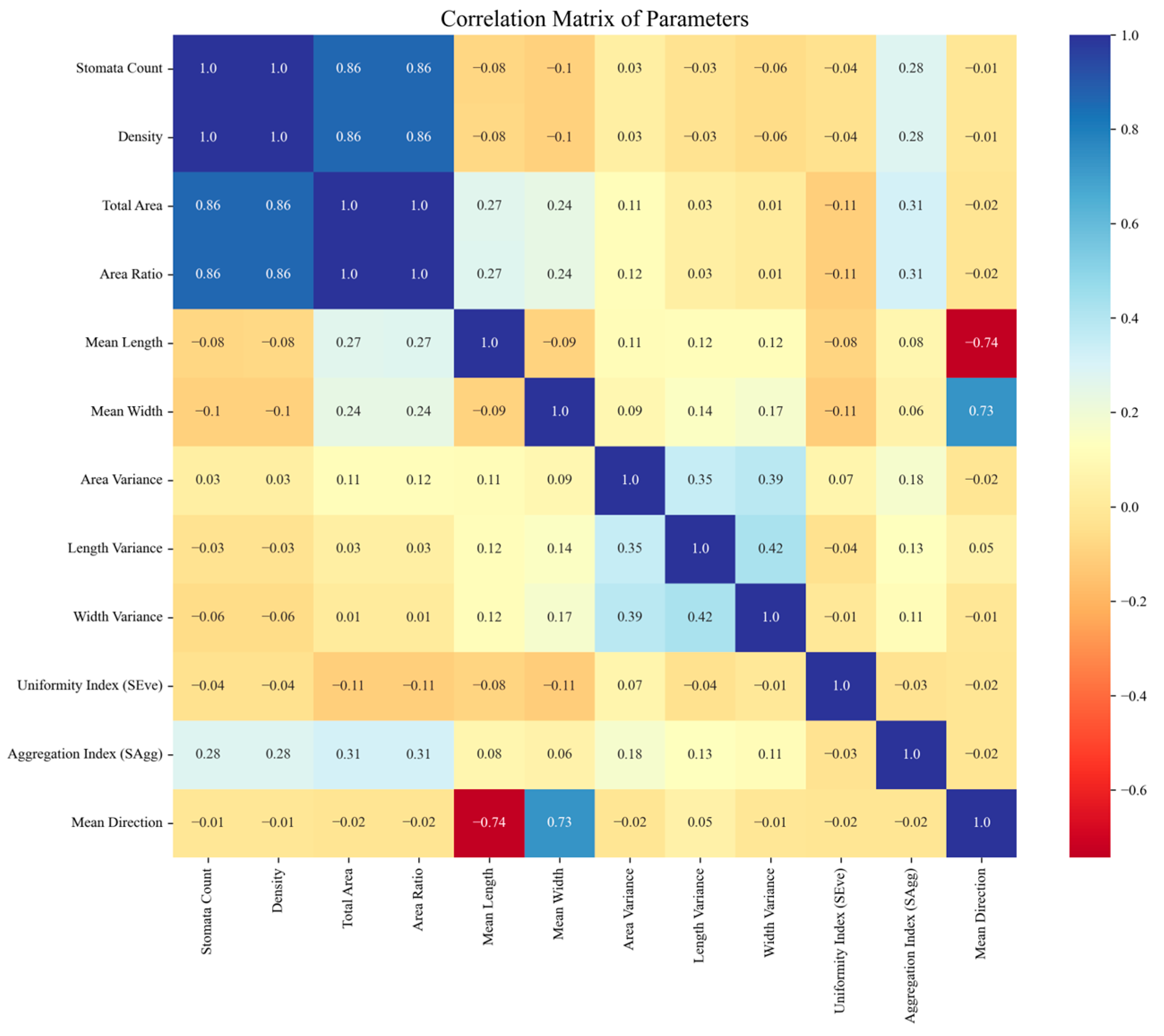
2.7. Calculation of Stomatal Characteristics
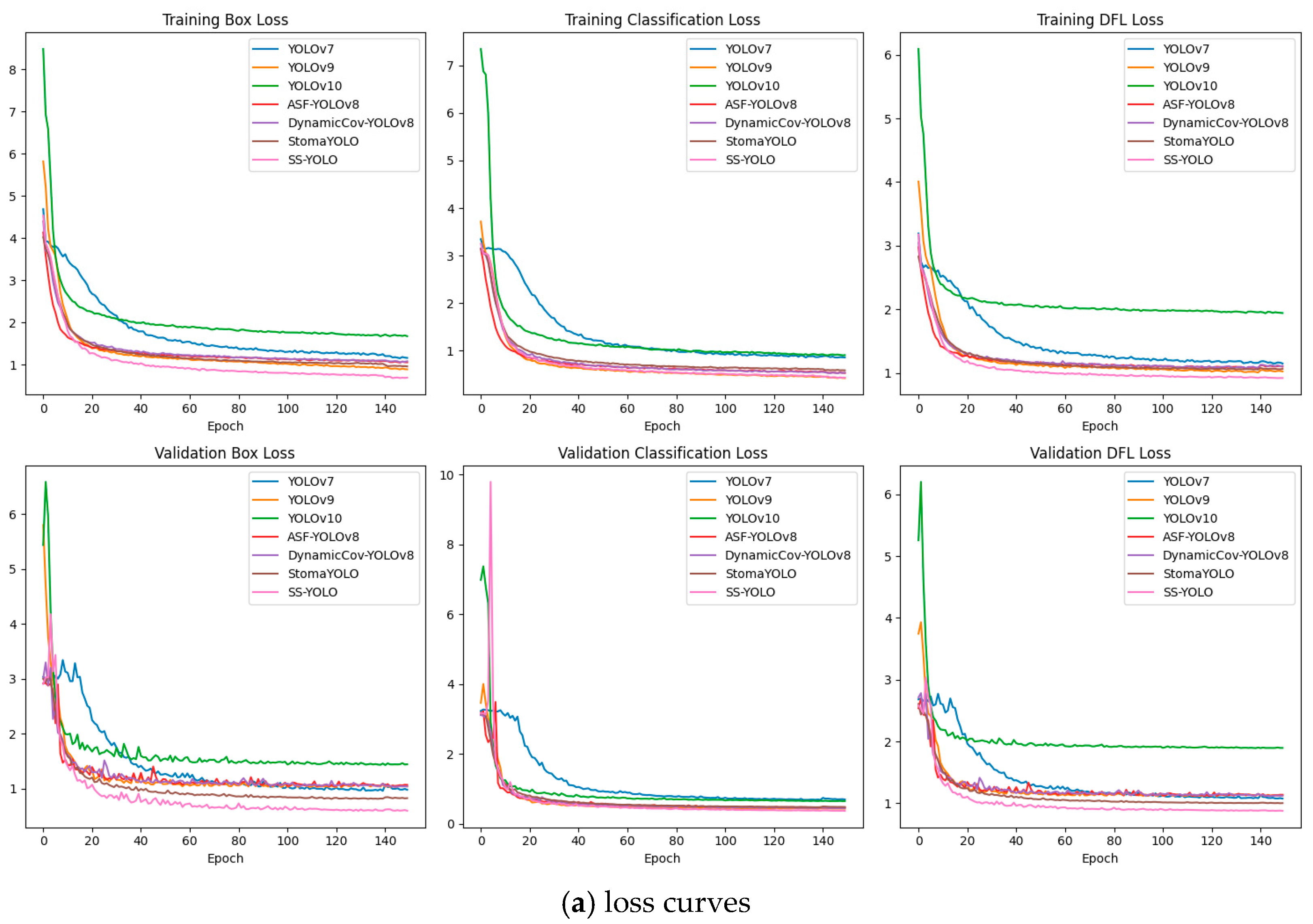
2.8. Model Training and Evaluation Indicators
3. Results
3.1. Ablation Experiment
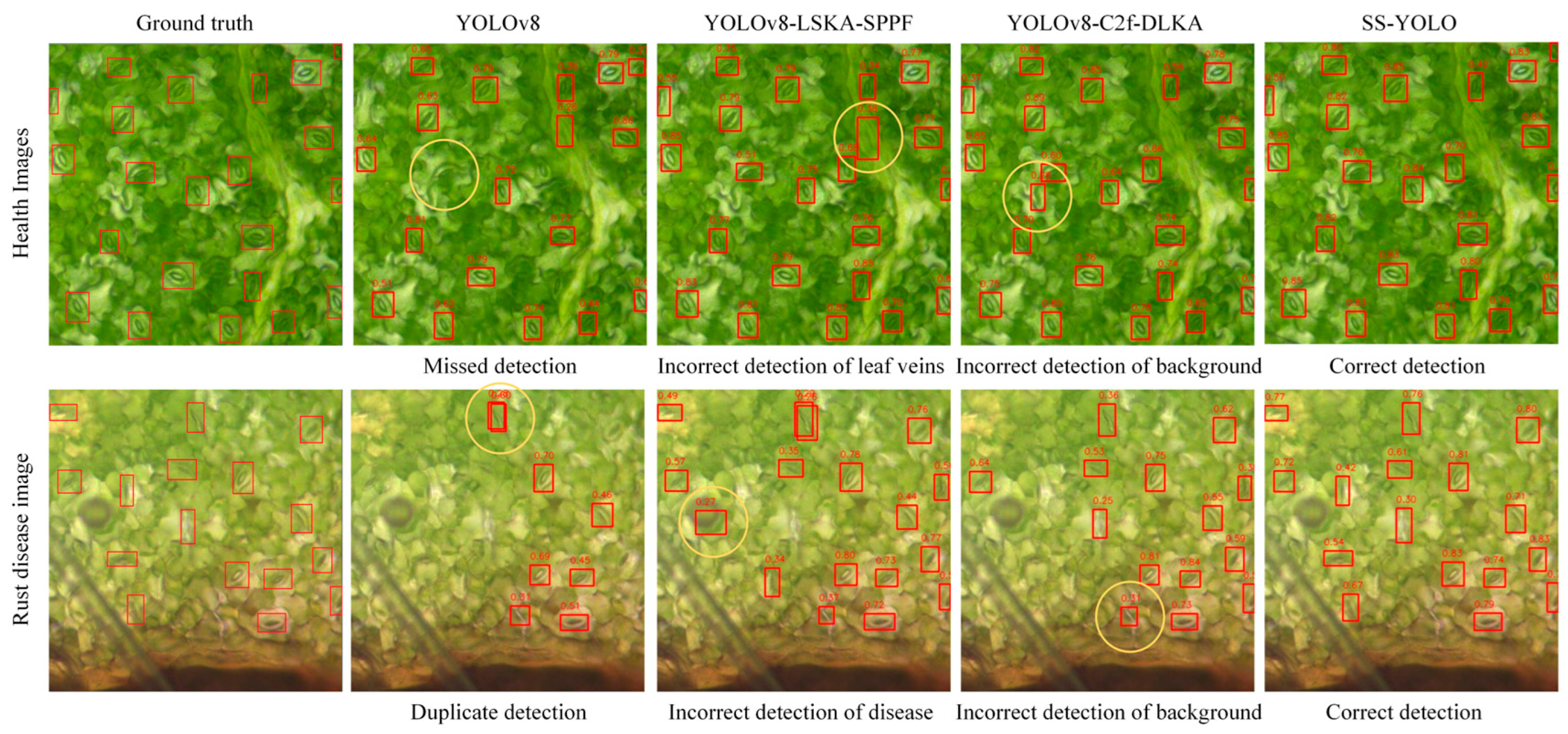
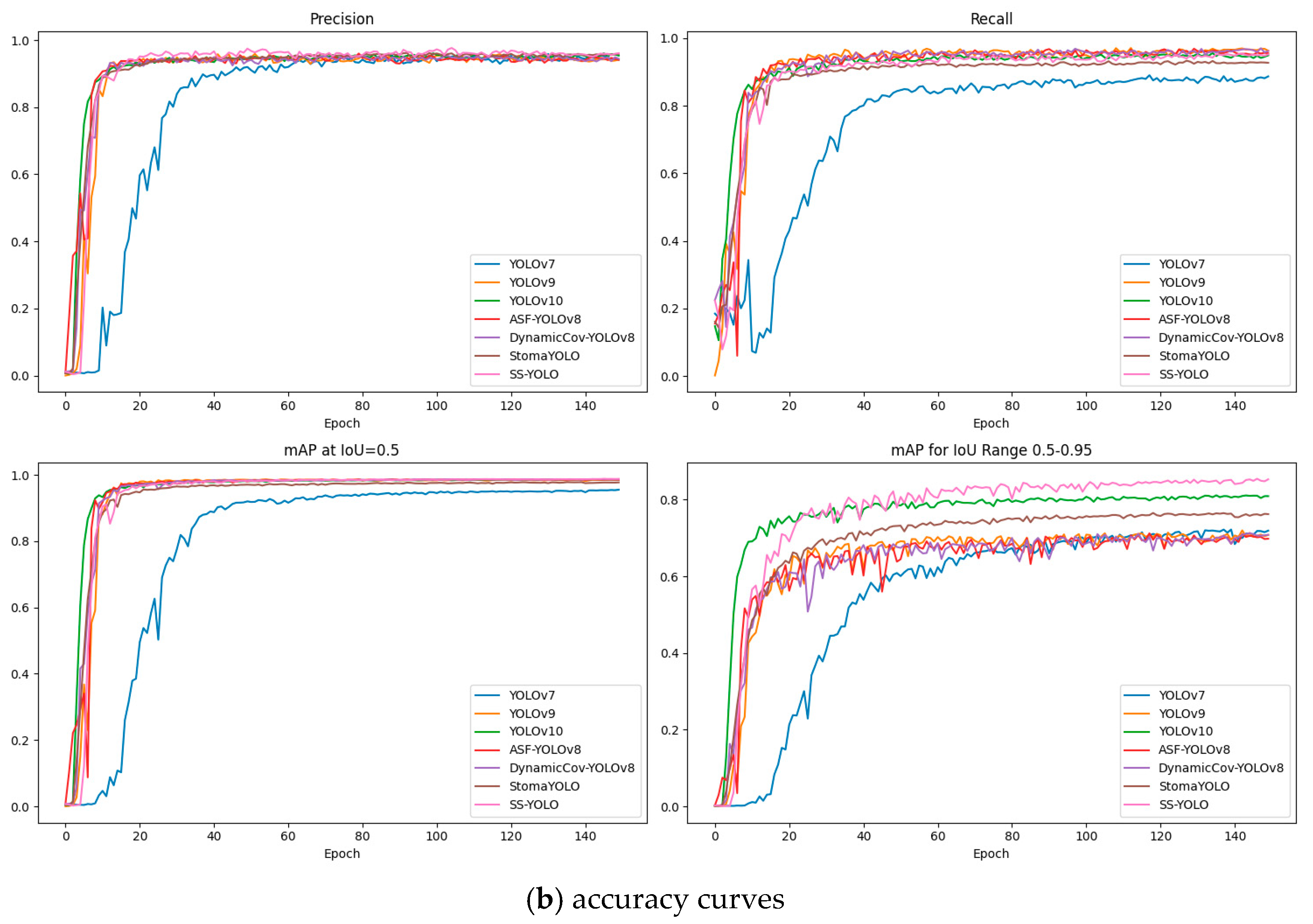
3.2. Comparative Experiments with Mainstream Models
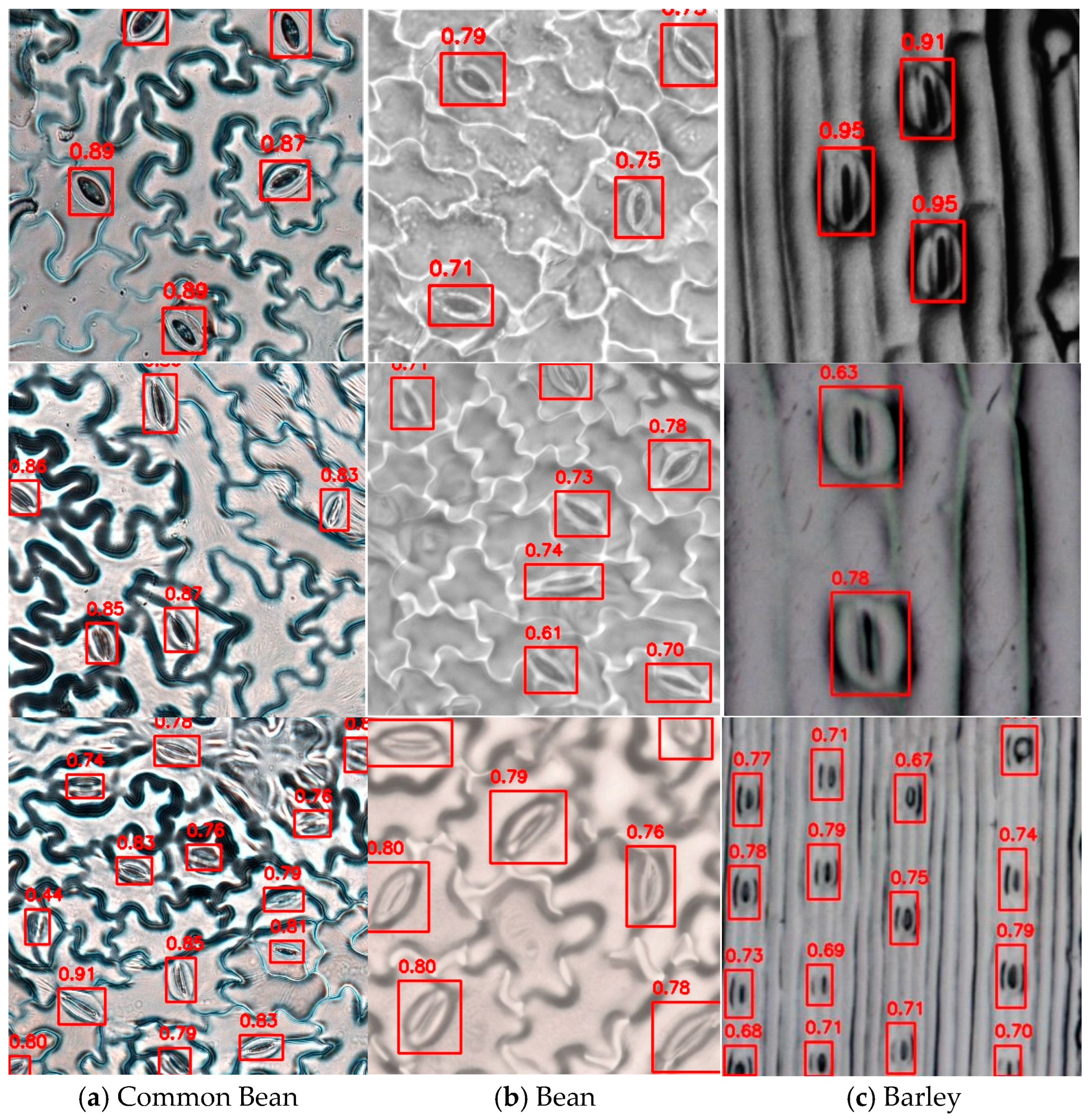
3.3. Model Generalization Experiment
3.4. Stomatal Characteristics Calculation Results and Disease Resistance Analysis
3.4.1. Stomatal Characteristics Calculation Results
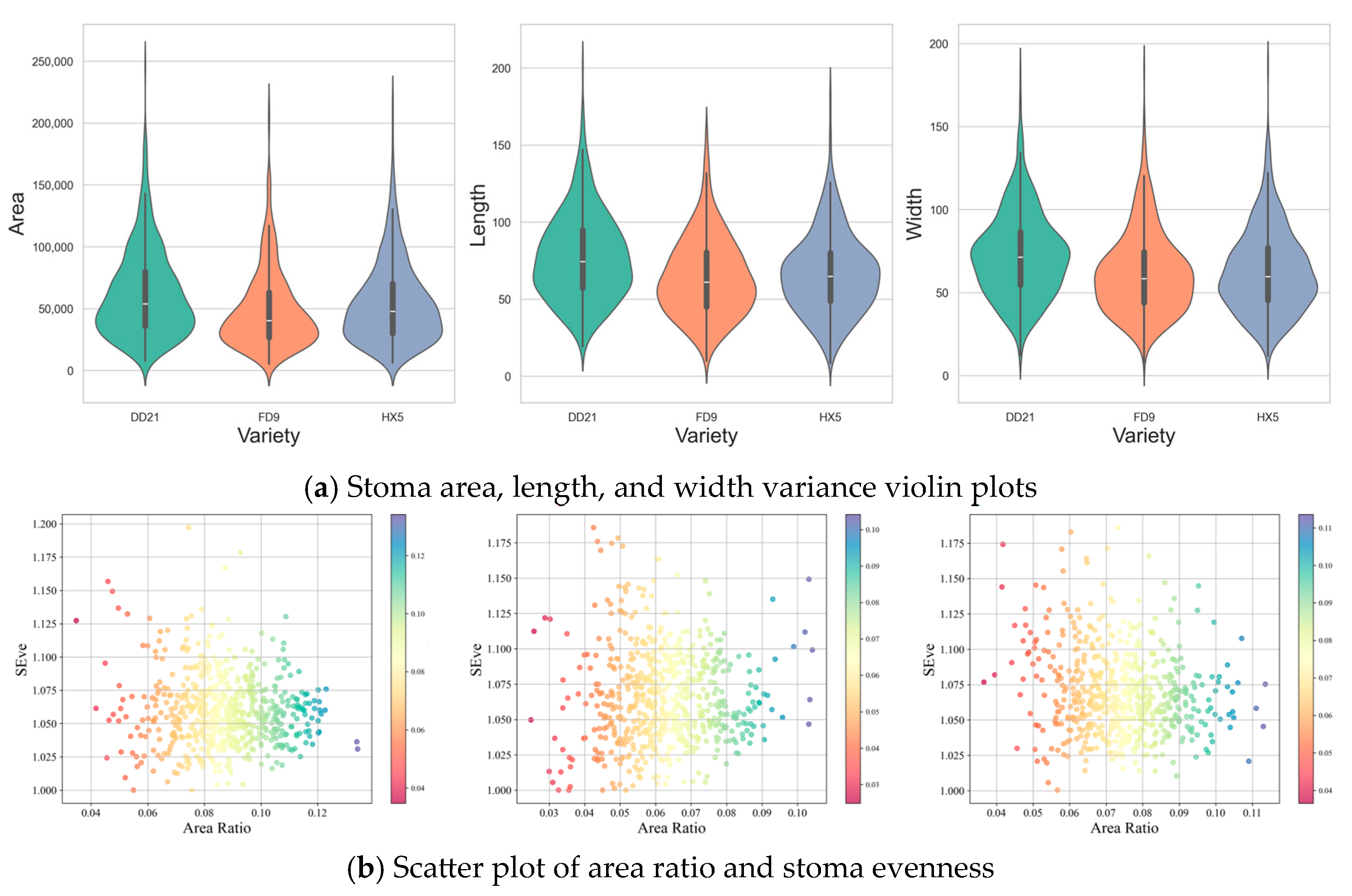
3.4.2. Analysis of Stomatal Parameters Among Different Soybean Varieties
3.4.3. Analysis of Stomatal Parameters at Different Disease Stages
3.4.4. Analysis of Stomatal Parameters and Disease Resistance
4. Discussion
4.1. Strengths of SS-YOLO
4.2. Relationship Between Stomatal Parameters and Disease Resistance
4.3. Limitations and Future Work
5. Conclusions
- (1)
- SS-YOLO achieved an accuracy of 98.7% on the self-constructed soybean stoma dataset. On the public datasets, common bean, bean, and barley, SS-YOLO achieved accuracies of 98.8%, 95.1%, and 94.6%, respectively. Especially in complex backgrounds and low-contrast images, SS-YOLO significantly improved the efficiency of soybean disease resistance screening and provided strong technical support for precision breeding and agricultural production.
- (2)
- Stoma phenotypic features, such as stoma length, width, number, and area of soybean leaves, were automatically extracted. The stomatal indices, such as stoma density, orientation, ratio of stoma area to image area, variance in area, variance in length, variance in width, uniformity, divergence, and aggregation, were calculated. These disease resistance evaluation indicators provided a new perspective for soybean disease resistance research.
- (3)
- By exploring the relationship between stomatal characteristics and disease resistance of soybean varieties at different disease stages, the results showed that disease resistance evaluation indicators can effectively distinguish between resistant and susceptible varieties. In the self-built soybean dataset, varieties with high resistance had more stable phenotypic characteristics.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumari, S.; Dambale, A.S.; Samantara, R.; Jincy, M.; Bains, G. Introduction, history, geographical distribution, importance, and uses of soybean (Glycine max L.). In Soybean Production Technology: Physiology, Production and Processing; Springer Nature: Singapore, 2025; pp. 1–17. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Yesmin, L.; Rubayet, M.T.; Abdullah, H.M.; Siddique, S.S.; Bhuiyan, M.A.B.; Yamanaka, N. Understanding Phakopsora Pachyrhizi in Soybean: Comprehensive Insights, Threats, and Interventions from the Asian Perspective. Front. Microbiol. 2023, 14, 1304205. [Google Scholar] [CrossRef]
- Murithi, H.M.; Beed, F.; Tukamuhabwa, P.; Thomma, B.P.H.J.; Joosten, M.H.A.J. Soybean Production in Eastern and Southern Africa and Threat of Yield Loss Due to Soybean Rust Caused by Phakopsora Pachyrhizi. Plant Pathol. 2016, 65, 176–188. [Google Scholar] [CrossRef]
- De Oliveira, T.B.; Peixoto, L.A.; Teodoro, P.E.; De Alvarenga, A.A.; Bhering, L.L.; Hoffmann-Campo, C.B. Relationship between Biochemical and Photosynthetic Traits with Asian Soybean Rust. An. Acad. Bras. Cienc. 2018, 90, 3925–3940. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R. Stomatal Size, Speed, and Responsiveness Impact on Photosynthesis and Water Use Efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Carins Murphy, M.R.; Jordan, G.J.; Brodribb, T.J. Cell Expansion Not Cell Differentiation Predominantly Co-Ordinates Veins and Stomata within and among Herbs and Woody Angiosperms Grown under Sun and Shade. Ann. Bot. 2016, 118, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Meddya, S.; Meshram, S.; Sarkar, D.; Rakesh, S.; Datta, R.; Singh, S.; Avinash, G.; Kumar Kondeti, A.; Savani, A.K.; Thulasinathan, T. Plant Stomata: An Unrealized Possibility in Plant Defense against Invading Pathogens and Stress Tolerance. Plants 2023, 12, 3380. [Google Scholar] [CrossRef] [PubMed]
- Fattah, A.; Yasin, M.; Suriani, S.; Nappu, M.B.; Mulia, S.; Hannan, M.F.I.; Wulanningtyas, H.S.; Saenong, S.; Dewayani, W.; Winanda, S.E.; et al. Performance and Morphology of Several Soybean Varieties and Responses to Pests and Diseases in South Sulawesi. Heliyon 2024, 10, e25507. [Google Scholar] [CrossRef]
- Bhaiswar, N.; Dixit, V.V.; Student, P.G. A Review: Methods of Automatic Stomata Detection and Counting Through Microscopic Images of a Leaf. Intl. J. Innov. Res. Sci. Eng. Technol. 2016, 5, 10612–10617. [Google Scholar]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A.; et al. Rice with Reduced Stomatal Density Conserves Water and Has Improved Drought Tolerance under Future Climate Conditions. New Phytol. 2019, 221, 371–384. [Google Scholar] [CrossRef]
- Li, X.; Guo, S.; Gong, L.; Lan, Y. An Automatic Plant Leaf Stoma Detection Method Based on YOLOv5. IET Image Process. 2023, 17, 67–76. [Google Scholar] [CrossRef]
- Dey, B.; Ahmed, R.; Ferdous, J.; Haque, M.M.U.; Khatun, R.; Hasan, F.E.; Uddin, S.N. Automated Plant Species Identification from the Stomata Images Using Deep Neural Network: A Study of Selected Mangrove and Fresh-water Swamp Forest Tree Species of Bangladesh. Ecol. Inform. 2023, 75, 102128. [Google Scholar] [CrossRef]
- Kaya, C. Optimizing Crop Production with Plant Phenomics Through High-Throughput Phenotyping and AI in Controlled Environments. Food Energy Secur. 2025, 14, e70050. [Google Scholar] [CrossRef]
- Minaee, S.; Boykov, Y.; Porikli, F.; Plaza, A.; Kehtarnavaz, N.; Terzopoulos, D. Image Segmentation Using Deep Learning: A Survey. IEEE Trans. Pattern Anal. Mach. Intell. 2022, 44, 3523–3542. [Google Scholar] [CrossRef]
- Wang, J.; Renninger, H.J.; Ma, Q.; Jin, S. Measuring Stomatal and Guard Cell Metrics for Plant Physiology and Growth Using StoManager1. Plant Physiol. 2024, 195, 378–394. [Google Scholar] [CrossRef]
- Liang, X.; Xu, X.; Wang, Z.; He, L.; Zhang, K.; Liang, B.; Ye, J.; Shi, J.; Wu, X.; Dai, M.; et al. StomataScorer: A Porta-ble and High-Throughput Leaf Stomata Trait Scorer Combined with Deep Learning and an Improved CV Model. Plant Biotechnol. J. 2022, 20, 577–591. [Google Scholar] [CrossRef]
- Meng, X.; Nakano, A.; Hoshino, Y. Automated Estimation of Stomatal Number and Aperture in Haskap (Lonicera caerulea L.). Planta 2023, 258, 77. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Fan, W.; Ma, S.; Zhang, C.; Kim, J.C.; Wang, K.; Russinova, E.; Zhu, Y.; Zhou, Y. LeafNet: A Tool for Segmenting and Quantifying Stomata and Pavement Cells. Plant Cell 2022, 34, 1171–1188. [Google Scholar] [CrossRef]
- Fetter, K.C.; Eberhardt, S.; Barclay, R.S.; Wing, S.; Keller, S.R. StomataCounter: A Neural Network for Automatic Stomata Identification and Counting. New Phytol. 2019, 223, 1671–1681. [Google Scholar] [CrossRef]
- Takagi, M.; Hirata, R.; Aihara, Y.; Hayashi, Y.; Mizutani-Aihara, M.; Ando, E.; Yoshimura-Kono, M.; Tomiyama, M.; Kinoshita, T.; Mine, A.; et al. Image-Based Quantification of Arabidopsis Thaliana Stomatal Aperture from Leaf Images. Plant Cell Physiol. 2023, 64, 1301–1310. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, B.; Lu, F.; Zhang, X. Rotating Stomata Measurement Based on Anchor-Free Object Detection and Stomata Conductance Calculation. Plant Phenomics 2023, 5, 0106. [Google Scholar] [CrossRef] [PubMed]
- Casado-García, A.; del-Canto, A.; Sanz-Saez, A.; Pérez-López, U.; Bilbao-Kareaga, A.; Fritschi, F.B.; Miran-da-Apodaca, J.; Muñoz-Rueda, A.; Sillero-Martínez, A.; Yoldi-Achalandabaso, A.; et al. LabelStoma: A Tool for Stomata Detection Based on the YOLO Algorithm. Comput. Electron. Agric. 2020, 178, 105751. [Google Scholar] [CrossRef]
- Yang, X.H.; Xi, Z.J.; Li, J.P.; Feng, X.L.; Zhu, X.H.; Guo, S.Y.; Song, C.P. Deep Transfer Learning-Based Multi-Object Detection for Plant Stomata Phenotypic Traits Intelligent Recognition. IEEE/ACM Trans. Comput. Biol. Bioinform. 2023, 20, 321–329. [Google Scholar] [CrossRef]
- Pathoumthong, P.; Zhang, Z.; Roy, S.J.; El Habti, A. Rapid Non-Destructive Method to Phenotype Stomatal Traits. Plant Methods 2023, 19, 36. [Google Scholar] [CrossRef]
- Wacker, T.S.; Smith, A.G.; Jensen, S.M.; Pflüger, T.; Hertz, V.G.; Rosenqvist, E.; Liu, F.; Dresbøll, D.B. Stomata Morphology Measurement with Interactive Machine Learning: Accuracy, Speed, and Biological Relevance? Plant Methods 2025, 21, 95. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liao, Y.; Chen, Z.; Lin, Z.; Huang, W.; Liu, Y.; Liu, Y.; Fan, Y.; Xu, J.; Xu, L.; et al. StomaYOLO: A Lightweight Maize Phenotypic Stomatal Cell Detector Based on Multi-Task Training. Plants 2025, 14, 2070. [Google Scholar] [CrossRef]
- Shi, X.; Song, Y.; Shi, X.; Lu, W.; Zhao, Y.; Zhou, Z.; Chai, J.; Liu, Z. Deep Learning for Stomatal Opening Recognition in Gynura formosana Kitam Leaves. Agronomy 2024, 14, 2622. [Google Scholar] [CrossRef]
- Wolter-Salas, S.; Canessa, P.; Campos-Vargas, R.; Opazo, M.C.; Sepulveda, R.V.; Aguayo, D. WS-YOLO: An Agronomical and Computer Vision-Based Framework to Detect Drought Stress in Lettuce Seedlings Using IR Imaging and YOLOv8. In Proceedings of the Communications in Computer and Information Science, Hyderabad, India, 27–28 December 2024; Volume 1935. [Google Scholar] [CrossRef]
- Ma, N.; Su, Y.; Yang, L.; Li, Z.; Yan, H. Wheat Seed Detection and Counting Method Based on Improved YOLOv8 Model. Sensors 2024, 24, 1654. [Google Scholar] [CrossRef]
- Sultana, S.N.; Park, H.; Choi, S.H.; Jo, H.; Song, J.T.; Lee, J.D.; Kang, Y.J. Optimizing the Experimental Method for Stomata-Profiling Automation of Soybean Leaves Based on Deep Learning. Plants 2021, 10, 2714. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, H.; Li, Y.; Zhou, W.; Ding, M. Segmentation and Phenotype Calculation of Rapeseed Pods Based on YOLO v8 and Mask R-Convolution Neural Networks. Plants 2023, 12, 3328. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Renninger, H.J.; Ma, Q. Labeled Temperate Hardwood Tree Stomatal Image Datasets from Seven Taxa of Populus and 17 Hardwood Species. Sci. Data 2024, 11, 1. [Google Scholar] [CrossRef]
- Wang, C.Y.; Bochkovskiy, A.; Liao, H.Y.M. YOLOv7: Trainable bag-of-freebies sets new state-of-the-art for real-time object detectors. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, Vancouver, BC, Canada, 17–24 June 2023; pp. 7464–7475. [Google Scholar] [CrossRef]
- Zhang, F.; Ren, F.; Li, J.; Zhang, X. Automatic Stomata Recognition and Measurement Based on Improved YOLO Deep Learning Model and Entropy Rate Superpixel Algorithm. Ecol. Inform. 2022, 68, 101521. [Google Scholar] [CrossRef]
- Sultana, S.N.; Jo, H.; Song, J.T.; Kim, K.; Lee, J.D. Stomatal Density Variation within and among Different Soybean Cultivars across Various Growth Stages. Agriculture 2024, 14, 2028. [Google Scholar] [CrossRef]
- Tanaka, Y.; Fujii, K.; Shiraiwa, T. Variability of Leaf Morphology and Stomatal Conductance in Soybean [Glycine max (L.) Merr.] Cultivars. Crop Sci. 2010, 50, 2525–2532. [Google Scholar] [CrossRef]
- Amaliah, N.; Zubaidah, S.; Kuswantoro, H. Trichomes and Stomata Diversity in Soybean (Glycine max L. Merill) Lines. IOP Conf. Ser. Earth Environ. Sci. 2019, 276, 012025. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, S.; Zhai, Z.; Yu, H.; Xu, H. DC2Net: An Asian Soybean Rust Detection Model Based on Hyperspectral Imaging and Deep Learning. Plant Phenomics 2024, 6, 0163. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.; Zhu, C.; Zheng, L.; Wang, H.; Ruan, C.; Wu, M.; Xu, K.; Liu, J. LSKA-YOLOv8: A Lightweight Steel Surface Defect Detection Algorithm Based on YOLOv8 Improvement. Alex Eng. J. 2024, 109, 201–212. [Google Scholar] [CrossRef]
- Huang, J.; Fang, C.; Zheng, X.; Liu, J. YOLOv8-UC: An Improved YOLOv8-Based Underwater Object Detection Algorithm. IEEE Access 2024, 12, 172186–172195. [Google Scholar] [CrossRef]
- Zhao, L.; Liang, G.; Hu, Y.; Xi, Y.; Ning, F.; He, Z. YOLO-RLDW: An Algorithm for Object Detection in Aerial Images under Complex Backgrounds. IEEE Access 2024, 12, 128677–128693. [Google Scholar] [CrossRef]
- Azad, R.; Niggemeier, L.; Hüttemann, M.; Kazerouni, A.; Aghdam, E.K.; Velichko, Y.; Bagci, U.; Merhof, D. Beyond Self-Attention: Deformable Large Kernel Attention for Medical Image Segmentation. In Proceedings of the IEEE/CVF Winter Conference on Applications of Computer Vision, Waikoloa, HI, USA, 3–8 January 2024; pp. 1287–1297. [Google Scholar] [CrossRef]
- Han, Z.; Cai, Y.; Liu, A.; Zhao, Y.; Lin, C. MS-YOLOv8-Based Object Detection Method for Pavement Diseases. Sensors 2024, 24, 4569. [Google Scholar] [CrossRef]
- Sakata, N.; Ishiga, Y. Prevention of Stomatal Entry as a Strategy for Plant Disease Control against Foliar Patho-genic Pseudomonas Species. Plants 2023, 12, 590. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Yu, R.; Yang, W.; Guo, S.; Liu, S.; Du, H.; Liang, J.; Zhang, X. Effect of Powdery Mildew on the Photosynthetic Parameters and Leaf Microstructure of Melon. Agriculture 2024, 14, 886. [Google Scholar] [CrossRef]
- Xiang, Q.; Lott, A.A.; Assmann, S.M.; Chen, S. Advances and Perspectives in the Metabolomics of Stomatal Movement and the Disease Triangle. Plant Sci. 2021, 302, 110697. [Google Scholar] [CrossRef]
- Song, W.; Li, J.; Li, K.; Chen, J.; Huang, J. An Automatic Method for Stomatal Pore Detection and Measurement in Microscope Images of Plant Leaf Based on a Convolutional Neural Network Model. Forests 2020, 11, 954. [Google Scholar] [CrossRef]
- Kang, M.; Ting, C.M.; Ting, F.F.; Phan, R.C.W. ASF-YOLO: A Novel YOLO Model with Attentional Scale Sequence Fusion for Cell Instance Segmentation. Image Vis. Comput. 2024, 147, 105057. [Google Scholar] [CrossRef]
- Han, K.; Wang, Y.; Guo, J.; Wu, E. ParameterNet: Parameters Are All You Need for Large-Scale Visual Pretraining of Mobile Networks. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Seattle, WA, USA, 16–22 June 2024. [Google Scholar] [CrossRef]
- Tateda, C.; Obara, K.; Abe, Y.; Sekine, R.; Nekoduka, S.; Hikage, T.; Nishihara, M.; Sekine, K.T.; Fujisaki, K. The Host Stomatal Density Determines Resistance to Septoria Gentianae in Japanese Gentian. Mol. Plant-Microb. Interact. 2019, 32, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.A.; Wade, R.N. Plant–pathogen Interactions and Elevated CO2: Morphological Changes in Favour of Pathogens. J. Exp. Bot. 2009, 60, 3123–3131. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Karimi, M.; Venditti, A.; Zahra, N.; Siddique, K.H.M.; Farooq, M. Plant Adaptation to Drought Stress: The Role of Anatomical and Morphological Characteristics in Maintaining the Water Status. J. Soil Sci. Plant Nutr. 2025, 25, 409–427. [Google Scholar] [CrossRef]
- Takahashi, Y.; Joo, H.; Pankasem, N.; Hsu, P.K.; Schroeder, J.I. Stomatal CO2 Sensing in Plants: Control of Gas Exchange and Interactions with Environmental Stimuli. Plant Cell Physiol. 2025, 66, 1259–1273. [Google Scholar] [CrossRef]
- Li, X.H.; Li, M.Z.; Li, J.Y.; Gao, Y.Y.; Liu, C.R.; Hao, G.F. Wearable Sensor Supports In-Situ and Continuous Monitoring of Plant Health in Precision Agriculture Era. Plant Biotechnol. J. 2024, 22, 1516–1535. [Google Scholar] [CrossRef]
- Attri, I.; Awasthi, L.K.; Sharma, T.P.; Rathee, P. A Review of Deep Learning Techniques Used in Agriculture. Ecol. Inform. 2023, 77, 102217. [Google Scholar] [CrossRef]
- de Melo Lima, B.P.; de Araújo Barbosa Borges, L.; Hirose, E.; Borges, D.L. A Lightweight and Enhanced Model for Detecting the Neotropical Brown Stink Bug, Euschistus Heros (Hemiptera: Pentatomidae) Based on YOLOv8 for Soybean Fields. Ecol. Inform. 2024, 80, 102543. [Google Scholar] [CrossRef]
- Zhou, H.; Kong, M.; Yuan, H.; Pan, Y.; Wang, X.; Chen, R.; Lu, W.; Wang, R.; Yang, Q. Real-Time Underwater Object Detection Technology for Complex Underwater Environments Based on Deep Learning. Ecol. Inform. 2024, 82, 102680. [Google Scholar] [CrossRef]



| Variety | Disease Stage | Number of Images | Training Set | Validation Set | Number of Stomata |
|---|---|---|---|---|---|
| DD21 | healthy | 138 | 110 | 28 | 8516 |
| asymptomatic | 156 | 125 | 31 | ||
| symptomatic | 306 | 245 | 61 | ||
| FD9 | healthy | 188 | 150 | 38 | 7969 |
| asymptomatic | 212 | 170 | 42 | ||
| symptomatic | 200 | 160 | 40 | ||
| HX5 | healthy | 232 | 185 | 47 | 8911 |
| asymptomatic | 208 | 167 | 41 | ||
| symptomatic | 160 | 128 | 32 |
| Category | Phenotypic Parameters | Symbols | Formulas | Description |
|---|---|---|---|---|
| basic parameters | quantity | Total number of detected stomata | ||
| density | Number of stomata per unit leaf area | |||
| area | Total area of stomata | |||
| ratio of total stoma area to image area | Proportion of stomata in the leaf area | |||
| variance of stoma parameters | Variability in area, width, and length | |||
| morphological parameters | length | Length of stoma | ||
| width | Width of stoma | |||
| direction | Rotation angle of the long axis of the stoma relative to the horizontal line | |||
| stomatal index | evenness | Regularity of stoma distribution | ||
| aggregation | Degree to which stoma distribution deviates from a random pattern |
| SPPF-LSKA | C2f-DLKA | Precision | Recall | mAP50 | mAP50:95 | FPS | Params(M) | GFLOPs |
|---|---|---|---|---|---|---|---|---|
| 0.925 | 0.850 | 0.936 | 0.464 | 39.42 | 3.01 | 8.12 | ||
| √ | 0.954 | 0.945 | 0.982 | 0.600 | 36.48 | 4.57 | 8.43 | |
| √ | 0.958 | 0.947 | 0.981 | 0.586 | 35.92 | 4.82 | 8.59 | |
| √ | √ | 0.959 | 0.950 | 0.987 | 0.854 | 34.03 | 4.95 | 10.21 |
| Model | Precision | Recall | mAP50 | mAP50:95 | FPS | Params (M) | GFLOPs |
|---|---|---|---|---|---|---|---|
| YOLOv7 | 0.946 | 0.879 | 0.952 | 0.722 | 37.82 | 3.63 | 8.57 |
| YOLOv9 | 0.938 | 0.970 | 0.985 | 0.720 | 48.53 | 1.97 | 6.21 |
| YOLOv10 | 0.956 | 0.946 | 0.986 | 0.811 | 44.21 | 2.71 | 7.38 |
| ASF-YOLOv8 | 0.925 | 0.850 | 0.936 | 0.464 | 41.53 | 3.06 | 7.65 |
| DynamicCov-YOLOv8 | 0.943 | 0.966 | 0.985 | 0.713 | 34.78 | 4.74 | 9.92 |
| StomaYOLO | 0.956 | 0.927 | 0.976 | 0.765 | 37.18 | 4.69 | 9.12 |
| SS-YOLO | 0.959 | 0.950 | 0.987 | 0.854 | 34.03 | 4.95 | 10.21 |
| Variety | Precision | Recall | mAP50 | mAP50:95 |
|---|---|---|---|---|
| Common Bean | 0.949 | 0.977 | 0.988 | 0.573 |
| Bean | 0.905 | 0.909 | 0.951 | 0.610 |
| Barley | 0.902 | 0.898 | 0.946 | 0.751 |
| Variety | Stage | Density Stomata/μm2 | Total Area /μm2 | Length /μm | Width /μm | Direction /° |
|---|---|---|---|---|---|---|
| DD21 | healthy | 70 | 25,763.71 | 38.42 | 37.74 | 45.44 |
| asymptomatic | 59 | 22,258.44 | 39.54 | 38.44 | 45.69 | |
| symptomatic | 57 | 21,704.21 | 39.31 | 38.88 | 45.24 | |
| FD9 | healthy | 55 | 17,082.91 | 36.03 | 34.45 | 46.18 |
| asymptomatic | 50 | 15,880.96 | 36.51 | 34.67 | 46.41 | |
| symptomatic | 48 | 16,521.08 | 37.39 | 36.27 | 45.80 | |
| HX5 | healthy | 58 | 20,350.39 | 37.75 | 36.74 | 45.69 |
| asymptomatic | 57 | 18,679.47 | 36.35 | 35.24 | 45.81 | |
| symptomatic | 54 | 19,484.81 | 38.45 | 37.06 | 46.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Wu, S.; Mu, R.; Xu, H.; Zhai, Z.; Hu, B. Stoma Detection in Soybean Leaves and Rust Resistance Analysis. Plants 2025, 14, 2994. https://doi.org/10.3390/plants14192994
Feng J, Wu S, Mu R, Xu H, Zhai Z, Hu B. Stoma Detection in Soybean Leaves and Rust Resistance Analysis. Plants. 2025; 14(19):2994. https://doi.org/10.3390/plants14192994
Chicago/Turabian StyleFeng, Jiarui, Shichao Wu, Rong Mu, Huanliang Xu, Zhaoyu Zhai, and Bin Hu. 2025. "Stoma Detection in Soybean Leaves and Rust Resistance Analysis" Plants 14, no. 19: 2994. https://doi.org/10.3390/plants14192994
APA StyleFeng, J., Wu, S., Mu, R., Xu, H., Zhai, Z., & Hu, B. (2025). Stoma Detection in Soybean Leaves and Rust Resistance Analysis. Plants, 14(19), 2994. https://doi.org/10.3390/plants14192994






