Efficient Tissue Culture Method Based on Clustered Bud Proliferation for Producing High-Quality Arundo donax Seedlings
Abstract
1. Introduction
2. Results
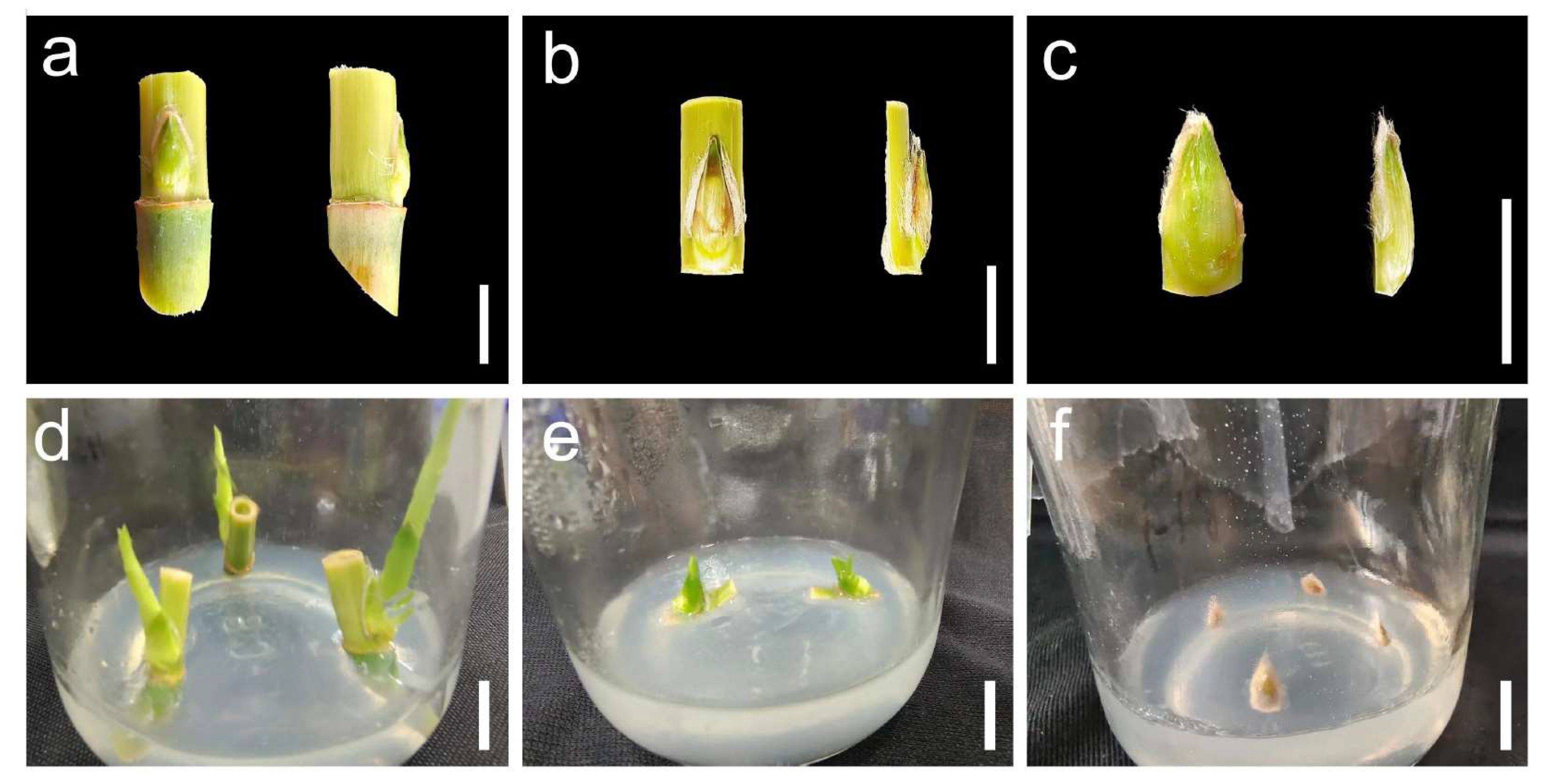
2.1. Effects of Various Explants on Bud Induction
2.2. Effects of Various Disinfectants on Explant Disinfection
2.3. Determination of Induction Medium
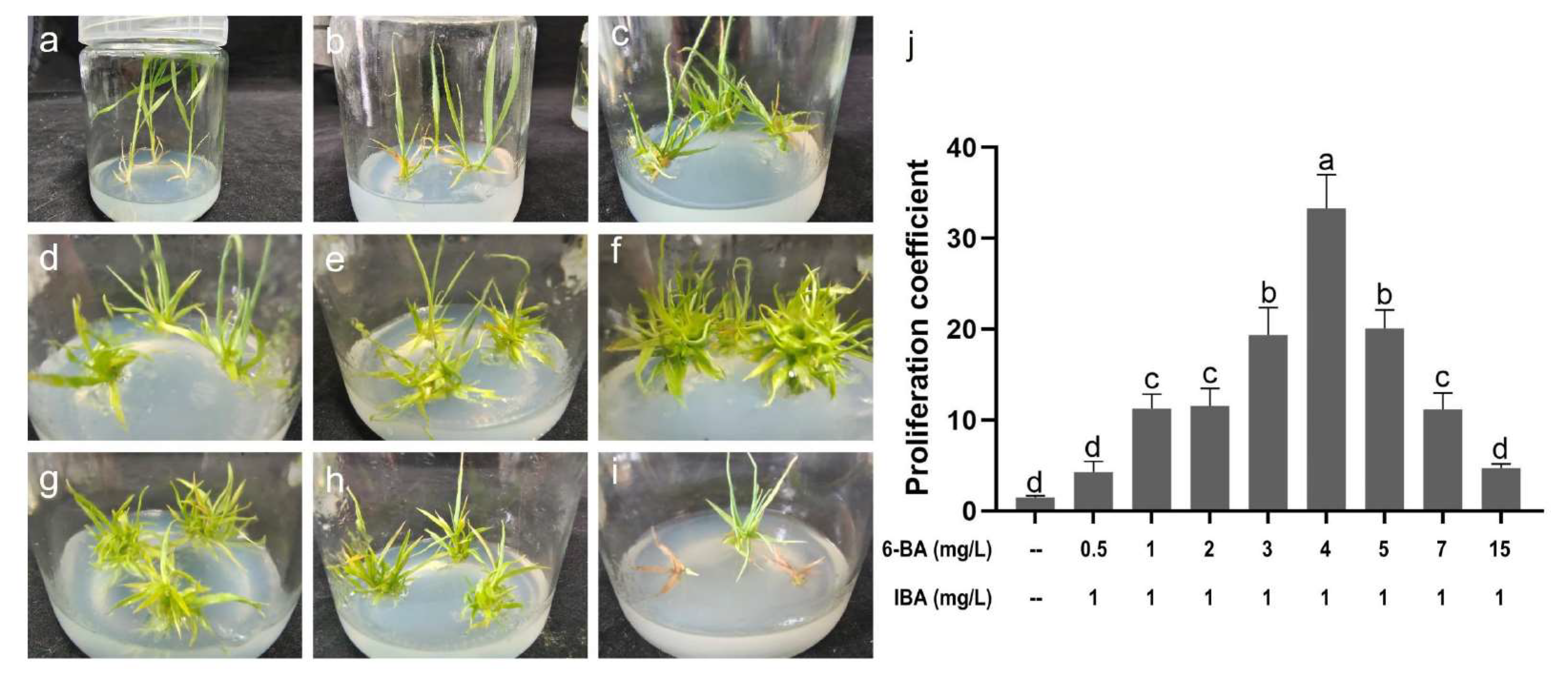
2.4. Determination of Proliferation Medium
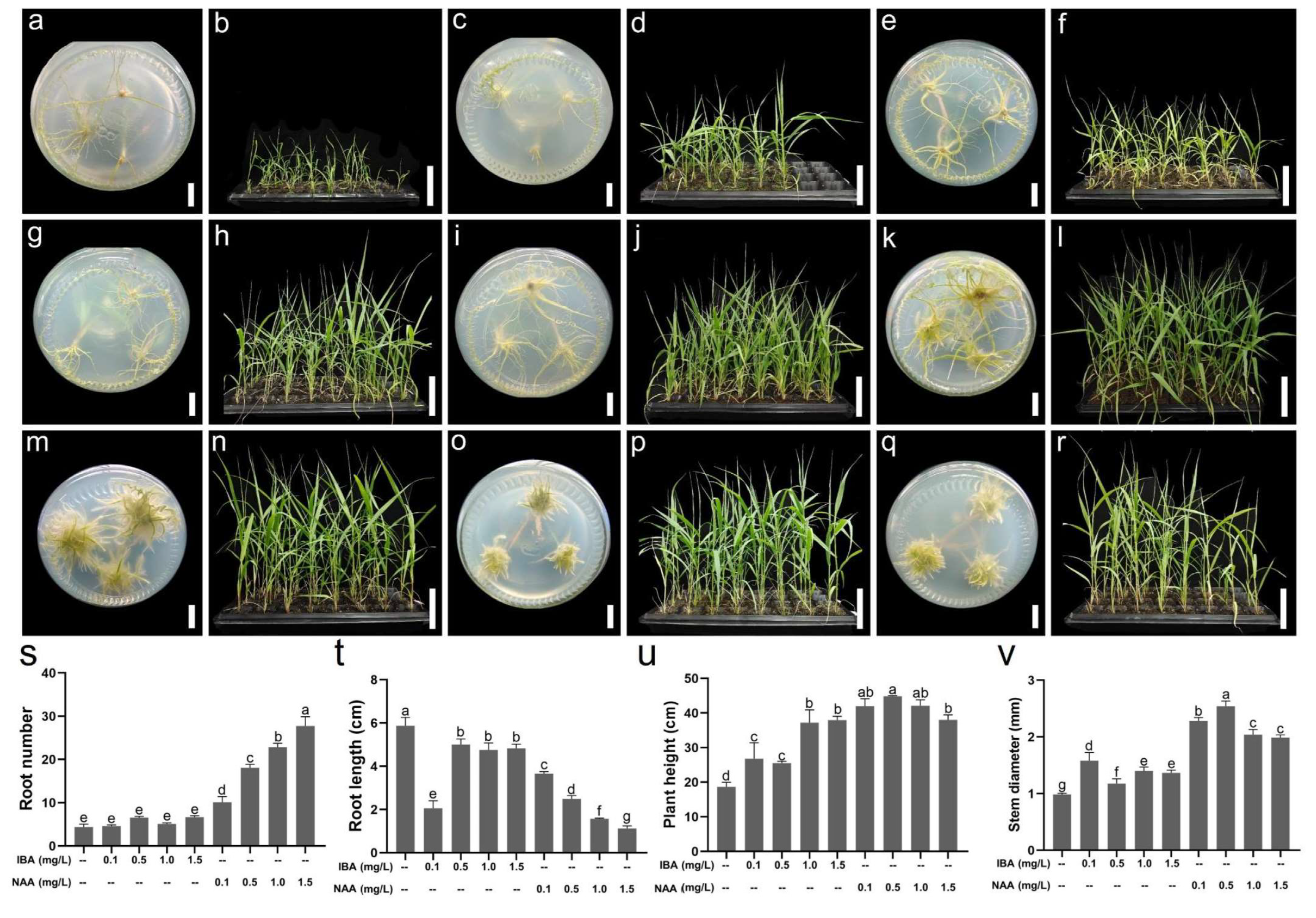
2.5. Determination of the Rooting Medium
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Explant Preparation and Disinfection
4.3. Induction of Clustered Buds
4.4. Proliferation of Clustered Buds
4.5. Rooting and Acclimation
4.6. Culture Condition
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cintura, E.; Faria, P.; Molari, L.; Barbaresi, L.; D’Orazio, D.; Nunes, L. Characterization of an Arundo donax-based composite: A solution to improve indoor comfort. Ind. Crops Prod. 2024, 208, 117756. [Google Scholar] [CrossRef]
- Jiménez-Ruiz, J.; Hardion, L.; Del Monte, J.P.; Vila, B.; Santín-Montanyá, M.I. Monographs on invasive plants in Europe N° 4: Arundo donax L. Bot. Lett. 2021, 168, 131–151. [Google Scholar] [CrossRef]
- SijiMol, K.; Dev, S.A.; Sreekumar, V.B. A review of the ecological functions of reed bamboo, genus in the Western Ghats of India: Implications for sustainable conservation. Trop. Conserv. Sci. 2016, 9, 389–407. [Google Scholar] [CrossRef]
- Seawright, E.K.; Rister, M.E.; Lacewell, R.D.; McCorkle, D.A.; Sturdivant, A.W.; Yang, C.; Goolsby, J.A. Economic implications for the biological control of Arundo donax: Rio Grande Basin. Southwest. Entomolog. 2009, 34, 377–394. [Google Scholar] [CrossRef]
- Allinson, G. Effect of increasing salinity on development of giant reed (Arundo donax) from rhizome and culms. Bull. Environ. Contam. Toxicol. 2017, 99, 743–747. [Google Scholar] [CrossRef]
- Docimo, T.; De Stefano, R.; De Palma, M.; Cappetta, E.; Villano, C.; Aversano, R.; Tucci, M. Transcriptional, metabolic and DNA methylation changes underpinning the response of Arundo donax ecotypes to NaCl excess. Planta 2020, 251, 34. [Google Scholar] [CrossRef]
- Shetty, R.; Vidya, C.S.-N.; Vaculík, M. Comparison of the single and combined effects of arsenic and antimony on growth and physiology of giant reed (Arundo donax L.). Environ. Sci. Pollut. Res. 2021, 28, 55476–55485. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, S.L.; Patanè, C.; Sanzone, E.; Testa, G.; Scordia, D. Leaf gas exchange, water status and radiation use efficiency of giant reed (Arundo donax L.) in a changing soil nitrogen fertilization and soil water availability in a semi-arid Mediterranean area. Eur. J. Agron. 2016, 72, 56–69. [Google Scholar] [CrossRef]
- Nassi o Di Nasso, N.; Roncucci, N.; Triana, F.; Tozzini, C.; Bonari, E. Productivity of giant reed (Arundo donax L.) and miscanthus (Miscanthus x giganteus Greef et Deuter) as energy crops: Growth analysis. Ital. J. Agron. 2011, 6, 141–147. [Google Scholar] [CrossRef]
- Idris, S.M.; Jones, P.L.; Salzman, S.A.; Croatto, G.; Allinson, G. Evaluation of the giant reed (Arundo donax) in horizontal subsurface flow wetlands for the treatment of dairy processing factory wastewater. Environ. Sci. Pollut. Res. 2012, 19, 3525–3537. [Google Scholar] [CrossRef]
- Islam, M.; Ferrarini, A.; Ali, A.; Kam, J.; Trindade, L.M.; Clifton-Brown, J.; Amaducci, S. Assessment of drought and zinc stress tolerance of novel Miscanthus hybrids and Arundo donax clones using physiological, biochemical, and morphological traits. Biology 2023, 12, 1525. [Google Scholar] [CrossRef]
- Lino, G.; Espigul, P.; Nogués, S.; Serrat, X. Arundo donax L. growth potential under different abiotic stress. Heliyon 2023, 9, e15521. [Google Scholar] [CrossRef]
- Riggi, E.; Avola, G.; Marino, G.; Haworth, M.; Cosentino, S.L.; Centritto, M. Open field experiment for the evaluation of Arundo donax ecotypes ecophysiology and yield as affected by soil water content. Ind. Crops Prod. 2019, 140, 111630. [Google Scholar] [CrossRef]
- Benbouzid, M.; Al-Jadabia, N.; Bensemlali, M.; El Hajjaji, S.; Labjar, N. Constructed wetland as a low-energy technique for wastewater treatment—Seasonal impact, performance and phytomanagement. J. Ecol. Eng. 2024, 25, 176–188. [Google Scholar] [CrossRef]
- Cappelli, G.A.; Ginaldi, F.; Fanchini, D.; Corinzia, S.A.; Cosentino, S.L.; Ceotto, E. Model-based assessment of giant reed (Arundo donax L.) energy yield in the form of diverse biofuels in marginal areas of Italy. Land 2021, 10, 548. [Google Scholar] [CrossRef]
- Mantziaris, S.; Iliopoulos, C.; Theodorakopoulou, I.; Petropoulou, E. Perennial energy crops vs. durum wheat in low input lands: Economic analysis of a Greek case study. Renew. Sustain. Energy Rev. 2017, 80, 789–800. [Google Scholar] [CrossRef]
- Curt, M.D.; Sanz, M.; Mauri, P.V.; Plaza, A.; Cano-Ruiz, J.; Sánchez, J.; Aguado, P.L.; Chaya, C.; Fernández, J. Effect of water regime change in a mature Arundo donax crop under a Xeric Mediterranean climate. Biomass Bioenergy 2018, 115, 203–209. [Google Scholar] [CrossRef]
- Panoutsou, C.; Chiaramonti, D. Socio-economic opportunities from miscanthus cultivation in marginal land for bioenergy. Energies 2020, 13, 2741. [Google Scholar] [CrossRef]
- Reinhardt, J.; Hilgert, P.; Von Cossel, M. Yield performance of dedicated industrial crops on low-temperature characterized marginal agricultural land in Europe—A review. Biofuels Bioprod. Biorefin. 2022, 16, 609–622. [Google Scholar] [CrossRef]
- Arai, Y.; Obataya, E.; Nakagawa-Izumi, A.; Okiyama, N. Acetylation of reed (Arundo donax) to prevent the contact dermatitis of woodwind musicians. Wood Sci. Technol. 2025, 59, 1. [Google Scholar] [CrossRef]
- Greco, S.; Molari, L.; Valdrè, G.; Garcia, J.J. Multilevel analysis of six species of Phyllostachys bamboo and Arundo donax: Preliminary survey on Italian grown stands. Wood Sci. Technol. 2024, 58, 1025–1049. [Google Scholar] [CrossRef]
- Liu, L.; Lin, Z.; Zheng, T.; Lin, L.; Zheng, C.; Lin, Z.; Wang, S.; Wang, Z. Fermentation optimization and characterization of the laccase from Pleurotus ostreatus strain 10969. Enzym. Microb. Technol. 2009, 44, 426–433. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, C.; Lin, D.; Lin, H.; Lin, Z. Effect of water extract from spent mushroom substrate after Ganoderma balabacense cultivation by using JUNCAO technique on production performance and hematology parameters of dairy cows. Anim. Sci. J. 2015, 86, 855–862. [Google Scholar] [CrossRef]
- Qiu, Y.; Lei, Y.; Zhao, H.; He, X.; Liu, B.; Huang, Y. Mesophilic anaerobic digestion of Arundo donax cv. Lvzhou No. 1 and Pennisetum giganteum for biogas production: Structure and functional analysis of microbial communities. Bioenergy Res. 2023, 16, 1205–1216. [Google Scholar] [CrossRef]
- Maduro Dias, C.S.A.M.; Nunes, H.; Vouzela, C.; Madruga, J.; Borba, A. In vitro rumen fermentation kinetics determination and nutritional evaluation of several non-conventional plants with potential for ruminant feeding. Fermentation 2023, 9, 416. [Google Scholar] [CrossRef]
- Zhang, K.; Yan, Y.; Zhao, R.; Song, X.; Du, L.; Zhang, B.; Yang, C.; Tang, X. Effects of substitution of wheat straw by giant reed on growth performance, serum biochemical parameters, nutrient digestibility, and antioxidant properties of sheep. Animals 2024, 14, 3678. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, A.; Debnath, K.; Gupta, M.K. Effects of alkaline-acid treatment on the physiochemical attributes of natural cellulosic fiber of Arundo donax L. J. Appl. Polym. Sci. 2023, 140, e54724. [Google Scholar] [CrossRef]
- Manniello, C.; Cillis, G.; Statuto, D.; Di Pasquale, A.; Picuno, P. Concrete blocks reinforced with Arundo donax natural fibers with different aspect ratios for application in bioarchitecture. Appl. Sci. 2022, 12, 2167. [Google Scholar] [CrossRef]
- Rodriguez, L.D.; Confalone, A.E.; Lazaro, L.; Pimentel, R.M.; Lyra, G.B.; de Oliveira Júnior, J.F.; Singh, S.K.; Pereira, C.R. Growth of the energy crop giant reed (Arundo donax L.) and optimization of the ARMIDA model in the south-central region of Buenos Aires, Argentina. Ind. Crops Prod. 2024, 211, 118190. [Google Scholar] [CrossRef]
- Verónica, C.; Alejandra, M.; Estela, S. Thermal behaviour and emission characteristics of Arundo donax L. as potential biofuel. Bioenergy Res. 2023, 16, 1618–1628. [Google Scholar] [CrossRef]
- Danelli, T.; Sepulcri, A.; Masetti, G.; Colombo, F.; Sangiorgio, S.; Cassani, E.; Anelli, S.; Adani, F.; Pilu, R. Arundo donax L. biomass production in a polluted area: Effects of two harvest timings on heavy metals uptake. Appl. Sci. 2021, 11, 1147. [Google Scholar] [CrossRef]
- Fagnano, M.; Impagliazzo, A.; Mori, M.; Fiorentino, N. Agronomic and environmental impacts of giant reed (Arundo donax L.): Results from a long-term field experiment in hilly areas subject to soil erosion. Bioenergy Res. 2015, 8, 415–422. [Google Scholar] [CrossRef]
- Speck, O. Field measurements of wind speed and reconfiguration in (Poaceae) with estimates of drag forces. Am. J. Bot. 2003, 90, 1253–1256. [Google Scholar] [CrossRef]
- Silva, G.H.d.; Renato, N.d.S.; Borges, A.C.; Martins, M.A.; Reis, A.J.D.d.; Otenio, M.H. Valorization and bioremediation of digestate from anaerobic co-digestion of giant reed (Arundo donax L.) and cattle wastewater using microalgae. Sustainability 2024, 16, 10328. [Google Scholar] [CrossRef]
- Balogh, E.; Herr, J.M.; Czakó, M.; Márton, L. Defective development of male and female gametophytes in Arundo donax L. (Poaceae). Biomass Bioenergy 2012, 45, 265–269. [Google Scholar] [CrossRef]
- Dragoni, F.; Volpi, I.; Lwin, A.K.; Triana, F.; Tozzini, C.; Ragaglini, G. Comparing different propagation methods for giant reed (Arundo donax L.) across three years from planting. Biomass Bioenergy 2021, 154, 106258. [Google Scholar] [CrossRef]
- Ceotto, E.; Di Candilo, M. Shoot cuttings propagation of giant reed (Arundo donax L.) in water and moist soil: The path forward? Biomass Bioenergy 2010, 34, 1614–1623. [Google Scholar] [CrossRef]
- Danelli, T.; Cantaluppi, E.; Tosca, A.; Cassani, E.; Landoni, M.; Bosio, S.; Adani, F.; Pilu, R. Influence of clonal variation on the efficiency of Arundo donax propagation methods. J. Plant Growth Regul. 2019, 38, 1449–1457. [Google Scholar] [CrossRef]
- Pilu, R.; Manca, A.; Landoni, M. Arundo donax as an energy crop: Pros and cons of the utilization of this perennial plant. Maydica 2013, 58, 54–59. [Google Scholar]
- Scordia, D.; Zanetti, F.; Varga, S.S.; Alexopoulou, E.; Cavallaro, V.; Monti, A.; Copani, V.; Cosentino, S.L. New insights into the propagation methods of switchgrass, miscanthus and giant reed. Bioenergy Res. 2015, 8, 1480–1491. [Google Scholar] [CrossRef]
- Spencer, D.F.; Ksander, G.G. Estimating Arundo donax ramet recruitment using degree-day based equations. Aquat. Bot. 2006, 85, 282–288. [Google Scholar] [CrossRef]
- Wijte, A.H.B.M.; Mizutani, T.; Motamed, E.R.; Merryfield, M.L.; Miller, D.E.; Alexander, D.E. Temperature and endogenous factors cause seasonal patterns in rooting by stem fragments of the invasive giant reed, Arundo donax (Poaceae). Int. J. Plant Sci. 2005, 166, 507–517. [Google Scholar] [CrossRef]
- Guo, J.; Li, W.; Cao, G.; Zhang, L.; Xie, Z.; Chen, W.; Shi, G.; Wei, F.; Tian, B. An efficient aqua-based culture method for the propagation of high-quality Arundo donax seedlings. Agronomy 2024, 14, 2047. [Google Scholar] [CrossRef]
- Antal, G.; Kurucz, E.; Fári, M.G.; Popp, J. Tissue culture and agamic propagation of winter-frost tolerant ‘Longicaulis’ Arundo donax L. Environ. Eng. Manag. J. 2014, 13, 2709–2715. [Google Scholar] [CrossRef]
- Cavallaro, V.; Patanè, C.; Cosentino, S.L.; Di Silvestro, I.; Copani, V. Optimizing in vitro large scale production of giant reed (Arundo donax L.) by liquid medium culture. Biomass Bioenergy 2014, 69, 21–27. [Google Scholar] [CrossRef]
- Ozudogru, E.A.; Karlik, E.; Elazab, D.; Lambardi, M. Establishment of an efficient somatic embryogenesis protocol for giant reed (Arundo donax L.) and multiplication of obtained shoots via semi-solid or liquid culture. Horticulturae 2023, 9, 735. [Google Scholar] [CrossRef]
- Valli, F.; Trebbi, D.; Zegada-Lizarazu, W.; Monti, A.; Tuberosa, R.; Salvi, S. In vitro physical mutagenesis of giant reed (Arundo donax L.). GCB Bioenergy 2017, 9, 1380–1389. [Google Scholar] [CrossRef]
- Cano-Ruiz, J.; Ruiz Galea, M.; Amorós, M.C.; Alonso, J.; Mauri, P.V.; Lobo, M.C. Assessing Arundo donax L. in vitro-tolerance for phytoremediation purposes. Chemosphere 2020, 252, 126576. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, H. Application of tissue culture for rapid propagation of Arundo donax. Chin. Agric. Sci. Bull. 2015, 31, 146–150. [Google Scholar]
- Permadi, N.; Akbari, S.I.; Prismantoro, D.; Indriyani, N.N.; Nurzaman, M.; Alhasnawi, A.N.; Doni, F.; Julaeha, E. Traditional and next-generation methods for browning control in plant tissue culture: Current insights and future directions. Curr. Plant Biol. 2024, 38, 100339. [Google Scholar] [CrossRef]
- Lin, Z.; Luo, Z.; Mei, L.; Liu, H.; Wu, J.; Lin, Z. Study on callus inductions of Lvzhou No. 3—A new material of Arundo. Pratacult. Sci. 2016, 33, 2012–2018. [Google Scholar]
- Takahashi, W.; Takamizo, T.; Kobayashi, M.; Ebina, M. Plant regeneration from calli in giant reed (Arundo donax L.). Grassl. Sci. 2010, 56, 224–229. [Google Scholar] [CrossRef]
- Hesami, M.; Naderi, R.; Tohidfar, M. Modeling and optimizing in vitro sterilization of chrysanthemum via multilayer perceptron-non-dominated sorting genetic algorithm-II (MLP-NSGAII). Front. Plant Sci. 2019, 10, 282. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Nhut, D.T.; Tanaka, M.; Fukai, S. The effect of antibiotics on the in vitro growth response of chrysanthemum and tobacco stem transverse thin cell layers (tTCLs). Sci. Hortic. 2003, 97, 397–410. [Google Scholar] [CrossRef]
- Kakani, A.; Li, G.; Peng, Z. Role of AUX1 in the control of organ identity during in vitro organogenesis and in mediating tissue specific auxin and cytokinin interaction in Arabidopsis. Planta 2009, 229, 645–657. [Google Scholar] [CrossRef]
- Li, D.; Kang, X. The status and prospect of exodogenous hormone and endogenous hormone impact in plant callus tissue culture. Lett. Biotechnol. 2007, 18, 546–548. [Google Scholar]
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The yin-yang of hormones: Cytokinin and auxin interactions in plant development. Plant Cell 2015, 27, 44–63. [Google Scholar] [CrossRef]
- Aslam, J.; Mujib, A.; Sharma, M.P. In vitro micropropagation of Dracaena sanderiana Sander ex Mast: An important indoor ornamental plant. Saudi J. Biol. Sci. 2013, 20, 63–68. [Google Scholar] [CrossRef]
- Shimabukuro, R.H.; Walsh, W.C.; Hoerauf, R.A. Reciprocal antagonism between the herbicides, diclofop-methyl and 2,4-D, in corn and soybean tissue-culture. Plant Physiol. 1986, 80, 612–617. [Google Scholar] [CrossRef]
- Singh, V.; Singh, K. Toxic effect of herbicide 2,4-D on the Earthworm eutyphoeus waltoni michaelsen. Environ. Process. 2015, 2, 251–260. [Google Scholar] [CrossRef]
- Gaspar, T.; Kevers, C.; Penel, C.; Greppin, H.; Reid, D.M.; Thorpe, T.A. Plant hormones and plant growth regulators in plant tissue culture. Vitr. Cell. Dev. Biol. Plant 1996, 32, 272–289. [Google Scholar] [CrossRef]
- Hu, G.; Dong, Y.; Zhang, Z.; Fan, X.; Ren, F. The effects of different hormones and concentration of major element stock solution on the proliferation of in vitro grapevine. Appl. Fruit Sci. 2025, 67, 4. [Google Scholar] [CrossRef]
- Pischke, M.S.; Huttlin, E.L.; Hegeman, A.D.; Sussman, M.R. A transcriptome-based characterization of habituation in plant tissue culture. Plant Physiol. 2006, 140, 1255–1278. [Google Scholar] [CrossRef]
- Fira, A.; Clapa, D.; Vescan, L.A. Direct ex vitro rooting and acclimation in blackberry cultivar ‘Loch Ness’. Bull. UASVM Anim. Sci. Biotechnol. 2012, 69, 247–254. [Google Scholar]
- Suzuki, N.; Miller, G.; Salazar, C.; Mondal, H.A.; Shulaev, E.; Cortes, D.F.; Shuman, J.L.; Luo, X.; Shah, J.; Schlauch, K.; et al. Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 2013, 25, 3553–3569. [Google Scholar] [CrossRef] [PubMed]
- Nkebiwe, P.M.; Weinmann, M.; Müller, T. Improving fertilizer-depot exploitation and maize growth by inoculation with plant growth-promoting bacteria: From lab to field. Chem. Biol. Technol. Agric. 2016, 3, 15. [Google Scholar] [CrossRef]
- Sharma, K.K.; Thorpe, T.A. In vitro propagation of mulberry (Morus alba L.) through nodal segments. Sci. Hortic. 1990, 42, 307–320. [Google Scholar] [CrossRef]
- Wang, T.; Li, H.; Zhao, J.; Huang, J.; Zhong, Y.; Xu, Z.; He, F. Exploration of suitable conditions for shoot proliferation and rooting of Quercus robur L. in plant tissue culture technology. Life 2025, 15, 348. [Google Scholar] [CrossRef]
- Cavallaro, V.; Tringali, S.; Patanè, C. Large-scale in vitro propagation of giant reed (Arundo donax L.), a promising biomass species. J. Hortic. Sci. Biotechnol. 2011, 86, 452–456. [Google Scholar] [CrossRef]
- Gubišová, M.; Čičková, M.; Klčová, L.; Gubiš, J. In vitro tillering—An effective way to multiply high-biomass plant Arundo donax. Ind. Crops Prod. 2016, 81, 123–128. [Google Scholar] [CrossRef]
| Disinfection Method | Contamination Rate (%) | Mortality Rate (%) |
|---|---|---|
| 2% sodium hypochlorite for 10 min | 74.4 ± 1.1 a | 0 d |
| 5% sodium hypochlorite for 10 min | 63.3 ± 1.9 b | 17.8 ± 2.9 c |
| 8% sodium hypochlorite for 10 min | 61.1 ± 2.9 b | 32.2 ± 2.9 b |
| 10% sodium hypochlorite for 10 min | 52.2 ± 2.2 c | 58.9 ± 2.2 a |
| 0.1% mercury chloride for 3 min | 35.6 ± 2.2 d | 0 d |
| 0.1% mercury chloride for 5 min | 17.8 ± 1.1 e | 22.2 ± 1.1 c |
| Combinations of Cytokinin and Auxin | Induction Rate (%) | Browning Rate (%) |
|---|---|---|
| 5.0 mg/L 6-BA + 1.0 mg/L 2,4-D | 3.3 ± 1.9 e | 25.6 ± 2.2 a |
| 5.0 mg/L 6-BA + 1.0 mg/L NAA | 72.2 ± 2.9 d | 4.4 ± 1.1 b |
| 5.0 mg/L 6-BA + 1.0 mg/L IBA | 97.8 ± 1.1 a | 1.1 ± 1.1 b |
| 5.0 mg/L KT + 1.0 mg/L IBA | 87.8 ± 1.1 b | 5.6 ± 1.1 b |
| 5.0 mg/L TDZ + 1.0 mg/L IBA | 81.1 ± 2.2 c | 4.4 ± 2.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Yu, M.; Li, W.; Cao, G.; Zhang, L.; Chen, W.; Xie, Z.; Shi, G.; Wei, F.; Tian, B. Efficient Tissue Culture Method Based on Clustered Bud Proliferation for Producing High-Quality Arundo donax Seedlings. Plants 2025, 14, 2978. https://doi.org/10.3390/plants14192978
Guo J, Yu M, Li W, Cao G, Zhang L, Chen W, Xie Z, Shi G, Wei F, Tian B. Efficient Tissue Culture Method Based on Clustered Bud Proliferation for Producing High-Quality Arundo donax Seedlings. Plants. 2025; 14(19):2978. https://doi.org/10.3390/plants14192978
Chicago/Turabian StyleGuo, Jialin, Mingchen Yu, Wei Li, Gangqiang Cao, Luyue Zhang, Weiwei Chen, Zhengqing Xie, Gongyao Shi, Fang Wei, and Baoming Tian. 2025. "Efficient Tissue Culture Method Based on Clustered Bud Proliferation for Producing High-Quality Arundo donax Seedlings" Plants 14, no. 19: 2978. https://doi.org/10.3390/plants14192978
APA StyleGuo, J., Yu, M., Li, W., Cao, G., Zhang, L., Chen, W., Xie, Z., Shi, G., Wei, F., & Tian, B. (2025). Efficient Tissue Culture Method Based on Clustered Bud Proliferation for Producing High-Quality Arundo donax Seedlings. Plants, 14(19), 2978. https://doi.org/10.3390/plants14192978





