Response Patterns and Mechanisms of Seed Germination and Mortality of Common Plants in Subalpine Wet Meadows to In Situ Burial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Seed Collection and Processing
2.3. In Situ Burial Experiment
2.4. Determination of Seed Germination Rate
2.5. Determination of Seed Mortality Rate
2.6. Data Analysis
2.6.1. Germination and Mortality Metrics: Calculation Methods
2.6.2. Statistical Processing and Analysis
3. Results
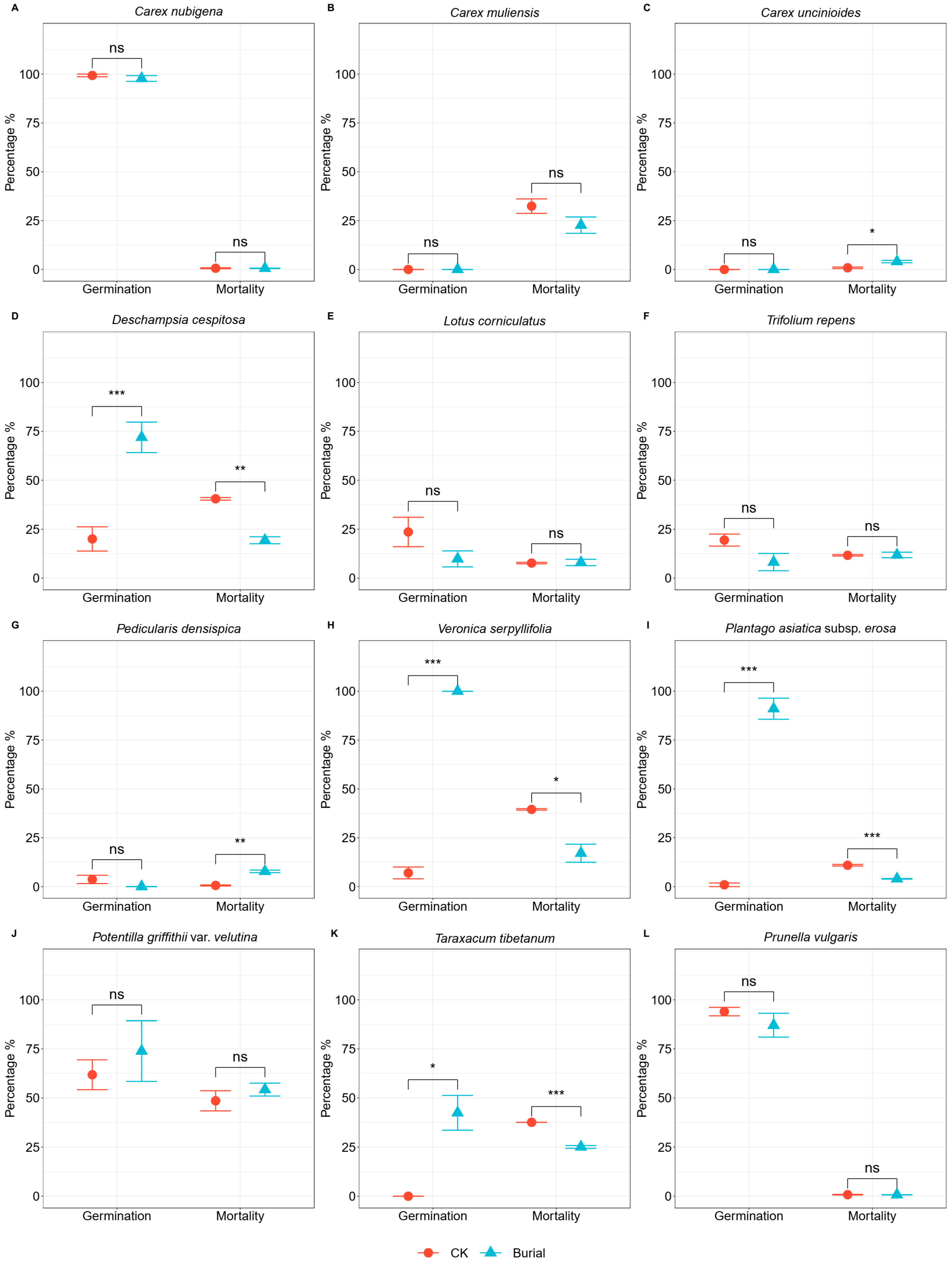
3.1. The Effect of Burial on Seed Germination Rate
3.2. The Effect of Burial on Seed Mortality Rate
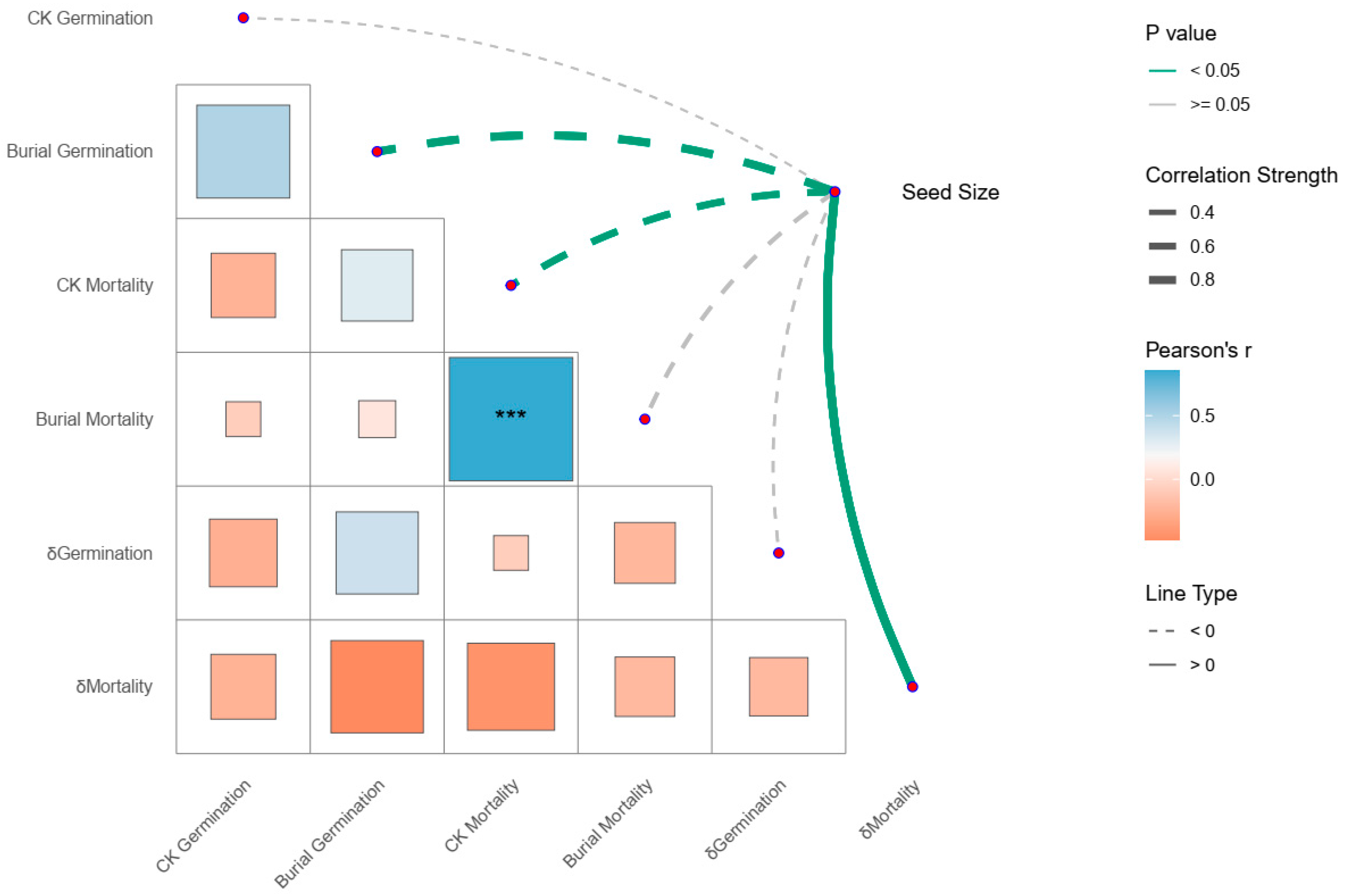
3.3. Relationships Between Seed Size and Germination/Mortality Rates Under Control and In Situ Burial Treatments
4. Discussion
4.1. Species—Specific Response Mechanisms
4.2. Relationships Between Seed Size and Response of Germination and Mortality Rates
4.3. Study Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rivera, D.; Jáuregui, B.M.; Peco, B. The fate of herbaceous seeds during topsoil stockpiling: Restoration potential of seed banks. Ecol. Eng. 2012, 44, 94–101. [Google Scholar] [CrossRef]
- Harrison, R.J.; Howieson, J.G.; Yates, R.J.; Nutt, B.J. Long-term storage of forage legumes greatly alters the hard seed breakdown pattern in situ. Grass Forage Sci. 2020, 76, 72–81. [Google Scholar] [CrossRef]
- Guzmán-Hernández, D.A.; Barbosa-Martínez, C.; Villa-Hernández, J.M.; Pérez-Flores, L.J. Redox imbalance accompanies loss of viability in seeds of two cacti species buried in situ. Seed Sci. Res. 2024, 34, 1–9. [Google Scholar] [CrossRef]
- Garcias-Morales, C.; Orozco-Segovia, A.; Soriano, D.; Zuloaga-Aguilar, S. Effects of In Situ Burial and Sub-Optimal Storage on Seed Longevity and Reserve Resources in Sub-Tropical Mountain Cloud Forest Tree Species of Mexico. Trop. Conserv. Sci. 2021, 14, 1940082921989196. [Google Scholar] [CrossRef]
- Wawrzyniak, M.K.; Michalak, M.; Chmielarz, P. Effect of different conditions of storage on seed viability and seedling growth of six European wild fruit woody plants. Ann. For. Sci. 2020, 77, 58. [Google Scholar] [CrossRef]
- Benvenuti, S. Weed seed movement and dispersal strategies in the agricultural environment. Weed Biol. Manag. 2007, 7, 141–157. [Google Scholar] [CrossRef]
- Chambers, J.C.; MacMahon, J.A. A Day in the Life of a Seed: Movements and Fates of Seeds and Their Implications for Natural and Managed Systems. Annu. Rev. Ecol. Syst. 1994, 25, 263–292. [Google Scholar] [CrossRef]
- Murdoch, A.J. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination. Crop Sci. 2000, 40, 564–565. [Google Scholar] [CrossRef]
- Santos, J.A.S.; Almeida, H.A.; Meiado, M.V.; Garcia, Q.S. Environmental and physiological factors influencing seed longevity of three Brazilian endemic cacti during in situ storage. Flora 2025, 326, 152711. [Google Scholar] [CrossRef]
- Omami; Haigh; Medd; Nicol. Changes in germinability, dormancy and viability of Amaranthus retroflexus as affected by depth and duration of burial. Weed Res. 1999, 39, 345–354. [Google Scholar] [CrossRef]
- Steckel, L.E.; Sprague, C.L.; Stoller, E.W.; Wax, L.M. Temperature effects on germination of nine Amaranthus species. Weed Sci. 2004, 52, 217–221. [Google Scholar] [CrossRef]
- Pons, T.L. Dormancy, germination and mortality of seeds in a chalk-grassland flora. J. Ecol. 1991, 79, 765–780. [Google Scholar] [CrossRef]
- Zhang, C.; Willis, C.G.; Ma, Z.; Ma, M.; Csontos, P.; Baskin, C.C.; Baskin, J.M.; Li, J.; Zhou, H.; Zhao, X.; et al. Direct and indirect effects of long-term fertilization on the stability of the persistent seed bank. Plant Soil 2019, 438, 239–250. [Google Scholar] [CrossRef]
- Li, C.; Ma, Z.; Wu, L.; Ren, Y.; Sun, J.; Fu, Y.; Meng, J.; Ning, X.; Zhou, H.; Zhang, C. Effects of seed size and in-situ burial on seed germination and mortality of 17 species of alpine meadow. Acta Agrestia Sin. 2021, 29, 2169–2175. [Google Scholar] [CrossRef]
- Jia, C.-Z.; Wang, J.-J.; Chen, D.-L.; Hu, X.-W. Seed Germination and Seed Bank Dynamics of Eruca sativa (Brassicaceae): A Weed on the Northeastern Edge of Tibetan Plateau. Front. Plant Sci. 2022, 13, 820925. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-L.; Mo, B.-T.; Wang, P.-C.; Zhang, Y.; Long, Z.-F. Relationship of Sophora davidii seed size to germination, dormancy, and mortality under water stress. S. Afr. J. Bot. 2015, 99, 12–16. [Google Scholar] [CrossRef]
- Luo, Y.; Cheng, J.; Yan, X.; Yang, H.; Shen, Y.; Ge, J.; Zhang, M.; Zhang, J.; Xu, Z. Density-Dependent Seed Predation of Quercus wutaishanica by Rodents in Response to Different Seed States. Animals 2023, 13, 1732. [Google Scholar] [CrossRef] [PubMed]
- Daws, M.I.; Ballard, C.; Mullins, C.E.; Garwood, N.C.; Murray, B.; Pearson, T.R.H.; Burslem, D.F.R.P. Allometric relationships between seed mass and seedling characteristics reveal trade-offs for neotropical gap-dependent species. Oecologia 2007, 154, 445–454. [Google Scholar] [CrossRef]
- Parker, V.T.; Ingalls, S.B. Seed size–seed number trade-offs: Influence of seed size on the density of fire-stimulated persistent soil seed banks. Am. J. Bot. 2022, 109, 486–493. [Google Scholar] [CrossRef]
- Cheng, J.; Yan, X.; Zhang, J.; Zhang, C.; Zhang, M.; Wei, S.; Wang, J.; Luo, Y. Seed traits and burial state affect plant seed secondary dispersal mediated by rodents. Heliyon 2024, 10, e32612. [Google Scholar] [CrossRef]
- Pearson, T.R.H.; Burslem, D.F.R.P.; Mullins, C.E.; Dalling, J.W. Germination Ecology of Neotropical Pioneers: Interacting Effects of Environmental Conditions and Seed Size. Ecology 2002, 83, 2798–2807. [Google Scholar] [CrossRef]
- Limón, Á.; Peco, B. Germination and emergence of annual species and burial depth: Implications for restoration ecology. Acta Oecologica 2016, 71, 8–13. [Google Scholar] [CrossRef]
- Espinar, J.L.; Thompson, K.; García, L.V. Timing of seed dispersal generates a bimodal seed bank depth distribution. Am. J. Bot. 2005, 92, 1759–1763. [Google Scholar] [CrossRef]
- Han, M.; Liu, C.; Liang, H.; Ma, H.; Ma, H.; Shen, Y.; Wang, G. The impact of different altitude on soil seed banks in temperate mountainous grassland. Acta Agrestia Sin. 2025, 1–16. Available online: https://link.cnki.net/urlid/11.3362.S.20250331.1603.002 (accessed on 14 August 2025).
- Zhang, L.; Liu, H.; Sheng, J. Effects of burial on seed germination traits of 10 ephemeral plant species. Arid Zone Res. 2018, 35, 633–639. [Google Scholar] [CrossRef]
- Guo, C.-r.; Wang, Z.-l.; Lu, J.-q. Seed germination and seedling development of Prunus armeniaca under different burial depths in soil. J. For. Res. 2010, 21, 492–496. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, H.; Yan, X.; Ma, Y.; Wei, S.; Wang, J.; Cao, Z.; Zuo, Z.; Yang, C.; Cheng, J. Response of Seed Germination and Seedling Growth of Six Desert Shrubs to Different Moisture Levels under Greenhouse Conditions. Biology 2024, 13, 747. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qian, L.; Zhang, Y.; Shi, H.; Sun, H.; Chen, J. Alpine community recruitment potential is determined by habitat attributes in the alpine ecosystems of the Himalaya-Hengduan Mountains, SW China. Ecol. Evol. 2021, 11, 17397–17408. [Google Scholar] [CrossRef]
- Wang, J.; Yan, Q. Effects of disturbances on animal-mediated seed dispersal effectiveness of forest plants:A review. Chin. J. Appl. Ecol. 2017, 28, 1716–1726. [Google Scholar] [CrossRef]
- Fontúrbel, F.E.; Candia, A.B.; Malebrán, J.; Salazar, D.A.; González-Browne, C.; Medel, R. Meta-analysis of anthropogenic habitat disturbance effects on animal-mediated seed dispersal. Glob. Change Biol. 2015, 21, 3951–3960. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Long, R.L.; Gorecki, M.J.; Renton, M.; Scott, J.K.; Colville, L.; Goggin, D.E.; Commander, L.E.; Westcott, D.A.; Cherry, H.; Finch-Savage, W.E. The ecophysiology of seed persistence: A mechanistic view of the journey to germination or demise. Biol. Rev. Camb. Philos. Soc. 2015, 90, 31–59. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Bu, Z.-J.; Poschlod, P.; Yusup, S.; Zhang, J.-Q.; Zhang, Z.-X. Seed dormancy types and germination response of 15 plant species in temperate montane peatlands. Ecol. Evol. 2024, 14, e11671. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.-L.; Yang, L.-E.; Yang, J.; Li, Z.-M. Seed Dormancy and Soil Seed Bank of the Two Alpine Primula Species in the Hengduan Mountains of Southwest China. Front. Plant Sci. 2021, 12, 582536. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, M.; Yan, X.; Zhang, C.; Zhang, J.; Luo, Y. Effects of Seed Size and Frequency on Seed Dispersal and Predation by Small Mammals. Biology 2024, 13, 353. [Google Scholar] [CrossRef] [PubMed]
- Moles, A.T.; Westoby, M. Seedling survival and seed size: A synthesis of the literature. J. Ecol. 2004, 92, 372–383. [Google Scholar] [CrossRef]
- Abbas, A.M.; Rubio-Casal, A.E.; De Cires, A.; Figueroa, E.M.; Pickart, A.J.; Castillo, J.M. Burial effects on seed germination and seedling emergence of two halophytes of contrasting seed size. Plant Ecol. Divers. 2020, 13, 339–349. [Google Scholar] [CrossRef]
- Mao, P.; Guo, L.; Gao, Y.; Qi, L.; Cao, B. Effects of Seed Size and Sand Burial on Germination and Early Growth of Seedlings for Coastal Pinus thunbergii Parl. in the Northern Shandong Peninsula, China. Forests 2019, 10, 281. [Google Scholar] [CrossRef]
- Berrached, R.; Kadik, L.; Ait Mouheb, H.; Prinzing, A. Deep roots delay flowering and relax the impact of floral traits and associated pollinators in steppe plants. PLoS ONE 2017, 12, e0173921. [Google Scholar] [CrossRef]
- Lu, J.; Yi, H.; Tan, D.; Baskin, C.C.; Baskin, J.M. Germination of Seeds from Flowers along a Continuum of Long to Short Styles in the Cold Desert Perennial Herb Ixiolirion songaricum. Plants 2022, 11, 1452. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Nan, Z.; Baskin, C.; Chen, T. Effect of seed size and fungicide on germination and survival of buried seeds of two grassland species on the Loess Plateau, China. Acta Oecologica 2021, 110, 103716. [Google Scholar] [CrossRef]
- Duncan, C.; Schultz, N.L.; Good, M.K.; Lewandrowski, W.; Cook, S. The risk-takers and -avoiders: Germination sensitivity to water stress in an arid zone with unpredictable rainfall. AoB Plants 2019, 11, plz066. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Guo, H.; Yin, J.; Ding, X.; Xu, X.; Wang, T.; Yang, C.; Xiong, W.; Zhong, S.; Tao, Q.; et al. Germination ecology of Chenopodium album L. and implications for weed management. PLoS ONE 2022, 17, e0276176. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.M.; Florentine, S.; Ali, H.H.; Weller, S. Effect of environmental factors on the germination and emergence of Salvia verbenaca L. cultivars (verbenaca and vernalis): An invasive species in semi-arid and arid rangeland regions. PLoS ONE 2018, 13, e0194319. [Google Scholar] [CrossRef] [PubMed]
| Species Name | Abbreviation | Family Name | Seed Mass/mg |
|---|---|---|---|
| Carex nubigena | C. nub | Cyperaceae | 0.35 ± 0.01 |
| Carex muliensis | C. mul | Cyperaceae | 0.69 ± 0.01 |
| Carex uncinioides | C. unc | Cyperaceae | 0.88 ± 0.02 |
| Deschampsia cespitosa | D. ces | Gramineae | 0.20 ± 0.01 |
| Lotus corniculatus | L. cor | Leguminosae | 0.93 ± 0.01 |
| Trifolium repens | T. rep | Leguminosae | 0.49 ± 0.02 |
| Pedicularis densispica | P. den | Scrophulariaceae | 1.49 ± 0.03 |
| Veronica serpyllifolia | V. ser | Scrophulariaceae | 0.05 ± 0.00 |
| Plantago asiatica subsp. erosa | P. asi | Plantaginaceae | 0.42 ± 0.01 |
| Potentilla griffithii var. velutina | P. grif | Rosaceae | 0.37 ± 0.00 |
| Taraxacum tibetanum | T.tib | Compositae | 0.51 ± 0.01 |
| Prunella vulgaris | P. vul | Labiatae | 0.63 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.; Cui, H.; Liu, Y.; Song, W.; Yu, J.; Li, J.; Zhao, X.; Wei, X.; Bi, X.; Zhang, P.; et al. Response Patterns and Mechanisms of Seed Germination and Mortality of Common Plants in Subalpine Wet Meadows to In Situ Burial. Plants 2025, 14, 2975. https://doi.org/10.3390/plants14192975
Yuan S, Cui H, Liu Y, Song W, Yu J, Li J, Zhao X, Wei X, Bi X, Zhang P, et al. Response Patterns and Mechanisms of Seed Germination and Mortality of Common Plants in Subalpine Wet Meadows to In Situ Burial. Plants. 2025; 14(19):2975. https://doi.org/10.3390/plants14192975
Chicago/Turabian StyleYuan, Suyao, Haijun Cui, Yuzhen Liu, Weifeng Song, Junbao Yu, Jie Li, Xuyan Zhao, Xiaoyan Wei, Xiaoting Bi, Putao Zhang, and et al. 2025. "Response Patterns and Mechanisms of Seed Germination and Mortality of Common Plants in Subalpine Wet Meadows to In Situ Burial" Plants 14, no. 19: 2975. https://doi.org/10.3390/plants14192975
APA StyleYuan, S., Cui, H., Liu, Y., Song, W., Yu, J., Li, J., Zhao, X., Wei, X., Bi, X., Zhang, P., Wang, T., & Pu, J. (2025). Response Patterns and Mechanisms of Seed Germination and Mortality of Common Plants in Subalpine Wet Meadows to In Situ Burial. Plants, 14(19), 2975. https://doi.org/10.3390/plants14192975





