Potential Therapeutic and Medicinal Applications of Four Invasive Non-Native Plant Species: A PRISMA-Guided Systematic Review of PubMed Studies
Abstract
1. Introduction
1.1. The Impact of Invasive Non-Native Plant Species and Their Possible Valorisation
1.2. Argumentation of the Study
1.3. Study Objectives
2. Methodology
2.1. Botanical Terminology
2.2. Data Collection
- 1.
- The cited research must utilise a traceable plant material or isolated phytocompound, with either a deposited voucher specimen or a clearly stated source.
- 2.
- The cited articles must be written in English or have an English translation available.
- 3.
- Only original research articles were considered; reviews, commentaries, and other non-original formats were excluded.
- 4.
- The study must evaluate at least one type of biological activity, conducted either in vitro, in vivo, or both.
2.3. Data Analysis
3. Results
3.1. Literature Screening
3.2. Studies Overview
3.3. INPS and Their Antimicrobial Potential
3.3.1. The Antibacterial Activity of INPS
3.3.2. The Antiviral Activity of INPS
3.3.3. The Antimalarial Activity of INPS
3.4. INPS and Their Antioxidant Potential
3.5. INPS and Their Cytotoxic Potential
3.6. INPS and Their Other Therapeutic Potentials
4. Study Limitations
- Most of the included research is based on in vitro assays, which may not accurately reflect therapeutic efficacy in vivo;
- The exclusion of non-English publications and reliance on a single database (PubMed) may have led to the omission of relevant studies.
- The lack of standardised methodologies across the reviewed articles limits direct comparability of results;
- The absence of toxicological and pharmacokinetic data for many compounds restricts the assessment of their clinical applicability.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 13-HODE | (9Z,11E)-13-hydroxy-9,11-octadecadienoic acid |
| 9-HODE | (10E,12Z)-9-hydroxy-10,12-octadecadienoic acid |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| AChE | Acetylcholinesterase |
| ACP | Apoptotic cells percentage |
| BBR | Bax/Bcl2 ratio |
| BI | Biofilm inhibition |
| BMMC | Primary bone marrow mononuclear spherical cells |
| BuChE | Butyrylcholinesterase |
| C | Concentration |
| CAT | Catalase |
| CCK-8 | Cell counting kit-8 |
| CDDP | Cisplatin |
| CDK4 | Cyclin-dependent kinase 4 |
| COX | Cyclooxygenase |
| CUPRAC | Cupric ion-reducing antioxidant capacity |
| CV | Cell viability |
| D | Dose |
| DGF | 5,7-dihydroxy-6-geranylflavanone |
| DMSO | Dimethyl sulfoxide |
| DNA | Deoxyribonucleic acid |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DW | Dry weight |
| EBV | Epstein–Barr virus |
| EBV-EA | Epstein–Barr virus early antigen |
| ERK | Extracellular signal-regulated kinase |
| EU | European Union |
| FIC | Ferrous ion chelating |
| FRAP | Ferric-reducing antioxidant power |
| GAS5 | Growth arrest specific 5 |
| GnRH-PAP | Pokeweed antiviral protein fused with gonadotropin-releasing hormone |
| GSH-Px | Glutathione peroxidase |
| HIV-1 | Human immunodeficiency virus 1 |
| HTLV-I | Human T-cell leukaemia virus I |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| INPS | Invasive non-native plant species |
| IP | Inhibition percentage |
| IPNI | International Plant Names Index |
| IR-Akt | Insulin receptor-protein kinase B |
| IZ | Inhibition zone |
| JAK | Janus kinase |
| JEV | Japanese encephalitis virus |
| JNK | c-Jun NH2-terminal kinase |
| LPS | Lipopolysaccharide |
| LTC4 | Leukotriene C4 |
| MAPK | Mitogen-activated protein kinase |
| MBC | Minimum bactericidal concentration |
| MDA | Malondialdehyde |
| MIC | Minimum inhibitory concentration |
| mRNA | Messenger ribonucleic acid |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide |
| N/A | Not applicable |
| NF-κB | Nuclear factor kappa B |
| NO | Nitric oxide |
| NSLCL | Non-small cell lung cancer |
| OH | Hydroxyl |
| PAP | Pokeweed antiviral protein |
| PPAR | Peroxisome proliferator-activated receptor |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RANKL | Receptor activator of nuclear factor kappa B ligand |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| SP | Survival percentage |
| SR | Scavenging rates |
| STAT3 | Signal transducer and activator of transcription 3 |
| TE | Trolox equivalents |
| TEAD | Transcription factor |
| TNF | Tumour necrosis factor |
| TUNEL | Terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labelling |
| UCHL1 | Ubiquitin carboxy-terminal hydrolase L1 |
| ULK1 | Unc51-like autophagy activating kinase 1 |
| UPF1 | Up-frameshift protein 1 |
| UV | Ultraviolet |
| WFO | World Flora Online |
| YAP | Yes-associated protein 1 |
| β-HEX | β-hexosaminidase |
References
- Royal Horticultural Society. Invasive Non-Native Plants: Prevention and Protection. Royal Horticultural Society. Available online: https://www.rhs.org.uk/prevention-protection/invasive-non-native-plants (accessed on 16 June 2025).
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vilà, M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species’ traits and environment. Glob. Change Biol. 2012, 18, 1725–1737. [Google Scholar] [CrossRef]
- Anderson, L.G.; Rocliffe, S.; Haddaway, N.R.; Dunn, A.M. The Role of Tourism and Recreation in the Spread of Non-Native Species: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0140833. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Dawson, W.; Essl, F.; Pergl, J.; Winter, M.; Weber, E.; Kreft, H.; Weigelt, P.; Kartesz, J.; Nishino, M.; et al. Global exchange and accumulation of non-native plants. Nature 2015, 525, 100–103. [Google Scholar] [CrossRef]
- Gioria, M.; Hulme, P.E.; Richardson, D.M.; Pyšek, P. Why Are Invasive Plants Successful? Annu. Rev. Plant Biol. 2023, 74, 635–670. [Google Scholar] [CrossRef] [PubMed]
- Weidlich, E.W.A.; Flórido, F.G.; Sorrini, T.B.; Brancalion, P.H.S. Controlling invasive plant species in ecological restoration: A global review. J. Appl. Ecol. 2020, 57, 1806–1817. [Google Scholar] [CrossRef]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Zeng, H.; Zhang, J.; Zhang, L. Public education improves farmers knowledge and management of invasive alien species. Biol. Invasions 2021, 23, 2003–2017. [Google Scholar] [CrossRef]
- Lorenzo, P.; Morais, M.C. Strategies for the Management of Aggressive Invasive Plant Species. Plants 2023, 12, 2482. [Google Scholar] [CrossRef]
- Invasive Alien Species—European Commission. Available online: https://environment.ec.europa.eu/topics/nature-and-biodiversity/invasive-alien-species_en (accessed on 16 June 2025).
- Dehnen-Schmutz, K.; Novoa, A. Advances in the Management of Invasive Plants. In Global Plant Invasions; Clements, D.R., Upadhyaya, M.K., Joshi, S., Shrestha, A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 317–330. ISBN 978-3-030-89684-3. [Google Scholar]
- Cuthbert, R.N.; Diagne, C.; Hudgins, E.J.; Turbelin, A.; Ahmed, D.A.; Albert, C.; Bodey, T.W.; Briski, E.; Essl, F.; Haubrock, P.J.; et al. Biological invasion costs reveal insufficient proactive management worldwide. Sci. Total Environ. 2022, 819, 153404. [Google Scholar] [CrossRef]
- Haubrock, P.J.; Turbelin, A.J.; Cuthbert, R.N.; Novoa, A.; Taylor, N.G.; Angulo, E.; Ballesteros-Mejia, L.; Bodey, T.W.; Capinha, C.; Diagne, C.; et al. Economic costs of invasive alien species across Europe. NeoBiota 2021, 67, 153–190. [Google Scholar] [CrossRef]
- Máximo, P.; Ferreira, L.M.; Branco, P.S.; Lourenço, A. Invasive Plants: Turning Enemies into Value. Molecules 2020, 25, 3529. [Google Scholar] [CrossRef]
- Peter, A.; Žlabur, J.Š.; Šurić, J.; Voća, S.; Purgar, D.D.; Pezo, L.; Voća, N. Invasive Plant Species Biomass—Evaluation of Functional Value. Molecules 2021, 26, 3814. [Google Scholar] [CrossRef]
- Ahmed, A.; Abu Bakar, M.S.; Hamdani, R.; Park, Y.-K.; Lam, S.S.; Sukri, R.S.; Hussain, M.; Majeed, K.; Phusunti, N.; Jamil, F.; et al. Valorization of underutilized waste biomass from invasive species to produce biochar for energy and other value-added applications. Environ. Res. 2020, 186, 109596. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.J.R.; Rodrigues, A.M.; Loureiro, L.M.E.F.; Sá, L.C.R.; Matias, J.C.O. Energy Recovery from Invasive Species: Creation of Value Chains to Promote Control and Eradication. Recycling 2021, 6, 21. [Google Scholar] [CrossRef]
- Míguez, C.; Cancela, Á.; Álvarez, X.; Sánchez, Á. The reuse of bio-waste from the invasive species Tradescantia fluminensis as a source of phenolic compounds. J. Clean. Prod. 2022, 336, 130293. [Google Scholar] [CrossRef]
- Banunle, A.; Fei-Baffoe, B.; Miezah, K.; Ewusi-Mensah, N.; Jørgensen, U.; Aidoo, R.; Amoah, A.; Addo-Fordjour, P.; Abaidoo, R.C. Valorisation of Biowaste and Aquatic Invasive Plants Through Compost Production for Agricultural Use. Waste Biomass Valorization 2023, 14, 4127–4139. [Google Scholar] [CrossRef]
- Lorenzo, P.; Morais, M.C. Repurposing Waste from Aggressive Acacia Invaders to Promote Its Management in Large Invaded Areas in Southwestern Europe. Plants 2024, 13, 1428. [Google Scholar] [CrossRef]
- Raudone, L.; Savickiene, N. Phytochemical Profiles of Plant Materials: From Extracts to Added-Value Ingredients. Plants 2024, 13, 964. [Google Scholar] [CrossRef]
- Vrabič-Brodnjak, U.; Možina, K. Invasive Alien Plant Species for Use in Paper and Packaging Materials. Fibers 2022, 10, 94. [Google Scholar] [CrossRef]
- Quinty, V.; Colas, C.; Nasreddine, R.; Nehmé, R.; Piot, C.; Draye, M.; Destandau, E.; Da Silva, D.; Chatel, G. Screening and Evaluation of Dermo-Cosmetic Activities of the Invasive Plant Species Polygonum cuspidatum. Plants 2022, 12, 83. [Google Scholar] [CrossRef]
- Chopra, B.; Dhingra, A.K. Natural products: A lead for drug discovery and development. Phytother. Res. 2021, 35, 4660–4702. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K. The Role of Natural Products as Sources of Therapeutic Agents for Innovative Drug Discovery. In Comprehensive Pharmacology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 408–422. ISBN 978-0-12-820876-2. [Google Scholar]
- Luo, Z.; Yin, F.; Wang, X.; Kong, L. Progress in approved drugs from natural product resources. Chin. J. Nat. Med. 2024, 22, 195–211. [Google Scholar] [CrossRef]
- Cappuccino, N.; Arnason, J.T. Novel chemistry of invasive exotic plants. Biol. Lett. 2006, 2, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Macel, M.; De Vos, R.C.H.; Jansen, J.J.; Van Der Putten, W.H.; Van Dam, N.M. Novel chemistry of invasive plants: Exotic species have more unique metabolomic profiles than native congeners. Ecol. Evol. 2014, 4, 2777–2786. [Google Scholar] [CrossRef] [PubMed]
- Skubel, S.A.; Su, X.; Poulev, A.; Foxcroft, L.C.; Dushenkov, V.; Raskin, I. Metabolomic differences between invasive alien plants from native and invaded habitats. Sci. Rep. 2020, 10, 9749. [Google Scholar] [CrossRef]
- Sirbu, C.; Miu, I.V.; Gavrilidis, A.A.; Gradinaru, S.R.; Niculae, I.M.; Preda, C.; Oprea, A.; Urziceanu, M.; Camen-Comanescu, P.; Nagoda, E.; et al. Distribution and pathways of introduction of invasive alien plant species in Romania. NeoBiota 2022, 75, 1–21. [Google Scholar] [CrossRef]
- Anastasiu, P.; Miu, I.V.; Gavrilidis, A.A.; Preda, C.; Rozylowicz, L.; Sirbu, C.; Oprea, A.; Urziceanu, M.; Camen-Comanescu, P.; Nagoda, E.; et al. Alien plant species distribution in Romania: A nationwide survey following the implementation of the EU Regulation on Invasive Alien Species. Biodivers. Data J. 2024, 12, e119539. [Google Scholar] [CrossRef]
- Dumitrascu, M.; Grigorescu, I.; Doroftei, M.; Kucsicsa, G.; Mierla, M.; Dragota, C.-S.; Nastase, M. Assessing Invasive Terrestrial Plan Species Amorpha fruticosa in Three Wetland Areas in Romania: Danube Delta Biosphere Reserve, Comana Natural Park and Mures Floodplain Natural Park. Int. Multidiscip. Sci. GeoConf. SGEM 2013, 1, 113–124. [Google Scholar]
- Plants of the World Online|Kew Science. Available online: https://powo.science.kew.org/ (accessed on 16 June 2025).
- International Plant Names Index. Available online: https://www.ipni.org/ (accessed on 16 June 2025).
- The WFO Plant List|World Flora Online. Available online: https://wfoplantlist.org/ (accessed on 16 June 2025).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Zhuo, Z.; Hu, J.; Yang, X.; Chen, M.; Lei, X.; Deng, L.; Yao, N.; Peng, Q.; Chen, Z.; Ye, W.; et al. Ailanthone Inhibits Huh7 Cancer Cell Growth via Cell Cycle Arrest and Apoptosis In Vitro and In Vivo. Sci. Rep. 2015, 5, 16185. [Google Scholar] [CrossRef]
- Wei, C.; Chen, C.; Cheng, Y.; Zhu, L.; Wang, Y.; Luo, C.; He, Y.; Yang, Z.; Ji, Z. Ailanthone induces autophagic and apoptotic cell death in human promyelocytic leukemia HL-60 cells. Oncol. Lett. 2018, 16, 3569–3576. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Liu, G.; Sun, C.; Wei, J. Ailanthone reverses multidrug resistance by inhibiting the P-glycoprotein-mediated efflux in resistant K562/A02 cells. Cell. Mol. Biol. 2018, 64, 55–61. [Google Scholar] [CrossRef]
- Daga, M.; Pizzimenti, S.; Dianzani, C.; Cucci, M.A.; Cavalli, R.; Grattarola, M.; Ferrara, B.; Scariot, V.; Trotta, F.; Barrera, G. Ailanthone inhibits cell growth and migration of cisplatin resistant bladder cancer cells through down-regulation of Nrf2, YAP, and c-Myc expression. Phytomedicine 2019, 56, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Cucci, M.A.; Grattarola, M.; Dianzani, C.; Damia, G.; Ricci, F.; Roetto, A.; Trotta, F.; Barrera, G.; Pizzimenti, S. Ailanthone increases oxidative stress in CDDP-resistant ovarian and bladder cancer cells by inhibiting of Nrf2 and YAP expression through a post-translational mechanism. Free Radic. Biol. Med. 2020, 150, 125–135. [Google Scholar] [CrossRef]
- Ding, H.; Yu, X.; Yan, Z. Ailanthone suppresses the activity of human colorectal cancer cells through the STAT3 signaling pathway. Int. J. Mol. Med. 2021, 49, 21. [Google Scholar] [CrossRef]
- Wang, S.; Cui, Q.; Chen, X.; Zhu, X.; Lin, K.; Zheng, Q.; Wang, Y.; Li, D. Ailanthone Inhibits Cell Proliferation in Tongue Squamous Cell Carcinoma via PI3K/AKT Pathway. Evid. Based Complement. Alternat. Med. 2022, 2022, 3859489. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, R.; Liu, Y.; Sun, X.; Liang, J.; Zhou, Y.; Wang, Y.; Yu, W.; Wang, Y.; Tang, L.; et al. Ailanthone Inhibits Proliferation, Migration and Invasion of Osteosarcoma Cells by Downregulating the Serine Biosynthetic Pathway. Front. Oncol. 2022, 12, 842406. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, Z.; Ma, M.; Zhao, Y.; Zhang, C.; Qian, Z.; Wang, B. The role played by ailanthone in inhibiting bone metastasis of breast cancer by regulating tumor-bone microenvironment through the RANKL-dependent pathway. Front. Pharmacol. 2023, 13, 1081978. [Google Scholar] [CrossRef]
- Fang, C.; Wu, W.; Ni, Z.; Liu, Y.; Luo, J.; Zhou, Y.; Gong, C.; Hu, D.; Yao, C.; Chen, X.; et al. Ailanthone inhibits non-small cell lung cancer growth and metastasis through targeting UPF1/GAS5/ULK1 signaling pathway. Phytomedicine 2024, 128, 155333. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, X.; Lu, Y.; Wang, X.; Zhang, Z.; Xu, H.; Li, F.; Chen, Q.; Bai, Y.; Bai, X.; et al. Ailanthone induces autophagy and ferroptosis in non-small cell lung cancer Lewis cells. Mol. Clin. Oncol. 2024, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Qiao, G.; Zhang, Y.; Yuan, Y.; Liu, Z.; Jiang, Y.; Zhang, Y.; Deng, Z.; Yu, L.; Lin, H.; et al. Ailanthone targets the KMT2A-MEN1 complex to suppress lung metastasis of osteosarcoma. Phytomedicine 2025, 136, 156258. [Google Scholar] [CrossRef]
- Li, J.; Lv, Y.; Xue, S.; Li, W.; Zhang, X. Ailanthone inhibits bladder cancer tumor and cell proliferation, epithelial-mesenchymal transition, and activation of the Janus kinase/signal transducer and activator of transcription 3 signaling pathway. Cytojournal 2025, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Fukamiya, N.; Okano, M.; Koyama, J.; Koike, K.; Tokuda, H.; Aoi, W.; Takayasu, J.; Kuchide, M.; Nishino, H. Three New Quassinoids, Ailantinol E, F, and G, from Ailanthus altissima. Chem. Pharm. Bull. 2003, 51, 385–389. [Google Scholar] [CrossRef]
- Okunade, A.L.; Bikoff, R.E.; Casper, S.J.; Oksman, A.; Goldberg, D.E.; Lewis, W.H. Antiplasmodial activity of extracts and quassinoids isolated from seedlings of Ailanthus altissima (Simaroubaceae). Phytother. Res. 2003, 17, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Woo, E. Korean medicinal plants inhibiting to Human Immunodeficiency Virus type 1 (HIV-1) fusion. Phytother. Res. 2003, 17, 426–429. [Google Scholar] [CrossRef] [PubMed]
- De Feo, V.; Martino, L.D.; Santoro, A.; Leone, A.; Pizza, C.; Franceschelli, S.; Pascale, M. Antiproliferative effects of tree-of-heaven (Ailanthus altissima Swingle). Phytother. Res. 2005, 19, 226–230. [Google Scholar] [CrossRef]
- Jin, M.H.; Yook, J.; Lee, E.; Lin, C.X.; Quan, Z.; Son, K.H.; Bae, K.H.; Kim, H.P.; Kang, S.S.; Chang, H.W. Anti-inflammatory Activity of Ailanthus altissima in Ovalbumin-Induced Lung Inflammation. Biol. Pharm. Bull. 2006, 29, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Ammirante, M.; Di Giacomo, R.; De Martino, L.; Rosati, A.; Festa, M.; Gentilella, A.; Pascale, M.C.; Belisario, M.A.; Leone, A.; Caterina Turco, M.; et al. 1-Methoxy-Canthin-6-One Induces c-Jun NH2-Terminal Kinase–Dependent Apoptosis and Synergizes with Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand Activity in Human Neoplastic Cells of Hematopoietic or Endodermal Origin. Cancer Res. 2006, 66, 4385–4393. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.-J.; Su, C.; Zhang, D.-M.; Xu, L.-P.; He, R.-R.; Wang, L.; Zhang, J.; Zhang, X.-Q.; Ye, W.-C. Cytotoxic quassinoids from Ailanthus altissima. Bioorg. Med. Chem. Lett. 2013, 23, 654–657. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, S.J.; Kim, H.-Y.; Ryu, B.; Kwak, H.; Hur, J.; Choi, J.-H.; Jang, D.S. Constituents of the stem barks of Ailanthus altissima and their potential to inhibit LPS-induced nitric oxide production. Bioorg. Med. Chem. Lett. 2015, 25, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, J.; Sezirahiga, J.; Kwon, J.; Jeong, M.; Lee, D.; Choi, J.-H.; Jang, D. A New Canthinone-Type Alkaloid Isolated from Ailanthus altissima Swingle. Molecules 2016, 21, 642. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Xiao, H.; Li, J.; Liu, Y.; Jia, M.; Wang, F.; Zhang, Y.; Wang, W.; Wang, S. Fingerprint analysis and pharmacological evaluation of Ailanthus altissima. Int. J. Mol. Med. 2018, 41, 3024–3032. [Google Scholar] [CrossRef]
- Wang, R.; Lu, Y.; Li, H.; Sun, L.; Yang, N.; Zhao, M.; Zhang, M.; Shi, Q. Antitumor activity of the Ailanthus altissima bark phytochemical ailanthone against breast cancer MCF-7 cells. Oncol. Lett. 2018, 15, 6022–6028. [Google Scholar] [CrossRef]
- Yan, Z.-Y.; Chen, J.-J.; Duan, Z.-K.; Yao, G.-D.; Lin, B.; Wang, X.-B.; Huang, X.-X.; Song, S.-J. Racemic phenylpropanoids from the root barks of Ailanthus altissima (Mill.) Swingle with cytotoxicity against hepatoma cells. Fitoterapia 2018, 130, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.; Kim, H.M.; Ahn, J.-H.; Lee, K.-T.; Jang, D.S.; Choi, J.-H. 9-Hydroxycanthin-6-one isolated from stem bark of Ailanthus altissima induces ovarian cancer cell apoptosis and inhibits the activation of tumor-associated macrophages. Chem. Biol. Interact. 2018, 280, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.M.A.; Rasool, M.F.; Imran, I. Pharmacological Studies Pertaining to Smooth Muscle Relaxant, Platelet Aggregation Inhibitory and Hypotensive Effects of Ailanthus altissima. Evid. Based Complement. Alternat. Med. 2019, 2019, 1871696. [Google Scholar] [CrossRef]
- Du, Y.-Q.; Lin, B.; Yan, Z.-Y.; Hou, Z.-L.; Guo, R.; Bai, M.; Zhou, L.; Huang, X.-X.; Song, S.-J. Enantiomeric 8,4′-type oxyneolignans from the root barks of Ailanthus altissima (Mill.) Swingle and their neuroprotective effects against H2O2-induced SH-SY5Y cells injury. Fitoterapia 2019, 139, 104403. [Google Scholar] [CrossRef]
- Yan, Z.-Y.; Lv, T.-M.; Wang, Y.-X.; Shi, S.-C.; Chen, J.-J.; Bin-Lin; Liu, Q.-B.; Huang, X.-X.; Song, S.-J. Terpenylated coumarins from the root bark of Ailanthus altissima (Mill.) Swingle. Phytochemistry 2020, 175, 112361. [Google Scholar] [CrossRef]
- Mo, Y.; Cheng, F.; Yang, Z.; Shang, X.; Liang, J.; Shang, R.; Hao, B.; Wang, X.; Zhang, H.; Wali, A.; et al. Antioxidant Activity and the Potential Mechanism of the Fruit from Ailanthus altissima Swingle. Front. Vet. Sci. 2021, 8, 784898. [Google Scholar] [CrossRef]
- Kim, S.R.; Park, Y.; Li, M.; Kim, Y.K.; Lee, S.; Son, S.Y.; Lee, S.; Lee, J.S.; Lee, C.H.; Park, H.H.; et al. Anti-inflammatory effect of Ailanthus altissima (Mill.) Swingle leaves in lipopolysaccharide-stimulated astrocytes. J. Ethnopharmacol. 2022, 286, 114258. [Google Scholar] [CrossRef]
- Gao, Z.-H.; Duan, Z.-K.; Ma, Z.-T.; Ye, L.; Yao, G.-D.; Huang, X.-X.; Song, S.-J. Chouchunsteride A–D, four new steroids from the leaves of Ailanthus altissima (Mill.) Swingle. Steroids 2022, 188, 109117. [Google Scholar] [CrossRef]
- Muhammad Abdur Rahman, H.; Javaid, S.; Ashraf, W.; Fawad Rasool, M.; Saleem, H.; Ali Khan, S.; Ul-Haq, Z.; Muhammad Muneeb Anjum, S.; Ahmad, T.; Alqahtani, F.; et al. Effects of long-term Ailanthus altissima extract supplementation on fear, cognition and brain antioxidant levels. Saudi Pharm. J. 2023, 31, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Boukhibar, H.; Laouani, A.; Touzout, S.N.; Alenazy, R.; Alqasmi, M.; Bokhari, Y.; Saguem, K.; Ben-Attia, M.; El-Bok, S.; Merghni, A. Chemical Composition of Ailanthus altissima (Mill.) Swingle Methanolic Leaf Extracts and Assessment of Their Antibacterial Activity through Oxidative Stress Induction. Antibiotics 2023, 12, 1253. [Google Scholar] [CrossRef]
- Andonova, T.; Muhovski, Y.; Slavov, I.; Vrancheva, R.; Georgiev, V.; Apostolova, E.; Naimov, S.; Mladenov, R.; Pavlov, A.; Dimitrova-Dyulgerova, I. Phenolic Profile, Antioxidant and DNA-Protective Capacity, and Microscopic Characters of Ailanthus altissima Aerial Substances. Plants 2023, 12, 920. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Duan, Z.-K.; Tan, Y.-N.; Gao, Z.-H.; Liu, D.; Hao, J.-L.; Lin, B.; Huang, X.-X.; Song, S.-J. Isolation of four new monoterpenes from Ailanthus altissima (Mill.) Swingle and their enzyme inhibitory effects. Fitoterapia 2024, 176, 105984. [Google Scholar] [CrossRef] [PubMed]
- Cocîrlea, M.D.; Soare, A.; Petrovici, A.R.; Silion, M.; Călin, T.; Oancea, S. Phenolic Composition and Bioactivities of Invasive Ailanthus altissima (Mill.) Swingle Leaf Extracts Obtained by Two-Step Sequential Extraction. Antioxidants 2024, 13, 824. [Google Scholar] [CrossRef]
- Cselőtey, A.; Baglyas, M.; Király, N.; Ott, P.G.; Glavnik, V.; Vovk, I.; Móricz, Á.M. Bioassay-Guided Isolation and Identification of Antibacterial Compounds from Invasive Tree of Heaven Stem and Trunk Bark. Molecules 2024, 29, 5846. [Google Scholar] [CrossRef]
- Weidner, C.; De Groot, J.C.; Prasad, A.; Freiwald, A.; Quedenau, C.; Kliem, M.; Witzke, A.; Kodelja, V.; Han, C.-T.; Giegold, S.; et al. Amorfrutins are potent antidiabetic dietary natural products. Proc. Natl. Acad. Sci. USA 2012, 109, 7257–7262. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ryu, Y.B.; Curtis-Long, M.J.; Yuk, H.J.; Cho, J.K.; Kim, J.Y.; Kim, K.D.; Lee, W.S.; Park, K.H. Flavanones and rotenoids from the roots of Amorpha fruticosa L. that inhibit bacterial neuraminidase. Food Chem. Toxicol. 2011, 49, 1849–1856. [Google Scholar] [CrossRef]
- Wu, X.; Liao, H.-B.; Li, G.-Q.; Liu, Y.; Cui, L.; Wu, K.-F.; Zhu, X.-H.; Zeng, X.-B. Cytotoxic rotenoid glycosides from the seeds of Amorpha fruticosa. Fitoterapia 2015, 100, 75–80. [Google Scholar] [CrossRef]
- Cui, X.; Guo, J.; Lai, C.-S.; Pan, M.-H.; Ma, Z.; Guo, S.; Liu, Q.; Zhang, L.; Ho, C.-T.; Bai, N. Analysis of bioactive constituents from the leaves of Amorpha fruticosa L. J. Food Drug Anal. 2017, 25, 992–999. [Google Scholar] [CrossRef]
- Lee, W.; Yoon, G.; Kim, M.C.; Kwon, H.C.; Bae, G.-U.; Kim, Y.K.; Kim, S.-N. 5,7-Dihydroxy-6-geranylflavanone improves insulin sensitivity through PPARα/γ dual activation. Int. J. Mol. Med. 2016, 37, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Jankovská, D.; Jurčová, N.; Kubínová, R.; Václavík, J.; Švajdlenka, E.; Mascellani, A.; Maršík, P.; Bouzková, K.; Malaník, M. Anticholinesterase Activity of Methanolic Extract of Amorpha fruticosa Flowers and Isolation of Rotenoids and Putrescine and Spermidine Derivatives. Plants 2024, 13, 1181. [Google Scholar] [CrossRef] [PubMed]
- Schlick, J.-L.; Dulieu, P.; Desvoyes, B.; Adami, P.; Radom, J.; Jouvenot, M. Cytotoxic activity of a recombinant GnRH-PAP fusion toxin on human tumor cell lines. FEBS Lett. 2000, 472, 241–246. [Google Scholar] [CrossRef]
- Ishag, H.Z.A.; Li, C.; Huang, L.; Sun, M.; Ni, B.; Guo, C.; Mao, X. Inhibition of Japanese encephalitis virus infection in vitro and in vivo by pokeweed antiviral protein. Virus Res. 2013, 171, 89–96. [Google Scholar] [CrossRef]
- Mansouri, S.; Choudhary, G.; Sarzala, P.M.; Ratner, L.; Hudak, K.A. Suppression of Human T-cell Leukemia Virus I Gene Expression by Pokeweed Antiviral Protein. J. Biol. Chem. 2009, 284, 31453–31462. [Google Scholar] [CrossRef]
- Zhabokritsky, A.; Mansouri, S.; Hudak, K.A. Pokeweed antiviral protein alters splicing of HIV-1 RNAs, resulting in reduced virus production. RNA 2014, 20, 1238–1247. [Google Scholar] [CrossRef]
- Takahasi, H.; Yanagi, K.; Ueda, M.; Nakade, K.; Fukuyama, Y. Structures of 1,4-Benzodioxane Derivatives from the Seeds of Phytolacca americana and Their Neuritogenic Activity in Primary Cultured Rat Cortical Neurons. Chem. Pharm. Bull. 2003, 51, 1377–1381. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D. Antioxidant and acetylcholinesterase inhibition properties of Amorpha fruticosa L. and Phytolacca americana L. Pharmacogn. Mag. 2013, 9, 109. [Google Scholar] [CrossRef]
- Patra, J.K.; Kim, E.S.; Oh, K.; Kim, H.-J.; Kim, Y.; Baek, K.-H. Antibacterial effect of crude extract and metabolites of Phytolacca americana on pathogens responsible for periodontal inflammatory diseases and dental caries. BMC Complement. Altern. Med. 2014, 14, 343. [Google Scholar] [CrossRef] [PubMed]
- Saleri, F.D.; Chen, G.; Li, X.; Guo, M. Comparative Analysis of Saponins from Different Phytolaccaceae Species and Their Antiproliferative Activities. Molecules 2017, 22, 1077. [Google Scholar] [CrossRef]
- Popovici, L.-F.; Brinza, I.; Gatea, F.; Badea, G.I.; Vamanu, E.; Oancea, S.; Hritcu, L. Enhancement of Cognitive Benefits and Anti-Anxiety Effects of Phytolacca americana Fruits in a Zebrafish (Danio rerio) Model of Scopolamine-Induced Memory Impairment. Antioxidants 2025, 14, 97. [Google Scholar] [CrossRef]
- Aslam, B.; Asghar, R.; Muzammil, S.; Shafique, M.; Siddique, A.B.; Khurshid, M.; Ijaz, M.; Rasool, M.H.; Chaudhry, T.H.; Aamir, A.; et al. AMR and Sustainable Development Goals: At a crossroads. Glob. Health 2024, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Bhojyawal, V.; Kesarwani, M.; Gupta, S. The Rise of Antibiotic Resistance: A Global Threat, Origin, and Evolution of Antibiotic Resistance: Current Scenario and Future Prospective. In Emerging Paradigms for Antibiotic-Resistant Infections: Beyond the Pill; Gangwar, M., Nath, G., Eds.; Springer Nature: Singapore, 2024; pp. 261–275. ISBN 978-981-9752-72-0. [Google Scholar]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the Role of Plant Natural Products in Antibiotic Drug Discovery. Chem. Rev. 2021, 121, 3495–3560. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Chandra, H.; Bishnoi, P.; Yadav, A.; Patni, B.; Mishra, A.P.; Nautiyal, A.R. Antimicrobial Resistance and the Alternative Resources with Special Emphasis on Plant-Based Antimicrobials—A Review. Plants 2017, 6, 16. [Google Scholar] [CrossRef]
- Arip, M.; Selvaraja, M.; Rajagopal, M.; Tan, L.F.; Leong, M.Y.; Tan, P.L.; Yap, V.L.; Chinnapan, S.; Tat, N.C.; Abdullah, M.; et al. Review on Plant-Based Management in Combating Antimicrobial Resistance—Mechanistic Perspective. Front. Pharmacol. 2022, 13, 879495. [Google Scholar] [CrossRef] [PubMed]
- Atta, S.; Waseem, D.; Fatima, H.; Naz, I.; Rasheed, F.; Kanwal, N. Antibacterial potential and synergistic interaction between natural polyphenolic extracts and synthetic antibiotic on clinical isolates. Saudi J. Biol. Sci. 2023, 30, 103576. [Google Scholar] [CrossRef]
- Alam, M.; Bano, N.; Ahmad, T.; Sharangi, A.B.; Upadhyay, T.K.; Alraey, Y.; Alabdallah, N.M.; Rauf, M.A.; Saeed, M. Synergistic Role of Plant Extracts and Essential Oils against Multidrug Resistance and Gram-Negative Bacterial Strains Producing Extended-Spectrum β-Lactamases. Antibiotics 2022, 11, 855. [Google Scholar] [CrossRef]
- Ramata-Stunda, A.; Petriņa, Z.; Valkovska, V.; Borodušķis, M.; Gibnere, L.; Gurkovska, E.; Nikolajeva, V. Synergistic Effect of Polyphenol-Rich Complex of Plant and Green Propolis Extracts with Antibiotics against Respiratory Infections Causing Bacteria. Antibiotics 2022, 11, 160. [Google Scholar] [CrossRef]
- Jin, X.; Ren, J.; Li, R.; Gao, Y.; Zhang, H.; Li, J.; Zhang, J.; Wang, X.; Wang, G. Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019. eClinicalMedicine 2021, 37, 100986. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Galvani, A.P. The global burden of HIV and prospects for control. Lancet HIV 2019, 6, e809–e811. [Google Scholar] [CrossRef]
- Michaud, C.M. Global Burden of Infectious Diseases. Encycl. Microbiol. 2009, 444–454. [Google Scholar] [CrossRef]
- Wong, Y.; Meehan, M.T.; Burrows, S.R.; Doolan, D.L.; Miles, J.J. Estimating the global burden of Epstein–Barr virus-related cancers. J. Cancer Res. Clin. Oncol. 2022, 148, 31–46. [Google Scholar] [CrossRef]
- Ali, S.I.; Sheikh, W.M.; Rather, M.A.; Venkatesalu, V.; Muzamil Bashir, S.; Nabi, S.U. Medicinal plants: Treasure for antiviral drug discovery. Phytother. Res. 2021, 35, 3447–3483. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Kıran, T.R.; Otlu, O.; Karabulut, A.B. Oxidative stress and antioxidants in health and disease. J. Lab. Med. 2023, 47, 1–11. [Google Scholar] [CrossRef]
- Pandhair, V.; Sekhon, B.S. Reactive Oxygen Species and Antioxidants in Plants: An Overview. J. Plant Biochem. Biotechnol. 2006, 15, 71–78. [Google Scholar] [CrossRef]
- Ahmad, P.; Sarwat, M.; Sharma, S. Reactive oxygen species, antioxidants and signaling in plants. J. Plant Biol. 2008, 51, 167–173. [Google Scholar] [CrossRef]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef]
- Ling, T.; Lang, W.H.; Maier, J.; Quintana Centurion, M.; Rivas, F. Cytostatic and Cytotoxic Natural Products against Cancer Cell Models. Molecules 2019, 24, 2012. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant derived substances with anti-cancer activity: From folklore to practice. Front. Plant Sci. 2015, 6, 799. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Otero, P.; Pereira, A.G.; Chamorro, F.; Carpena, M.; Echave, J.; Fraga-Corral, M.; Simal-Gandara, J.; Prieto, M.A. Status and Challenges of Plant-Anticancer Compounds in Cancer Treatment. Pharmaceuticals 2021, 14, 157. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef]
- ALNasser, M.N.; Alboraiy, G.M.; Alsowig, E.M.; Alqattan, F.M. Cholinesterase Inhibitors from Plants and Their Potential in Alzheimer’s Treatment: Systematic Review. Brain Sci. 2025, 15, 215. [Google Scholar] [CrossRef]
- dos Santos, T.C.; Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; de Andrade Paes, A.M. Naturally Occurring Acetylcholinesterase Inhibitors and Their Potential Use for Alzheimer’s Disease Therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Ghalib, R.M.; Sasikala, P.; Ahmed, K.K.M. Cholinesterase inhibitors from botanicals. Pharmacogn. Rev. 2013, 7, 121–130. [Google Scholar] [CrossRef] [PubMed]
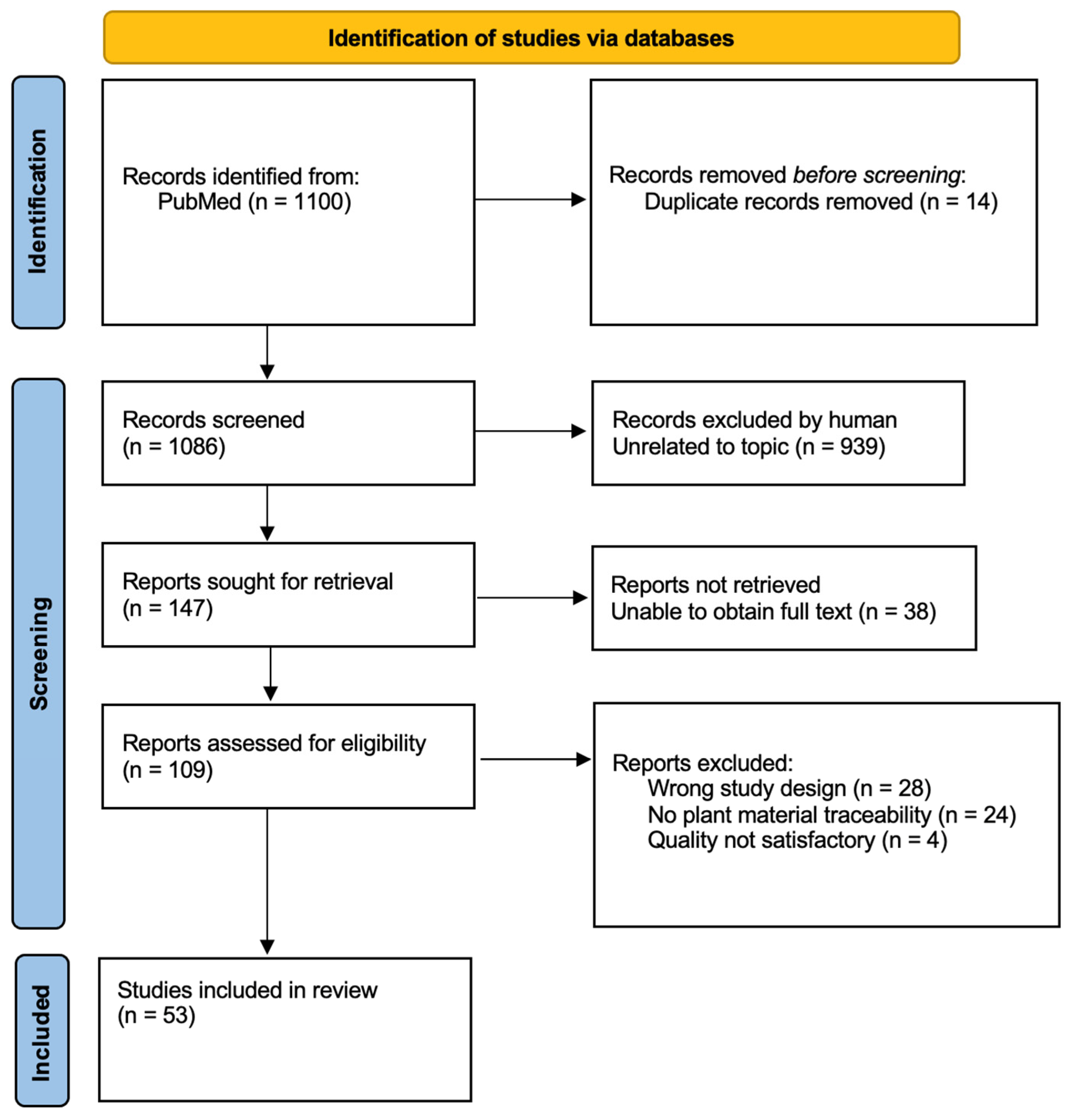
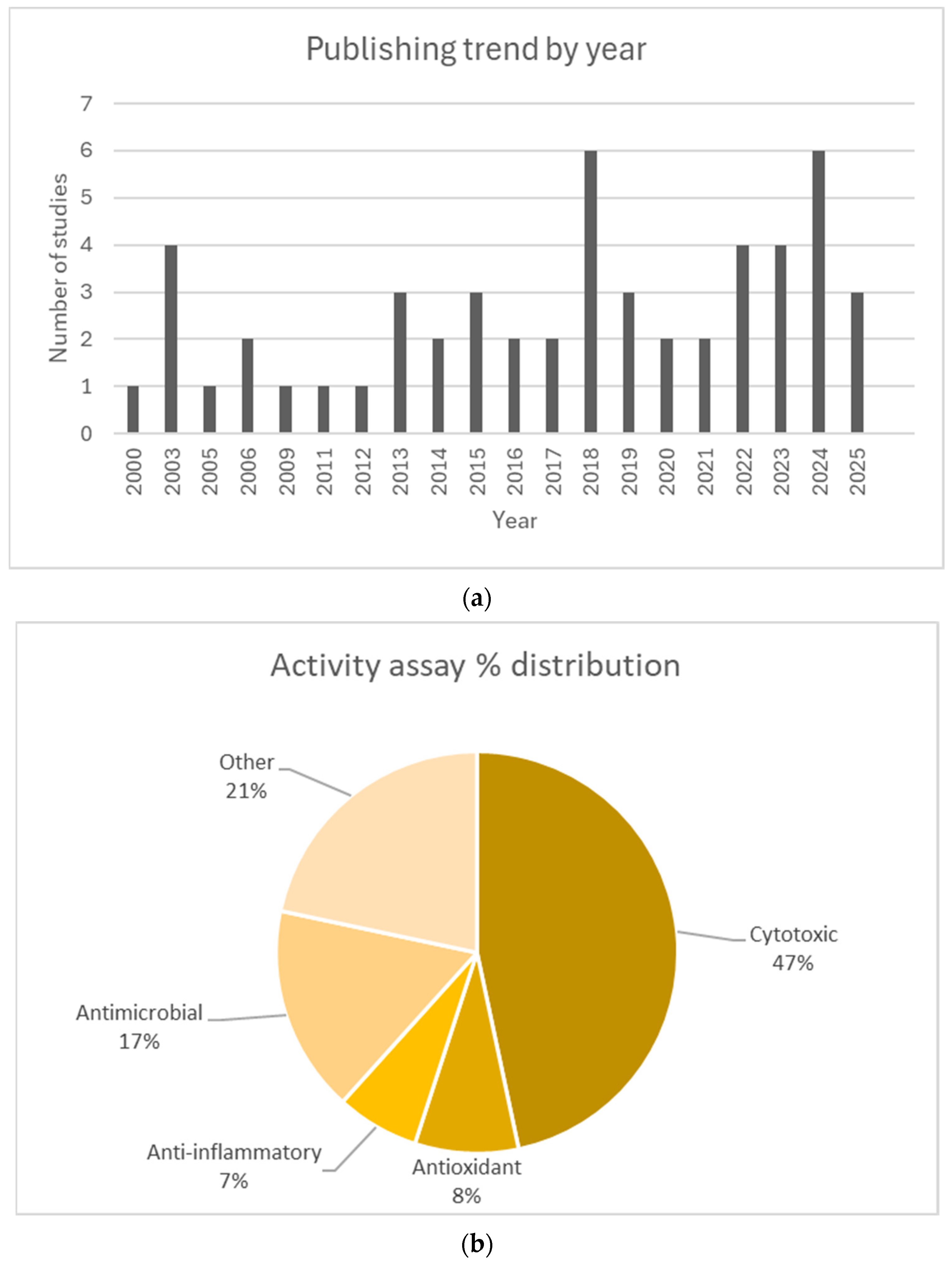
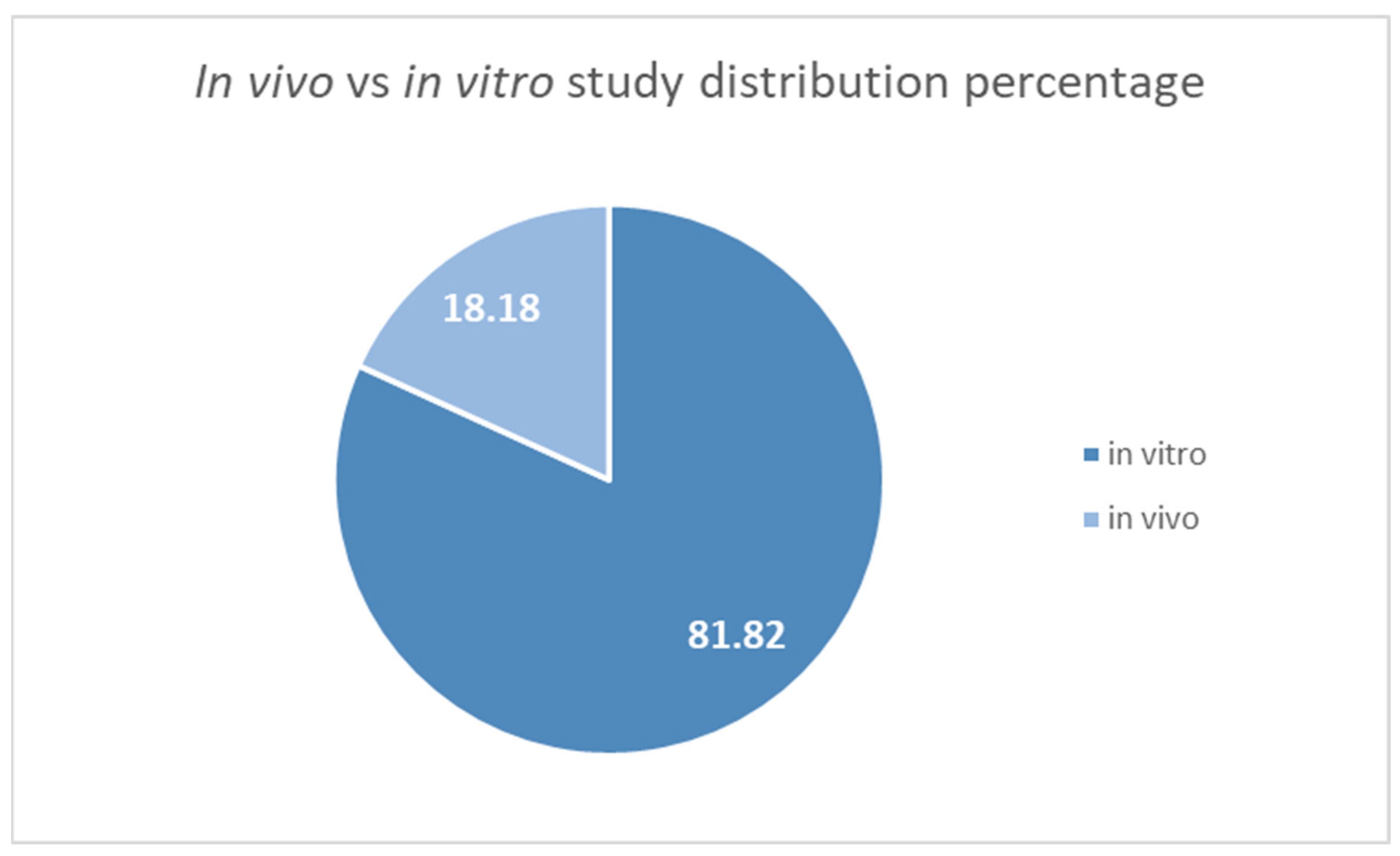
| Author, Year | Plant/Compound | Activity | Origin | Non-Native (Yes/No) | Reference |
|---|---|---|---|---|---|
| Zhuo Z et al., 2015 | Ailanthone | Cytotoxic | Jinan University, Guangzhou, China | N/A | [37] |
| Wei C et al., 2018 | Ailanthone | Cytotoxic | Jinan University, Guangzhou, China | N/A | [38] |
| Han F et al., 2018 | Ailanthone | Cytotoxic | Puruifa Science & Technology Development Co., Chengdu, China | N/A | [39] |
| Daga M et al., 2019 | Ailanthone | Cytotoxic | Baoji Herbest, Bio-Tech Co., Ltd., Baoji, China | N/A | [40] |
| Cucci MA et al., 2020 | Ailanthone | Cytotoxic | Baoji Herbest, Bio-Tech Co., Baoji, China | N/A | [41] |
| Ding H et al., 2021 | Ailanthone | Cytotoxic | Shanghai Yiyan Biotechnology Co., Ltd., Shanghai, China | N/A | [42] |
| Wang S et al., 2022 | Ailanthone | Cytotoxic | Jiangxi Herb Tiangong Technology, Nanchang, China | N/A | [43] |
| Zhang Y et al., 2022 | Ailanthone | Cytotoxic | GlpBio Technology, Montclair, CA, USA | N/A | [44] |
| Wang Y et al., 2023 | Ailanthone | Cytotoxic | MedChemExpress Ltd., Monmouth Junction, NJ, USA | N/A | [45] |
| Fang C et al., 2024 | Ailanthone | Cytotoxic | Dasfbio Nanjing, Nanjing, China | N/A | [46] |
| Yang H et al., 2024 | Ailanthone | Cytotoxic | Chengdu Alfa Biological Technology Co., Ltd., Chengdu, China | N/A | [47] |
| Liang J et al., 2025 | Ailanthone | Cytotoxic | GlpBio Technology, Montclair, CA, USA | N/A | [48] |
| Li J et al., 2025 | Ailanthone | Cytotoxic | ChemFaces, Wuhan, China | N/A | [49] |
| Tamura S et al., 2003 | Ailanthus altissima | Antiviral | Taiwan | No | [50] |
| Okunade AL et al., 2003 | Ailanthus altissima | Antimalarial | United States | Yes | [51] |
| Chang Y et al., 2003 | Ailanthus altissima | Antiviral | South Korea | Yes | [52] |
| De Feo V et al., 2005 | Ailanthus altissima | Cytotoxic | Italy | Yes | [53] |
| Jin MH et al., 2006 | Ailanthus altissima | Anti-inflammatory | South Korea | Yes | [54] |
| Ammirante M et al., 2006 | Ailanthus altissima | Cytotoxic | Italy | Yes | [55] |
| Wang Y et al., 2013 | Ailanthus altissima | Cytotoxic | China | No | [56] |
| Kim HM et al., 2015 | Ailanthus altissima | Anti-inflammatory | South Korea | Yes | [57] |
| Kim H et al., 2016 | Ailanthus altissima | Anti-inflammatory | South Korea | Yes | [58] |
| He Q et al., 2018 | Ailanthus altissima | Cytotoxic | China | No | [59] |
| Wang R et al., 2018 | Ailanthus altissima | Cytotoxic | China | No | [60] |
| Yan ZY et al., 2018 | Ailanthus altissima | Cytotoxic | China | No | [61] |
| Jeong M et al., 2018 | Ailanthus altissima | Cytotoxic | South Korea | Yes | [62] |
| Rahman HMA et al., 2019 | Ailanthus altissima | Hypotensive Anticoagulant Smooth muscle relaxant | Pakistan | Yes | [63] |
| Du YQ et al., 2019 | Ailanthus altissima | Neuroprotective | China | No | [64] |
| Yan ZY et al., 2020 | Ailanthus altissima | Cytotoxic | China | No | [65] |
| Mo Y et al., 2021 | Ailanthus altissima | Antioxidant | Bozhou Baohua Pharmaceutical Co., Ltd. Bozhou, China | N/A | [66] |
| Kim SR et al., 2022 | Ailanthus altissima | Anti-inflammatory | South Korea | Yes | [67] |
| Gao ZH et al., 2022 | Ailanthus altissima | Cytotoxic | China | No | [68] |
| Muhammad Abdur Rahman H et al., 2023 | Ailanthus altissima | Neuroprotective Antioxidant Anti-enzymatic | Pakistan | Yes | [69] |
| Boukhibar H et al., 2023 | Ailanthus altissima | Antibacterial | Algeria Tunisia | Yes | [70] |
| Andonova T et al., 2023 | Ailanthus altissima | Antioxidant DNA protective | Bulgaria | Yes | [71] |
| Song Q et al., 2024 | Ailanthus altissima | Anti-enzymatic | China | No | [72] |
| Cocîrlea MD et al., 2024 | Ailanthus altissima | Antioxidant Antibacterial | Romania | Yes | [73] |
| Cselőtey A et al., 2024 | Ailanthus altissima | Antibacterial | Hungary | Yes | [74] |
| Weidner C et al., 2012 | Amorfrutin | Antidiabetic | Analyticon Discovery | N/A | [75] |
| Kim YS et al., 2011 | Amorpha fruticosa | Antibacterial | South Korea | Yes | [76] |
| Wu X et al., 2015 | Amorpha fruticosa | Cytotoxic | China | Yes | [77] |
| Cui X et al., 2017 | Amorpha fruticosa | Cytotoxic | China | Yes | [78] |
| Lee W et al., 2016 | Amorpha fruticosa | Antidiabetic | South Korea | Yes | [79] |
| Jankovská D et al., 2024 | Amorpha fruticosa | Anti-enzymatic | Czech Republic | Yes | [80] |
| Schlick J et al., 2000 | GnRH-PAP | Cytotoxic | N/A | N/A | [81] |
| Ishag HZ et al., 2013 | PAP | Antiviral | Huazhong University of Science and Technology, Wuhan, China | N/A | [82] |
| Mansouri S et al., 2009 | PAP | Antiviral | N/A | N/A | [83] |
| Zhabokritsky A et al., 2014 | PAP | Antiviral | N/A | N/A | [84] |
| Takahasi H et al., 2003 | Phytolacca americana | Neurotrophic | Japan | Yes | [85] |
| Zheleva-Dimitrova Dzh et al., 2013 | Phytolacca americana | Antioxidant Anti-enzymatic | Bulgaria | Yes | [86] |
| Patra JK et al., 2014 | Phytolacca americana | Antibacterial | South Korea | Yes | [87] |
| Saleri FD et al., 2017 | Phytolacca americana | Cytotoxic | China | Yes | [88] |
| Popovici LF et al., 2025 | Phytolacca americana | Anxiolytic, Anti-enzymatic | Romania | Yes | [89] |
| Bacterial Strain | Growth Inhibition Capability | Extract Type/Isolated Compound | Plant Material/Origin | In Vivo/In Vitro | Reference |
|---|---|---|---|---|---|
| Bacillus subtilis ATCC 6633 | IZ = 8.00 | Hydroethanolic crude extract of dried autumn leaves. | Ailanthus altissima, leaf, Romania | In vitro | [74] |
| Bacillus subtilis F1276 | MIC = 0.07 | 13-HODE isolated from fractionated methanolic crude young stem bark extract. | Ailanthus altissima, bark, Hungary | In vitro | [74] |
| MIC = 0.07 | 9-HODE isolated from fractionated methanolic crude young stem bark extract. | ||||
| MIC = 0.07 | Juniperic acid isolated from fractionated methanolic outer trunk bark extract. | ||||
| MIC = 0.01 | Canthin-6-one isolated from fractionated methanolic inner trunk bark extract. | ||||
| Clostridium perfringens Neuraminidase (E.C. 3.2.1.18.) | IC50 = 4.15 | Amoradicin isolated from the hexane–acetone fraction. | Amorpha fruticosa, root, South Korea | In vitro | [76] |
| IC50 = 0.12 | Amorisin isolated from the hexane–acetone subfraction. | ||||
| IC50 = 7.86 | Isoamoritin isolated from the hexane–acetone subfraction. | ||||
| IC50 = 22.03 | Amoricin from the hexane–acetone subfraction. | ||||
| IC50 = 12.94 | Amorphigenin from the 80% methanolic subfraction. | ||||
| IC50 = 16.74 | Dalbinol isolated from the hexane–acetone subfraction. | ||||
| IC50 = 8.34 | 6-ketodehydroamorphigenin isolated from the hexane–acetone subfraction. | ||||
| Enterococcus faecalis ATCC 29212 | IZ = 8.00 | Hydroethanolic crude extract of frozen summer leaves. | Ailanthus altissima, leaf, Romania | In vitro | [73] |
| IZ = 9.00 | Hydroethanolic crude extract of frozen autumn leaves. | ||||
| IZ = 9.00 | Hydroethanolic crude extract of dried summer leaves. | ||||
| IZ = 10.00 | Hydroethanolic crude extract of dried autumn leaves. | ||||
| Escherichia coli ATCC 25922 | MIC = 31.25 | Crude 80% methanolic extract, macerated. | Ailanthus altissima, leaf, Blida, Algeria | In vitro | [70] |
| MIC = 8.00 | Ailanthus altissima, leaf, Tlemcen, Algeria | ||||
| MIC = 31.25 | Ailanthus altissima, leaf, Bizerte, Tunisia | ||||
| MIC = 31.25 | Ailanthus altissima, leaf, Sousse, Tunisia | ||||
| MBC = 250.00 | Ailanthus altissima, leaf, Blida, Algeria | ||||
| MBC = 250.00 | Ailanthus altissima, leaf, Tlemcen, Algeria | ||||
| IZ = 9.00 | Hydroethanolic crude extract of frozen summer leaves. | Ailanthus altissima, leaf, Romania | In vitro | [73] | |
| IZ = 10.00 | Hydroethanolic crude extract of dried autumn leaves. | ||||
| Porphyromonas gingivalis W83 ATCC BAA-1703 | MIC = 0.20 | Crude 80% methanolic | Phytolacca americana, leaf and soft stem, South Korea | In vitro | [87] |
| MIC = 0.20 | Hexane fraction | ||||
| MIC = 0.20 | CHCl3 fraction | ||||
| Pseudomonas aeruginosa | BI: 29.60 | Isoamoritin isolated from the hexane–acetone subfraction. | Amorpha fruticosa, root, South Korea | In vivo | [76] |
| BI: 21.00 | Dalbinol isolated from the hexane–acetone subfraction. | ||||
| Pseudomonas aeruginosa ATCC 27853 | MIC = 31.25 | Crude 80% methanolic extract, macerated. | Ailanthus altissima, leaf, Blida, Algeria | In vitro | [70] |
| MIC = 16.00 | Ailanthus altissima, leaf, Tlemcen, Algeria | ||||
| MIC = 125.00 | Ailanthus altissima, leaf, Bizerte, Tunisia | ||||
| MIC = 72.50 | Ailanthus altissima, leaf, Sousse, Tunisia | ||||
| MBC = 250.00 | Ailanthus altissima, leaf, Tlemcen, Algeria | ||||
| Staphylococcus aureus ATCC 25923 | MIC = 4.00 | Crude 80% methanolic | Ailanthus altissima, leaf, Blida, Algeria | In vitro | [70] |
| MIC = 16.00 | Ailanthus altissima, leaf, Tlemcen, Algeria | ||||
| MIC = 4.00 | Ailanthus altissima, leaf, Bizerte, Tunisia | ||||
| MIC = 4.00 | Ailanthus altissima, leaf, Sousse, Tunisia | ||||
| MBC = 16.00 | Ailanthus altissima, leaf, Blida, Algeria | ||||
| MBC = 72.00 | Ailanthus altissima, leaf, Tlemcen, Algeria | ||||
| MBC = 16.00 | Ailanthus altissima, leaf, Bizerte, Tunisia | ||||
| IZ = 8.00 | Hydroethanolic crude extract of dried summer leaves. | Ailanthus altissima, leaf, Romania | In vitro | [73] | |
| IZ = 8.00 | Hydroethanolic crude extract of dried autumn leaves. | ||||
| Staphylococcus aureus Clinical isolate | IZ = 8.00 | Hydroethanolic crude extract of frozen summer leaves. | Ailanthus altissima, leaf, Romania | In vitro | [73] |
| IZ = 8.00 | Hydroethanolic crude extract of frozen autumn leaves. | ||||
| IZ = 10.00 | Hydroethanolic crude extract of dried summer leaves. | ||||
| IZ = 10.00 | Hydroethanolic crude extract of dried autumn leaves. | ||||
| Staphylococcus epidermidis ATCC 2059 | MIC = 72.25 | Crude 80% methanolic extract, macerated. | Ailanthus altissima, leaf, Blida, Algeria | In vitro | [70] |
| MIC = 8.00 | Ailanthus altissima, leaf, Tlemcen, Algeria | ||||
| MIC = 16.00 | Ailanthus altissima, leaf, Bizerte, Tunisia | ||||
| MIC = 16.00 | Ailanthus altissima, leaf, Sousse, Tunisia | ||||
| MBC = 125.00 | Ailanthus altissima, leaf, Blida, Algeria | ||||
| MBC = 125.00 | Ailanthus altissima, leaf, Tlemcen, Algeria | ||||
| Streptococcus mutans UA159 ATCC 700610 | MIC = 1.80 (44%) | Crude 80% methanolic | Phytolacca americana, leaf and soft stem, South Korea | In vitro | [87] |
| MIC = 0.20 | Hexane fraction | ||||
| MIC = 0.20 | CHCl3 fraction | ||||
| Streptococcus pyogenes ATCC 19615 | IZ = 8.00 | Hydroethanolic crude extract of dried autumn leaves. | Ailanthus altissima, leaf, Romania | In vitro | [73] |
| Virus | Result | Extract Type/Isolated Compound | Tested Cell Line/Organism | In Vivo/In Vitro | Reference |
|---|---|---|---|---|---|
| EBV | IC50 = 221.00 | Ailantinol E from the methanolic extract of aerial part of Ailanthus altissima. | EBV-EA-positive Raji cells. | In vitro | [50] |
| IC50 = 180.00 | Ailantinol F from the methanolic extract of aerial part of Ailanthus altissima. | ||||
| IC50 = 285.00 | Ailantinol G from the methanolic extract of aerial part of Ailanthus altissima. | ||||
| HIV-1 | 450-fold reduction in virus production due to altering the splicing of RNAs | PAP (0.50, 1.00, or 2.50 μg) | Human embryonic kidney 293T cells transfected with a proviral clone of HIV-1. | In vitro | [84] |
| HIV-1 fusion inhibition of 74.90% | Ailanthus altissima stem bark methanolic extract (D = 100.00 μg/mL). | HeLa-CD4+ cell lines, recombinant vaccinia virus vPE 16 with the expressed HIV-1 envelope protein gp120/41. | In vitro | [52] | |
| HTLV-I | Suppression of HTLV-I gene expression at translational and transcriptional levels, diminishing virus production. Absence of cytotoxicity. | PAP (10.00, 25.00 and 50.00 ng) | Human embryonic kidney 293Tcells, Jur- KAT cells, and HTLV-I-infected human T-cell line. | In vitro | [83] |
| JEV | IC50 = 23.10 (inhibitory value of JEV titre) | PAP (0.10–50.00 μg/mL) | JEV-infected BHK-21 cells. | In vitro | [82] |
| SP = 87.50 | PAP (pre-infection, intraperitoneally, D = 1.00 mg/kg) | 2-week-old BALB/c mice challenged with a lethal dose of JEV. | In vivo | ||
| SP = 85.70 | PAP (post-infection, intraperitoneally, D = 1.00 mg/kg) |
| Assay Method | Result | Extract Type/Isolated Compound | Plant Material/Origin | In Vivo/In Vitro | Reference |
|---|---|---|---|---|---|
| ABTS | IC50 = 643.21 μg/mL | Methanolic extract | Ailanthus altissima, bark, Pakistan | In vitro | [69] |
| c = 299.54 mmol TE/g DW | Ethanolic extract | Ailanthus altissima, leaf, Bulgaria | In vitro | [71] | |
| c = 893.14 mmol TE/g DW | Ailanthus altissima, flower, Bulgaria | ||||
| c = 31.24 mmol TE/g DW | Ailanthus altissima, stem bark, Bulgaria | ||||
| IC50 = 18.43 μg/mL | Methanolic extract | Phytolacca americana, leaf, Bulgaria | In vitro | [86] | |
| IC50 = 112.49 μg/mL | Phytolacca americana, fruit, Bulgaria | ||||
| IC50 = 2.93 μg/mL | Methanolic extract | Amorpha fruticosa, leaf, Bulgaria | In vitro | [86] | |
| IC50 = 2.90 μg/mL | Amorpha fruticosa, fruit, Bulgaria | ||||
| CUPRAC | c = 548.07 mmol TE/g DW | Ethanolic extract | Ailanthus altissima, leaf, Bulgaria | In vitro | [71] |
| c = 789.54 mmol TE/g DW | Ailanthus altissima, flower, Bulgaria | ||||
| c = 10.22 mmol TE/g DW | Ailanthus altissima, stem bark, Bulgaria | ||||
| DPPH | IC50 = 88.79 μg/mL | Methanolic extract | Phytolacca americana, leaf, Bulgaria | In vitro | [86] |
| IC50 = 412.06 μg/mL | Phytolacca americana, fruit, Bulgaria | ||||
| IC50 = 16.00 | Americanoic Acid A Methyl Ester | Phytolacca americana, seed, Japan | In vitro | [85] | |
| IC50 = 38.00 | Isoamericanoic Acid A Methyl Ester | ||||
| IC50 = 9.00 | 9′-O-Methylamericanol A | ||||
| IC50 = 11.00 | Americanin-type 4 | ||||
| IC50 = 39.00 | Isoamericanin-type 5 | ||||
| IC50 = 5.00 | Americanol A | ||||
| IC50 = 16.00 | Isoamericanol A | ||||
| IC50 = 10.00 | Americanin A | ||||
| IC50 = 16.00 | Isoamericanin A | ||||
| IC50 = 741.74 μg/mL | Methanolic extract | Ailanthus altissima, bark, Pakistan | In vitro | [69] | |
| c = 225.62 mmol TE/g DW | Ethanolic extract | Ailanthus altissima, leaf, Bulgaria | In vitro | [71] | |
| c = 729.72 mmol TE/g DW | Ailanthus altissima, flower, Bulgaria | ||||
| c = 24.96 mmol TE/g DW | Ailanthus altissima, stem bark, Bulgaria | ||||
| SR = 20.95% (c = 15.63 µg/mL) | Ethanolic extract | Ailanthus altissima, fruit, Bozhou Baohua Pharmaceutical Co., Ltd. | In vitro | [66] | |
| SR = 91.97% (c = 0.50 mg/mL) | |||||
| SR = 97.90% (c = 1.00 mg/mL) | |||||
| IC50 = 11.23 μg/mL | Methanolic extract | Amorpha fruticosa, leaf, Bulgaria | In vitro | [86] | |
| IC50 = 9.83 μg/mL | Amorpha fruticosa, fruit, Bulgaria | ||||
| FIC | IP = 54.94% (c = 1 mg/mL) | Ethanolic extract | Ailanthus altissima, fruit, Bozhou Baohua Pharmaceutical Co., Ltd. | In vitro | [66] |
| FRAP | c = 906.01 mmol TE/g DW | Ethanolic extract | Ailanthus altissima, leaf, Bulgaria | In vitro | [71] |
| c = 661.48 mmol TE/g DW | Ailanthus altissima, flower, Bulgaria | ||||
| c = 16.65 mmol TE/g DW | Ailanthus altissima, stem bark, Bulgaria | ||||
| IC50 = 508.81 μg/mL | Methanolic extract | Amorpha fruticosa, leaf, Bulgaria | In vitro | [86] | |
| IC50 = 642.95 μg/mL | Amorpha fruticosa, fruit, Bulgaria | ||||
| O2− | IC50 = 412.06 μg/mL | Americanoic Acid A Methyl Ester | Phytolacca americana, fruit, Bulgaria | In vitro | [85] |
| IC50 = 64.00 | Isoamericanoic Acid A Methyl Ester | ||||
| IC50 = 8.00 | 9′-O-Methylamericanol A | ||||
| IC50 = 9.00 | Americanin-type 4 | ||||
| IC50 = 29.00 | Isoamericanin-type 5 | ||||
| IC50 = 24.00 | Americanol A | ||||
| IC50 = 23.00 | Isoamericanol A | ||||
| IC50 = 9.00 | Americanin A | ||||
| IC50 = 58.00 | Isoamericanin A | ||||
| OH | IP = 42.34% (c = 1.00 mg/mL) | Ethanolic extract | Ailanthus altissima, fruit, Bozhou Baohua Pharmaceutical Co., Ltd. | In vitro | [66] |
| Tested Cell Line/Organism | Result | Extract Type/Isolated Compound | Assay | Plant Material/Origin | In Vivo/In Vitro | Reference |
|---|---|---|---|---|---|---|
| 2008/MRP1 | IC50 = 7.08 | Ailanthone | MTT | Puruifa Science & Technology Development Co., Chengdu, China | In vitro | [39] |
| A2780 | IC50 = 10.60 μg/mL | Crude ethanolic | MTT | Ailanthus altissima, bark, South Korea | In vitro | [62] |
| IC50 = 89.50 μg/mL | n-Hexane fraction | |||||
| IC50 = 8.60 μg/mL | EtOAc fraction | |||||
| IC50 = 7.10 μg/mL | n-BuOH fraction | |||||
| IC50 = 25.70 μg/mL | Water fraction | |||||
| Cal-27 | ACP = 39.00 (24 h) | Ailanthone (D = 4.00 µM) | Apoptosis | Jiangxi Herb Tiangong Technology, Jiangxi, China | In vitro | [43] |
| IC50 = 0.84 | Ailanthone | MTT | ||||
| HCT-116 | IC50 = 1.79 | Dalbin | MTT | Amorpha fruticosa, seed, China | In vitro | [77] |
| IC50 = 1.98 | 8′-O-β-D-glucopyranosyl-amorphigenin | |||||
| IC50 = 0.6 | Ailanthone | CCK-8 | Shanghai Yiyan Biotechnology Co., Ltd., Shanghai, China | In vitro | [42] | |
| HEK293/R2 | IC50 = 4.77 | Ailanthone | MTT | Puruifa Science & Technology Development Co., Chengdu, China | In vitro | [39] |
| HeLa | CV = 6.00 | Chloroform crude extract | Trypan blue staining | Ailanthus altissima, root, Italy | In vitro | [53] |
| CV = 6.00 | Chloroform fraction | |||||
| CV = 9.00 | 1-Methoxy-canthin-6-one isolated from the chloroform fraction | |||||
| ACP = 41.00 (48 h) | Chloroform crude extract | Cell apoptosis | ||||
| ACP = 28.00 (48 h) | Chloroform fraction | |||||
| ACP = 27.00 (48 h) | 1-methoxy-canthin-6-one isolated from the chloroform fraction | |||||
| Hep3B | IC50 = 0.37 | Altissinol A | MTT | Ailanthus altissima, bark, China | In vitro | [56] |
| IC50 = 0.48 | Ailanthone | |||||
| IC50 = 0.98 | 13,18-Dehydroglaucarubinone | |||||
| IC50 = 2.17 | (-)-Cha-parrinone | |||||
| IC50 = 0.05 | 6a-Tigloyloxychaparrinone | |||||
| IC50 = 8.01 | Shinjulactone A | |||||
| IC50 = 2.36 | Altissinol B | |||||
| IC50 = 2.43 | 6a-Tigloyloxychaparrin | |||||
| IC50 = 23.46 | Glaucarubin | |||||
| IC50 = 47.08 | Chouchunsteride B | MTT | Ailanthus altissima, leaf, China | In vitro | [68] | |
| IC50 = 31.52 | Chouchunsteride D | |||||
| IC50 = 31.49 | 6-dehydropregnenolone | |||||
| IC50 = 44.52 | 20S- Hydroxyergosta-4,6,24(28)-trien-3-one | |||||
| IC50 = 39.88 | 3-O-β-D-glucopyranosyl-16-dehydropregnenolone | |||||
| IC50 = 45.21 | Altissimacoumarin C | MTT | Ailanthus altissima, root bark, China | In vitro | [65] | |
| IC50 = 0.54 | Ailanthone | MTT | Jinan University, Guangzhou, China | In vitro | [37] | |
| HepG2 | IC50 = 0.28 | Altissinol A | MTT | Ailanthus altissima, bark, China | In vitro | [56] |
| IC50 = 0.24 | Ailanthone | |||||
| IC50 = 1.15 | 13,18-Dehydroglaucarubinone | |||||
| IC50 = 1.20 | (-)-Cha-parrinone | |||||
| IC50 = 0.55 | 6a-Tigloyloxychaparrinone | |||||
| IC50 = 4.67 | Shinjulactone A | |||||
| IC50 = 1.22 | Altissinol B | |||||
| IC50 = 10.54 | 6a-Tigloyloxychaparrin | |||||
| IC50 = 35.57 | Glaucarubin | |||||
| IC50 = 4.03 | Chouchunsteride A | MTT | Ailanthus altissima, leaf, China | In vitro | [68] | |
| IC50 = 7.62 | 6-dehydropregnenolone | |||||
| IC50 = 13.43 | 20S- Hydroxyergosta-4,6,24(28)-trien-3-one | |||||
| IC50 = 66.47 | (+)-7S,8R-ailanthussin A | MTT | Ailanthus altissima, bark, China | In vitro | [61] | |
| IC50 = 29.53 | (−)-7R,8S-ailanthussin A | |||||
| IC50 = 0.63 | Ailanthone | MTT | Jinan University, Guangzhou, China | In vitro | [37] | |
| HepG2/ADM | IC50 = 4.03 | Chouchunsteride A | MTT | Ailanthus altissima, leaf, China | In vitro | [56] |
| IC50 = 7.62 | 6-Dehydropregnenolone | |||||
| IC50 = 13.43 | 20S- Hydroxyergosta-4,6,24(28)-trien-3-one | |||||
| HL-60 | IC50 = 5.99 | Ailanthone | MTT | Jinan University, Guangzhou, China | In vitro | [38] |
| ACP = 42.02 | Ailanthone (5.00 μM) | Cell apoptosis | ||||
| ACP = 52.05 | Ailanthone (10.00 μM) | |||||
| ACP = 56.69 | Ailanthone (20.00 μM) | |||||
| Huh7 | IC50 = 0.35 | Ailanthone | MTT | Jinan University, Guangzhou, China | In vitro | [37] |
| Ishikawa cell line | IC50 = 3.00 nM | GnRH-PAP | Translation | N/A | In vitro | [81] |
| K562/A02 | IC50 = 2.21 | Ailanthone | MTT | Puruifa Science & Technology Development Co., Chengdu, China | In vitro | [39] |
| MCF-7 | IC50 = 3.90 | Amorphasidase | MTT | Amorpha fruticosa, seed, China | In vitro | [86] |
| IC50 = 1.50 | Dalbin | |||||
| IC50 = 0.45 | 8′-O-β-D-glucopyranosyl-amorphigenin | |||||
| IC50 = 0.95 | Amorphin | |||||
| IC50 = 34.08 | 6′-O-β-D-glucopyranosyl-12a-hydroxydalpanol | |||||
| CV = 64.36 | Ailanthone (0.50 µg/mL) | MTT | Ailanthus altissima, bark, China | In vitro | [60] | |
| CV = 62.48 | Ailanthone (1.00 µg/mL) | |||||
| CV = 57.64 | Ailanthone (2.00 µg/mL) | |||||
| CV = 50.24 | Ailanthone (4.00 µg/mL) | |||||
| CV = 43.24 | Ailanthone (8.00 µg/mL) | |||||
| ACP = 22.68 | Ailanthone (0.50 µg/mL) | Cell apoptosis | ||||
| ACP = 27.99 | Ailanthone (1.00 µg/mL) | |||||
| ACP = 35.88 | Ailanthone (2.00 µg/mL) | |||||
| ACP = 49.77 | Ailanthone (4.00 µg/mL) | |||||
| ACP = 75.51 | Ailanthone (8.00 µg/mL) | |||||
| BBR = 0.53 | Ailanthone (0.50 µg/mL) | Bax/Bcl-2 protein expression levels | ||||
| BBR = 0.56 | Ailanthone (1.00 µg/mL) | |||||
| BBR = 0.80 | Ailanthone (2.00 µg/mL) | |||||
| BBR = 0.93 | Ailanthone (4.00 µg/mL) | |||||
| BBR = 1.25 | Ailanthone (8.00 µg/mL) | |||||
| NCM460 | IC50 = 1.76 | Ailanthone | CCK-8 | Shanghai Yiyan Biotechnology Co., Ltd., Shanghai, China | In vitro | [42] |
| NSCLC Lewis cells | IC50 = 7.70 (24 h) | Ailanthone | MTT | Chengdu Alfa Biological Technology Co., Ltd., Chengdu, China | In vitro | [47] |
| OVCAR3 | IC50 = 34.70 μg/mL | Crude ethanolic | MTT | Ailanthus altissima, bark, South Korea | In vitro | [62] |
| IC50 = 28.00 μg/mL | EtOAc fraction | |||||
| IC50 = 22.50 μg/mL | n-BuOH fraction | |||||
| IC50 = 44.20 μg/mL | Water fraction | |||||
| SKOV3 | IC50 = 34.70 μg/mL | Crude ethanolic | MTT | Ailanthus altissima, bark, South Korea | In vitro | [62] |
| IC50 = 28.00 μg/mL | EtOAc fraction | |||||
| IC50 = 22.50 μg/mL | n-BuOH fraction | |||||
| IC50 = 44.20 μg/mL | Water fraction | |||||
| SW620 | IC50 = 1.01 | Ailanthone | CCK-8 | Shanghai Yiyan Biotechnology Co., Ltd., Shanghai, China | In vitro | [42] |
| Tca8113 | ACP = 17.00 (24 h) | Ailanthone (D = 4.00 µM) | Apoptosis | Jiangxi Herb Tiangong Technology, Jiangxi, China | In vitro | [43] |
| IC50 = 0.79 | Ailanthone | MTT | ||||
| U87MG | ACP = 20.00 (48 h) | Chloroform crude extract | Cell apoptosis | Ailanthus altissima, root, Italy | In vitro | [53] |
| ACP = 8.00 (48 h) | Chloroform fraction | |||||
| ACP = 9.00 (48 h) | 1-methoxy-canthin-6-one isolated from the chloroform fraction | |||||
| U937 | ACP = 19.00 (48 h) | Chloroform crude extract | Cell apoptosis | |||
| ACP = 11.00 (48 h) | Chloroform fraction | |||||
| ACP = 11.00 (48 h) | 1-methoxy-canthin-6-one isolated from the chloroform fraction |
| Activity | Assay | Result | Extract Type/Isolated Compound | Plant Material/Origin | In Vivo/In Vitro | Reference |
|---|---|---|---|---|---|---|
| Neuroprotective | MTT assay on H2O2-induced SH-SY5Y cells | CV = 70.50% | 7S,8R-Guaiacylglycerol-8-acetovanillone ether (50.00 μM) | Ailanthus altissima root bark, China | In vitro | [64] |
| Enzyme inhibition | AChE inhibition | IP = 76.00 | Methanolic crude extract | Amorpha fruticosa, flower, Czech Republic | In vitro | [80] |
| IP = 9.10 | (E)-N6-(Z)-di-p-Coumaroylputrescine (100.00 μM) | |||||
| IP = 1.10 | N1,N6-(E)-di-p-Coumaroylputrescine (100.00 μM) | |||||
| IP = 14.10 | N1-(E)-N5,N10-(Z)-tri-p-Coumaroylspermidine (100.00 μM) | |||||
| IP = 26.60 | N1,N5-(Z)-N10-(E)-tri-p-Coumaroylspermidine (100.00 μM) | |||||
| IP = 47.90 | N1,N5,N10-(E)-tri-p-Coumaroylspermidine (100.00 μM) | |||||
| IP = 17.90 | cis-12a-Hydroxymunduserone (100.00 μM) | |||||
| IP = 37.90 | 6-Deoxyclitoriacetal (100.00 μM) | |||||
| IP = 22.40 | 12α-Hydroxy-α-toxicarol (100.00 μM) | |||||
| IP = 25.43 | Methanolic crude extract (0.17 mg/mL) | Amorpha fruticosa, leaf, Bulgaria | In vitro | [86] | ||
| IP = 48.86 | Amorpha fruticosa, fruit, Bulgaria | |||||
| IC50 = 3.28 | Chouchunionone A | Ailanthus altissima, leaf, China | In vitro | [72] | ||
| IC50 = 16.31 μg/mL | Methanolic crude extract | Ailanthus altissima, bark, Pakistan | In vitro | [69] | ||
| BuChE inhibition | IP = 90.00 | Methanolic crude extract | Amorpha fruticosa, flower, Czech Republic | In vitro | [80] | |
| IP = 8.10 | (E)-N6-(Z)-di-p-Coumaroylputrescine (100.00 μM) | |||||
| IP = 4.80 | N1,N6-(E)-di-p-Coumaroylputrescine (100.00 μM) | |||||
| IP = 4.50 | N1-(E)-N5,N10-(Z)-tri-p-Coumaroylspermidine (100.00 μM) | |||||
| IP = 19.10 | N1,N5-(Z)-N10-(E)-tri-p-Coumaroylspermidine (100.00 μM) | |||||
| IP = 43.80 | N1,N5,N10-(E)-tri-p-Coumaroylspermidine (100.00 μM) | |||||
| IP = 23.80 | cis-12a-Hydroxymunduserone (100.00 μM) | |||||
| IP = 25.60 | 6-Deoxyclitoriacetal (100.00 μM) | |||||
| IP = 37.60 | Amorphispironone (100.00 μM) | |||||
| IP = 23.60 | Tephrosin (100.00 μM) | |||||
| IP = 22.80 | 12α-Hydroxy-α-toxicarol (100.00 μM) | |||||
| Tyrosinase inhibition | IC50 = 20.28 | Chouchunionone C | Ailanthus altissima, leaf, China | In vitro | [72] | |
| Anti-inflammatory | NO production inhibition in LPS-induced RAW 264.7 cells | IC50 = 15.09 | (R)-5-(1-Hydroxyethyl)-canthin-6-on | Ailanthus altissima, stem bark, South Korea | In vitro | [58] |
| IC50 = 9.09 | Canthin-6-one | |||||
| IC50 = 7.73 | 9-Hydroxycanthin-6-one | |||||
| IC50 = 12.01 | 10-Hydroxycanthin-6-one | |||||
| IC50 = 5.92 | Sinapaldehyde | |||||
| IC50 = 10.69 | Erythro-guaiacylglycerol-β-O-41-coniferyl ether | |||||
| IC50 = 63.50 | Canthin-6-one-1-O-b-D-apiofuranosyl-(1->2)-b-D-glucopyranoside | Ailanthus altissima, stem bark, South Korea | In vitro | [57] | ||
| IC50 = 85.00 | Canthin-6-one-1-O-[6-O-(3-hydroxy-3-methylglutaryl)]-b- D-glucopyranoside | |||||
| IC50 = 63.10 | Shinjudilactone | |||||
| IC50 = 5.18 | Ailanthone | |||||
| IC50 = 56.40 | Shinjulactone A | |||||
| IC50 = 72.80 | 4-Hydroxybenzoic acid | |||||
| IC50 = 23.20 | Vanillic acid | |||||
| IC50 = 43.80 | 3-Hydroxy-1-(4-hydroxy-3-methoxyphenyl)-propan-1-one | |||||
| IC50 = 73.40 | p-coumaric acid | |||||
| IC50 = 71.60 | trans-4-O-b-D-Glucopyranosyl ferulic acid | |||||
| IC50 = 21.60 | Syringaresinol | |||||
| COX-2 inhibition in BMMC cells | IC50 = 47.40 µg/mL | Ethanol crude extract | Ailanthus altissima, leaf and branch, South Korea | In vitro | [54] | |
| COX-1 inhibition in BMMC cells | IC50 = 131.66 µg/mL | |||||
| LTC4 suppression in BMMC cells | IC50 = 25.70 µg/mL | |||||
| β-HEX release inhibition in BMMC cells | IC50 = 27.30 µg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nădășan, I.; Babotă, M.; Rusu, A.; Tanase, C. Potential Therapeutic and Medicinal Applications of Four Invasive Non-Native Plant Species: A PRISMA-Guided Systematic Review of PubMed Studies. Plants 2025, 14, 2966. https://doi.org/10.3390/plants14192966
Nădășan I, Babotă M, Rusu A, Tanase C. Potential Therapeutic and Medicinal Applications of Four Invasive Non-Native Plant Species: A PRISMA-Guided Systematic Review of PubMed Studies. Plants. 2025; 14(19):2966. https://doi.org/10.3390/plants14192966
Chicago/Turabian StyleNădășan, Ingrid, Mihai Babotă, Aura Rusu, and Corneliu Tanase. 2025. "Potential Therapeutic and Medicinal Applications of Four Invasive Non-Native Plant Species: A PRISMA-Guided Systematic Review of PubMed Studies" Plants 14, no. 19: 2966. https://doi.org/10.3390/plants14192966
APA StyleNădășan, I., Babotă, M., Rusu, A., & Tanase, C. (2025). Potential Therapeutic and Medicinal Applications of Four Invasive Non-Native Plant Species: A PRISMA-Guided Systematic Review of PubMed Studies. Plants, 14(19), 2966. https://doi.org/10.3390/plants14192966








